Abstract
Less than a third of adults with acute myeloid leukemia (AML) are cured by current treatments, emphasizing the need for new approaches to therapy. The discovery over a decade ago that myeloid leukemias originate from rare stem-like cells that can transfer the disease to immunodeficient mice suggested that these ‘leukemia stem cells’ (LSCs) are responsible for relapse of leukemia following conventional or targeted cancer therapy and that eradication of LSCs might be necessary to cure the disease permanently. Several recent studies have provided insight into the signaling pathways underlying the LSC phenotype and have also described approaches to eliminate LSCs with antibodies. Here, we review recent advances in LSC research and discuss novel therapeutic strategies to specifically target LSCs.
Keywords: AML, BCR-ABL, CML, LIC, LSC, NOD-SCID, xenotransplantation
The leukemia (cancer) stem cell hypothesis
Leukemia is a group of malignant diseases of the blood system characterized by uncontrolled overproduction of leukocytes (white blood cells) that are either immature (acute leukemia) or terminally differentiated (chronic leukemia) (Box 1 and Figure 1). Leukemia can be further subdivided by whether the malignant leukocytes are of myeloid origin (cells of granulocyte, monocyte, erythroid or megakaryocyte lineage) or lymphoid origin (B-cell, T-cell or natural killer-cell lineage). Currently, acute leukemia is treated by administration of cytotoxic chemotherapy, which is intended to kill the majority of the leukemic cells. For certain high-risk patients, allogeneic hematopoietic stem-cell (HSC) transplantation is indicated to achieve more extensive eradication of leukemia and to induce immune responses against the disease. By contrast, generally, the chronic leukemias are controlled by drugs that suppress leukocyte production; in the case of chronic myeloid leukemia (CML; see Glossary) and other conditions that are associated with dysregulated tyrosine kinases, agents that inhibit the activity of the relevant kinase are the mainstay of therapy.
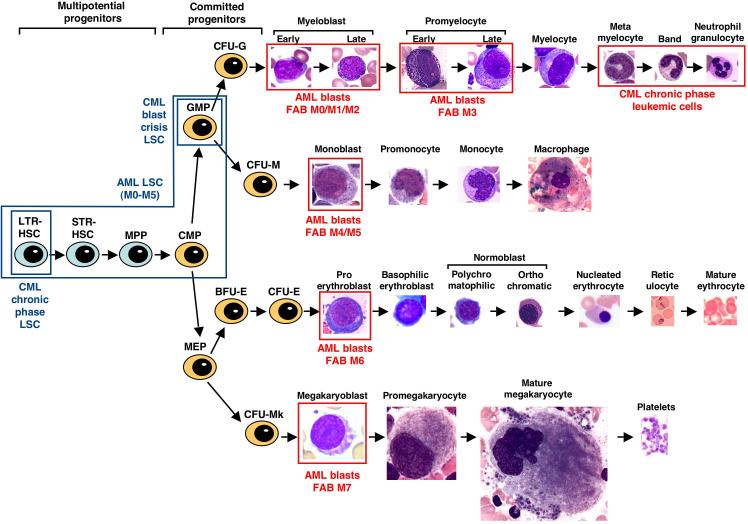
Figure 1.
Diagram of normal myeloid development and the relationship to leukemic cells and LSCs. Multipotential stem and progenitor cells, including long-term repopulating (LTR) and short-term repopulating (STR) stem cells and multipotential progenitors (MPPs), are depicted in blue on the left. Committed progenitors, including common myeloid progenitors (CMPs), granulocyte–macrophage progenitors (GMPs), megakaryocyte–erythroid progenitors (MEPs) and the colony-forming units (CFUs) for granulocytes (CFU-Gs), macrophages (CFU-Ms), erythroid (CFU-E) and megakaryocytes (CFU-Mks), are depicted in orange in the middle. Differentiating myeloid cells, recognizable by their distinct morphology, are shown at the right. The malignant cells in acute and chronic myeloid leukemia are indicated by red boxes; leukemic blasts for the different FAB subclasses of AML (M0 through M7) correspond approximately to the different normal blasts in each lineage, whereas CML chronic-phase cells correspond to terminally differentiated granulocytes. By contrast, the LSCs for acute and chronic myeloid leukemias are restricted to rare multi-potential and committed progenitors, as indicated by the blue boxes.
For many years, it has been appreciated that most cancers, including leukemia, are heterogeneous in their growth potential. Only a minor fraction of leukemia blasts (immature malignant hematopoietic cells) isolated from patients can grow or form colonies in vitro or can proliferate in vivo [1]. Two possibilities could explain this: either, all leukemic cells have the same low growth potential or the clonogenic and leukemogenic activity might reside in a minor, more primitive fraction of the leukemia cell population. In 1994, a seminal paper from John Dick and colleagues showed that, for human acute myeloid leukemia (AML), the latter was true: only leukemic blasts with the immature cell-surface phenotype CD34+CD38_ (Table 1), comprising a minority of the total population, were capable of transferring AML to immunodeficient severe combined immunodeficiency (SCID) mice [2]. This and related observations led to the cancer stem-cell hypothesis, which postulates that many tumors are maintained by a small population of stem-like cancer cells that have the capacity for indefinite self-renewal [3]. If this hypothesis is correct, cancer must either originate from normal tissue stem cells that already possess the capacity for self-renewal or else this property is acquired aberrantly during tumorigenesis [4,5]. Cancer stem cells are defined functionally by their ability to transfer the malignancy on xenotransplantation into an immunodeficient recipient animal, usually non-obese diabetic (NOD) –SCID mice; hence, the terms ‘cancer-initiating’ or ‘tumorigenic’ cells might be more accurate. Since the description of AML stem cells, leukemia- initiating cells have been described in acute lymphoblastic leukemia (ALL) [6] and multiple myeloma [7]. Recently, similar tumorigenic cells have also been identified in breast [8], central nervous system [9], colorectal [10] and pancreatic [11] cancers, although we understand far more of the biology of leukemia stem cells (LSCs) than of their counterparts from solid tumors.
Table 1.
Molecular and phenotypic characteristics of normal stem or progenitors and LSCs
| Sp | Molecular markers | Cell-surface phenotype | |
|---|---|---|---|
| Normal stem or progenitor cells | |||
| LTR-HSC | Hu | ND | Lin−CD34+Thy-1+ [23]; CD33+/− [27] |
| Mo | Activated Notch [43]; activated Hedgehog [42]; activated Wnt [44]; Bmi-1 [45]; HoxB4 [40] | Lin−c-Kit+Sca-1+Thy-1lo [22] | |
| STR-HSC | Hu | ND | |
| Mo | Lin−c-Kit+Sca-1+Thy-1loFlk-2+ [22] | ||
| MPP | Hu | ND | |
| Mo | Lin−c-Kit+Sca-1+Thy-1−Flk-2+ [22] | ||
| CMP | Hu | Lin−CD34+CD38+CD123loCD45RA−FcγR− [99] | |
| Mo | Lin−c-Kit+Sca-1−CD34+FcγRlo [49] | ||
| GMP | Hu | Lin−CD34+CD38+CD123loCD45RA+FcγR− [99] | |
| Mo | Lin−c-Kit+Sca-1−CD34+FcγRhi [49] | ||
| MEP | Hu | Lin−CD34+CD38+CD123−CD45RA−FcγR− [99] | |
| Mo | Lin−c-Kit+Sca-1−CD34−FcγRlo [49] | ||
| Leukemia Stem Cells | |||
| CML CP LSC | Hu | BCR-ABL+ | CD34+CD38_ [33,34] |
| Mo | BCR-ABL+ | Lin−Sca-1+c-Kit+ [55,56]; CD44hi [18] | |
| CML mBC LSC | Hu | BCR-ABL+; β-catenin+ [32] | ND |
| Mo | BCR-ABL+; NUP98-HOXA9+ [65] | Sca-1+CD34+c-KitloFlt3+CD150− [56] | |
| AML LSC | Hu | Activated NF-κB [35]; activated PI3K [70]; activated mTOR [71] | CD34+CD38− [2,20]; CD123+ [26]; CD33+/− [27]; CLL-1+/− [15] |
| Mo | MLL-AF9+ | Lin−c-Kit+CD34+FcγR+ [17,61]; CD11b+ [61] | |
| Mo | CALM-AF10+ | B220+CD11b−Gr-1− [64] | |
| Mo | Over-expressed HoxA9/Meis1; Bmi-1 [51] | ND | |
| Mo | Pten−/−; activated mTOR [16] | Lin−Sca1+c-Kit+Flk-2− [16] | |
Abbreviations: AML, acute myeloid leukemia; CML, chronic myeloid leukemia; CMP, common myeloid progenitor; CP, chronic phase; GMP, granulocyte-macrophage progenitor; HSC, hematopoietic stem cell; Hu, human; Lin, lineage; LTR, long-term repopulating; mBC, myeloid blast crisis; MEP, megakaryocyte-erythroid progenitor; Mo, mouse; MPP, multipotential progenitor; ND, not determined; Sp, species; STR, short-term repopulating.
LSCs are also postulated, but not proven, to be responsible for a familiar and frustrating clinical scenario – the relapse of disease following a remission induced by cytotoxic or targeted therapy. In AML, conventional chemotherapy will produce remission (in which bone-marrow morphology and cytogenetics are normal and blasts comprise <5% of marrow cells) in over 70% of patients, although the majority will relapse within 5 years despite continued treatment [12]. In CML, the breakpoint cluster region (BCR)–Abelson (ABL) tyrosine-kinase inhibitor imatinib mesylate induces hematological and cytogenetic remissions in nearly every patient treated in the early (chronic) phase of the disease, although molecular remissions (defined by the absence of BCR–ABL transcripts detected by reverse-transcriptase PCR) are observed rarely [13] and most patients relapse if imatinib treatment is stopped [14]. Hence, LSC eradication might be necessary to cure leukemia.
There have been several recent and exciting advances in LSC research. New markers for AML stem cells should enable better quantitation of persistent or minimal residual disease (MRD) following therapy [15]. Studies in mouse model systems have revealed the importance of the phosphatidylinositol 3-kinase (PI3K) signaling pathway in LSCs [16] and have shed light on their self-renewal pathways [17]. New approaches to target the engraftment and survival of LSCs specifically with antibodies have been developed [18,19]. This review will have a principal focus on myeloid LSCs. We will review the properties of myeloid LSCs and describe their signaling and self-renewal pathways. We will discuss recent LSC studies in mouse model systems and conclude by considering strategies to eliminate LSCs specifically through the use of drugs and antibodies.
Properties of myeloid LSCs
The development of a xenotransplantation assay for AML stem cells enabled them to be characterized physically. Subsequent work from the Dick laboratory demonstrated that NOD– SCID mice were superior to SCID mice for the engraftment of human AML and also confirmed that LSCs were found exclusively in the immature CD34+CD38– fraction, regardless of the morphological subtype of AML. LSCs proliferate and renew themselves in vivo and generate clonogenic [blast or AML colony-forming units (AML–CFU)] and more differentiated (CD34+CD38+) AML cells, suggesting that AML is organized as a hierarchy originating from a primitive hematopoietic progenitor [20] (Figure 1). Using a lentivirus (which marks cells clonally by provirus integration) to track individual LSCs that were transplanted serially from primary to secondary to tertiary recipient mice, some LSCs that contributed to long-term (i.e. persisting for >12 weeks) leukemogenesis in primary recipients gave only short-term (4–8-week) leukemic repopulation in secondary recipients, whereas some long-term LSCs (‘quiescent’ LSCs) arose only in secondary or tertiary recipients [21]. The normal HSC population contains a similar hierarchy of cells with long- and short-term repopulating potential [22], which suggested that, in AML, the initial target cell for transformation might have originated from within the HSC compartment. However, other studies have demonstrated that, unlike normal HSCs [23], LSCs generally lack expression of Thy-1 (CD90) [24] and c- Kit (CD117) [25] but do express interleukin (IL)-3 receptor α (CD123) [26] (Table 1). In addition, recent studies suggest that LSCs from most AMLs express the early myeloid antigen CD33 [27] and the novel antigen C-type lectin-like molecule-1 (CLL-1) [28]. Although CD33 might also be expressed on some normal HSCs [27], CLL-1 is present exclusively on malignant but not normal CD34+CD38– cells [15]. Hence, there are phenotypic differences between normal HSCs and LSCs in AML, which have important therapeutic implications. The burden of LSCs at the time of diagnosis of AML has prognostic value: AML patients whose blasts at initial diagnosis fail to engraft NOD–SCID mice at high cell doses (suggesting low LSC content) have superior long-term survival [29], whereas a high frequency of phenotypic LSCs at diagnosis correlates with MRD following therapy and with inferior survival [30].
In CML, by contrast, the LSCs appear to be very similar if not identical biologically and functionally to the normal HSCs. In chronic phase CML patients, fluorescence in situ hybridization (FISH) demonstrates the presence of the Philadelphia (Ph) chromosome in nearly all myeloid and some B- and T-lymphoid cells [31], indicating that the cell of origin in CML has multilineage differentiation capacity. BCR–ABL transcripts are detected in CD34+CD38–CD90+ cells purified from CML bone marrow [32]; in normal marrow, the CD34+CD38–CD90+ population contains virtually all the HSC activity [23]. Chronic-phase CML patient cells engraft NOD–SCID mice poorly relative to normal HSCs [33], which has hampered the functional characterization of LSCs from this stage of the disease, however, the LSC activity is found within the CD34+CD38– compartment [34].
In both AML and CML, at least a portion of the LSC population is quiescent and not cycling actively. In AML, quiescent LSCs can be detected in vivo in serially transplanted mice, as described earlier [21]. The vast majority (93–99%) of CD34+CD38– AML cells are in the G0 phase of the cell cycle, as determined by flow cytometric measurement of DNA and RNA content [35,36], and these LSCs are resistant to in vitro treatment with 5-fluorouracil or tritiated thymidine, which kill cells that are progressing actively through the cell cycle, including the majority of AML–CFUs [36,37]. In CML samples, BCR–ABL-expressing CD34+ progenitors in the G0 phase also exist and are capable of engrafting NOD–SCID mice [38]. The quiescent, noncycling state of these LSCs might help to explain their relative resistance to chemotherapy. Both LSCs and normal HSCs also produce high levels of the ATP-binding cassette (ABC) family of drug transporters, including MDR1 (multi-drug resistance-1) and ABCG2, which mediate the active efflux of many cancer drugs and might also contribute to the relative chemoresistance of LSCs [39].
Mechanisms of stem-cell self-renewal
Self-renewal reflects the ability of a stem cell to divide asymmetrically, yielding one daughter cell that can differentiate and another that maintains pluripotent stem-cell function. For both normal HSCs and LSCs, self-renewal is rigorously defined by serial transplantation, enabling the regeneration of cells with the capacity for multi-lineage hematopoietic repopulation (for HSCs) or for the propagation of leukemia (for LSCs). At the molecular level, the mechanisms controlling self-renewal of normal and leukemic stem cells are understood poorly and what little we know is based primarily on studies in mouse model systems (Table 2 and Figure 2). Several genes and signaling pathways have a role in maintaining the ‘stemness’ of normal murine HSCs. Over-expression of the homeodomain genes HoxB4 or HoxA9 in bone marrow causes expansion of functional HSCs in vitro and in vivo [40,41]. Activators of the hedgehog [42], Notch [43] and Wnt [44] signaling pathways also stimulate HSC proliferation in vitro. In the Wnt pathway, stimulation of cells with Wnt ligands leads to stabilization of β-catenin, a protein with dual function as a cytoskeletal component and latent transcription factor, which subsequently translocates to the nucleus and activates specific gene transcription. Overexpression of a stabilized β-catenin mutant in HSCs induces their expansion, whereas expression of the β-catenin antagonist axin impairs HSC proliferation and their repopulating function [44]. Lastly, the polycomb group protein Bmi-1 has been implicated in HSC self-renewal through loss-of-function and over-expression studies [45], possibly acting through modulation of the cell cycle and the apoptosis regulators p16 and ARF.
Table 2.
Molecular markers of normal and malignant hematopoietic cells
| Molecule | Description | Function |
|---|---|---|
| Cell surface markers | ||
| β1 integrin | CD29; adhesion receptor for fibronectin, vascular cell adhesion molecule-1 (VCAM-1) | Required for homing and engraftment of normal HSC; adhesion of AML LSC |
| CD11b | Myeloid-specific αM integrin | Trafficking and adhesion of mature myeloid cells |
| CD33 | Sialoadhesin transmembrane protein | Unknown; cell adhesion molecule |
| CD34 | Sialomucin transmembrane protein | Unknown; cell adhesion molecule |
| CD38 | Transmembrane protein with cyclic ADP-ribose hydrolase ectoenzyme activity | Positive and negative regulator of cell proliferation; cell adhesion molecule |
| CD44 | Receptor for hyaluronan and osteopontin; increased on BCR-ABL+ LSC | Required for homing/engraftment of CML LSC; negative regulator of AML LSC self-renewal |
| CD45RA | B220 (mouse); splice variant of CD45, a transmembrane tyrosine phosphatase | B-lymphoid marker; negative regulator of B cell receptor signaling |
| CD123 | Interleukin-3 receptor a chain | Survival and proliferation signaling in normal cells; no known role in LSC |
| CD150 | SLAM (signaling lymphocyte activation molecule) family | Enhances proliferation and Ig production in activated B cells; role in HSC unknown |
| CLL-1 | C-type lectin-like molecule 1 | Unknown; potential adhesion/signaling molecule |
| c-Kit | CD117; receptor TK for stem cell factor | Survival, proliferation, and differentiation signaling |
| FcγR | Receptor for Fc portion of IgG; expressed on phagocytes and lymphocytes | Mediates phagocytosis; antibody-dependent cell cytotoxicity by T/NK cells |
| Flk-2/Flt3 | Receptor tyrosine kinase for Flt3 ligand; mutated and activated in ~35% of AML | Survival and proliferation signaling |
| Gr-1 | Ly-6; specifically expressed on neutrophils | Unknown; ?neutrophil activation |
| Sca-1 | Stem cell antigen-1; lymphocyte activation protein-6A | Positively regulates HSC self-renewal |
| Thy-1 | CD90; GPI-linked Ig superfamily member | Unknown |
| Intracellular markers | ||
| β-catenin | Dual function as adherens junction protein and latent transcription factor in Wnt pathway | Active/nuclear in CML blast crisis LSC; proliferation and ?self-renewal signaling |
| Bmi-1 | Polycomb group transcription factor | Regulates self-renewal of HSC and LSC through repression of p16 and ARF |
| Hedgehog | Ligand for Patched, a transmembrane receptor | Proliferation signaling in normal HSC |
| HoxA9 | Homeodomain transcription factor | Proliferation of HSC; over-expression in AML |
| HoxB4 | Homeodomain transcription factor | Proliferation of HSC |
| mTOR | Mammalian target of rapamycin; serine-threonine kinase | Regulates cell metabolic and protein synthesis responses; survival and proliferation in LSC |
| NF-κB | Nuclear factor-kappa B; transcription factor activated by inflammation and lymphoid development | Active in LSC but not HSC; role in LSC unknown but ?survival factor |
| Notch | Transmembrane receptor/transcription factor activated by proteolysis following ligand (Jagged) binding | Proliferation signaling in normal HSC and many T-cell leukemias |
| PTEN | Phosphatase and Tensin homolog; lipid phosphatase | Antagonist of PI3K; regulates HSC self-renewal; leukemia suppressor |
| PI3K | Phosphatidylinositol 3-kinase; lipid kinase | Activated in AML LSC; proliferation and survival signaling |
| Wnt | Ligand for Frizzled receptor, mediates stabilization and activation of β-catenin | Proliferation signaling in normal HSC |
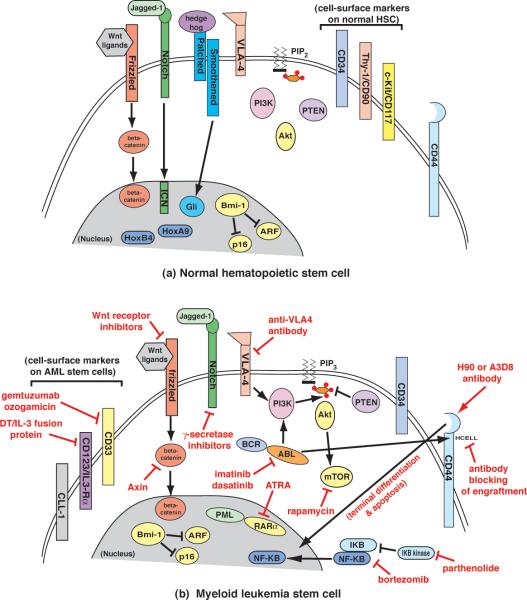
Figure 2.
Cell-surface markers and therapeutic targets of myeloid leukemia stem cells. (a) Diagram of a normal hematopoietic stem cell (HSC), which expresses the cell surface markers CD34, CD90 and CD117 (right) [23]. Some of the signaling pathways known to promote expansion and self-renewal of HSCs include WNT–frizzled–b-catenin [44], Jagged-Notch [43], Hedgehog–Patched-–Smoothened–Gli [42] and HoxA9/HoxB4 [40,41]. The polycomb group protein Bmi-1 promotes HSC maintenance through inhibition of the cell cycle and apoptosis regulators p16 and ARF [45]. (b) Diagram of a hypothetical myeloid leukemia stem cell (LSC), some of which can express the cellsurface markers CD33 [27], CD123 [26] and CLL-1 [28] (left). Potential agents and strategies for the eradication of LSCs are depicted in red and include antibodies and fusion proteins as well as small-molecule inhibitors. For details, see text.
Origins of myeloid leukemias
It is important to recognize that the cell of origin of AML (defined here as the cell in which the first leukemia-specific genetic event, such as a chromosomal translocation, has occurred) might or might not be the same as the LSCs that are present once that AML is established. Several chimeric transcription factors produced by chromosomal translocations in human AML, including NUP98 (nucleoporin 98)–HOXA9 (homeobox A9), MOZ (monocytic leukemia zinc-finger)–TIF2 (transcription intermediary factor-2) and fusions involving the MLL (mixed lineage leukemia) protein products [MLL–ELL (eleven-nineteen lysine-rich leukemia), MLL–ENL (eleven-nineteen leukemia), MLL–AF9 (ALL fused gene from chromosome 9)], have transforming and leukemogenic activity when expressed in primary mouse bone-marrow cells by retroviral transduction. These oncogenic transcription factors can immortalize murine myeloid progenitors in serial replating assays in vitro [46] and the transduced cells can induce AML-like leukemia on transplantation into irradiated recipient mice, providing a powerful assay for analyzing the leukemia-initiating cells and their signaling pathways [47].
When purified HSCs, common myeloid progenitors (CMPs) or granulocyte–macrophage progenitors (GMPs) are transduced with MLL–ENL retrovirus, the progenitors are immortalized in vitro and induce similar phenotypically and transplantable AMLs in recipient mice [48], despite the fact that normal CMPs and GMPs lack self-renewal capacity [49]. By contrast, neither common lymphoid progenitors nor restricted megakaryocyte–erythroid progenitors could induce AML following transduction with MLL–ENL [48]. This leukemogenic transcription factor can therefore induce self-renewal capacity in myeloid progenitors with granulocyte– macrophage differentiation potential, which suggests that human AML might also arise from committed myeloid progenitors. Similar observations have been made with MOZ–TIF2 [50] and MLL– AF9 [17]. As with normal HSCs, Bmi-1 expression might be required within LSCs for the self-renewal of AMLs induced by dysregulated transcription factors, which have been assessed by serial transplantation [51]. These studies provide convincing evidence that AML can be initiated from mutations that arise either in committed myeloid progenitors or in HSCs and also suggest that the cell of origin of human AMLs might be similarly promiscuous. However, not all AML-associated transcription factors appear capable of inducing self-renewal: AML1–ETO, the product of the t(8;21) translocation in M2 AML, is found in phenotypically normal HSCs in some patients in remission [52] and does not confer efficient secondary transplantation when expressed in hematopoietic progenitors [53].
In a similar mouse model of CML, transduction of marrow with retrovirus expressing BCR–ABL followed by transplantation into irradiated recipients induces CML-like myeloproliferative disease (MPD) in all recipients. The murine disease is similar to human CML: the leukemia-initiating cells have multi-lineage repopulating ability [54] and the leukemia is transplantable by transfer of Lin–Kit+Sca1+ cells to secondary recipients [55,56]. Transgenic expression of BCR–ABL in CMPs and their differentiated progeny is sufficient to induce MPD [57], whereas transduction of purified CMPs or GMPs with BCR–ABL retrovirus does not yield sustained MPD in transplant recipients [50]. In a similar vein, the CML-like MPD that develops in mice with hematopoietic deficiency of JunB can be transferred to recipient mice only by the transplantation of phenotypic HSCs and not by committed myeloid progenitors [58]. Collectively, these observations suggest that hematopoietic mutations that cause myeloproliferative phenotypes (overproduction of maturing myelo-erythroid cells with normal differentiation), in particular those that produce dysregulated tyrosine kinases, do not activate self-renewal pathways in committed progenitors. This, in turn, helps to explain an old paradox in MPD. Clonality studies in CML, polycythemia vera (PV) and essential thrombocythemia (ET) indicate that the cell of origin is always a pluripotent HSC [59], however, each MPD predominantly affects only a single myeloid lineage (neutrophils in CML, red cells in PV and platelets in ET). Our current knowledge suggests that MPD-causative mutations might be found exclusively in self-renewing HSCs because they cannot be sustained if they occur in committed progenitors that lack self-renewal capacity.
When CML progresses from chronic phase to myeloid blast crisis, a condition resembling AML, the situation might be different. Relative to normal or chronic-phase CML marrow, the phenotypic GMP compartment in patients with CML myeloid-blast crisis was expanded and expressed transcriptionally active nuclear β-catenin aberrantly [32]. Leukemic GMP from CML blast-crisis patients also exhibited self-renewal capacity (in a replating assay) that was reduced by enforced expression of axin, an inhibitor of β-catenin signaling [32]. These observations suggest that activation of β-catenin in CML GMP might confer self-renewal potential and contribute to disease progression, although the existence of bona fide LSCs in the GMP compartment of these patients (by xenotransplantation) has not yet been documented. The mechanism of β-catenin stabilization and activation in CML blast crisis is not clear, although direct tyrosyl phosphorylation of β-catenin by BCR–ABL might have a role [60].
Characterization of LSCs in murine myeloid leukemia models
Mouse retroviral models are also useful for studying the properties of the LSCs that are present in established murine AML that is induced by these mutant transcription factors. These myeloid leukemias express the myeloid integrin CD11b predominantly and have low levels of the granulocyte marker Gr-1, with variable expression of c-Kit (the tyrosine-kinase receptor for stem-cell factor) [48,50]. In AML induced by MLL–AF9, limiting-dilution secondary-transplantation analysis estimates the frequency of LSCs in leukemic spleen or bone marrow to be 0.6–0.8% [17,61]. However, AML can also be induced efficiently in secondary recipients of single blast colonies grown from the marrow or spleen of primary leukemic mice and the frequency of these clonogenic cells is considerably higher (7–30%) [17,61]. This suggests that limiting- dilution transplantation might underestimate the actual LSC frequency, perhaps owing to inefficient homing and engraftment of single LSCs [61]. It should also be recognized that the frequency of LSCs in some hematological malignancies might be considerably higher than in AML. For example, nearly every malignant cell in mice with established B-lymphoma induced by a MYC or NRAS transgene was capable of transferring disease to non-irradiated congenic recipients [62].
The immunophenotype of LSCs induced by MLL–AF9 is similar to normal GMPs, which lack lineage markers and express c-Kit, the stem or progenitor marker CD34 and the Fc receptor for IgG (Lin–Kit+Sca1+FcγRII/III+) [17], except that the LSCs also express CD11b (Mac-1) [61], placing them somewhat downstream of the GMP compartment. Expression of more differentiated myeloid antigens (CD34, FcγRII/III and CD11b) by these murine LSCs is in contrast to human AML, in which the LSCs are CD34+CD38– (and in which CD34 is a marker of stem cells and not of myeloid progenitors) [20]. However, this might be characteristic of AML expressing MLL–AF9 because CD34– cells from patients with AML–M5 and t(9;11) (the chromosomal translocation that generates MLL–AF9) were able to engraft NOD–SCID mice [24,25]. Experiments with additional transcription-factor oncogenes are needed to determine if these findings can be generalized to other molecular classes of AML. Quantitative transcriptional profiling of LSCs in both studies indicated that the LSC population had reactivated a set of genes expressed at high levels in HSCs, including multiple HoxA cluster genes, the transcription factor genes Meis1 and Mef2c and the gene for the Slam-family cell-surface protein CD48 [17,61]. Interestingly, HoxA genes are required for the induction of AML by MLL-fusion proteins [63] and shRNA knockdown of Mef2c impairs leukemogenesis by clonogenic MLL–AF9+ cells [17]. Hence, transcriptional profiling of LSCs might provide insights into pathways of LSC self-renewal that can be mined for potential therapeutic targets.
In contrast to MLL fusions, in murine AML induced by a CALM (clathrin assembly lymphoid myeloid leukemia)– AF10 (ALL fused gene from chromosome 10) fusion transcription factor, the LSCs predominantly had the phenotype of early B-lymphoid progenitors (B220+CD11b–Gr-1–) with clonal immunoglobulin heavy-chain gene rearrangements, whereas the bulk of the leukemic cells expressed CD11b and Gr-1 with or without B220 [64]. Similar CALM–AF10+ B-lymphoid progenitors were identified in several patients with CALM–AF10-associated AML, although these cells were not assessed for LSC activity by xenotransplantation. These observations suggest that a transformed progenitor with B-lymphoid characteristics can propagate CALM–AF10+ AML, emphasizing the potential LSC diversity that might be present in human AML.
Myeloid blast crisis of CML can be modeled in mice by co-transduction of progenitors with retroviruses expressing BCR–ABL and a mutant transcription factor, such as NUP98– HOXA9 [65], providing a promising new model for the analysis of blast-crisis stem cells [56]. The LSCs in this disease are predominantly Sca-1+CD34+c-Kitlo and express the Flt3 receptor but lack expression of the SLAM (signaling lymphocytic activation molecule)-family member CD150 [56]. Although these LSCs are sensitive to imatinib in vitro [65], in vivo they appear to be relatively resistant to either imatinib or ionizing radiation [56], in agreement with the high rate of relapse of CML blast-crisis patients treated with kinase inhibitors [66].
Targeting LSCs with drugs
One approach to eliminating LSCs is to target pathways regulating stem-cell self-renewal. For example, inhibitors of Wnt signaling might be beneficial in CML myeloid blast crisis [32]. Approximately half of human T-cell ALLs (TALLs) have activating mutations in Notch1 and treatment with a γ-secretase inhibitor, which blocks ligand-induced Notch proteolysis and signaling, induces growth arrest and apoptosis of T-ALL cells [67], although effects on LSCs have not been assessed. However, treatments directed at self-renewal pathways (such as Wnt and Notch) that are shared between normal and leukemic stem cells might have unacceptable toxicity to normal HSCs, particularly when combined with cytotoxic chemotherapy. Our increasing understanding of differences between normal HSCs and LSCs suggests the exciting possibility of selectively impairing the proliferation, survival or self-renewal of LSCs with targeted drugs, while sparing normal HSCs.
One plausible molecular target in LSCs is NF-κB, a transcription factor normally activated by inflammatory stimuli and during lymphoid development, which is active constitutively in most AML LSCs but not in normal, non-stimulated hematopoietic progenitors [35]. The proteasome- inhibitor MG-132, which inhibits NF-κB activation through stabilization of its cellular inhibitor IκB, induced apoptosis in CD34+CD38– AML cells while sparing normal primitive progenitors [35,68]. Phase I/II trials of the role of a US FDA-approved proteasome inhibitor, bortezomib, in AML induction and maintenance therapy are in progress. Another approach to blocking NF-κB is through inhibition of IκB kinase (IKK), which phosphorylates and inactivates IκB. Parthenolide, a novel sesquiterpene lactone natural product with IKK-inhibitory activity, induces selective apoptosis in AML stem cells coincident with NF-κB inhibition, p53 activation and reactive oxygen species induction [69], although its precise mechanism of action is unclear. Studies with more specific and potent IKK inhibitors in AML consolidation and maintenance therapy are warranted.
Another attractive therapeutic target in AML stem cells is the PI3K–Akt–mammalian target of rapamycin (mTOR) pathway. The kinases Akt and mTOR are activated in human AML blasts and treatment with inhibitors of PI3K or mTOR, in combination with cytarabine or etoposide, induced apoptosis in AML cells and decreased the abundance of LSCs [70,71]. Recent studies in mouse models also implicate mTOR as an attractive drug target for the selective elimination of LSCs. Conditional hematopoietic deletion of phosphatase and tensin homolog (PTEN), a lipid phosphatase that regulates PI3K signaling negatively, causes a MPD-like syndrome in mice, characterized by initial proliferation and expansion of HSCs followed by HSC depletion through decreased self-renewal [16]. Similar to CML, the MPD in mice with hematopoietic PTEN deficiency progresses to transplantable AML or ALL within 6 weeks. In leukemic mice, LSCs were rare in total marrow but enriched greatly in a population that expressed HSC markers (Lin–Sca1+c-Kit+Flk-2–). Treatment of mice with rapamycin, an mTOR inhibitor, prevented the development of leukemia if treatment was initiated before PTEN deletion. In mice with established leukemia, rapamycin prolonged survival while reducing leukemic-cell proliferation and impairing the efficiency of transplantation of leukemia to secondary recipients. Although the effect of rapamycin on LSC frequency was not measured directly, these results suggest that rapamycin can eliminate PTEN-deficient LSCs [16]. By contrast, rapamycin had the opposite effect on non-leukemic HSCs that are present immediately following PTEN loss, reducing their proliferation but enhancing their self-renewal capacity [16]. Rapamycin and other mTOR inhibitors are also in clinical trials in leukemia.
In acute promyelocytic leukemia (APL), the addition of all-trans retinoic acid (ATRA) or arsenic trioxide to anthracycline chemotherapy has increased dramatically the frequency of complete remission and cure [72]. This implies that these agents, which target the oncogenic transcription factor PML (promyelocytic leukemia)–RARα (retinoic acid receptor-α) specifically, must contribute to the elimination of the LSCs. However, APL cells are typically unable to engraft NOD–SCID mice [20], so a direct assessment of the frequency and phenotype of LSCs in APL has not been possible [73]. Correlative studies suggest that the effect of the two agents on primitive APL cells is distinct, with ATRA inducing proliferation and differentiation and arsenic inducing growth arrest and apoptosis [74].
In CML, the fact that most patients treated with imatinib have residual disease detected by RT–PCR and FISH [13,75] suggests that ABL kinase inhibitors might not eradicate all CML stem cells. Quiescent CML stem cells are resistant to killing by imatinib in vitro [76,77], providing a potential explanation for disease persistence in imatinib-treated patients. The resistant LSCs have a single BCR–ABL gene by FISH analysis but produce high levels of BCR– ABL and its phosphorylated substrate pCrkL, whereas BCR–ABL kinase-domain mutations associated with imatinib resistance are not detected [78]. Dasatinib (which is ~300 times more potent for inhibition of BCR–ABL than imatinib) decreased pCrkL levels in quiescent CML stem cells whereas imatinib did not; however, neither drug eliminated the quiescent LSC population [78]. Similar results were obtained with nilotinib, a more potent version of imatinib [79]. Together, these studies suggest that CML stem cells are resistant to kinase inhibitors because they do not require BCR–ABL signaling for survival in the quiescent state. Indeed, these quiescent CML progenitors are resistant to multiple drugs used alone or in combination with imatinib [80,81], including cytarabine, etoposide, PI3K inhibitors, heat-shock protein (HSP)90 antagonists and arsenic, all of which induce apoptosis in cycling leukemia cells. Only the combination of lonafarnib (a farnesyltransferase inhibitor) and imatinib reduced significantly but did not eliminate the quiescent CML LSC population [80]. This suggests that, if quiescent CML stem cells could be induced to enter the cell cycle, this might increase their sensitivity to BCR– ABL kinase inhibitors. In agreement with this, two recent studies have shown that pretreatment of CD34+ CML progenitors with myeloid cytokines (granulocyte colony-stimulating factor or granulocyte–macrophage colony-stimulating factor) decreases the quiescent CML LSC fraction and promotes their elimination by imatinib [82,83]. These results should motivate clinical trials that test the effect of intermittent treatment with myeloid cytokines and imatinib on residual disease in CML.
Targeting LSCs with antibodies
An alternative strategy to eliminate LSCs is to target cell-surface molecules expressed on LSCs using antibodies or other proteins. A crucial concern with this approach is the specificity of expression of the molecule to be targeted – ideally, it would be expressed on LSCs and perhaps also differentiated normal hematopoietic cells but not on normal HSCs. CD33, a sialylated adhesion protein found on the surface of normal immature myelomonocytic cells and on many AML blasts, is also present on LSCs from some AMLs [27]. Gemtuzumab ozogamicin (Mylotarg®), a recombinant humanized anti-CD33 monoclonal antibody conjugated with the cytotoxic antibiotic calicheamicin, induces remissions in relapsed CD33+ AML and can eliminate MRD in APL [84]. Although it has not been studied directly, it is likely that some of the therapeutic activity of gemtuzumab is a result of direct killing of CD33+ AML stem cells [85]. This, in turn, might help to explain the efficacy of gemtuzumab in AML patients in whom the blasts are CD33– predominantly [86]. However, prolonged cytopenias (low blood counts) have been observed in some AML patients treated with gemtuzumab, which might reflect CD33 expression by some normal HSCs [27]. An ongoing randomized clinical trial is testing the effect of gemtuzumab treatment of AML patients in remission before autologous stem-cell harvest and transplantation.
Another rational cell-surface target on AML stem cells is CD123, the receptor for IL-3 [26]. A diptheria toxin–IL-3 fusion protein was toxic selectively to AML versus normal hematopoietic progenitors in vitro and also decreased the efficiency of AML engraftment of NOD–SCID mice, indicative of an effect on LSCs. In combination with the chemotherapeutic agent cytarabine, the fusion protein was also effective in reducing the leukemic burden of NOD– SCID mice engrafted with human AML [87].
VLA-4 (α4β1 integrin) is expressed on the cell surface of a subset of human AMLs, where it mediates adhesive interactions of leukemic cells with fibronectin and vascular- cell adhesion molecule-1 within the bone marrow niche. VLA-4+ AML has a higher relapse rate following induction chemotherapy than does VLA-4– AML [88], suggesting that VLA-4 expression might insulate LSCs from cytotoxic chemotherapy. Binding of VLA-4+ AML blasts to fibronectin triggers PI3K–Akt signaling and protects these blasts from chemotherapy-induced apoptosis in vitro, whereas the combination of anti-VLA-4 antibody and cytarabine can eradicate residual AML in xenografted mice [88]. Addition of anti-VLA-4 antibodies to standard AML consolidation therapy might help to eliminate LSCs and lead to lower relapse rates in VLA-4+ AML.
CLL-1 (C-type lectin-like molecule-1), an adhesion molecule of unknown function, is expressed preferentially on many LSCs [15]. In normal marrow, CLL-1 is expressed on CD38+ myeloid progenitors but not on CD34+CD38– cells, making it an attractive therapeutic target. Although antibody to CLL-1 apparently does not block engraftment or impair viability of LSCs, at least some anti-CLL-1 antibodies are internalized following cell-surface binding [89], raising the possibility that they could be coupled to toxins like calicheamicin to produce a cytotoxic response.
Yet another adhesion molecule, CD44, has emerged recently as a promising target that is over-expressed on both AML and CML stems cells relative to normal HSCs [19]. CD44 is a widely expressed transmembrane glycoprotein that exists in more than 20 different isoforms, binds to hyaluronan, selectins and osteopontin and has pleiotropic functions in organogenesis, cell homing and migration as well as tumor cell metastasis [90]. Certain anti-CD44 monoclonal antibodies (clones H90 and A3D8) can trigger terminal differentiation and apoptosis of AML blasts [91], particularly the M1 to M5 subtypes (Box 1). A recent study demonstrated that the H90 anti-CD44 antibody also targets LSCs in human AML [19]. Injection of H90 into NOD– SCID mice engrafted with human AML reduced the leukemic burden and abolished secondary transplantation of leukemia, indicative of LSC elimination, whereas H90 had no effect on the engraftment of normal human marrow or cord blood. Part of the effect of H90 on LSCs was mediated by the disruption of LSC–niche interactions in primary or secondary recipients, although there was also evidence of LSC differentiation and of loss of LSC self-renewal capacity [19].
In a complementary study, CD44 was found to be required for homing and engraftment of LSCs in the mouse retroviral transplant model of CML [18]. BCR–ABL induces defects in β1 integrin function, which are required for homing and engraftment of normal HSCs [92]. CML stem cells rely instead on selectins and their ligands for efficient engraftment [93] and CD44 contributes selectin counter-receptors on CML stem cells through a specialized glycoform known as hematopoietic E- and L-selectin ligand (HCELL) [94]. In transplants with CD44-deficient mice, CD44 was required specifically on donor BCR–ABL+ cells for efficient homing and engraftment of cells initiating CML-like leukemia but not for engraftment of normal HSCs or of lymphoid progenitors that initiate BCR– ABL+ B-cell ALL. Engraftment and leukemogenesis were restored by direct intrafemoral transplantation of CD44-deficient LSCs or by ectopic expression of human CD44 in the LSCs, whereas in vitro treatment with a different CD44 antibody blocked engraftment of CD44+ LSCs partially [18]. Whereas CML stem cells can engraft and contribute to leukemic relapse in patients treated by autologous HSC transplantation [95], these results suggest that blocking CD44 or HCELL might interfere preferentially with homing and engraftment of CML stem cells without affecting repopulation by normal HSCs.
Summary and future directions
The era of targeted therapy for leukemia and other cancers has arrived. The targeted eradication of leukemia stem cells, which might be essential for permanent cure, is a much more challenging task (Box 2). Owing to the efforts of many different laboratories, we are beginning to understand the properties of LSCs and how they differ from normal HSCs. Going forward, we should expect biochemical and genetic studies of LSCs derived from human samples and mouse models to contribute additional rational targets for specific therapy towards their elimination. We should appreciate that the LSCs from different blood cancers might be different in their phenotypic properties and self-renewal pathways and these will need to be defined for each disease. There will be a major focus on discovering genetic lesions in LSCs that render them uniquely sensitive to inhibition of other pathways, leading to ‘synthetic lethality’. This concept is illustrated by the selective response of PTEN-deficient AML to rapamycin [16] and the profound sensitivity of BRCA-1/ 2-mutant breast cancers to PARP (poly ADP-ribose polymerase) inhibition [96]. Because of the close similarities between normal stem cells and LSCs, we should not expect too many of these ‘magic bullets’ and, when LSC-specific therapy finds it way to the clinic, it is probable that a careful balance of targeted and cytotoxic therapies will be necessary to maximize LSC killing and minimize toxicity. This, in turn, will require robust methods to monitor LSC frequency and MRD in clinical samples and the identification of leukemic progenitors in AML [28] and ALL [97] by flow cytometry is an important step forward. Finally, we should not forget that the immune system is responsible for the eradication of residual LSCs in patients treated by allogeneic HSC transplantation and probably also in those whom are ‘cured’ by conventional chemotherapy as well. Although outside the scope of this review, immunotherapeutic strategies to eliminate LSCs will probably become an important part of the post-remission care of patients with leukemia [98].
Box 1. Types of leukemia and their treatment.
Acute myeloid leukemias (AMLs)
Classification: old French American British (FAB) classification – M0 (undifferentiated), M1 (myeloblastic, without maturation), M2 (myeloblastic, with maturation), M3 (promyelocytic), M4 (myelomonocytic), M5a (monoblastic), M5a (monocytic), M6 (erythrocytic), M7 (megakaryoblastic)
Immunophenotype: generally CD13+, CD33+, CD15+, myeloperoxidase (MPO) +, CD117+
Cytogenetic and molecular characteristics:
M2 and others: t(8;21)(q22;q22); AML1–ETO fusion
M4 eo: inv(16) or t(16;16)(p13;22); CBFβ–MYH11 fusion
M3: t(15;17)(q22;q12); PML–RARα fusion (and other variant 17q12 translocations)
M4/M5 and others: 11q23 (MLL) abnormalities
M0–M5: activating mutations in FLT3, a receptor tyrosine kinase
Treatment: cytarabine or daunorubicin, all-trans retinoic acid (ATRA) in M3, hematopoietic stem-cell transplantation, gemtuzumab ozogamicin (Mylotarg®), FLT3 kinase inhibitors
Clinical challenges and problems: multi-drug resistance, relapse of leukemia following remission
Chronic myeloid leukemia (CML)
Classification: chronic phase (CP), accelerated phase (AP), blast crisis (BC)
Cytogenetic and molecular characteristics: t(9;22)(q34;q11); BCR– ABL fusion
Treatment: ABL kinase inhibitors (imatinib (Gleevec®), dasatinib, nilotinib); interferon-α; hematopoietic stem-cell transplantation
Clinical challenges and problems: acquired resistance to kinaseinhibitor therapy, lack of molecular remissions (imatinib does not target quiescent leukemic stem cells harboring BCR–ABL)
Acute lymphoblastic leukemias (ALL)
Classification: FAB classification– small uniform cells (L1), large varied cells (L2), large varied cells with vacuoles (L3)
Immunophenotype: generally CD19+, CD10+, CD20+/–, terminaldeoxynucleotidyl transferase (TdT)+
Cytogenetic and molecular characteristics:
t(9;22) (q34;q11.2); BCR–ABL fusion
t(v;11q23); MLL fusions
t(12;21) (p13;q22); TEL–AML1 fusion
t(1;19)(q23;p13.3); E2A–PBX1 fusion
Treatment: cytarabine/daunorubicin/corticosteroids, hematopoietic stem-cell transplantation
Clinical challenges and problems: multi-drug resistance, relapse of leukemia following remission
Chronic lymphocytic leukemia (CLL)
Classification: B-lymphoid (majority), T-lymphoid (uncommon)
Immunophenotype: B-lymphoid – CD19+, CD20+, CD23+, CD43+, CD5+, monotypic light-chain expression; T-lymphoid – CD2+, CD3+, CD5+, CD7+, variable expression of CD4 and/or CD8
Cytogenetic and molecular characteristics: del(13q14); del(11q); del(17p); CD38 over-expression
Treatment: chemotherapy (chlorambucil, fludarabine), rituximab, alemtuzumab
Clinical challenges and problems: eventual disease progression that is refractory to therapy
Box 2. Outstanding issues.
It is postulated, but not proven, that leukemia stem cells (LSCs) are responsible for the persistence and relapse of leukemia following cytotoxic or targeted therapy. As a corollary, eradication of LSCs might be necessary to cure leukemia. To achieve this in the clinic, progress must be made in several areas.
The cell-surface immunophenotype of LSCs must be further defined by xenotransplantation assays. Of particular importance are markers expressed specifically on LSCs and not on normal HSCs.
The signaling pathways underlying the survival, cell-cycle control and self-renewal ability of LSCs and how they differ from normal HSCs should be better understood
Targeted therapies against these pathways must be developed. These could include small molecules and biologicals (antibodies and fusion proteins). High-throughput drug screening and chemical genetic approaches might be indicated.
We need facile methods to quantify LSCs in clinical samples (e.g. flow cytometry) that do not rely on limiting-dilution xenotransplantation.
Acknowledgements
Supported by NIH grants CA090576 and CA105043, and by a SCOR grant from the Leukemia and Lymphoma Society. We thank Craig Jordan and Mike Cleary for critically reading the manuscript. We apologize that, owing to space constraints, not all the relevant publications in each area could be cited.
Glossary
- Acute myeloid leukemia (AML)
a clonal hematopoietic malignancy characterized by overproduction of immature myeloid cells (blasts) with a block in differentiation.
- Blast
the malignant cell in acute leukemia, characterized by a non-terminally differentiated hematopoietic cell.
- Cancer stem cell
rare cells present in tumors that are defined functionally by their ability to transfer the malignancy on xenotransplantation into an immunodeficient recipient animal (usually NOD-SCID mice). Synonyms include cancer-initiating cells and tumorigenic cells.
- Cancer stem-cell hypothesis
this states that tumors are maintained in their host by a small population of stem-like cancer cells that have the capacity for indefinite self-renewal. Cancer must either originate from cells, such as normal tissue stem cells, that already possess the capacity for self-renewal or else self-renewal must be acquired aberrantly during tumorigenesis.
- Cell of origin of leukemia
defined as the cell in which the first leukemia-specific genetic event, such as a chromosomal translocation or mutation, has occurred. The cell of origin of a leukemia might be different from the leukemic stem cell that is present once the leukemia is established because the phenotype and properties might change during leukemogenesis.
- Chronic myeloid leukemia (CML)
a myeloproliferative disease characterized by clonal overproduction of maturing myeloid cells and disease progression towards blast crisis, a condition resembling AML. CML is caused by the product of the Philadelphia chromosome, the BCR–ABL fusion tyrosine kinase.
- Committed myeloid progenitor
a hematopoietic cell that has the capacity to differentiate into one or more myeloid cell lineages based on colony-forming assays and transplantation but has limited self-renewal capacity.
- Leukemia stem cell (LSC)
cancer stem cells were first identified in AML and designated as leukemia-initiating cells or leukemia stem cells.
- Minimal residual disease (MRD)
evidence for the presence of residual malignant cells, even when so few cancer cells are present that they cannot be detected by histopathology or cytogenetics. Some methods for detecting MRD include flow cytometry and reverse-transcriptase PCR for fusion transcripts.
- Molecular remission
the absence of leukemia-specific fusion transcripts detected by reverse-transcriptase PCR.
- Myeloproliferative disease (MPD)
a group of clonal hematopoietic diseases originating in multipotent hematopoietic stem/progenitor cells that is characterized by overproduction of mature myeloid cells of particular lineages. MPDs include CML (overproduction of neutrophils), polycythemia vera [(PV) overproduction of erythrocytes], essential thrombocythemia [(ET) overproduction of platelets] and primary myelofibrosis (marrow fibrosis with extramedullary hematopoiesis).
- NOD–SCID mice
non-obese diabetic–severe combined immunodeficiency mice. These mice lack mature B and T lymphocytes and functional natural killer cells and will accept hematopoietic cell grafts from humans.
- Self-renewal
self-renewal reflects the ability of a stem cell to divide asymmetrically, yielding one daughter cell that can differentiate and another that maintains pluripotent stem-cell function. For both normal HSCs and LSCs, self-renewal is defined rigorously by serial transplantation, enabling regeneration of cells with the ability for multi-lineage hematopoietic repopulation (for HSCs) or propagation of leukemia (for LSCs). It is distinct from proliferation, which might generate daughter cells that both lack pluripotency.
- Xenotransplantation
transplantation of human hematopoietic cells intoimmunodeficient mice (such as NOD–SCID mice).
References
- 1.Bruce WR, Van Der Gaag H. A quantitative assay for the number of murine lymphoma cells capable of proliferation in vivo. Nature. 1963;199:79–80. doi: 10.1038/199079a0. [DOI] [PubMed] [Google Scholar]
- 2.Lapidot T, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 3.Reya T, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 4.Pardal R, et al. Applying the principles of stem-cell biology to cancer. Nat. Rev. Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 5.Passegue E, et al. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc. Natl. Acad. Sci. U. S. A. 2003;100(Suppl. 1):11842–11849. doi: 10.1073/pnas.2034201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castor A, et al. Distinct patterns of hematopoietic stem cell involvement in acute lymphoblastic leukemia. Nat. Med. 2005;11:630–637. doi: 10.1038/nm1253. [DOI] [PubMed] [Google Scholar]
- 7.Matsui W, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332–2336. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Hajj M, et al. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh SK, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 10.O'Brien CA, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 11.Li C, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 12.Appelbaum FR, et al. Acute myeloid leukemia. Hematology Am. Soc. Hematol. Educ. Program. 2001:62–86. doi: 10.1182/asheducation-2001.1.62. [DOI] [PubMed] [Google Scholar]
- 13.Hughes TP, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N. Engl. J. Med. 2003;349:1423–1432. doi: 10.1056/NEJMoa030513. [DOI] [PubMed] [Google Scholar]
- 14.Cortes J, et al. Discontinuation of imatinib therapy after achieving a molecular response. Blood. 2004;104:2204–2205. doi: 10.1182/blood-2004-04-1335. [DOI] [PubMed] [Google Scholar]
- 15.van Rhenen A, et al. The novel AML stem cell associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood. 2007;110:2659–2666. doi: 10.1182/blood-2007-03-083048. [DOI] [PubMed] [Google Scholar]
- 16.Yilmaz OH, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 17.Krivtsov AV, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- 18.Krause DS, et al. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat. Med. 2006;12:1175–1180. doi: 10.1038/nm1489. [DOI] [PubMed] [Google Scholar]
- 19.Jin L, et al. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat. Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 20.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 21.Hope KJ, et al. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat. Immunol. 2004;5:738–743. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- 22.Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 23.Baum CM, et al. Isolation of a candidate human hematopoietic stem-cell population. Proc. Natl. Acad. Sci. U. S. A. 1992;89:2804–2808. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blair A, et al. Lack of expression of Thy-1 (CD90) on acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo. Blood. 1997;89:3104–3112. [PubMed] [Google Scholar]
- 25.Blair A, Sutherland HJ. Primitive acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo lack surface expression of c-kit (CD117). Exp. Hematol. 2000;28:660–671. doi: 10.1016/s0301-472x(00)00155-7. [DOI] [PubMed] [Google Scholar]
- 26.Jordan CT, et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14:1777–1784. doi: 10.1038/sj.leu.2401903. [DOI] [PubMed] [Google Scholar]
- 27.Taussig DC, et al. Hematopoietic stem cells express multiple myeloid markers: implications for the origin and targeted therapy of acute myeloid leukemia. Blood. 2005;106:4086–4092. doi: 10.1182/blood-2005-03-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Rhenen A, et al. Aberrant marker expression patterns on the CD34+CD38– stem cell compartment in acute myeloid leukemia allows to distinguish the malignant from the normal stem cell compartment both at diagnosis and in remission. Leukemia. 2007;21:1700–1707. doi: 10.1038/sj.leu.2404754. [DOI] [PubMed] [Google Scholar]
- 29.Pearce DJ, et al. AML engraftment in the NOD/SCID assay reflects the outcome of AML: implications for our understanding of the heterogeneity of AML. Blood. 2006;107:1166–1173. doi: 10.1182/blood-2005-06-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Rhenen A, et al. High stem cell frequency in acute myeloid leukemia at diagnosis predicts high minimal residual disease and poor survival. Clin. Cancer Res. 2005;11:6520–6527. doi: 10.1158/1078-0432.CCR-05-0468. [DOI] [PubMed] [Google Scholar]
- 31.Haferlach T, et al. Which compartments are involved in Philadelphia-chromosome positive chronic myeloid leukemia? An answer at the single cell level by combining May-Grünwald-Giemsa staining and fluorescence in situ hybridization techniques. Br. J. Haematol. 1997;97:99–106. doi: 10.1046/j.1365-2141.1997.9662656.x. [DOI] [PubMed] [Google Scholar]
- 32.Jamieson CHM, et al. Granulocyte–macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N. Engl. J. Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 33.Wang JCY, et al. High level engraftment of NOD/SCID mice by primitive normal and leukemic hematopoietic cells from patients with chronic myeloid leukemia in chronic phase. Blood. 1998;91:2406–2414. [PubMed] [Google Scholar]
- 34.Eisterer W, et al. Different subsets of primary chronic myeloid leukemia stem cells engraft immunodeficient mice and produce a model of the human disease. Leukemia. 2005;19:435–441. doi: 10.1038/sj.leu.2403649. [DOI] [PubMed] [Google Scholar]
- 35.Guzman ML, et al. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–2317. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 36.Guan Y, et al. Detection, isolation, and stimulation of quiescent primitive leukemic progenitor cells from patients with acute myeloid leukemia (AML). Blood. 2003;101:3142–3149. doi: 10.1182/blood-2002-10-3062. [DOI] [PubMed] [Google Scholar]
- 37.Terpstra W, et al. Fluorouracil selectively spares acute myeloid leukemia cells with long-term growth abilities in immunodeficient mice and in culture. Blood. 1996;88:1944–1950. [PubMed] [Google Scholar]
- 38.Holyoake T, et al. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood. 1999;94:2056–2064. [PubMed] [Google Scholar]
- 39.Dean M, et al. Tumour stem cells and drug resistance. Nat. Rev. Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 40.Antonchuk J, et al. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002;109:39–45. doi: 10.1016/s0092-8674(02)00697-9. [DOI] [PubMed] [Google Scholar]
- 41.Thorsteinsdottir U, et al. Overexpression of the myeloid leukemia-associated Hoxa9 gene in bone marrow cells induces stem cell expansion. Blood. 2002;99:121–129. doi: 10.1182/blood.v99.1.121. [DOI] [PubMed] [Google Scholar]
- 42.Bhardwaj G, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat. Immunol. 2001;2:172–180. doi: 10.1038/84282. [DOI] [PubMed] [Google Scholar]
- 43.Varnum-Finney B, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat. Med. 2000;6:1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- 44.Reya T, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 45.Park IK, et al. Bmi-1 is required for maintenance of adult selfrenewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 46.Lavau C, et al. Immortalization and leukemic transformation of a myelomonocytic precursor by retrovirally transduced HRX-ENL. EMBO J. 1997;16:4226–4237. doi: 10.1093/emboj/16.14.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ayton PM, Cleary ML. Molecular mechanisms of leukemogenesis mediated by MLL fusion proteins. Oncogene. 2001;20:5695–5707. doi: 10.1038/sj.onc.1204639. [DOI] [PubMed] [Google Scholar]
- 48.Cozzio A, et al. Similar MLL-associated leukemias arising from self-renewing stem cells and short-lived myeloid progenitors. Genes Dev. 2003;17:3029–3035. doi: 10.1101/gad.1143403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akashi K, et al. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 50.Huntly BJ, et al. MOZ-TIF2, but not BCR-ABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell. 2004;6:587–596. doi: 10.1016/j.ccr.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 51.Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- 52.Miyamoto T, et al. AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc. Natl. Acad. Sci. U. S. A. 2000;97:7521–7526. doi: 10.1073/pnas.97.13.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Guzman CG, et al. Hematopoietic stem cell expansion and distinct myeloid developmental abnormalities in a murine model of the AML1-ETO translocation. Mol. Cell. Biol. 2002;22:5506–5517. doi: 10.1128/MCB.22.15.5506-5517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li S, et al. The P190, P210, and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leukemogenic activity. J. Exp. Med. 1999;189:1399–1412. doi: 10.1084/jem.189.9.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu Y, et al. Targeting multiple kinase pathways in leukemic progenitors and stem cells is essential for improved treatment of Ph+ leukemia in mice. Proc. Natl. Acad. Sci. U. S. A. 2006;103:16870–16875. doi: 10.1073/pnas.0606509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neering SJ, et al. Leukemia stem cells in a genetically defined murine model of blast crisis CML. Blood. 2007;110:2578–2585. doi: 10.1182/blood-2007-02-073031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jaiswal S, et al. Expression of BCR/ABL and BCL2 in myeloid progenitors leads to myeloid leukemias. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10002–10007. doi: 10.1073/pnas.1633833100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Passegue E, et al. JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic stem cells. Cell. 2004;119:431–443. doi: 10.1016/j.cell.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 59.Fialkow PJ, et al. Chronic myelocytic leukemia: clonal origin in a stem cell common to the granulocyte, erythrocyte, platelet and monocyte/macrophage. Am. J. Med. 1977;63:125–130. doi: 10.1016/0002-9343(77)90124-3. [DOI] [PubMed] [Google Scholar]
- 60.Coluccia AM, et al. Bcr-Abl stabilizes beta-catenin in chronic myeloid leukemia through its tyrosine phosphorylation. EMBO J. 2007;26:1456–1466. doi: 10.1038/sj.emboj.7601485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Somervaille TC, Cleary ML. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell. 2006;10:257–268. doi: 10.1016/j.ccr.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 62.Kelly PN, et al. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 63.Ayton PM, Cleary ML. Transformation of myeloid progenitors by MLL oncoproteins is dependent on Hoxa7 and Hoxa9. Genes Dev. 2003;17:2298–2307. doi: 10.1101/gad.1111603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deshpande AJ, et al. Acute myeloid leukemia is propagated by a leukemic stem cell with lymphoid characteristics in a mouse model of CALM/AF10-positive leukemia. Cancer Cell. 2006;10:363–374. doi: 10.1016/j.ccr.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 65.Dash AB, et al. A murine model of CML blast crisis induced by cooperation between BCR/ABL and NUP98/HOX49. Proc. Natl. Acad. Sci. U. S. A. 2002;99:7622–7627. doi: 10.1073/pnas.102583199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Druker BJ, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med. 2001;344:1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 67.Weng AP, et al. Growth suppression of pre-T acute lymphoblastic leukemia cells by inhibition of notch signaling. Mol. Cell. Biol. 2003;23:655–664. doi: 10.1128/MCB.23.2.655-664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guzman ML, et al. Preferential induction of apoptosis for primary human leukemic stem cells. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16220–16225. doi: 10.1073/pnas.252462599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guzman ML, et al. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105:4163–4169. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu Q, et al. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102:972–980. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- 71.Xu Q, et al. mTOR regulates cell survival after etoposide treatment in primary AML cells. Blood. 2005;106:4261–4268. doi: 10.1182/blood-2004-11-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frankfurt O, Tallman MS. Strategies for the treatment of acute promyelocytic leukemia. J. Natl. Compr. Canc. Netw. 2006;4:37–50. doi: 10.6004/jnccn.2006.0005. [DOI] [PubMed] [Google Scholar]
- 73.Grimwade D, Enver T. Acute promyelocytic leukemia: where does it stem from? Leukemia. 2004;18:375–384. doi: 10.1038/sj.leu.2403234. [DOI] [PubMed] [Google Scholar]
- 74.Zheng X, et al. Arsenic but not all-trans retinoic acid overcomes the aberrant stem cell capacity of PML/RARα-positive leukemic stem cells. Haematologica. 2007;92:323–331. doi: 10.3324/haematol.10541. [DOI] [PubMed] [Google Scholar]
- 75.Bhatia R, et al. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003;101:4701–4707. doi: 10.1182/blood-2002-09-2780. [DOI] [PubMed] [Google Scholar]
- 76.Graham SM, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- 77.Holtz MS, et al. Imatinib mesylate (STI571) inhibits growth of primitive malignant progenitors in chronic myelogenous leukemia through reversal of abnormally increased proliferation. Blood. 2002;99:3792–3800. doi: 10.1182/blood.v99.10.3792. [DOI] [PubMed] [Google Scholar]
- 78.Copland M, et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006;107:4532–4539. doi: 10.1182/blood-2005-07-2947. [DOI] [PubMed] [Google Scholar]
- 79.Jorgensen HG, et al. Nilotinib exerts equipotent antiproliferative effects to imatinib and does not induce apoptosis in CD34+ CML cells. Blood. 2007;109:4016–4019. doi: 10.1182/blood-2006-11-057521. [DOI] [PubMed] [Google Scholar]
- 80.Jorgensen HG, et al. Lonafarnib reduces the resistance of primitive quiescent CML cells to imatinib mesylate in vitro. Leukemia. 2005;19:1184–1191. doi: 10.1038/sj.leu.2403785. [DOI] [PubMed] [Google Scholar]
- 81.Holtz MS, et al. Nonproliferating CML CD34+ progenitors are resistant to apoptosis induced by a wide range of proapoptotic stimuli. Leukemia. 2005;19:1034–1041. doi: 10.1038/sj.leu.2403724. [DOI] [PubMed] [Google Scholar]
- 82.Jorgensen HG, et al. Intermittent exposure of primitive quiescent chronic myeloid leukemia cells to granulocyte-colony stimulating factor in vitro promotes their elimination by imatinib mesylate. Clin. Cancer Res. 2006;12:626–633. doi: 10.1158/1078-0432.CCR-05-0429. [DOI] [PubMed] [Google Scholar]
- 83.Holtz M, et al. Growth factor stimulation reduces residual quiescent chronic myelogenous leukemia progenitors remaining after imatinib treatment. Cancer Res. 2007;67:1113–1120. doi: 10.1158/0008-5472.CAN-06-2014. [DOI] [PubMed] [Google Scholar]
- 84.Tsimberidou AM, et al. The role of gemtuzumab ozogamicin in acute leukaemia therapy. Br. J. Haematol. 2006;132:398–409. doi: 10.1111/j.1365-2141.2005.05872.x. [DOI] [PubMed] [Google Scholar]
- 85.Sperr WR, et al. CD 33 as a target of therapy in acute myeloid leukemia: current status and future perspectives. Leuk. Lymphoma. 2005;46:1115–1120. doi: 10.1080/10428190500126075. [DOI] [PubMed] [Google Scholar]
- 86.Jedema I, et al. Internalization and cell cycle-dependent killing of leukemic cells by Gemtuzumab Ozogamicin: rationale for efficacy in CD33-negative malignancies with endocytic capacity. Leukemia. 2004;18:316–325. doi: 10.1038/sj.leu.2403205. [DOI] [PubMed] [Google Scholar]
- 87.Hogge DE, et al. The efficacy of diphtheria-growth factor fusion proteins is enhanced by co-administration of cytosine arabinoside in an immunodeficient mouse model of human acute myeloid leukemia. Leuk. Res. 2004;28:1221–1226. doi: 10.1016/j.leukres.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 88.Matsunaga T, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat. Med. 2003;9:1158–1165. doi: 10.1038/nm909. [DOI] [PubMed] [Google Scholar]
- 89.Bakker AB, et al. C-type lectin-like molecule-1: a novel myeloid cell surface marker associated with acute myeloid leukemia. Cancer Res. 2004;64:8443–8450. doi: 10.1158/0008-5472.CAN-04-1659. [DOI] [PubMed] [Google Scholar]
- 90.Ponta H, et al. CD44: from adhesion molecules to signaling regulators. Nat. Rev. Mol. Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 91.Charrad R-S, et al. Ligation of the CD44 adhesion molecule reverses blockage of differentiation in human acute myeloid leukemia. Nat. Med. 1999;5:669–676. doi: 10.1038/9518. [DOI] [PubMed] [Google Scholar]
- 92.Salesse S, Verfaillie CM. Mechanisms underlying abnormal trafficking and expansion of malignant progenitors in CML: BCR/ABL-induced defects in integrin function in CML. Oncogene. 2002;21:8605–8611. doi: 10.1038/sj.onc.1206088. [DOI] [PubMed] [Google Scholar]
- 93.Krause DS, et al. Selectins and their ligands are required for homing and engraftment of BCR-ABL+ leukemia-initiating cells. Blood. 2005;106:206a. doi: 10.1182/blood-2013-11-538694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dagia NM, et al. G-CSF induces E-selectin ligand expression on human myeloid cells. Nat. Med. 2006;12:1185–1190. doi: 10.1038/nm1470. [DOI] [PubMed] [Google Scholar]
- 95.Deisseroth AB, et al. Genetic marking shows that Ph+ cells present in autologous transplants of chronic myelogenous leukemia (CML) contribute to relapse after autologous bone marrow in CML. Blood. 1994;83:3068–3076. [PubMed] [Google Scholar]
- 96.Farmer H, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 97.Krampera M, et al. Methodological approach to minimal residual disease detection by flow cytometry in adult B-lineage acute lymphoblastic leukemia. Haematologica. 2006;91:1109–1112. [PubMed] [Google Scholar]
- 98.Guinn BA, et al. Immunotherapy of myeloid leukaemia. Cancer Immunol. Immunother. 2007;56:943–957. doi: 10.1007/s00262-006-0267-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Manz MG, et al. Prospective isolation of human clonogenic common myeloid progenitors. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11872–11877. doi: 10.1073/pnas.172384399. [DOI] [PMC free article] [PubMed] [Google Scholar]