Abstract
Background
Neuroimaging measures of behavioral and emotional dysregulation can yield biomarkers denoting developmental trajectories of psychiatric pathology in youth. We aimed to identify functional abnormalities in emotion regulation (ER) neural circuitry associated with different behavioral and emotional dysregulation trajectories using Latent Class Growth Analyses (LCGA) and neuroimaging.
Methods
61 youth (9-17 years) from The Longitudinal Assessment of Manic Symptoms (LAMS) study, and 24 healthy control youth, completed an emotional face n-back ER task during scanning. LCGA was performed on 12 biannual reports completed over five years of the Parent General Behavior Inventory-10 Item Mania Scale (PGBI-10M), a parental report of the child’s difficulty regulating positive mood and energy.
Results
There were 2 latent classes of PBGI-10M trajectories: high and decreasing (HighD; n=22) and low and decreasing (LowD; n=39) course of behavioral and emotional dysregulation over the 12 time points. Task performance was >89% in all youth, but more accurate in healthy controls and LowD versus HighD (p<.001). During ER, LowD had greater activity than HighD and healthy controls in dorsolateral prefrontal cortex, a key ER region, and greater functional connectivity than HighD between amygdala and ventrolateral prefrontal cortex (ps<0.001, corrected).
Conclusions
Patterns of function in lateral prefrontal cortical-amygdala circuitry in youth denote the severity of the developmental trajectory of behavioral and emotional dysregulation over time, and may be biological targets to guide differential treatment and novel treatment development for different levels of behavioral and emotional dysregulation in youth.
Keywords: fMRI, latent class growth analysis, youth, behavioral and emotional dysregulation, emotional nback, emotion regulation
Introduction
Psychiatric disorders characterized by behavioral and emotional dysregulation in youth are often difficult to disentangle nosologically. Behavioral and emotional dysregulation are common among youth seeking treatment, and youth with these behaviors may be diagnosed with a variety of disorders such as bipolar spectrum disorder(BPSD) depressive disorders, attention deficit hyperactivity disorder(ADHD), and disruptive disorders, or remain undiagnosed (Brotman et al., 2006, Findling et al., 2010, Lewinsohn et al., 2000, Stringaris and Goodman, 2009). The high rates of comorbid disorders add challenges to diagnosis and treatment. These factors suggest that behavioral and emotional dysregulation is not well characterized using current diagnostic nomenclature, and may represent a behavioral dimension(s) that cut across different diagnostic categories. Adopting a dimensional approach to the study of behavioral and emotional dysregulation in youth parallels the approach advocated by the NIMH RDoC(Insel et al., 2010).
Identifying objective biomarkers that reflect pathophysiologic processes underlying behavioral and emotional dysregulation(Charney and Babich, 2002, Hasler et al., 2006) may ultimately provide biological targets to guide treatment choice and treatment development for different levels of severity of behavioral and emotion dysregulation in youth(Phillips and Frank, 2006). The use of neuroimaging to identify measures of dysfunctional neural circuitry associated with behavioral and emotional dysregulation may be a way to identify such biomarkers. Combining neuroimaging with methodologies such as Latent Class Growth Analysis(LCGA) that can identify subgroups of youth defined by different underlying trajectories of behavioral and emotional dysregulation over time may provide a way to identify biomarkers associated with these different subgroups. This approach may lead to better understanding of pathophysiological processes underlying different trajectories of behavioral and emotional dysregulation in youth.
The Longitudinal Assessment of Manic Symptoms(LAMS) study ((Horwitz et al., 2010) for a complete description) is a multisite study of youth initially aged 6-12 years who at enrollment were seeking treatment for behavioral and emotional dysregulation. The aim of LAMS is to assess relationships among longitudinal symptom course, clinical, and functional outcomes in youth with behavioral and emotional dysregulation who have a variety of diagnoses. For five years, youth in the first LAMS phase(LAMS1) were assessed every six months in order to characterize developmental trajectories on a range of clinical dimensions. One especially important measure is the Parent General Behavior Inventory-10-Item Mania Scale(PGBI-10M), a ten-item parental report of observed child behaviors associated with difficulty regulating positive mood and energy(Youngstrom et al., 2008). Families with PGBI-10M scores of ≥12, plus a demographically matched subset of lower scoring youth were invited to participate in LAMS1. At baseline assessment, PGBI-10M scores were associated with risk of having BPSD (Frazier et al., 2011), behavioral extremes, poor overall functioning, and high risk for developing severe psychopathology other than BPSD (e.g., other mood disorders, anxiety disorders, ADHD, and disruptive disorders)(Findling et al., 2010, Horwitz et al., 2010). LAMS2, the second phase, is an ongoing study that includes neuroimaging and neurocognitive evaluations. A goal of LAMS2 is to examine relationships between functional integrity of neural circuitry supporting emotion regulation(ER) and developmental trajectories of behavioral and emotional dysregulation in youth.
ER neural circuitry includes regions implicated in early appraisal of emotional information during “automatic” or implicit sub-processes of ER: rostral and subgenual regions of the anterior cingulate cortex (ACC; Brodmann Areas, BA24/25, respectively), orbitofrontal cortex (OFC:BA11), and dorsomedial prefrontal cortex (DMPFC: medial BA9/10); and regions involved in more demanding executive and attentional control processes that support effortful, ER processes: dorsal-ACC (dorsal BA24/32), ventrolateral prefrontal cortex (VLPFC; BA47), and dorsolateral prefrontal cortex (DLPFC: BA44/46 and lateral BA9)(Ochsner and Gross, 2005, Phillips et al., 2008). An increasing number of studies have examined ER neural circuitry in youth characterized by behavioral and emotional dysregulation(Ladouceur et al., 2011, Passarotti et al., 2010b, Pavuluri et al., 2008, Rich et al., 2011). For example, abnormally reduced DLPFC and VLPFC activity was reported during a variety of ER tasks, including emotional-face gender labeling, response inhibition and emotional-color-word task in youth with BPSD versus healthy control youth(Ladouceur et al., 2011, Passarotti et al., 2010a, Pavuluri et al., 2008). Reduced connectivity relative to healthy youth within prefrontal cortical-amygdalar circuitry was shown in bipolar youth during ER tasks, including a working memory(WM) task with emotional distracters, gender labeling, and emotional-face identification (Ladouceur et al., 2011, Passarotti et al., 2012, Rich et al., 2008); in depressed youth during an ER task(Perlman et al., 2012); and in youth at risk for psychosis during emotion processing(Gee et al., 2012).
Our overarching goal in the present study was to identify biomarkers associated with trajectories of behavioral and emotional dysregulation in LAMS youth, to lead to a better understanding of pathophysiological processes underlying these trajectories. We had two main aims:
Aim 1
Identify in LAMS youth, subgroups with different developmental trajectories of behavioral and emotional dysregulation symptoms using PGBI-10M scores and LCGA. LCGA is an established technique for classifying longitudinal data into homogenous and distinct classes within the larger heterogeneous group, based on latent (unobserved) trajectories within the data (Muthén and Muthén, 1998-2011, Nylund et al., 2007).
Hypothesis 1
LCGA would identify distinct classes of PGBI-10M developmental trajectories in LAMS youth during the five-year course of LAMS1.
Aim 2
Identify functional abnormalities in ER neural circuitry that differentiate LCGA derived subgroups in LAMS youth in Hypothesis1, and that also differentiate LAMS subgroups from healthy control youth(HC). The following hypothesis was guided by reports of reduced activity in prefrontal cortical regions and reduced prefrontal cortical-amygdala connectivity in behaviorally and emotionally dysregulated (BPSD, depressed, ADHD) youth versus HC during ER tasks (Halari et al., 2009, Hulvershorn et al., 2011, Ladouceur et al., 2011, Passarotti et al., 2010a).
Hypothesis 2
LAMS youth with more severe PGBI-10M developmental trajectory would show significantly reduced activity in prefrontal cortical regions in ER circuitry and significantly reduced prefrontal cortical-amygdala connectivity, during task performance than LAMS youth with less severe PGBI-10M trajectory and HC.
In exploratory analyses, we aimed to examine how patterns of activity and functional connectivity in ER circuitry were associated with other clinical factors (e.g., diagnosis, medication, other symptoms) and demographic factors (age, gender, SES), and task performance.
Methods
Participants
One hundred twenty eight youth, recruited from the LAMS1 cohort of 707 youth, and thirty-four newly recruited HC, participated in the neuroimaging component of LAMS2. All HC were free of any psychiatric disorder; first-degree relatives were free of mood disorders and psychosis, and second-degree relatives were free of BPSD and psychosis. All 128 youth from LAMS1 entered LAMS1 with a variety of symptoms and diagnoses. Inclusion criteria for the LAMS1 cohort were: no outpatient treatment at a LAMS clinic in the last 12 months; 6-12 years of age; and without a sibling who was screened for LAMS1. Families of eligible children completed the PGBI-10M. Children who scored ≥12 on this scale, and an age-sex-matched group of those who scored <12, were invited to participate in LAMS1. The 128 youth in the LAMS2 neuroimaging component were selected to include approximately equal numbers of youth: 1) with high(≥12) versus low(<12) PGBI-10M scores; 2) who were older(≥13 years) versus younger(≤12 years); 3) who were male versus female (2. and 3. for each PGBI-10M subgroup per site). HC were recruited using local advertising at the three sites: Case Western Reserve University(n=32, LAMS; 13, HC); Cincinnati Children’s Hospital(n=48, LAMS; 6, HC); and University of Pittsburgh Medical Center(n=48, LAMS; 15, HC). Institutional Review Boards approved the study at each site. Parents/guardians provided written informed consent. Youth performed three different neuroimaging tasks: for results from the reward task see Bebko et al. (2013).
Yearly assessments throughout LAMS1 and LAMS2 included the parent/guardian’s reported PGBI-10M over the last six months (Youngstrom et al., 2008, Youngstrom et al., 2005), parent and child reported Screen for Child Anxiety Related Emotional Disorders(SCARED) to assess anxiety symptoms (Birmaher et al., 1999) over the last six months, and parent and child report of manic and depressive symptom severity, respectively, using the Schedule for Affective Disorders and Schizophrenia for School-Age Children Mania Rating Scale(KMRS) (Axelson et al., 2003), and Depression Rating Scale(KDRS) (Kaufman et al., 1997). The PGBI-10M and SCARED were also reported biannually. Additionally, SCARED, KDRS, and KMRS were performed on the day of magnetic resonance (MR) scan.
See Supplemental for Exclusion criteria.
Data loss on the challenging EFNBACK task was due to head movement >4 mm during scanning (Morgan et al., 2013), task accuracy <75%, and inability to complete both task runs. Sixty-one LAMS and 24 HC successfully completed the task (Mean age: LAMS=13.41(2.21), HC=14.11(1.93), females: LAMS=26, HC=11). Clinical measures, medication use, and demographic variables for participants who successfully completed the scan appear in Table 1. Over half (33/61, 54%) of LAMS participants were using one or more medications on the scan date, including antidepressants, antipsychotic medication, mood stabilizers, non-stimulant ADHD medications, and stimulant medications (Table 1). As a whole, completers and non-completers did not differ on sex, socioeconomic status (SES), clinical variables (PGBI-10M score, SCARED, KDRS, KMRS), or site (all p≥0.12, Supplemental Table 1). Completers were, however, significantly older (p=.001) and had higher IQs (p=.039). Individual class completion statistics: see Supplemental Table 2.
Table 1.
Demographic information, clinical measures, and current medication usage (Mean ± Standard Deviation or Proportion) describing latent classes of LAMS2 imaging sample and healthy control youth.
| HighD n = 22 |
LowD n=39 |
HC n=24 |
Statistic | ||
|---|---|---|---|---|---|
| Demographic Information | |||||
| Age | 13.71(2.02) | 14.34(1.87) | 13.41(2.21) | F(2, 82)=1.73 | .18 |
| Gender (females) | 12/22 | 14/39 | 13/24 | χ2=2.89 | .24 |
| IQ | 101.41(17.03) | 104.72(15.23) | 105.21(12.48) | F(2, 82)=.450 | .64 |
| SES (primary caregiver education) | Fisher’s exact | .24 | |||
| No/some HS | 1/22 | 2/39 | 0/24 | ||
| GED or HS Diploma | 4/22 | 11/39 | 1/24 | ||
| Some post HS | 7/22 | 8/39 | 7/24 | ||
| Associate’s Degree | 6/22 | 10/39 | 6/24 | ||
| Bachelor’s Degree or higher | 4/22 | 8/39 | 10/24 | ||
| Clinical Measures | |||||
| Lams1 baseline assessment | |||||
| PGBIM10 | 18.36(5.41) | 8.57(5.39) | NA | t(58)=-6.77 | .001** |
| Biannual assessment closest to scan | |||||
| PGBIM10 | 10.55(6.92) | 3.00(3.63) | NA | t(59)=-5.60 | .001** |
| Scan day assessments | |||||
| KDRS | 4.86(4.25) | 3.92(5.05) | NA | t(59)=-.738 | .46 |
| KMRS | 7.96(9.65) | 2.87(5.29) | NA | t(59)=-2.28 | .03* |
| SCARED | 11.48(10.31) | 9.84(10.07) | NA | t(59)=-.592 | .56 |
| Current Medication Use | NA | ||||
| Antidepressant | 4/22 | 5/39 | NA | .71 | |
| Antipsychotic | 8/22 | 5/39 | NA | .05* | |
| Benzodiazepine | 1/22 | 0/39 | NA | .36 | |
| Mood Stabilizer | 5/22 | 1/39 | NA | .02* | |
| Non-stimulant | 1/22 | 1/39 | NA | 1.0 | |
| Stimulant | 9/22 | 15/39 | NA | 1.0 | |
Abbreviations: HighD= latent class with high and decreasing trajectory of behavioral and emotional dysregulation, LowD= latent class with low and decreasing trajectory of behavioral and emotional dysregulation, HC = healthy control youth,
=significant at p≤.01;
=significant at p≤.01;
PGBIM10 LAMS1 screen score=Parental Behavior Inventory 10 item scale; HS=high school; IQ=intelligence quotient Wechsler Intelligence test; KDRS= Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Present Episode Depression Rating Scale; KMRS=Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Mania Rating Scale; SCARED=Screen for Child Anxiety Related Emotional Disorders (child rating); SES=socio-economic status based on primary caregiver level of education. NA = not assessed/applicable. Chi square for age and SES computed using Pearson chi square. Chi square for medications computed using Fisher’s exact test
Latent Class Growth Analyses
Patterns of PGBI-10M scores were evaluated to determine class membership using Latent Class Growth Analysis(LCGA) in Mplus6(Muthén and Muthén, 1998-2011), by defining the number of subgroups within the data that were distinct from each other. Twelve biannual PGBI-10M scores collected over five years of LAMS1 were used to define class membership for the total sample of 128 LAMS youth with neuroimaging. Three model fit indices were used: Bayesian Information Criterion(BIC), a measure of relative fit (Nylund et al., 2007); Vuong-Lo-Mendell-Rubin Likelihood Ratio, a test of improvement of k from k-1 classes; and entropy (range:0-1), to determine distinctiveness of the classes. Convergence was aided by increasing number of iterations and using random start values. The 61 LAMS participants who successfully completed neuroimaging were assigned to their appropriate latent classes according to these analyses.
Diagnoses: LCGA Subgroups
Paradigm
The emotional-n-back(EFNBACK) task was used to examine recruitment of prefrontal cortical systems in the context of simultaneously-presented emotionally-salient distracting stimuli during WM (Ladouceur et al., 2009a, Ochsner and Gross, 2005). (Supplemental).
Neuroimaging Data Acquisition and Preprocessing
See Supplemental.
Neuroimaging Data Analysis: Activity
Using Statistical Parametric Mapping software(SPM8), http://www.fil.ion.ucl.ac.uk/spm, a two level random-effects ROI analysis was conducted. At the first level, a mixed model was used with each trial event modeled separately, given the jittered nature of the ISI between trails in each block; global signal normalization was also performed to improve model fit assumptions (see Combining data across sites). Individual wholebrain statistical maps were then constructed to evaluate main 2-back conditions of interest: 2-back with fear-face-distracter, 2-back with happy-face-distracter, and 2-back with neutral-face-distracter. Movement parameters from the realignment stage served as no interest covariates.
At the second level of BOLD fMRI data analysis, a 3-Group (2 LAMS subgroups derived from LCGA [see below] and HC) x3-(Conditions: 2-back:fear, 2-back:happy, and 2-back:neutral) ANOVA examined neural activity during ER within one single large ROI mask, comprising bilateral amygdala, DLPFC(BA9/46), dACC(BA24/32), and VLPFC(BA47). Anatomical masks for these bilateral ROIs were created from the WFUPickAtlas (Wake Forest University, Winston-Salem)(Maldjian et al., 2003). Covariates were: age, sex, IQ, and scanning site. A voxelwise threshold of p≤0.01, with an AlphaSim cluster level correction threshold of p≤0.01(Ward, 2002) to correct for multiple voxelwise comparisons across the entire mask, were used.
Significant effects from the model above were further examined using post-hoc, pairwise between-group comparisons on activity in the bilateral ROI mask, using Bonferroni-corrected voxelwise thresholds as appropriate. For example, to control for three post-hoc pairwise group comparisons to interpret any significant overall main effect of group, we used a voxelwise threshold of p≤0.003(0.01/3), AlphaSim cluster level corrected p≤0.01.
Neuroimaging Data Analysis: PPI
PPI analysis was conducted in SPM8 to examine connectivity of the amygdala seed region with bilateral prefrontal-anterior cingulate target regions (described above) during ER. For each task condition, we created a PPI vector by multiplying mean time series from the seed region by task condition vector. Single subject first level analyses were then run for each 2-back:emotion condition with the following regressors: PPI vector, seed region time-course vector, and task-condition vector. Resulting contrast maps, weighted 1(positive modulation) were used in a 3-Group (2 LAMS subgroups and HC) × 3-PPI-(Conditions: 2-back:fear, 2-back:happy, and 2-back:neutral) full-factorial model at the second level to examine functional connectivity during ER within our single ROI target mask: (bilateral DLPFC(BA9/46), dACC(BA24/32), and VLPFC(BA47). Covariates were: age, sex, IQ, and site. A voxelwise threshold of p≤0.01, and p≤0.01 cluster level correction, were used.
Significant main effects of group, emotion, or group×emotion interaction, were further examined using post-hoc, pairwise between-group comparisons on PPIs in the bilateral ROI target mask, using Bonferroni-corrected voxelwise thresholds as appropriate.
FurtherAnalyses
In parallel analyses we performed a full-factorial 3-groups (LowD, HighD, HC) × 2-cognitive loads (0-back and 2-back) × 3-emotional conditions (fear, happy, neutral) ANOVA model. Here we, used the same voxelwise and clusterwise thresholds as in the above 2-(group) ×3-(emotional condition) ANOVA.
Exploratory Analyses
Exploratory analyses examined wholebrain activity and connectivity to 2-back conditions:(voxelwise threshold of p≤.005, cluster-level corrected threshold of p≤0.01). Significant main effects of group, emotion, or group×emotion interaction, were examined using post-hoc tests, using Bonferroni-corrected voxelwise thresholds as appropriate.
We also examined relationships between clusters of activity and measures of functional connectivity showing a significant main effect of group from the main analyses focusing on the 2-back conditions and: diagnosis, medication use, KMRS, KDRS, SCARED scores, age, sex, IQ, SES, and task performance.
Combining Data across Sites
Studies report that merging neuroimaging data from multiple sites is feasible (Magnotta and Friedman, 2006, Segall et al., 2009). We used the following procedures to control for inter-site scanner variability and to combine neuroimaging data across our three sites. First, to improve the degree to which the first-level models met model assumptions at each site, global normalization was implemented (Eklund et al., 2012). Normality of the residuals was calculated using the Shapiro-Wilk test separately for each first-level model with and without global normalization, averaged over all voxels in the single a priori bilateral ROI. Nonparametric tests showed significant improvement in normality of residuals after global normalization (Z=-5.133, p<.001); and the Durbin Watson test showed improvements in serial independence of the residuals (χ2=9.276, p=.002). Second, standards published by the Biomedical Informatics Research Network(BIRN; http://www.nbirn.net) for data acquisition and information sharing were implemented. Using a BIRN phantom, scanner signal-to-noise-ratio(SNR) was collected and monitored for stability monthly at each scanner site (Friedman and Glover, 2006, Friedman et al., 2006) (Supplemental Figure 1). Third, we used scanning site as a covariate in all analyses.
Results
Latent Class Growth Analysis
A two class model was revealed as acceptable and compatible with neuroimaging analysis sample requirements (Table 2), where power analyses suggest that a group of at least 12 is needed to provide 80% power at p<.01 for fMRI data analysis (Desmond and Glover, 2002). In the total sample of 128 LAMS, we identified two latent class subgroups of PGBI-10M trajectory: youth with a high and decreasing developmental trajectory of behavioral and emotional dysregulation (HighD; n=49, 22: successfully completed the neuroimaging protocol); and youth with low and decreasing developmental trajectory of behavioral and emotional dysregulation (LowD; n=79, 39:successfully completed the neuroimaging protocol; Figure 1). HighD and LowD did not differ significantly on age, sex, IQ, SES, KDRS, SCARED, antidepressant, stimulant, or non-stimulant-ADHD medication use. The two subgroups who completed neuroimaging differed on PGBI-10M at baseline (entry into LAMS1; p=.001), PGBI-10M nearest to scan (p=.001), KMRS (p=.012), and use of antipsychotic (p=.031) and mood stabilizer medications (p=.011; Table 1). Of note, prior analyses (Findling et al., 2013) using the complete LAMS1 cohort (N=707) and four PGBI-10M time points identified four latent LAMS classes, with the two largest classes defined as high and decreasing (38.5%) and low and decreasing (47.2%), reflecting class distinctions observed in the present analysis Figure 1.
Table 2.
Latent class growth analysis model fit indices.
| Number of classes | BIC | Vuong-Lo-Mendell-Rubin likelihood ratio test | Entropy |
|---|---|---|---|
| 1 | 8309.766 | ||
| 2 | 7855.745 | 0.0019 | 0.907 |
| 3 | 7684.071 | 0.0019 | 0.926 |
| 4 | 7632.772 | 0.1853 | 0.872 |
| 5 | 7631.240 | 0.1891 | 0.822 |
Linear model in all n=128 LAMS youth using twelve six-monthly PGBI-10M scores collected over the five years of LAMS1
Figure 1.
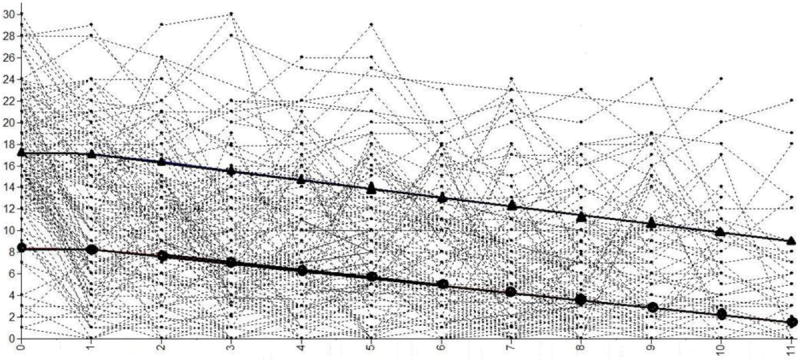
Spaghetti plot of latent class models based on latent class growth analysis of 12 PGBIM10 reports over 5 years of LAMS1. Triangles mark the latent trajectory of the high and decreasing (HighD) behavioral and emotional dysregulation trajectory. Circles mark the latent trajectory of the low and decreasing (LowD) behavioral and emotional dysregulation trajectory.
Behavioral Data
Performance on the 2-back with emotional faces task was good (mean accuracy=89.4%). Performance differed by group, with HC (accuracy=92%) and LowD (accuracy=91%) performing more accurately than HighD (accuracy=84%) (F(2,82)=6.32, p=.003). LowD and HC did not differ significantly on task performance. Performance for the entire neuroimaging sample showed the same pattern of between group differences in accuracy(Supplemental).
Activity
There was a significant main effect of group in two clusters in bilateral DLPFC (peak voxel: right: F(2,241)=9.92, p<.001, corrected; left: F(2,241)=6.40, p=.002, corrected). There was no significant main effect of emotion or group×emotion interaction (Table 3; Figure 2).
Table 3.
Between Group Differences in Activity and PPI functional connectivity during a working memory task with emotional distracters.
| BOLD Activation | |||||
|---|---|---|---|---|---|
| Comparison | Area | BA | Cluster | MNI Peak Voxel |
p |
| Main effect of Group | |||||
| Right DLPFC | 9 | 66 | 36 28 42 | .001 | |
| Left DLPFC | 9 | 30 | -30 30 38 | .002 | |
| Left DLPFC | 9 | 27 | -22 42 40 | .003 | |
| LowD >HighD | |||||
| Left DLPFC | 9 | 49 | -30 30 38 | .001 | |
| LowD>HC | |||||
| Right DLPFC | 9 | 62 | 36 28 42 | .001 | |
| Left DLPFC | 9 | 34 | -22 42 40 | .001 | |
|
| |||||
| PPI functional connectivity with Amygdala seed | |||||
| Comparison | Area | BA | Cluster |
MNI Peak voxel |
p |
| Main effect of Group | |||||
| Left VLPFC | 47 | 48 | -42 30 -14 | .001 | |
| LowD>HighD | |||||
| Left VLPFC | 47 | 67 | -42 30 -14 | .001 | |
| Left dACC | 24 | 106 | -2 6 40 | .001 | |
| Left dACC | 24 | 30 | -2 38 8 | .001 | |
| Main effect of emotion | Right DLPFC | 9 | 43 | 40 38 38 | .001 |
| Left DLPFC | 9 | 37 | -40 36 36 | .001 | |
| Fear>Neutral | Right DLPFC | 9 | 55 | 40 38 38 | .001 |
| Left DLPFC | 9 | 50 | -40 36 36 | .001 | |
A single mask ROI analyses including bilateral amygdala, BA 9, 46, 24, 32, 47, with a voxelwise threshold of p<.01, and p<0.01 cluster level corrected. Each line in the table represents the voxel of peak activity difference within the specified region.
p = pvalue, uncorrected, HighD= latent class with high and decreasing trajectory of behavioral and emotional dysregulation, LowD= latent class with low and decreasing trajectory of behavioral and emotional dysregulation, HC = healthy control youth
Figure 2.
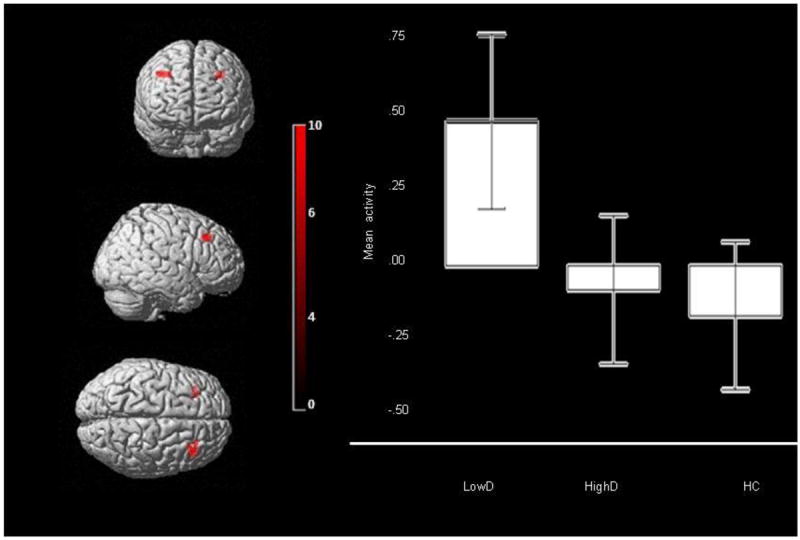
Bilateral DLPFC (BA9) activity for main effect of group on neural activity across all emotional distracters in the entire bilateral ROI mask. Peak voxel Right DLPFC: mni: 36 28 42, k=66, p<.001, Left DLPFC: mni: -28, 32, 40, k=30, p=.002, Left DLPFC: mni: -22, 42, 40, k=27, p=.003. Color bar represents F values. Bars represent the 95% confidence interval.
Post-hoc analyses, using a Bonferroni-corrected voxelwise threshold of p≤0.003 (0.01/3) to control for three pairwise between-group comparisons, revealed that LowD showed greater bilateral DLPFC activity than HC (right: t(241)=4.20, p=.001; left: t(241)=3.46, p=.001, corrected) and greater left DLPFC activity than HighD (t(241)=3.46, p=.001, corrected; Table 3). HC and HighD did not differ significantly.
PPI
PPI analysis revealed a significant main effect of group on functional connectivity between the amygdala and left VLPFC (F(2,241)=7.58, p=.001, corrected). Post-hoc analyses, using a Bonferroni-corrected voxelwise threshold of p≤0.003 (0.01/3) to control for three pairwise between-group comparisons, revealed significantly reduced positive functional connectivity in HighD than LowD between bilateral amygdala and left VLPFC (t(241)=3.87, p<.001, corrected), and between bilateral amygdala and two clusters in the left dACC (t(241)=3.49 and t(241)=3.05, p=.001, corrected; Table 4; Figure 3A and B). The magnitude of functional connectivity among these regions in HC was intermediate between that shown by the two LAMS subgroups, but did not differ significantly from either LowD or HighD.
Figure 3.
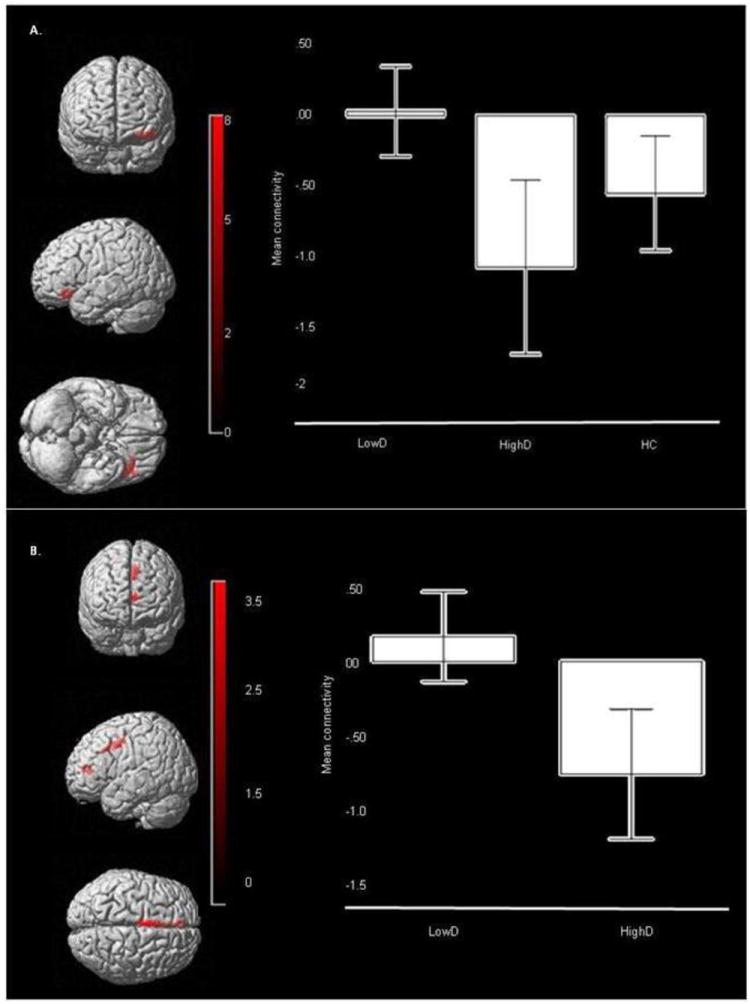
Functional Connectivity between amygdala seed (not shown) and bilateral ROI mask target regions. A. Main effect of group for functional connectivity across all emotional distracters: amygdala- left VLPFC (BA47) connectivity: (Peak voxel mni: -42 30 -14, k=117, p=.001, corrected). Color bar represents F values. Bars represent the 95% confidence interval. B. Post hoc analysis of LowD versus HighD for amygdala- left dACC connectivity: (Peak voxel mni: -2 6 40, k=106, p<.001, corrected). Color bar represents t values. Bars represent the 95% confidence interval
There was also a main effect of emotional condition on functional connectivity between bilateral amygdala and bilateral DLPFC (right: F(2,241)=8.70, p<.001; left: F(2,241)=8.42, p<.001, corrected; Table 4; Figure 3). Post-hoc analyses, using a Bonferroni-corrected voxelwise threshold of p≤0.003 (0.01/3) to control for three pairwise between-emotion condition comparisons revealed significantly greater functional connectivity between bilateral amygdala and bilateral DLPFC to the fear distracter than to neutral distracter across all participants (right: t(241)=4.14, p<.001; left: t(241)=4.08, p<.001, corrected; Table 4).
Further Analysis
Findings from the full-factorial 3-groups (LowD, HighD, HC) × 2-cognitive loads (0-back and 2-back) × 3-emotional conditions (fear, happy, neutral) ANOVA for activity revealed a similar pattern of a significant main effect of group in right DLPFC(BA9; F(2, 487) =9.57, p<.001, corrected, 64voxels, mni:34, 26, 42). Group comparisons: see Supplemental.
Findings from the full factorial model for functional connectivity revealed a similar pattern of a significant main effect of group on functional connectivity between bilateral amygdala and left VLPFC (BA47; F(2, 487) =11.65, p<.001, corrected, 67voxels, mni:-34, 32, -14) and between bilateral amygdala and bilateral dACC (BA 24; left: F(2, 487) =9.25, p<.001, corrected, 140voxels, mni:-2, 6, 40 right: F(2, 487) =8.44, p<.001, corrected, 171voxels, mni:4, 8, 38). Group comparisons: see Supplemental.
Exploratory Analysis
Given the between-group difference in task accuracy, LAMS not-taking versus LAMS taking mood stabilizer medication (p=.03) and LAMS without versus those with a BPSD diagnosis (p=.03) (Supplemental Table 4), we covaried for these in additional analyses. See Supplemental materials for results with significant covariates.
Wholebrain results: see Supplemental data/Tables 5-6.
Discussion
The goal of this study was identifying biomarkers associated with different trajectories of behavioral and emotional dysregulation in LAMS to lead to a better understanding of pathophysiological processes underlying developmental trajectories. We used LCGA and neuroimaging measures of functional integrity of ER neural circuitry in a large group of LAMS and HCs. In support of our first hypothesis, LCGA of 12 PGBI-10M reports over five years revealed two latent class subgroups: LAMS participants with an initially high, then gradually decreasing(HighD) developmental trajectory of behavioral and emotional dysregulation symptoms; and LAMS participants with an initially low yet also decreasing(LowD) developmental trajectory of behavioral and emotional dysregulation symptoms. In partial support of our second hypothesis, these two groups were differentiated by patterns of activity and functional connectivity in our a priori regions of interest involved in emotion regulation. The results of the analyses converged showing a common pattern of greater activity and functional connectivity by LowD relative to HighD in important prefrontal and cingular regions as predicted. These findings provide a novel, data-driven understanding of previous developmental trajectories of behavioral and emotional dysregulation and associated patterns of activity and functional connectivity in ER neural circuitry.
LowD showed significantly greater bilateral DLPFC activity during ER task performance than either HighD or HC to the demanding 2-back cognitive load. By contrast, HighD not only showed significantly less DLPFC activity than LowD during ER task performance, but also failed to complete the task at the same performance level as either HC or LowD. These findings suggest that recruiting DLPFC to a greater than normal extent during ER task performance may be necessary to help compensate for behavioral and emotional dysregulation and equate task performance with that of HC in LAMS youth. Thus, LowD recruited DLPFC to a greater extent than HC to maximize task performance, HighD failed to do this, resulting in poorer task performance than either of the other groups. Although differences observed in HighD may alternatively reflect inattention to task, the high accuracy rate for this group, and the fact that they succeeded in remaining still for this fMRI paradigm, suggests that HighD did, in fact, attend to task. Furthermore, analyses covarying for accuracy revealed similar patterns of between-group differences in activity. Previous reports of significantly decreased DLPFC activity on ER tasks in youth with severe pathology evidenced by BPSD diagnoses in these samples (Ladouceur et al., 2011, Passarotti et al., 2010a) provide further support for this interpretation of findings, and suggest that more severely behaviorally and emotionally dysregulated youth may be less able to recruit prefrontal cortical regions during cognitive task performance.
PPI analysis similarly showed that prefrontal and anterior cingulate cortical regions were differentially connected with the amygdala during task performance across the two LAMS subgroups. Here, HighD showed significantly reduced positive amygdala-left VLPFC and reduced positive amygdala-left dACC functional connectivity than LowD, even after covarying for task accuracy. Furthermore, this between-group difference in functional connectivity resulted from HighD showing significantly greater inverse functional connectivity between these regions than LowD, while the magnitude of functional connectivity among these regions in HC was intermediate between that shown by LowD and HighD (Figure 3A). In the context of emotionally distracting material, a combination of decreased positive/increased inverse functional coupling among amygdala, VLPFC and dACC and decreased DLPFC activity may thus represent a neural mechanism for impaired ER task performance that may in turn be associated with more severe behavioral and emotional dysregulation in youth. By contrast, greater positive functional coupling and activity in this circuitry than HC may represent a compensatory response to help optimize ER task performance, but is shown only by youth with less severe behavioral and emotional dysregulation. Again, evidence of decreased positive amygdala-prefrontal functional connectivity was previously reported in youth with severe dysregulation such as mood disorders and at-risk for psychosis has been reported (Cusi et al., 2012, Gee et al., 2012, Passarotti et al., 2012). The present study is the first to our knowledge to examine dimensions of dysregulation across diagnoses and to use LCGA to characterize subgroups of youth based on previous developmental trajectories of behavioral and emotional regulation symptoms. Further it is the first to our knowledge to examine how these subgroups are differentiated by patterns of activity and functional connectivity in ER neural circuitry.
Interestingly, similar patterns of between group differences in DLPFC activity and amygdala-VLPFC and amygdala-dACC functional connectivity were shown across both 0-back and 2-back cognitive loads in the full factorial analyses. The 0-back condition, while less difficult than the 2-back condition, still requires an ability to redirect attention from emotional distracters toward the task-relevant stimulus, and thus requires intact attentional resources. Our findings suggest between-group differences in recruitment of neural circuitry for performance of the 0-back condition as for the 2-back condition.
Critically, we were able to show significant differences in both activity and functional connectivity between LAMS subgroups, even though at the time of scanning, PGBI-10M severity had decreased since study entry in both subgroups. Furthermore, findings remained after covarying for clinical measures that differed between LAMS subgroups on the scan date: mood stabilizer medication and having a BPSD diagnosis, with greater amygdala-left VLPFC and amygdala-dACC functional connectivity still observed in LowD than HighD. Together, these findings suggest that previous developmental trajectories of behavioral and emotional dysregulation impact the functional integrity of ER neural circuitry, irrespective of present diagnosis or medication, and highlight the importance of examining the contribution of developmental trajectories in neuroimaging studies of behaviorally and emotionally dysregulated youth.
The significance of the left-lateralized nature of bilateral amygdala-prefrontal cortical functional connectivity across groups is unclear. The VLPFC has a specific role in supporting reversal learning and set shifting (Rygula et al., 2010) and the left hemisphere is involved in activities requiring attention to distinctive features and judgment (Haxby et al., 1995). Thus, recruitment of the left VLPFC during this task may be required to allow redirection of attention away from facial features during facial emotion processing to facilitate task performance.
Interestingly, all youth showed greater functional connectivity between bilateral amygdala and bilateral DLPFC to fearful than to neutral distracter. Given our previous report that youth are slower to perform the task in the presence of fearful than other distracters (Ladouceur et al., 2009b), these findings suggest that greater amygdala-prefrontal cortical functional connectivity was required by all youth to maintain 2-back WM performance in the presence of fearful face distracters.
Limitations include the inability to determine the temporal sequence of neuroimaging measure differentiation and development of behavioral and emotional dysregulation. Future research should directly test this question by performing longitudinal clinical assessments after neuroimaging assessments in youth. Data loss was significant, although accuracy on the task for the entire group was similar to the subset successfully completing neuroimaging, and youth who were able to complete the task, versus those who were not, differed only in age and IQ: older and higher IQ youth were more successful at task completion, suggesting that generalizability was not compromised by the data loss. A careful comparison of completers and non-completers in each subgroup (LowD, HighD, and HC) showed that, in each group, age was related to completion, with older youth being more successful. LowD completers had higher depression scores than LowD non-completers, however, suggesting that LowD completers may in fact have been more depressed at the time of scanning than LowD non-completers. Future neuroimaging studies of these high-risk populations may benefit by limiting the scanning session length. We employed an ROI approach for activity and functional connectivity analyses. We used a single, large bilateral ROI for analyses. Exploratory wholebrain analyses provided findings largely in support of these ROI analyses, however. Multiple sites were included, allowing for recruitment of larger numbers of youth, and greater generalizability. We accounted for potential effect of scanner site upon neuroimaging measures by following BIRN recommendations for multi-site data collection and SNR monitoring, by ensuring model assumptions were met, and co-varying for site in analyses.
Identifying objective biological markers that reflect underlying pathophysiologic processes in pediatric psychiatric disorders is vital to identify biological targets to guide treatment choices and novel treatment development. The opportunity to recruit a subset of youth from the large LAMS study of youth with behavioral and emotional dysregulation symptoms provided a unique opportunity to examine neural correlates of the developmental trajectories of these symptoms, regardless of diagnosis, an approach that parallels the dimensional approach of the RDoC. Our findings suggest differential patterns of underlying prefrontal cortical activity and prefrontal cortical-amygdala connectivity associated with developmental trajectories of behavioral and emotional dysregulation. These findings may ultimately provide biological targets to guide treatment for different levels of severity of behavioral and emotional dysregulation in youth.
Supplementary Material
Acknowledgments
This research was supported by the National Institute of Mental Health grant 2R01 MH73953-06A1 (Birmaher and Phillips, University of Pittsburgh)
Dr. Findling receives or has received research support, acted as a consultant and/or served on a speaker’s bureau for Alexza Pharmaceuticals, American Psychiatric Press, AstraZeneca, Bracket, Bristol-Myers Squibb, Cognition Group, Forest, GlaxoSmithKline, Guilford Press, Johns Hopkins University Press, Johnson & Johnson, KemPharm, Lilly, Lundbeck, Merck, NIH, Novartis, Noven, Otsuka, Pfizer, Physicians Postgraduate Press, Rhodes Pharmaceuticals, Roche, Sage, Seaside Pharmaceuticals, Shire, Stanley Medical Research Institute, Sunovion, Supernus Pharmaceuticals, Transcept Pharmaceuticals, Validus, and WebMD.
Dr. Arnold has had research funding from Curemark, Forest, Lilly, and Shire, advisory board honoraria from Biomarin, Novartis, Noven, Roche, Seaside Therapeutics, & Shire, consulting with Tris Pharma, and travel support from Noven.
Dr. Youngstrom has consulted with Lundbeck and received travel support from Bristol-Myers Squibb, as well as grant support from the NIH.
Dr. Fristad receives royalties from Guilford Press, Inc., APPI, CFPSI
Dr. Birmaher has or will receive royalties from for publications from Random House, Inc (New hope for children and teens with bipolar disorder) and Lippincott Williams & Wilkins (Treating Child and Adolescent Depression). He is employed by the University of Pittsburgh and the University of Pittsburgh Medical Center and receives research funding from NIMH
Dr. Kowatch is a consultant for Forest Pharmaceutical, Astra-Zeneca and the REACH Foundation. He receives research support from NIMH. He is employed by Ohio State University and an editor for Current Psychiatry
Dr. Sunshine receives research support from Siemens Healthcare.
Footnotes
Disclosures:
Drs Bertocci, Bebko, Olino, Fournier, Horwitz, Phillips, Axelson, Holland, Schirda, Versace, Almeida, Perlman, Diwadkar, Travis, as well as Christine Demeter, Lisa Bonar, Mary Kay Gill, Amanda Hinze, and Richard White have no financial interests or potential conflicts of interest.
References
- Axelson DA, Birmaher B, Brent DA, Wassick S, Hoover C, Bridge J, Ryan ND. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. Journal of Child and Adolescent Psychopharmacology. 2003;13:463–70. doi: 10.1089/104454603322724850. [DOI] [PubMed] [Google Scholar]
- Bebko G, Bertocci MA, Fournier JC, et al. Parsing dimensional vs diagnostic category–related patterns of reward circuitry function in behaviorally and emotionally dysregulated youth in the longitudinal assessment of manic symptoms study. JAMA Psychiatry. 2013 doi: 10.1001/jamapsychiatry.2013.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M. Psychometric Properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): A Replication Study. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:1230–1236. doi: 10.1097/00004583-199910000-00011. [DOI] [PubMed] [Google Scholar]
- Brotman M, Schmajuk M, Rich B, Dickstein D, Guyer A, Costello E, Egger H, Angold A, Pine D, Leibenluft E. Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biological Psychiatry. 2006;60:991–7. doi: 10.1016/j.biopsych.2006.08.042. [DOI] [PubMed] [Google Scholar]
- Charney DS, Babich KS. Foundation for the NIMH strategic plan for mood disorders research. Biological Psychiatry. 2002;52:455–456. doi: 10.1016/s0006-3223(02)01543-3. [DOI] [PubMed] [Google Scholar]
- Cusi A, Nazarov A, Holshausen K, Macqueen G, McKinnon M. Systematic review of the neural basis of social cognition in patients with mood disorders. Journal of Psychiatry Neuroscience. 2012;37:154–69. doi: 10.1503/jpn.100179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond J, Glover G. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. Journal of Neuroscience Methods. 2002;118:115–28. doi: 10.1016/s0165-0270(02)00121-8. [DOI] [PubMed] [Google Scholar]
- Eklund A, Andersson M, Josephson C, Johannesson M, Knutsson H. Does parametric fMRI analysis with SPM yield valid results? An empirical study of 1484 rest datasets. NeuroImage. 2012;61:565–78. doi: 10.1016/j.neuroimage.2012.03.093. [DOI] [PubMed] [Google Scholar]
- Findling RL, Jo B, Frazier TW, Youngstrom EA, Demeter CA, Fristad MA, Birmaher B, Kowatch RA, Arnold E, Axelson DA, Ryan N, Hauser JC, Brace DJ, Marsh LE, Gill MK, Depew J, Rowles BM, Horwitz SM. The 24-month course of manic symptoms in children. Bipolar Disord. 2013;15:669–79. doi: 10.1111/bdi.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL, Youngstrom EA, Fristad M, Birmaher B, Kowatch R, Arnold LE, Frazier TW, Axelson DA, Ryan ND, Demeter C, Gill MK, Fields B, Depew J, Kennedy S, Marsh L, Rowles B, Horwitz SM. Characteristics of children with elevated symptoms of mania: the Longitudinal Assessment of Manic Symptoms LAMS study. The journal of clinical psychiatry. 2010;71:1664–72. doi: 10.4088/JCP.09m05859yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Youngstrom EA, Horwitz SM, Demeter C, Fristad M, Arnold LE, Birmaher B, Kowatch R, Axelson DA, Ryan ND, Gill MK, Findling RL. Relationship of persistent manic symptoms to the diagnosis of pediatric bipolar spectrum disorders. Journal of Clinical Psychiatry. 2011;72:846–53. doi: 10.4088/JCP.10m06081yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman L, Glover G. Report on a multicenter fMRI quality assurance protocol. Journal of Magnetic Resonance Imaging. 2006;23:827–839. doi: 10.1002/jmri.20583. [DOI] [PubMed] [Google Scholar]
- Friedman L, Glover G The, F.C. Reducing interscanner variability of activation in a multicenter fMRI study: Controlling for signal-to-fluctuation-noise-ratio (SFNR) differences. NeuroImage. 2006;33:471–481. doi: 10.1016/j.neuroimage.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Gee D, Karlsgodt K, van Erp T, Bearden C, Lieberman M, Belger A, Perkins D, Olvet D, Cornblatt B, Constable T, Woods S, Addington J, Cadenhead K, McGlashan T, Seidman L, Tsuang M, Walker E, Cannon T. Altered age-related trajectories of amygdala-prefrontal circuitry in adolescents at clinical high risk for psychosis: a preliminary study. Schizophrenia Research. 2012;134:1–9. doi: 10.1016/j.schres.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halari R, Simic M, Pariante C, Papadopoulos A, Cleare A, Brammer M, Fombonne E, Rubia K. Reduced activation in lateral prefrontal cortex and anterior cingulate during attention and cognitive control functions in medication-naive adolescents with depression compared to controls. Journal of Child Psychology and Psychiatry. 2009;50:307–16. doi: 10.1111/j.1469-7610.2008.01972.x. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK. Toward constructing an endophenotype strategy for bipolar disorders. Biological Psychiatry. 2006;60 doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Haxby J, Ungerleider L, Horwitz B, Rapoport S, Grady C. Hemispheric differences in neural systems for face working memory: A PET-rCBF study. Human Brain Mapping. 1995;3:68–82. [Google Scholar]
- Horwitz SM, Demeter C, Pagano M, Youngstrom EA, Fristad M, Arnold LE, Birmaher B, Gill MK, Axelson DA, Kowatch R, Frazier T, Findling RL. Longitudinal Assessment of Manic Symptoms (LAMS) study: background, design, and initial screening results. The journal of clinical psychiatry. 2010;71:1511–7. doi: 10.4088/JCP.09m05835yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulvershorn L, Cullen K, Anand A. Toward dysfunctional connectivity: a review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging Behavior. 2011;5:307–28. doi: 10.1007/s11682-011-9134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine D, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Rao U, Flynn C, Moreci P, Williamson D, Ryan ND. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Ladouceur C, Silk JS, Dahl RE, Ostapenko L, Kronhaus DM, Phillips ML. Fearful Faces Influence Attentional Control Processes in Anxious Youth and Adults. Emotion. 2009a;9:855–864. doi: 10.1037/a0017747. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Farchione T, Diwadkar V, Pruitt P, Radwan J, Axelson D, Birmaher B, Phillips ML. Differential Patterns of Abnormal Activity and Connectivity in the Amygdala–Prefrontal Circuitry in Bipolar-I and Bipolar-NOS Youth. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50:1275–1289.e2. doi: 10.1016/j.jaac.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD, Silk JS, Dahl RE, Ostapenko L, Kronhaus D, Phillips ML. Fearful faces influence attentional control processes in anxious youth and adults. Emotion. 2009b;9:855–64. doi: 10.1037/a0017747. [DOI] [PubMed] [Google Scholar]
- Lewinsohn P, Klein D, Seeley J. Bipolar disorder during adolescence and young adulthood in a community sample. Bipolar Disorders. 2000;2:281–293. doi: 10.1034/j.1399-5618.2000.20309.x. [DOI] [PubMed] [Google Scholar]
- Magnotta V, Friedman L. Measurement of Signal-to-Noise and Contrast-to-Noise in the fBIRN Multicenter Imaging Study. Journal of Digital Imaging. 2006;19:140–7. doi: 10.1007/s10278-006-0264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Morgan J, Olino TM, McMakin D, Ryan ND, Forbes EE. Neural response to reward as a predictor of increases in depressive symptoms in adolescence. Neurobiological Disorders. 2013;52:66–74. doi: 10.1016/j.nbd.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén B. Mplus User’s Guide. Sixth Edition. Muthen & Muthen; Los Angeles, CA: 1998-2011. [Google Scholar]
- Nylund K, Asparouhov T, Muthen B. Deciding on the Number of Classes in Latent Class Analysis and Growth Mixture Modeling: A Monte Carlo Simulation Study. Structural Equation Modeling: A Multidisciplinary Journal. 2007;14:535–569. [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Passarotti A, Ellis J, Wegbreit E, Stevens M, Pavuluri M. Recudeced Functioanl Connectivity of Prefontal Regions and Amygdala Within Affect and Working Memory Networks in Pediatric Bipolar Disorder. Brain Connectivity. 2012;2:320–334. doi: 10.1089/brain.2012.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti A, Sweeney J, Pavuluri M. Emotion Processing Influences Working Memory Circuits in Pediatric Bipolar Disorder and Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2010a;49:1064–1080. doi: 10.1016/j.jaac.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti A, Sweeney J, Pavuluri M. Neural correlates of response inhibition in pediatric bipolar disorder and attention deficit hyperactivity disorder. Psychiatry Research: Neuroimaging. 2010b;181:36–43. doi: 10.1016/j.pscychresns.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri M, O’Connor M, Harral E, Sweeney J. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Research: Neuroimaging. 2008;162:244–255. doi: 10.1016/j.pscychresns.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman G, Simmons A, Wu J, Hahn K, Tapert S, Max J, Paulus M, Brown G, Frank G, Campbell-Sills L, Yang T. Amygdala response and functional connectivity during emotion regulation: a study of 14 depressed adolescents. Journal of Affective Disorders. 2012;139:75–84. doi: 10.1016/j.jad.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M, Ladouceur C, Drevets W. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Frank E. Redefining bipolar disorder: Toward DSM5. American Journal of Psychiatry. 2006;163 doi: 10.1176/ajp.2006.163.7.1135. [DOI] [PubMed] [Google Scholar]
- Rich B, Carver F, Holroyd T, Rosen H, Mendoza J, Cornwell B, Fox N, Pine D, Coppola R, Leibenluft E. Different neural pathways to negative affect in youth with pediatric bipolar disorder and severe mood dysregulation. Journal of Psychiatry Research. 2011;45:1283–94. doi: 10.1016/j.jpsychires.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich B, Fromm S, Berghorst L, Dickstein D, Brotman M, Pine D, Leibenluft E. Neural connectivity in children with bipolar disorder: impairment in the face emotion processing circuit. Journal of Child Psychology & Psychiatry. 2008;49:88–96. doi: 10.1111/j.1469-7610.2007.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygula R, Walker S, Clarke H, Robbins T, Roberts A. Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. Journal of Neuroscience. 2010;30:14552–9. doi: 10.1523/JNEUROSCI.2631-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall J, Turner J, van Erp T, White T, Bockholt H, Gollub R, Ho B, Magnotta V, Jung R, McCarley R, Schulz S, Lauriello J, Clark V, Voyvodic J, Diaz M, Calhoun V. Voxel-based morphometric multisite collaborative study on schizophrenia. Schizophrenia Bulletin. 2009;35:82–95. doi: 10.1093/schbul/sbn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Goodman R. Mood lability and psychopathology in youth. Psychological Medicine. 2009;39:1237–45. doi: 10.1017/S0033291708004662. [DOI] [PubMed] [Google Scholar]
- Ward B. AlphaSim. National Institute of Mental Health; 2002. [Google Scholar]
- Youngstrom EA, Frazier T, Demeter C, Calabrese J, Findling RL. Developing a 10-item mania scale from the Parent General Behavior Inventory for children and adolescents. Journal of Clinical Psychiatry. 2008;69:831–9. doi: 10.4088/jcp.v69n0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstrom EA, Meyers O, Demeter C, Youngstrom J, Morello L, Piiparinen R, Feeny N, Calabrese J, Findling RL. Comparing diagnostic checklists for pediatric bipolar disorder in academic and community mental health settings. Bipolar Disorders. 2005;7:507–17. doi: 10.1111/j.1399-5618.2005.00269.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


