Abstract
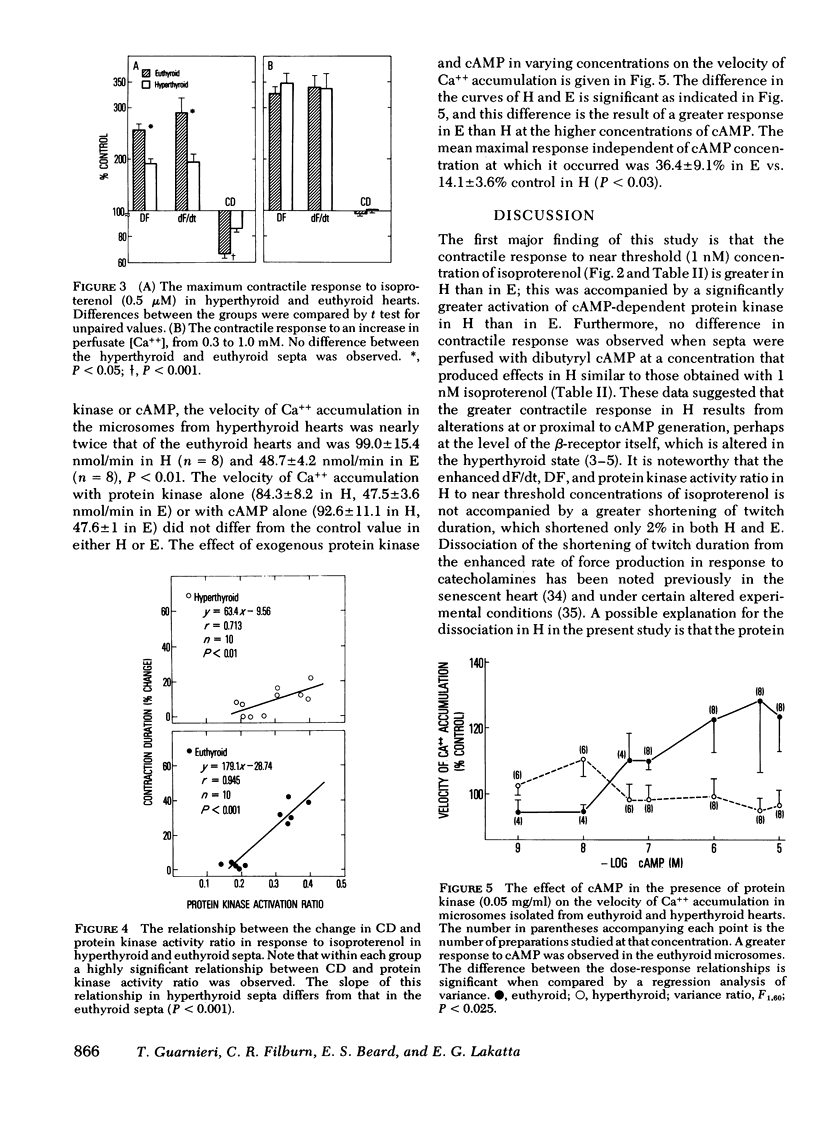
The contractile response measured as maximum rate of force development to a near threshold concentration of isoproterenol (1 nM) was enhanced in perfused interventricular septa from hyperthyroid (128±4% control) compared with euthyroid rats (105±2%, P < 0.01). This enhanced contractile response was accompanied by a significant activation of cyclic (c)AMP-dependent protein kinase (protein kinase activity ratio increased from 0.159±0.008 to 0.218±0.019, P < 0.005, although no significant changes from base line occurred in euthyroid septa, 0.152±0.007-0.179±0.012). No difference between hyperthyroid and euthyroid hearts was observed in the contractile response to 0.1 mM dibutyryl cAMP (126.5±2.5% and 122.0±9.2% in hyperthyroid and euthyroid, respectively), and the magnitude of the response to dibutyryl cAMP was comparable with that observed in the hyperthyroid group with 1 nM isoproterenol. These results suggest that the mechanism for enhanced protein kinase activation and contractile response to low concentrations of isoproterenol in the hyperthyroid heart is at or proximal to cAMP generation. The maximum contractile response to isoproterenol (0.5 μM), however, was decreased in hyperthyroid myocardium (192±13%) compared with euthyroid (291±37%, P < 0.05). Both protein kinase activity ratio (0.356±0.017 and 0.344±0.013) and the maximum contractile response to Ca++ (335±15 and 340±12% control in hyperthyroid and euthyroid, respectively) were similar, suggesting that the mechanism of the diminished maximum response was distal to protein kinase activation but not a function of an altered Ca++-troponin interaction. The diminished maximum rate of force development response in the hyperthyroid hearts was accompanied by significantly less shortening of the contraction duration that was 85.6±2.1% control in hyperthyroid vs. 66±2.8% control in euthyroid, P < 0.001. Although the basal rate of Ca++ accumulation was greater in microsomes isolated from hyperthyroid than from euthyroid hearts, there was significantly less additional stimulation of Ca++ accumulation in response to exogenous cAMP and protein kinase in hyperthyroid compared with euthyroid hearts. This reduction may explain the diminished effect of isoproterenol on the shortening of contraction duration in hyperthyroid compared with the euthyroid myocardium, and may explain, at least in part, the diminished maximum contractile response to isoproterenol.
Full text
PDF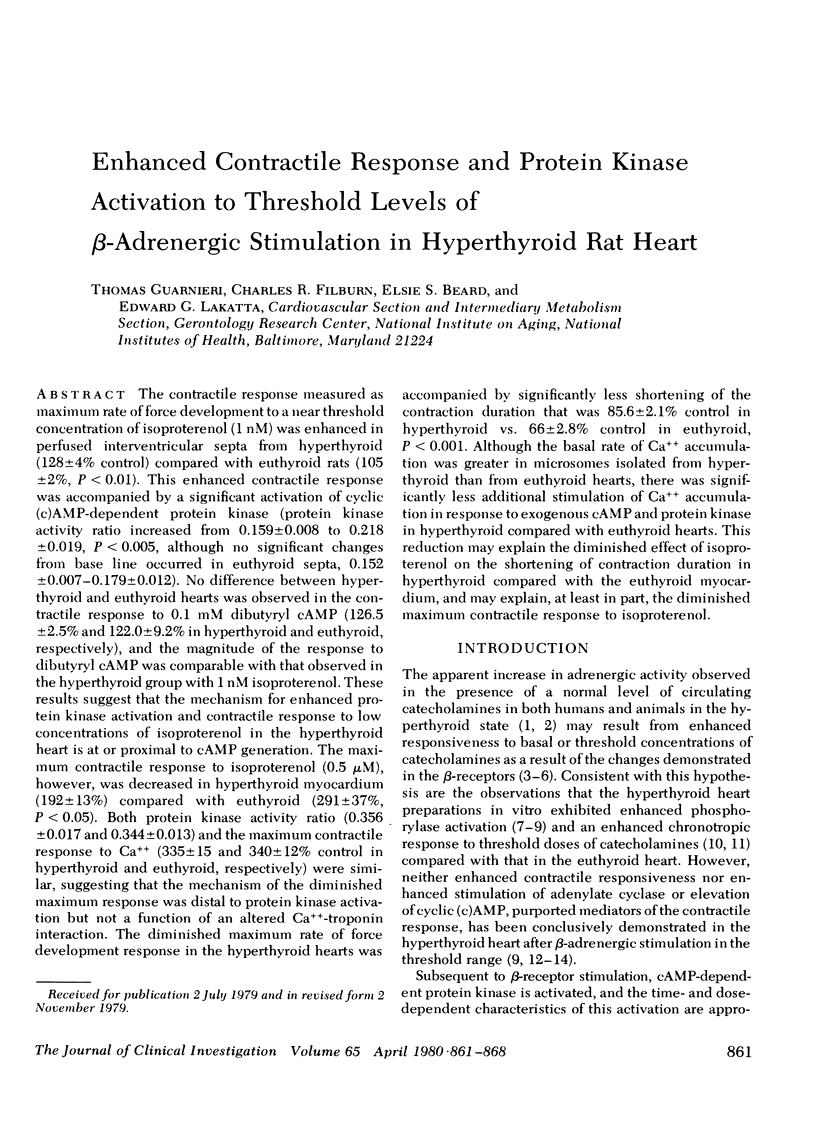



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby C. D., Walsh D. A. Characterization of the interaction of a protein inhibitor with adenosine 3',5'-monophosphate-dependent protein kinases. II. Mechanism of action with the holoenzyme. J Biol Chem. 1973 Feb 25;248(4):1255–1261. [PubMed] [Google Scholar]
- Banerjee S. P., Kung L. S. beta-Adrenergic receptors in rat heart: effects of thyroidectomy. Eur J Pharmacol. 1977 May 15;43(2):207–208. doi: 10.1016/0014-2999(77)90134-0. [DOI] [PubMed] [Google Scholar]
- Briggs F. N., Poland J. L., Solaro R. J. Relative capabilities of sarcoplasmic reticulum in fast and slow mammalian skeletal muscles. J Physiol. 1977 Apr;266(3):587–594. doi: 10.1113/jphysiol.1977.sp011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccino R. A., Spann J. F., Jr, Pool P. E., Sonnenblick E. H., Braunwald E. Influence of the thyroid state on the intrinsic contractile properties and energy stores of the myocardium. J Clin Invest. 1967 Oct;46(10):1669–1682. doi: 10.1172/JCI105658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Stull J. T. Calcium binding to cardiac troponin and the effect of cyclic AMP-dependent protein kinase. FEBS Lett. 1977 Jan 15;73(1):101–104. [PubMed] [Google Scholar]
- Ciaraldi T., Marinetti G. V. Thyroxine and propylthiouracil effects of vivo on alpha and beta adrenergic receptors in rat heart. Biochem Biophys Res Commun. 1977 Feb 7;74(3):984–991. doi: 10.1016/0006-291x(77)91615-1. [DOI] [PubMed] [Google Scholar]
- Cooke B. A., Lindh M. L., Janszen F. H. Correlation of protein kinase activation and testosterone production after stimulation of Leydig cells with luteinizing hormone. Biochem J. 1976 Dec 15;160(3):439–446. doi: 10.1042/bj1600439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson J. G., Jr Protein kinase regulation of cardiac phosphorylase activity and contractility. Am J Physiol. 1978 May;234(5):H638–H645. doi: 10.1152/ajpheart.1978.234.5.H638. [DOI] [PubMed] [Google Scholar]
- Ezrailson E. G., Potter J. D., Michael L., Schwartz A. Positive inotropy induced by ouabain, by increased frequency, by X537A (RO2-2985), by calcium and by isoproterenol: the lack of correlation with phosphorylation of TnI. J Mol Cell Cardiol. 1977 Aug;9(8):693–698. doi: 10.1016/s0022-2828(77)80364-7. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calcium release from the sarcoplasmic reticulum. Circ Res. 1977 Feb;40(2):119–129. doi: 10.1161/01.res.40.2.119. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Relaxing and inotropic effects of cyclic AMP on skinned cardiac cells. Nature. 1975 Feb 13;253(5492):556–558. doi: 10.1038/253556b0. [DOI] [PubMed] [Google Scholar]
- Froehlich J. P., Lakatta E. G., Beard E., Spurgeon H. A., Weisfeldt M. L., Gerstenblith G. Studies of sarcoplasmic reticulum function and contraction duration in young adult and aged rat myocardium. J Mol Cell Cardiol. 1978 May;10(5):427–438. doi: 10.1016/0022-2828(78)90364-4. [DOI] [PubMed] [Google Scholar]
- Gerstenblith G., Spurgeon H. A., Froehlich J. P., Weisfeldt M. L., Lakatta E. G. Diminished inotropic responsiveness to ouabain in aged rat myocardium. Circ Res. 1979 Apr;44(4):517–523. doi: 10.1161/01.res.44.4.517. [DOI] [PubMed] [Google Scholar]
- HARRISON T. S. ADRENAL MEDULLARY AND THYROID RELATIONSHIPS. Physiol Rev. 1964 Apr;44:161–185. doi: 10.1152/physrev.1964.44.2.161. [DOI] [PubMed] [Google Scholar]
- Harigaya S., Schwartz A. Rate of calcium binding and uptake in normal animal and failing human cardiac muscle. Membrane vesicles (relaxing system) and mitochondria. Circ Res. 1969 Dec;25(6):781–794. doi: 10.1161/01.res.25.6.781. [DOI] [PubMed] [Google Scholar]
- Hornbrook K. R., Cabral A. Enhancement by thyroid hormone treatment of norepinephrine-induced phosphorylase activation in the rat heart. Biochem Pharmacol. 1972 Apr 1;21(7):897–907. doi: 10.1016/0006-2952(72)90395-4. [DOI] [PubMed] [Google Scholar]
- Ito Y., Suko J., Chidsey C. A. Intracellular calcium and myocardial contractility. V. Calcium uptake of sarcoplasmic reticulum fractions in hypertrophied and failing rabbit hearts. J Mol Cell Cardiol. 1974 Jun;6(3):237–247. doi: 10.1016/0022-2828(74)90053-4. [DOI] [PubMed] [Google Scholar]
- Keely S. L., Corbin J. D. Involvement of cAMP-dependent protein kinase in the regulation of heart contractile force. Am J Physiol. 1977 Aug;233(2):H269–H275. doi: 10.1152/ajpheart.1977.233.2.H269. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lakatta E. G., Gerstenblith G., Angell C. S., Shock N. W., Weisfeldt M. L. Diminished inotropic response of aged myocardium to catecholamines. Circ Res. 1975 Feb;36(2):262–269. doi: 10.1161/01.res.36.2.262. [DOI] [PubMed] [Google Scholar]
- Limas C. J. Enhanced phosphorylation of myocardial sarcoplasmic reticulum in experimental hyperthyroidism. Am J Physiol. 1978 Apr;234(4):H426–H431. doi: 10.1152/ajpheart.1978.234.4.H426. [DOI] [PubMed] [Google Scholar]
- Ling W. Y., Marsh J. M. Reevaluation of the role of cyclic adenosine 3',5'-monophosphate and protein kinase in the stimulation of steroidogenesis by luteinizing hormone in bovine corpus luteum slices. Endocrinology. 1977 Jun;100(6):1571–1578. doi: 10.1210/endo-100-6-1571. [DOI] [PubMed] [Google Scholar]
- McClellan G. B., Winegrad S. The regulation of the calcium sensitivity of the contractile system in mammalian cardiac muscle. J Gen Physiol. 1978 Dec;72(6):737–764. doi: 10.1085/jgp.72.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill J. H., Brody T. M. The effect of triiodothyronine pretreatment on amine-induced rat cardiac phosphorylase activation. J Pharmacol Exp Ther. 1968 May;161(1):40–46. [PubMed] [Google Scholar]
- Morad M., Weiss J., Cleemann L. The inotropic action of adrenaline on cardiac muscle: does it relax or potentiate tension? Eur J Cardiol. 1978 Jun;7 (Suppl):53–62. [PubMed] [Google Scholar]
- Neely J. R., Liebermeister H., Morgan H. E. Effect of pressure development on membrane transport of glucose in isolated rat heart. Am J Physiol. 1967 Apr;212(4):815–822. doi: 10.1152/ajplegacy.1967.212.4.815. [DOI] [PubMed] [Google Scholar]
- Poole-Wilson P. A., Langer G. A. Glycoside inotropy in the absence of an increase in potassium efflux in the rabbit heart. Circ Res. 1975 Sep;37(3):390–395. doi: 10.1161/01.res.37.3.390. [DOI] [PubMed] [Google Scholar]
- Skelton C. L., Levey G. S., Epstein S. E. Positive inotropic effects of dibutyryl cyclic adenosine 3',5'-monophosphate. Circ Res. 1970 Jan;26(1):35–43. doi: 10.1161/01.res.26.1.35. [DOI] [PubMed] [Google Scholar]
- Suko J. Alterations of Ca 2 uptake and Ca 2+ activated ATPase of cardiac sarcoplasmic reticulum in hyper- and hypothyroidism. Biochim Biophys Acta. 1971 Nov 12;252(2):324–327. doi: 10.1016/0304-4165(71)90013-4. [DOI] [PubMed] [Google Scholar]
- Tada M., Kirchberger M. A., Repke D. I., Katz A. M. The stimulation of calcium transport in cardiac sarcoplasmic reticulum by adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1974 Oct 10;249(19):6174–6180. [PubMed] [Google Scholar]
- Tsai J. S., Chen A. Effect of L-triiodothyronine on (--)3H-dihydroalprenolol binding and cyclic AMP response to (--)adrenaline in cultured heart cells. Nature. 1978 Sep 14;275(5676):138–140. doi: 10.1038/275138a0. [DOI] [PubMed] [Google Scholar]
- Waldstein S. S. Thyroid-catecholamine interrelations. Annu Rev Med. 1966;17:123–132. doi: 10.1146/annurev.me.17.020166.001011. [DOI] [PubMed] [Google Scholar]
- Watanabe A. M., Besch H. R., Jr Interaction between cyclic adenosine monophosphate and cyclic gunaosine monophosphate in guinea pig ventricular myocardium. Circ Res. 1975 Sep;37(3):309–317. doi: 10.1161/01.res.37.3.309. [DOI] [PubMed] [Google Scholar]
- Weber H., Rosen O. M. Purification of a protein inhibitor of adenosine 3':5'-monophosphate-dependent protein kinase from bovine myocardium by a non-denaturing procedure. J Cyclic Nucleotide Res. 1977 Dec;3(6):415–424. [PubMed] [Google Scholar]
- Wildenthal K. Studies of fetal mouse hearts in organ culture: influence of prolonged exposure to triiodothyronine on cardiac responsiveness to isoproterenol, glucagon, theophylline, acetylcholine and dibutyryl cyclic 3',5'-adenosine monophosphate. J Pharmacol Exp Ther. 1974 Aug;190(2):272–279. [PubMed] [Google Scholar]
- Wildenthal K. Studies of isolated fetal mouse hearts in organ culture. Evidence for a direct effect of triiodothyronine in enhancing cardiac responsiveness to norepinephrine. J Clin Invest. 1972 Oct;51(10):2702–2709. doi: 10.1172/JCI107089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. T., Lefkowitz R. J., Watanabe A. M., Hathaway D. R., Besch H. R., Jr Thyroid hormone regulation of beta-adrenergic receptor number. J Biol Chem. 1977 Apr 25;252(8):2787–2789. [PubMed] [Google Scholar]
- Young B. A., McNeill J. H. The effect of noradrenaline and tyramine on cardiac contractility, cyclic AMP, and phosphorylase a in normal and hyperthyroid rats. Can J Physiol Pharmacol. 1974 Jun;52(3):375–383. doi: 10.1139/y74-053. [DOI] [PubMed] [Google Scholar]



