Abstract
Viral hepatitis is responsible for great health, social and economic burden both globally and in the UK. This study aimed to assess the research funding awarded to UK institutions for viral hepatitis research and the relationship of funded research to clinical and public health burden of viral hepatitis. Databases and websites were systematically searched for information on infectious disease research studies funded for the period 1997–2010. Studies specifically related to viral hepatitis research were identified and categorized in terms of funding by pathogen, disease and by a research and development value chain describing the type of science. The overall data set included 6165 studies (total investment £2.6 billion) of which £76.9 million (3.0%) was directed towards viral hepatitis across 323 studies (5.2%). By pathogen, there were four studies specifically investigating hepatitis A (£3.8 million), 69 studies for hepatitis B (21.4%) with total investment of £14.7 million (19.1%) and 236 (73.1%) hepatitis C studies (£62.7 million, 81.5%). There were 4 studies investigating hepatitis G, and none specifying hepatitis D or E. By associated area, viral hepatitis and therapeutics research received £17.0 million, vaccinology £3.1 million and diagnostics £2.9 million. Preclinical research received £50.3 million (65.4%) across 173 studies, whilst implementation and operational research received £19.4 million (25.3%) across 128 studies. The UK is engaged in much hepatology research, but there are areas where the burden is great and may require greater focus, such as hepatitis E, development of a vaccine for hepatitis C, and further research into hepatitis-associated cancers. Private sector data, and funding information from other countries, would also be useful in priority setting.
Keywords: hepatitis A, hepatitis B, hepatitis C, funding, research
Introduction
A range of factors makes preventing and managing hepatitis infections across the different viruses, a challenging and complex task, and these include susceptibility of different populations, the availability of vaccines, the length of the latent period, severity of symptoms and secondary outcomes such as carcinomas and the multiple modes of transmission. These factors are highlighted in prevalence and mortality data across different world regions and countries. Prevalence of hepatitis A is highest in sub-Saharan Africa and parts of south-east Asia, where infection is often at a young age (and asymptomatic), with the lowest prevalence found in some high-income countries where few individuals become infected in childhood [1].
Mortality of hepatitis A, B and C in the United States is most common in the 45–64 age group, with much higher mortality rates in males compared with females, and significant differences by ethnic group; there were a total of 18 473 viral hepatitis-related deaths in 2010, with 17 113 of these related to hepatitis C [2]. Approximately 14 million people in Europe are estimated to be infected with hepatitis B and 9 million with hepatitis C, causing 36 000 and 86 000 deaths, respectively [3]. Worldwide, hepatitis B causes 1 million deaths annually with the majority of these in sub-Saharan Africa [4]. The 2005 global burden of hepatitis E was estimated to include 20.1 million incident infections with 3.4 million symptomatic cases, 70 000 deaths and 3000 stillbirths [5]. Within the UK, prevalence of hepatitis A is considered to be very low compared with global data [1], as is hepatitis B prevalence [6]. Hepatitis C prevalence is estimated at 1.38% in men and 0.42% in women, with mean number of 7571 new infections per year between 1986 and 2000 [7].
UK research institutions received at least £2.6 billion of public and philanthropic funding to carry out infectious disease research between 1997 and 2010 from a variety of national and international funding sources [8]. These included the Wellcome Trust, Medical Research Council, Department of Health, Bill & Melinda Gates Foundation, European Commission, and a range of other bodies and departments and research charities. This funding was spent on all types of science along the research pipeline, from laboratory studies to operational research and translational medicine. We report here, the research funding that was awarded to UK institutions specifically for viral hepatitis-associated infection research, along with temporal trends and the relative proportions allocated. We assess how closely the topics funded relate to the clinical and public health burden of viral hepatitis, seeking to identify potential funding gaps that policy makers and funders can be encouraged to focus on in future, and areas where the UK has clear research strengths.
Methods
The analyses in this paper focussed on studies funded in a 14-year period (1997–2010 inclusive) that were clearly relevant to, or had specific mention of, viral hepatitis disease. Several studies specifically referred to more than one type of viral hepatitis (e.g. hepatitis B and C); these were counted in all relevant categories. No private sector funding was included in this analysis as the publicly available data are very limited from these sources and were considered to be under-representative.
The methods have been described in detail elsewhere [8] and are reiterated here. The overarching data set was obtained from the major sources of public and charitable funding for infectious disease research studies, including the Wellcome Trust, Medical Research Council and other research councils, UK government departments, European Commission, Bill and Melinda Gates Foundation and other research charities (Fig.1). Data collection was performed by (i) downloading all data from the funder website and manually filtering the infectious disease studies; or (ii) searching open access databases on the funder website for infection-related keyword terms or (iii) contacting the funder directly and requesting details of their infection studies. Funders were identified through author's knowledge of the research and development (R&D) landscape, searches of the internet, the Infectious Disease Research Network and databases such as the National Research Register, Clinicaltrials.gov and the Association for Medical Research Charities. Author MGH performed the majority of data extraction, with support from author JRF. Each study was assigned to as many primary disease categories as appropriate [9]. Within each category, topic-specific subsections (including specific pathogen or disease) were documented. Studies were also allocated to one of four R&D categories: preclinical; phase 1, 2, or 3; product development and implementation and operational research (including surveillance, epidemiology and statistical and modelling projects). Funders were either considered in their own right, or for convenience, some were grouped into categories, such as in-house university funding, research charities, and government departments. A total of 26 funder categories were used [9]. Studies were excluded if (i) they were not immediately relevant to infection; (ii) they were veterinary infectious disease research studies; (iii) they concerned the use of viral vectors to investigate noncommunicable diseases; (iv) they were grants for symposia or meetings or (v) they included UK researchers, but with the funding awarded to and administered through a non-UK institution. Studies were categorized as viral hepatitis research where there was specific mention or a clear implication of relevance to viral hepatitis in the project title or abstract. Unfunded studies were excluded. Grants awarded in a currency other than pounds sterling were converted to UK pounds using the mean exchange rate in the year of the award. All awards were adjusted for inflation and reported in 2010 UK pounds. Analysis was carried out in Microsoft Excel and Access (versions 2000 and 2007) and Stata (version 11) (StataCorp, College Station, Texas, USA). Further information on methods and lists of categories are available online (http://www.researchinvestments.org/data). Data were compared with disease burden using disability-adjusted life years (DALYs), sourced from the Global Burden of Disease study carried out by the Institute for Health Metrics and Evaluation (Washington, Seattle) [10].
Fig 1.
Flowchart of study methodology.
Results
We identified 6165 studies funded within the 14-year study period and covering all infectious disease research, representing a total investment of £2.6 billion. There were 323 studies of relevance to viral hepatitis research, comprising 5.2% of total infectious disease research projects. These were awarded £76.9 million, 3.0% of the total spend, with a median award of £114 943 (interquartile range £40 075–246 841) and mean award of £238 161 (standard deviation £404 983) (Table 1). Mean total annual investment was £5.5 million. The ten studies awarded the greatest funding are shown in Appendix S1.
Table 1.
Investments in viral hepatitis and associated disciplines by sum, number of studies and mean and median award size
| Disease | Number of studies | Percentage of viral hepatitis study numbers (%) | Total funding | Percentage of viral hepatitis funding (%) | Mean award, £ (SD) | Median award, £ (IQR) | Top funder, millions (%) |
|---|---|---|---|---|---|---|---|
| All viral hepatitis | 323 | n/a | £76 925 993 | n/a | 238 161 (404 983) | 114 943 (40 075–246 841) | MRC, 27.7 (35.9) |
| Hepatitis A | 4 | 1.2 | £3843 889 | 5.0 | 960 972 (1 388 394) | 435 239 (19 703–1 902 242) | MRC, 3.0 (77.0) |
| Hepatitis B | 69 | 21.4 | £14 728 372 | 19.1 | 213 454 (440 536) | 66 056 (19 703–213 039) | MRC, 8.0 (54.4) |
| Hepatitis C | 236 | 73.1 | £62 688 106 | 81.5 | 265 628 (453 444) | 116 998 (41 675–271 596) | MRC, 23.9 (38.2) |
| Therapeutics | 35 | 10.8 | £17 017 224 | 22.1 | 486 206 (734 331) | 105 021 (38 625–717 547) | European Commission, 6.2 (36.4) |
| Immunology | 60 | 18.6 | £12 647 273 | 16.4 | 210 787 (268 020) | 115 760 (37 113–226 278) | MRC, 7.7 (61.1) |
| Drug addiction | 36 | 11.1 | £4520 610 | 5.9 | 125 572 (153 569) | 64 343 (45 177–156 805) | Department of Health, 1.6 (35.1) |
| Global health | 13 | 4.0 | £4264 556 | 5.5 | 328 042 (282 327) | 235 233 (121 493–428 346) | Wellcome, 2.5 (57.8) |
| HIV | 13 | 4.0 | £4193 189 | 5.5 | 322 553 (425 368) | 138 921 (42 009–365 967) | MRC, 1.4 (32.9) |
| Vaccinology | 14 | 4.3 | £3123 395 | 4.1 | 223 100 (277 098) | 93 721 (15 445–256 237) | MRC, 1.8 (56.1) |
| Diagnostics | 31 | 9.6 | £2934 127 | 3.8 | 94 649 (165 073) | 63 013 (25 133–111 784) | Wellcome, 1.2 (42.0) |
| HCAI | 13 | 4.0 | £2032 137 | 2.6 | 156 318 (118 904) | 153 325 (66 747–197 873) | Department of Health, 0.5 (24.9) |
| Economics | 8 | 2.5 | £1331 778 | 1.7 | 166 472 (276 556) | 78 274 (46 488–114 767) | European Commission, 0.8 (63.4) |
| Paediatrics | 10 | 3.1 | £1284 598 | 1.7 | 128 460 (188 829) | 67 510 (5890–155 937) | Wellcome, 0.6 (49.6) |
| Cancer | 5 | 1.5 | £469 344 | 0.6 | 93 869 (107 729) | 42 475 (38 625–68 645) | Wellcome, 0.3 (68.1) |
| Antimicrobial resistance | 4 | 1.2 | £176 003 | 0.2 | 44 000 (44 063) | 38 250 (7608–80 393) | Charity 0.1 (38.7) |
| Prison health | 5 | 1.5 | £103 380 | 0.1 | 20 676 (27 587) | 7818 (3769–22 900) | Scottish government funding, 0.07 (65.5) |
| Primary care | 1 | 0.3 | £70 146 | 0.1 | 70 146 (n/a) | 70 145 (n/a) | NHS 0.1 (100) |
HCAI, Healthcare-associated infections; MRC, Medical Research Council. Much of the rest of the funding was directed towards preclinical research for which there has not yet been further categorization.
Of the 323 viral hepatitis projects (Table 1), there were four studies (1.2% of all hepatology projects) specifically investigating hepatitis A, with total investment of £3.8 million (5.0% of total hepatology investment). Hepatitis B was a focus in 69 studies (21.4%) with total investment of £14.7 million (19.1%). There were 236 (73.1%) hepatitis C studies, receiving investment of £62.7 million (81.5%). A comparison of funding for these pathogens against selected other infectious diseases is shown in Appendix S2. There were four studies investigating hepatitis G, and none specifying hepatitis D or E. Not all studies specified a pathogen; some merely referred to viral hepatitis as an area of focus (e.g. one study title was ‘Determining the prevalence of chronic viral hepatitis in Tower Hamlets Bengali population’).
By cross-cutting theme across viral hepatitis studies (Table 1), there were 35 studies (10.8%) investigating hepatitis and therapeutics, with total investment of £17.0 million (22.1%). There were 14 studies (4.3%) focusing on vaccinology, receiving investment of £3.1 million (4.1%). Diagnostics was a focus of 31 studies (9.6%), with investment of £2.9 million (3.8%). Research incorporating associated cancers received funding of £0.5 million across five studies, and intravenous drug use was studied in 36 projects (funding received £4.5 million). Viral hepatitis with a global health focus was evident from 13 studies (4.0%), with investment of £4.3 million (5.5%); this differs greatly from the entire infectious disease data set where global health studies account for 35.6% of the funding [8].
Along the R&D value chain (Table 2), preclinical research received £50.3 million (65.4%) across 173 studies (53.6%), phase I to III studies received just £0.5 million (0.7%) from 3 studies (0.9%), product development research received £6.7 million (8.7%) across 19 studies (5.9%) and implementation and operational research received £19.4 million (25.3%) across 128 studies (39.6%). There were no clear temporal trends in the levels of overall funding (Fig.2a), and specifically there was reduced funding for hepatitis B and C from 1997–2004 to 2005–2010 (Table 3). This decrease was also reflected in the relative investment in relation to DALYs from 2004 and 2010 (Table 3). The proportion of funding for preclinical research appears to have broadly increased in recent years (Fig.2b). The Medical Research Council provided the greatest quantities of investment in viral hepatitis research (£27.7 million, 35.9%).
Table 2.
Investments in viral hepatitis and associated disciplines by type of science
| Disease | Number of studies | Total funding | Preclinical | Phase I–III | Product development | Operational | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study numbers | Funding | Study numbers | Funding | Study numbers | Funding | Study numbers | Funding | |||
| All viral hepatitis | 323 | £76 925 993 | 173 | £50 277 580 | 3 | £518 577 | 19 | £6688 902 | 128 | £19 440 934 |
| Hepatitis A | 4 | £3843 889 | 2 | £2986 547 | 0 | £0 | 0 | £0 | 2 | £857 341 |
| Hepatitis B | 69 | £14 728 372 | 43 | £10 445 301 | 1 | £256 237 | 5 | £1559 360 | 20 | £2467 474 |
| Hepatitis C | 236 | £62 688 106 | 123 | £41 004 478 | 3 | £518 577 | 16 | £6080 822 | 94 | £15 084 228 |
| Hepatology + selected disciplines | ||||||||||
| Therapeutics | 35 | £17 017 224 | 14 | £9583 300 | 1 | £13 582 | 9 | £3774 814 | 11 | £3645 528 |
| Immunology | 60 | £12 647 273 | 49 | £10 773 459 | 2 | £504 995 | 0 | £0 | 9 | £1 368 817 |
| Drug addiction | 36 | £4520 610 | 1 | £58 143 | 0 | £0 | 5 | £531 712 | 30 | £3930 755 |
| Global health | 13 | £4264 556 | 10 | £2964 130 | 0 | £0 | 1 | £428 347 | 2 | £872 078 |
| HIV | 13 | £4193 189 | 6 | £1844 006 | 0 | £0 | 1 | £912 655 | 6 | £1436 528 |
| Vaccinology | 14 | £3123 395 | 8 | £1657 786 | 2 | £504 995 | 0 | £0 | 4 | £960 613 |
| Diagnostics | 31 | £2934 127 | 4 | £223 576 | 0 | £0 | 6 | £1253 397 | 21 | £1457 154 |
| HCAI | 13 | £2032 137 | 5 | £729 893 | 0 | £0 | 1 | £231 830 | 7 | £1070 413 |
| Economics | 8 | £1331 778 | 0 | £0 | 0 | £0 | 0 | £0 | 8 | £1331 779 |
| Paediatrics | 10 | £1284 598 | 0 | £0 | 0 | £0 | 0 | £0 | 10 | £1284 599 |
| Cancer | 5 | £469 344 | 2 | £81 101 | 0 | £0 | 0 | £0 | 3 | £388 244 |
| Antimicrobial resistance | 4 | £176 003 | 4 | £176 003 | 0 | £0 | 0 | £0 | 0 | £0 |
| Prison health | 5 | £103 380 | 0 | £0 | 0 | £0 | 0 | £0 | 5 | £103 380 |
| Primary care | 1 | £70 146 | 0 | £0 | 0 | £0 | 0 | £0 | 1 | £70 146 |
Fig 2.
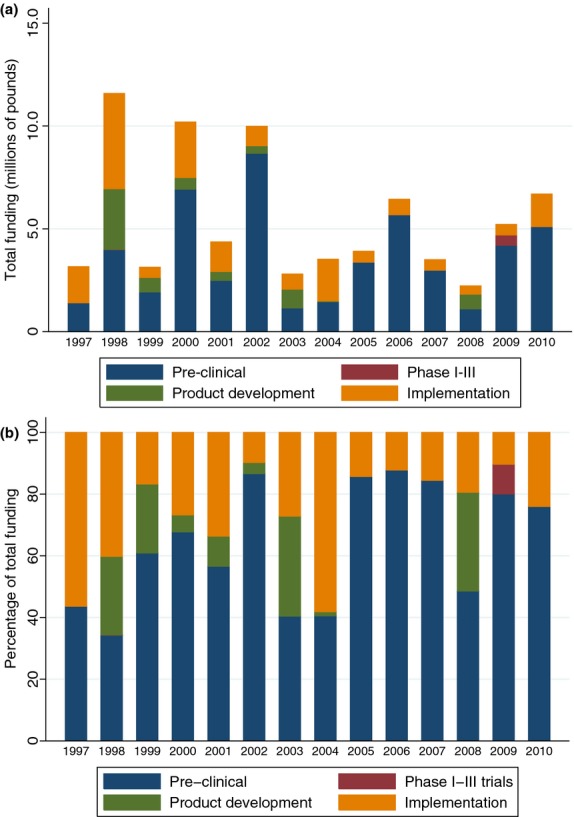
Investments on viral hepatitis research awarded to the UK over time and by type of science, (a) by sum and (b) by proportion.
Table 3.
Relative levels of research investment, changes in investment over time and associated burden (2010 DALYs) of viral hepatitis
| No. of studies | Total funding | DALY 2004 | DALY 2010* | Investment 1997–2004 | Investment 2005–2010 | Change in investment | Proportional change in investment (%) | Total investment relative to 2004 burden (£ per DALY) | Total investment relative to 2010 burden (£ per DALY) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Hepatitis A | 4 | £3843 889 | − | 4 351 000 | £883 612 | £2960 277 | £2076 665 | 70.2 | n/a | £0.88 |
| Hepatitis B | 69 | £14 728 372 | 2 067 533 | 22 602 000 | £9829 207 | £4899 165 | £ −4930 042 | −100.6 | £7.12 | £0.65 |
| Hepatitis C | 236 | £62 688 106 | 954 622 | 12 111 000 | £38 124 617 | £24 563 489 | £ −13 561 128 | −55.2 | £65.67 | £5.18 |
Disability-adjusted life years (DALYs) include burden attributable to acute infection, cirrhosis and hepatocellular carcinoma.
Discussion
This study is the first systematic analysis of research funding for viral hepatitis. Over the 14-year study period analysed, 323 studies were identified that related to viral hepatitis where public or charitable funding had been awarded to a UK institution. The most studied pathogen was hepatitis C (236 studies, £62.7 million). Preclinical science received greatest investment along the R&D value chain, followed by implementation research, and very little for phase I–III trials and product development studies. The large difference between mean and median award (and wide interquartile ranges and associated standard deviation) demonstrates there were a large number of relatively small grants, set alongside a few large investments. The Medical Research Council invested the greatest amount of funding.
Using the 2004 burden data (which is the temporal mid-point of this study), the investment in hepatitis C research compared with DALYs is actually relatively high (£65.67), as compared with hepatitis B (£7.12) and also other disease areas within the data set (for example, tuberculosis with investment of £4.54 per DALY) [11] so perhaps the UK has strengths in viral hepatitis research (particularly preclinical studies). However, any current measure of burden will only take into account currently diagnosed cases, and not adequately convey the likely greatly increased future burden of hepatitis C in particular. The current levels of investment are likely not an over-investment. It also appears that investment in both hepatitis B and C are declining over this time period, and the relative investment in these pathogens against the burden of disease has dropped – a worrying situation if this is an actual trend. The global acute burden of hepatitis E is likely to be similar to that each of hepatitis A and B [12] so the clear lack of any notable investment in this data set is surprising and arguably should be addressed.
There are demonstrably different ways to present the hepatology-related data from the Global Burden of Disease study, and caution is appropriately advised when interpreting the study findings; viral hepatitis burden can be presented by just the acute infection, or including the chronic burdens of cirrhosis and cancers [12]. There are also other measures of burden that could be used to assess research investments, such as mortality, incidence, prevalence or economic burden. It would be useful also to quantify investments into viral hepatitis awarded since 2010. The limitations of this study are described fully elsewhere [8] and reiterated here. There was little publicly available data from the pharmaceutical industry. Hence, there is a data gap in relation to funding of clinical trials and development of vaccines and diagnostics, which the pharmaceutical and biotechnology industry are financing. Beyond disease burden, other measures, such as economic burden should also be utilized when prioritizing limited resources, but little information is available regarding the economic impact of viral hepatitis infections. We rely on the original data being complete and accurate and were unable to take into account distribution of funds from the lead institution to collaborating partners, nor could we realistically assess quantity of each award given to overheads, the impact of the introduction of full-economic costing, or distribution of funding along each year of the award (for simplicity, all funding was assumed to have been awarded at the start of the grant). Also, assigning studies to categories is a subjective process – although we used at least two researchers to do this to reduce interobserver error. Our study focuses on UK-led investments – we do not know if similar patterns (e.g. a dominance of preclinical research and lack of public or charitably funded clinical trials) would also emerge if the analysis was repeated for other high-income countries. We have not here measured either the outputs or impact of funded research.
The UK is well-placed to contribute to many of the priority research areas that need additional funding, given particular focuses on preclinical science as well as operational and implementation research. However, there is a need for funders in other countries to provide similar and detailed information on funded studies, and so build a global research funding database – the World Health Organisation are encouraging efforts in this area in creating a global R&D observatory [13]. This could be used for analytical work to identify gaps in research funding, reduce unnecessary duplication of research investments, prioritize health and social policy decisions and help inform resource allocation for global research priorities. Recent years have seen huge progress in the treatment of HBV and HCV. With effective vaccination, diagnostics and treatment, there are still important questions about how to eradicate human reservoirs of HBV infection requiring preclinical research. However, developments of highly active, well tolerated and curative HCV treatments demand more investment in strategic clinical research to understand best how these drugs may abate the HCV epidemic. Preclinical R&D for HCV vaccination remains an important area of potential development but progress has been slow to date and the prospects of effective vaccination remain distant. Given the global burden of hepatitis E, there may be some valid research questions surrounding the distribution and uptake of a vaccine within the regions of highest burden.
Acknowledgments
We thank the Infectious Disease Research Network for their contribution to this work, and acknowledge the assistance of the research and development funding agencies for provision of data.
Glossary
- DALY
disability-adjusted life years
- HBV
hepatitis B virus
- HCAI
Healthcare-associated infections
- HCV
hepatitis C virus
- IQR
interquartile range
- MRC
Medical Research Council
Funding
This study was carried out alongside routine duties.
Contributors
MGH designed the study and collated the data set. JRF checked and refined the data set. MGH and JRF undertook data analysis and created the graphs and figures with input from GSC, GF and RA. MGH interpreted the data and wrote the draft and final versions. GSC, GF, JRF and RA commented on the data set, draft paper and final version. All authors reviewed and approved the final version. MGH is guarantor of the paper.
Conflict of Interest Statement
MGH works for the Infectious Disease Research Network, which has supported this work and is funded by the UK Department of Health. JRF has received funds from the Wellcome Trust and is a steering group member for the Infectious Disease Research Network. RA has received research funding from the Medical Research Council, the National Institute for Health Research and the UK Department for International Development. RA is a member of the Medical Research Council Global Health Group. GF has received funding from companies that market products for the treatment of viral hepatitis including BI, BMS, GSK, Merck, Roche, Janssen, Novartis and Gilead.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
Appendix S1: The ten viral hepatitis studies awarded the greatest funding.
Appendix S2: Comparison of funding for hepatitis A, B and C with other selected infectious diseases.
References
- 1.Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010;28(41):6653–6657. doi: 10.1016/j.vaccine.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 2.Ly KN, Xing J, Klevens RM, et al. Causes of death and characteristics of decedents with viral hepatitis, United States, 2010. Clin Infect Dis. 2014;58(1):40–49. doi: 10.1093/cid/cit642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van de Laar MJW, Lopalco PL. World Hepatitis Day: a timely reminder of the challenges ahead. Euro Surveill. 2008;13(21) doi: 10.2807/ese.13.21.18883-en. pii: 18883. [DOI] [PubMed] [Google Scholar]
- 4.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11(2):97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 5.Rein DB, Stevens GA, Theaker J, et al. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55(4):988–997. doi: 10.1002/hep.25505. [DOI] [PubMed] [Google Scholar]
- 6.European Centre for Disease Prevention and Control. Hepatitis B and C in the EU Neighbourhood: Prevalence, Burden of Disease and Screening Policies. Stockholm: European Centre for Disease Prevention and Control; 2010. [Google Scholar]
- 7.Balogun MA, Vyse AJ, Hesketh LM, et al. Estimating hepatitis C infection acquired in England, 1986–2000. Epidemiol Infect. 2009;137(9):1249–1254. doi: 10.1017/S0950268809002143. [DOI] [PubMed] [Google Scholar]
- 8.Head MG, Fitchett JR, Cooke MK, et al. UK investments in global infectious disease research 1997–2010: a case study. Lancet Infect Dis. 2013;13(1):55–64. doi: 10.1016/S1473-3099(12)70261-X. [DOI] [PubMed] [Google Scholar]
- 9. ResIn - Research Investments in Global Health. Available at: http://www.researchinvestments.org (accessed 4 July 2014)
- 10.World Health Organisation. The Global Burden of Disease: 2004 update. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 11.Head MG, Fitchett JR, Cooke MK, et al. Investments in respiratory infectious disease research 1997–2010: a systematic analysis of UK funding. BMJ Open. 2014;4(3):e004600. doi: 10.1136/bmjopen-2013-004600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooke GS, Lemoine M, Thursz M, et al. Viral hepatitis and the Global Burden of Disease: a need to regroup. J Viral Hepat. 2013;20(9):600–601. doi: 10.1111/jvh.12123. [DOI] [PubMed] [Google Scholar]
- 13.Røttingen J-A, Regmi S, Eide M, et al. Mapping of available health research and development data: what's there, what's missing, and what role is there for a global observatory? Lancet. 2013;382(9900):1286–307. doi: 10.1016/S0140-6736(13)61046-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: The ten viral hepatitis studies awarded the greatest funding.
Appendix S2: Comparison of funding for hepatitis A, B and C with other selected infectious diseases.


