Abstract
Objectives
To determine whether greater burden of geriatric conditions would have contrasting effects on quality of care (QOC) than nongeriatric, general medical conditions.
Design
Cross-sectional observation over 1 year of ambulatory care
Setting
The Assessing Care of Vulnerable Elders-2 study
Participants
Older adults prospectively screened for falls, incontinence, and dementia (N=644).
Measurements
Participant-level QOC in absolute percentage points calculated using 65 ambulatory care care-process quality indicators (QIs) for 13 general medical and geriatric conditions (#QIs provided/# QIs eligible). Secondary outcomes were geriatric QOC (a subset of 38 geriatric care QIs) and medical QOC (the 27 remaining nongeriatric QIs). Exposure variables were number of six medical conditions (medical comorbidity) and six geriatric conditions (geriatric comorbidity), controlling for age, sex, number of primary care visits, and site.
Results
Medical and geriatric comorbidity were unrelated to each other (correlation coefficient=0.04, p=.27) yet had opposite effects on QOC. Each additional medical condition was associated with a 3.2-percentage point (95% confidence interval (CI)=2.3–4.2 percentage point) increment in QOC, and each additional geriatric condition was associated with 4.9-percentage point (95% CI=3.5–6.5 percentage point) decrement in QOC. Participants with greater geriatric comorbidity received poorer medical and geriatric QOC.
Conclusion
Greater burden of geriatric conditions, or geriatric multimorbidity, is associated with poorer QOC. Geriatric multimorbidity should be targeted for better care using a comprehensive approach.
Keywords: multi-morbidity, aging, quality of care
Because of the increasing number of older adults and the complexity of chronic condition care, there is much interest in improving the quality of care (QOC) provided to older individuals with multimorbidity.1 In 2004, people aged 65 and older represented 11% of the U.S. population but consumed 31% of healthcare expenses.2 Furthermore, the top 5% of older spenders account for one-third of all expenses by older individuals.3 Most healthcare expenditures are on chronic condition care,2 but despite concern that the QOC provided to older persons in the community is inadequate4,5 and that multiple chronic conditions could result in adverse care,6,7 individuals with a greater burden of chronic conditions receive better QOC than those with fewer conditions, even after accounting for healthcare use.8,9
Geriatric conditions such as dementia and falls are increasingly being recognized as measurable conditions that are prevalent in aging populations and coexist with “traditional” comorbidities such as diabetes mellitus and cardiovascular heart disease.10,11 Geriatric conditions are underdiagnosed in primary care.4,12–14 Research has shown that medical care of geriatric conditions is costly in terms of time15–17 and complex18 for multifactorial physical, cognitive, and social reasons. In the Assessing the Care of Vulnerable Elders Study (ACOVE-1), the QOC provided for geriatric conditions was worse than that for general medical conditions in community practice.4
Therefore, this study investigated whether having multiple geriatric conditions would have a greater effect on QOC than having multiple nongeriatric, medical conditions. To evaluate this question, a new study of QOC provided to a separate cohort of individuals receiving ambulatory care participating in the ACOVE-2 study was performed. In contrast to the original ACOVE-1 cohort (older individuals in managed care not selected according to medical conditions),8,9 ACOVE-2 studied individuals selected for one or more geriatric conditions (falls, urinary incontinence, and dementia). Therefore, the ACOVE-2 cohort was more likely than the general older population to have geriatric and general medical multimorbidity. Similar to ACOVE-1, ACOVE-2 measured comprehensive QOC according to detailed process-of-care quality indicators (QIs), permitting this study of medical and geriatric condition burdens and their contrasting effects on QOC.
METHODS
Sample
This is a cross-sectional observational analysis of data from ACOVE-2, a practice-based intervention that enrolled 649 individuals in two large multispecialty, multisite practices in southern California. The institutional review boards of RAND Health and the University of California at Los Angeles approved ACOVE-2. The ACOVE-2 intervention improved the QOC for falls and urinary incontinence but not dementia 5 and did not cause an unintended decrement in QOC for other conditions. 19 One site in each multisite practice received a practice-improvement intervention for dementia, falls, and urinary incontinence. 20 The other sites (1 site in one practice, 4 sites in the other practice) served as controls (screening for eligible individuals without intervention). In ACOVE-2, individuals aged 75 and older were screened at intervention and control sites for symptoms of three conditions (bothersome urinary or bladder symptoms; problems with memory; concern about falling, ≥2 falls, or any fall requiring medical care) over 13 months in 2002–03.
In contrast to the original ACOVE-2 study, which focused on care provided by practices, this is a new analysis at the level of individual participants: 644 ACOVE-2 participants whose overall QOC and comorbid conditions were measured as part of the ACOVE-2 study.
Primary predictor measure: Condition counts
Using medical record review of primary and specialty ambulatory medical records from the 1-year study period, ACOVE-2 considered documentation of atrial fibrillation, coronary artery disease, congestive heart failure, cerebrovascular disease, diabetes mellitus, hypertension, hearing impairment, dementia, falls, urinary incontinence, malnutrition, and osteoporosis (definitions in online Appendix S1). Although ACOVE-2 used self-reported symptoms of three geriatric conditions as inclusion criteria (e.g., concern about falling), participants were considered not to have the condition if information to the contrary was documented in the medical record (e.g., a fall had not actually occurred, or the individual had no gait impairment on physical examination). Therefore, it was possible to have no comorbid conditions.
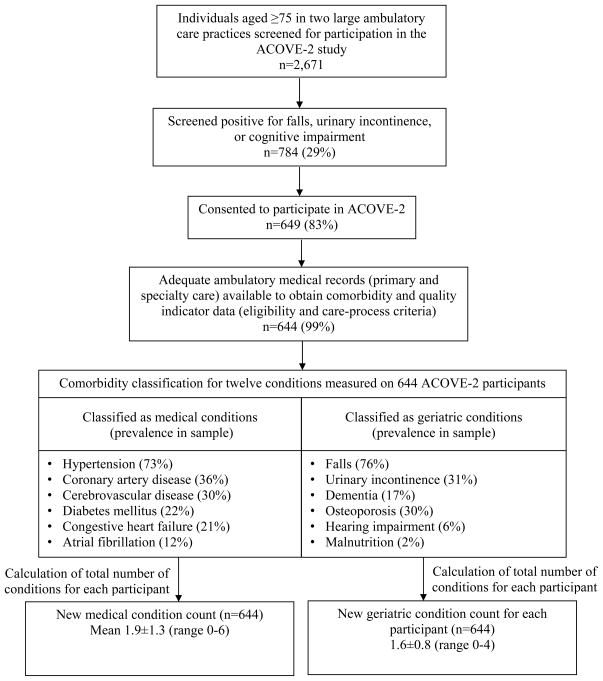
For the current analysis, the 12 conditions were categorized into general medical versus geriatric types of conditions—the first six conditions as general medical comorbidities (tallied as medical comorbidity) and the last six conditions as geriatric comorbidities (tallied as a separate geriatric comorbidity count) (Figure 1). Therefore, each participant had a geriatric and a medical comorbidity count, and the sum of the two counts was the total comorbidity count. The decision to categorize a condition as a geriatric condition was based on older age of onset and multifactorial etiology (e.g., psychosocial in addition to medical). Categorization also aimed to balance the two counts with a similar number of conditions. Two conditions, hearing impairment and osteoporosis, could have been classified in either category, but classifying either as general medical conditions did not change the findings of the study.
Figure 1.
Data flow. ACOVE=Assessing Care of Vulnerable Elders
Outcome measure: QOC
ACOVE-2 used medical record review of all primary and specialty care visits during the study period to measure 65 ambulatory care process QIs and to calculate a comprehensive QOC score for each participant. Care-process QI development for vulnerable elderly adults, 21 including appropriateness criteria (< 6-month life expectancy, advanced dementia, and global preferences for avoiding hospital care or surgery),22 have been previously described. In short, the ACOVE QIs first specify which individuals are eligible for the QI, depending on their condition or clinical circumstance.4 The QIs span comprehensive areas of care (dementia, depression, diabetes mellitus, falls and fear of falling, hearing impairment, cardiovascular disease, malnutrition, osteoporosis, pain, urinary incontinence, medication use, screening and prevention). Individuals with more conditions qualified for more QIs, but all were eligible for at least four preventive measures. Second, the QIs specify the recommended care process that should be provided. Based on medical record review, if an eligible individual receives the recommended care, the QI is scored as 1, whereas not receiving the care is scored as 0. An overall QOC score can be calculated for each individual as the number of QIs passed divided by the number of QIs eligible, expressed as a percentage.
The current analysis used the overall QOC score as the primary outcome. Then two new quality scores, geriatric QOC and general medical QOC, were developed as secondary outcomes so that they could be tested separately against the new geriatric and medical comorbidity counts. To calculate the two new scores, each of the 65 QIs was differentiated into one of two types of care processes: geriatric versus general medical care. (See online Appendix S2 for QI definitions and how they corresponded with geriatric versus medical conditions.) Of the 65 ACOVE-2 QIs, 27 were determined to be geriatric: those applying to previously designated geriatric conditions (dementia, falls, osteoporosis, urinary incontinence, hearing impairment, malnutrition) and any QIs concerning advance care planning. These geriatric QIs were used to calculate a geriatric QOC score (number of geriatric QIs passed/number of geriatric QIs eligible). The remaining 38 general medical QIs (cardiovascular, cerebrovascular, diabetes mellitus, depression, pain management and QIs concerning influenza and pneumonia vaccinations) were used to calculate the medical QOC score (number of medical QIs passed/number of medical QIs eligible). Because of ACOVE-2 inclusion criteria (symptoms of falling, incontinence, or memory impairment), participants were eligible for at least one geriatric QI. Therefore, all participants had a geriatric QOC score. Because preventive QIs were classified as general medical QIs, all participants also had a medical QOC score.
Primary care visits
Using available administrative data, ACOVE-2 identified a primary care provider for each participant based on the greatest number of primary care visits during the study year. For this study, the number of visits with this provider was tabulated for each participant.
Analysis
Unadjusted linear regression was first used to test the relationship between the original total comorbidity count (12 conditions) and overall QOC. Next, allowing the geriatric and medical conditions to vary independently with QOC, geriatric and general medical condition counts (instead of the original count) were used to predict overall QOC. The two regressions (total comorbidity model and the model with the two new comorbidity counts) were compared for incremental improvement in model variance (R-squared).
Initial exploration included testing for nonlinear relationships between the two types of comorbidity counts and overall QOC (using quadratic and nonlinear dummy indicators). Interaction effects were investigated between geriatric comorbidity counts and the intervention and control groups. To address concerns that a particular geriatric condition (e.g., advanced dementia) might be driving the findings, participants with specific combinations of geriatric conditions were identified using a method developed in prior comorbidity work (Table 1).8 This method was used to examine whether the mean unadjusted overall QOC increased over increasingly complex combinations (Table 1) of the four most-prevalent medical (hypertension, coronary artery disease, diabetes mellitus, atrial fibrillation) and the three most-prevalent geriatric (falls, urinary incontinence, dementia) conditions in the dataset.
Table 1.
Overall Quality of Care Across Combinations of Specific Medical versus Geriatric Conditions
| Condition | |||||
| General medical | |||||
| Hypertension | Absent | Present | Present | Present | Present |
| Coronary artery disease | Absent | Absent | Present | Present | Present |
| Diabetes mellitus | Absent | Absent | Absent | Present | Present |
| Atrial fibrillation or congestive heart failure | Absent | Absent | Absent | Absent | Present |
| N | 100 | 202 | 105 | 49 | 11 |
| Mean overall quality of care, %a | 53 | 56 | 63 | 67 | 76 |
| Geriatric | |||||
| Falls and fear of falling | Absent | Present | Present | Present | |
| Urinary incontinence | Absent | Absent | Present | Present | |
| Dementia | Absent | Absent | Absent | Present | |
| N | 42 | 326 | 86 | 20 | |
| Mean overall quality of care, %a | 51 | 42 | 31 | 33 |
P<.001 for test of trend
The multivariable models predicting overall QOC included geriatric and medical comorbidity counts and covariables (age, sex, number of primary care clinician visits, whether a participant was from a control or intervention site). Because participants were clustered at the level of the primary care provider (39 clinician clusters, ranging from 1 to 49 study participants per provider), final fully adjusted models also included physician-level random intercepts.
Next, the analysis focused on medical and geriatric QOC as separate outcomes. Because participants with greater burden of medical comorbidity had medical QOC scores that approached 100%, errors were not normally distributed and resulted in out-of-sample predictions greater than 100%. Therefore, linear regression was inappropriate. Generalized linear regression (binomial, logit link, a functional form that constrains the outcome to between 0% and 100%) 23 with robust standard errors was used for these analyses. Results of these analyses were expressed as marginal effect at the mean of the predictor variables and graphically as predicted scores; 95% confidence intervals (CIs) were obtained for model predictions using bootstrapping techniques. All analyses were performed using Stata 12 (Stata Corp., College Station, TX).
RESULTS
ACOVE-2 screened 2,671 individuals, 784 (29%) of whom screened positively for one or more geriatric conditions (Figure 1); 649 (83%) of these consented to participate, and 644 (99%) had adequate medical records to evaluate the 65 QIs (Appendix 2). During the study, the 644 ACOVE-2 participants were eligible for 7,856 QIs (3,582 geriatric QIs and 4,274 medical QIs). Participants were eligible for a mean of 12.2±4.4 QIs (range 4–27). On average, participants were eligible for 5.6 (range 2–17) geriatric QIs and 6.6 (range 0–19) medical QIs. Overall QOC (# QIs passed/# QIs eligible) was normally distributed, with a mean score of 59±18% (range 0–100%). The geriatric QOC score was also normally distributed, with a mean of 41±23% (range 0–100%). The medical QOC score was negatively skewed (long left tail), with a modal score of 100% (mean 73±23%, range 0–100%).
The sample was one-third male and had a mean age of 81. The total comorbidity count (geriatric plus medical comorbidity) was 3.6±1.6 (range 0–9). Ninety-five percent had one or more geriatric and medical conditions, and 29% had two or more. Medical (mean 1.9, range 0–6) and geriatric (mean 1.6, range 0–4) comorbidity counts were uncorrelated (correlation coefficient (r)=0.04, p=.27). Thirty participants had no geriatric conditions despite screening positive for potential symptoms (16 for falls or gait impairment, 12 for incontinence, 6 for dementia). The mean number of primary care visits over the 13-month study was 5.4±3.6 (range 1–20) (Figure 1).
Results are expressed in absolute percentage of QIs passed (of those eligible) or percentage points. On average, each additional condition (using the total count of 12 conditions) was associated with an improvement of 1.5 percentage points in overall QOC (unadjusted R2=0.02), although when conditions were split into medical and geriatric types and both counts used as the only predictors of overall QOC, each medical comorbidity was associated with a 4.0–percentage point increase in overall QOC, whereas each additional geriatric comorbidity was associated with a 4.9–percentage point decrease in overall QOC, independent of each other (p<.001 for both, R2 =0.14). Unadjusted number of visits also predicted overall QOC (0.8–percentage point increase per visit, p<.001).
Similar trends in unadjusted mean overall QOC scores were observed across increasingly more medically complex individuals according to combinations of specific conditions; more complex combinations of general medical conditions (top, Table 1) were associated with better QOC (p<.001) regardless of which condition was added to the combinations. Conversely, more complex combinations of geriatric conditions (bottom, Table 2) were associated with poorer QOC (p<.001), suggesting that no single geriatric condition (e.g., dementia) was the dominant condition driving results.
Table 2.
Multivariable Models Predicting Overall, Medical, and Geriatric Quality of Care (QOC)
| Predictor Variable | Dependent Variable, Effect on QOC, Absolute Percentage Points (95% Confidence Interval) | ||
|---|---|---|---|
| Overall QOC | Medical QOC | Geriatric QOC | |
| Comorbidity count | |||
| Geriatric (per comorbidity) | −4.9 (−6.5 to −3.5) | −2.3 (−4.5 to −0.1) | −3.4 (−5.5 to −1.2) |
| Medical (per comorbidity) | 3.2 (2.3–4.2) | 3.0 (1.6–4.3) | −0.04 (−1.4–1.3) |
| Age (per 10 years) | 0.7 (−1.9–3.3) | −5.3 (−26–15) | 2.9 (−0.6–6.5) |
| Male | 2.7 (−0.05–5.5) | 10.7 (−9.6–31) | 3.7 (−0.3–7.7) |
| Intervention site (vs control) | 6.1 (3.6–8.6) | 0.6 (−18–19) | 13.3 (9.8–17) |
| Number of primary care provider visits per year (each additional visit) | 0.6 (0.2–1.0) | 4.3 (1.3–7.2) | 0.02 (−0.6–0.6) |
The effect sizes reported for Overall QOC) are beta-coefficients from a multivariable linear regression model. The effect sizes reported for medical and geriatric QOC are marginal effect sizes from a fractional logistic regression model (interpretable like a beta coefficient in the middle of the distribution).
In the multivariable models that added age, sex, intervention site, primary care visits, and physician-level random effects, each medical condition was associated with a 3.2–percentage point increase in overall QOC (p<.001), and each geriatric condition was associated with a 4.9–percentage point decrease in overall QOC (p<.001) (Table 2). Predicted overall QOC is displayed in Figure 2 using increasing values of geriatric and medical condition counts and holding other covariates constant. The relationship was monotonic and linear in the intervention and control groups and confirmed using alternative nonlinear analyses (not displayed).
Figure 2.
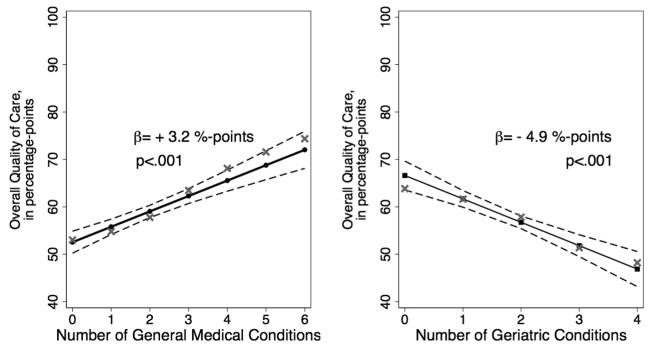
Contrasting effects of geriatric and general medical comorbidity counts on overall quality of care (N=644 older adults in ambulatory care).
● Medical Comorbidity Count
■ Geriatric Comorbidity Count
Dashed lines=bootstrapped 95% confidence intervals.
X = unadjusted mean values; β = beta-coefficient of the medical comorbidity count on left and geriatric comorbidity count on right. Medical comorbidity count associated with better overall quality of care, but geriatric comorbidity count associated with poorer quality of care in this linear regression controlling for both comorbidity counts, age, sex, site, visits, and random effect of provider.
When examining the effect of comorbidity on different types of QOC, better medical QOC was delivered to those with multiple medical conditions (adjusted marginal effect per medical condition, 3.0 percentage points, 95% CI=1.6–4.3), but worse medical QOC was delivered to those with multiple geriatric conditions (adjusted marginal effect per geriatric condition, 2.3 percentage points, 95% CI=−4.5 to −0.1 percentage points) (Figure 3, top). In contrast, geriatric QOC was poor for all participants, regardless of number of medical conditions (no marginal effect (p>.9) in fully adjusted model). Furthermore, geriatric QOC was worse per additional geriatric condition (marginal effect, −3.4 percentage points, 95% CI=−5.5 to −1.2 percentage points, in fully adjusted model) (Figure 3, bottom).
Figure 3.
Effects of geriatric comorbidity on geriatric quality of care (top) and general medical quality of care (bottom).
● Medical Comorbidity Count
■ Geriatric Comorbidity Count
Dashed lines=bootstrapped 95% confidence intervals.
X = unadjusted mean values; Dy/dx = marginal effect of comorbidity counts (medical on the left, geriatric on the right). These results show that medical condition at 100%. The marginal effect (dy/dx) is the approximate slope in the middle of the distribution (~2 conditions) and can be interpreted like the slope in a linear model.
Consistent with prior reports, the ACOVE-2 intervention was associated with better geriatric QOC and did not result in substantial change in medical QOC. Number of primary care visits was associated with better overall QOC and better medical QOC but not geriatric QOC (Table 2). Sex and age did not affect any of the QOC scores.
DISCUSSION
This study furthers understanding of how geriatric conditions differ from general medical conditions: their cumulative effect on ambulatory care quality. Prior research on comorbidity found that a simple count of conditions was related to better overall QOC,8,9,24 but in the current study, when conditions were differentiated into geriatric versus general medical type, number of geriatric conditions had an opposite and equally strong effect on overall QOC as number of medical conditions. The most prevalent geriatric conditions were falls and urinary incontinence, with a small number of participants with cognitive impairment. No single geriatric condition explained the findings. Rather, a decrement in care quality was observed in a dose-response fashion with additional geriatric comorbidity burden. Individuals with multiple geriatric conditions receive poor care not only for their geriatric conditions, but also for their general medical conditions.
There are several possible reasons for the findings. First, geriatric conditions themselves are costly to treat in time and effort,5–17 for example, a high-quality history and examination for weight loss, falls, or cognitive impairment. Greater burden of more-severe geriatric conditions is related to a greater number of complex care processes that primary care clinicians are responsible for.18 If the proportion of effort spent on a individual for a condition determines complexity of care, then perhaps the complexity of care that geriatric conditions contribute interferes with providing high QOC for the individual’s nongeriatric conditions and preventive care processes. The negative effect, or discordance, of a complex condition on the QOC for another less-complex condition has been shown in other clinical situations.25,26
Second, individuals with geriatric conditions may also simply require more time to deliver high QOC (in addition to condition counts themselves), for example, because of additional time needed to communicate information clearly with the individual, family, or caregivers. Capturing these time-related factors would be a potential direction for future research. Third, this study found that having more primary clinician visits was associated with more recommended care, consistent with prior studies,8,9,27 although doctor visits only partly explain why those with multimorbidity receive better QOC; as shown in prior studies,8,9,27 the effect of comorbidity count is independent of primary care visits. Increasing the expected frequency of primary care visits for medically complex individuals (e.g., for a clinician with a complex patient panel) might be a future strategy for medical systems to improve care of multimorbidity.
These findings have implications for the provision of care to medically complex older adults with geriatric conditions. First, although practice-based quality improvement interventions can somewhat mitigate poor QOC for geriatric conditions,5,28,29 whether any intervention can mitigate the negative effect of geriatric conditions on QOC for other conditions is not known. These findings demonstrate a clear need to develop better systems to treat older adults with multiple geriatric conditions. Interventions such as Guided Care,28 aimed at coordinating care for multimorbid older adults, exist. One potential opportunity to improve care in generalist practices might be increasing awareness of the requirements for providing a “Welcome to Medicare” visit, which includes screening for falls and function. Medical homes that strive to improve care and outcomes should focus on models to address the needs of individuals with geriatric multimorbidity at the greatest risk of receiving poor care.
Second, although ACOVE-2 was a primary care study, any care provided during an ambulatory care visit—including specialty care—was evaluated for crediting QIs. It is possible that individuals with general medical conditions such as heart disease may have benefitted from seeing additional specialists. Further research in the care of older adults with multimorbidity, for example, within health systems, might also target specialty care visits as valuable opportunities to boost geriatric QOC, such as neurology (for falls or memory impairment) or urology or gynecology (for incontinence).
Third, predicting receipt of recommended health services is challenging; even good models of utilization predict only approximately 10% of overall model variance.30 In this study predicting overall QOC, the magnitude of each effect of geriatric (β=−4.9) and medical counts (β=3.2) was more than double the effect of the original total comorbidity count (β=1.5). Also, the overall model fit using the two new comorbidity counts was 7 times greater (R2=0.14 vs R2=0.02) as when using the original total condition count alone. Both of these findings suggest that the original method of simply counting chronic conditions mixed the opposite effects of medical and geriatric conditions, obfuscating the effect of comorbidity on QOC. Therefore, our health care system may need new ways to appropriately measure quality provided by hospitals and plans, measuring complexity due to multiple geriatric conditions separately from traditional general medical co-morbidity measures.
Last, prior work had suggested that risk adjustment for providing recommended care-processes was unnecessary because comorbidity did not negatively affect care-process QOC.8,24 The findings of this study suggest that adjustment is needed for number of geriatric conditions, suggesting that it is appropriate to offer risk adjustment (a comparative advantage) to plans or providers serving older adults with multiple geriatric conditions.
A strength of this study is that it used an ambulatory care population that was older and medically complex, with a high degree of complexity with respect to geriatric and medical comorbidities. No other ambulatory care dataset includes geriatric comorbidity and geriatric QOC measured in as detailed a fashion as ACOVE-2.
This study has several limitations. Although ACOVE-2 applied appropriateness criteria (e.g., exclusions for individuals with limited life expectancy or advanced dementia) to many of the general medical QIs,22 it is possible that physicians or participants did not believe that some of the general medical preventive measures would be beneficial if the individual had a great burden of geriatric comorbidity. Therefore, it is possible that some participants with geriatric multimorbidity had lower general medical QOC scores that were appropriate. The second limitation is that criteria for QOC (the ACOVE-2 QIs) were appropriate in 2003, but standards for some care processes have evolved. Cardiovascular care standards have become more stringent, but it is likely that this trend will further accentuate the negative relationship between geriatric multimorbidity and medical QOC. Other limitations are related to study design. This analysis took place in the setting of a quality improvement study for geriatric conditions (falls, incontinence, dementia). It is possible that the screening protocol identified milder geriatric conditions that the provider or participant dismissed, resulting in lower-quality care, although this would not account for the negative effect that these conditions had on the QOC provided for nonstudy geriatric conditions (osteoporosis, hearing impairment, malnutrition). The number of conditions does not account for disease severity, although the ACOVE-2 QIs were designed for all individuals with conditions regardless of severity. In addition, the study design screened out two-thirds of older adults with no self-reported symptoms of geriatric conditions, and ACOVE-2 was conducted in two communities in California with little racial or economic diversity. Therefore, the results may not be generalizable to other groups of older adults and other settings.
In conclusion, medically complex older adults with multiple general medical conditions receive good QOC, but those with greater geriatric comorbidity burden receive poorer care across all conditions, geriatric and general medical. It is imperative to achieve a better understanding of the effect of geriatric conditions on care and improve the QOC of medically complex older adults with geriatric morbidities. These results suggest a critical need for more-systematic approaches to improving QOC for geriatric conditions and for individuals with those conditions. Further research on geriatric multimorbidity, such as how to individualize care and improve clinical outcomes, is needed.
Supplementary Material
Acknowledgments
Sponsor’s Role: This project was supported by the Agency for Healthcare Research and Quality (Min R21 HS017621). Dr. Min was supported by the National Institute on Aging (NIA), Claude Pepper Older Americans Independence Centers at the University of California at Los Angeles (NIA-K12 AG001004, 2006–10) and the University of Michigan (AG024824, 2010–12). These results were presented at the American Geriatrics Society Annual Scientific Meeting (2012) and the Society for General Internal Medicine Annual Scientific Conference (2011). The original ACOVE-2 study was supported by a contract from Pfizer, Inc. to RAND. Pfizer did not serve a role in the design, analysis, or preparation of this study. The authors conducted this research independently of all of the funders. The original ACOVE-2 study was approved for Human Subjects Research through RAND Health with the University of California at Los Angeles through a Memorandum of Understanding. The secondary data analysis performed for this manuscript was conducted at the University of Michigan (LM), where the University of Michigan Institutional Review Board for Human Subjects Research reviewed it as nonhuman subjects research.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Min: final approval, whole content, conception and design, analysis and interpretation of data, drafting and critical revision of manuscript, statistical analysis. Kerr: final approval, whole content, interpretation of data, critical revision of manuscript, supervision. Blaum: final approval, part content, interpretation of data, critical revision of manuscript, supervision. Reuben: final approval, part content, interpretation of data, critical revision of manuscript, supervision. Cigolle: final approval, part content, interpretation of data, critical revision of manuscript, supervision. Wenger: final approval, whole content, interpretation of data, drafting and critical revision of manuscript, supervision.
References
- 1.Boyd CM, McNabney MK, Brandt N, et al. Guiding principles for the care of older adults with multimorbidity: An approach for clinicians: American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. J Am Geriatr Soc. 2012;60:E1–E25. doi: 10.1111/j.1532-5415.2012.04188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moses H, III, Matheson DH, Dorsey ER, et al. The anatomy of health care in the United States. JAMA. 2013;310:1947–1963. doi: 10.1001/jama.2013.281425. [DOI] [PubMed] [Google Scholar]
- 3.Yu WW, Ezzati-Rice TM. Concentration of Health Care Expenses in the US Civilian Noninstitutionalized PopulationStatistical Brief #81. Agency for Healthcare Research and Quality; Rockville MD: 2005. [Accessed October 8, 2012]. [on-line]Available at http://meps.ahrq.gov/mepsweb/data_files/publications/st81/stat81.pdf. [Google Scholar]
- 4.Wenger NS, Solomon DH, Roth CP, et al. The quality of medical care provided to vulnerable community-dwelling older patients. Ann Intern Med. 2003;139:740–747. doi: 10.7326/0003-4819-139-9-200311040-00008. [DOI] [PubMed] [Google Scholar]
- 5.Wenger NS, Roth CP, Shekelle PG, et al. A practice-based intervention to improve primary care for falls, urinary incontinence, and dementia. J Am Geriatr Soc. 2009;57:547–555. doi: 10.1111/j.1532-5415.2008.02128.x. [DOI] [PubMed] [Google Scholar]
- 6.Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: Implications for pay for performance. JAMA. 2005;294:716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 7.Tinetti ME, Bogardus ST, Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351:2870–2874. doi: 10.1056/NEJMsb042458. [DOI] [PubMed] [Google Scholar]
- 8.Min LC, Wenger NS, Fung C, et al. Multimorbidity is associated with better quality of care among vulnerable elders. Med Care. 2007;45:480–488. doi: 10.1097/MLR.0b013e318030fff9. [DOI] [PubMed] [Google Scholar]
- 9.Higashi T, Wenger NS, Adams JL, et al. Relationship between number of medical conditions and quality of care. N Engl J Med. 2007;356:2496–2504. doi: 10.1056/NEJMsa066253. [DOI] [PubMed] [Google Scholar]
- 10.Lee PG, Cigolle C, Blaum C. The co-occurrence of chronic diseases and geriatric syndromes: The health and retirement study. J Am Geriatr Soc. 2009;57:511–516. doi: 10.1111/j.1532-5415.2008.02150.x. [DOI] [PubMed] [Google Scholar]
- 11.Cigolle CT, Langa KM, Kabeto MU, et al. Geriatric conditions and disability: The Health and Retirement Study. Ann Intern Med. 2007;147:156–164. doi: 10.7326/0003-4819-147-3-200708070-00004. [DOI] [PubMed] [Google Scholar]
- 12.Valcour VG, Masaki KH, Curb JD, et al. The detection of dementia in the primary care setting. Arch Intern Med. 2000;160:2964–2968. doi: 10.1001/archinte.160.19.2964. [DOI] [PubMed] [Google Scholar]
- 13.Callahan CM, Hendrie HC, Tierney WM. Documentation and evaluation of cognitive impairment in elderly primary care patients. Ann Intern Med. 1995;122:422–429. doi: 10.7326/0003-4819-122-6-199503150-00004. [DOI] [PubMed] [Google Scholar]
- 14.Boise L, Neal MB, Kaye J. Dementia assessment in primary care: Results from a study in three managed care systems. J Gerontol A Biol Sci Med Sci. 2004;59A:M621–M626. doi: 10.1093/gerona/59.6.m621. [DOI] [PubMed] [Google Scholar]
- 15.Mann E, Koller M, Mann C, et al. Comprehensive Geriatric Assessment (CGA) in general practice: Results from a pilot study in Vorarlberg, Austria. BMC Geriatr. 2004;4:4. doi: 10.1186/1471-2318-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lachs MS, Feinstein AR, Cooney LM, Jr, et al. A simple procedure for general screening for functional disability in elderly patients. Ann Intern Med. 1990;112:699–706. doi: 10.7326/0003-4819-112-9-699. [DOI] [PubMed] [Google Scholar]
- 17.Rockwood K, Silvius JL, Fox RA. Comprehensive geriatric assessment. Helping your elderly patients maintain functional well-being. Postgrad Med. 1998;103:247–249. 254–258, 264. doi: 10.3810/pgm.1998.03.424. [DOI] [PubMed] [Google Scholar]
- 18.Min L, Wenger N, Walling AM, et al. When comorbidity, aging, and complexity of primary care meet: Development and validation of the Geriatric CompleXity of Care Index. J Am Geriatr Soc. 2013;61:542–550. doi: 10.1111/jgs.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganz DA, Wenger NS, Roth CP, et al. The effect of a quality improvement initiative on the quality of other aspects of health care: The law of unintended consequences? Med Care. 2007;45:8–18. doi: 10.1097/01.mlr.0000241115.31531.15. [DOI] [PubMed] [Google Scholar]
- 20.Reuben DB, Roth C, Kamberg C, et al. Restructuring primary care practices to manage geriatric syndromes: The ACOVE-2 intervention. J Am Geriatr Soc. 2003;51:1787–1793. doi: 10.1046/j.1532-5415.2003.51565.x. [DOI] [PubMed] [Google Scholar]
- 21.Shekelle PG, MacLean CH, Morton SC, et al. Assessing care of vulnerable elders: Methods for developing quality indicators. Ann Intern Med. 2001;135:647–652. doi: 10.7326/0003-4819-135-8_part_2-200110161-00003. [DOI] [PubMed] [Google Scholar]
- 22.Solomon DH, Wenger NS, Saliba D, et al. Appropriateness of quality indicators for older patients with advanced dementia and poor prognosis. J Am Geriatr Soc. 2003;51:902–907. doi: 10.1046/j.1365-2389.2003.513331.x. [DOI] [PubMed] [Google Scholar]
- 23.Papke LE, Wooldridge JM. Econometric methods for fractional response variables with an application to 401(k) plan participation rates. J Appl Econ. 1996;11:619–632. [Google Scholar]
- 24.Min LC, Reuben DB, MacLean CH, et al. Predictors of overall quality of care provided to vulnerable older people. J Am Geriatr Soc. 2005;53:1705–1711. doi: 10.1111/j.1532-5415.2005.53520.x. [DOI] [PubMed] [Google Scholar]
- 25.Kerr EA, Heisler M, Krein SL, et al. Beyond comorbidity counts: How do comorbidity type and severity influence diabetes patients’ treatment priorities and self-management? J Gen Intern Med. 2007;22:1635–1640. doi: 10.1007/s11606-007-0313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redelmeier DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med. 1998;338:1516–1520. doi: 10.1056/NEJM199805213382106. [DOI] [PubMed] [Google Scholar]
- 27.Anger JT, Saigal CS, Stothers L, et al. The prevalence of urinary incontinence among community dwelling men: Results from the National Health and Nutrition Examination Survey. J Urol. 2006;176:2103–2108. doi: 10.1016/j.juro.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 28.Boult C, Reider L, Leff B, et al. The effect of guided care teams on the use of health services: Results from a cluster-randomized controlled trial. Arch Intern Med. 2011;171:460–466. doi: 10.1001/archinternmed.2010.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen KR, Hazelett SE, Radwany S, et al. The Promoting Effective Advance Care for Elders (PEACE) randomized pilot study: Theoretical framework and study design. Popul Health Manag. 2012;15:71–77. doi: 10.1089/pop.2011.0004. [DOI] [PubMed] [Google Scholar]
- 30.Pope GC, Kautter J, Ellis RP, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25:119–141. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.