Abstract
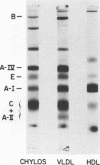
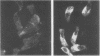
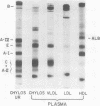
The role of the human intestine has been explored as a site of synthesis of apoA-IV, a major apoprotein of human intestinal triglyceride-rich lipoproteins. Intestinal biopsies were performed on normal volunteers while fasting and after lipid ingestion. Indirect immunofluorescence demonstrated a marked increase in immunofluorescence for apoA-IV during lipid absorption consistent with an increased intracellular content. ApoA-IV comprised 10-13% of chylomicron apoprotein and 24-30% of intestinal very low density lipoprotein (VLDL) as assessed by densitometry of sodium dodecyl sulfate gels of lipoproteins from chylous urine (mesenteric lymphatic-urinary fistula) and thoracic duct lymph (postoperative fistula). After one subject with chyluria ingested 40 g of corn oil, triglyceride excretion in urine was accompanied by an increased excretion of apoA-IV. 11.5 g of triglyceride and 81 mg of apoA-IV were recovered in the urine. In chylous urine 56% of apoA-IV was in the triglyceride-rich lipoproteins (chylomicrons and intestinal VLDL) and 44% in the d > 1.006-g/ml fraction.
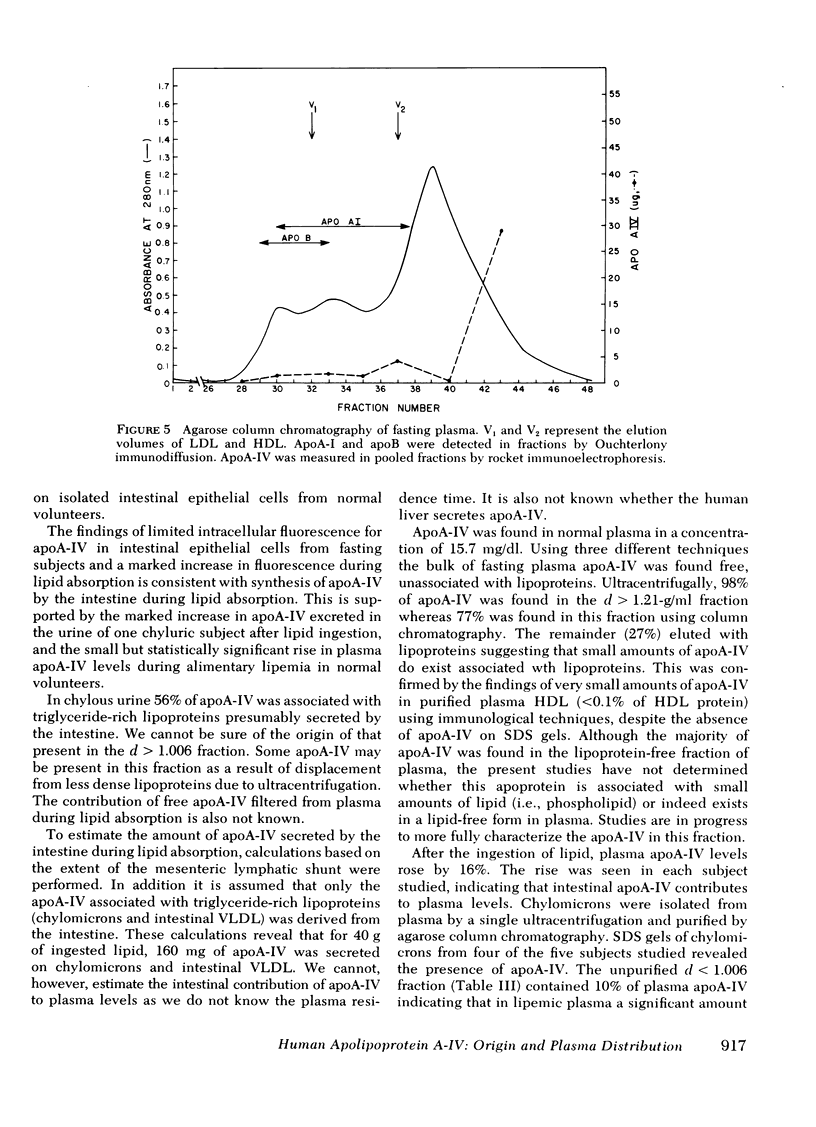
Normal plasma apoA-IV was 15.7±0.9 mg/dl (n = 14) whereas four subjects with abetalipoproteinemia had reduced levels 1.2, 7.6, 9.6, and 8.3 mg/dl, respectively. Lipid feeding in normal volunteers resulted in a rise in plasma apoA-IV (16.1±0.7 mg/dl to 18.5±0.7 mg/dl, n = 5, P < 0.01). In fasting plasma, 98% of apoA-IV was in the d > 1.21-g/ml fraction. In lipemic plasma, 10% of apoA-IV was associated with triglyceride-rich lipoproteins and 90% with the d > 1.21-g/ml fraction. Agarose column chromatography of fasting plasma confirmed that the bulk of plasma apoA-IV is free, unassociated with lipoproteins.
These results demonstrate that apoA-IV is present in human intestinal epithelial cells and is secreted as a chylomicron and VLDL apoprotein. Within fasting plasma most of the apoA-IV is found free, unassociated with lipoproteins. After lipid ingestion apoA-IV is also found in plasma chylomicrons indicating that some apoA-IV remains associated with chylomicrons in plasma during chylomicron metabolism, although some may be transferred from the chylomicron surface.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOMSTRAND R., THORN N. A., AHRENS E. H., Jr The absorption of fats, studied in a patient with chyluria. I. Clinical investigation. Am J Med. 1958 Jun;24(6):958–966. doi: 10.1016/0002-9343(58)90348-6. [DOI] [PubMed] [Google Scholar]
- Beisiegel U., Utermann G. An apolipoprotein homolog of rat apolipoprotein A-IV in human plasma. Isolation and partial characterisation. Eur J Biochem. 1979 Feb 1;93(3):601–608. doi: 10.1111/j.1432-1033.1979.tb12860.x. [DOI] [PubMed] [Google Scholar]
- Brook J. G., Lees R. S., Yules J. H., Cusack B. Tangier disease (alpha-lipoprotein deficiency). JAMA. 1977 Jul 25;238(4):332–334. [PubMed] [Google Scholar]
- Curry M. D., McConathy W. J., Alaupovic P. Quantitative determination of human apolipoprotein D by electroimmunoassay and radial immunodiffusion. Biochim Biophys Acta. 1977 Mar 28;491(1):232–241. doi: 10.1016/0005-2795(77)90059-9. [DOI] [PubMed] [Google Scholar]
- Downing D. T. Photodensitometry in the thin-layer chromatographic analysis of neutral lipids. J Chromatogr. 1968 Nov 5;38(1):91–99. doi: 10.1016/0021-9673(68)85011-3. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Levy R. I. Lipoprotein metabolism. Adv Lipid Res. 1975;13:1–89. [PubMed] [Google Scholar]
- Fielding C. J., Shore V. G., Fielding P. E. A protein cofactor of lecithin:cholesterol acyltransferase. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1493–1498. doi: 10.1016/0006-291x(72)90776-0. [DOI] [PubMed] [Google Scholar]
- Glickman R. M., Green P. H., Lees R. S., Lux S. E., Kilgore A. Immunofluorescence studies of apolipoprotein B in intestinal mucosa. Absence in abetalipoproteinemia. Gastroenterology. 1979 Feb;76(2):288–292. [PubMed] [Google Scholar]
- Glickman R. M., Green P. H., Lees R. S., Tall A. Apoprotein A-I synthesis in normal intestinal mucosa and in Tangier disease. N Engl J Med. 1978 Dec 28;299(26):1424–1427. doi: 10.1056/NEJM197812282992602. [DOI] [PubMed] [Google Scholar]
- Glickman R. M., Khorana J., Kilgore A. Localization of apolipoprotein B in intestinal epithelial cells. Science. 1976 Sep 24;193(4259):1254–1255. doi: 10.1126/science.183265. [DOI] [PubMed] [Google Scholar]
- Glickman R. M., Kirsch K. Lymph chylomicron formation during the inhibition of protein synthesis. Studies of chylomicron apoproteins. J Clin Invest. 1973 Nov;52(11):2910–2920. doi: 10.1172/JCI107487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Gotto A. M., Levy R. I., John K., Fredrickson D. S. On the protein defect in abetalipoproteinemia. N Engl J Med. 1971 Apr 15;284(15):813–818. doi: 10.1056/NEJM197104152841503. [DOI] [PubMed] [Google Scholar]
- Green P. H., Glickman R. M., Saudek C. D., Blum C. B., Tall A. R. Human intestinal lipoproteins. Studies in chyluric subjects. J Clin Invest. 1979 Jul;64(1):233–242. doi: 10.1172/JCI109444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. H., Tall A. R., Glickman R. M. Rat intestine secretes discoid high density lipoprotein. J Clin Invest. 1978 Feb;61(2):528–534. doi: 10.1172/JCI108963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi K., Fainaru M., Havel R. J. Composition of proteins of mesenteric lymph chylomicrons in the rat and alterations produced upon exposure of chylomicrons to blood serum and serum proteins. J Lipid Res. 1978 Aug;19(6):712–722. [PubMed] [Google Scholar]
- Imaizumi K., Havel R. J., Fainaru M., Vigne J. L. Origin and transport of the A-I and arginine-rich apolipoproteins in mesenteric lymph of rats. J Lipid Res. 1978 Nov;19(8):1038–1046. [PubMed] [Google Scholar]
- Innerarity T. L., Mahley R. W. Enhanced binding by cultured human fibroblasts of apo-E-containing lipoproteins as compared with low density lipoproteins. Biochemistry. 1978 Apr 18;17(8):1440–1447. doi: 10.1021/bi00601a013. [DOI] [PubMed] [Google Scholar]
- Katz S. S., Shipley G. G., Small D. M. Physical chemistry of the lipids of human atherosclerotic lesions. Demonstration of a lesion intermediate between fatty streaks and advanced plaques. J Clin Invest. 1976 Jul;58(1):200–211. doi: 10.1172/JCI108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayden H. J. Abetalipoproteinemia. Annu Rev Med. 1972;23:285–296. doi: 10.1146/annurev.me.23.020172.001441. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaRosa J. C., Levy R. I., Herbert P., Lux S. E., Fredrickson D. S. A specific apoprotein activator for lipoprotein lipase. Biochem Biophys Res Commun. 1970 Oct 9;41(1):57–62. doi: 10.1016/0006-291x(70)90468-7. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Olofsson S. O., McConathy W. J., Alaupovic P. Isolation and partial characterization of a new acidic apolipoprotein (apolipoprotein F) from high density lipoproteins of human plasma. Biochemistry. 1978 Mar 21;17(6):1032–1036. doi: 10.1021/bi00599a014. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D., Albers J. J., Saunders D. R. Apoprotein B in fasting and postprandial human jejunal mucosa. J Clin Invest. 1976 Feb;57(2):530–533. doi: 10.1172/JCI108307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmilewitz D., Albers J. J., Saunders D. R., Fainaru M. Apoprotein synthesis by human duodenojejunal mucosa. Gastroenterology. 1978 Oct;75(4):677–682. [PubMed] [Google Scholar]
- Redgrave T. G., Small D. M. Quantitation of the transfer of surface phospholipid of chylomicrons to the high density lipoprotein fraction during the catabolism of chylomicrons in the rat. J Clin Invest. 1979 Jul;64(1):162–171. doi: 10.1172/JCI109435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roheim P. S., Edelstein D., Pinter G. G. Apolipoproteins in rat serum and renal lymph. Proc Natl Acad Sci U S A. 1976 May;73(5):1757–1760. doi: 10.1073/pnas.73.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanu A. M., Aggerbeck L. P., Kruski A. W., Lim C. T., Kayden H. J. A study of the abnormal lipoproteins in abetalipoproteinemia. J Clin Invest. 1974 Feb;53(2):440–453. doi: 10.1172/JCI107578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer E. J., Blum C. B., Levy R. I., Jenkins L. L., Alaupovic P., Foster D. M., Brewer H. B., Jr Metabolism of high-density lipoprotein apolipoproteins in Tangier disease. N Engl J Med. 1978 Oct 26;299(17):905–910. doi: 10.1056/NEJM197810262991701. [DOI] [PubMed] [Google Scholar]
- Schaefer E. J., Eisenberg S., Levy R. I. Lipoprotein apoprotein metabolism. J Lipid Res. 1978 Aug;19(6):667–687. [PubMed] [Google Scholar]
- Shore V. G., Shore B., Lewis S. B. Isolation and characterization of two threonine-poor apolipoproteins of human plasma high density lipoproteins. Biochemistry. 1978 May 30;17(11):2174–2179. doi: 10.1021/bi00604a023. [DOI] [PubMed] [Google Scholar]
- Stein Y., Glangeaud M. C., Fainaru M., Stein O. The removal of cholesterol from aortic smooth muscle cells in culture and Landschutz ascites cells by fractions of human high-density apolipoprotein. Biochim Biophys Acta. 1975 Jan 24;380(1):106–118. doi: 10.1016/0005-2760(75)90049-1. [DOI] [PubMed] [Google Scholar]
- Swaney J. B., Braithwaite F., Eder H. A. Characterization of the apolipoproteins of rat plasma lipoproteins. Biochemistry. 1977 Jan 25;16(2):271–278. doi: 10.1021/bi00621a018. [DOI] [PubMed] [Google Scholar]
- Tall A. R., Green P. H., Glickman R. M., Riley J. W. Metabolic fate of chylomicron phospholipids and apoproteins in the rat. J Clin Invest. 1979 Oct;64(4):977–989. doi: 10.1172/JCI109564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisgraber K. H., Bersot T. P., Mahley R. W. Isolation and characterization of an apoprotein from the d less than 1.006 lipoproteins of human and canine lymph homologous with the rat A-IV apoprotein. Biochem Biophys Res Commun. 1978 Nov 14;85(1):287–292. doi: 10.1016/s0006-291x(78)80041-2. [DOI] [PubMed] [Google Scholar]
- Wu A. L., Windmueller H. G. Identification of circulating apolipoproteins synthesized by rat small intestine in vivo. J Biol Chem. 1978 Apr 25;253(8):2525–2528. [PubMed] [Google Scholar]