Abstract
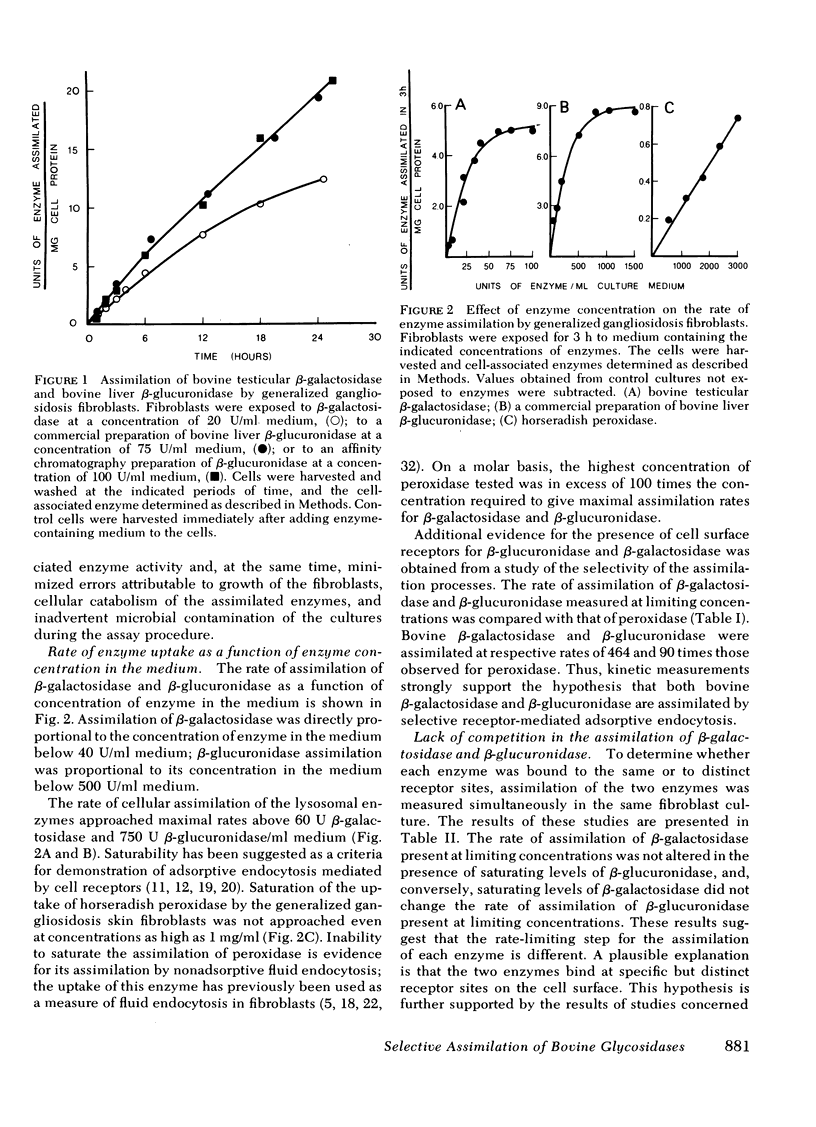
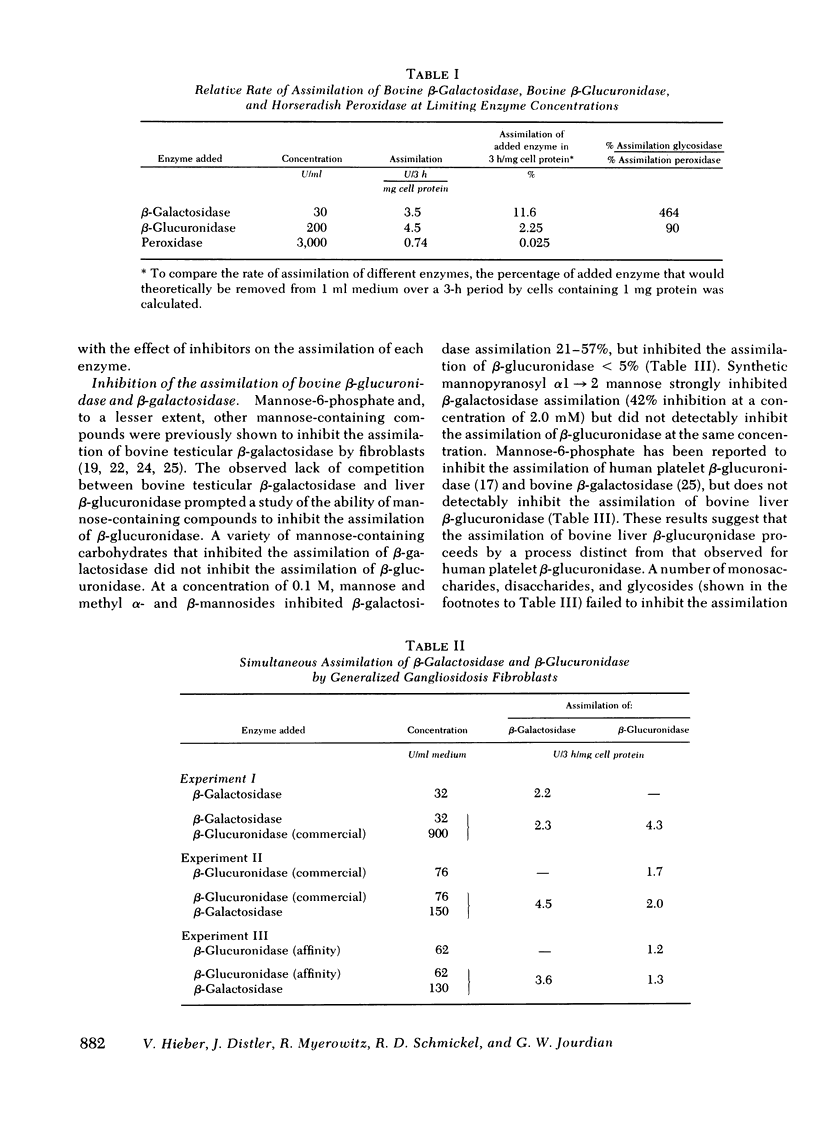
Bovine liver beta-glucuronidase and testicular beta-galactosidase were assimilated by generalized gangliosidosis fibroblasts at respectively rates of 90 and 464 times the rate of assimilation of horseradish peroxidase. Assimilation of either of the two enzymes by the fibroblasts was saturable, suggesting the participation of receptor-mediated adsorptive endocytosis for internalization. The rate of assimilation of either enzyme was not affected by high levels of the other enzyme, suggesting that distinct receptors for each enzyme occur on the fibroblasts' cell surface. Furthermore, although assimilation of beta-galactosidase was inhibited by mannose, methyl mannosides, mannosyl alpha 1 leads to 2 mannose, and mannose-6-phosphate, these compounds did not detectably inhibit the assimilation of beta-glucuronidase. These results suggest that testicular beta-galactosidase was assimilated by the well-established phosphomannosyl recognition system. However, liver beta-glucuronidase was assimilated by a distinct, noncompeting, and as yet undefined, recognition system.
Full text
PDF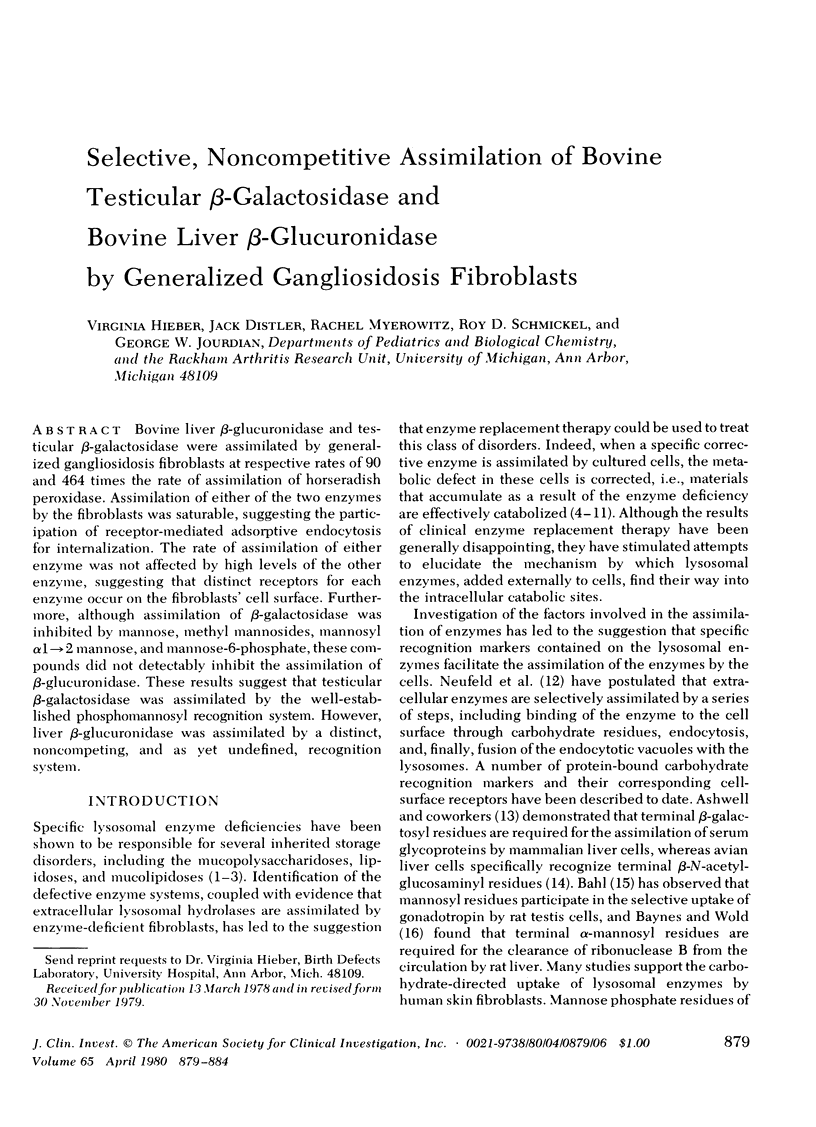



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahl O. P. Human chorionic gonadotropin, its receptor and mechanism of action. Fed Proc. 1977 Jul;36(8):2119–2127. [PubMed] [Google Scholar]
- Baynes J. W., Wold F. Effect of glycosylation on the in vivo circulating half-life of ribonuclease. J Biol Chem. 1976 Oct 10;251(19):6016–6024. [PubMed] [Google Scholar]
- Cantz M., Kresse H. Sandhoff disease: defective glycosaminoglycan catabolism in cultured fibroblasts and its correction by beta-N-acetylhexosaminidase. Eur J Biochem. 1974 Sep 16;47(3):581–590. doi: 10.1111/j.1432-1033.1974.tb03729.x. [DOI] [PubMed] [Google Scholar]
- Distler J. J., Jourdian G. W. beta-Galactosidase from bovine testes. Methods Enzymol. 1978;50:514–520. doi: 10.1016/0076-6879(78)50055-4. [DOI] [PubMed] [Google Scholar]
- Distler J., Hieber V., Sahagian G., Schmickel R., Jourdian G. W. Identification of mannose 6-phosphate in glycoproteins that inhibit the assimilation of beta-galactosidase by fibroblasts. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4235–4239. doi: 10.1073/pnas.76.9.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. W., Cantz M., Neufeld E. F. A -glucuronidase deficiency mucopolysaccharidosis: studies in cultured fibroblasts. Arch Biochem Biophys. 1973 Mar;155(1):32–38. doi: 10.1016/s0003-9861(73)80006-2. [DOI] [PubMed] [Google Scholar]
- Harris R. G., Rowe J. J., Stewart P. S., Williams D. C. Affinity chromatography of -glucuronidase. FEBS Lett. 1973 Jan 15;29(2):189–192. doi: 10.1016/0014-5793(73)80558-7. [DOI] [PubMed] [Google Scholar]
- Hieber V., Distler J., Myerowitz R., Schmickel R. D., Jourdian G. W. The role of glycosidically bound mannose in the assimilation of beta-galactosidase by generalized gangliosidosis fibroblasts. Biochem Biophys Res Commun. 1976 Dec 6;73(3):710–717. doi: 10.1016/0006-291x(76)90868-8. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Weissmann G. Genetic disorders of lysosomes. Prog Med Genet. 1976;1:49–101. [PubMed] [Google Scholar]
- Kaplan A., Achord D. T., Sly W. S. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc Natl Acad Sci U S A. 1977 May;74(5):2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A., Fischer D., Achord D., Sly W. Phosphohexosyl recognition is a general characteristic of pinocytosis of lysosomal glycosidases by human fibroblasts. J Clin Invest. 1977 Nov;60(5):1088–1093. doi: 10.1172/JCI108860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lagunoff D., Nicol D. M., Pritzi P. Uptake of beta-glucuronidase by deficient human fibroblasts. Lab Invest. 1973 Oct;29(4):449–453. [PubMed] [Google Scholar]
- Lunney J., Ashwell G. A hepatic receptor of avian origin capable of binding specifically modified glycoproteins. Proc Natl Acad Sci U S A. 1976 Feb;73(2):341–343. doi: 10.1073/pnas.73.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell A. G., Gregoriadis G., Scheinberg I. H., Hickman J., Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971 Mar 10;246(5):1461–1467. [PubMed] [Google Scholar]
- Neufeld E. F., Lim T. W., Shapiro L. J. Inherited disorders of lysosomal metabolism. Annu Rev Biochem. 1975;44:357–376. doi: 10.1146/annurev.bi.44.070175.002041. [DOI] [PubMed] [Google Scholar]
- Neufeld E. F., Sando G. N., Garvin A. J., Rome L. H. The transport of lysosomal enzymes. J Supramol Struct. 1977;6(1):95–101. doi: 10.1002/jss.400060108. [DOI] [PubMed] [Google Scholar]
- O'Brien J. S., Miller A. L., Loverde A. W., Veath M. L. Sanfilippo disease type B: enzyme replacement and metabolic correction in cultured fibroblasts. Science. 1973 Aug 24;181(4101):753–755. doi: 10.1126/science.181.4101.753. [DOI] [PubMed] [Google Scholar]
- Plapp B. V., Cole R. D. Purification and characterization of bovine liver beta-glucuronidase. Arch Biochem Biophys. 1966 Sep 26;116(1):193–206. doi: 10.1016/0003-9861(66)90027-0. [DOI] [PubMed] [Google Scholar]
- Sando G. N., Neufeld E. F. Recognition and receptor-mediated uptake of a lysosomal enzyme, alpha-l-iduronidase, by cultured human fibroblasts. Cell. 1977 Nov;12(3):619–627. doi: 10.1016/0092-8674(77)90262-8. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Silver J. M., Cohn Z. A. Pinocytosis in fibroblasts. Quantitative studies in vitro. J Cell Biol. 1974 Dec;63(3):949–969. doi: 10.1083/jcb.63.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawser L. D., Touster O. Demonstration of a rat liver microsomal binding protein specific for beta-glucuronidase. J Biol Chem. 1979 May 25;254(10):3716–3719. [PubMed] [Google Scholar]
- Ullrich K., Mersmann G., Weber E., Von Figura K. Evidence for lysosomal enzyme recognition by human fibroblasts via a phosphorylated carbohydrate moiety. Biochem J. 1978 Mar 15;170(3):643–650. doi: 10.1042/bj1700643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura K., Kresse H. Quantitative aspects of pinocytosis and the intracellular fate of N-acetyl-alpha-D-glucosaminidase in Sanfilippo B fibroblasts. J Clin Invest. 1974 Jan;53(1):85–90. doi: 10.1172/JCI107563. [DOI] [PMC free article] [PubMed] [Google Scholar]


