Abstract
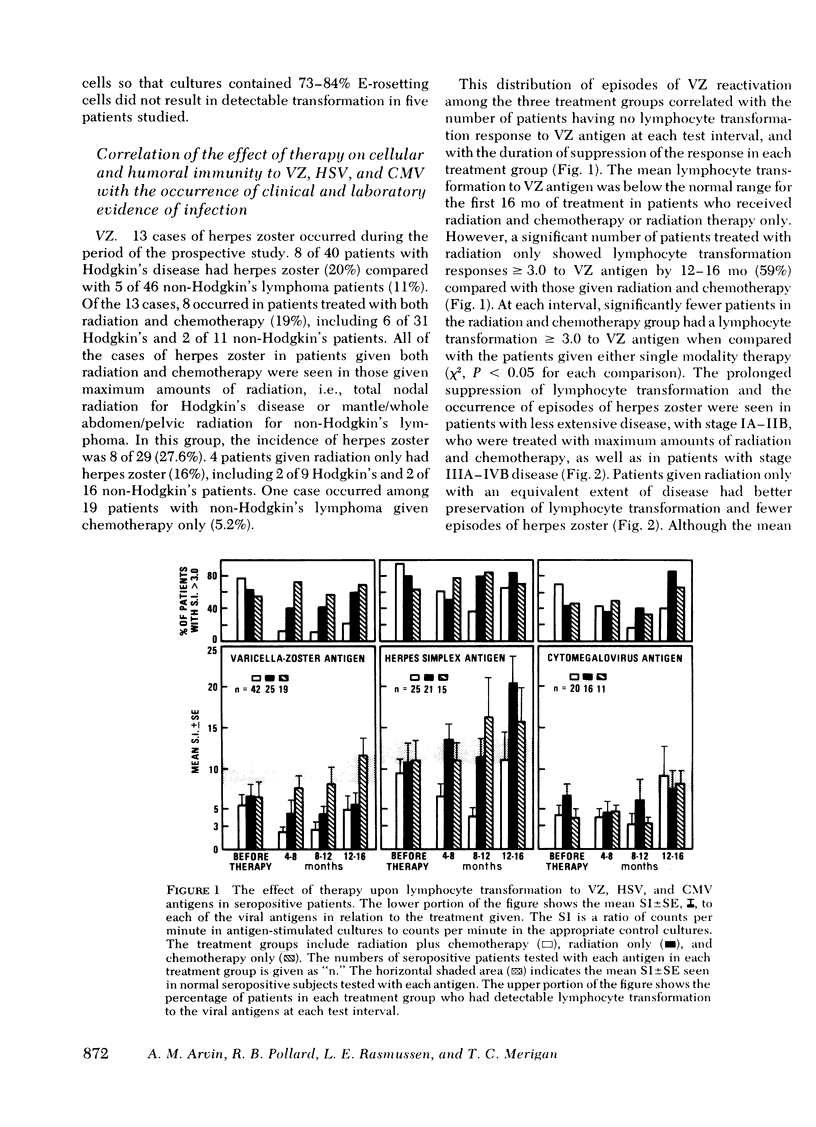
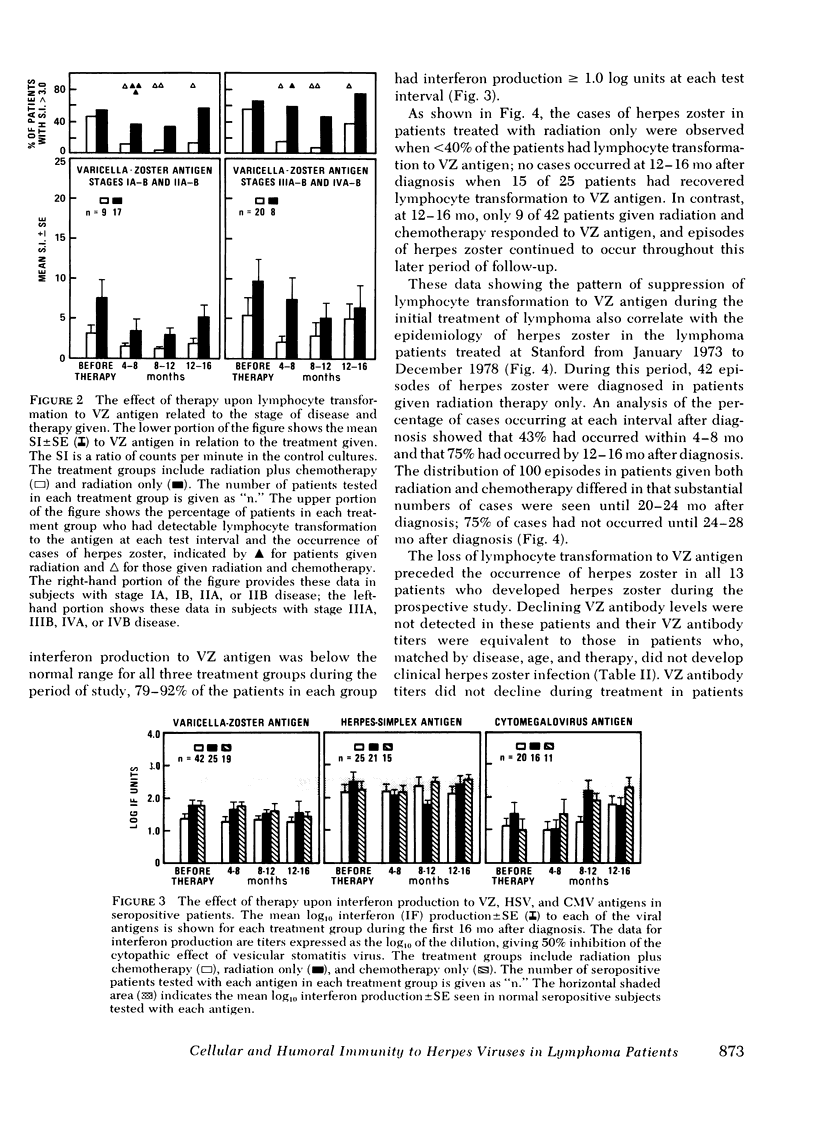
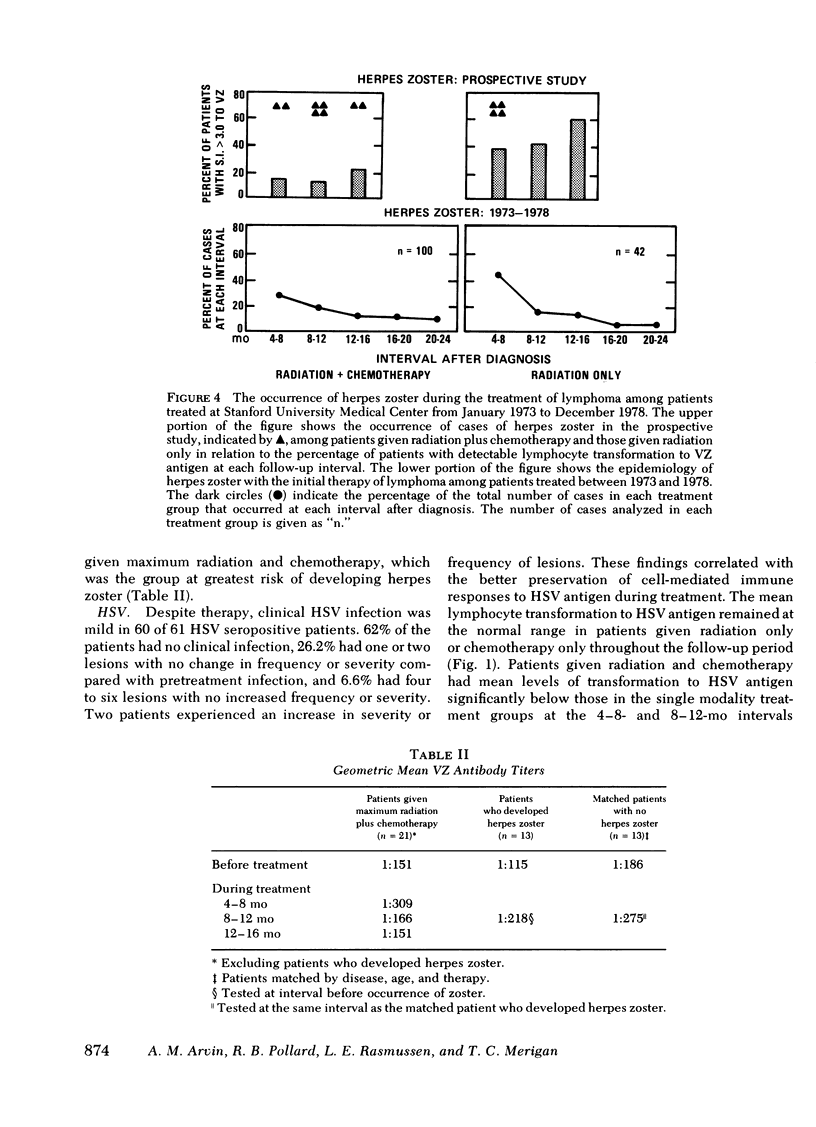
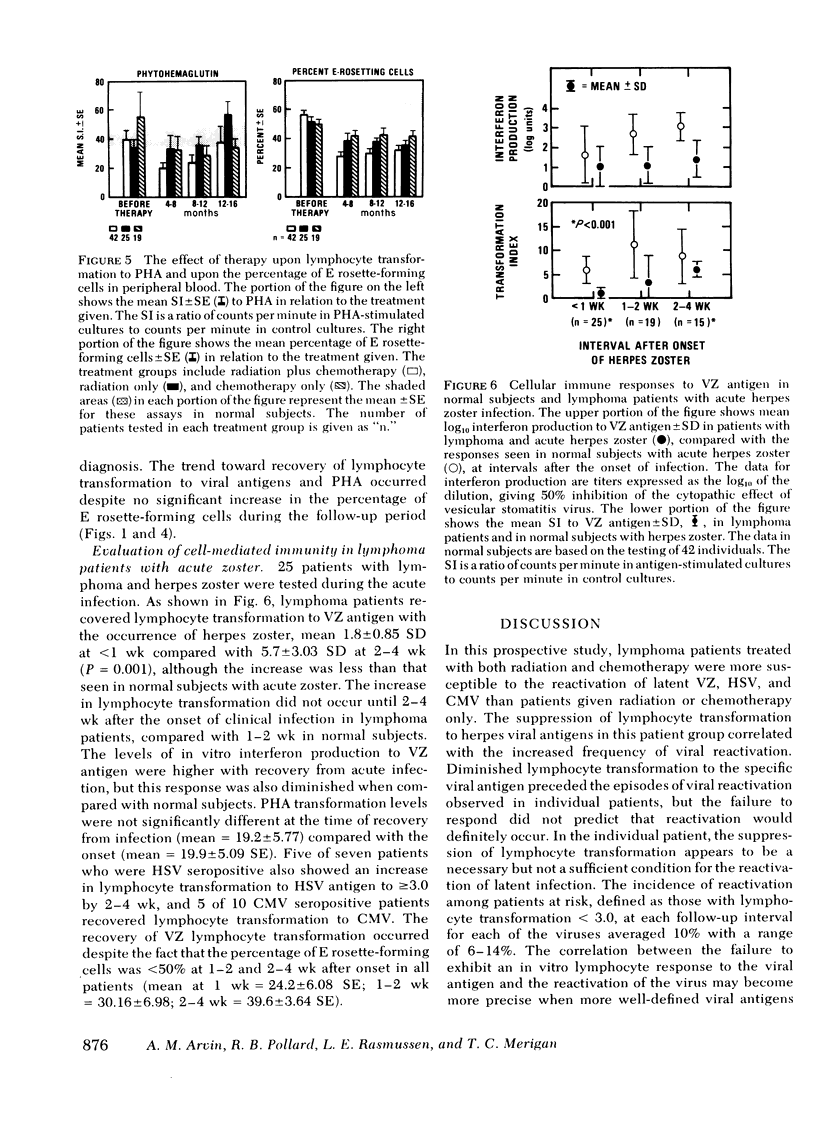
86 patients with lymphoma were evaluated prospectively for clinical and laboratory evidence of recurrent varicella-zoster, herpes simplex, and cytomegalovirus infections during the first 16 mo of treatment. Cellular immunity to the viral antigens was measured by in vitro lymphocyte transformation and interferon production. Antibody titers and nonspecific measures of cellular immunity, including T-cell quantitation and transformation to phytohemagglutinin, were also assessed. The patients treated with radiation and chemotherapy had the highest incidence of reactivation of each of the viruses (15-19%). Greater susceptibility to herpes viral reactivation in these patients correlated with suppression of cell-mediated immunity to the specific virus. In individual patients, suppression of cellular immunity to the specific herpes viral antigen preceded each episode of reactivation, but recurrent infection did not occur in all patients with diminished specific lymphocyte transformation. Absence of the response appears to be a necessary but not a sufficient condition for the recrudescence of latent infection. Better preservation of cellular immunity to herpes simplex antigen during treatment was associated with infrequent reactivation of herpes simplex. In 25 patients with acute herpes zoster, uncomplicated recovery from the infection was accompanied by the development of lymphocyte transformation and interferon production to varicella-zoster antigen. Quantitation of T-cell numbers and phytohemagglutinin transformation did not correlate with the presence of viral cellular immunity in treated patients. Responses returned while T-cell numbers were low, and the recovery of phytohemagglutinin transformation often preceded recovery of the responses to viral antigens. Although some patients had deficiencies in viral cellular immunity at diagnosis, the duration of the suppression of specific antiviral responses resulting from treatment appears to be the most important factor predisposing to the recurrence of herpes infections in lymphoma patients.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvin A. M., Pollard R. B., Rasmussen L. E., Merigan T. C. Selective impairment of lymphocyte reactivity to varicella-zoster virus antigen among untreated patients with lymphoma. J Infect Dis. 1978 May;137(5):531–540. doi: 10.1093/infdis/137.5.531. [DOI] [PubMed] [Google Scholar]
- Aston D. L., Cohen A., Spindler M. A. Herpesvirus hominis infection in patients with myeloproliferative and lymphoproliferative disorders. Br Med J. 1972 Nov 25;4(5838):462–465. doi: 10.1136/bmj.4.5838.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts R. F., Hanshaw J. B. Cytomegalovirus (CMV) in the compromised host(s). Annu Rev Med. 1977;28:103–110. doi: 10.1146/annurev.me.28.020177.000535. [DOI] [PubMed] [Google Scholar]
- Bobrove A. M., Strober S., Herzenberg L. A., DePamphilis J. D. Identification and quantitation of thymus-derived lymphocytes in human peripheral blood. J Immunol. 1974 Feb;112(2):520–527. [PubMed] [Google Scholar]
- Dolin R., Reichman R. C., Mazur M. H., Whitley R. J. NIH conference. Herpes zoster-varicella infections in immunosuppressed patients. Ann Intern Med. 1978 Sep;89(3):375–388. doi: 10.7326/0003-4819-89-3-375. [DOI] [PubMed] [Google Scholar]
- Fuks Z., Strober S., Bobrove A. M., Sasazuki T., McMichael A., Kaplan H. S. Long term effects of radiation of T and B lymphocytes in peripheral blood of patients with Hodgkin's disease. J Clin Invest. 1976 Oct;58(4):803–814. doi: 10.1172/JCI108532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuks Z., Strober S., Kaplan H. S. Interaction between serum factors and T lymphocytes in Hodgkin's disease. Use as a diagnostic test. N Engl J Med. 1976 Dec 2;295(23):1273–1278. doi: 10.1056/NEJM197612022952301. [DOI] [PubMed] [Google Scholar]
- Gallagher J. G., Merigan T. C. Prolonged herpes-zoster infection associated with immunosuppressive therapy. Ann Intern Med. 1979 Dec;91(6):842–846. doi: 10.7326/0003-4819-91-6-842. [DOI] [PubMed] [Google Scholar]
- Goffinet D. R., Glatstein E. J., Merigan T. C. Herpes Zoster-Varicella infections and lymphoma. Ann Intern Med. 1972 Feb;76(2):235–240. doi: 10.7326/0003-4819-76-2-235. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Bankhurst A. D., Peake G. T., Saiki J. H., Williams R. C., Jr Prostaglandin-producing suppressor cells in Hodgkin's disease. N Engl J Med. 1977 Nov 3;297(18):963–968. doi: 10.1056/NEJM197711032971802. [DOI] [PubMed] [Google Scholar]
- Haahr S., Rasmussen L., Merigan T. C. Lymphocyte transformation and interferon production in human mononuclear cell microcultures for assay of cellular immunity to herpes simplex virus. Infect Immun. 1976 Jul;14(1):47–54. doi: 10.1128/iai.14.1.47-54.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren K. M., Douglas R. G., Jr, Couch R. B. Significance of Herpesvirus hominis in respiratory secretions of man. N Engl J Med. 1968 Mar 7;278(10):517–523. doi: 10.1056/NEJM196803072781001. [DOI] [PubMed] [Google Scholar]
- Luby J. P., Ramirez-Ronda C., Rinner S., Hull A., Vergne-Marini P. A longitudinal study of varicella-zoster virus infections in renal transplant recipients. J Infect Dis. 1977 Apr;135(4):659–663. doi: 10.1093/infdis/135.4.659. [DOI] [PubMed] [Google Scholar]
- Muller S. A., Herrmann E. C., Jr, Winkelmann R. K. Herpes simplex infections in hematologic malignancies. Am J Med. 1972 Jan;52(1):102–114. doi: 10.1016/0002-9343(72)90012-5. [DOI] [PubMed] [Google Scholar]
- Patel P. A., Yoonessi S., O'Malley J., Freeman A., Gershon A., Ogra P. L. Cell-mediated immunity to varicella-zoster virus infection in subjects with lymphoma or leukemia. J Pediatr. 1979 Feb;94(2):223–230. doi: 10.1016/s0022-3476(79)80828-8. [DOI] [PubMed] [Google Scholar]
- Pollard R. B., Rand K. H., Arvin A. M., Merigan T. C. Cell-mediated immunity of cytomegalovirus infection in normal subjects and cardiac transplant patients. J Infect Dis. 1978 May;137(5):541–549. doi: 10.1093/infdis/137.5.541. [DOI] [PubMed] [Google Scholar]
- Rand K. H., Rasmussen L. E., Pollard R. B., Arvin A., Merigan T. C. Cellular immunity and herpesvirus infections in cardiac-transplant patients. N Engl J Med. 1977 Jun 16;296(24):1372–1377. doi: 10.1056/NEJM197706162962402. [DOI] [PubMed] [Google Scholar]
- Rasmussen L., Merigan T. C. Role of T lymphocytes in cellular immune responses during herpes simplex virus infection in humans. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3957–3961. doi: 10.1073/pnas.75.8.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckdeschel J. C., Schimpff S. C., Smyth A. C., Mardiney M. R., Jr Herpes zoster and impaired cell-associated immunity to the varicella-zoster virus in patients with Hodgkin's disease. Am J Med. 1977 Jan;62(1):77–85. doi: 10.1016/0002-9343(77)90352-7. [DOI] [PubMed] [Google Scholar]
- Schimpff S., Serpick A., Stoler B., Rumack B., Mellin H., Joseph J. M., Block J. Varicella-Zoster infection in patients with cancer. Ann Intern Med. 1972 Feb;76(2):241–254. doi: 10.7326/0003-4819-76-2-241. [DOI] [PubMed] [Google Scholar]
- Stratton J. A., Byfield P. E., Byfield J. E., Small R. C., Benfield J., Pilch Y. A comparison of the acute effects of radiation therapy, including or excluding the thymus, on the lymphocyte subpopulations of cancer patients. J Clin Invest. 1975 Jul;56(1):88–97. doi: 10.1172/JCI108084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]



