Abstract
The role of Rho family GTPases in controlling the actin cytoskeleton and thereby regulating cell migration has been well studied for cells migrating on 2D surfaces. In vivo, cell migration occurs within three dimensional matrices and along aligned collagen fibers with rather different spatial requirements. Recently, a handful of studies coupled with new approaches have demonstrated that Rho GTPases have unique regulation and roles during cell migration within 3D matrices, along collagen fibers, and in vivo. Here we propose that migration on aligned matrices facilitates spatial organization of Rho family GTPases to restrict and stabilize protrusions in the principle direction of alignment, thereby maintaining persistent migration. The result is coordinated cell movement that ultimately leads to higher rates of metastasis n vivo.
Keywords: Cell Migration, Rho GTPases, 3D Extracellular Matrix, Matrix Alignment, Metastasis
1. INTRODUCTION
Although cell migration has been studied for multiple decades, there is still a lack of understanding of how cell migration is regulated in the context of complex, three dimensional matrices in vivo. There are several aspects of the in vivo extracellular matrix that differ from classic studies on 2D surfaces, and likely affect cell migration, including the greatly lower stiffness of many 3D matrices, as well as the composition, cross-linking, pore size, and topography. Recently, we demonstrated that collagen alignment accompanies tumor progression and facilitates local invasion (Provenzano et al., 2006, Provenzano et al., 2008). In breast cancer patient samples, the presence of aligned collagen fibers oriented radially to the tumor/stromal boundary is associated with poor prognosis (Conklin et al., 2011). The presence of aligned collagen facilitates invasion, creating a sort of “highway” that may serve to provide tumor cells with a means to escape a primary tumor and direct their migration to a nearby blood vessel. In this context, aligned collagen represents an aspect of cancer progression that requires further study, not only to better understand the mechanisms underlying the formation of aligned collagen fibers in vivo, but also to determine the molecular players involved in cell recognition of and migration along aligned fibers. Despite data demonstrating cell migration along collagen fibers in vivo, little is known of how cells recognize and track along aligned extracellular matrices. Here we consider signaling pathways that may control cell migration in 3D matrices and along aligned collagen fibers. A wealth of literature investigating cell migration on 2D coated surfaces demonstrates a role for integrin-mediated activation of the Rho family of GTPases Rho, Rac, and Cdc42. The coordinated action of these GTPases controls the steps required for a cell to translocate, beginning with actin-induced protrusion generation, adhesion formation and maturation, and resulting in leading edge traction force generation and trailing edge detachment (Lauffenburger and Horwitz, 1996). While the role of these molecules in mediating cell migration on 2D surfaces has been well established, recent data suggests that these pathways are regulated somewhat differently in the context of 3D cell migration.
2. FUNCTIONS
The Rho family members belong to the Ras superfamily of small GTPases, characterized by their ability to cycle between an inactive GDP and an active GTP-bound state. In the GTP-bound state, conformational changes allow binding of the GTPase to effectors and activation of downstream signaling pathways. For each GTPase, there are over a dozen possible effectors, and the regulation of these pathways is still under investigation. Among the effectors, several mediate the classic role for Rho GTPases as regulators of actin structure, first described in two classic papers by Ridley and Hall (Ridley and Hall, 1992, Ridley et al., 1992) and reviewed recently (Ridley, 2013). These initial observations demonstrated a role for Rac in membrane ruffling and protrusion, and a role for Rho in actin stress fiber formation. Since that time, thousands of papers have been published on the Rho family GTPases and their role in actin dynamics and cell migration. Effectors of Rho GTPases that are particularly relevant to cell migration have been reviewed in (Hanna and El-Sibai, 2013) and include WASP/Wave complex, PAK, PIP5K, ROCK, LIMK, and the formin family of proteins including mDia.
Adding to the complexity is the fact that there are multiple isoforms of these molecules. Thus, while Rho A, Rho B, and Rho C all share several effectors, they have different roles in cancer (Ridley, 2013). Much work is focused on understanding the unique effectors that account for these differing functions. For example, Rho C has been implicated in enhancing metastasis and promotes invasiveness through effects on an alternate formin, FMNL3 downstream of RhoC but not RhoA (Vega, Ridley, 2011) (Ridley, 2013)). Moreover, the effectors themselves are complex. For example, Rho A works to limit cell invasiveness by restricting protrusions through ROCK1. However, the closely related Rho effector, ROCK 2, enhances protrusions through Rac.
The choice of which effector is bound by Rho isoforms is likely determined in part by location. In 2D migration, RhoA localizes to the rear of the cell where it is thought to promote rear retraction. At the front of the cell, Rho A is found at the border of the lamellipod and lamella, while RhoC is found at the leading edge (Zawistowski et al., 2013)(Bravo-Cordero et. al., 2014).
2.1 RHO GTPASES IN 2D DIRECTIONAL MIGRATION
A wealth of knowledge exists about the spatial organization of Rho GTPase signaling pathways in 2D migration, making this knowledge base a springboard to understand regulation of cell migration in 3D. In 2D scratch wound assays, persistently protruding cells align their actin network relative to the axis of migration (Lim et al., 2010). Actin polymerization at the leading edge forces membrane deformation and expansion, which results in a growing protrusion. Protrusions are stabilized by adhesion to an underlying surface, which serve to anchor the contractile forces to allow forward translocation of the cell. The control of directional migration, in part through the regulation of actin, is likely dictated by the spatial and temporal activation of Rho GTPases.
Rac is localized to protrusion tips (Kraynov et al., 2000) and is known to increase actin polymerization by activating Arp2/3 and increasing actin network branching, as well as inhibiting actin-capping proteins. This initial protrusion of the membrane facilitates new contacts with the substratum at the protruding edge via integrin-mediated adhesions. The ability of active Rac to spatially and temporally control membrane protrusions has been demonstrated by the use of a photoactivatable protein switch coupled to a constitutively active Rac1. Wu, et al. showed that local photoactivation of Rac1 induced protrusions at the site of light irradiation within seconds. Additionally, repeated activations sustained cell polarity by maintaining extension and coordinated retraction of the rear, allowing persistent migration (Wu et al., 2009). These results demonstrate not only that Rac activation precisely controls the spatial and temporal localization of protrusions, but that consistent localization and activation of Rac1 facilitates persistent directed migration.
Cdc42 is also involved in the regulation of directional migration. It is well established that Cdc42 regulates actin and filopodial extension through mechanisms that are similar to Rac. Additional evidence suggests an additional role for Cdc42 in stabilizing protrusions through the involvement of microtubules. Cdc42 regulates the orientation of the microtubule-organizing centers and maintains microtubule plus end polymerization in the direction of a growing lamellipod (Etienne-Manneville and Hall, 2002). Microtubules regulate local Rac and Rho GTPase activation (Waterman-Storer et al., 1999, Wittmann and Waterman-Storer, 2001) and appear to determine the localization of nascent adhesions (Stehbens and Wittmann, 2012). In addition to potentially stabilizing a growing protrusion in a similar manner to actin, microtubules provide tracts for kinesin-based delivery of membrane vesicles, signaling proteins, and matrix altering proteases (Stehbens and Wittmann, 2012). Thus, the prolonged organization of microtubules also likely serves to more efficiently shuttle proteins involved in migration to the leading edge, thereby enhancing directional persistence.
Rho provides intracellular contractility through actin-myosin force generation. Both RhoA and RhoC play roles in the extension of membrane protrusions during migration on a 2D surface, with RhoC preceding protrusion formation (Pertz et al., 2006, Zawistowski, Sabouri-Ghomi, 2013). Rho A also regulates the contraction at the lateral and rear of the cell, further supporting forward migration. Rho C has emerged as an important spatial regulator of protrusions and invadopodia in 3D matrices and in vivo (Bravo-Cordero et al., 2014).
Integrin engagement at the leading edge at nascent adhesions spatially activates RhoA, a mechanism dependent on c-Src and p190RhoGAP, but does not affect Rac1 or Cdc42. Following initial activation, RhoA is transiently suppressed via p190RhoGAP, allowing a subsequent cycle of Rac induced protrusion generation (Arthur and Burridge, 2001). The temporal regulation of Rho and Rac activity suggests they are mutually opposed, and highlights the importance of their precise timing to allow for efficient migration through coordinated protrusion and contraction cycles (Ridley, 2013).
2.2 RHO GTPASES IN 3D MIGRATION
Membrane protrusions are thought to be driven largely by the forward force of actin polymerization at the barbed end, which overcomes the tension of the membrane, or by the forward protrusion of membrane blebs due to contractility near the rear of the cell with a force sufficient to displace local collagen fibers (Wyckoff et al., 2006). In 2D migration, protrusions based on actin polymerization dominate, while in 3D environments cells make switches between actin-based protrusions and contraction-driven blebs. Accordingly, the spatial and temporal use of Rho GTPases appears to differ when cells are migrating within 3D matrices compared to on 2D substrata. When Rho activity is inhibited in cells cultured in 3D, the cells exhibit increased cytoskeletal remodeling that is dependent on cofilin, which leads to an increase in cellular protrusions. Unlike on 2D surfaces, where increased protrusions lead to faster cell migration (Arthur and Burridge, 2001), this increase in protrusions has the effect of reducing motility in 3D (Worthylake and Burridge, 2003). Furthermore, cells within 3D matrices have inherently lower levels of Rac activity compared to cells on 2D surfaces, which correlates with fewer peripheral lamellae, and thus more directional migration (Pankov, Yamada 2005). However, even in a 3D matrix, Rac maintains its role in driving forward protrusion, as shown in live zebrafish embryos (Yoo, Huttenlocher, 2012).
As in 2D migration, Cdc42 is also a key regulator of migration within 3D matrices. A recent finding is that spatial activation of Cdc42 at protrusions is coordinated in part through βPix, a GEF for Cdc42 and Rac1. βPix localizes to focal adhesions in cells migrating on fibronectin-based 3D matrices, but is released and activates Cdc42 within 3D collagen matrices. Moreover, the resulting Pix/Cdc42 complex leads to local Rho inactivation through srGAP1 (Kutys and Yamada, 2014).
The disparity between migration in 2D and 3D implicates the importance of the physical properties of the matrix. A 3D environment is inherently less stiff and more confining than a 2D surface, and may limit motility even when protrusions are enhanced. Furthermore, in 3D there are many more possible adhesions that may stabilize protrusions, which may oppose one another and thereby impede migration. Based on these observations, the most effective strategy for a migrating cell to employ in a 3D environment may be to limit protrusion generation by coordinated spatial and temporal activation of Rho GTPases. Thus, cross talk between Rho and Rac emerges as an important regulator of not only migration speed, but in regulating persistence via protrusions, and further suggests that efficient migration in 3D occurs when relative Rho activity is high and Rac activity is low (Figure 1). Rho contractility in 3D also results in reorganization and alignment of matrix fibers (Provenzano, Inman, 2008), which may locally increase matrix stiffness and may further strengthen adhesions, thereby enhancing downstream migratory signals. In considering the enhanced directionality of cells migrating within aligned collagen, the relative levels of Rho and Rac activity as well as their localization may serve to maintain even greater persistence in aligned matrices.
Figure 1.
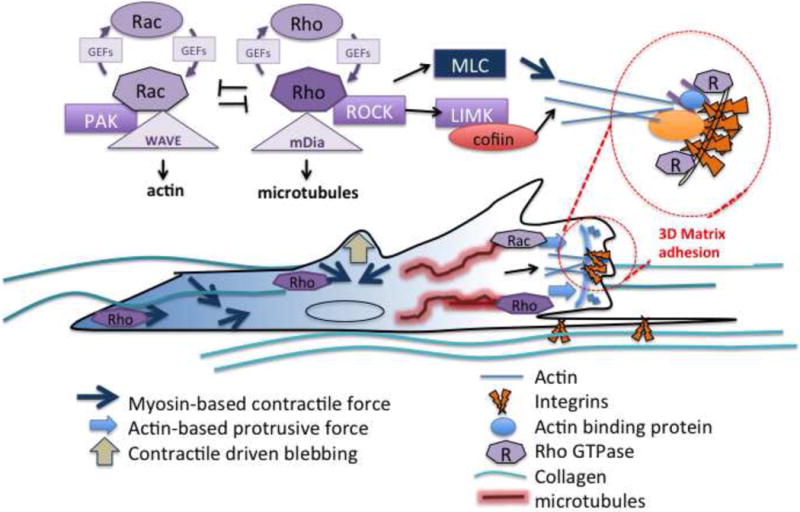
Relationship of signaling pathways that are hypothesized to control the spatial regulation of cell migration in 3D and aligned matrices, based in part on their role in 2D matrices. The appropriate spatial and temporal regulation of Rho family GTPases relative to the organization of the surrounding extracellular matrix is proposed to spatially regulate cellular protrusions, leading to persistent migration.
2.3 MIGRATION IN 3D ALIGNED MATRICES
In vivo, cell migration on aligned collagen fibers results in efficient migration out from a tumor (Wycoff et al, 2006). In order to study the effects of matrix alignment on cell migration, many groups have taken advantage of fibroblasts’ ability to deposit 3D extracellular matrix (also reviewed in Doyle, Petrie 2013). Within these cell-derived matrices (CDMs), regions of high fiber alignment are prevalent. Petrie, et al. (Petrie et al., 2012) made use of these CDMs and identified a new type of cell migration, termed lobopodial migration. Cells employing lobopodial migration maintained the ability to migrate directionally and were morphologically polarized, but did not contain polarized Rac and Cdc42 signaling. This type of migration was found to be specific to 3D linearly elastic matrices, and was distinct from either mesenchymal or amoeboid strategies. Despite the nonpolarized signaling of Rac and Cdc42 in lobopodial migration, Rac and Cdc42 still influenced migration speed; cells expressing siRNA for Rac had reduced cell migration speeds while those expressing siRNA for Cdc42 had enhanced migration speeds. Furthermore, lobopodial migration was dependent on Rho, as siRNA or pharmacologic inhibition of either Rho or ROCK resulted in a switch to lamellipodial-based migration with polarized signaling (Petrie, Gavara, 2012). While these results are intriguing, the mechanisms establishing polarity and maintaining directional migration in aligned 3D matrices remain unclear (reviewed in (Friedl and Alexander, 2011). In addition, it is not clear whether lobopodial migration confers an advantage to maintaining directional persistence in aligned 3D matrices, and whether this mode of migration is present in metastatic cells. Because lobopodial migration contains characteristics reminiscent of both mesenchymal and amoeboid migration strategies, lobopodial migration may represent an intermediary in the mesenchymal to amoeboid transition (Petrie, Gavara, 2012).
Another study investigating the mechanisms of directional migration in response to matrix topography made use of cell derived matrices to show that Rac localization and signaling is required for cells to recognize and migrate within regions of alignment. Cells lacking syndecan-4 or expressing a syndecan-4 mutant that disrupted PKC alpha signaling failed to localize Rac to points of engagement with the matrix. As a result, cells lose the ability to migrate directionally along aligned fibers (Bass et al., 2007). These findings are some of the first to show that persistent directional cell migration in 3D aligned matrices is dependent on proper Rac localization and activity, and likely involves the ability of cells to stabilize protrusions in the direction of alignment by restricting signals that control Rac activity.
While cellular responses to defined changes in ECM organization have not been extensively studied due in part to inherent complexities in engineering matrices with precise topographies, one approach to mimic aligned collagen matrices involves coating a defined line of substrate onto a surface to generate “1D” migration conditions. When cells are cultured and allowed to migrate on these 1D lines of matrix, cells migrate directionally and at faster rates similar to those observed for 3D matrices and in contrast to those on 2D substrates (reviewed in (Doyle et al., 2013)). Additionally, adhesions are more stable, and faster rates of protrusion and retraction cycles occur, which results in more efficient migration that is dependent on myosin IIA compared to migration on traditional 2D substrates (Doyle, Petrie, 2013). This reductionist approach provides a means to study directional migration purely in response to topographic cues without the competing effects of other 3D matrix properties. Furthermore this approach may also provide the means to better understand how spatial and temporal regulation of Rho GTPases facilitates directional migration in response to topography, which can then be extrapolated to more complex 3D environments.
Aligned collagen is likely stiffer than randomly organized matrices ((Xu et al., 2011) and unpublished data), and therefore may promote elevated Rho at cell-matrix contacts that are further restricted by the organization of the matrix. Indeed, we find in vivo that active Rho is organized within elongated protrusions by the orientation of the underlying collagen matrix (Figure 2). This spatially controlled signaling could allow for more coordinated traction forces, providing additional support for the enhanced persistence of cells migrating in aligned matrices. Moreover, aligned matrices may promote even lower levels of active Rac, further limiting protrusion generation peripheral to the leading edge, allowing greater directional migration relative to a random matrix.
Figure 2.
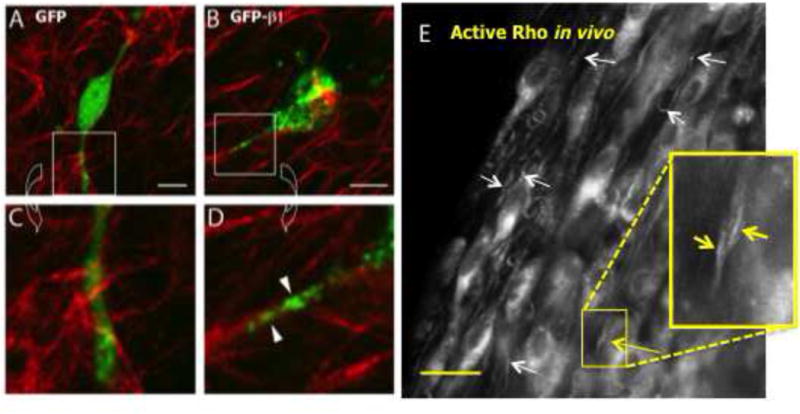
Adhesions and Rho organize along collagen fibers. A–D) MDA-MB-231 breast carcinoma cells transfected with β1 integrin-GFP, or GFP as a control were cultured in 3D collagen gels. Note that the β1 integrin-GFP organizes along collagen fibers in a punctate manner. (β1 integrin-GFP courtesy of Dr. Maddy Parsons). Scale bar in B = 10 μm. E) Active Rho organizes along the axis of collagen alignment in vivo. MDA-MB-231 breast carcinoma cells were stably transfected with the Rho reporter, GFP-RBD, and injected into the mammary fat pad of a nude mouse. Seven days later, cells migrating out on aligned collagen were imaged (short white arrows: collagen). While the reporter is typically diffuse in cells, it localizes to sites of Rho activation. Inset: GFP-RBD localizes to a cell protrusion along the axis of collagen alignment (yellow arrows). Scale bar in E = 30 μm
3. CASCADES
Downstream of the Rho family effectors are molecules that bind to and regulate actin. LIMK, activated by ROCK, in turns phosphorylates ADF/cofilin to prevent it from capping growing actin filaments. The result is that actin polymerization is enhanced. Indeed, release of caged cofilin is enough to induce local protrusions (reviewed in (Bravo-Cordero, Hodgson, 2014)). WASP and its related protein, Wave, bind to the Arp2/3 complex, which binds into the growing actin filament to create branch points. This branched actin network creates additional free barbed ends, changes the structure of the actin cytoskeleton, and is important for the production of a lamellipodium. Interestingly, Arp2/3 is required for adhesive cues, but not polarized responses to chemotaxis (Wu et al., 2012).
In addition to actin, the microtubule network is regulated by Rho family GTPases through the formin proteins including the mammalian homologue of diaphanous, mDia (Wen et al., 2004). (reviewed in (Narumiya et al., 2009)). Intriguingly, microtubules also regulate activation of Rho through GEFH1, and targeting of RhoGTPases to adhesive sites to provide spatial cues (Waterman-Storer, Worthylake, 1999).
One of the key outcomes of Rho activation is the induction of intracellular contractility through actin-myosin interactions. ROCK directly regulates this process by phosphorylating the regulatory myosin light chain, MLC, leading to increased interaction of myosin with actin. In addition, ROCK inhibits the activity of the phosphatase that acts at these sites (Amano et al., 2010). The end result is enhanced contractility of the actin network. This contractility generates forces thought to move the rear and cell body forward, and restricts lateral protrusions to help coordinate the front-to-rear polarity of the cell (Worthylake and Burridge, 2003). Contractility serves to cluster nascent adhesions at the leading edge, leading to maturation to a focal complex which scaffold dozens of signaling molecules (Chrzanowska-Wodnicka and Burridge, 1996). Integrin clustering, however, occurs only if this contractile event works against an external counter-force, in this case provided by the resistance of a sufficiently stiff substratum (Choquet et al., 1997, Wozniak et al., 2003). Thus, a model that emerges is one in which Rho-mediated contractility serves to locally align the ECM (Provenzano, Inman, 2008), creating topographical cues that then organize adhesions along the axis of alignment. Indeed, we find that integrins cluster along collagen fibers within 3D matrices (Figure 2).
The role of integrins and GTPases is cyclical. Integrins acting as force transducers can directly couple the actin cytoskeleton with the ECM, and can organize other signaling molecules via stretch-induced exposure of binding sites, thereby facilitating transmission of external mechanical signals. In this manner, integrins are capable of regulating changes in intracellular signaling in response to changes in ECM stiffness. Increased matrix stiffness, such as an aligned matrix, may therefore act to strengthen integrin adhesions, promote clustering, and enhance intracellular tension through increased actin-myosin contractility. This enhanced contractility in stiffer matrices is driven by elevated levels of active Rho (Wozniak, Desai, 2003), which is regulated by Rho-specific GAPs and GEFs.
4. KEY MOLECULES
Rho GTPases are regulated by GAPs and GEFs
In compliant matrices, the Rho GEF, GEF-H1, is held inactive by its association with microtubules, but is released during microtubule destabilization in stiff matrices, thus allowing greater activation of Rho. When GEF-H1 is knocked down in tumor cells, migration is disrupted (Heck et al., 2012); (Guilluy et al., 2011). The Rho-specific p114RhoGEF regulates the spatial activation of Rho and inactivation of Rac, coordinating MLC phosphorylation and invasion into 3D matrices and in vivo (Terry et al., 2012).
5. ASSOCIATED PATHOLOGIES AND THERAPEUTIC IMPLICATIONS
In breast cancer the presence of collagen alignment correlates to poor outcome (Conklin, Eickhoff, 2011). Metastasis has been a difficult and intractable aspect of cancer treatment. It is attractive to consider GTPases as possible targets for therapy, however attempts to target these molecules has been complex—both because of their ubiquitous roles in all aspects of cell behavior, and because of their redundancy. One approach has been to target downstream effectors, many of which are kinases. For example, ROCK kinase inhibitors have been used for cardiovascular pathologies (Olson, 2008). However, the ubiquitous nature of ROCK-driven contractility again creates issues of toxicity and specificity. An alternative approach has been rational drug design, leading to identification a selective inhibitor for activated Rac1, which works by interfering with binding to a subset of Rac GEFs (Gao et al., 2004). The promise of this approach is that it may become possible to target only some of the functions of Rac or other GTPases within a cell, allowing other functions activated by non-targeted GEFs to remain intact. As we understand more about the regulation of RhoGTPases in cell migration, it will become more feasible to select interactions that target specific events, such as cancer invasion and metastasis.
FACTS.
Cell migration requires precisely controlled signaling and cytoskeletal dynamic events at the leading and trailing edges to set up front-to-rear polarity.
Rho GTPases are key regulators of cell migration, providing spatial and temporal control of the actin cytoskeleton, microtubules, and cell adhesions.
Rho GTPase act as molecular switches that cycle between GDP-bound (off) and GTP-bound (on).
Rho family GTPases are comprised of the Rho family, RhoA, B, and C, the Rac family, Rac1, 2, 3, RhoG, and Cdc42.
Effectors of Rho GTPases regulate the actin cytoskeleton by affecting polymerization dynamics, branching and bundling, and contraction via actin-myosin interactions.
For more information, see the Cell Migration Consortium Gateway: https://www.cellmigration.org/
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010;67:545–54. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur WT, Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol Biol Cell. 2001;12:2711–20. doi: 10.1091/mbc.12.9.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass MD, Roach KA, Morgan MR, Mostafavi-Pour Z, Schoen T, Muramatsu T, et al. Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix. J Cell Biol. 2007;177:527–38. doi: 10.1083/jcb.200610076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-Cordero JJ, Hodgson L, Condeelis JS. Spatial regulation of tumor cell protrusions by RhoC. Cell Adh Migr. 2014:8. doi: 10.4161/cam.28405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin- cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–15. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol. 2011;178:1221–32. doi: 10.1016/j.ajpath.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle AD, Petrie RJ, Kutys ML, Yamada KM. Dimensions in cell migration. Curr Opin Cell Biol. 2013;25:642–9. doi: 10.1016/j.ceb.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004;101:7618–23. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilluy C, Swaminathan V, Garcia-Mata R, O’Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011;13:722–7. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna S, El-Sibai M. Signaling networks of Rho GTPases in cell motility. Cell Signal. 2013;25:1955–61. doi: 10.1016/j.cellsig.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Heck JN, Ponik SM, Garcia-Mendoza MG, Pehlke CA, Inman DR, Eliceiri KW, et al. Microtubules regulate GEF-H1 in response to extracellular matrix stiffness. Mol Biol Cell. 2012;23:2583–92. doi: 10.1091/mbc.E11-10-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraynov VS, Chamberlain C, Bokoch GM, Schwartz MA, Slabaugh S, Hahn KM. Localized Rac activation dynamics visualized in living cells. Science. 2000;290:333–7. doi: 10.1126/science.290.5490.333. [DOI] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: A physically integrated molecular process. Cell. 1996;84:359–69. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Lim JI, Sabouri-Ghomi M, Machacek M, Waterman CM, Danuser G. Protrusion and actin assembly are coupled to the organization of lamellar contractile structures. Exp Cell Res. 2010;316:2027–41. doi: 10.1016/j.yexcr.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- Olson MF. Applications for ROCK kinase inhibition. Curr Opin Cell Biol. 2008;20:242–8. doi: 10.1016/j.ceb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankov R, Endo Y, Even-Ram S, Araki M, Clark K, Cukierman E, Matsumoto K, Yamada KM. A Rac switch regulates random versus directionally persistent cell migration. J Cell Biol. 2005;170(5):793–802. doi: 10.1083/jcb.200503152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–72. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- Petrie RJ, Gavara N, Chadwick RS, Yamada KM. Nonpolarized signaling reveals two distinct modes of 3D cell migration. J Cell Biol. 2012;197:439–55. doi: 10.1083/jcb.201201124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Trier SM, Keely PJ. Contact Guidance Mediated Three-Dimensional Cell Migration is Regulated by Rho/ROCK-Dependent Matrix Reorganization. Biophys J. 2008;95:5374–84. doi: 10.1529/biophysj.108.133116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ. RhoA, RhoB and RhoC have different roles in cancer cell migration. J Microsc. 2013;251:242–9. doi: 10.1111/jmi.12025. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–99. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–10. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Stehbens S, Wittmann T. Targeting and transport: how microtubules control focal adhesion dynamics. J Cell Biol. 2012;198:481–9. doi: 10.1083/jcb.201206050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry SJ, Elbediwy A, Zihni C, Harris AR, Bailly M, Charras GT, et al. Stimulation of cortical myosin phosphorylation by p114RhoGEF drives cell migration and tumor cell invasion. PLoS One. 2012;7:e50188. doi: 10.1371/journal.pone.0050188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega FM, Fruhwirth G, Ng T, Ridley AJ. RhoA and RhoC have distinct roles in migration and invasion by acting through different targets. J Cell Biol. 2011;193(4):655–65. doi: 10.1083/jcb.201011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Worthylake RA, Liu BP, Burridge K, Salmon ED. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts [see comments] Nat Cell Biol. 1999;1:45–50. doi: 10.1038/9018. [DOI] [PubMed] [Google Scholar]
- Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, et al. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol. 2004;6:820–30. doi: 10.1038/ncb1160. [DOI] [PubMed] [Google Scholar]
- Wittmann T, Waterman-Storer CM. Cell motility: can Rho GTPases and microtubules point the way? J Cell Sci. 2001;114:3795–803. doi: 10.1242/jcs.114.21.3795. [DOI] [PubMed] [Google Scholar]
- Worthylake RA, Burridge K. RhoA and ROCK Promote Migration by Limiting Membrane Protrusions. J Biol Chem. 2003;278:13578–84. doi: 10.1074/jbc.M211584200. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol. 2003;163:583–95. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Asokan SB, Berginski ME, Haynes EM, Sharpless NE, Griffith JD, et al. Arp2/3 is critical for lamellipodia and response to extracellular matrix cues but is dispensable for chemotaxis. Cell. 2012;148:973–87. doi: 10.1016/j.cell.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–8. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol. 2006;16(15):1515–23. doi: 10.1016/j.cub.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Xu B, Chow MJ, Zhang Y. Experimental and modeling study of collagen scaffolds with the effects of crosslinking and fiber alignment. Int J Biomater. 2011;2011:172389. doi: 10.1155/2011/172389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SK, Lam PY, Eichelberg MR, Zasadil L, Bement WM, Huttenlocher A. The role of microtubules in neutrophil polarity and migration in live zebrafish. J Cell Sci. 2012;125(Pt 23):5702–10. doi: 10.1242/jcs.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawistowski JS, Sabouri-Ghomi M, Danuser G, Hahn KM, Hodgson L. A RhoC biosensor reveals differences in the activation kinetics of RhoA and RhoC in migrating cells. PLoS One. 2013;8:e79877. doi: 10.1371/journal.pone.0079877. [DOI] [PMC free article] [PubMed] [Google Scholar]


