Abstract
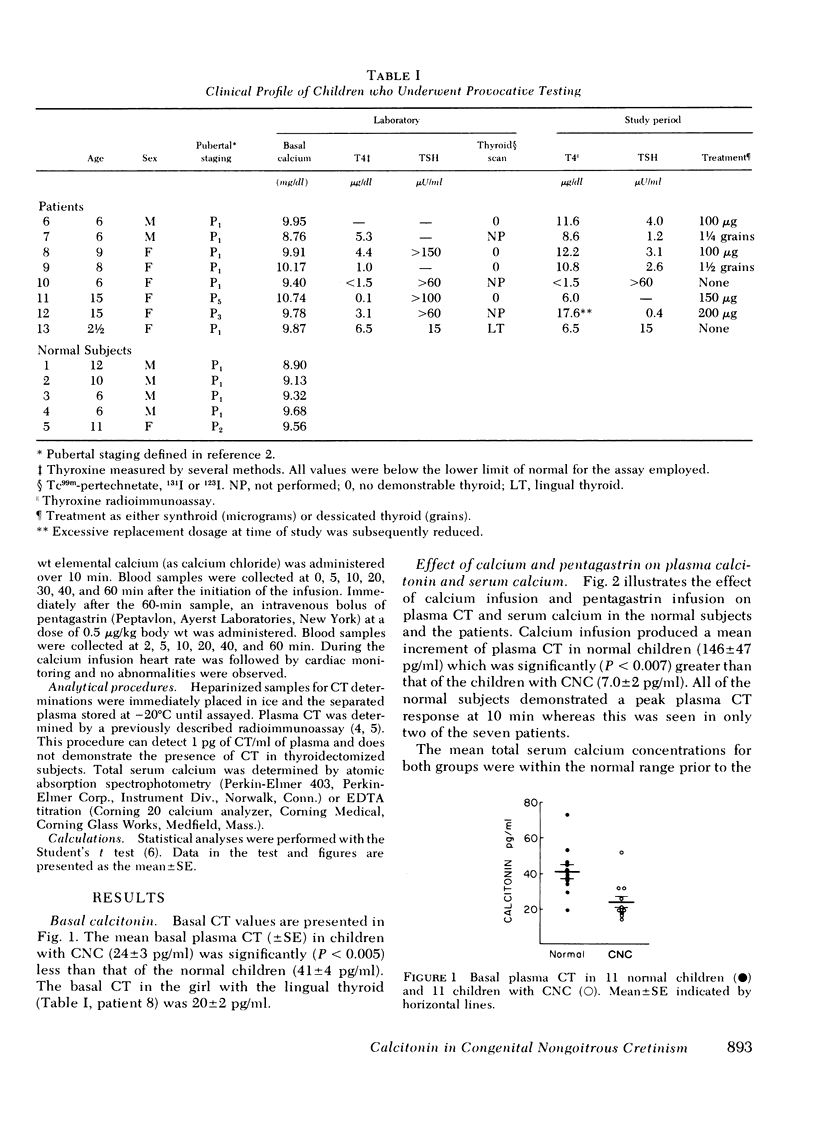
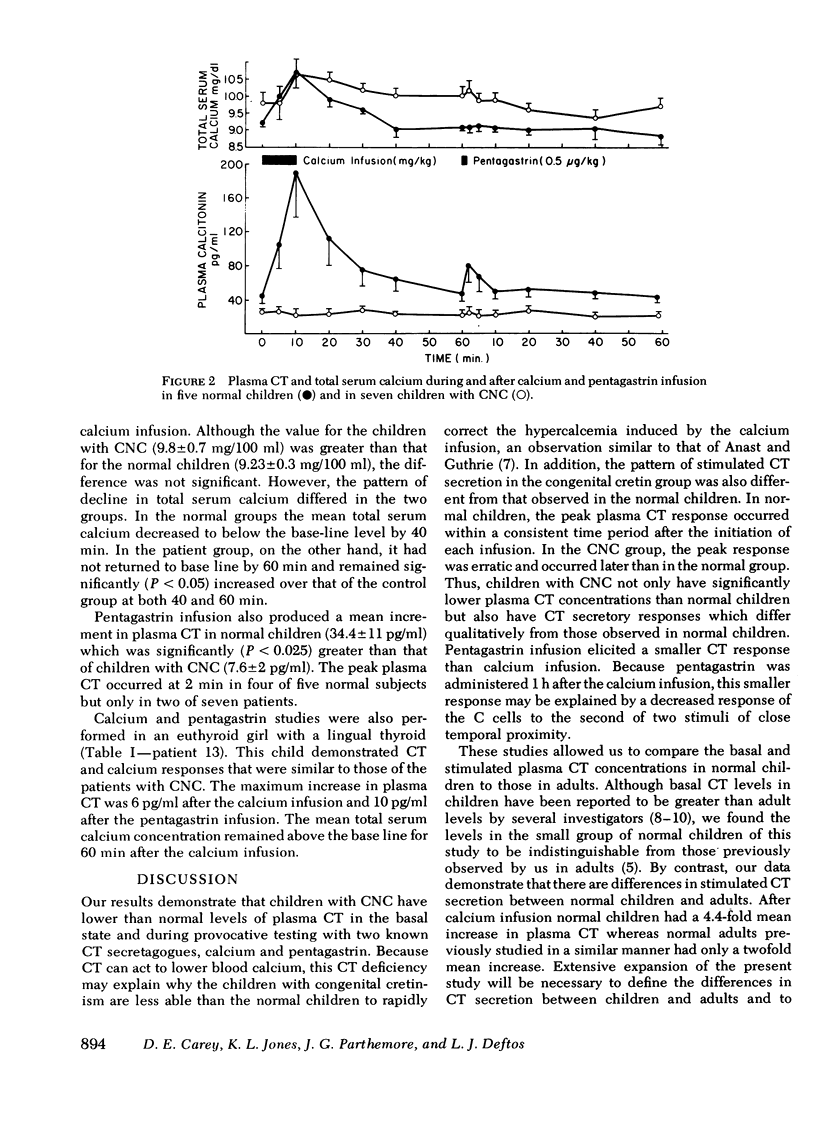
Plasma calcitonin (CT) was measured in the basal state and/or during provocative tests of hormone secretion in 11 children with congenital non-goitrous cretinism (CNC), in 1 girl with a lingual thyroid, and in 11 normal children. Basal and stimulated CT concentrations were significantly lower in the patients with CNC than in the normal subjects. Mean basal CT (+/- SE) was 41 +/- 4 pg/ml in the normal children, 24 +/- 3 pg/ml in the children with CNC, and 20 +/- 2 pg/ml in the patient with the lingual thyroid. The mean incremental CT responses to calcium infusion were 7.0 +/- 2 pg/ml in the children with CNC, 6.0 pg/ml in the patient with the lingual thyroid, and 146 +/- 47 pg/ml in the normal children. The children with CNC also demonstrated a significant delay in the return of the total serum calcium to basal level after the calcium infusion. The mean incremental CT response after infusion of pentagastrin was 7.6 +/- 2 pg/ml in the children with CNC, 10.0 pg/ml in the child with the lingual thyroid, and 34.4 +/- 11 pg/ml in the normal children. These data indicate that CT deficiency is present in children with CNC and suggest that the deficiency is a consequence of the defective embryologic development of the thyroid gland.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergman L., Kjellmer I., Selstam U. Calcitonin and parathyroid hormone--relation to early neonatal hypocalcemia in infants of diabetic mothers. Biol Neonate. 1974;24(1):151–160. doi: 10.1159/000240646. [DOI] [PubMed] [Google Scholar]
- Hayek A., Bauman R. A., Crawford J. D. 99m Tc pertechnetate for detection of cryptic thyroid tissue in childhood hypothyroidism. J Pediatr. 1971 Sep;79(3):466–468. doi: 10.1016/s0022-3476(71)80158-0. [DOI] [PubMed] [Google Scholar]
- Nunez E. A., Gershon M. D. Cytophysiology of thyroid parafollicular cells. Int Rev Cytol. 1978;52:1–80. doi: 10.1016/s0074-7696(08)60753-6. [DOI] [PubMed] [Google Scholar]
- Parthemore J. G., Deftos L. J. Calcitonin secretion in normal human subjects. J Clin Endocrinol Metab. 1978 Jul;47(1):184–188. doi: 10.1210/jcem-47-1-184. [DOI] [PubMed] [Google Scholar]
- Parthemore J. G., Deftos L. J. Calcitonin secretion in primary hyperparathyroidism. J Clin Endocrinol Metab. 1979 Aug;49(2):223–226. doi: 10.1210/jcem-49-2-223. [DOI] [PubMed] [Google Scholar]
- Samaan N. A., Anderson G. D., Adam-Mayne M. E. Immunoreactive calcitonin in the mother, neonate, child and adult. Am J Obstet Gynecol. 1975 Mar 1;121(5):622–625. doi: 10.1016/0002-9378(75)90462-7. [DOI] [PubMed] [Google Scholar]
- Shainkin-Kerstenbaum R., Funkenstein B., Conforti A., Shani S., Berlyne G. M. Serum calcitonin and blood mineral interrelationships in normal children aged six to twelve years. Pediatr Res. 1977 Feb;11(2):112–116. doi: 10.1203/00006450-197702000-00006. [DOI] [PubMed] [Google Scholar]
- Weichert R. F., 3rd The neural ectodermal origin of the peptide-secreting endocrine glands. A unifying concept for the etiology of multiple endocrine adenomatosis and the inappropriate secretion of peptide hormones by nonendocrine tumors. Am J Med. 1970 Aug;49(2):232–241. doi: 10.1016/s0002-9343(70)80079-1. [DOI] [PubMed] [Google Scholar]
- Wolfe H. J., DeLellis R. A., Voelkel E. F., Tashjian A. H., Jr Distribution of calcitonin-containing cells in the normal neonatal human thyroid gland: a correlation of morphology with peptide content. J Clin Endocrinol Metab. 1975 Dec;41(06):1076–1081. doi: 10.1210/jcem-41-6-1076. [DOI] [PubMed] [Google Scholar]


