Abstract
The way species affect one another in ecological communities often depends on the order of species arrival. The magnitude of such historical contingency, known as priority effects, varies across species and environments, but this variation has proven difficult to predict, presenting a major challenge in understanding species interactions and consequences for community structure and function. Here, we argue that improved predictions can be achieved by decomposing species' niches into three components: overlap, impact and requirement. Based on classic theories of community assembly, three hypotheses that emphasise related, but distinct influences of the niche components are proposed: priority effects are stronger among species with higher resource use overlap; species that impact the environment to a greater extent exert stronger priority effects; and species whose growth rate is more sensitive to changes in the environment experience stronger priority effects. Using nectar-inhabiting microorganisms as a model system, we present evidence that these hypotheses complement the conventional hypothesis that focuses on the role of environmental harshness, and show that niches can be twice as predictive when separated into components. Taken together, our hypotheses provide a basis for developing a general framework within which the magnitude of historical contingency in species interactions can be predicted.
Keywords: Alternative states, chemical ecology, community assembly, ecological niche, microbial ecology, nectar yeast, niche components, niche overlap, priority effect, resource competition
Introduction
One difficulty in predicting how species affect one another in ecological communities is the common occurrence of priority effects, where the order in which species arrive at local sites dictates the effect of species on one another (Gleason 1926; Lewontin 1969; MacArthur 1972; Gilpin & Case 1976; Drake 1991; Chase & Leibold 2003; Fukami & Morin 2003; Petraitis 2013). In many cases, species arrival order is highly stochastic and impossible to know, making the outcome of species interactions essentially unpredictable when priority effects are strong. For this reason, understanding factors that determine the magnitude of historical contingency due to priority effects is important not only to improving basic knowledge of how species assemble into communities (Chase 2003; Fukami 2010) but also to applying this knowledge to environmental, agricultural, medical and other problems that involve management of ecological systems (Temperton & Zirr 2004; Young et al. 2005; Grman & Suding 2010; Costello et al. 2012; Verbruggen et al. 2013).
Besides primary productivity, disturbance rate and other environmental factors (e.g. Chase 2003, 2007; Kardol et al. 2013), characteristics of potential colonists, including dispersal ability (e.g. Shorrocks & Bingley 1994; Porensky et al. 2012), organism lifespan (e.g. Munguia et al. 2010; Young & Peffer 2010) and ecological similarity among species (e.g. Fargione et al. 2003; Fukami et al. 2005), have been hypothesised to determine the magnitude of historical contingency by priority effects. For example, priority effects have been thought to act strongly when early-arriving species deplete local resources and inhibit colonisation by late-arriving species that have similar resource requirements (Fox 1987; Weiher et al. 1998; Wilson 1999; Fargione et al. 2003; Fukami et al. 2005). However, attempts to link the strength of priority effects to resource requirements and other characteristics of species have met with limited success (Peay et al. 2012; Tan et al. 2012). Moreover, although consideration of phylogenetic relatedness between species has shown some promise (Jiang et al. 2010; Peay et al. 2012; Tan et al. 2012), phylogeny is an imperfect proxy for functional traits and lacks a mechanistic basis in predicting priority effects (Mayfield & Levine 2010; Best et al. 2013).
The goal of this article is to suggest that mechanistic predictions of the strength of priority effects can be accomplished by separating species' niches into basic components. To support this claim, we will demonstrate the utility of the approach with a model experimental system involving nectar-inhabiting yeasts. To stimulate further development in future research, we will then discuss ways to expand the scope of the approach and outline possible applications for management of ecological communities.
Niche components and priority effects
A species' niche can be viewed as consisting of three components, including niche overlap, impact niche and requirement niche (Fig.1). Derived from Gause's (1932) competitive exclusion principle and MacArthur & Levins's (1967) limiting similarity concept, niche overlap refers to resource use similarity among co-occurring species, independent of their rate of resource consumption (Pianka 1973; Petraitis 1989). Based on Elton's (1927) niche concept as a species' role in the environment and Tilman's (1982) theory of resource competition, the impact niche is defined as a species' per capita influence on the environment through resource consumption and other modes of environmental modification (Leibold 1995; Chase & Leibold 2003). Finally, originating from Grinnell's (1917) limiting factors and Hutchinson's (1957) fundamental niche, the requirement niche describes the environmental conditions that affect a species' survival, growth and reproduction (Leibold 1995; Chase & Leibold 2003). The contrast between the impact niche and the requirement niche is similar to that of ‘effects traits’ and ‘response traits’ (sensu Lavorel & Garnier 2002; Suding et al. 2008), where species with high impact and those with low requirement may be strong ‘effect competitors’ and ‘response competitors’, respectively (sensu Goldberg & Landa 1991).
Figure 1.
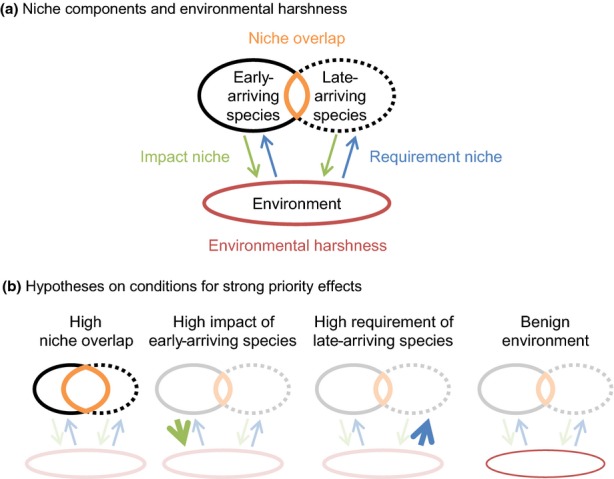
Schematic depiction of niche components and environmental harshness (a) and how they are hypothesised to influence the strength of priority effects (b).
Recognising that these related, but distinct niche components may differentially determine how species interact, and assuming that interactions among species are generally weaker than interactions within species, one can propose the following hypotheses (Fig.1): priority effects should be strong when (1) species display a high degree of similarity in resource use (high overlap), (2) early-arriving species strongly affect the environment (high impact) and (3) the growth rate of late-arriving species is highly dependent on the environment (high requirement). Emphasising multiple niche components, these hypotheses are firmly founded on classic niche-based theories of community assembly (Grinnell 1917; Elton 1927; Gause 1932; Hutchinson 1957; MacArthur & Levins 1967; Pianka 1973; Tilman 1982; Leibold 1995; Chase & Leibold 2003). Yet, to our knowledge, niche components have never been distinguished in empirical studies of priority effects.
The lack of relevant empirical studies is rather surprising because, as we argue, decomposing niches into components can be essential to explaining priority effects. For example, given the same species that arrives late, the strength of priority effects should depend not only on the requirement niche of that species but also on the impact niche of the species that arrives early. In a similar vein, even with high niche overlap, no strong priority effects are expected when the impact of early-arriving species is low, whereas even with low niche overlap, a species of high impact can cause strong priority effects. Consequently, it seems plausible that the role of niches in priority effects is obscured and left undetected if not decomposed into components.
Experimental test of the niche component hypotheses
To evaluate the niche-component hypotheses empirically, we conducted a simple experiment using four species of yeast that inhabit floral nectar (Lachance et al. 2001). In this experiment, we assessed the effect of an early-arriving species on late-arriving species in multiple pairs of species in multiple environments that differed in resource richness (either a rich or poor supply of amino acids) and environmental harshness (either benign or harsh osmotic conditions). By measuring population growth and the changes imposed by yeast species on each environment, we quantified the niche components and linked these metrics to the measured strength of priority effects. To place our results in a broad context, data were analysed to determine the amount of variation that our hypotheses could explain over and above one conventional hypothesis that focuses on characteristics of the environment rather than those of the species. According to this hypothesis, priority effects will be stronger when the environment is less harsh, in the sense that species show higher growth rates under benign environmental conditions (e.g. Chase 2003, 2007; Kardol et al. 2013).
Materials and methods
Study organisms
In the field, nectar yeasts are dispersed from flower to flower by pollinators and other floral visitors, and comprise communities that are relatively species-poor within flowers (Herrera et al. 2010). Within these ephemeral habitats, nectar yeasts are introduced at low densities but grow rapidly, consuming amino acids and altering chemical characteristics of nectar, including pH, H2O2 and sugars (Vannette et al. 2013). Previous work has indicated that phylogenetically more closely related yeast species may be more ecologically similar and exert stronger inhibitory priority effects than distantly related species within the nectar of the sticky monkeyflower, Mimulus aurantiacus, a hummingbird-pollinated shrub in California (Peay et al. 2012). Yeast species can inhabit the nectar of different plant species that vary in the chemical properties of nectar (Baker & Baker 1973), and may vary in their response to, and effect on, nectar chemistry (Peay et al. 2012; Pozo et al. 2012).
Yeast species used in this experiment were Candida rancensis, Hanseniaspora valbyensis, Metschnikowia reukaufii and Starmerella bombicola. These species were commonly observed in the nectar of M. aurantiacus at the Jasper Ridge Biological Preserve in the Santa Cruz Mountains of California (Belisle et al. 2012; Peay et al. 2012). Colonies formed by these species are morphologically distinguishable on yeast media agar (YMA; Difco, Sparks, MD, USA) and cell morphology is also easily differentiated (Fig. S1). Strains from all species were isolated from the nectar of M. aurantiacus except for H. valbyensis, which was acquired from the Phaff Yeast Culture collections at the University of California, Davis (strain # 60-360, collected in California). Yeast strains were cultured on YMA and grown at 25 °C. Yeast suspensions for inoculation were diluted to about 400 cells μL−1 using a haemocytometer immediately before the beginning of the experiment described below.
Nectar environment
We designed four nectar environments to mimic the variation in sugar and amino acid concentrations in floral nectar that yeasts might encounter in natural situations. Although sugars are consumed by yeasts, the concentration of nectar sugars affects yeast growth primarily as a determinant of the osmotic harshness of the environment (Herrera et al. 2010). Nectar also varies in the concentration of amino acids, and previous work in M. aurantiacus nectar indicates that yeast strongly reduce amino acid concentration in nectar (Peay et al. 2012). The synthetic nectar solutions were 15& sucrose (0.15 g mL−1), which we refer to as benign, or 50& sucrose (0.5 g mL−1), which we refer to as harsh. We used amino acids from digested casein, which is similar in composition to nectar amino acid composition (Baker & Baker 1973), to manipulate initial resource levels: 3.16 mM total amino acids, referred to as rich, or 0.0316 mM, referred to as poor, spanning the range of previously measured nectar amino acid concentrations among plant species (Baker & Baker 1973). All synthetic nectar solutions were stored at −80 °C and filtered through a 0.2 μm filter immediately prior to use to ensure sterile conditions. We verified that these concentrations of sugar levels impose environmental harshness and that these concentrations of amino acids influence yeast carrying capacity in a pilot experiment (Fig. S2).
Experimental design
We conducted a pairwise, sequential inoculation experiment in four different synthetic nectar environments. To prepare the synthetic nectar environments, we manipulated nectar sugar and amino acid levels in a full factorial design as described above, resulting in four different nectar environments: harsh, rich; harsh, poor; benign, rich; and benign, poor. In each nectar environment, we assessed the strength of priority effects between all pairs of the four yeast species. The experiment was performed in 200-μL polymerase chain reaction (PCR) tubes (BioExpress, UT, USA) and lasted for 5 days, which approximates the lifespan of a single M. aurantiacus flower (Peay et al. 2012). To each tube, we added 9 μL of synthetic nectar and 0.5 μL of a suspension containing a single yeast species (∼200 cells) or a water control on day 0, closely mimicking the amount of nectar found in M. aurantiacus flowers (Peay et al. 2012). After 48 h, which is a realistic time interval between immigration events in this system (Peay et al. 2012), we added 0.5 μL of yeast suspension of the invader species or a water control, in all pairwise combinations, plus one all-water control, for a total of 21 treatment combinations in each nectar environment. Five days after the first inoculation, the experiment was ended and nectar from each tube was divided for chemical analysis and determining yeast abundance. All treatment combinations were performed in each of four nectar environments, and the entire experiment was performed four times, with 366 microcosms used in total.
Measurements
A subset of nectar from each sample was serially diluted in sterile sugar water, plated on YMA at final concentrations of 0.5 and 0.05 μL of nectar, incubated at 25 °C for 6 days, and colony forming units (CFUs) of each species counted. To further verify species identities, cell morphology from a subset of the colonies was examined at 20× magnification. In addition, 24 colonies were analysed using molecular methods (Peay et al. 2012). Briefly, DNA from individual colonies was extracted, the D1/D2 region amplified by PCR, and fragment patterns following digestion with restriction enzymes were assessed (Peay et al. 2012). Both cell structure and molecular sequencing indicated that species could be accurately identified based on colony morphology.
From the remaining nectar, we quantified both non-resource and resource chemical properties of nectar to assess the effects of yeast on the nectar environment. To quantify H2O2 concentration, we used a Peroxide Assay Kit for aqueous samples (Thermo Scientific, Rockford, IL, USA). Briefly, 2 μL of nectar or H2O2 standard solution was added to 100 μL of reaction solution and absorbance measured at 560 nm using a plate reader (TECAN, San Jose, CA, USA). To quantify pH, we applied 0.5 μL of nectar to each of three sections of a pH strip (EMD Millipore, Darmstadt, Germany). Remaining nectar was diluted 1:10 in diH20 for later analysis of sugars and amino acids and frozen at −80 °C until analyses could be completed.
To measure sucrose, glucose and fructose concentrations, samples were further diluted in 50:50 acetonitrile:water containing 0.5 mg mL−1 maltose (Sigma-Aldrich, St. Louis, MO, USA) as an internal standard. Sugars were then separated by UPLC (Waters, Milford, MA, USA) on a Luna amide column (50 × 2 mm, 3 μm, Phenomenex, Torrance, CA, USA). An acetonitrile:water (MeCN:H2O) mobile phase with a 4.5 min linear gradient at 170 μL min−1, beginning at 80:20 MeCN:H2O and ending at 30:70 MeCN:H2O was used, with a 10 min equilibration at initial conditions between samples. Mono- and disaccharides were quantified using an ELS Detector (Waters), and the concentration of sucrose, glucose and fructose in each sample was calculated using the internal standard and a series of external standards. Glucose and fructose were rarely detected in nectar samples, so we restrict our analyses to sucrose concentration. To quantify amino acids in individual nectar samples, diluted samples were prepared using an AccQ-Tag Kit (Waters) following the manufacturer's instructions. Briefly, 1 μL of derivatised sample was injected onto an AccQTag Ultra Column (2.1 × 100 mm) (Waters Corporation, Milford, MA, USA) at 43 °C using UPLC. Each gradient run was 10 min long, with a flow rate of 700 μL min−1 and began with an aqueous mobile phase with increasing concentration of organics, following Waters AccQTag Protocol for H-Class. Derivatised compounds were detected using UV absorbance at 260 nm, identified by comparing retention times of a series of known standards, and the concentration of each compound was calculated based on a series of external standards. All chemical analyses were performed on at least two of the four replicates for each treatment.
Quantifying priority effects
To quantify the strength of priority effects, Pij, we calculated the log of the ratio between the density of species i, D(i), measured in the number of CFU per μL nectar + 1, when it was introduced after species j and when it was introduced before species j, i.e.
where subscripts indicate introduction order. This calculation was repeated for all pairwise combinations of yeast species in each nectar environment for all replicates.
We also tried an alternative metric,
where ln [D(i)ji/D(i)0i] estimates the effect of early-arriving species j on late-arriving species i and ln [D(i)ij/D(i)i0] the effect of late-arriving species j on early-arriving species i. The two metrics were tightly correlated (Fig. S3) and gave qualitatively the same regression results.
The term priority effect is sometimes used to refer to cases where early-arriving species completely exclude late-arriving species. Here, because we are interested in quantitative predictions of the strength of the effects of species arrival order, we take a broader view and regard any significant effect of arrival order on the abundance, not just the presence or absence, of species as a priority effect.
Quantifying niche components
To quantify the degree of niche overlap among species, we compared the per capita rate of amino acid consumption for each of 22 measured amino acids among yeast species in each environment. We calculated the scalar product of unit resource use vectors following Pianka's (1973) method, between all pairs of species in all environments. This metric is equivalent to the cosine of the angle between resource utilisation vectors in Tilman's model of resource competition (Tilman 1982; Petraitis 1989) and gives a single overlap value for each species pair. The scalar product of unit vectors was calculated for all species pairs (N = 12) in the four different environments, because we suspected that resource use would vary depending on the environment. Importantly, this metric uses a unit vector to standardise total amino acid consumption for each species, so the scalar product is a metric of overlap and is distinct from the rate of resource consumption.
As a measure of the impact niche, we calculated the per capita amino acid consumption by each yeast species, using nectar conditioned for 3 days after species inoculation. To account for non-resource changes in nectar chemistry, we calculated per capita effects of each yeast species on pH, H2O2 and sucrose concentrations for each environment. Because yeast effects on non-resource components were highly correlated, we used a principle component analysis (PCA) to generate two independent axes (95& var. cumulatively explained by first two axes, Fig. S4) to use in subsequent analyses.
Each species' tolerance of environmental conditions (requirement niche) was quantified using an additional experiment. We grew each yeast species separately in nectar that varied in amino acid concentration, and characterised yeast growth. Nectar conditions in this experiment mimicked the conditions used in the original experiment, but for each nectar environment in the original experiment, we assessed the growth of each yeast species in nectar that varied in resource levels, using 100, 75, 50 and 25& of initial amino acid concentrations in the resource-poor and resource-rich environments. Assays were conducted in both sugar environments. Yeasts were inoculated as described previously, grown for 3 days, plated and counted. Best-fit linear or quadratic lines were used to predict the response of late-arriving species to measured resource reduction by the first species (Fig. S5). The predicted difference in final cell density due to resource reduction by the early-arriving species was used to represent the tolerance to environmental conditions (requirement niche) of late-arriving species in the full linear model described in the next section.
Data analysis
To determine if the measured niche components influenced the strength of priority effects, Pij, across all nectar environments, we began with the full linear model:
where Oij is the scalar product, quantifying the niche overlap between species i and j; Aj is the per capita effect of species j on total amino acid concentration, quantifying the rate of change in amino acid concentration (impact niche) imposed by species j; B1j and B2j are the first two principle component axes that represent the per capita effect of species j on non-resource nectar chemistry, quantifying the rate of change in nectar properties (impact niche) of species j; Rij is the predicted reduction in population growth of species i, given reduction in total amino acid concentration imposed by species j, quantifying the tolerance of low resources (requirement niche) of species i; H is a term representing environmental harshness; and E is an error term. Species j is the early-arriving species, and species i the late-arriving species. None of the predictors was highly correlated (all r < 0.5, Fig. S6), and all had variance inflation factors below 4, so all terms were included in the initial full model.
Non-significant predictors were sequentially removed using the likelihood ratio test, with P < 0.05 as a cut-off. To assess the relative importance of retained predictors in the final model, we sequentially dropped each predictor and compared the change in the variance explained by the model (adjusted R2) to the full model. Two additional analyses were conducted. First, we included all possible interactions among niche predictors in the original full model (i.e. all two-way interactions among Oij, Aj, B1j, B2j and Rij) and sequentially removed non-significant terms as described above. Second, we repeated the analysis for each nectar environment separately, omitting the predictor for environmental harshness, to determine if the relative importance of each niche component depended on the environmental conditions.
We also assessed if the niche components were better predictors of priority effects than either phylogenetic relatedness or ecological similarity, two metrics previously used to predict the strength of priority effects (Peay et al. 2012; Tan et al. 2012). Note that many definitions of ecological similarity in the literature aggregate multiple traits (e.g. Weiher et al. 1998; Kraft et al. 2008), including those that may determine the species' impact on, and response to, environmental conditions, in contrast to the measure of niche overlap used in our analysis, which quantifies the similarity of resource use independently of the impact niche and the requirement niche. To assess phylogenetic relatedness as a predictor, we calculated the patristic distance between each pair of species (Peay et al. 2012). We regressed patristic distance between each pair against the strength of priority effects among species, including environmental harshness as a covariate. This analysis was repeated among all environments separately, as above. To assess ecological similarity as a predictor of priority effects, we used PCA to summarise measured species traits from each environment and extracted three axes to represent independent measures of these ecological traits. We included consumption of all individual amino acids, changes in non-resource nectar chemistry (i.e. pH, H2O2 and sucrose), and coefficients that describe species growth rates calculated in the requirement niche experiment (Fig. S5). The PCA was implemented using the rda function in R package vegan (Oksanen et al. 2012), with values standardised during analysis. The first three principal component axes cumulatively explained 98& of variation in the included data. These axes and environmental harshness were used as predictors of priority effects using multiple regression. Model selection was performed using the likelihood ratio test, as described above. To further compare among the three models (niche components, phylogenetic relatedness and ecological similarity) and account for variation in the number of predictors among models, we extracted Akaike's information criterion (AIC) from each model (Table S1).
All analyses were performed in R v.2.15.2 (R Development Core Team 2012).
Results
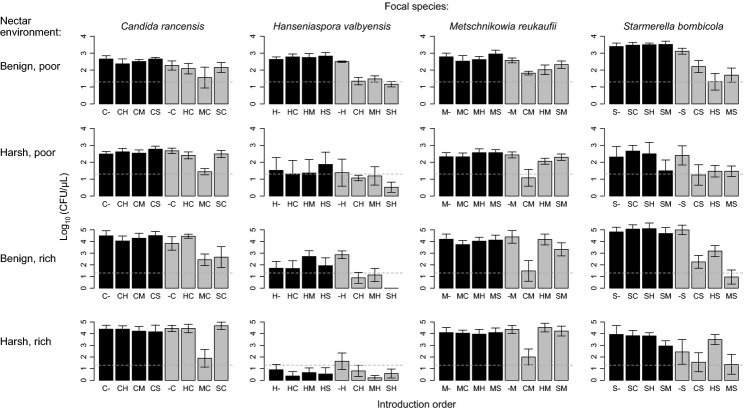
We found that the direction of priority effects was consistent across the experiment, with early-arriving species negatively affecting late-arriving species in most cases (Figs2 and 3). However, the strength of these effects varied considerably among species pairs and among environments (Fig.2). Of the predictors retained in the final model reached by our statistical analysis (Table1), environmental harshness explained 38& of this variation. Priority effects were stronger in benign environments (Fig.3d), in concordance with the conventional hypothesis we focused on (Chase 2003, 2007; Kardol et al. 2013). Over and above the influence of environmental harshness, all niche components were found to be significant predictors of the strength of priority effects, lending support to our hypotheses. Niche overlap explained 14& of the total variation, with species pairs with higher resource use overlap exhibiting stronger inhibitory priority effects (Fig.3a). The rate of change in nectar properties (impact niche) explained 11&, with early-arriving species that caused larger declines in nectar pH and sucrose concentration exerting stronger priority effects (Figs3b and S4). Species' tolerance of low resources (requirement niche) explained 10&, with late-arriving species that were more sensitive to amino acid reduction experiencing stronger inhibitory priority effects (Fig.3c). None of the interactions among niche components was a significant predictor. Separate analyses for different environments indicated that whether a particular niche component was a significant predictor depended on the environment (Table2). However, whenever significant, the direction of their effect was always consistent with our hypotheses (Fig.1).
Figure 2.
Effect of introduction order and nectar environment on the abundance (mean log10(CFU μL−1 nectar +1) measured on day 5± SE of 4 replicates for each treatment) of Candida rancensis (C), Hanseniaspora valbyensis (H), Metschnikowia reukaufii (M) and Starmerella bombicola (S). Introduction order is indicated below bars, where letters indicate species and hyphens (-) indicate no introduction (sterile control). For example, C- means that C. rancensis was introduced on day 0 and no species on day 2; CH means that C. rancensis was introduced on day 0 and H. valbyensis on day 2; and -C means no species was introduced on day 0 and C. rancensis on day 2. Bars are black when focal species was introduced first, and grey when introduced second. Dotted lines indicate the initial density.
Figure 3.
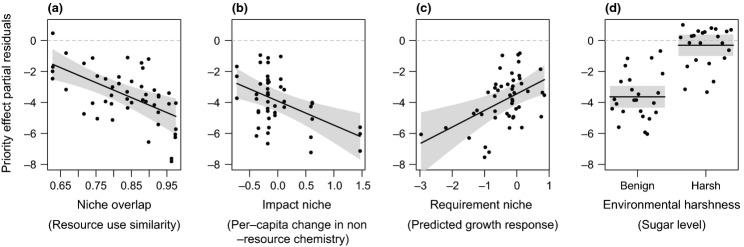
Relationships between the strength of priority effects among yeast species and the variables retained in the multiple regression model, including niche overlap (a), impact niche (b), requirement niche (c) and environmental harshness (d). The y-axis displays the partial residuals of the response variable conditioned on the median value of all other retained predictors. Grey bands represent 95& confidence intervals around the predicted fit line. Dotted line represents zero priority effect. Figures were generated using package visreg in R v.2.15.2.
Table 1.
Regression coefficients from the final model predicting the effects of multiple niche components on the strength of priority effects
| Predictors | Coefficient | Standard error | P-value | Reduction in R2 |
|---|---|---|---|---|
| Intercept | 7.79 | 2.23 | 0.001 | – |
| Niche overlap (scalar product) | −9.66 | 2.47 | <0.001 | 0.14 |
| Impact niche (principal component 2) | −1.56 | 0.45 | 0.0013 | 0.11 |
| Requirement niche (predicted growth) | 1.07 | 0.32 | 0.0018 | 0.10 |
| Environmental harshness (sugar level) | −1.63 | 0.65 | <0.001 | 0.38 |
Because priority effects were largely inhibitory, negative coefficients indicate stronger priority effects with an increase in the value of the predictor. Coefficient indicates the unstandardised partial regression coefficient for each predictor. Reduction in R2 was calculated by dropping each term from the final model and comparing the change in R2. Model adjusted R2 = 0.57, P < 0.001. See Materials and methods for full descriptions of each niche component and metric used.
Table 2.
Regression coefficients from the final model for each environment, where sucrose concentration was high (harsh environment) or low (benign environment) and resource (amino acid) concentration was high (rich environment) or low (poor environment)
| Nectar environment | Predictors | Coefficient | Standard error | P-value | R2adj |
|---|---|---|---|---|---|
| Harsh, rich | 0.008 | 0.58 | |||
| Niche overlap (scalar product) | −13.61 | 5.73 | 0.04 | ||
| Requirement niche (predicted growth) | 1.54 | 0.49 | 0.01 | ||
| Harsh, poor | 0.01 | 0.54 | |||
| Niche overlap (scalar product) | −14.90 | 5.16 | 0.02 | ||
| Impact niche (principal component 2) | −1.40 | 0.48 | 0.02 | ||
| Benign, rich | 0.003 | 0.64 | |||
| Requirement niche (predicted growth) | 2.12 | 0.78 | 0.02 | ||
| Impact niche (principal component 2) | −2.98 | 1.00 | 0.01 | ||
| Benign, poor | 0.02 | 0.33 | |||
| Impact niche (principal component 2) | −4.29 | 1.17 | 0.0083 |
Adjusted R2 (R2adj) indicates the variance explained in the full model. N = 12 for each regression analysis.
In additional analyses, we assessed how much variation in the strength of priority effects could be explained by phylogenetic relatedness or overall ecological similarity between species without their niches separated into multiple components. Phylogenetic relatedness was not significantly associated with priority effects when data from all environments were analysed together (Table S1). When analysed separately for each environment, closely related species exhibited stronger priority effects in one of the four environments (harsh, resource-rich environment), but with only 35& of the total variation explained (Table S1), as opposed to 58& explained with decomposition of niche components (Table2). Overall, the ecological similarity model explained only 31& of the total variation, even when used in combination with environmental harshness (Table S1). Phylogenetic relatedness and ecological similarity were not correlated with each other (P > 0.16).
Discussion
Taken together, these results show that niche components can collectively explain a large fraction of variation in the strength of priority effects. By decomposing niche components, it was possible to predict nearly twice as much variation as explained by phylogenetic relatedness or overall ecological similarity (cf. Peay et al. 2012). Furthermore, niche components were fairly robust and consistent predictors of priority effects even in the face of large differences in species growth and resource use among environments.
Some species-specific patterns may have existed in our data. For example, priority effects tended to be strong when imposed by C. rancensis and M. reukaufii and when experienced by H. valbyensis, whereas S. bombicola tended to cause strong effects only in benign environments (Fig.2). For the most part, however, species-specific effects appeared too complicated to provide systematic explanation (Fig.2). Our analysis demonstrates that consideration of niche components across species helps to discern patterns in seemingly idiosyncratic variation in the strength of priority effects (Fig.3).
The potential scope of the niche-component hypotheses is broader than can be captured by the specific data from our experiment. For example, priority effects were mostly inhibitory in our experiment, but facilitative priority effects, where early-arriving species promote the growth of late colonisers, may also be common in natural communities (Callaway & Walker 1997; Bruno et al. 2003). We suggest that both facilitative and inhibitory priority effects can be considered within the same niche-component framework. For instance, just as early-arriving species with high negative impact would cause strong inhibitory priority effects, those with high positive impact should cause strong facilitative priority effects. In addition, the data that we used to analyse priority effects in nectar yeasts mostly concerned interactions via changes in abiotic environmental conditions, such as nectar pH and resource availability. However, the niche-component hypotheses should be equally applicable to interactions via changes in biotic environmental conditions, such as predator and mutualist densities, caused by early-arriving species.
Extending the niche framework
The hypotheses that we examined and the experimental results that supported them provide a basis for developing a new, multiple-niche-component approach for empirically studying priority effects. We identify four research areas in which the approach can be developed further in the future. First, we suggest that phylogenetic information should be better integrated. In our experiment, phylogenetic relatedness was a poorer predictor of priority effects than niche components, as we suspected. For many types of communities, however, detailed ecological data needed to quantify niche components may be harder to obtain than phylogenetic data, which are now readily available for many taxa owing to the recent advances in molecular phylogenetics (Mouquet et al. 2012). It may be possible to make the multiple niche component approach more generally practical by exploring the potentials and limitations of estimating niche components from phylogenetic information (Martiny et al. 2013). In this effort, it should be kept in mind that traits that underlie niche components may sometimes be correlated, although no correlation was found in our nectar yeasts. In plants, for example, there may be a trade-off between low requirement and high impact, such that slow-growing species have low requirement and can readily tolerate the effects of other species, whereas fast-growing species have high impact and can rapidly change environmental conditions (Goldberg & Landa 1991).
A second area of development concerns the relative strength of intra- and interspecific competition. We assumed in this study that intraspecific competition was generally stronger than interspecific competition, or that each species had a greater impact on the resources that were more limiting to themselves than to other species (Tilman 1982). However, this may not always be the case. In some systems, certain species may have a greater impact on others than on themselves. In freshwater ecosystems, for example, light may be more limiting to submerged plants than to floating plants, yet light availability may be more greatly reduced by floating plants than by submerged plants. Conversely, another resource, water-column nutrients, may be more limiting to floating plants, but more greatly reduced by submerged plants (Scheffer et al. 2003). In cases like this, theory predicts that priority effects can be stronger when niche overlap is less, contrary to the hypothesis we focused on in this study (Tilman 1988; Reynolds & Pacala 1993). The multiple niche component approach can be made more inclusive by clarifying how predictions about the influence of niche components on priority effects differs between cases where intraspecific competition is greater than interspecific competition and those where the opposite is true.
A third area of development involves consideration of labile niches. In our experiment, we assumed that niche components were fixed. However, some niche components may be labile because of phenotypic plasticity (e.g. Ashton et al. 2010) or evolutionary response (e.g. Bohannan & Lenski 2000), potentially with substantial consequences for community assembly (Silvertown et al. 2006; Agosta & Klemens 2008). For example, early-arriving species may rapidly diversify or adapt to local conditions, resulting in a stronger priority effect than expected without evolution (De Meester et al. 2002; Knope et al. 2012). In other cases, species may evolve to minimise niche overlap with competitors, resulting in character displacement (Brown & Wilson 1956). Quantifying the extent to which niche components are labile may help to predict greater variation in priority effects than is possible under the assumption of fixed niches (see also Losos 2008).
Finally, the niche component approach can also be extended by considering how higher-order interactions and their effects on priority effects may be associated with each niche component. We have focused on pairwise species interactions in our experiment because field observations indicate that most individual flowers contain only a few species of yeast at most (Pozo et al. 2011; Belisle et al. 2012). However, in other, more diverse systems, species may engage in higher-order indirect interactions in addition to pairwise interactions (Werner & Peacor 2003; Hoverman & Relyea 2008). Inclusion of these interactions into the niche component framework through experimental tests would make the framework more general.
Applying the niche framework
We suggest that the framework outlined here has the potential to inform any applied discipline in which historical contingency can be used to manage ecological systems. For example, early introduction of native plants and animals that are resistant to biological invasion may benefit ecosystem restoration (Young et al. 2001, 2005; Temperton & Zirr 2004; Grman & Suding 2010; Wainwright et al. 2012). Evidence indicates that niche-based assembly rules may govern plant succession in restored sites even when the succession appears sensitive to priority effects (Fukami et al. 2005), suggesting that consideration of niche components may improve our ability to restore ecosystems via sequential introduction and/or removal of species. These efforts may be particularly useful if niche components are integrated into existing approaches such as community assembly maps (Warren et al. 2003) and state-and-transition models of community assembly (Westoby et al. 1989; Jackson & Bartolome 2002).
Similar strategies, with application of symbiotic microbes to prevent pest outbreaks, may help crop production, but only with the right application timing in relation to the phenology of the host plants and other microbes associated with them (Verbruggen et al. 2013). In this case, many mechanisms can underlie protective effects of non-pathogenic microbes, including the production of antibiotics by endophytes, direct competition for shared resources within the plant, and indirect competition mediated by plant resistance (Sturz et al. 2000). Determining the extent to which the microbial impact niche, requirement niche, and niche overlap determine the strength of protective priority effects may help to devise more effective, species-specific inoculations for crop protection.
The niche framework may also be useful in preventing human disease. For example, recent medical research suggests that an effective treatment of harmful Clostridium difficile infections may be a combined use of antibiotic treatment followed by transplantation of faecal microbiota from healthy individuals to establish resistant communities (Borody & Khoruts 2012). However, difficulty in the identification of healthy donors with appropriate microbiota limits the application of this treatment (Borody & Khoruts 2012). Determining which niche components are more critical in structuring gut microbial communities may aid in the development of microbial communities that impose strong priority effects and resist invasion by C. difficile to avoid medically important dysfunctions of the gut microbial community. In these applications, accurate predictions of historically contingent species interactions are essential. The framework emphasising distinct roles of multiple niche components may serve as a new foundation for improved management of such systems.
Conclusion
Over the past decade, the controversy surrounding the neutral theory of biodiversity (Hubbell 2001) has led to a resurgence of interest in using classic niche-based theories to explain species interactions and their implications for species diversity (e.g. Chase & Leibold 2003; Fargione et al. 2003; HilleRisLambers et al. 2012). However, despite the intensive research that ensued, the role of niches in species interactions remains elusive. Our experimental findings suggest that substantial progress will come from explicit consideration of multiple niche components, which can greatly improve our ability to explain even the most difficult aspect of species interactions, historical contingency. The relative importance of niche components will likely vary from system to system, but the approach we have taken here provides a basis for developing a general framework within which their relative importance can be determined in different types of communities.
Acknowledgments
We thank Mathew Leibold and the members of the community ecology group at Stanford University, particularly Ben Callahan, Marie-Pierre Gauthier, Holly Moeller, Kabir Peay, and Caroline Tucker, for comments, and the Phaff Yeast Culture Collection at UC Davis for yeast. The manuscript was improved by comments from Truman Young and three anonymous referees. The Department of Biology and the Terman Fellowship of Stanford University and the National Science Foundation (award number: DEB1149600) supported this research. RLV is funded by the Gordon and Betty Moore Foundation through Grant GBMF 2550.02 to the Life Sciences Research Foundation.
Authorship
RLV and TF conceived the study and designed the experiment. RLV conducted the experiment and analysed the data. RLV and TF wrote the manuscript.
Supporting Information
Additional Supporting Information may be downloaded via the online version of this article at Wiley Online Library (www.ecologyletters.com).
Supplementary
References
- Agosta SJ. Klemens JA. Ecological fitting by phenotypically flexible genotypes: implications for species associations, community assembly and evolution. Ecol. Lett. 2008;11:1123–1134. doi: 10.1111/j.1461-0248.2008.01237.x. [DOI] [PubMed] [Google Scholar]
- Ashton IW, Miller AE, Bowman WD. Suding KN. Niche complementarity due to plasticity in resource use: plant partitioning of chemical N forms. Ecology. 2010;91(3252–3260):3. doi: 10.1890/09-1849.1. [DOI] [PubMed] [Google Scholar]
- Baker HG. Baker I. Amino acids in nectar and their evolutionary significance. Nature. 1973;241:543–545. [Google Scholar]
- Belisle M, Peay KG. Fukami T. Flowers as islands: spatial distribution of nectar-inhabiting microfungi among plants of Mimulus aurantiacus, a hummingbird-pollinated shrub. Microb. Ecol. 2012;63:711–718. doi: 10.1007/s00248-011-9975-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best RJ, Caulk NC. Stachowicz JJ. Trait vs. phylogenetic diversity as predictors of competition and community composition in herbivorous marine amphipods. Ecol. Lett. 2013;16:72–80. doi: 10.1111/ele.12016. [DOI] [PubMed] [Google Scholar]
- Bohannan BJM. Lenski RE. Linking genetic change to community evolution: insights from studies of bacteria and bacteriophage. Ecol. Lett. 2000;3:362–377. [Google Scholar]
- Borody TJ. Khoruts A. Fecal microbiota transplantation and emerging applications. Nat. Rev. Gastroenterol. Hepatol. 2012;9:88–96. doi: 10.1038/nrgastro.2011.244. [DOI] [PubMed] [Google Scholar]
- Brown WL., Jr Wilson EO. Character displacement. Syst. Zool. 1956;5:49–64. [Google Scholar]
- Bruno JF, Stachowicz JJ. Bertness MD. Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 2003;18:119–125. [Google Scholar]
- Callaway RM. Walker LR. Competition and facilitation: a synthetic approach to interactions in plant communities. Ecology. 1997;78:1958–1965. [Google Scholar]
- Chase JM. Community assembly: when should history matter? Oecologia. 2003;136:489–498. doi: 10.1007/s00442-003-1311-7. [DOI] [PubMed] [Google Scholar]
- Chase JM. Drought mediates the importance of stochastic community assembly. Proc. Natl Acad. Sci. 2007;104:17430–17434. doi: 10.1073/pnas.0704350104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase JM. Leibold MA. Ecological Niches: Linking Classical and Contemporary Approaches. Chicago: University of Chicago Press; 2003. [Google Scholar]
- Costello EK, Stagaman K, Dethlefsen L, Bohannan BJM. Relman DA. The application of ecological theory toward an understanding of the human microbiome. Science. 2012;336:1255–1262. doi: 10.1126/science.1224203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meester L, Gomez A, Okamura B. Schwenk K. The Monopolization Hypothesis and the dispersal-gene flow paradox in aquatic organisms. Acta Oecol. 2002;23:121–135. [Google Scholar]
- Drake JA. Community-assembly mechanics and the structure of an experimental species ensemble. Am. Nat. 1991;137:1–26. [Google Scholar]
- Elton CS. Animal Ecology. London: Sidgewick and Jackson; 1927. [Google Scholar]
- Fargione J, Brown CS. Tilman D. Community assembly and invasion: an experimental test of neutral versus niche processes. Proc. Natl Acad. Sci. USA. 2003;100:8916–8920. doi: 10.1073/pnas.1033107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox B. Species assembly and the evolution of community structure. Evol. Ecol. 1987;1:201–213. [Google Scholar]
- Fukami T. Community assembly dynamics in space. In: Verhoef HA, Morin PJ, editors. Community Ecology: Processes, Models, and Applications. Oxford: Oxford University Press; 2010. pp. 45–54. [Google Scholar]
- Fukami T. Morin PJ. Productivity-biodiversity relationships depend on the history of community assembly. Nature. 2003;424:423–426. doi: 10.1038/nature01785. [DOI] [PubMed] [Google Scholar]
- Fukami T, Bezemer TM, Mortimer SR. van der Putten WH. Species divergence and trait convergence in experimental plant community assembly. Ecol. Lett. 2005;8:1283–1290. [Google Scholar]
- Gause GF. Experimental studies on the struggle for existence: I. Mixed population of two species of yeast. J. Exp. Biol. 1932;9:389–402. [Google Scholar]
- Gilpin ME. Case TJ. Multiple domains of attraction in competition communities. Nature. 1976;261:40–42. doi: 10.1038/261040a0. [DOI] [PubMed] [Google Scholar]
- Gleason HA. The individualistic concept of the plant association. Bull. Torry Bot. Club. 1926;53:7–26. [Google Scholar]
- Goldberg DE. Landa K. Competitive effect and response: hierarchies and correlated traits in the early stages of competition. J. Ecol. 1991;79:1013–1030. [Google Scholar]
- Grinnell J. The niche-relationships of the California thrasher. Auk. 1917;34:427–433. [Google Scholar]
- Grman E. Suding KN. Within-year soil legacies contribute to strong priority effects of exotics on native California grassland communities. Restor. Ecol. 2010;18:664–670. [Google Scholar]
- Herrera CM, Canto A, Pozo MI. Bazaga P. Inhospitable sweetness: nectar filtering of pollinator-borne inocula leads to impoverished, phylogenetically clustered yeast communities. Proc. Biol. Sci. 2010;277:747–754. doi: 10.1098/rspb.2009.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HilleRisLambers J, Adler PB, Harpole WS, Levine JM. Mayfield MM. Rethinking community assembly through the lens of coexistence theory. Annu. Rev. Ecol. Evol. Syst. 2012;43:227–248. [Google Scholar]
- Hoverman JT. Relyea RA. Temporal environmental variation and phenotypic plasticity: a mechanism underlying priority effects. Oikos. 2008;117:23–32. [Google Scholar]
- Hubbell SP. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton, NJ, USA: Princeton University Press; 2001. [Google Scholar]
- Hutchinson GE. Concluding remarks. Population Studies: Animal Ecology and Demography. Cold Spring Harbor Symp. Quant. Biol. 1957;22:415–27. [Google Scholar]
- Jackson R. Bartolome J. A state-transition approach to understanding nonequilibrium plant community dynamics in Californian grasslands. Plant Ecol. 2002;162:49–65. [Google Scholar]
- Jiang L, Tan J. Pu Z. An experimental test of Darwin's naturalization hypothesis. Am. Nat. 2010;175:415–423. doi: 10.1086/650720. [DOI] [PubMed] [Google Scholar]
- Kardol P, Souza L. Classen AT. Resource availability mediates the importance of priority effects in plant community assembly and ecosystem function. Oikos. 2013;122:84–94. [Google Scholar]
- Knope ML, Forde SE. Fukami T. Evolutionary history, immigration history, and the extent of diversification in community assembly. Front. Microbiol. 2012;2:273. doi: 10.3389/fmicb.2011.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft NJB, Valencia R. Ackerly DD. Functional traits and niche-based tree community assembly in an Amazonian forest. Science. 2008;322:580–582. doi: 10.1126/science.1160662. [DOI] [PubMed] [Google Scholar]
- Lachance M-A, Starmer WT, Rosa CA, Bowles JM, Barker JSF. Janzen DH. Biogeography of the yeasts of ephemeral flowers and their insects. FEMS Yeast Res. 2001;1:1–8. doi: 10.1111/j.1567-1364.2001.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Lavorel S. Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct. Ecol. 2002;16:545–556. [Google Scholar]
- Leibold MA. The niche concept revisited: mechanistic models and community context. Ecology. 1995;76:1371–1382. [Google Scholar]
- Lewontin RC. The meaning of stability. Brookhaven Symp. Biol. 1969;22:13–24. [PubMed] [Google Scholar]
- Losos JB. Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol. Lett. 2008;11:995–1003. doi: 10.1111/j.1461-0248.2008.01229.x. [DOI] [PubMed] [Google Scholar]
- MacArthur RH. Geographical Ecology: Patterns in the Distribution of Species. Princeton, NJ: Princeton University Press; 1972. [Google Scholar]
- MacArthur RH. Levins R. The limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 1967;101:377–385. [Google Scholar]
- Martiny AC, Treseder K. Pusch G. Phylogenetic conservatism of functional traits in microorganisms. ISME J. 2013;7:830–838. doi: 10.1038/ismej.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield MM. Levine JM. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010;13:1085–1093. doi: 10.1111/j.1461-0248.2010.01509.x. [DOI] [PubMed] [Google Scholar]
- Mouquet N, Devictor V, Meynard CN, Munoz F, Bersier L-F, Chave J, et al. Ecophylogenetics: advances and perspectives. Biol. Rev. 2012;87:769–785. doi: 10.1111/j.1469-185X.2012.00224.x. [DOI] [PubMed] [Google Scholar]
- Munguia P, Osman R, Hamilton J, Whitlatch R. Zajac R. Modeling of priority effects and species dominance in Long Island Sound benthic communities. Mar. Ecol. Prog. Ser. 2010;413:229–240. [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Legendre P, O'Hara RB, Simpson GL, et al. 2012. vegan: Community Ecology Package. R package version 2.0-4. Available at: http://CRAN.R-project.org/package=vegan.
- Peay KG, Belisle M. Fukami T. Phylogenetic relatedness predicts priority effects in nectar yeast communities. Proc. Biol. Sci. 2012;279:749–758. doi: 10.1098/rspb.2011.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraitis PS. The representation of niche breadth and overlap on Tilman's consumer-resource graphs. Oikos. 1989;56:289–292. [Google Scholar]
- Petraitis PS. Multiple Stable States in Natural Ecosystems. 1. Oxford: Oxford University Press; 2013. [Google Scholar]
- Pianka ER. The structure of lizard communities. Annu. Rev. Ecol. Syst. 1973;4:53–74. [Google Scholar]
- Porensky LM, Vaughn KJ. Young TP. Can initial intraspecific spatial aggregation increase multi-year coexistence by creating temporal priority? Ecol. Appl. 2012;22:927–36. doi: 10.1890/11-0818.1. [DOI] [PubMed] [Google Scholar]
- Pozo MI, Herrera CM. Bazaga P. Species richness of yeast communities in floral nectar of southern Spanish plants. Microb. Ecol. 2011;61:82–91. doi: 10.1007/s00248-010-9682-x. [DOI] [PubMed] [Google Scholar]
- Pozo MI, Lachance M-A. Herrera CM. Nectar yeasts of two southern Spanish plants: the roles of immigration and physiological traits in community assembly. FEMS Microbiol. Ecol. 2012;80:281–293. doi: 10.1111/j.1574-6941.2011.01286.x. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- Reynolds HL. Pacala SW. An analytical treatment of root-to-shoot ratio and plant competition for soil nutrient and light. Am. Nat. 1993;141:51–70. doi: 10.1086/285460. [DOI] [PubMed] [Google Scholar]
- Scheffer M, Szabo S, Gragnani A, van Nes EH, Rinaldi S, Kautsky N, et al. Floating plant dominance as a stable state. Proc. Natl Acad. Sci. 2003;100:4040–4045. doi: 10.1073/pnas.0737918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorrocks B. Bingley M. Priority effects and species coexistence: experiments with fungal-breeding Drosophila. J. Anim. Ecol. 1994;63:799–806. [Google Scholar]
- Silvertown J, Dodd M, Gowing D, Lawson C. McConway K. Phylogeny and the hierarchical organization of plant diversity. Ecology. 2006;87:S39–S49. doi: 10.1890/0012-9658(2006)87[39:pathoo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Sturz AV, Christie BR. Nowak J. Bacterial endophytes: potential role in developing sustainable systems of crop production. Crit. Rev. Plant Sci. 2000;19:1–30. [Google Scholar]
- Suding KN, Lavorel S, Chapin FS, Cornelissen JHC, Diaz S, Garnier E, et al. Scaling environmental change through the community-level: a trait-based response-and-effect framework for plants. Glob. Change Biol. 2008;14:1125–1140. [Google Scholar]
- Tan J, Pu Z, Ryberg WA. Jiang L. Species phylogenetic relatedness, priority effects, and ecosystem functioning. Ecology. 2012;93:1164–1172. doi: 10.1890/11-1557.1. [DOI] [PubMed] [Google Scholar]
- Temperton VM. Zirr K. Order of arrival and availability of safe sites: an example of their importance for plant community assembly in stressed ecosystems. In: Temperton VM, Hobbs R, Nuttle T, Halle S, editors; Assembly Rules and Restoration Ecology: Bridging the Gap Between Theory and Practice. Washington D.C: Island Press; 2004. pp. 285–304. [Google Scholar]
- Tilman D. Resource Competition and Community Structure. Princeton, NJ: Princeton University Press; 1982. [PubMed] [Google Scholar]
- Tilman D. Plant Strategies and the Dynamics and Structure of Plant Communities. Princeton, NJ: Princeton University Press; 1988. [Google Scholar]
- Vannette RL, Gauthier M-PL. Fukami T. Nectar bacteria, but not yeast, weaken a plant-pollinator mutualism. Proc. Biol. Sci. 2013;280:20122601. doi: 10.1098/rspb.2012.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen E, van der Heijden MGA, Rillig MC. Kiers ET. Mycorrhizal fungal establishment in agricultural soils: factors determining inoculation success. New Phytol. 2013;197:1104–1109. doi: 10.1111/j.1469-8137.2012.04348.x. [DOI] [PubMed] [Google Scholar]
- Wainwright CE, Wolkovich EM. Cleland EE. Seasonal priority effects: implications for invasion and restoration in a semi-arid system. J. Appl. Ecol. 2012;49:234–241. [Google Scholar]
- Warren PH, Law R. Weatherby AJ. Mapping the assembly of protist communities in microcosms. Ecology. 2003;84:1001–1011. [Google Scholar]
- Weiher E, Clarke GDP. Keddy PA. Community assembly rules, morphological dispersion, and the coexistence of plant species. Oikos. 1998;81:309–322. [Google Scholar]
- Werner EE. Peacor SD. A review of trait-mediated indirect interactions in ecological communities. Ecology. 2003;84:1083–1100. [Google Scholar]
- Westoby M, Walker B. Noy-Meir I. Opportunistic management for rangelands not at equilibrium. J. Range Manag. 1989;42:266–274. [Google Scholar]
- Wilson JB. Assembly rules in plant communities. In: Weiher E, Keddy P, editors. Ecological Assembly Rules: Perspectives, Advances, Retreats. Press: Cambridge University; 1999. pp. 130–164. [Google Scholar]
- Young TP. Peffer E. “Recalcitrant understory layers” revisited: arrested succession and the long life-spans of clonal mid-successional species. Can. J. For. Res. 2010;40:1184–1188. [Google Scholar]
- Young TP, Chase JM. Huddleston RT. Community succession and assembly comparing, contrasting and combining paradigms in the context of ecological restoration. Ecol. Restor. 2001;19:5–18. [Google Scholar]
- Young TP, Petersen DA. Clary JJ. The ecology of restoration: historical links, emerging issues and unexplored realms. Ecol. Lett. 2005;8:662–673. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary