Abstract
Circadian rhythms are physiological and behavioural cycles generated by an endogenous biological clock, the suprachiasmatic nucleus. The circadian system influences the majority of physiological processes, including sleep–wake homeostasis. Impaired sleep and alertness are common symptoms of neurodegenerative disorders, and circadian dysfunction might exacerbate the disease process. The pathophysiology of sleep–wake disturbances in these disorders remains largely unknown, and is presumably multifactorial. Circadian rhythm dysfunction is often observed in patients with Alzheimer disease, in whom it has a major impact on quality of life and represents one of the most important factors leading to institutionalization of patients. Similarly, sleep and circadian problems represent common nonmotor features of Parkinson disease and Huntington disease. Clinical studies and experiments in animal models of neurodegenerative disorders have revealed the progressive nature of circadian dysfunction throughout the course of neurodegeneration, and suggest strategies for the restoration of circadian rhythmicity involving behavioural and pharmacological interventions that target the sleep–wake cycle. In this Review, we discuss the role of the circadian system in the regulation of the sleep–wake cycle, and outline the implications of disrupted circadian timekeeping in neurodegenerative diseases.
Introduction
Circadian rhythms—physiological and behavioural cycles with a periodicity of approximately 24 h—are generated by an endogenous biological clock, the suprachiasmatic nucleus (SCN). In synchrony with the solar time, the circadian system dictates the 24 h rhythmicity in rest–activity behaviour, feeding, body temperature, hormonal levels and many other biological processes of the organism. Any disruption of this system can, therefore, negatively affect sleep quality, alertness, cognitive performance, motor control, mental health and metabolism.1 Many of these functions become impaired in neurodegenerative disorders such as Alzheimer disease (AD), Parkinson disease (PD) and Huntington disease (HD), in which several brain areas—including the nuclei involved in circadian and sleep regulation—are affected by neurodegenerative processes. It is not surprising, therefore, that these disorders often entail progressive breakdown of the normal cycles of rest–activity, sleep and alertness; this disruption of circadian rhythms not only contributes to morbidity and poor quality of life, but could also be involved in driving the disease process itself.
In this Review, we provide a brief overview of the circadian system, and a comprehensive summary of the current understanding of the function of the circadian system in three common neurodegenerative disorders: AD, PD, and HD.
Human circadian system
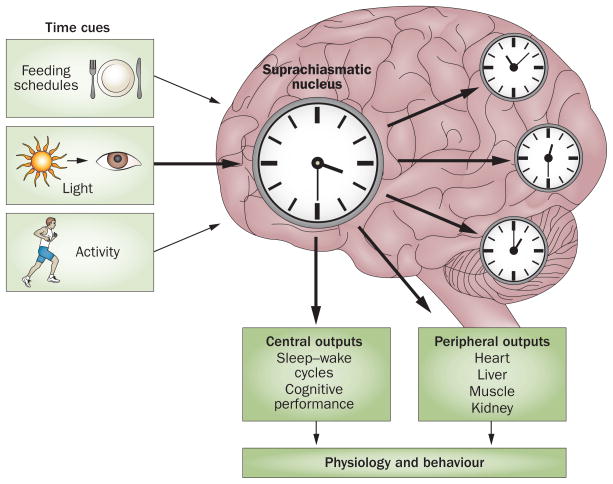
Circadian timekeeping is orchestrated by sophisticated molecular loops. The circadian timing system has three distinct components: a pacemaker (SCN), afferent pathways for light and other stimuli that synchronize the pacemaker to the environment, and efferent output rhythms that are regulated by the SCN (Figure 1).
Figure 1.
A simplified scheme of the circadian system. The timing of human biological rhythms is synchronized to the rotation of the Earth, and is influenced by numerous external and internal time cues. These stimuli are known as ’zeitgebers’ (German for ‘time giver’). Light is the most important and potent zeitgeber. In addition to light, activity, feeding schedules, and the hormone melatonin also influence circadian timing. This synchronization can become disrupted, which eventually leads to misalignment or internal desynchronization. This loss of coordination of circadian rhythms can have negative consequences for sleep–wake cycles and numerous other biological functions.
The SCN represents the core of the circadian system, and contains approximately 10,000 neurons in mice, and about 50,000 neurons in humans.2,3 The SCN is the main clock of the circadian system, and is composed of ‘core’ and ‘shell’ subnuclei. Both subnuclei have distinct neurochemical properties.4 γ-Aminobutyric acid (GABA) is the main neurotransmitter in nearly all neurons of the SCN; neurons that secrete vasoactive intestinal polypeptide are preferentially distributed in the SCN core, and neurons that secrete arginine vasopressin are located mostly in the SCN shell.
The main afferent pathways emerge from the melanopsin-containing retinal ganglion cells and reach the SCN directly via the retinohypothalamic tract, or indirectly via retinogeniculate pathways.5 The SCN also receives nonphotic information from the raphe nuclei, basal forebrain, pons, medulla and posterior hypothalamus. The main efferents project to the sub-paraventricular zone and paraventricular nucleus of the hypothalamus, as well as the dorsomedial hypothalamus, thalamus, preoptic and retrochiasmatic areas, stria terminalis, lateral septum, and intergeniculate nucleus. In addition, the SCN communicates using humoral signals such as transforming growth factor α, cardiotrophin-like cytokine factor 1, and prokineticin receptor 2.
Direct and indirect connections of the SCN with the autonomic nervous system regulate melatonin synthesis and corticosteroid secretion. These hormonal rhythms are well-accepted markers of endogenous rhythmicity. Circadian regulation of the autonomic nervous system has an important role in the regulation of peripheral tissues.6
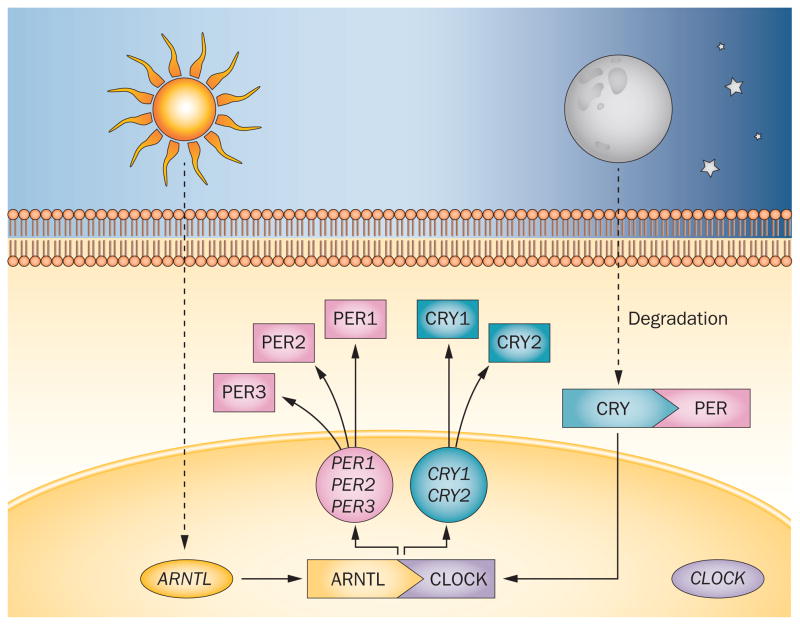
Circadian rhythms are regulated by a core set of clock genes (Figure 2), including three PER genes (PER1, PER2 and PER3), CLOCK, ARNTL (also known as BMAL1), and two plant cryptochrome gene homologues (CRY1 and CRY2). These genes control a substantial proportion of the genome: approximately 10% of all expressed genes estimated to be regulated by the clock genes. Furthermore, almost all peripheral tissues, as well as brain regions outside the SCN, contain autonomous circadian clocks. These clocks are influenced by their own distinct circadian synchronizers, but are probably synchronized by SCN-mediated messages, or by a direct input from the SCN.
Figure 2.
Molecular organization of the circadian system. The proteins encoded by the core set of clock genes—PER, CLOCK, ARNTL (also known as BMAL1) and CRY—interact to create a self-sustaining negative transcription–translation feedback loop.171 The intracellular level of CLOCK remains steady throughout the 24 h period. The high level of ARNTL at the beginning of the day promotes the formation of ARNTL–CLOCK heterodimers, which in turn activate transcription of PER and CRY. As PER accumulates in the cytoplasm, it becomes phosphorylated and degraded by ubiquitylation. CRY accumulates in the cytoplasm late in the subjective day, and translocates to the nucleus to inhibit ARNTL–CLOCK-mediated transcription. At night, the PER–CRY complex is degraded, and the cycle starts again. This feedback loop ensures a high level of ARNTL and low levels of PER and CRY at the beginning of a new circadian day.
Circadian dysfunction in AD
AD is clinically characterized by a slowly progressive cognitive decline, with predominant dysfunction in anterograde memory, along with aphasia, apraxia and executive dysfunction. The disease is pathologically defined by the accumulation of amyloid-β (Aβ) peptides in extracellular senile plaques, and of tau proteins in intracellular neurofibrillary tangles.7,8 These changes lead to neuronal death, spreading from the entorhinal cortex to the hippocampus and surrounding areas and eventually to the neocortex.9 Circadian rhythm dysfunction is common in patients with AD, and has a major impact on quality of life; it is also one of the most important factors leading to institutionalization of individuals with AD.10,11
Functional indicators of circadian disruption
The circadian disturbances seen in AD are comparable to the ones seen with normal ageing, but are much more pronounced and severe. Sleep is often highly fragmented, with frequent awakenings. A phase delay of the nocturnal sleep period is a typical indicator of circadian rhythm disturbance in AD, and these changes increase with disease duration and progression,12 often eventually leading to a virtual reversal of the day–night sleep pattern.
Alterations in the periodic changes in the core body temperature have also been described as an indicator of circadian dysfunction in AD.13,14 To some extent, these alterations, such as dampening of the amplitude in the 24 h body temperature rhythm, are also seen in normal ageing; however, phase delay, which can be observed in AD, is not seen in normal ageing.14 A recent study has shown an increase in distal skin temperature during the day in patients with AD, possibly as a result of altered circadian autonomic control.15
Important information on changes in circadian activity has been obtained by wrist-worn actigraphy devices that enable monitoring of rest–activity patterns.13,16,17 Patients with AD are more active during the night than are healthy controls, which is in line with the clinical picture of fragmented sleep. Changes in rest–activity patterns correlate with the severity of the dementia; in addition, the amplitude of the daily variation in the activity cycle was shown to be diminished in AD. A prospective study in over 1,200 participants demonstrated that these altered activity patterns could predict the development of mild cognitive impairment and/or AD,18 suggesting that impaired circadian rhythm is a preclinical finding, and might even contribute to the development of dementia. An actigraphy study in home-dwelling patients with early AD showed that whereas participants with mild dementia had activity patterns comparable to healthy controls, participants with moderate dementia showed a substantially disrupted rest–activity cycle.19 The most fragmented rhythms were found in institutionalized patients, and within this group, circadian rhythm fragmentation was associated with decreased levels of daytime activity.17
Activity patterns in humans display a temporal organization with so-called scale invariance, meaning that the statistical properties of fluctuations remain similar over different timescales. Such scale invariance was subsequently demonstrated in rats, and was shown to be critically dependent on the SCN.20 In healthy older adults, scale-invariant correlations in activity rhythms decline with age.21 The greatest reduction in scale invariance—similar to that seen in experimental animal models with SCN lesions—has been found in patients with late-stage AD.
Neuroendocrine and neuropathological markers
The dynamics of melatonin secretion in AD have been studied extensively. Reductions in total melatonin levels are more profound in AD than in normally ageing individuals.22–24 In a postmortem study, a marked decrease in cerebrospinal fluid melatonin levels was found in patients with AD;25 furthermore, the rhythmicity in melatonin secretion disappeared at the early—possibly already pre-clinical—stage of the illness,26,27 and the pattern became very irregular.28
Postmortem studies have demonstrated a region-specific reduction in histidine decarboxylase mRNA levels in the tuberomammillary nucleus of patients with AD,29 and diurnal expression of this histamine production rate-limiting enzyme was found to be reduced in neurodegenerative disease.30
Several lines of research indicate the SCN is directly affected by the pathophysiological processes that underlie AD. Atrophy of the SCN, involving both volume reduction and decreased total cell count, has been demonstrated.2 The number of neurons in the SCN that express melatonin receptor type MT1 decreases in late-stage AD, but not in the earlier disease stages.31 Besides loss of neurons, neurofibrillary tangles were observed in SCN neurons in patients with severe AD, even though amyloid plaques were scarce in this region.32 Within the SCN, neurons that express vasoactive intestinal polypeptide (VIP), arginine vasopressin (AVP) or neurotensin have been shown to be depleted in AD.32–35 Consequently, AVP levels are strikingly lower in AD, and vasopressin mRNA levels are already decreased in the preclinical stage of AD—these findings are thought to be related to the disappearance of the normal diurnal rhythm.34 Importantly, progression of AD neuropathology correlates with increased circadian dysfunction, indicating a functional link between the two.12
Chronogenetics
The ‘chronogenetics’ of AD is complex, and our understanding of it is still in its infancy. The genetic underpinnings of circadian system disruption in AD are discussed in more detail elsewhere.36 Although AD pathology seems to affect the pineal gland only to a limited degree,37 melatonin secretion is disrupted and the diurnal pattern in the expression of the clock genes ARNTL, PER1 and CRY1 is lost in the pineal gland of patients with AD.38 The periodic fluctuations in the expression of several clock genes are preserved in brain regions outside the SCN in patients with AD,39 but abnormalities have been found in both the phase of the clock gene rhythms and the phase relationships between brain regions. These alterations reflect the disruption of the ‘master control’ by the SCN.
Circadian dysfunction in animal models
Precise assessment of circadian parameters (such as circadian period length) in patients with AD is virtually impossible due to difficulties in applying experimental designs such as constant routine studies in this population. Animal models of AD are useful, therefore, to disentangle the mechanisms responsible for circadian dysfunction.
The triple-transgenic (3xTg) mouse model contains three mutations that have been associated with AD in humans (APP LysMet670–671AspLeu, MAPT Pro301Leu and PSEN1 Met146Val). In line with postmortem findings in patients with AD, the 3xTg mouse model exhibits decreased numbers of AVP and VIP neurons in the SCN.40 In addition, circadian patterns of locomotor activity are altered: nocturnal activity is decreased and daytime activity is increased, and the mice have shorter free-running periods (circadian rhythms not entrained to external time cues). These alterations were observed even before the onset of AD pathology.40 The 3xTg mice also showed age-dependent changes in core body temperature rhythms (phase advance and increased mean body temperatures) similar to those seen in healthy older adults.41 Mice that were transgenic only for human APP (Tg2576) showed no changes in circadian activity rhythms;42 however, marked extension of the-free running period was seen, although normal entrainment by light remained possible.43 A mouse model with gross overexpression of human APP (TgCRND8), considered to be a model for ‘aggressive amyloidosis’ with widespread plaque formation, exhibited disorganization of diurnal activity even before the emergence of AD pathology.44
Circadian dysfunction and AD pathology
So far, we have discussed circadian disruption as a consequence of AD; however, increasing evidence suggests that circadian and/or sleep dysfunction can aggravate AD pathology, indicating a reciprocal relationship between the two. Evidence that the sleep–wake cycle influences amyloid dynamics,45 and the possible neuroprotective effects of melatonin, one of the most important circadian regulators, support this hypothesis.46
The bidirectional interaction between sleep and AD pathology has been discussed in detail elsewhere.47 Mouse experiments showing that sleep deprivation resulted in a marked increase in Aβ accumulation led to the exciting hypothesis that disrupted sleep could be a causal contributor to the development of AD.48 In 2013, sleep was shown to contribute to the clearance of metabolites such as Aβ from the brain in mice.49 More recently, sleep deprivation has been shown to increase Aβ levels in healthy humans.50 These experimental data are backed by several epidemiological studies showing that sleep restriction increases the risk of cognitive impairment.47
Several in vitro and animal model studies have assessed the potential antioxidant, neuroprotective and anti-amyloidogenic effects of melatonin These studies have been extensively reviewed recently.23,24 Melatonin inhibits the formation of β-sheets and amyloid fibrils,51 and analysis of mouse brain extracts revealed that treatment with melatonin leads to a modest reduction in cortical APP and Aβ levels.52 Treating Tg2576 mice with melatonin at an early age reduced the expected age-related increase in Aβ concentrations and, more importantly, increased survival.53
Treatment of circadian dysfunction in AD
A number of double-blind controlled trials and several open-label studies have shown that melatonin can improve sleep quality and reduce sundowning.54–57 However, a large double-blind, randomized, placebo-controlled trial in 157 patients with AD failed to show a beneficial effect of melatonin on sleep patterns measured by actigraphy.58 Another recent study also found no effects of melatonin on agitation, sleep or circadian rhythms in AD.59 These negative findings are in line with an earlier meta-analysis, which found no significant effects of melatonin on cognitive impairment.60
Several controlled trials have shown that bright light therapy can improve sleep quality and restore diurnal activity rhythms in patients with dementia.61–64 In two other studies, light therapy was found to stabilize diurnal activity rhythms without direct effects on sleep quality.65,66
Circadian dysfunction in PD
PD is the second most common neurodegenerative disorder after AD.67,68 The cardinal features of PD result from degeneration of dopaminergic neurons within the substantia nigra, and include tremor, bradykinesia, rigidity, and gait impairment. Other neurotransmitter systems are also affected, and are responsible for numerous nonmotor manifestations of PD.
A growing body of evidence suggests alterations of the circadian system in PD.69,70 Dopamine is a neurotransmitter of major importance at several levels of the circadian system, and its metabolism and signalling activity are strongly influenced by the circadian clock, as reviewed elsewhere.71 Impaired circadian function in PD is implicated not only in dysregulation of the sleep–wake cycle, but also in autonomic, cognitive, psychiatric and motor manifestations of the disease.
Diurnal clinical fluctuations
Numerous studies have reported that the behaviours, symptoms and signs associated with PD, such as disruptions in motor activity,72–75 autonomic function,76–78 sleep–wake cycles79–81 and visual performance,82 as well as responsiveness to dopaminergic treatments,72,83 show diurnal fluctuation. These observations suggest circadian influences on the expression of clinical features of PD.
Fluctuations of motor symptoms in PD are very common. Actigraphy studies demonstrate lower peak activity level and lower amplitude of the rest–activity cycle in patients in PD than in healthy older adults.80,81 Increased physical activity and shorter periods of immobility during the night result in an almost flat diurnal pattern of motor activity in PD.84 Patients with PD also have a more fragmented pattern of activity, with transitions from high-activity to low-activity periods, leading to less-predictable rest–activity rhythms than are seen in healthy older people.85 In both stable patients with PD and those with ‘wearing off ’ phenomena, motor symptoms tend to be more prominent in the afternoon and evening.72,83 This daily pattern seems to be independent of the timing of dopaminergic medications, and could be related to circadian regulation of dopaminergic systems. In support of this notion, the responsiveness of PD motor symptoms to dopaminergic treatments declines throughout the day, despite the absence of significant changes in levodopa pharmacokinetics.72
Alterations in the circadian regulation of the autonomic system have also been reported in PD. 24 h ambulatory blood pressure monitoring in patients with PD demonstrates reversal of the normal circadian rhythm of blood pressure, increased diurnal blood pressure variability, postprandial hypotension, and high nocturnal blood pressure;86,87 Holter electrocardiographic monitoring reveals a decrease in sympathetic activity during the day, combined with loss of circadian heart rate variability and disappearance of the sympathetic morning peak.88 These abnormalities could arise within the peripheral autonomic ganglia, but the central networks, including the hypothalamus, might also be involved.89,90
Circadian fluctuations in visual performance, as measured by contrast sensitivity, are altered in PD.82 Impaired regulation of retinal dopamine, which exhibits an endogenous circadian rhythm that is independent of light–dark cycles, is most likely to be responsible for these changes.91 Because circadian changes in contrast sensitivity can occur independently of circadian oscillations in motor symptoms, it is possible that the retina, striatum, cortex and other anatomical networks involved in visual processing can have differential threshold responses to the circadian signalling of dopamine.92
Sleep disturbances are among the most common and disabling nonmotor symptoms of PD, and affect as many as 90% of patients.93 The impact of circadian dysregulation on sleep and alertness in the PD population has not been systematically assessed. The aetiology of sleep disturbance is likely to be multifactorial, and to include the effects of PD-associated motor features on sleep, adverse effects of antiparkinsonian medications, and neurodegeneration of central areas that regulate sleep, such as the raphe nucleus and locus coeruleus; this degeneration is likely to underlie sleep impairment that emerges before the onset of the motor symptoms of PD.94
Markers of circadian function
Only a few studies have attempted to identify markers of circadian function in PD. In a cohort of 26 individuals with PD, patients—with or without motor complications—who were treated with dopaminergic agents had a decreased amplitude of melatonin fluctuation, and the phase of secretion was advanced in comparison with recently diagnosed patients who were not yet receiving treatment.95 These results suggest a trend toward a shift in the phase and a reduction in the amplitude of melatonin secretion during the evolution of PD, and emphasize a need to investigate the effects of dopaminergic medication of circadian function. Circadian fluctuation of body temperature and plasma cortisol did not differ between the groups in this study. In another study, melatonin secretion in nine patients with recently diagnosed PD was similar to healthy controls.96 In a study of 12 patients with PD, 24 h mean cortisol production rate was higher and the mean secretory cortisol curve was flatter—indicating reduced diurnal variation—in the PD group than in the control group.97 In a small study of eight recently diagnosed patients who were not receiving medication, no significant alterations were found in diurnal secretion profiles of growth hormone, thyrotropin and prolactin.98
24 h rhythms of core body temperature are preserved in PD,99 but basal body temperature is lowered in patients with parkinsonian symptoms.100 Patients with PD and comorbid depression exhibit altered circadian rhythms of rectal temperature and decreased amplitudes of core body temperature.101
Although these investigations suggest altered circadian rhythmicity in PD, they should be interpreted with caution due to small sample sizes and study designs that reflect influences of both endogenous circadian and exogenous (light exposure, physical activity, meals, social schedules) rhythms on PD.
In a recent study that employed a modified constant routine experimental protocol in 20 patients with mild to moderate PD and 12 age-matched controls, significant blunting of the circadian rhythm of melatonin secretion and reduced amplitude of the melatonin rhythm was observed.102 Patients with excessive daytime somnolence had a particularly prominent reduction of the melatonin rhythm amplitude. In another study, notable reductions in the amplitude of melatonin secretion were also demonstrated in patients with de novo PD.103 In contrast to these findings, an additional study reported a threefold increase in melatonin secretion in a cohort of medicated patients with PD.104 Although further investigations are needed to confirm the role of neurodegenerative changes in disruption of circadian function, these observations support a role for circadian disruption in excessive daytime sleepiness associated with PD.
Several studies have demonstrated robust oscillatory patterns in the of core clock genes in human whole blood and peripheral blood mononuclear cells,105–107 demonstrating the feasibility of using clock gene expression as a marker of circadian function. In a study of 17 individuals with PD and 16 age-matched controls, the relative abundance of the clock gene ARNTL was notably lower during the night in patients with PD than in controls. Expression levels of ARNTL correlated with PD severity, as assessed by Unified PD Rating Scale scores (UPDRS).108 The lack of diurnal variation in ARNTL expression was recently confirmed in another study.103
Circadian entrainment
Numerous studies have demonstrated beneficial effects of supplemental wide-spectrum bright light therapy on sleep quality, daytime sleepiness, mood and quality of life in healthy elderly individuals and patients with dementia. Several lines of evidence provide a rationale for exploring supplemental light exposure in PD. For example, dopamine is a probable mediator of light signalling to the retinal circadian clock that provides direct input to the SCN.109 Exposure to light facilitates the recovery of motor function in an experimental rat model of chronic PD.110
To date, only a few exploratory studies have examined the effects of bright light in patients with PD. In a case series of 12 patients, daily evening exposure to supplemental bright light of 1,000–1,500 lux improved sleep quality and mood, as well as bradykinesia and rigidity.111 Similar beneficial effects of supplemental bright light on sleep, mood, anxiety and motor performance were reported in an open-label retrospective study of 129 levodopa-treated patients followed up for 8 years.112 In another study, exposure to bright light of 7,500 lux was associated with improvements in UPDRS part I, II and IV scores, and alleviated daytime sleepiness.113 Although these studies demonstrated good feasibility and positive effects of bright light therapy on nonmotor and motor manifestations of PD, further validation studies involving larger cohorts and employing objective outcome measures are needed.
Circadian dysregulation in animal models
Animal models of PD provide an opportunity to further explore the functioning of the circadian system in PD and to address questions that cannot be investigated within the clinical research protocols in the PD population. Primates treated with the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exhibit immediate disruption of the rest–activity rhythms, alterations in rapid eye movement (REM) sleep, and increased daytime sleepiness.114–116 In rats, dopaminergic depletion induced by bilateral striatal injection of 6-hydroxydopamine (6-OHDA) results in disturbances of circadian rhythms of heart rate, temperature and locomotor activity.117–119 In addition to disruption of behavioural rhythms, dopaminergic depletion induced by 6-OHDA has been demonstrated to be followed by blunting of diurnal fluctuation in the expression of the clock gene PER2 in the striatum.120 A mouse model overexpressing α-synuclein, a protein that has been implicated in the neuropathology of PD, exhibits altered circadian rest–activity rhythms and reduced daytime neuronal firing rate in the SCN.121 Animal models of circadian disruption in PD have been reviewed in more detail elsewhere.122
Circadian dysfunction in HD
HD is a fatal autosomal dominant neurodegenerative condition that affects approximately 14–16 individuals per 100,000,123 and typically becomes manifest in midlife.124 The disease is caused by abnormal expansion of the trinucleotide CAG repeat125 in exon 1 of the huntingtin gene (HTT),126 leading to ubiquitous expression of mutant HTT.126–128 HD is characterized by progressively worsening motor and nonmotor deficits, including cognitive abnormalities that eventually lead to dementia,129 neuropsychiatric disorders,130 weight loss,131 and severe sleep132–137 and circadian disturbances.138,139
Limitations and potential for research
Clinical experience and epidemiological and experimental data all indicate that the circadian system is affected in HD; however, patient data are limited. This lack of data could be attributable to the relatively low incidence of HD in the general population, and the methodological challenges of putting patients who exhibit complex combinations of motor, cognitive and psychiatric features through relatively intense protocols for circadian and sleep research. Furthermore, sleep problems in HD tend to go undiagnosed by physicians and underreported by patients, possibly due to a lack of insight or perceived relative unimportance of sleep problems when compared with the motor, cognitive and affective features that are recognized as key features of this disorder. In spite of these limitations, the study of patients with HD has great research potential by virtue of the genetic basis of the disease, which enables the identification and follow-up of premanifest individuals, and the ability to predict disease onset. These factors create a unique opportunity to investigate and establish causality between the pre-morbid structural and functional degeneration of the brain and the clinical features, including circadian and sleep disruption. Such longitudinal studies could aid in elucidating the role of the circadian system and sleep behaviour in normal physiological processes, as well as in other neurodegenerative disorders.
Another important research opportunity in HD stems from the ability to make transgenic animal models of the disease. These models, especially mice, can recapitulate many of the main features of HD, and have been essential to our current understanding of the role of sleep and circadian disturbances in the condition. A need exists, however, for detailed prospective observational and interventional sleep and circadian studies in HD to confirm that the deficits reported in these animals are relevant for human disease.
Sleep–wake timing and sleep quality
Delayed sleep–wake timing and diurnal preference have been repeatedly linked to mental health problems, in particular depression.140 Depression is a major problem in HD, along with apathy, anxiety and irritability.141 In a focused questionnaire-based sleep survey, wake-up time was markedly delayed in patients with manifest HD as compared with both premanifest mutation carriers and noncarrier controls, but no differences in bedtimes were observed.142 Self-reported sleep quality was also considerably reduced in the patients with manifest disease, whereas premanifest mutation carriers had intermediate sleep quality. These findings were confirmed in another cohort of manifest patients and premanifest mutation carriers; moreover, the later wake-up times were negatively associated with cognitive scores and total functional capacity, and positively associated with depressive symptoms. The degree of sleep phase delay in premani-fest mutation carriers was intermediate between that of HD patients and controls.143
In line with the self reports, a small polysomnography study involving nine patients with early-stage HD showed reduced sleep efficiency and increased time spent in bed, although the authors did not report whether this latter finding was due to an earlier bedtime or to a delayed get-up time.132 Another polysomnography study, however, reported that sleep onset before 9 pm was much more frequent in manifest and premanifest HD than in controls.137 According to a large questionnaire-based community survey, sleep problems were highly prevalent (>87%) among the 292 individuals with HD, with early waking time being the most important sleep complaint.144 Several other studies have shown that self-reported daytime sleepiness and sleep complaints are more prevalent in patients with HD than in controls.142,143,145 although it is not known how these subjective sleep reports relate to the objective measures of vigilance and sleep quality. The earliest objective sleep study using polysomnography was published in 1967146 as a case report on a 25-year-old patient with HD who showed suppression of rapid eye movements during REM sleep as an early symptom. A later study, published in 1985, described increased sleep latency, worse sleep efficiency and interspersed wakefulness in a small cohort of patients with HD.133 The few subsequent polysomnography studies have confirmed these findings;132,135–137,147 in addition, decreased slow-wave sleep,135,136,147 REM sleep disturbances132,135,137 and an increased number of sleep spindles have been documented.134,147
Rest–activity rhythms
The initial continuous actigraphic assessments in patients with HD aimed to examine involuntary movements in the daytime, rather than the rest–activity cycle, as a core feature of HD. Despite the presence of chorea, these studies reported a decreased level in daytime activity148,149 that correlated with disease penetrance.149 In another study, patients with mild to moderate disease exhibited a robust increase in total and maximal activity and spent longer times performing high-acceleration movements while they were awake compared with controls. These patients also showed more overnight activity than did the control individuals.150
The first study that employed actigraphy specifically to assess circadian rest–activity rhythms in patients with HD found evidence of increased nocturnal activity, while daytime activity remained similar to controls. The increased activity during the night-time was primarily driven by having a sleep–rest period interspersed with activity bouts, and could not have been caused by the involuntary movement disorder, which subsides during sleep.138 These results are reminiscent of the subjective and objective sleep reports in patients with HD, which show an increased number of awakenings and an overall decrease in sleep quality.132,133,135–137,142,143,147
Rest–activity rhythms in animal models of HD
The genetic basis of HD enables the generation of transgenic models of disease that can recapitulate many of the main features of HD151 and, despite fundamental interspecies differences in sleep–wake behaviour, studies of circadian function in these animal models have helped us to understand the basis and progression of circadian rhythm disruption in HD. In the early stages of disease, the diurnal rest–activity cycle is fairly well conserved, and becomes increasingly disrupted as the disease progresses, with total disintegration in end-stage disease.138,139,152–158 This circadian disruption depends not only on age but also on gene dose:159 the rest–activity disorganization becomes more prominent in animals that are homozygous for the mutation than in heterozygotes. The homozygotes also exhibited a more severely abnormal circadian behavioural response to light, and a steeper decline in locomotor rhythms.159 Interestingly, in a transgenic sheep model of HD, the circadian abnormalities also depend on social factors: abnormalities of circadian behaviour emerged in sheep kept in an ‘HD-only’ flock, whereas the behaviour of HD sheep kept mixed with nontransgenic sheep remained relatively normal.152
In most studies, the circadian disruption is associated with progressive impairment of sleep architecture and sleep EEG153,154,156,157 suggesting an interaction between disruption of circadian behaviour and fragmented sleep architecture, but with no exact indication on any causal relationship between the two. Restoration of circadian rhythmicity and sleep has been shown to alleviate the disease course in HD: reconsolidation of circadian entrainment by pharmacological agents or other interventions slows down cognitive decline and normalizes circadian gene expression.160–163 These effects are likely to be modulated by sex and genotype.163
The central circadian pacemaker
Surprisingly few data are available on the integrity and function of the SCN in patients with HD. One study that measured blood melatonin reported that although patients with HD had similar overall melatonin concentrations to healthy controls, they displayed a delayed melatonin secretion rhythm, and the severity of the delay correlated with impairments in motor and other functions.164 More recently, blood melatonin was shown to be already decreased in premanifest patients, with manifest patients having a more marked reduction. In both manifest and premanifest HD, timing of melatonin secretion onset was much more variable than in controls.165
Several studies have found altered levels of cortisol in blood and urine in HD.152,160,166,167 Furthermore, changes in the pattern of pulsatile release of adrenocorticotropic hormone, followed by increased pulsatile secretion of cortisol, correlated with CAG repeat size and could, thus, be caused directly by the central HD pathology rather than being secondary to any neuropsychiatric disease.167 The VIP-containing cells of the SCN probably help to regulate cortisol secretion by inhibiting its release,168 and postmortem studies in patients with HD have revealed loss of VIP and AVP expression within the SCN.169 This decrease in VIP levels has also been associated with circadian disruption in transgenic animal models.155
Besides the progressive circadian disruption of the rest–activity rhythm in transgenic animal models of HD, progressive disruption of circadian patterns of body temperature and heart rate has been found.139 One study in a mouse model of HD reported abnormal cellular gene expression in the SCN and several other brain regions, indicative of dysfunction of the genetic underpinnings of the molecular clock,138 but other studies reported no change in expression of the genes associated with this clock.139,159 The former study connected the impaired circadian rhythm to spontaneous electrical activity of SCN neurons, suggesting that the electrical activity within the central clock itself could be altered by the disease.139 However, in vitro studies have described normal electrophysiological output and endogenous rhythms of circadian gene expression in the SCN, indicating that in vivo circadian disturbances in transgenic mice might be caused by abnormalities in the afferent pathways to the SCN.161 This idea is further supported by the normalization of circadian gene expression in vivo after re-entrainment of circadian rhythms by behavioural interventions in a transgenic mouse model of HD,161 although the study provided no direct evidence for normal electrophysiological activity of the SCN in vivo. Experimentally induced mitochondrial dysfunction leads to striatal lesions and can also contribute to sleep and/or circadian dysfunction, suggesting a mechanistic link that is relevant to HD.170
In summary, although reports from patients and transgenic animal models are inconsistent, there is convincing evidence for major disruption of the circadian system in HD. This might be reversible, and could provide a strategy to slow down the disease process.
Conclusions
The circadian system controls a wide range of physiological and behavioural processes and coordinates their synchronicity. Increasing evidence shows suggests that the circadian rhythms are dysregulated in neurodegenerative disorders. In this Review, we have highlighted the literature that spans clinical and animal studies of circadian function in three common neurodegenerative disorders: AD, PD and HD. While each of these disorders has its distinct phenotype–genotype signature, each also exhibits overlapping symptoms that could, at least in part, be based on circadian dysfunction. One of the best examples is disruption of the sleep–wake cycle, which represents one of the most robust output rhythms of the circadian system. Both human and animal studies have documented disruption of circadian timekeeping from physiological, molecular and behavioural perspectives in these three disorders. Impairments in circadian timekeeping are likely not only to influence regulation of sleep and alertness, but also affect mood, cognition, autonomic function and motor function in these disorders.
One of the key questions that remains unanswered is whether circadian disruption associated with neurodegeneration represents one of its consequences, or whether it could be one of the causative factors leading and/or contributing to the neurodegenerative cascade. In the future, longitudinal studies aimed at simultaneously examining circadian function and other disease manifestations throughout the course of these disorders will be critical in attempting to answer this question. These studies should include premanifest carriers of the HD mutant allele, individuals carrying mutations that increase the risk of AD, and individuals at high risk of development of PD, such as those with REM sleep behaviour disorder.
Regardless of whether circadian disruption represents a cause or a consequence of neurodegeneration, we argue that AD, PD and HD share ‘chronodegeneration’ as a common feature. This is an important notion, as interventions directed at normalizing circadian rhythmicity in these disorders might provide novel opportunities for much-needed therapies. These therapies could not only improve symptoms and quality of life, but also slow down disease progression. The circadian system, therefore, could be a new diagnostic and therapeutic target in neurodegenerative disorders.
Review criteria.
A search for original articles was performed in MEDLINE and PubMed. The search terms used were “circadian”, “suprachiasmatic nucleus”, “Parkinson’s”, “Alzheimer’s” and “Huntington’s”, alone and in combination. All articles identified were English-language, full-text papers. We also searched the reference lists of identified articles for further relevant papers.
Key points.
Sleep and circadian disruption are common in Alzheimer disease, Parkinson disease and Huntington disease
Symptoms of circadian disruption range from subjective sleep complaints and alterations of sleep timing to severe disruptions in rest–activity cycles and sleep architecture
Behavioural, physiological and molecular markers of circadian system function suggest progressive deterioration of circadian rhythmicity as the disease progresses
The relationship between circadian dysfunction and neurodegenerative processes might be bidirectional: circadian dysfunction might exacerbate the disease processes
Behavioural or pharmaceutical interventions that target sleep–wake cycles might reverse circadian disruption
Acknowledgments
A.V. has received research grant support from the NIH (K23 NS072283). A.S.L. has received research grant support from the CHDI Foundation (RG50786). R.A.B. has received research grant support from the CHDI Foundation (RG50786), Evelyn Trust (RG66030), Butterfield Trust (RG68592), and the NIHR Biomedical Research Centre and Biomedical Research Unit Award to Addenbrooke’s Hospital/University of Cambridge (RG68592). S.O. has received research grant support from the Netherlands Organization for Scientific Research (grant no. 016.116.371).
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
A.V., A.S.L. and S.O. researched data for the article. All authors provided substantial contributions to discussion of the content, wrote the article and reviewed and/or edited the manuscript before and after submission.
References
- 1.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- 3.Cassone VM, Speh JC, Card JP, Moore RY. Comparative anatomy of the mammalian hypothalamic suprachiasmatic nucleus. J Biol Rhythms. 1988;3:71–91. doi: 10.1177/074873048800300106. [DOI] [PubMed] [Google Scholar]
- 4.Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90:1063–1102. doi: 10.1152/physrev.00009.2009. [DOI] [PubMed] [Google Scholar]
- 5.Dardente H, Cermakian N. Molecular circadian rhythms in central and peripheral clocks in mammals. Chronobiol Int. 2007;24:195–213. doi: 10.1080/07420520701283693. [DOI] [PubMed] [Google Scholar]
- 6.Moore RY. The suprachiasmatic nucleus and the circadian timing system. Prog Mol Biol Transl Sci. 2013;119:1–28. doi: 10.1016/B978-0-12-396971-2.00001-4. [DOI] [PubMed] [Google Scholar]
- 7.Ballard C, et al. Alzheimer’s disease. Lancet. 2011;377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 8.Ittner LM, Götz J. Amyloid-β and tau-—a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci. 2011;12:65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- 9.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 10.Bianchetti A, et al. Predictors of mortality and institutionalization in Alzheimer disease patients 1 year after discharge from an Alzheimer dementia unit. Dementia. 1995;6:108–112. doi: 10.1159/000106930. [DOI] [PubMed] [Google Scholar]
- 11.Pollak CP, Perlick D. Sleep problems and institutionalization of the elderly. J Geriatr Psychiatry Neurol. 1991;4:204–210. doi: 10.1177/089198879100400405. [DOI] [PubMed] [Google Scholar]
- 12.Harper DG, et al. Dementia severity and Lewy bodies affect circadian rhythms in Alzheimer disease. Neurobiol Aging. 2004;25:771–781. doi: 10.1016/j.neurobiolaging.2003.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Satlin A, Volicer L, Stopa EG, Harper D. Circadian locomotor activity and core-body temperature rhythms in Alzheimer’s disease. Neurobiol Aging. 1995;16:765–771. doi: 10.1016/0197-4580(95)00059-n. [DOI] [PubMed] [Google Scholar]
- 14.Harper DG, et al. Disturbance of endogenous circadian rhythm in aging and Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:359–368. doi: 10.1176/appi.ajgp.13.5.359. [DOI] [PubMed] [Google Scholar]
- 15.Most EI, Scheltens P, Van Someren EJ. Increased skin temperature in Alzheimer’s disease is associated with sleepiness. J Neural Transm. 2012;119:1185–1194. doi: 10.1007/s00702-012-0864-1. [DOI] [PubMed] [Google Scholar]
- 16.Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the circadian rest–activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry. 1990;27:563–572. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]
- 17.van Someren EJ, et al. Circadian rest–activity rhythm disturbances in Alzheimer’s disease. Biol Psychiatry. 1996;40:259–270. doi: 10.1016/0006-3223(95)00370-3. [DOI] [PubMed] [Google Scholar]
- 18.Tranah GJ, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70:722–732. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatfield CF, Herbert J, van Someren EJ, Hodges JR, Hastings MH. Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer’s dementia. Brain. 2004;127:1061–1074. doi: 10.1093/brain/awh129. [DOI] [PubMed] [Google Scholar]
- 20.Manconi M, Hutchins W, Feroah TR, Zucconi M, Ferini-Strambi L. On the pathway of an animal model for restless legs syndrome. Neurol Sci. 2007;28 (Suppl 1):S53–S60. doi: 10.1007/s10072-007-0738-8. [DOI] [PubMed] [Google Scholar]
- 21.Hu K, Van Someren EJ, Shea SA, Scheer FA. Reduction of scale invariance of activity fluctuations with aging and Alzheimer’s disease: Involvement of the circadian pacemaker. Proc Natl Acad Sci USA. 2009;106:2490–2494. doi: 10.1073/pnas.0806087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skene DJ, Swaab DF. Melatonin rhythmicity: effect of age and Alzheimer’s disease. Exp Gerontol. 2003;38:199–206. doi: 10.1016/s0531-5565(02)00198-5. [DOI] [PubMed] [Google Scholar]
- 23.Rosales-Corral SA, et al. Alzheimer’s disease: pathological mechanisms and the beneficial role of melatonin. J Pineal Res. 2012;52:167–202. doi: 10.1111/j.1600-079X.2011.00937.x. [DOI] [PubMed] [Google Scholar]
- 24.Lin L, et al. Melatonin in Alzheimer’s disease. Int J Mol Sci. 2013;14:14575–14593. doi: 10.3390/ijms140714575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu RY, Zhou JN, van Heerikhuize J, Hofman MA, Swaab DF. Decreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer’s disease, and apolipoprotein E-ε4/4 genotype. J Clin Endocrinol Metab. 1999;84:323–327. doi: 10.1210/jcem.84.1.5394. [DOI] [PubMed] [Google Scholar]
- 26.Zhou JN, Liu RY, Kamphorst W, Hofman MA, Swaab DF. Early neuropathological Alzheimer’s changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J Pineal Res. 2003;35:125–130. doi: 10.1034/j.1600-079x.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 27.Wu YH, et al. Molecular changes underlying reduced pineal melatonin levels in Alzheimer disease: alterations in preclinical and clinical stages. J Clin Endocrinol Metab. 2003;88:5898–5906. doi: 10.1210/jc.2003-030833. [DOI] [PubMed] [Google Scholar]
- 28.Mishima K, et al. Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer’s type with disturbed sleep–waking. Biol Psychiatry. 1999;45:417–421. doi: 10.1016/s0006-3223(97)00510-6. [DOI] [PubMed] [Google Scholar]
- 29.Shan L, Bossers K, Unmehopa U, Bao AM, Swaab DF. Alterations in the histaminergic system in Alzheimer’s disease: a postmortem study. Neurobiol Aging. 2012;33:2585–2598. doi: 10.1016/j.neurobiolaging.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 30.Shan L, et al. Diurnal fluctuation in histidine decarboxylase expression, the rate limiting enzyme for histamine production, and its disorder in neurodegenerative diseases. Sleep. 2012;35:713–715. doi: 10.5665/sleep.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu YH, Zhou JN, Van Heerikhuize J, Jockers R, Swaab DF. Decreased MT1 melatonin receptor expression in the suprachiasmatic nucleus in aging and Alzheimer’s disease. Neurobiol Aging. 2007;28:1239–1247. doi: 10.1016/j.neurobiolaging.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Stopa EG, et al. Pathologic evaluation of the human suprachiasmatic nucleus in severe dementia. J Neuropathol Exp Neurol. 1999;58:29–39. doi: 10.1097/00005072-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Coogan AN, Rawlings N, Luckman SM, Piggins HD. Effects of neurotensin on discharge rates of rat suprachiasmatic nucleus neurons in vitro. Neuroscience. 2001;103:663–672. doi: 10.1016/s0306-4522(00)00583-2. [DOI] [PubMed] [Google Scholar]
- 34.Zhou JN, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer’s disease. Neurobiol Aging. 1995;16:571–576. doi: 10.1016/0197-4580(95)00043-e. [DOI] [PubMed] [Google Scholar]
- 35.Liu RY, et al. Decreased vasopressin gene expression in the biological clock of Alzheimer disease patients with and without depression. J Neuropathol Exp Neurol. 2000;59:314–322. doi: 10.1093/jnen/59.4.314. [DOI] [PubMed] [Google Scholar]
- 36.Coogan AN, et al. The circadian system in Alzheimer’s disease: disturbances, mechanisms, and opportunities. Biol Psychiatry. 2013;74:333–339. doi: 10.1016/j.biopsych.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 37.Friedland RP, Luxenberg JS, Koss E. A quantitative study of intracranial calcification in dementia of the Alzheimer type. Int Psychogeriatr. 1990;2:36–43. [PubMed] [Google Scholar]
- 38.Wu YH, et al. Pineal clock gene oscillation is disturbed in Alzheimer’s disease, due to functional disconnection from the “master clock”. FASEB J. 2006;20:1874–1876. doi: 10.1096/fj.05-4446fje. [DOI] [PubMed] [Google Scholar]
- 39.Cermakian N, Lamont EW, Boudreau P, Boivin DB. Circadian clock gene expression in brain regions of Alzheimer’s disease patients and control subjects. J Biol Rhythms. 2011;26:160–170. doi: 10.1177/0748730410395732. [DOI] [PubMed] [Google Scholar]
- 40.Sterniczuk R, Dyck RH, Laferla FM, Antle MC. Characterization of the 3xTg-AD mouse model of Alzheimer’s disease: part 1. Circadian changes. Brain Res. 2010;1348:139–148. doi: 10.1016/j.brainres.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 41.Knight EM, et al. Age-related changes in core body temperature and activity in triple-transgenic Alzheimer’s disease (3xTgAD) mice. Dis Model Mech. 2013;6:160–170. doi: 10.1242/dmm.010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorman MR, Yellon S. Lifespan daily locomotor activity rhythms in a mouse model of amyloid-induced neuropathology. Chronobiol Int. 2010;27:1159–1177. doi: 10.3109/07420528.2010.485711. [DOI] [PubMed] [Google Scholar]
- 43.Wisor JP, et al. Sleep and circadian abnormalities in a transgenic mouse model of Alzheimer’s disease: a role for cholinergic transmission. Neuroscience. 2005;131:375–385. doi: 10.1016/j.neuroscience.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 44.Ambree O, et al. Activity changes and marked stereotypic behavior precede Aβ pathology in TgCRND8 Alzheimer mice. Neurobiol Aging. 2006;27:955–964. doi: 10.1016/j.neurobiolaging.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Slats D, Claassen JA, Verbeek MM, Overeem S. Reciprocal interactions between sleep, circadian rhythms and Alzheimer’s disease: focus on the role of hypocretin and melatonin. Ageing Res Rev. 2013;12:188–200. doi: 10.1016/j.arr.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Wang JZ, Wang ZF. Role of melatonin in Alzheimer-like neurodegeneration. Acta Pharmacol Sin. 2006;27:41–49. doi: 10.1111/j.1745-7254.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- 47.Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology —a bidirectional relationship. Nat Rev Neurol. 2014;10:115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang JE, et al. Amyloid-β dynamics are regulated by orexin and the sleep–wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie L, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ooms S, et al. Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol. 2014;71:971–977. doi: 10.1001/jamaneurol.2014.1173. [DOI] [PubMed] [Google Scholar]
- 51.Pappolla M, et al. Inhibition of Alzheimer β-fibrillogenesis by melatonin. J Biol Chem. 1998;273:7185–7188. doi: 10.1074/jbc.273.13.7185. [DOI] [PubMed] [Google Scholar]
- 52.Lahiri DK, Chen D, Ge YW, Bondy SC, Sharman EH. Dietary supplementation with melatonin reduces levels of amyloid beta-peptides in the murine cerebral cortex. J Pineal Res. 2004;36:224–231. doi: 10.1111/j.1600-079X.2004.00121.x. [DOI] [PubMed] [Google Scholar]
- 53.Matsubara E, et al. Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer’s disease. J Neurochem. 2003;85:1101–1108. doi: 10.1046/j.1471-4159.2003.01654.x. [DOI] [PubMed] [Google Scholar]
- 54.Asayama K, et al. Double blind study of melatonin effects on the sleep–wake rhythm, cognitive and non-cognitive functions in Alzheimer type dementia. J Nippon Med Sch. 2003;70:334–341. doi: 10.1272/jnms.70.334. [DOI] [PubMed] [Google Scholar]
- 55.Cardinali DP, Furio AM, Brusco LI. Clinical aspects of melatonin intervention in Alzheimer’s disease progression. Curr Neuropharmacol. 2010;8:218–227. doi: 10.2174/157015910792246209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dowling GA, et al. Melatonin and bright-light treatment for rest–activity disruption in institutionalized patients with Alzheimer’s disease. J Am Geriatr Soc. 2008;56:239–246. doi: 10.1111/j.1532-5415.2007.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahlberg R, Walther S. Actigraphy in agitated patients with dementia. Monitoring treatment outcomes. Z Gerontol Geriatr. 2007;40:178–184. doi: 10.1007/s00391-007-0420-z. [DOI] [PubMed] [Google Scholar]
- 58.Singer C, et al. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer’s disease. Sleep. 2003;26:893–901. doi: 10.1093/sleep/26.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gehrman PR, et al. Melatonin fails to improve sleep or agitation in double-blind randomized placebo-controlled trial of institutionalized patients with Alzheimer disease. Am J Geriatr Psychiatry. 2009;17:166–169. doi: 10.1097/JGP.0b013e318187de18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jansen SL, Forbes DA, Duncan V, Morgan DG. Melatonin for cognitive impairment. Cochrane Database of Systematic Reviews. (1):Art. No.: CD003802. doi: 10.1002/14651858.CD003802.pub3. http://dx.doi.org/10.1002/14651858.CD003802.pub3. [DOI] [PMC free article] [PubMed]
- 61.Lyketsos CG, Lindell Veiel L, Baker A, Steele C. A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. Int J Geriatr Psychiatry. 1999;14:520–525. [PubMed] [Google Scholar]
- 62.Ancoli-Israel S, et al. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients. Behav Sleep Med. 2003;1:22–36. doi: 10.1207/S15402010BSM0101_4. [DOI] [PubMed] [Google Scholar]
- 63.Burns A, Allen H, Tomenson B, Duignan D, Byrne J. Bright light therapy for agitation in dementia: a randomized controlled trial. Int Psychogeriatr. 2009;21:711–721. doi: 10.1017/S1041610209008886. [DOI] [PubMed] [Google Scholar]
- 64.McCurry SM, et al. Increasing walking and bright light exposure to improve sleep in community-dwelling persons with Alzheimer’s disease: results of a randomized, controlled trial. J Am Geriatr Soc. 2011;59:1393–1402. doi: 10.1111/j.1532-5415.2011.03519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dowling GA, et al. Effect of morning bright light treatment for rest–activity disruption in institutionalized patients with severe Alzheimer’s disease. Int Psychogeriatr. 2005;17:221–236. doi: 10.1017/S1041610205001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dowling GA, Mastick J, Hubbard EM, Luxenberg JS, Burr RL. Effect of timed bright light treatment for rest–activity disruption in institutionalized patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 2005;20:738–743. doi: 10.1002/gps.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alves G, Forsaa EB, Pedersen KF, Dreetz Gjerstad M, Larsen JP. Epidemiology of Parkinson’s disease. J Neurol. 2008;255 (Suppl 5):18–32. doi: 10.1007/s00415-008-5004-3. [DOI] [PubMed] [Google Scholar]
- 68.Dorsey ER, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 69.Bruguerolle B, Simon N. Biologic rhythms and Parkinson’s disease: a chronopharmacologic approach to considering fluctuations in function. Clin Neuropharmacol. 2002;25:194–201. doi: 10.1097/00002826-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 70.Willis GL. Parkinson’s disease as a neuroendocrine disorder of circadian function: dopamine-melatonin imbalance and the visual system in the genesis and progression of the degenerative process. Rev Neurosci. 2008;19:245–316. doi: 10.1515/revneuro.2008.19.4-5.245. [DOI] [PubMed] [Google Scholar]
- 71.Videnovic A, Golombek D. Circadian and sleep disorders in Parkinson’s disease. Exp Neurol. 2013;243:45–56. doi: 10.1016/j.expneurol.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bonuccelli U, et al. Diurnal motor variations to repeated doses of levodopa in Parkinson’s disease. Clin Neuropharmacol. 2000;23:28–33. doi: 10.1097/00002826-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 73.Nutt JG, Woodward WR, Carter JH, Trotman TL. Influence of fluctuations of plasma large neutral amino acids with normal diets on the clinical response to levodopa. J Neurol Neurosurg Psychiatry. 1989;52:481–487. doi: 10.1136/jnnp.52.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Hilten JJ, et al. Assessment of response fluctuations in Parkinson’s disease by ambulatory wrist activity monitoring. Acta Neurol Scand. 1993;87:171–177. doi: 10.1111/j.1600-0404.1993.tb04096.x. [DOI] [PubMed] [Google Scholar]
- 75.van Hilten JJ, Middelkoop HA, Kerkhof GA, Roos RA. A new approach in the assessment of motor activity in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1991;54:976–979. doi: 10.1136/jnnp.54.11.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mihci E, Kardelen F, Dora B, Balkan S. Orthostatic heart rate variability analysis in idiopathic Parkinson’s disease. Acta Neurol Scand. 2006;113:288–293. doi: 10.1111/j.1600-0404.2006.00580.x. [DOI] [PubMed] [Google Scholar]
- 77.Pathak A, Senard JM. Blood pressure disorders during Parkinson’s disease: epidemiology, pathophysiology and management. Expert Rev Neurother. 2006;6:1173–1180. doi: 10.1586/14737175.6.8.1173. [DOI] [PubMed] [Google Scholar]
- 78.Pursiainen V, et al. Circadian heart rate variability in Parkinson’s disease. J Neurol. 2002;249:1535–1540. doi: 10.1007/s00415-002-0884-0. [DOI] [PubMed] [Google Scholar]
- 79.Comella CL. Sleep disorders in Parkinson’s disease: an overview. Mov Disord. 2007;22 (Suppl 17):S367–S373. doi: 10.1002/mds.21682. [DOI] [PubMed] [Google Scholar]
- 80.Porter B, Macfarlane R, Walker R. The frequency and nature of sleep disorders in a community-based population of patients with Parkinson’s disease. Eur J Neurol. 2008;15:50–54. doi: 10.1111/j.1468-1331.2007.01998.x. [DOI] [PubMed] [Google Scholar]
- 81.Verbaan D, van Rooden SM, Visser M, Marinus J, van Hilten JJ. Nighttime sleep problems and daytime sleepiness in Parkinson’s disease. Mov Disord. 2008;23:35–41. doi: 10.1002/mds.21727. [DOI] [PubMed] [Google Scholar]
- 82.Struck LK, Rodnitzky RL, Dobson JK. Circadian fluctuations of contrast sensitivity in Parkinson’s disease. Neurology. 1990;40:467–470. doi: 10.1212/wnl.40.3_part_1.467. [DOI] [PubMed] [Google Scholar]
- 83.Piccini P, et al. Diurnal worsening in Parkinson patients treated with levodopa [Italian] Riv Neurol. 1991;61:219–224. [PubMed] [Google Scholar]
- 84.van Hilten B, et al. Sleep disruption in Parkinson’s disease. Assessment by continuous activity monitoring. Arch Neurol. 1994;51:922–928. doi: 10.1001/archneur.1994.00540210094018. [DOI] [PubMed] [Google Scholar]
- 85.Whitehead DL, Davies AD, Playfer JR, Turnbull CJ. Circadian rest–activity rhythm is altered in Parkinson’s disease patients with hallucinations. Mov Disord. 2008;23:1137–1145. doi: 10.1002/mds.22057. [DOI] [PubMed] [Google Scholar]
- 86.Kallio M, et al. Heart rate variability in patients with untreated Parkinson’s disease. Eur J Neurol. 2000;7:667–672. doi: 10.1046/j.1468-1331.2000.00127.x. [DOI] [PubMed] [Google Scholar]
- 87.Senard JM, Chamontin B, Rascol A, Montastruc JL. Ambulatory blood pressure in patients with Parkinson’s disease without and with orthostatic hypotension. Clin Auton Res. 1992;2:99–104. doi: 10.1007/BF01819664. [DOI] [PubMed] [Google Scholar]
- 88.Devos D, et al. Heart rate variability and Parkinson’s disease severity. J Neural Transm. 2003;110:997–1011. doi: 10.1007/s00702-003-0016-8. [DOI] [PubMed] [Google Scholar]
- 89.Wakabayashi K, Takahashi H. Neuropathology of autonomic nervous system in Parkinson’s disease. Eur Neurol. 1997;38 (Suppl 2):2–7. doi: 10.1159/000113469. [DOI] [PubMed] [Google Scholar]
- 90.Langston J. The hypothalamus in Parkinson’s disease. Ann Neurol. 1978;3:129–133. doi: 10.1002/ana.410030207. [DOI] [PubMed] [Google Scholar]
- 91.Wirz-Justice A, Da Prada M, Reme C. Circadian rhythm in rat retinal dopamine. Neurosci Lett. 1984;45:21–25. doi: 10.1016/0304-3940(84)90323-9. [DOI] [PubMed] [Google Scholar]
- 92.Dearry A, Burnside B. Dopaminergic regulation of cone retinomotor movement in isolated teleost retinas: I. Induction of cone contraction is mediated by D2 receptors. J Neurochem. 1986;46:1006–1021. doi: 10.1111/j.1471-4159.1986.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 93.Raggi A, Bella R, Pennisi G, Neri W, Ferri R. Sleep disorders in Parkinson’s disease: a narrative review of the literature. Rev Neurosci. 2013;24:279–291. doi: 10.1515/revneuro-2013-0002. [DOI] [PubMed] [Google Scholar]
- 94.Braak H, Del Tredici K. Invited Article: Nervous system pathology in sporadic Parkinson disease. Neurology. 2008;70:1916–1925. doi: 10.1212/01.wnl.0000312279.49272.9f. [DOI] [PubMed] [Google Scholar]
- 95.Bordet R, et al. Study of circadian melatonin secretion pattern at different stages of Parkinson’s disease. Clin Neuropharmacol. 2003;26:65–72. doi: 10.1097/00002826-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 96.Fertl E, Auff E, Doppelbauer A, Waldhauser F. Circadian secretion pattern of melatonin in de novo parkinsonian patients: evidence for phase-shifting properties of l-dopa. J Neural Transm Park Dis Dement Sect. 1993;5:227–234. doi: 10.1007/BF02257677. [DOI] [PubMed] [Google Scholar]
- 97.Hartmann A, Veldhuis JD, Deuschle M, Standhardt H, Heuser I. Twenty-four hour cortisol release profiles in patients with Alzheimer’s and Parkinson’s disease compared to normal controls: ultradian secretory pulsatility and diurnal variation. Neurobiol Aging. 1997;18:285–289. doi: 10.1016/s0197-4580(97)80309-0. [DOI] [PubMed] [Google Scholar]
- 98.Aziz NA, Pijl H, Frolich M, Roelfsema F, Roos RA. Diurnal secretion profiles of growth hormone, thyrotrophin and prolactin in Parkinson’s disease. J Neuroendocrinol. 2011;23:519–524. doi: 10.1111/j.1365-2826.2011.02134.x. [DOI] [PubMed] [Google Scholar]
- 99.Pierangeli G, et al. Nocturnal body core temperature falls in Parkinson’s disease but not in multiple-system atrophy. Mov Disord. 2001;16:226–232. doi: 10.1002/mds.1039. [DOI] [PubMed] [Google Scholar]
- 100.Cagnacci A, et al. Effect of naloxone on body temperature in postmenopausal women with Parkinson’s disease. Life Sci. 1990;46:1241–1247. doi: 10.1016/0024-3205(90)90499-h. [DOI] [PubMed] [Google Scholar]
- 101.Suzuki K, et al. Circadian variation of core body temperature in Parkinson disease patients with depression: a potential biological marker for depression in Parkinson disease. Neuropsychobiology. 2007;56:172–179. doi: 10.1159/000119735. [DOI] [PubMed] [Google Scholar]
- 102.Videnovic A, et al. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol. 2014;71:463–469. doi: 10.1001/jamaneurol.2013.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Breen DP, et al. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol. 2014;71:589–595. doi: 10.1001/jamaneurol.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bolitho SJ, et al. Disturbances in melatonin secretion and circadian sleep–wake regulation in Parkinson disease. Sleep Med. 2014;15:342–347. doi: 10.1016/j.sleep.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 105.Boivin DB, et al. Circadian clock genes oscillate in human peripheral blood mononuclear cells. Blood. 2003;102:4143–4145. doi: 10.1182/blood-2003-03-0779. [DOI] [PubMed] [Google Scholar]
- 106.Takimoto M, et al. Daily expression of clock genes in whole blood cells in healthy subjects and a patient with circadian rhythm sleep disorder. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1273–R1279. doi: 10.1152/ajpregu.00126.2005. [DOI] [PubMed] [Google Scholar]
- 107.Teboul M, et al. Atypical patterns of circadian clock gene expression in human peripheral blood mononuclear cells. J Mol Med. 2005;83:693–699. doi: 10.1007/s00109-005-0697-6. [DOI] [PubMed] [Google Scholar]
- 108.Cai Y, Liu S, Sothern RB, Xu S, Chan P. Expression of clock genes Per1 and Bmal1 in total leukocytes in health and Parkinson’s disease. Eur J Neurol. 2009;17:550–554. doi: 10.1111/j.1468-1331.2009.02848.x. [DOI] [PubMed] [Google Scholar]
- 109.Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- 110.Harrell LE, Balagura S. The effects of dark and light on the functional recovery following lateral hypothalamic lesions. Life Sci. 1974;15:2079–2087. doi: 10.1016/0024-3205(74)90024-1. [DOI] [PubMed] [Google Scholar]
- 111.Willis GL, Turner EJ. Primary and secondary features of Parkinson’s disease improve with strategic exposure to bright light: a case series study. Chronobiol Int. 2007;24:521–537. doi: 10.1080/07420520701420717. [DOI] [PubMed] [Google Scholar]
- 112.Willis GL, Moore C, Armstrong SM. A historical justification for and retrospective analysis of the systematic application of light therapy in Parkinson’s disease. Rev Neurosci. 2012;23:199–226. doi: 10.1515/revneuro-2011-0072. [DOI] [PubMed] [Google Scholar]
- 113.Paus S, et al. Bright light therapy in Parkinson’s disease: a pilot study. Mov Disord. 2007;22:1495–1498. doi: 10.1002/mds.21542. [DOI] [PubMed] [Google Scholar]
- 114.Barraud Q, et al. Sleep disorders in Parkinson’s disease: the contribution of the MPTP non-human primate model. Exp Neurol. 2009;219:574–582. doi: 10.1016/j.expneurol.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 115.Fifel K, et al. Alteration of daily and circadian rhythms following dopamine depletion in MPTP treated non-human primates. PLoS ONE. 2014;9:e86240. doi: 10.1371/journal.pone.0086240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vezoli J, et al. Early presymptomatic and long-term changes of rest activity cycles and cognitive behavior in a MPTP-monkey model of Parkinson’s disease. PLoS ONE. 2011;6:e23952. doi: 10.1371/journal.pone.0023952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ben V, Bruguerolle B. Effects of bilateral striatal 6-OHDA lesions on circadian rhythms in the rat: a radiotelemetric study. Life Sci. 2000;67:1549–1558. doi: 10.1016/s0024-3205(00)00751-7. [DOI] [PubMed] [Google Scholar]
- 118.Isobe Y, Nishino H. Circadian rhythm of drinking and running-wheel activity in rats with 6-hydroxydopamine lesions of the ventral tegmental area. Brain Res. 2001;899:187–192. doi: 10.1016/s0006-8993(01)02223-5. [DOI] [PubMed] [Google Scholar]
- 119.Baier PC, et al. Circadian distribution of motor-activity in unilaterally 6-hydroxydopamine lesioned rats. Exp Brain Res. 2006;169:283–288. doi: 10.1007/s00221-005-0343-0. [DOI] [PubMed] [Google Scholar]
- 120.Gravotta L, Gavrila AM, Hood S, Amir S. Global depletion of dopamine using intracerebroventricular 6-hydroxydopamine injection disrupts normal circadian wheel-running patterns and PERIOD2 expression in the rat forebrain. J Mol Neurosci. 2011;45:162–171. doi: 10.1007/s12031-011-9520-8. [DOI] [PubMed] [Google Scholar]
- 121.Kudo T, Loh DH, Truong D, Wu Y, Colwell CS. Circadian dysfunction in a mouse model of Parkinson’s disease. Exp Neurol. 2011;232:66–75. doi: 10.1016/j.expneurol.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 122.Willison LD, Kudo T, Loh DH, Kuljis D, Colwell CS. Circadian dysfunction may be a key component of the non-motor symptoms of Parkinson’s disease: insights from a transgenic mouse model. Exp Neurol. 2013;243:57–66. doi: 10.1016/j.expneurol.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Morrison PJ. Accurate prevalence and uptake of testing for Huntington’s disease. Lancet Neurol. 2010;9:1147. doi: 10.1016/S1474-4422(10)70287-8. [DOI] [PubMed] [Google Scholar]
- 124.Harper PS. The epidemiology of Huntington’s disease. Hum Genet. 1992;89:365–376. doi: 10.1007/BF00194305. [DOI] [PubMed] [Google Scholar]
- 125.Snell RG, et al. Relationship between trinucleotide repeat expansion and phenotypic variation in Huntington’s disease. Nat Genet. 1993;4:393–397. doi: 10.1038/ng0893-393. [DOI] [PubMed] [Google Scholar]
- 126.Gusella JF, et al. A polymorphic DNA marker genetically linked to Huntington’s disease. Nature. 1983;306:234–238. doi: 10.1038/306234a0. [DOI] [PubMed] [Google Scholar]
- 127.Gilliam TC, et al. A DNA segment encoding two genes very tightly linked to Huntington’s disease. Science. 1987;238:950–952. doi: 10.1126/science.2890209. [DOI] [PubMed] [Google Scholar]
- 128.A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. No authors listed. [DOI] [PubMed] [Google Scholar]
- 129.Bamford KA, Caine ED, Kido DK, Cox C, Shoulson I. A prospective evaluation of cognitive decline in early Huntington’s disease: functional and radiographic correlates. Neurology. 1995;45:1867–1873. doi: 10.1212/wnl.45.10.1867. [DOI] [PubMed] [Google Scholar]
- 130.Paulsen JS, Ready RE, Hamilton JM, Mega MS, Cummings JL. Neuropsychiatric aspects of Huntington’s disease. J Neurol Neurosurg Psychiatry. 2001;71:310–314. doi: 10.1136/jnnp.71.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stoy N, McKay E. Weight loss in Huntington’s disease. Ann Neurol. 2000;48:130–131. [PubMed] [Google Scholar]
- 132.Goodman AO, et al. Asymptomatic sleep abnormalities are a common early feature in patients with Huntington’s disease. Curr Neurol Neurosci Rep. 2011;11:211–217. doi: 10.1007/s11910-010-0163-x. [DOI] [PubMed] [Google Scholar]
- 133.Hansotia P, Wall R, Berendes J. Sleep disturbances and severity of Huntington’s disease. Neurology. 1985;35:1672–1674. doi: 10.1212/wnl.35.11.1672. [DOI] [PubMed] [Google Scholar]
- 134.Emser W, Brenner M, Stober T, Schimrigk K. Changes in nocturnal sleep in Huntington’s and Parkinson’s disease. J Neurol. 1988;235:177–179. doi: 10.1007/BF00314313. [DOI] [PubMed] [Google Scholar]
- 135.Silvestri R, et al. Sleep features in Tourette’s syndrome, neuroacanthocytosis and Huntington’s chorea. Neurophysiol Clin. 1995;25:66–77. doi: 10.1016/0987-7053(96)81034-3. [DOI] [PubMed] [Google Scholar]
- 136.Cuturic M, Abramson RK, Vallini D, Frank EM, Shamsnia M. Sleep patterns in patients with Huntington’s disease and their unaffected first-degree relatives: a brief report. Behav Sleep Med. 2009;7:245–254. doi: 10.1080/15402000903190215. [DOI] [PubMed] [Google Scholar]
- 137.Arnulf I, et al. Rapid eye movement sleep disturbances in Huntington disease. Arch Neurol. 2008;65:482–488. doi: 10.1001/archneur.65.4.482. [DOI] [PubMed] [Google Scholar]
- 138.Morton AJ, et al. Disintegration of the sleep–wake cycle and circadian timing in Huntington’s disease. J Neurosci. 2005;25:157–163. doi: 10.1523/JNEUROSCI.3842-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kudo T, et al. Dysfunctions in circadian behavior and physiology in mouse models of Huntington’s disease. Exp Neurol. 2011;228:80–90. doi: 10.1016/j.expneurol.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lazar AS, et al. Sleep, diurnal preference, health, and psychological well-being: a prospective single-allelic-variation study. Chronobiol Int. 2012;29:131–146. doi: 10.3109/07420528.2011.641193. [DOI] [PubMed] [Google Scholar]
- 141.Epping EA, Paulsen JS. Depression in the early stages of Huntington disease. Neurodegener Dis Manag. 2011;1:407–414. doi: 10.2217/nmt.11.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goodman AO, Morton AJ, Barker RA. Identifying sleep disturbances in Huntington’s disease using a simple disease-focused questionnaire. PLoS Curr. 2010;2:RRN1189. doi: 10.1371/currents.RRN1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Aziz NA, Anguelova GV, Marinus J, Lammers GJ, Roos RA. Sleep and circadian rhythm alterations correlate with depression and cognitive impairment in Huntington’s disease. Parkinsonism Relat Disord. 2010;16:345–350. doi: 10.1016/j.parkreldis.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 144.Taylor N, Bramble D. Sleep disturbance and Huntingdon’s disease. Br J Psychiatry. 1997;171:393. doi: 10.1192/bjp.171.4.393c. [DOI] [PubMed] [Google Scholar]
- 145.Videnovic A, Leurgans S, Fan W, Jaglin J, Shannon KM. Daytime somnolence and nocturnal sleep disturbances in Huntington disease. Parkinsonism Relat Disord. 2009;15:471–474. doi: 10.1016/j.parkreldis.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 146.Starr A. A disorder of rapid eye movements in Huntington’s chorea. Brain. 1967;90:545–564. doi: 10.1093/brain/90.3.545. [DOI] [PubMed] [Google Scholar]
- 147.Wiegand M, et al. Nocturnal sleep in Huntington’s disease. J Neurol. 1991;238:203–208. doi: 10.1007/BF00314781. [DOI] [PubMed] [Google Scholar]
- 148.van Vugt JP, van Hilten BJ, Roos RA. Hypokinesia in Huntington’s disease. Mov Disord. 1996;11:384–388. doi: 10.1002/mds.870110406. [DOI] [PubMed] [Google Scholar]
- 149.van Vugt JP, et al. Quantitative assessment of daytime motor activity provides a responsive measure of functional decline in patients with Huntington’s disease. Mov Disord. 2001;16:481–488. doi: 10.1002/mds.1097. [DOI] [PubMed] [Google Scholar]
- 150.Hurelbrink CB, Lewis SJ, Barker RA. The use of the Actiwatch-Neurologica system to objectively assess the involuntary movements and sleep–wake activity in patients with mild-moderate Huntington’s disease. J Neurol. 2005;252:642–647. doi: 10.1007/s00415-005-0709-z. [DOI] [PubMed] [Google Scholar]
- 151.Pouladi MA, Morton AJ, Hayden MR. Choosing an animal model for the study of Huntington’s disease. Nat Rev Neurosci. 2013;14:708–721. doi: 10.1038/nrn3570. [DOI] [PubMed] [Google Scholar]
- 152.Shirbin CA, et al. Cortisol and depression in pre-diagnosed and early stage Huntington’s disease. Psychoneuroendocrinology. 2013;38:2439–2447. doi: 10.1016/j.psyneuen.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 153.Kantor S, Szabo L, Varga J, Cuesta M, Morton AJ. Progressive sleep and electroencephalogram changes in mice carrying the Huntington’s disease mutation. Brain. 2013;136:2147–2158. doi: 10.1093/brain/awt128. [DOI] [PubMed] [Google Scholar]
- 154.Fisher SP, et al. Longitudinal analysis of the electroencephalogram and sleep phenotype in the R6/2 mouse model of Huntington’s disease. Brain. 2013;136:2159–2172. doi: 10.1093/brain/awt132. [DOI] [PubMed] [Google Scholar]
- 155.Fahrenkrug J, Popovic N, Georg B, Brundin P, Hannibal J. Decreased VIP and VPAC2 receptor expression in the biological clock of the R6/2 Huntington’s disease mouse. J Mol Neurosci. 2007;31:139–148. doi: 10.1385/jmn/31:02:139. [DOI] [PubMed] [Google Scholar]
- 156.Rudenko O, Tkach V, Berezin V, Bock E. Detection of early behavioral markers of Huntington’s disease in R6/2 mice employing an automated social home cage. Behav Brain Res. 2009;203:188–199. doi: 10.1016/j.bbr.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 157.Bode FJ, et al. Increased numbers of motor activity peaks during light cycle are associated with reductions in adrenergic α-receptor levels in a transgenic Huntington’s disease rat model. Behav Brain Res. 2009;205:175–182. doi: 10.1016/j.bbr.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 158.Pietropaolo S, Delage P, Cayzac S, Crusio WE, Cho YH. Sex-dependent changes in social behaviors in motor pre-symptomatic R6/1 mice. PLoS ONE. 2011;6:e19965. doi: 10.1371/journal.pone.0019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Loh DH, Kudo T, Truong D, Wu Y, Colwell CS. The Q175 mouse model of Huntington’s disease shows gene dosage- and age-related decline in circadian rhythms of activity and sleep. PLoS ONE. 2013;8:e69993. doi: 10.1371/journal.pone.0069993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Heuser IJ, Chase TN, Mouradian MM. The limbic-hypothalamic-pituitary-adrenal axis in Huntington’s disease. Biol Psychiatry. 1991;30:943–952. doi: 10.1016/0006-3223(91)90007-9. [DOI] [PubMed] [Google Scholar]
- 161.Pallier PN, et al. Pharmacological imposition of sleep slows cognitive decline and reverses dysregulation of circadian gene expression in a transgenic mouse model of Huntington’s disease. J Neurosci. 2007;27:7869–7878. doi: 10.1523/JNEUROSCI.0649-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pallier PN, Morton AJ. Management of sleep/wake cycles improves cognitive function in a transgenic mouse model of Huntington’s disease. Brain Res. 2009;1279:90–98. doi: 10.1016/j.brainres.2009.03.072. [DOI] [PubMed] [Google Scholar]
- 163.Wood NI, et al. Responses to environmental enrichment differ with sex and genotype in a transgenic mouse model of Huntington’s disease. PLoS ONE. 2010;5:e9077. doi: 10.1371/journal.pone.0009077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Aziz NA, et al. Delayed onset of the diurnal melatonin rise in patients with Huntington’s disease. J Neurol. 2009;256:1961–1965. doi: 10.1007/s00415-009-5196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kalliolia E, et al. Plasma melatonin is reduced in Huntington’s disease. Mov Disord. 2014 doi: 10.1002/mds.26003. [DOI] [PubMed] [Google Scholar]
- 166.Saleh N, et al. Neuroendocrine disturbances in Huntington’s disease. PLoS ONE. 2009;4:e4962. doi: 10.1371/journal.pone.0004962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Aziz NA, et al. Increased hypothalamic–pituitary–adrenal axis activity in Huntington’s disease. J Clin Endocrinol Metab. 2009;94:1223–1228. doi: 10.1210/jc.2008-2543. [DOI] [PubMed] [Google Scholar]
- 168.Kalsbeek A, Buijs RM, van Heerikhuize JJ, Arts M, van der Woude TP. Vasopressin-containing neurons of the suprachiasmatic nuclei inhibit corticosterone release. Brain Res. 1992;580:62–67. doi: 10.1016/0006-8993(92)90927-2. [DOI] [PubMed] [Google Scholar]
- 169.van Wamelen DJ, et al. Suprachiasmatic nucleus neuropeptide expression in patients with Huntington’s Disease. Sleep. 2013;36:117–125. doi: 10.5665/sleep.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kudo T, et al. Circadian dysfunction in response to in vivo treatment with the mitochondrial toxin 3-nitropropionic acid. ASN Neuro. 2014;6:e00133. doi: 10.1042/AN20130042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Robinson I, Reddy AB. Molecular mechanisms of the circadian clockwork in mammals. FEBS Lett. 2014;588:2477–2483. doi: 10.1016/j.febslet.2014.06.005. [DOI] [PubMed] [Google Scholar]