Abstract
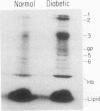
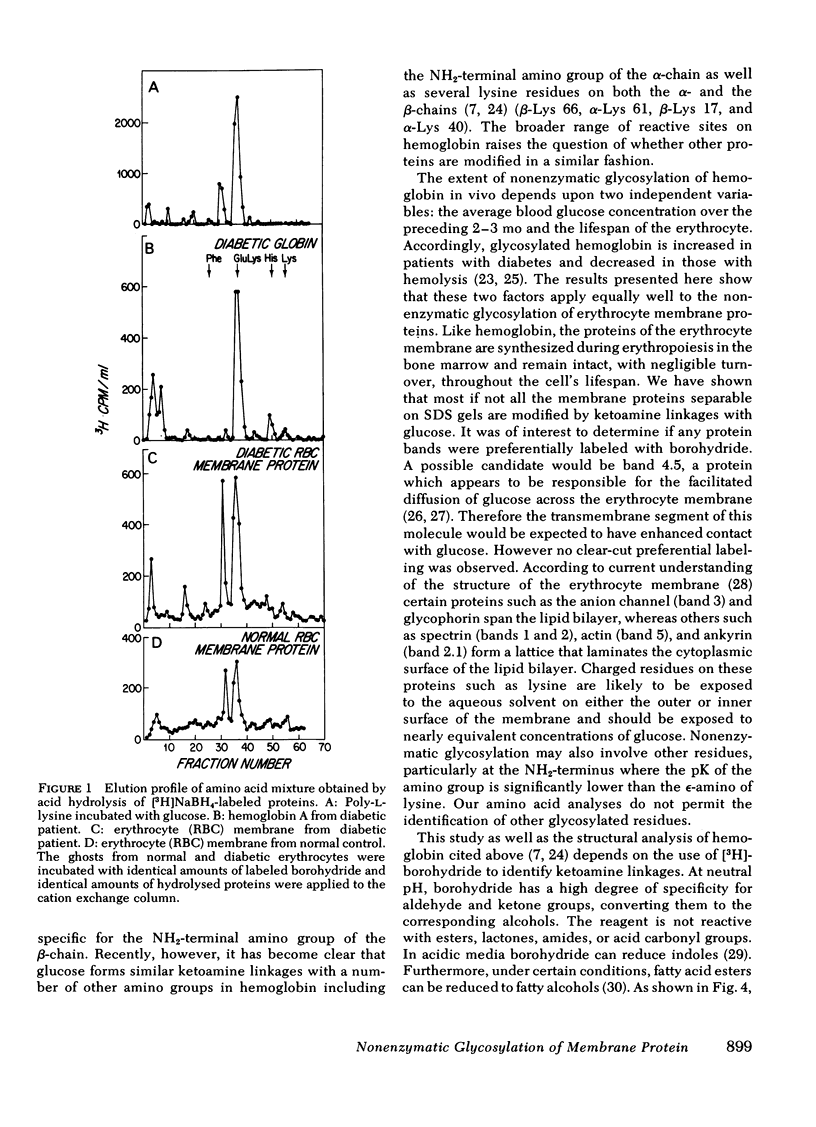
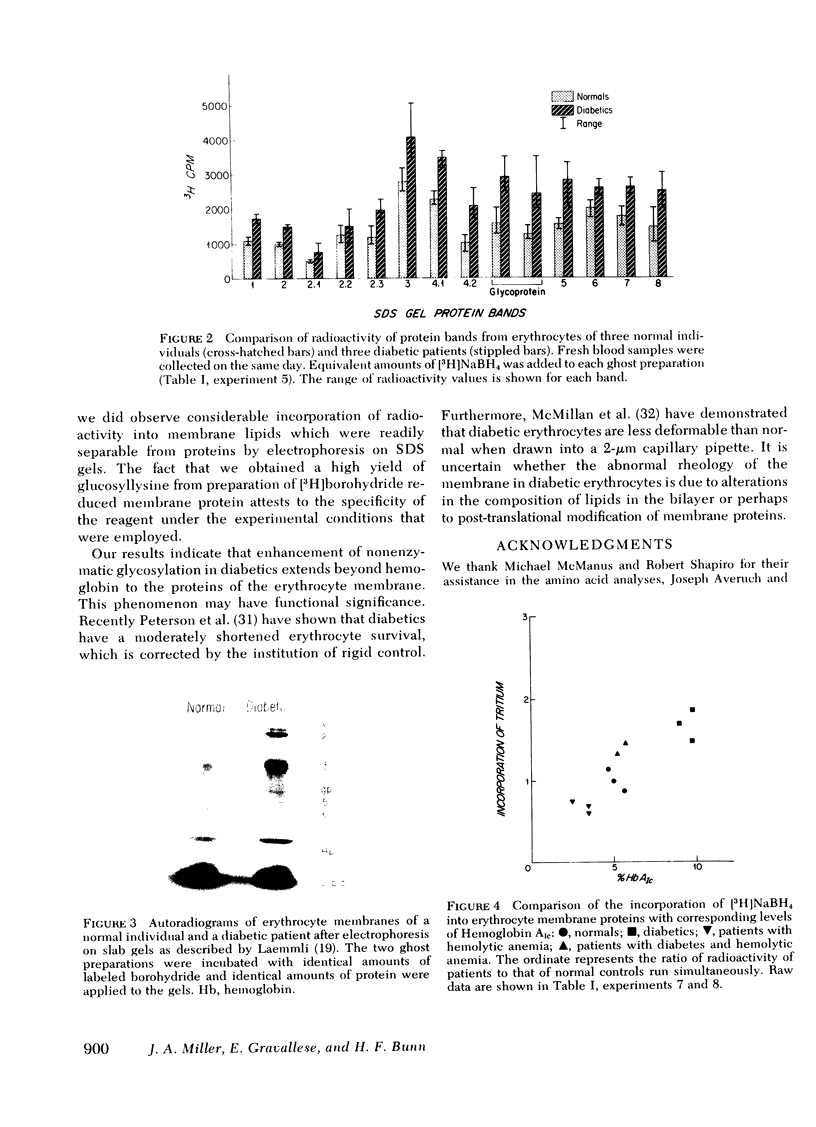
Nonenzymatic glycosylation of proteins of the erythrocyte membrane was determined by incubating erythrocyte ghosts with [3H]borohydride. The incorporation of tritium into protein provides a reliable assay of ketoamine linkages. The membrane proteins from 18 patients with diabetes incorporated twice as much radioactivity as membrane proteins from normal erythrocytes. After acid hydrolysis, amino acid analysis showed that the majority of radioactivity was localized to glucosyllysine. Autoradiograms showed that all of the major proteins of the erythrocyte membrane, separated by electrophoresis on sodium dodecyl sulfate gels, contained ketoamine linkages. No protein bands in either normal or diabetic erythrocytes showed significant preferential labeling. Erythrocyte membranes from three patients with hemolytic anemia showed reduced incorporation of tritium from [3H]-borohydride, indicating decreased nonenzymatic glycosylation. Two patients with diabetes and hemolytic anemia had incorporation of radioactivity similar to that of normal individuals. In these groups of patients the incorporation of tritium into erythrocyte membrane proteins correlated with levels of hemoglobin AIc. Thus the modification of membrane proteins like that of hemoglobin depends on blood glucose levels as well as erythrocyte age. These studies show that the enhanced nonenzymatic glycosylation of proteins in diabetics extends beyond hemoglobin to the proteins of the erythrocyte membrane and probably affects other proteins that have slow turnover and are exposed to high concentrations of glucose.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J., Robins S. P., Tanner M. J. Reducible components in the proteins of human erythrocyte membrane. Biochim Biophys Acta. 1976 May 20;434(1):51–57. doi: 10.1016/0005-2795(76)90034-9. [DOI] [PubMed] [Google Scholar]
- Bookchin R. M., Gallop P. M. Structure of hemoglobin AIc: nature of the N-terminal beta chain blocking group. Biochem Biophys Res Commun. 1968 Jul 11;32(1):86–93. doi: 10.1016/0006-291x(68)90430-0. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Gabbay K. H., Gallop P. M. The glycosylation of hemoglobin: relevance to diabetes mellitus. Science. 1978 Apr 7;200(4337):21–27. doi: 10.1126/science.635569. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Haney D. N., Gabbay K. H., Gallop P. M. Further identification of the nature and linkage of the carbohydrate in hemoglobin A1c. Biochem Biophys Res Commun. 1975 Nov 3;67(1):103–109. doi: 10.1016/0006-291x(75)90289-2. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Haney D. N., Kamin S., Gabbay K. H., Gallop P. M. The biosynthesis of human hemoglobin A1c. Slow glycosylation of hemoglobin in vivo. J Clin Invest. 1976 Jun;57(6):1652–1659. doi: 10.1172/JCI108436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F., Shapiro R., McManus M., Garrick L., McDonald M. J., Gallop P. M., Gabbay K. H. Structural heterogeneity of human hemoglobin A due to nonenzymatic glycosylation. J Biol Chem. 1979 May 25;254(10):3892–3898. [PubMed] [Google Scholar]
- Cerami A., Koenig R., Peterson C. M. Haemoglobin Alc and diabetes mellitus. Br J Haematol. 1978 Jan;38(1):1–4. doi: 10.1111/j.1365-2141.1978.tb07101.x. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Day J. F., Thornburg R. W., Thorpe S. R., Baynes J. W. Nonenzymatic glucosylation of rat albumin. Studies in vitro and in vivo. J Biol Chem. 1979 Oct 10;254(19):9394–9400. [PubMed] [Google Scholar]
- Day J. F., Thorpe S. R., Baynes J. W. Nonenzymatically glucosylated albumin. In vitro preparation and isolation from normal human serum. J Biol Chem. 1979 Feb 10;254(3):595–597. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Flückiger R., Winterhalter K. H. In vitro synthesis of hemoglobin AIc. FEBS Lett. 1976 Dec 1;71(2):356–360. doi: 10.1016/0014-5793(76)80969-6. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H., Hasty K., Breslow J. L., Ellison R. C., Bunn H. F., Gallop P. M. Glycosylated hemoglobins and long-term blood glucose control in diabetes mellitus. J Clin Endocrinol Metab. 1977 May;44(5):859–864. doi: 10.1210/jcem-44-5-859. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H., Sosenko J. M., Banuchi G. A., Mininsohn M. J., Flückiger R. Glycosylated hemoglobins: increased glycosylation of hemoglobin A in diabetic patients. Diabetes. 1979 Apr;28(4):337–340. doi: 10.2337/diab.28.4.337. [DOI] [PubMed] [Google Scholar]
- Gonen B., Rubenstein A., Rochman H., Tanega S. P., Horwitz D. L. Haemoglobin A1: An indicator of the metabolic control of diabetic patients. Lancet. 1977 Oct 8;2(8041):734–737. doi: 10.1016/s0140-6736(77)90237-9. [DOI] [PubMed] [Google Scholar]
- HORTON B. F., HUISMAN T. H. STUDIES ON THE HETEROGENEITY OF HAEMOGLOBIN. VII. MINOR HAEMOGLOBIN COMPONENTS IN HAEMATOLOGICAL DISEASES. Br J Haematol. 1965 May;11:296–304. doi: 10.1111/j.1365-2141.1965.tb06589.x. [DOI] [PubMed] [Google Scholar]
- Holmquist W. R., Schroeder W. A. A new N-terminal blocking group involving a Schiff base in hemoglobin AIc. Biochemistry. 1966 Aug;5(8):2489–2503. doi: 10.1021/bi00872a002. [DOI] [PubMed] [Google Scholar]
- Kasahara M., Hinkle P. C. Reconstitution and purification of the D-glucose transporter from human erythrocytes. J Biol Chem. 1977 Oct 25;252(20):7384–7390. [PubMed] [Google Scholar]
- Koenig R. J., Peterson C. M., Jones R. L., Saudek C., Lehrman M., Cerami A. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N Engl J Med. 1976 Aug 19;295(8):417–420. doi: 10.1056/NEJM197608192950804. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T. Functional proteins of the human red blood cell membrane. Semin Hematol. 1979 Jan;16(1):3–20. [PubMed] [Google Scholar]
- McDonald M. J., Shapiro R., Bleichman M., Solway J., Bunn H. F. Glycosylated minor components of human adult hemoglobin. Purification, identification, and partial structural analysis. J Biol Chem. 1978 Apr 10;253(7):2327–2332. [PubMed] [Google Scholar]
- McMillan D. E., Utterback N. G., La Puma J. Reduced erythrocyte deformability in diabetes. Diabetes. 1978 Sep;27(9):895–901. doi: 10.2337/diab.27.9.895. [DOI] [PubMed] [Google Scholar]
- Nichols B. W., Safford R. Conversion of lipids to fatty alcohols and lysolipids by NaBH4. Chem Phys Lipids. 1973 Oct;11(3):222–227. doi: 10.1016/0009-3084(73)90024-8. [DOI] [PubMed] [Google Scholar]
- Peterson C. M., Jones R. L., Koenig R. J., Melvin E. T., Lehrman M. L. Reversible hematologic sequelae of diabetes mellitus. Ann Intern Med. 1977 Apr;86(4):425–429. doi: 10.7326/0003-4819-86-4-425. [DOI] [PubMed] [Google Scholar]
- Stevens V. J., Rouzer C. A., Monnier V. M., Cerami A. Diabetic cataract formation: potential role of glycosylation of lens crystallins. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2918–2922. doi: 10.1073/pnas.75.6.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer M. L., Fairweather R., Gallop P. M. Collagen crosslinks: isolation of reduced N -hexosylhydroxylysine from borohydride-reduced calf skin insoluble collagen. Arch Biochem Biophys. 1972 Jul;151(1):137–141. doi: 10.1016/0003-9861(72)90482-1. [DOI] [PubMed] [Google Scholar]