Preface
The field of nickel catalysis has made tremendous advances in the past decade. There are several key properties of nickel that have allowed for a broad range of innovative reaction development, such as facile oxidative addition and ready access to multiple oxidation states. In recent years, these properties have been increasingly understood and leveraged to perform transformations long considered exceptionally challenging. Herein, we discuss some of the most recent and significant developments in homogeneous nickel catalysis with an emphasis on both synthetic outcome and mechanism.
Introduction
To the uninitiated, nickel might seem like just the impoverished younger sibling of palladium in the field of transition metal catalysis. After all, the use of palladium-catalyzed cross-coupling has skyrocketed over the past half century: honored with the 2010 Nobel Prize in Chemistry and ubiquitous in applications ranging from complex natural product synthesis to drug discovery to manufacturing. Nickel lies just above palladium in the periodic table, meaning as a group 10 metal it can perform many of the same elementary reactions as palladium or platinum. What, then, is the purpose of using nickel for catalysis? Is the value of nickel, as a common misconception expresses, simply as a low-cost replacement catalyst for cross-coupling reactions? In short, the answer is a resounding negative. Currently, homogenous nickel catalysis is experiencing a period of intensified interest. In this Review, we aim to use recent developments in organonickel chemistry to illustrate how the intrinsic properties of nickel have enabled its use as an effective catalyst for many intriguing, valuable, and difficult transformations.
Historically, the use of nickel in organometallic reactions predates many other examples of transition metal catalysis.1,2 Nickel was isolated in 1751 and named after the “devil Nick ore,” a copper-nickel ore resistant to copper extraction. In the 1890s, Ludwig Mond observed one of the unusual reactivity patterns of nickel: elemental nickel and CO reacted at room temperature to form Ni(CO)4, an extremely toxic, low-boiling liquid, which could be used to purify the metal. Shortly thereafter, Sabatier performed the first hydrogenation of ethylene using nickel, for which he was awarded the 1912 Nobel Prize in Chemistry. But undoubtedly, one of the most prominent and prolific early contributors to organonickel chemistry was Günther Wilke.1 Wilke made seminal contributions in the structure and reactivity of nickel complexes including the synthesis of the ubiquitous Ni(0) source Ni(cod)2, and investigation of olefin oligomerization reactions.
Beginning in the 1970s, nickel found extensive use both for cross-coupling and reactions of alkenes/alkynes such as nucleophilic allylation, oligomerization, cycloisomerization, and reductive coupling. Many excellent books and reviews of organonickel chemistry in general,2 as well as of specific transformations (e.g., reductive coupling3 and cross-coupling4), already exist. Consequently, we have chosen to focus on key advances in nickel-catalyzed reactions since 2005 and to highlight how researchers can take advantage of nickel’s characteristic properties and reactivity to perform innovative and useful transformations. Due to the short nature of this Review and the breadth of nickel chemistry, we are unable to include discussions of all the exemplary nickel catalysis developed in the past decade. However, we hope that the selected reactions and mechanistic studies presented herein spark further investigation into the full range of nickel-catalyzed reactions.
Mechanism and Elementary Steps
Before discussing each class of transformations, a survey of nickel’s characteristic modes of reactivity, particularly in regard to some of the elementary steps of transition metal catalysis (Box 1) is needed. First, nickel is a relatively electropositive late transition metal. Therefore, oxidative addition,5 which results in loss of electron density around nickel and oxidation, for example from Ni(0) to Ni(II), tends to occur quite readily (though conversely, this characteristic makes reductive elimination more difficult).6 This property allows for the use of cross-coupling electrophiles that would be considerably less reactive under palladium catalysis, such as phenol derivatives, 7,8,9 aromatic nitriles, 10 or even aryl fluorides.11
Box 1.
Nickel fundamentals: comparison to palladium and elementary reactions.
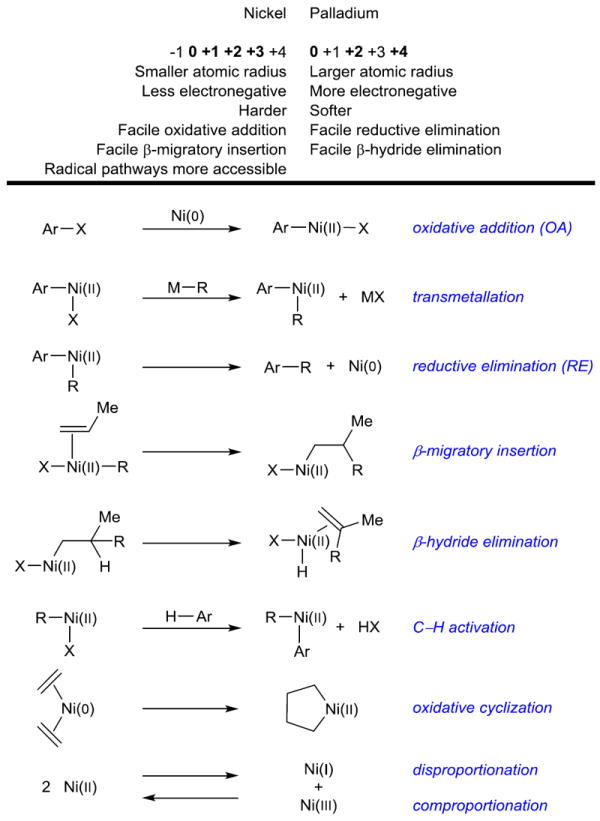
|
Nickel also has a number of readily available oxidation states commonly invoked in catalysis. The majority of palladium-catalyzed reactions are based on a Pd(0)/Pd(II) catalytic cycle, and most often proceed through polar (i.e. non-radical) mechanisms. Likewise, Ni(0)/Ni(II) catalytic cycles are widespread, but the easy accessibility of Ni(I) and Ni(III) oxidation states allows different modes of reactivity and radical mechanisms. As a result, many transformations are based on Ni(I)/Ni(III), Ni(0)/Ni(II)/Ni(I), or even cycles in which nickel remains in the Ni(I) state for the entire catalytic cycle.12
Many nickel complexes have long been known as privileged catalysts for reactions of alkenes and alkynes, such as oligomerization 13 or reductive coupling. Nickel readily donates d-electrons to π-acceptors, so olefin bonding is generally strong.14 β-Hydride elimination tends to be slower with nickel relative to palladium; specifically, the energy barrier to Ni–C bond rotation to form a β-agostic hydride complex is normally significantly higher for comparable nickel than palladium species.15
Finally, there are a few more obvious differences between nickel and its group 10 counterparts. Practically speaking, the cost of nickel in its elemental form is roughly 2,000 times lower than palladium and 10,000 times lower than platinum on a mole for mole basis, though the price of commonly used nickel sources for catalysis can be less favorable. As a first row transition metal, nickel has a small atomic radius, and Ni–ligand bond lengths are often relatively short.16 Researchers have been taking advantage of all of the above features to develop new reactions, which are demonstrated in the specific examples that follow.
Cross-Coupling
Building on discoveries and developments from the 1970s, nickel has proven to be an extremely effective catalyst for Suzuki–Miyaura and Negishi cross-couplings, in particular.17 However, as will be demonstrated in the following sections, catalyst systems based on nickel have been developed for an incredible variety of transformations.
Cross-Coupling of Aryl Halides
The ability of nickel to oxidatively insert into carbon–heteroatom bonds with ease is of particular advantage in the Suzuki–Miyaura reaction of heteroaromatic boronic acids, which readily undergo protodeboronation under the basic (and usually hydrous) reaction conditions, particularly at elevated reaction temperatures. One useful contribution comes from Hartwig and Ge, who have developed a method for the synthesis of heterobiaryls that takes advantage of this rapid oxidative addition by combining a lowered reaction temperature with readily-activated precatalyst 3 to afford the traditionally difficult to access heterobiaryls (Figure 1a).18 Additionally, precatalyst 3 possesses adequate air-stability to be handled in air, and only 0.5 mol % of the precatalyst is required, making this a highly efficient reaction. This method is applicable to both heteroaryl chlorides and bromides and gives high yields of products across an impressive range of substrates. A related method for the synthesis of heterobiaryls was disclosed by Garg and coworkers in 2013 (Figure 1b).19 This method focused on further improving the efficiency of Suzuki–Miyaura couplings as well as employing green solvents such as 2-MeTHF and t-amyl alcohol.
Figure 1. Recent nickel-catalyzed Suzuki–Miyaura arylations.
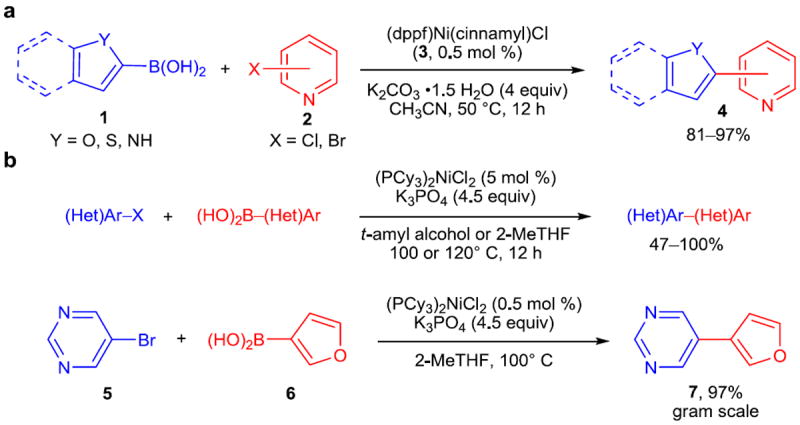
a, Cross-coupling of heteroaryl boronic acids (1) with heteroaryl halides (2) to form heterobiaryls (4) is a longstanding challenge. This method, employing an air-stable nickel catalyst precursor 3, provides the desired heterobiaryls in excellent yields. b, Cross-couplings developed for small-scale use are often carried out in solvents poorly suited to industrial or large-scale use. As such, the adaptation of the Suzuki–Miyaura cross-coupling to form (hetero)biaryls such as 7 using green solvents while still obtaining the products in high yield is a valuable development. dppf, 1,1’-bis(diphenylphosphino)ferrocene; cinnamyl = trans-C6H5CHCHCH2-; (Het)Ar, heteroaryl; Me, methyl; THF, tetrahydrofuran; Cy, cyclohexyl.
Cross-Coupling of Phenol Derivatives
Although using aryl halides as cross-coupling partners is the de facto standard, accessing the desired halide coupling partner is not always trivial and can sometimes be extremely challenging. One well-established solution is the use of aryl triflates (Figure 2a). They typically possess extremely high reactivity in cross-coupling reactions, and because they are obtained by reaction of a phenol with triflic anhydride, are derived from an entirely separate pool of materials than aryl halides. For these two reasons, triflates are valuable coupling partners. However, triflates are prone to hydrolysis, especially under basic conditions, making their use for certain reactions challenging. Tosylates and mesylates (Figure 2b), close relatives of triflates, have found use in cross-coupling reactions, as their greater stability reduces or eliminates the problem of hydrolysis. However, their reactivity towards oxidative addition by metals is also considerably reduced, often leading to the need for harsher reaction conditions. For these reasons, catalyst systems capable of activating the C–O bonds of functional groups other than triflates, such as ethers, esters, carbamates, and carbonates (Figure 2c) are highly desired. Reactions based on such catalyst systems would represent an ideal combination of ready access to coupling partners and good reactivity of the electrophilic coupling partner.
Figure 2. Halogen alternatives used in cross-coupling reactions.
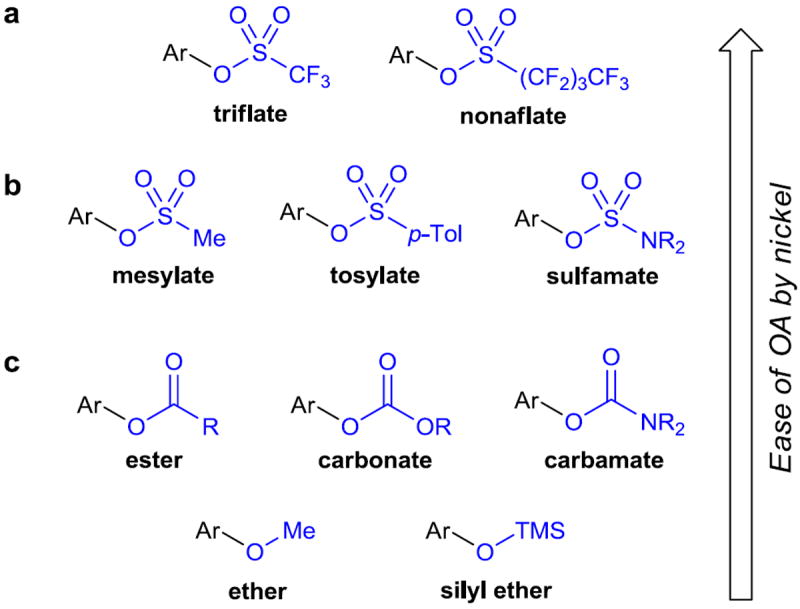
a, Aryl triflates have long been used as replacements for halogens in cross-coupling reactions. Aryl nonaflates were developed later to address some of the issues encountered when working with aryl triflates, but their use is less widespread. b, The use of aryl mesylates, tosylates, and sulfamates presents many advantages over triflates and related fluorinated sulfonates due to their increased stability. c, Like sulfonate derivatives, the use of carboxylic esters, carbonates, carbamates, ethers, and silyl ethers can be advantageous in many situations. Ar, aryl; p-Tol, para-tolyl (4-methylphenyl); TMS, trimethylsilyl.
Nickel has been known to activate C–O bonds since as early as 1977,20 and since this time, a number of advancements to this field have been reported. For example, Kocienski and Dixon developed the first effective Ni(0)-catalyzed cross-coupling of vinyl carbamates with organomagnesium reagents in 1989,21 and several years later Snieckus and coworkers described the Ni(0)-catalyzed cross-coupling of aryl carbamates and organomagnesium reagents in concert with further functionalization by directed ortho-metallation.22 Subsequently, Snieckus also reported the Ni(0)-catalyzed cross-coupling of tertiary aryl sulfamates with organomagnesium reagents.23
Around this time, the field experienced an increased interest in the nickel-mediated activation of these “inert” C–O bonds. A seminal development in the field came from Dankwardt,24 which described the cross-coupling of aryl ethers (8) with arylmagnesium reagents (9) in a Kumada–Corriu-type coupling (Figure 3a). Another critical advance in this area of research came from Chatani and coworkers in 2008, when they reported the first Suzuki–Miyaura cross-coupling using aryl ethers (8) and boronic esters (10) (Figure 3b).25 Previously, all couplings of this type had employed Grignard reagents, which are generally poorly compatible with many common functional groups; the change to boronic esters, however, provided a considerable improvement to the substrate scope and allowed much easier implementation of this chemistry.
Figure 3. Milestones in cross-coupling reactions of aryl ethers and esters.
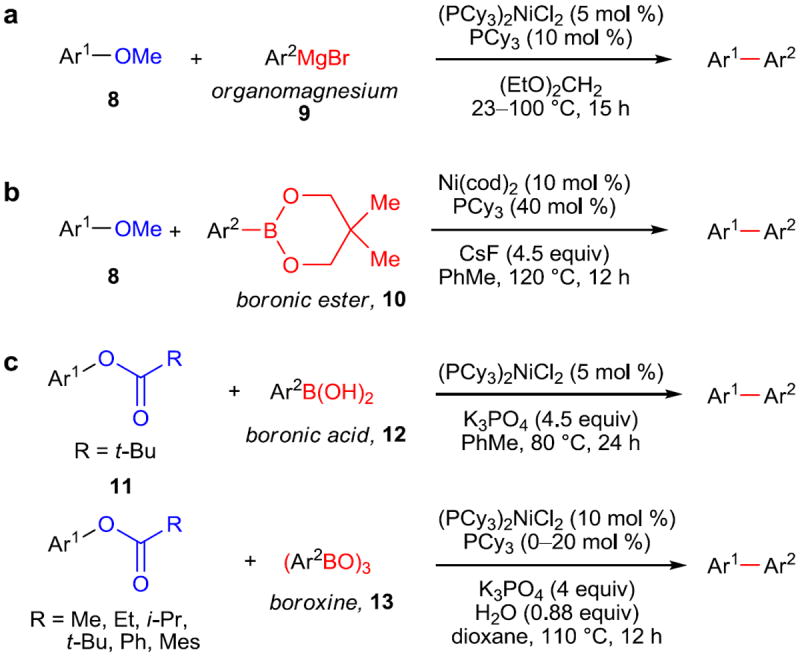
a, Negishi-type, nickel-catalyzed biaryl formation from aryl ethers (8) and organomagnesium (Grignard) reagents (9). b, Suzuki–Miyaura-type, nickel-catalyzed biaryl synthesis from aryl ethers (8) and boronic esters (10). c, Suzuki–Miyaura-type, nickel-catalyzed biaryl synthesis utilizing aryl esters (11) and aryl boronic acids (12) or aryl boroxines (13). Ph, phenyl; t-Bu, tert-butyl; Et, ethyl; cod, 1,5-cyclooctadiene; i-Pr, isopropyl; Mes, 2,4,6-trimethylphenyl.
In 2008, in nearly simultaneous reports, Shi and coworkers reported the Suzuki–Miyaura cross-coupling of aryl acetates and pivalates with boroxines (13),26 while Garg and coworkers reported the Suzuki–Miyaura cross-coupling of aryl pivalates (11) with boronic acids (12) (Figure 2c).27 Both methods are capable of producing a wide variety of biaryls, demonstrating their viability as valuable alternatives to the use of traditional aryl halides and sulfonate esters. Following these initial reports, it was demonstrated that organozinc reagents28 and aryl alkoxides29,30 can be used, further expanding the range of available coupling partners. Additional advances were realized through a collaborative experimental and theoretical investigation carried out by Houk, Sneickus, Garg, and coworkers.31
In the period since these seminal reports, many fruitful developments have been made to allow the transformation of many types of readily accessible phenol derivatives into valuable biaryls,32 and other types of reactions beginning from phenol derivatives have since followed, such as the Mizoroki–Heck reaction reported by Watson and coworkers.33 Aside from C–C bond forming reactions, methods for the amination34,35 and reduction12,36 of phenol derivatives have been disclosed.
Benzylic Cross-Coupling
Another mode of bond activation that has come to prominence, at least in part thanks to nickel catalysis, is the activation of benzylic C–O bonds.37 Benzylic ethers, esters, carbonates, carbamates, and in some instances, even free alcohols (via the corresponding magnesium alkoxide) can be activated by low-valent nickel. With proper choice of starting material and organometallic reagent, the reaction products can be di- or triarylalkanes, both of which are ubiquitous motifs in drug targets, natural products, and materials applications. Perhaps the most significant feature of these transformations is their high stereospecificity, which allows access to these products in highly enantioenriched form from the (readily available) corresponding enantioenriched mono- or diarylmethanol.
In 2011, Jarvo and coworkers disclosed the first stereospecific nickel-catalyzed alkyl–alkyl cross-coupling reaction (Figure 4a).38 This method, in contrast to previous nickel-catalyzed cross-couplings using sp3 electrophiles, does not racemize the alkyl electrophile and in this instance provides clean inversion of the starting stereochemistry. Therefore, the existing stereochemistry of the starting material (14) can be used as the only source of chiral information, rather than relying on catalyst control with or without a directing group that must be later removed from the molecule. Jarvo demonstrated the synthesis of several interesting and useful molecules using this method, including the anti-cancer agent 16 from the corresponding methyl ether. The product was obtained with 96% ee beginning from material of 98% ee, which demonstrates the extremely high stereospecificity of this method. A subsequent publication demonstrated the use of the 2-methoxyethyl ether moiety as a directing/activating group, which greatly increases the ease with which oxidative addition into the C–O bond takes place.39
Figure 4. Reactions of benzylic alcohols and alcohol derivatives.
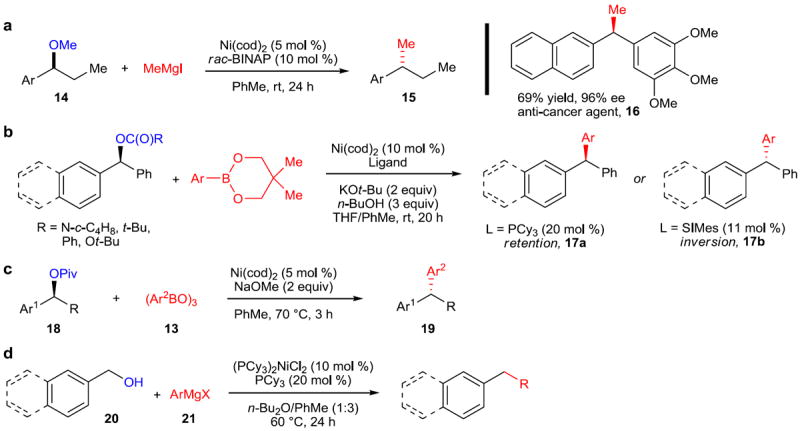
a, Stereospecific methylation of benzylic ethers. A nickel catalyst comprising Ni(cod)2 and rac-BINAP was found to catalyze the methylation of benzylic methyl ethers (14) to form alkyl-substituted arenes (15). A modification for the synthesis of diarylethanes was also devised, allowing the synthesis of the anti-cancer agent 16 in 69% yield and 96% ee. b, A Suzuki–Miyaura-type arylation of benzylic esters, carbonates, and carbamates. The synthesis of triarylmethanes (17) can be achieved by catalytic Ni(cod)2 and PCy3 or SIMes—the stereoselectivity (retention or inversion) is determined by the identity of the ligand. c, A phosphine- and carbene-free nickel catalyst was also developed, yielding inversion of the stereochemistry of benzylic pivalates (18) to provide access to diarylalkanes (22). d, Cross-coupling of free benzylic alcohols. An excess of organomagnesium reagent (21) can be added to form a magnesium alkoxide, which is then a competent coupling partner for the Kumada-type coupling with organomagnesium reagents. rac, racemic; BINAP, 2,2’-bis(diphenylphosphino)-1,1’-binaphthyl; SIMes, (1,3-Bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidene); Bu, butyl; Piv, pivaloyl.
Furthermore, in a subsequent publication, the Jarvo group demonstrated the Suzuki–Miyaura cross-coupling of benzylic esters, carbonates, and carbamates with arylboronic esters (Figure 4b).40 A striking feature of this method is that it affords retention (17a) or inversion (17b) of the starting stereochemistry based on the ligand employed—the use of tricyclohexylphosphine provides retention of stereochemistry, whereas using an N-heterocyclic carbene ligand (SIMes) provides inversion. In both cases, the enantiospecificity is greater than 97% across a wide range of substrates. Simultaneous with the disclosure from the Jarvo group, Watson and coworkers published a closely related method for the synthesis of diarylalkanes and triarylmethanes beginning from benzylic pivalates (18) and boroxines (13) (Figure 4c).41 In contrast to Jarvo’s method, however, it was found that no external phosphine or carbene ligand was necessary to obtain good stereospecificity—Ni(cod)2 alone was demonstrated to be an effective catalyst and provided inversion of configuration at the benzylic position.
In 2012, Shi and coworkers reported the direct cross-coupling of benzylic alcohols (20) with Grignard reagents (21) to provide diarylmethanes or alkyl arenes with a catalyst system comprised of (PCy3)2NiCl2 and PCy3 (Figure 4d).42 The magnesium alkoxide, the active coupling partner, is pre-formed by addition of MeMgBr, which is also used to activate the Ni(II) precatalyst by reducing it to Ni(0) via a sequence of two successive transmetallations and reductive elimination of ethane. Subsequent addition of the Grignard reagent initiates the reaction to form the desired coupling product.
While all reactions so far described involve cleavage of C–O bonds, benzylic halides are also useful substrates for this type of activation. One clever example is from Martin and coworkers, which transformed benzylic halides to the corresponding phenylacetic acids using carbon dioxide as the carbon source.43 Subsequently, this methodology was extended to aryl and benzylic pivalates to synthesize benzoates and phenylacetic acids.44 Additionally, the activation of benzylic ammonium salts has recently been demonstrated.45
Cross-Coupling of Aziridines
The activation of C–N bonds, specifically those of aziridines, by zerovalent nickel has been known for a number of years. 46 This activation is facile, and intriguingly, it can be reversed upon exposure to molecular oxygen to again afford the original aziridine. However, it was not until a full decade later that this mode of activation was successfully incorporated into a catalytic coupling reaction. Doyle and coworkers succeeded in coupling alkyl and arylzinc halides (23) to styrene-derived N-tosyl aziridines (22) to yield aziridine ring opening with excellent specificity for addition at the more substituted position of the aziridine (24) (Figure 5a).47 Critical to the success of this approach was the use of dimethyl fumarate as an additive in place of the phosphine, carbene, and/or amine ligands often used in nickel catalysts. Dimethyl fumarate is believed to accelerate reductive elimination through π-coordination to nickel. Alkyl-substituted aziridines, unfortunately, were found to be unsuitable substrates for this set of conditions. However, the use of the dual-purpose cinsyl group, which functions both as a removable protecting group and a directing group, enabled the use of aliphatic aziridines (25).48 In this way, good selectivity (2.5:1 to 4.7:1) for substitution at the less substituted position could be achieved with moderate to high yields (Figure 5b).
Figure 5. Nickel-catalyzed Negishi-type cross-coupling of aromatic and aliphatic aziridines.
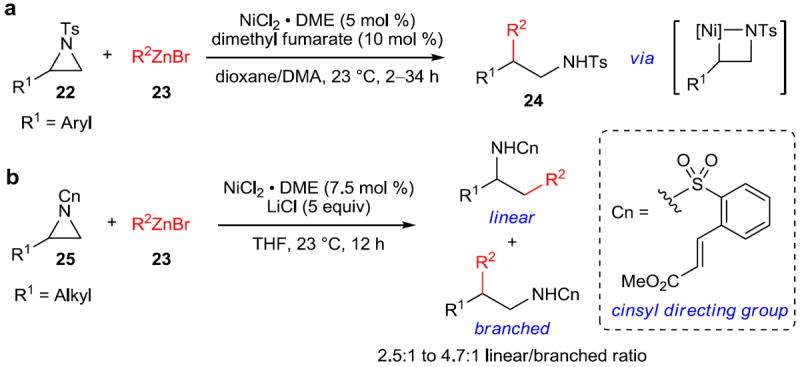
a, Nickel-catalyzed addition of organozinc halides to styrenyl aziridines (22) occurs with incorporation of the nucleophile (23) at the substituted position of the aziridine to furnish β,β-disubstituted amines (24). b, Nickel-catalyzed addition of organozinc halides (23) to aliphatic aziridines (25) directed by the cinsyl group, which imparts a preference for addition at the less substituted position of the aziridine. Ts, (4-methyl)phenylsulfonyl; DME, 1,2-dimethoxyethane; DMA, dimethylacetamide.
Cross-Coupling of sp3 Electrophiles
The utility of cross-coupling of aryl or vinyl electrophiles is unquestioned with regard to scope, functional group compatibility, and relevance to the arene-rich pharmacophores ubiquitous in modern drug molecules. However, the number of Csp3–Csp3 linkages found in natural products and other complex organic molecules far outstrips the number of arene carbon linkages. However, Csp3–Csp3 bond forming reactions, particularly those involving tertiary or quaternary stereocenters, can be challenging, even with modern methods, though are essential for building up molecular complexity. In the past decade, an enormous amount of progress has been made toward an ultimate goal of the capability to form aliphatic carbon–carbon bonds stereoselectively at will, just as the formation of biaryl or aryl–heteroatom bonds has already been revolutionized by the field of cross-coupling.
Some key challenges are associated with any cross-coupling in which the electrophile (i.e. the component that undergoes oxidative addition to the metal catalyst) is sp3 hybridized.49,50 First, oxidative addition can be difficult, given that Csp3–X bonds are more electron-rich than Csp2–X bonds. For primary electrophiles, oxidative addition can proceed by an SN2-like inversion pathway, but for secondary or tertiary electrophiles, this pathway is very slow.51 Then, once the carbon–metal bond has been formed, the challenge is to suppress intramolecular β-hydride elimination, which would produce an alkene product. Transmetallation and/or reductive elimination, therefore, must proceed more rapidly than β-hydride elimination. Cases in which β-hydrogens are not available or of the correct geometry for elimination, such as in benzyl or adamantyl electrophiles, as discussed previously, are also known but do not represent the same level of difficulty.
Despite these difficulties, reactions of sp3 electrophiles were investigated in the early days of cross-coupling.52 Suzuki reported the first Pd- or Ni-catalyzed Csp3–Csp3 cross-coupling reaction, primary alkyl iodides with alkyl boranes using Pd(PPh3)4, but significant amounts of reduction and elimination products were also formed (reduction/elimination/desired coupling: 27:9:50).53 Knochel then showed that nickel could be used to successfully couple primary iodides with organozinc reagents using either an intramolecularly tethered alkene54 or exogenous electron-poor alkene55 to facilitate reductive elimination. Kambe reported an olefin-assisted Kumada coupling of primary alkyl halides and tosylates, proposed to proceed via a bis(η3-allyl)nickel catalyst formed by the coupling of two equivalents of butadiene.56
However, the modern era of Csp3–Csp3 cross-coupling began with a seminal paper by Fu and coworkers in 2003, in which the coupling of secondary alkyl bromides with β-hydrogens and organozinc reagents was reported (Figure 6a). 57 The transition from primary electrophiles used previously to secondary electrophiles was groundbreaking, since it opened the possibility for asymmetric synthesis of tertiary or quaternary all-carbon stereocenters. The chelating tridentate PyBOX ligand (28) was found to be essential, likely in order to slow the rate of β-hydride elimination, which would require a vacant coordination site. In addition to the Negishi reaction, the cross-coupling of secondary electrophiles with aryl and vinyl boronic acids,58 aryl silicon reagents,59 and aryl tin reagents60 have been disclosed. Cross-coupling reactions with a variety of Csp3–metal reagents can also be accomplished using this chemistry, for example the mild Suzuki coupling carried out at room temperature shown in Figure 6b.61 Finally, Fu and coworkers provided the first examples of the cross-coupling of a tertiary alkyl electrophile (e.g., 32) with B2pin2 (Figure 6c) 62 and later in a Suzuki reaction.63 In stark contrast, palladium has only been reported to cross-couple secondary sp3 electrophiles in a handful of very specialized cases.64
Figure 6. Key representative examples of cross-coupling reactions involving oxidative addition to sp3 carbon electrophiles.
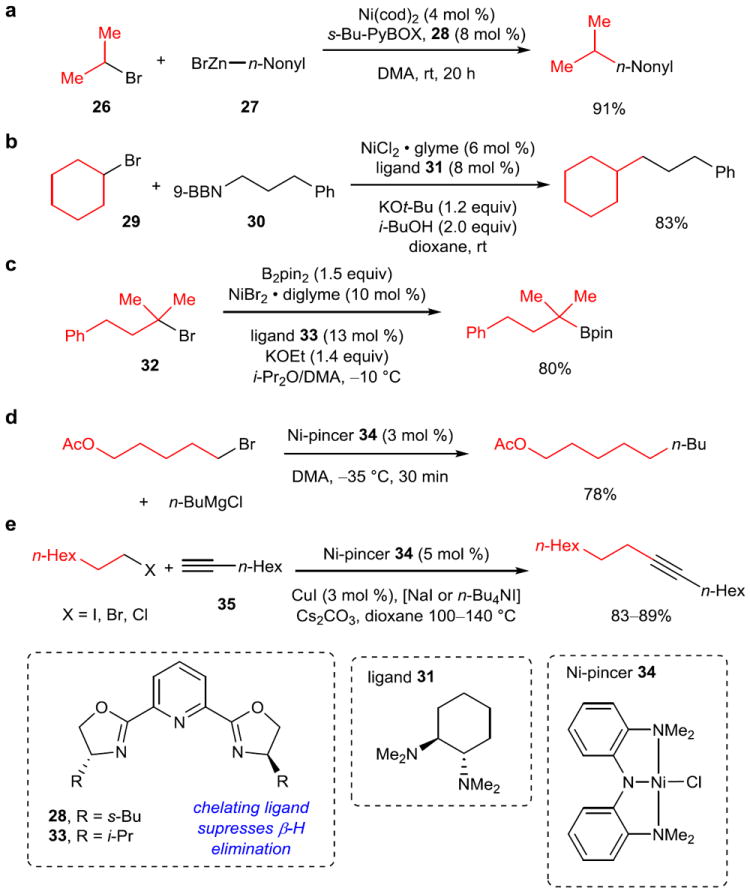
a, The first example of a Csp3–Csp3 cross-coupling reaction using a secondary electrophile. Secondary bromides and iodides, such as 26, were coupled in a Negishi reaction with primary alkyl zinc reagents, such as 27. It was proposed the chelating PyBOX ligand (28) blocks the open coordination site needed for undesired β-hydride elimination. b, A mild (room temperature) Suzuki reaction of secondary bromides (29) and primary alkylboranes (30). Previously used bipyridyl or PyBOX ligands were unable to promote the transformation, so diamino ligand (31) was utilized. c, The first cross-coupling reaction to use an unactivated tertiary electrophile (32). In contrast to previous results, tertiary electrophiles reacted with faster rates than secondary or primary electrophiles. d, The Kumada coupling of primary alkyl bromides and iodides, and some secondary alkyl iodides, with Grignard reagents was accomplished with the Ni-pincer complex 34 with an amidobis(amine) ligand. Low temperatures allow for a wide range of functional groups (ketones, esters, etc) to be tolerated. e, The first example of a Ni-catalyzed Sonogashira reaction. β-Hydrogen-containing alkyl iodides, bromides, and chlorides could be used as the electrophile with a variety of terminal alkynes (35). 9-BBN, 9-borabicyclo[3.3.1]nonane; pin, pinacolato; glyme, bis(2-methoxyethyl) ether; Pr, propyl; Hex, hexyl.
Preliminary mechanistic investigations of these reactions are consistent with an inner-sphere electron-transfer pathway for oxidative addition. For these coupling reactions, Fu proposes a Ni(I)/(III) cycle with a radical oxidative addition pathway (Figure 7a), although the Vicic group has demonstrated that with redox active ligands such as terpyridine, the transmetallated species prior to oxidative addition is perhaps better thought of as Ni(II) cationic species 39 bound to reduced radical anion ligands (Figure 7a, in brackets). 65 However, the first isolable and well-characterized Ni(III) complex arising from a Ni(I)/Ni(III) oxidative addition was also recently reported.66 In any case, these mechanistic manifolds clearly demonstrate the ability of Ni to access various oxidation states in order to facilitate reactions otherwise inaccessible with group 10 metals.
Figure 7. Proposed mechanisms for Csp3–Csp3 cross-coupling.
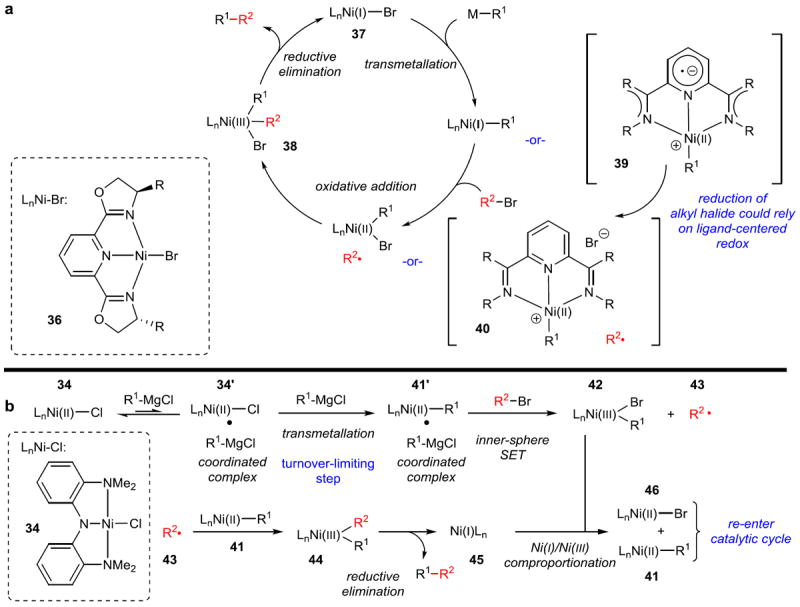
a, Mechanism proposed for Fu-type cross-coupling reactions in which ligands are typically PyBOX or similar as shown (36). A Ni(I) complex (37) undergoes transmetallation, then a radical oxidative addition of the electrophile, to eventually form a Ni(III) complex (38), which can reductively eliminate the coupled product. Nickel complexes with redox active ligands have been shown to be perhaps better thought of as the species in brackets (39, 40), in which the oxidative addition to the electrophile proceeds through ligand-centered rather than metal-centered redox. Ni(II) compounds are used as precatalysts, allowing for reactions to be set up outside of the glovebox. Reduction to Ni(I) presumably occurs prior to the beginning of the catalytic cycle via reduction of Ni(II) to Ni(0) (transmetallation/reductive elimination), then comproportionation of Ni(0)/Ni(II). b, Mechanism proposed for the Csp3–Csp3 Kumada cross-coupling with Ni-pincer 34. Extensive mechanistic studies have shown that a more complex bimetallic oxidative addition is operative, in which a Ni(II) complex bound to an equivalent of Grignard reagent (34’) is the active complex for the turnover-limiting transmetallation. SET, single electron transfer.
Another prominent contributor to the field of Csp3–Csp3 cross-coupling has been Xile Hu, who developed Ni-pincer complex 34.67 This complex has proven catalytically active for Csp3–Csp3 Kumada cross-couplings (Figure 6d)68 as well as the Sonogashira coupling of alkynes with primary alkyl iodides, bromides, and chlorides (Figure 6e).69 Extensive mechanistic investigation has been conducted with the former reaction (Figure 7b), revealing a number of interesting features.70 The active complex for the turnover-limiting step of transmetallation appears to be the Ni-pincer complex coordinated to the Grignard reagent (34’). A bimetallic oxidative addition of the primary alkyl halide occurs via a persistent alkyl radical (43), which reacts with a different Ni(II)-alkyl complex (41), and then undergoes reductive elimination to form the product. The remaining unstable Ni(I) complex (45) transfers an electron to a Ni(III) complex (42) to form two Ni(II) complexes (41, 46), which can rejoin the catalytic cycle.
These examples of cross-coupling are impressive, but the promise of secondary Csp3–Csp3 cross-couplings lies in asymmetric reactions providing tertiary and quaternary stereocenters with high enantioselectivity. The Fu group has published numerous examples of this type of reactivity. These approaches rely on a tethered directing group that can coordinate to the nickel catalyst upon oxidative addition to form a rigid complex such that, upon reductive elimination, enantioenriched products are generated (Figure 8). Since the electrophile likely undergoes a radical oxidative addition, both enantiomers of the starting alkyl halide are converted through a common planar radical intermediate. Therefore, a racemic alkyl halide can be used to produce a highly enantioenriched product.
Figure 8. Asymmetric Csp3–Csp3 cross-coupling reactions.
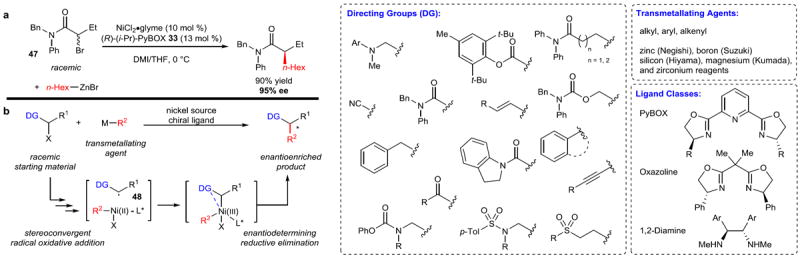
a, The first example of an asymmetric Csp3–Csp3 cross-coupling between a racemic α-bromoamide (47) and aliphatic organozinc reagent. b, Proposed mechanism for the coupling reaction. A racemic aliphatic halide adjacent to a directing group (in box) undergoes oxidative addition. Since it is proposed to proceed an unligated aliphatic radical (48) as shown in Figure 7a, the overall oxidative addition is stereoconvergent, and thus the chiral ligand on nickel dictates the stereochemistry of the product. Many classes of directing groups and transmetallating reagents have been successfully reacted, generally relying on one of the three chiral ligand classes shown in the box. Bn, benzyl; DMI, 1,3-dimethyl-2-imidazolidinone.
The first example of an asymmetric secondary Csp3–Csp3 cross-coupling was published in 2005: a Negishi reaction of α-bromo amides (47) with organozinc reagents (Figure 8a).71 The use of an (R)-PyBOX ligand (33) provided good yields and excellent enantioselectivities (>90% ee in nearly all cases). These conditions not only conferred good enantioselectivity, but also provided selectivity for oxidative addition to the α-bromide in the presence of other primary or secondary alkyl bromides.
Although amides are useful building blocks for further synthetic elaboration, the need for a directing group does limit the generality of this approach. The Fu group has shown that a wide variety of directing groups can be used (Figure 8b). Not only do various coordinating functional groups such as amides, esters, sulfones, and alkenes work well under these reactions, but the tether length can vary as well. In fact, the Fu group has shown that an amide in conjunction with the chiral nickel catalyst can confer high levels of enantioselectivity even when the halide is in the δ-position, a full five atoms away from the amide nitrogen.72 Doubtless, as it develops, the asymmetric cross-coupling reaction will become more and more general and find use in applications of challenging bond formations in the synthesis of natural products and other more complex molecules.73,74
Reductive Cross-Coupling
Traditional cross-coupling reactions join electrophilic (e.g., aryl bromide) and nucleophilic (e.g., aryl zinc) components. However, nucleophilic reagents can be unstable, costly, or cause undesired side reactions. In contrast, reductive cross-coupling reactions join two electrophilic components without the formation of a stoichiometric organometallic species. The challenge inherent in reductive cross-coupling processes is the differentiation between the two electrophiles in order to suppress homodimerization of either component.
Recently, Weix and coworkers demonstrated an impressive solution to these difficulties by performing the first catalytic reductive cross-coupling to show high selectivities for cross-coupled products over dimerization without resorting to the use of a large excess of one of the components (Figure 9a).75 By coupling an aryl iodide (49) and an alkyl iodide (50) electrophile with NiI2·xH2O and a bipyridyl and phosphine ligand, as well as a stoichiometric manganese reducing agent, high reactivity and selectivity for cross-coupled products were observed. Later, a range of aryl bromides and chlorides and alkyl bromides were shown to be reactive with zinc as the reducing agent.76 Though Zn(0) is present, no organozinc species are formed; rather, zinc (or manganese) acts as a reductant directly to the nickel center, accounting for the excellent functional group compatibility.
Figure 9. Reductive cross-coupling reactions.
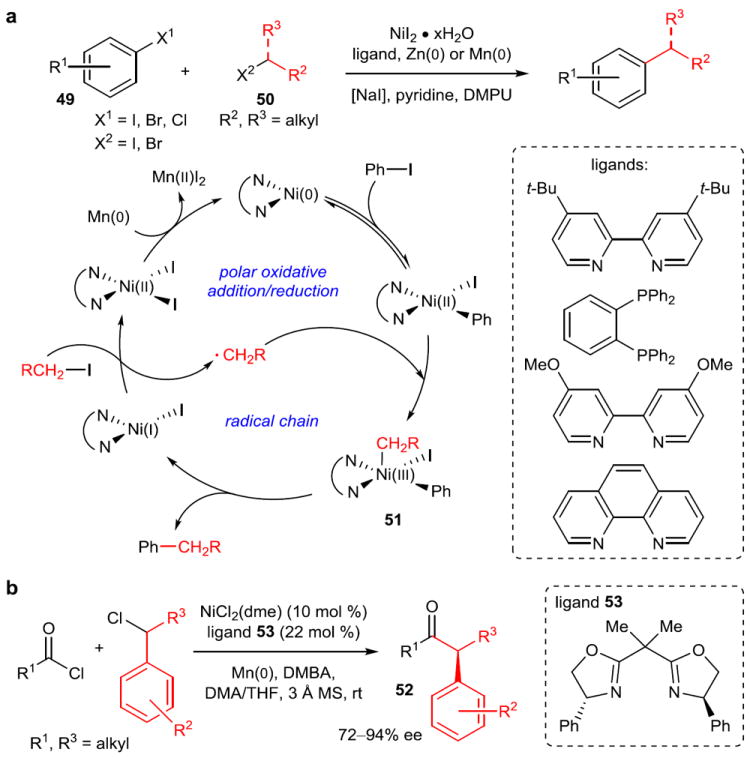
a, The reductive cross-coupling reaction of an aryl halide (49) with an alkyl halide (50) without the intermediacy of an organozinc or organomanganese species. Extensive mechanistic studies have suggested that this method combines both polar (aryl halide) and radical chain (alkyl halide) formal oxidative addition mechanisms. Because the oxidation state of nickel is matched to each electrophile, homodimerization is suppressed. For details on possible methods of radical chain initiation, see reference 77. b, First asymmetric acyl reductive cross-coupling. High enantioselectivities are obtained with bisoxazoline ligands such as 53. DMPU, 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; DMBA, 2,6-dimethylbenzoic acid; MS, molecular sieve.
After extensive mechanistic investigation, Weix proposes the mechanism in Figure 9a.77 Nickel undergoes a polar oxidative addition to the aryl halide followed by a radical chain generation of an alkyl radical. This radical then undergoes coordination to form Ni(III) species 51 competent for reductive elimination. In this way, the full potential of readily available nickel oxidation states (vide supra) is realized, and the use of alkyl electrophiles is allowed. Additionally, the properties of the halides are matched to the capabilities of the catalyst to avoid side reactions: aryl halides more readily undergo polar oxidative addition (Ni(0)/Ni(II)), and alkyl radicals are more stable than aryl radicals (Ni(I)/Ni(III)). Close comparison of this mechanism with the one shown in Figure 7b also demonstrates that a number of complex elementary steps are available with radical pathways, and the factors that lead to certain patterns of reactivity have not been fully understood.
The Reisman group has also developed an enantioselective reductive coupling that affords α,α-disubstituted ketones (52) from the coupling of acyl chlorides and secondary benzylic chlorides (Figure 9b).78 Although extensive mechanistic investigations have not been carried out, Reisman also proposes a mechanism in which Mn(0) acts as a direct reductant to Ni. In any case, further developments of asymmetric reductive cross-coupling would be a valuable alternative to the use of traditional transmetallating agents for cross-coupling.
C–H Activation
C–H activation, the direct functionalization of a hydrocarbon, presents several potential advantages over traditional cross-coupling in that pre-functionalization of the substrate, for example with a halogen, is not necessary. At the same time, however, controlling site-selectivity with C–H activation can be very challenging, as molecules often contain many C–H bonds with similar chemical properties. Though examples of nickel-mediated C–H activation date to at least 1963,79 the development of catalytic C–H activation methods using nickel are more recent.
One such example comes from the research group of Itami, which describes the decarbonylative coupling of aryl and heteroaryl esters (55) and (benz)oxazoles (54) (Figure 10a).80, 81 This reaction provides an unusual means of arylating azoles, and adds to the existing decarbonylative/decarboxylative approaches for C–H arylation. The yields of the isolated products are good to excellent across a wide range of substituted esters and azoles, and furthermore, the air-stable precatalyst (dcype)Ni(CO)2 (56) can be used in place of Ni(cod)2/dycpe, making this reaction operationally simple. Itami used this method to execute an expedient formal synthesis of muscoride A (58) in excellent yield. Furthermore, a subsequent report from Itami and coworkers describes the isolation of the key arylnickel(II) pivalate intermediate formed in couplings of this type.82
Figure 10. Selected examples of nickel-catalyzed C–H activation reactions.
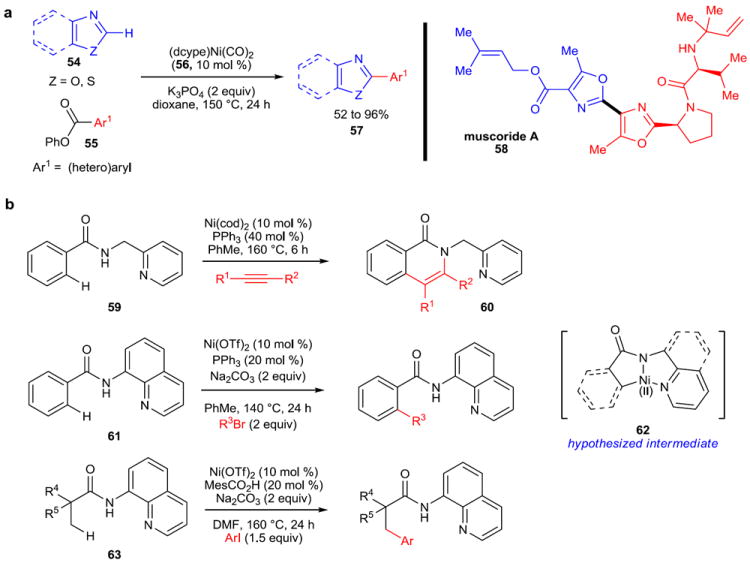
a, Benzoxazoles and benzothiazoles (54) are useful substrates for this nickel-catalyzed C–H activation reaction, which uses aryl esters (55) as the electrophilic coupling partners to produce (hetero)biaryls (57). This methodology was applied to the formal synthesis of muscoride A (58) to great effect. b, Nickel-catalyzed, chelation-assisted C–H activation reactions have recently been developed. These reactions rely on a directing group to facilitate addition of nickel into the C–H bond in the ortho position of a benzamide (59, 61) or into the C–H bond of an adjacent aliphatic substituent (63). dcype, 1,2-bis(dicyclohexylphosphino)ethane; OTf, triflate (trifluoromethanesulfonate); DMF, dimethylformamide.
In 2011, Chatani and coworkers disclosed the first example of a nickel-catalyzed, chelation-assisted C–H activation reaction.83 The reaction, an oxidative cycloaddition of alkynes to aromatic amides to form 1-isoquinolones 60 (Figure 10b), relies on a 2-pyridylmethyl group on the amide nitrogen to function as a directing group. Subsequently, in 2013, Chatani and Tobisu published the ortho C–H activation of a similar aryl amide system (61)84 and the arylation of aliphatic C–H substituents (62),85 further expanding the repertoire of nickel-catalyzed C–H activation reactions. All three reactions are hypothesized to occur by pre-coordination of nickel to the 1,2-diamine moiety of the directing group, followed by metallation to form 62, after which the reaction paths diverge.
Heck Reaction
Many of the advantages of nickel catalysis can also be applied to the Heck reaction.86 The Heck reaction is similar to cross-coupling reactions, but rather than undergo transmetallation, the oxidative addition complex coordinates an alkene. Subsequent migratory insertion and β-hydride elimination then furnishes a more substituted alkene. Computational work comparing Ni and Pd for use in the Heck reaction has been carried out, with Guo and coworkers proposing lower energy barriers for oxidative addition and migratory insertion for Ni in contrast to faster β-hydride elimination and catalyst regeneration for Pd.15 However, relatively little has been done to truly capitalize on these features until quite recently.
Questions of regioselectivity are critical in the Heck reaction, since in theory migratory insertion can occur at either end of the alkene coupling partner. Traditionally, electron-poor alkenes such as styrenes and acrylates have been used, which confer high selectivity for the electrophilic addition to the terminal position of the olefin. Conditions under which a cationic Pd or Ni species is formed by dissociation of the halide or triflate component, rather than the phosphine, after oxidative addition can provide high selectivity for electron-rich alkenes.87 An example of the latter using Ni-catalysis is given by Skrydstrup and coworkers who demonstrated good selectivity for coupling of aryl triflates (64) and enol ethers (65), which following subsequent hydrolysis formed methyl ketones (66) (Figure 11a).88
Figure 11. Nickel-catalyzed Heck reactions.
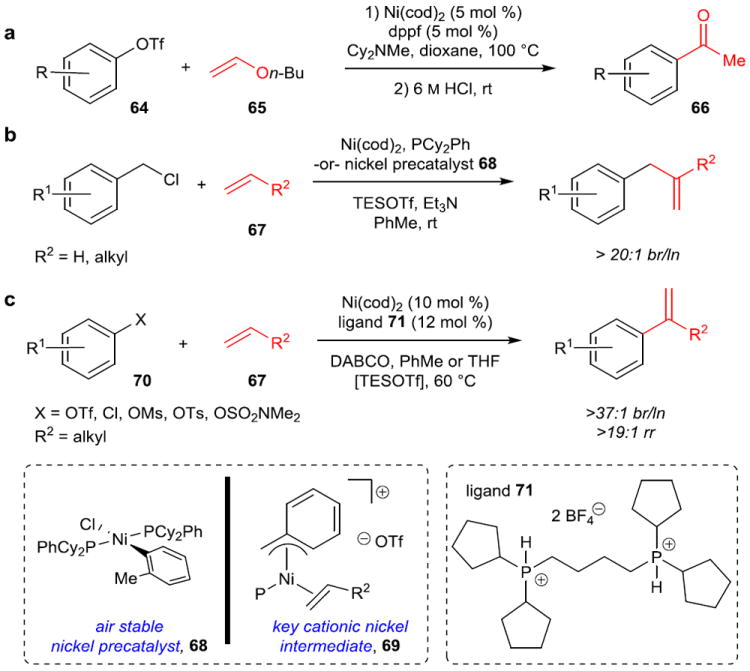
a, Coupling of aryl triflates (64) with electron-rich enol ethers (65) to obtain high selectivity for branched products, which upon acidic hydrolysis form ketones. Computational work supports a cationic Heck pathway with catalyst regeneration as turnover-limiting. b, The first Heck reaction highly selective for branched products with electronically unbiased (aliphatic) and non-chelating alkenes (67). Again, it was proposed to proceed through a cationic Ni species (69) to give high regioselectivity, and an air stable precatalyst (68) was developed to eliminate the need for air-free technique. c, Branch-selective Heck reaction for aryl electrophiles (70) with aliphatic olefins (67). Bidentate ligand 71 was key to both reactivity of aryl electrophiles and suppression of undesired isomerization. Aryl chlorides and other phenol-derived electrophiles can be utilized with the use of TESOTf, which is proposed to perform a counterion exchange in order to enter the cationic Heck pathway. TESOTf, triethylsilyl trifluoromethylsulfonate; DABCO, 1,4-diazabicyclo[2.2.2]octane.
However, electronically unbiased olefins had always given mixtures of branched and linear product isomers arising from addition of the electrophile to the internal and terminal position of the alkene, respectively. In 2011, Jamison and coworkers reported the first highly-selective Heck reaction for these olefins including ethylene and terminal aliphatic alkenes (67) (Figure 11b).89 This reaction is proposed to proceed via a cationic Heck pathway; it is suggested that the shorter Ni–ligand bond lengths make steric differentiation between the H and alkyl substituents of the alkene feasible. Later, an air-stable nickel precatalyst (68) was developed for this reaction, which obviates the need for Ni(cod)2 (and therefore the use of a glovebox) and increases reaction rates.90 Finally, a branch-selective Heck reaction of the more typically used aryl electrophiles (70) with electronically unbiased olefins has recently been reported (Figure 11c).91 A wide range of electrophiles including aryl triflates, chlorides, and other less reactive sulfonates (mesylates, tosylates, sulfamates) can be used, underscoring the utility of nickel to undergo oxidative addition to a broad range of cheap, stable, traditionally unreactive electrophiles.
Reductive Coupling
In addition to cross-coupling, one of the prototypical nickel-catalyzed reactions is reductive coupling. Reductive coupling involves the joining of two π-components with a reducing agent to form a new σ-bond between the coupling partners and a new C–H σ-bond arising from the reducing agent (Figure 12a). The reaction is generally accepted to proceed via a concerted oxidative cyclization, followed by either coordination of the reducing agent to Ni or σ-bond metathesis to provide a nickel hydride, and terminated by reductive elimination. Several excellent reviews3 of the state of the field prior to 2005 exist including couplings of both alkenes92 and alkynes.93
Figure 12. Prototypical reductive coupling reactions and use of new reducing agents.
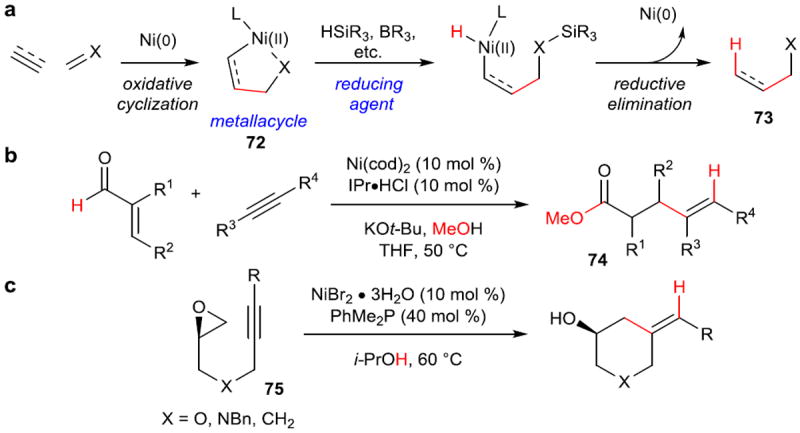
a, Standard reductive coupling reaction. Oxidative cyclization of two π-components forms a nickallacycle (72), which upon formation of a Ni–H bond with a reducing agent, undergoes reductive elimination to form a new C–C σ-bond and a new C–H bond overall (73). b, Use of methanol as a mild reducing agent via the intermediacy of a hemiacetal. c, Use of isopropanol as a mild, external reducing agent, allowing for the use of air-stable Ni(II) salts as precatalysts. IPr, 1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-imidazol-2-ylidene.
In more recent years, there have been two key developments in this field. The first development involves the search for milder reducing agents. Traditionally, reactive hydride donors such as alkyl silanes or trialkyl boranes have been used. However, in 2008, Montgomery and coworkers reported that methanol could be used to facilitate β-hydride elimination and provide the nickel hydride directly from an aldehyde in internal redox to form 74 (Figure 12b).94 A few years later, Jamsion and Beaver reported that by using alcohols directly as the source of the requisite hydride, air-stable Ni(II) salts (reduced under the reaction conditions) could be used to carry out reductive couplings between epoxides and tethered alkynes (75) (Figure 12c).95
The second major challenge inherent in reductive coupling reactions is the question of regioselectivity (Figure 13). For strongly electronically-biased coupling components, such as aryl–alkyl alkynes, regioselectivity is controlled by these elements. However, dialkyl alkynes pose a much tougher problem. The first high levels of regiocontrol were achieved by the use of alkynes with a pendant alkene, which could act as a ligand to Ni after coordination to the alkyne (Figure 13a).96 Then, by adjusting the ligand sphere around Ni by the addition of phosphine ligands, either position of the alkyne (A or B) could undergo reaction with the aldehyde. 97 Montgomery and coworkers subsequently discovered that by alteration of the steric bulk of carbene ligand substituents, the selectivity in the reductive coupling of internal dialkyl alkynes and aldehydes could be reversed (Figure 13b). 98 Subsequent computational studies suggested that the orientation of the groups bound to nitrogen was important, particularly in filling the quadrant proximal to the alkyne below the plane of the Ni–ligand bond.99 These impressive results demonstrate the fine-tuned control over these systems that can now be achieved.
Figure 13. Regiocontrol in reductive coupling reactions: two strategies.
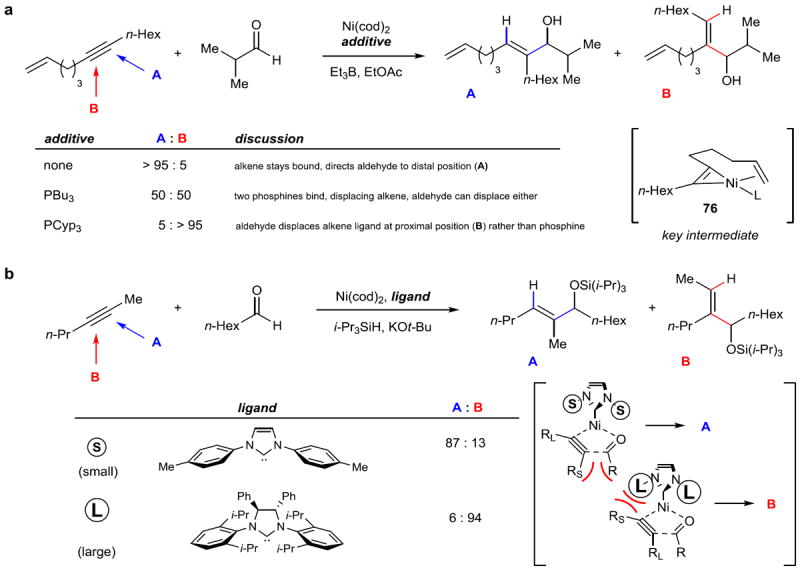
a, A tethered alkene can be used to control regioselectivity in coupling an alkyne with an aldehyde. The alkyne oxidatively adds to Ni to produce nickallacyclopropene 76. Then, by selective displacement by added phosphine ligands, or with no additive, the binding and orientation of the aldehyde is controlled to produce either linear (A) or branched (B) products. b, The steric profile of carbene ligands can be used to control the regioselectivity of an alkyne–aldehyde reductive coupling. Computational work suggests that unfavorable steric interactions between the groups on the alkyne with either the group on the aldehyde or the groups on the ligand dictate the orientation of the forming five-membered nickallacycle intermediate (shown in brackets). EtOAc, ethyl acetate; Cyp, cyclopentyl.
Looking Forward
Although the field of nickel catalysis has rapidly expanded over the last decade, there are many challenges that remain to be overcome. Through extensive mechanistic studies, including those described above, researchers now understand a great deal more about the elementary steps and oxidation states of nickel in a number of varying reaction manifolds. In many instances, the picture presented is more complex than originally envisioned, often due to the easy access of nickel to multiple oxidation states, and thus catalytic pathways. With this increased understanding, we expect future efforts to be directed toward the design of new catalysts and the development of new transformations that accomplish even more complex bond-forming reactions. The activation of simple electrophiles for cross-coupling such as C–H bonds or phenol derivatives, the formation of difficult bonds such as carbon–fluorine bonds, and the use of coupling partners currently considered challenging, such as carbon dioxide (CO2) are all areas that will benefit from further development and mechanistic understanding.
In addition, we expect to see further developments in the area of Csp3–Csp3 bond formation, particularly in expansion of substrate scope and application to the synthesis of complex molecules. Although there are a few examples of the cross coupling of sp3 electrophiles used in total synthesis, the adjustment of synthetic strategy has not perhaps yet occurred to enable their use as a foundational tool in organic chemistry. Another conspicuous absence is a general method for the Heck reaction of sp3 electrophiles.100 This reaction is particularly challenging since β-H elimination of the electrophile prior to coupling must be suppressed, but in order to form the desired alkene product, the measures taken to do so must not prohibit a β-H elimination after C–C bond formation.
Finally, we expect great strides in the development of low-cost, air-stable, and easier to handle sources of nickel for catalysis. The use of the most common Ni(0) source, Ni(cod)2, requires the use of a glovebox, and while in some reactions inexpensive nickel halide sources can be used with subsequent reduction accomplished in situ, the development of different modes of activation for nickel precatalysts could lead to wider adoption of nickel catalysis, in both academic and industrial laboratories. All in all, we expect that nickel catalysis will prove a fertile field of study well into the future as chemists continue to address even more challenging problems of reactivity and rapid assembly of complex molecules. We also hope that nickel will continue to gain recognition, not as an inexpensive substitute for palladium, but rather as possessing a number of inherent properties that provide a complement to catalysis by other metals.
Acknowledgments
Financial support was provided by the NIGMS (GM62755) and NSF (Graduate Research Fellowship to S.Z.T. and E.A.S.)
Footnotes
Author Contributions. S.Z.T. and E.A.S. worked together to outline and write the content, while T.F.J. helped edit the manuscript, references and figures.
The authors declare no competing financial interests.
References
- 1.Wilke G. Contributions to organo-nickel chemistry. Angew Chem Int Ed Engl. 1988;27:185–206. [Google Scholar]
- 2.Tamaru Y, editor. Modern Organonickel Chemistry. Wiley-VCH; 2005. This book provides an excellent discussion of nickel catalysis in general. [Google Scholar]
- 3.Montgomery J. Nickel-catalyzed reductive cyclizations and couplings. Angew Chem Int Ed. 2004;43:3890–3908. doi: 10.1002/anie.200300634. This paper provides a comprehensive review of reductive coupling prior to 2004. [DOI] [PubMed] [Google Scholar]
- 4.Diederich F, Stang PJ, editors. Metal-catalyzed cross-coupling reactions. Wiley-VCH; 1998. [Google Scholar]
- 5.Tsou TT, Kochi JK. Mechanism of oxidative addition. Reaction of nickel(0) complexes with aromatic halides. J Am Chem Soc. 1979;101:6319–6332. This article presents an excellent investigation into the mechanism of oxidative addition of Ni(0) into aryl halides and the factors determining rate, selectivity, and solvent effects of such reactions. [Google Scholar]
- 6.Lanni EL, McNeil AJ. Mechanistic studies on Ni(dppe)Cl2-catalyzed polymerizations: evidence for rate-determining reductive elimination. J Am Chem Soc. 2009;131:16573–16579. doi: 10.1021/ja904197q. [DOI] [PubMed] [Google Scholar]
- 7.Li B-J, Yu D-G, Sun C-L, Shi Z-J. Activation of “inert” alkenyl/aryl C–O bond and its application in cross-coupling reactions. Chem –Eur J. 2011;17:1728–1759. doi: 10.1002/chem.201002273. This review provides a detailed account of cross-coupling reactions of phenol derivatives of all types, including those catalyzed by metals other than nickel. [DOI] [PubMed] [Google Scholar]
- 8.Rosen BM, et al. Nickel-catalyzed cross-couplings involving carbon–oxygen bonds. Chem Rev. 2011;111:1346–1416. doi: 10.1021/cr100259t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mesganaw T, Garg NK. Ni- and Fe-catalyzed cross-coupling reactions of phenol derivatives. Org Process Res Dev. 2013;17:29–39. [Google Scholar]
- 10.Garcia JJ, Brunkan NM, Jones WD. Cleavage of carbon–carbon bonds in aromatic nitriles using nickel(0) J Am Chem Soc. 2002;124:9547–9555. doi: 10.1021/ja0204933. [DOI] [PubMed] [Google Scholar]
- 11.Tobisu M, Xu T, Shimasaki T, Chatani N. Nickel-catalyzed Suzuki–Miyaura reaction of aryl fluorides. J Am Chem Soc. 2011;133:19505–19511. doi: 10.1021/ja207759e. [DOI] [PubMed] [Google Scholar]
- 12.Cornella J, Gómez-Bengoa E, Martin R. Combined experimental and theoretical study on the reductive cleavage of inert C–O bonds with silanes: ruling out a classical Ni(0)/Ni(II) catalytic couple and evidence for Ni(I) intermediates. J Am Chem Soc. 2013;135:1997–2009. doi: 10.1021/ja311940s. This paper provides a rigorous experimental and computational study of the hypothesized nickel(I)-based catalytic cycle. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor CT, Kojima M. Alkene oligomerization. Catal Today. 1990;6:329–349. [Google Scholar]
- 14.Massera C, Frenking G. Energy partitioning analysis of the bonding in L2TM–C2H2 and L2TM–C2H4 (TM = Ni, Pd, Pt; L2 = (PH3)2, (PMe3)2, H2PCH2PH2, H2P(CH2)2PH2) Organometallics. 2003;22:2758–2765. [Google Scholar]
- 15.Lin B-L, et al. Comparing nickel- and palladium-catalyzed Heck reactions. Organometallics. 2004;23:2114–2123. [Google Scholar]
- 16.Cordero B, et al. Covalent radii revisited. Dalton Trans. 2008:2832–2838. doi: 10.1039/b801115j. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi J, Muto K, Itami K. Recent progress in nickel-catalyzed biaryl coupling. Eur J Org Chem. 2013:19–30. This short review is a concise overview of the state of biaryl synthesis by a variety of nickel-catalyzed methods. [Google Scholar]
- 18.Ge S, Hartwig JF. Highly reactive, single-component nickel catalyst precursor for Suzuki–Miyuara cross-coupling of heteroaryl boronic acids with heteroaryl halides. Angew Chem Int Ed. 2012;51:12837–12841. doi: 10.1002/anie.201207428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramgren SD, Hie L, Ye Y, Garg NK. Nickel-catalyzed Suzuki–Miyaura couplings in green solvents. Org Lett. 2013;15:3950–3953. doi: 10.1021/ol401727y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chuit C, Felkin H, Frajerman C, Roussi G, Swierczewski G. Action des organomagnesiens sur les alcools allyliques en presence de complexes du nickel: I. Synthese d’olefines. J Organomet Chem. 1977;127:371–384. [Google Scholar]
- 21.Kocienski P, Dixon NJ. Stereoselective synthesis of homoallylic alcohols by migratory insertion reactions of higher-order cyanocuprates and nickel-catalysed coupling reactions involving enol carbamates. Synlett. 1989;1989:53–54. [Google Scholar]
- 22.Sengupta S, Leite M, Raslan DS, Quesnelle C, Snieckus V. Nickel(0)-catalyzed cross coupling of aryl O-carbamates and aryl triflates with Grignard reagents. Directed ortho metalation-aligned synthetic methods for polysubstituted aromatics via a 1,2-dipole equivalent. J Org Chem. 1992;57:4066–4068. [Google Scholar]
- 23.Milburn RR, Snieckus V. The tertiary sulfonamide as a latent directed-metalation group: Ni0-catalyzed reductive cleavage and cross-coupling reactions of aryl sulfonamides with Grignard reagents. Angew Chem Int Ed. 2004;43:888–891. doi: 10.1002/anie.200352633. [DOI] [PubMed] [Google Scholar]
- 24.Dankwardt JW. Nickel-catalyzed cross-coupling of aryl Grignard reagents with aromatic alkyl ethers: an efficient synthesis of unsymmetrical biaryls. Angew Chem Int Ed. 2004;43:2428–2432. doi: 10.1002/anie.200453765. [DOI] [PubMed] [Google Scholar]
- 25.Tobisu M, Shimasaki T, Chatani N. Nickel-catalyzed cross-coupling of aryl methyl ethers with aryl boronic esters. Chem Int Ed. 2008;47:4866–4869. doi: 10.1002/anie.200801447. [DOI] [PubMed] [Google Scholar]
- 26.Guan B-T, Wang Y, Li B-J, Yu D-G, Shi Z-J. Biaryl construction via Ni-catalyzed C–O activation of phenolic carboxylates. J Am Chem Soc. 2008;130:14468–14470. doi: 10.1021/ja8056503. [DOI] [PubMed] [Google Scholar]
- 27.Quasdorf KW, Tian X, Garg NK. Cross-coupling reactions of aryl pivalates with boronic acids. J Am Chem Soc. 2008;130:14422–14423. doi: 10.1021/ja806244b. [DOI] [PubMed] [Google Scholar]
- 28.Li B-J, et al. Cross-coupling of aryl/alkenyl pivalates with organozinc reagents through nickel-catalyzed C–O bond activation under mild reaction conditions. Angew Chem Int Ed. 2008;47:10124–10127. doi: 10.1002/anie.200803814. [DOI] [PubMed] [Google Scholar]
- 29.Yu D-G, et al. Direct application of phenolic salts to nickel-catalyzed cross-coupling reactions with aryl Grignard reagents. Angew Chem Int Ed. 2010;49:4566–4570. doi: 10.1002/anie.200907359. [DOI] [PubMed] [Google Scholar]
- 30.Yu D-G, Shi Z-J. Mutual activation: Suzuki–Miyaura coupling through direct cleavage of the sp2 C–O bond of naphtholate. Angew Chem Int Ed. 2011;50:7097–7100. doi: 10.1002/anie.201101461. [DOI] [PubMed] [Google Scholar]
- 31.Quasdorf KW, et al. Suzuki–Miyaura cross-coupling of aryl carbamates and sulfamates: experimental and computational studies. J Am Chem Soc. 2011;133:6352–6363. doi: 10.1021/ja200398c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antoft-Finch A, Blackburn T, Snieckus V. N, N-diethyl O-carbamate: directed metalation group and orthogonal Suzuki–Miyaura cross-coupling partner. J Am Chem Soc. 2009;131:17750–17752. doi: 10.1021/ja907700e. [DOI] [PubMed] [Google Scholar]
- 33.Ehle AR, Zhou Q, Watson MP. Nickel(0)-catalyzed Heck cross-coupling via activation of aryl C–OPiv bonds. Org Lett. 2013;14:1202–1205. doi: 10.1021/ol203322v. [DOI] [PubMed] [Google Scholar]
- 34.Tobisu M, Yasutome A, Yamakawa K, Shimasaki T, Chatani N. Ni(0)/NHC-catalyzed amination of N-heteroaryl methyl ethers through the cleavage of carbon–oxygen bonds. Tetrahedron. 2012;68:5157–5161. [Google Scholar]
- 35.Hie L, Ramgren SD, Mesganaw T, Garg NK. Nickel-catalyzed amination of aryl sulfamates and carbamates using an air-stable precatalyst. Org Lett. 2012;14:4182–4185. doi: 10.1021/ol301847m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Álvarez-Bercedo P, Martin R. Ni-catalyzed reduction of inert C−O bonds: a new strategy for using aryl ethers as easily removable directing groups. J Am Chem Soc. 2010;132:17352–17353. doi: 10.1021/ja106943q. [DOI] [PubMed] [Google Scholar]
- 37.Guan B-T, et al. Direct benzylic alkylation via Ni-catalyzed selective benzylic sp3 C–O activation. J Am Chem Soc. 2008;130:3268–3269. doi: 10.1021/ja710944j. [DOI] [PubMed] [Google Scholar]
- 38.Taylor BLH, Swift EC, Waetzig JD, Jarvo ER. Stereospecific nickel-catalyzed cross-coupling reactions of alkyl ethers: enantioselective synthesis of diarylethanes. J Am Chem Soc. 2011;133:389–391. doi: 10.1021/ja108547u. [DOI] [PubMed] [Google Scholar]
- 39.Greene MA, Yonova IM, Williams FJ, Jarvo ER. Traceless directing group for stereospecific nickel-catalyzed alkyl−alkyl cross-coupling reactions. Org Lett. 2012;14:4293–4296. doi: 10.1021/ol300891k. [DOI] [PubMed] [Google Scholar]
- 40.Harris MR, Hanna LE, Green MA, Moore CE, Jarvo ER. Retention or inversion in stereospecific nickel-catalyzed cross-coupling of benzylic carbamates with arylboronic esters: control of absolute stereochemistry with an achiral catalyst. J Am Chem Soc. 2013;135:3303–3306. doi: 10.1021/ja311783k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Q, Srinivas HD, Dasgupta S, Watson MP. Nickel-catalyzed cross-couplings of benzylic pivalates with arylboroxines: stereospecific formation of diarylalkanes and triarylmethanes. J Am Chem Soc. 2013;135:3307–3310. doi: 10.1021/ja312087x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu D-G, et al. Direct arylation/alkylation/magnesiation of benzyl alcohols in the presence of Grignard reagents via Ni-, Fe-, or Co-catalyzed sp3 C–O bond activation. J Am Chem Soc. 2012;134:14638–14641. doi: 10.1021/ja307045r. [DOI] [PubMed] [Google Scholar]
- 43.León T, Correa A, Martin R. Ni-catalyzed direct carboxylation of benzyl halides with CO2. J Am Chem Soc. 2013;135:1221–1224. doi: 10.1021/ja311045f. [DOI] [PubMed] [Google Scholar]
- 44.Correa A, León T, Martin R. Ni-catalyzed carboxylation of C(sp2)– and C(sp3)–O bonds with CO2. J Am Chem Soc. 2014;136:1062–1069. doi: 10.1021/ja410883p. [DOI] [PubMed] [Google Scholar]
- 45.Maity P, Shacklady-McAtee DM, Yap GPA, Sirianni ER, Watson MP. Nickel-catalyzed cross couplings of benzylic ammonium salts and boronic acids: stereospecific formation of diarylethanes via C−N bond activation. J Am Chem Soc. 2013;135:280–285. doi: 10.1021/ja3089422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin BL, Clough CR, Hillhouse GL. Interactions of aziridines with nickel complexes: oxidative-addition and reductive-elimination reactions that break and make C−N bonds. J Am Chem Soc. 2002;124:2890–2891. doi: 10.1021/ja017652n. [DOI] [PubMed] [Google Scholar]
- 47.Huang C-Y, Doyle AG. Nickel-catalyzed Negishi alkylations of styrenyl aziridines. J Am Chem Soc. 2012;134:9541–9544. doi: 10.1021/ja3013825. [DOI] [PubMed] [Google Scholar]
- 48.Nielsen DK, Huang C-Y, Doyle AG. Directed nickel-catalyzed Negishi cross coupling of alkyl aziridines. J Am Chem Soc. 2013;135:13605–13609. doi: 10.1021/ja4076716. [DOI] [PubMed] [Google Scholar]
- 49.Frisch AC, Beller M. Catalysts for cross-coupling reactions with non-activated alkyl halides. Angew Chem Int Ed. 2005;44:674–688. doi: 10.1002/anie.200461432. [DOI] [PubMed] [Google Scholar]
- 50.Hu X. Nickel-catalyzed cross coupling of non-activated alkyl halides: a mechanistic perspective. Chem Sci. 2011;2:1867–1886. [Google Scholar]
- 51.Hills ID, Netherton MR, Fu GC. Toward an improved understanding of the unusual reactivity of Pd0/trialkylphosphane catalysts in cross-couplings of alkyl electrophiles: quantifying the factors that determine the rate of oxidative addition. Angew Chem Int Ed. 2003;42:5749–5752. doi: 10.1002/anie.200352858. [DOI] [PubMed] [Google Scholar]
- 52.Netherton MR, Fu GC. Nickel-catalyzed cross-couplings of unactivated alkyl halides and pseudohalides with organometallic compounds. Adv Synth Catal. 2004;346:1525–1532. [Google Scholar]
- 53.Ishiyama T, Abe S, Miyaura N, Suzuki A. Palladium-catalyzed alkyl-alkyl cross-coupling reaction of 9-alkyl-9-BBN derivatives with iodoalkanes possessing β-hydrogens. Chem Lett. 1992;21:691–694. [Google Scholar]
- 54.Devasagayaraj A, Stüdemann T, Knochel P. A new nickel-catalyzed cross-coupling reaction between sp3 carbon centers. Angew Chem Int Ed. 1995;34:2723–2725. [Google Scholar]
- 55.Giovannini R, Knochel P. Ni(II)-catalyzed cross-coupling between polyfunctionalized arylzinc derivatives and primary alkyl iodides. J Am Chem Soc. 1998;120:11186–11187. [Google Scholar]
- 56.Terao J, Watanabe H, Ikumi A, Kuniyasu H, Kambe N. Nickel-catalyzed cross-coupling reaction of Grignard reagents with alkyl halides and tosylates: remarkable effect of 1,3-butadienes. J Am Chem Soc. 2002;124:4222–4223. doi: 10.1021/ja025828v. [DOI] [PubMed] [Google Scholar]
- 57.Zhou J, Fu GC. Cross-couplings of unactivated secondary alkyl halides: room-temperature nickel-catalyzed Negishi reactions of alkyl bromides and iodides. J Am Chem Soc. 2003;125:14726–14727. doi: 10.1021/ja0389366. This paper represents the first secondary Csp3–Csp3 cross-coupling. [DOI] [PubMed] [Google Scholar]
- 58.Zhou J, Fu GC. Suzuki cross-couplings of unactivated secondary alkyl bromides and iodides. J Am Chem Soc. 2004;126:1340–1341. doi: 10.1021/ja039889k. [DOI] [PubMed] [Google Scholar]
- 59.Powell DA, Fu GC. Nickel-catalyzed cross-couplings of organosilicon reagents with unactivated secondary alkyl bromides. J Am Chem Soc. 2004;126:7788–7789. doi: 10.1021/ja047433c. [DOI] [PubMed] [Google Scholar]
- 60.Powell DA, Maki T, Fu GC. Stille cross-couplings of unactivated secondary alkyl halides using monoorganotin reagents. J Am Chem Soc. 2005;127:510–511. doi: 10.1021/ja0436300. [DOI] [PubMed] [Google Scholar]
- 61.Saito B, Fu GC. Alkyl–alkyl Suzuki cross-couplings of unactivated secondary alkyl halides at room temperature. J Am Chem Soc. 2007;129:9602–9603. doi: 10.1021/ja074008l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dudnik AS, Fu GC. Nickel-catalyzed coupling reactions of alkyl electrophiles, including unactivated tertiary halides, to generate carbon–boron bonds. J Am Chem Soc. 2012;134:10693–10697. doi: 10.1021/ja304068t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zultanski SL, Fu GC. Nickel-catalyzed carbon–carbon bond-forming reactions of unactivated tertiary alkyl halides: Suzuki arylations. J Am Chem Soc. 2013;135:624–627. doi: 10.1021/ja311669p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rudolph A, Lautens M. Secondary alkyl halides in transition-metal-catalyzed cross-coupling reactions. Angew Chem Int Ed. 2009;48:2656–2670. doi: 10.1002/anie.200803611. [DOI] [PubMed] [Google Scholar]
- 65.Jones GD, et al. Ligand redox effects in the synthesis, electronic structure, and reactivity of an alkyl–alkyl cross-coupling catalyst. J Am Chem Soc. 2006;128:13175–13183. doi: 10.1021/ja063334i. [DOI] [PubMed] [Google Scholar]
- 66.Lipschutz MI, Yang X, Chatterjee R, Tilley TD. A structurally rigid bis(amido) ligand framework in low-coordinate Ni(I), Ni(II), and Ni(III) analogues provides access to a Ni(III) methyl complex via oxidative addition. J Am Chem Soc. 2013;135:15298–15301. doi: 10.1021/ja408151h. [DOI] [PubMed] [Google Scholar]
- 67.Csok Z, Vechorkin O, Harkins SB, Scopelliti R, Hu X. Nickel complexes of a pincer NN2 ligand: multiple carbon–chloride activation of CH2Cl2 and CHCl3 leads to selective carbon–carbon bond formation. J Am Chem Soc. 2008;130:8156–8157. doi: 10.1021/ja8025938. [DOI] [PubMed] [Google Scholar]
- 68.Vechorkin O, Hu X. Nickel-catalyzed cross-coupling of non-activated and functionalized alkyl halides with alkyl Grignard reagents. Angew Chem Int Ed. 2009;48:2937–2940. doi: 10.1002/anie.200806138. [DOI] [PubMed] [Google Scholar]
- 69.Vechorkin O, Barmaz D, Proust V, Hu X. Ni-catalyzed Sonogashira coupling of nonactivated alkyl halides: orthogonal functionalization of alkyl iodides, bromides, and chlorides. J Am Chem Soc. 2009;131:12078–12079. doi: 10.1021/ja906040t. [DOI] [PubMed] [Google Scholar]
- 70.Breitenfeld J, Ruiz J, Wodrich MD, Hu X. Bimetallic oxidative addition involving radical intermediates in nickel-catalyzed alkyl–alkyl Kumada coupling reactions. J Am Chem Soc. 2013;135:12004–12012. doi: 10.1021/ja4051923. [DOI] [PubMed] [Google Scholar]
- 71.Fischer C, Fu GC. Asymmetric nickel-catalyzed Negishi cross-couplings of secondary α-bromo amides with organozinc reagents. J Am Chem Soc. 2005;127:4594–4595. doi: 10.1021/ja0506509. [DOI] [PubMed] [Google Scholar]
- 72.Zultanski SL, Fu GC. Catalytic asymmetric γ-alkylation of carbonyl compounds via stereoconvergent Suzuki cross-couplings. J Am Chem Soc. 2011;133:15362–15364. doi: 10.1021/ja2079515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gong H, Gagné MR. Diastereoselective Ni-catalyzed Negishi cross-coupling approach to saturated, fully oxygenated C-alkyl and C-aryl glycosides. J Am Chem Soc. 2008;130:12177–12183. doi: 10.1021/ja8041564. [DOI] [PubMed] [Google Scholar]
- 74.Son S, Fu GC. Nickel-catalyzed asymmetric Negishi cross-couplings of secondary allylic chlorides with alkylzincs. J Am Chem Soc. 2008;130:2756–2757. doi: 10.1021/ja800103z. [DOI] [PubMed] [Google Scholar]
- 75.Everson DA, Shrestha R, Weix DJ. Nickel-catalyzed reductive cross-coupling of aryl halides with alkyl halides. J Am Chem Soc. 2010;132:920–921. doi: 10.1021/ja9093956. [DOI] [PubMed] [Google Scholar]
- 76.Everson DA, Jones BA, Weix DJ. Replacing conventional carbon nucleophiles with electrophiles: nickel-catalyzed reductive alkylation of aryl bromides and chlorides. J Am Chem Soc. 2012;134:6146–6159. doi: 10.1021/ja301769r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Biswas S, Weix DJ. Mechanism and selectivity in nickel-catalyzed cross-electrophile coupling of aryl halides with alkyl halides. J Am Chem Soc. 2013;135:16192–16197. doi: 10.1021/ja407589e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cherney AH, Kadunce NT, Reisman SE. Catalytic asymmetric reductive acyl cross-coupling: synthesis of enantioenriched acyclic α,α-disubstituted ketones. J Am Chem Soc. 2013;135:7442–7445. doi: 10.1021/ja402922w. [DOI] [PubMed] [Google Scholar]
- 79.Kleiman JP, Dubeck M. The preparation of cyclopentadienyl [o-(phenylazo)phenyl]nickel. J Am Chem Soc. 1963;85:1544–1545. [Google Scholar]
- 80.Muto K, Yamaguchi J, Itami K. Nickel-catalyzed C–H/C–O coupling of azoles with phenol derivatives. J Am Chem Soc. 2012;134:169–172. doi: 10.1021/ja210249h. [DOI] [PubMed] [Google Scholar]
- 81.Amaike K, Muto K, Yamaguchi J, Itami K. Decarbonylative C–H coupling of azoles and aryl esters: unprecedented nickel catalysis and application to the synthesis of muscoride A. J Am Chem Soc. 2012;134:13573–13576. doi: 10.1021/ja306062c. [DOI] [PubMed] [Google Scholar]
- 82.Muto K, Yamaguchi J, Lei A, Itami K. Isolation, structure, and reactivity of an arylnickel(II) pivalate complex in catalytic C–H/C–O biaryl coupling. J Am Chem Soc. 2013;135:16384–16387. doi: 10.1021/ja409803x. [DOI] [PubMed] [Google Scholar]
- 83.Shiota H, Ano Y, Aihara Y, Fukumoto Y, Chatani N. Nickel-catalyzed chelation-assisted transformations involving ortho C–H bond activation: regioselective oxidative cycloaddition of aromatic amides to alkynes. J Am Chem Soc. 2011;133:14952–14955. doi: 10.1021/ja206850s. [DOI] [PubMed] [Google Scholar]
- 84.Aihara Y, Chatani N. Nickel-catalyzed direct alkylation of C–H bonds in benzamides and acrylamides with functionalized alkyl halides via bidentate-chelation assistance. J Am Chem Soc. 2013;135:5308–5311. doi: 10.1021/ja401344e. [DOI] [PubMed] [Google Scholar]
- 85.Aihara Y, Chatani N. Nickel-catalyzed direct arylation of C(sp3)–H bonds in aliphatic amides via bidentate-chelation assistance. J Am Chem Soc. 2014;136:898–901. doi: 10.1021/ja411715v. [DOI] [PubMed] [Google Scholar]
- 86.Oestreich M, editor. The Mizoroki–Heck Reaction. Wiley; 2009. [Google Scholar]
- 87.Cabri W, Candiani I. Recent developments and new perspectives in the Heck reaction. Acc Chem Res. 1995;28:2–7. [Google Scholar]
- 88.Gøgsig TM, et al. Mild and efficient nickel-catalyzed Heck reactions with electron-rich olefins. J Am Chem Soc. 2012;134:443–452. doi: 10.1021/ja2084509. [DOI] [PubMed] [Google Scholar]
- 89.Matsubara R, Gutierrez AC, Jamison TF. Nickel-catalyzed Heck-type reactions of benzyl chlorides and simple olefins. J Am Chem Soc. 2011;133:19020–19023. doi: 10.1021/ja209235d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Standley EA, Jamison TF. Simplifying nickel(0) catalysis: an air-stable nickel precatalyst for the internally selective benzylation of terminal alkenes. J Am Chem Soc. 2013;135:1585–1592. doi: 10.1021/ja3116718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tasker SZ, Gutierrez AC, Jamison TF. Nickel-catalyzed Mizoroki–Heck reaction of aryl sulfonates and chlorides with electronically unbiased terminal olefins: high selectivity for branched products. Angew Chem Int Ed. 2014 doi: 10.1002/anie.201308391. Online early access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ng S-Z, Ho C-Y, Schleicher KD, Jamison TF. Nickel-catalyzed coupling reactions of alkenes. Pure Appl Chem. 2008;80:929–939. doi: 10.1351/pac200880050929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moslin RM, Miller-Moslin K, Jamison TF. Regioselectivity and enantioselectivity in nickel-catalysed reductive coupling reactions of alkynes. Chem Commun. 2007:4441–4449. doi: 10.1039/b707737h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Herath A, Li W, Montgomery J. Fully intermolecular nickel-catalyzed three-component couplings via internal redox. J Am Chem Soc. 2008;130:469–471. doi: 10.1021/ja0781846. [DOI] [PubMed] [Google Scholar]
- 95.Beaver MG, Jamison TF. Ni(II) salts and 2-propanol effect catalytic reductive coupling of epoxides and alkynes. Org Lett. 2011;13:4140–4143. doi: 10.1021/ol201702a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miller KM, Jamison TF. Ligand-switchable directing effects of tethered alkenes in nickel-catalyzed additions to alkynes. J Am Chem Soc. 2004;126:15342–15343. doi: 10.1021/ja0446799. [DOI] [PubMed] [Google Scholar]
- 97.Moslin RM, Jamison TF. Mechanistic implications of nickel-catalyzed reductive coupling of aldehydes and chiral 1,6-enynes. Org Lett. 2006;8:455–458. doi: 10.1021/ol052719n. [DOI] [PubMed] [Google Scholar]
- 98.Malik HA, Sormunen GJ, Montgomery J. A general strategy for regiocontrol in nickel-catalyzed reductive couplings of aldehydes and alkynes. J Am Chem Soc. 2010;132:6304–6305. doi: 10.1021/ja102262v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu P, Montgomery J, Houk KN. Ligand steric contours to understand the effects of N-heterocyclic carbene ligands on the reversal of regioselectivity in Ni-catalyzed reductive couplings of alkynes and aldehydes. J Am Chem Soc. 2011;133:6956–6959. doi: 10.1021/ja202007s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Firmansjah L, Fu GC. Intramolecular Heck reactions of unactivated alkyl halides. J Am Chem Soc. 2007;129:11340–11341. doi: 10.1021/ja075245r. [DOI] [PubMed] [Google Scholar]


