Figure 10. Selected examples of nickel-catalyzed C–H activation reactions.
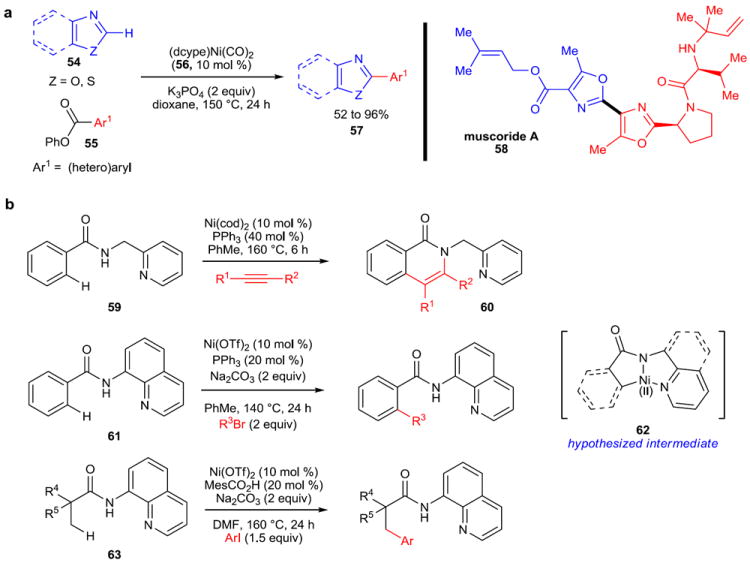
a, Benzoxazoles and benzothiazoles (54) are useful substrates for this nickel-catalyzed C–H activation reaction, which uses aryl esters (55) as the electrophilic coupling partners to produce (hetero)biaryls (57). This methodology was applied to the formal synthesis of muscoride A (58) to great effect. b, Nickel-catalyzed, chelation-assisted C–H activation reactions have recently been developed. These reactions rely on a directing group to facilitate addition of nickel into the C–H bond in the ortho position of a benzamide (59, 61) or into the C–H bond of an adjacent aliphatic substituent (63). dcype, 1,2-bis(dicyclohexylphosphino)ethane; OTf, triflate (trifluoromethanesulfonate); DMF, dimethylformamide.
