Abstract
Over the past decade, there has been increasing biochemical evidence that the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway is aberrantly activated in malignant cells from patients with a wide spectrum of cancers of the blood and immune systems. The emerging availability of small molecule inhibitors of JAK kinases and other signaling molecules in the JAK-STAT pathway has allowed preclinical studies validating an important role of this pathway in the pathogenesis of many hematologic malignancies, and provided motivation for new strategies for treatment of these diseases. Here, a roundtable panel of experts reviews the current preclinical and clinical landscape of the JAK-STAT pathway in acute lymphoid and myeloid leukemias, lymphomas and myeloma, and chronic myeloid neoplasms.
Keywords: JAK/STAT pathway, JAK inhibitors, hematological malignancies
Introduction
Though patients with hematological malignancies appear to have been well served by translational research since the early 1980s, it is has only been the past two decades when the unraveling of some of the principal molecular events paved the way to define rationale molecular targets for treatment [1]. For some rare malignancies, such as chronic myeloid leukemia (CML), the identification of a definitive initiating molecular event, the BCR-ABL1 fusion gene, in 1985 led to efforts to develop a remarkably successful treatment with the ABL1 tyrosine kinase inhibitors (TKIs) [2]. Today, this is considered one of the most significant successes in cancer medicine. It is, however, of some interest, that despite this claim, there remains some debate as to whether the ‘initiating molecular event’ is indeed the first molecular event in all patients with CML or not [3].
Around the same time as the aberrant BCR-ABL1 fusion gene was discovered, another molecular pathway, the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway that regulate cell differentiation, proliferation and survival, was also described [4]. Further elucidation and insights into potential initiating events in candidate hematological and other cancers, however, occurred only in the past decade, following the seminal discovery of the JAK2V617F mutation in patients with myeloproliferative neoplasms (MPN) in 2005 [5-8]. The JAK2V617F mutation confers constitutive kinase activity resulting in cytokine hypersensitivity and abnormal hematopoiesis in such patients. These observations led to efforts in developing JAK inhibitors as targeted therapies for patients with MPN.
Much of what we have learned about the JAK-STAT pathway stems from the work conducted on the molecular basis of the effects of various cytokines. Preclinical research on cytokines, such as interferons (IFN), erythropoietins, and diverse growth factors (GFs) confirmed their importance for hematopoiesis, cell proliferation, survival, differentiation, and immune and inflammatory responses [9,10]. It is now well established that the JAK-STAT pathway is pivotal to signaling by cytokine receptors and select GFs, and centrally implicated in diverse myeloid and lymphoid malignancies as well as several solid tumors (Figure 1) [11]. This understanding has now paved the way for new targeted treatments to be developed for diseases that appear dependent on the JAK-STAT signaling [12,13].
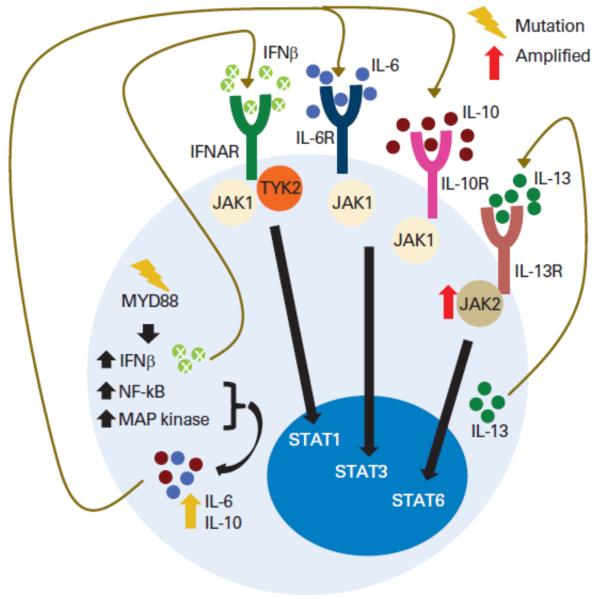
Figure 1.
Pathologic activation of autocrine JAK signaling pathways in hematologic malignancies. Schematic depiction of the multiple autocrine signaling loops identified in B-lymphoma cells: enhanced interleukin-13 (IL-13) signaling via amplified JAK2 with downstream activation of STAT6, MYD88 mutations activating JAK-STAT3 signaling through IL-6 secretion, and activation of IL-6 and IL-10 secretion and activation of JAK-STAT1 signaling by type I interferons (IFN). Abbreviations: IFNAR, interferon alpha receptor; MAP, mitogen-activated protein; NF-κB, nuclear factor κB; RTK, receptor tyrosine kinase; TYK2, tyrosine kinase 2. Adapted with permission from [28].
This review, based in part on a roundtable discussion amongst academic experts at the 54th American Society of Hematology Annual Meeting in Atlanta, Georgia, focuses on recent advances in the understanding of the biology of the JAK-STAT pathway in hematological malignancies, and discusses the potential therapeutic benefits of JAK inhibitors for such patients.
Cytokines and the JAK-STAT Signaling Pathway
The JAK family is comprised of four cytoplasmic tyrosine kinases, JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2), which exhibit considerable diversity in their functions: JAK1 and JAK2 having a broader role in hematopoiesis, neural development, host defense and now considered to have a causal role in a number of hematological malignancies; JAK3 and TYK2 are implicated principally in immune responses. The STAT family comprise of seven DNA-binding proteins, STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b and STAT 6. Of the STAT proteins, STAT3, STAT5a and STAT5b have a broad and critical transcription role in survival, proliferation and self-renewal; in contrast, STAT1, STAT2, STAT4 and STAT6 appear to have a more restricted role, primarily in immunoregulation [14,15].
Both JAKs and STATs mediate signaling by binding with the cytoplasmic domains of various cytokine and GF receptors. Recent efforts have confirmed the notion of the previously inactive JAKs, which are in close proximity to the cytokine/GF receptor’s cytoplasmic region, being activated upon binding with the cognate cytokine/GF and resulting in cross-phosphorylation and receptor tyrosine phosphorylation. This in turn creates selective binding sites for the STAT proteins. Upon binding, these proteins become tyrosine-phosphorylated, then dimerize and translocate to the nucleus, where they function as transcription factors by regulating gene expression and affect the molecularly distinct disease phenotypes [11,14]. In addition to STAT activation, JAK signaling also activates other molecular pathways, such as the mitogen-activated protein kinase (MAPK), AKT/mammalian target of rapamycin (mTOR) and phosphatidylinositol-3′-kinase (P13K) cascades [16]. STAT proteins can also be activated by other kinases, in particular SRC family kinases [17].
The notion of an aberrant activation of the JAK-STAT pathway being associated with malignancy was suggested by the landmark observations of constitutive JAK-STAT activation in human T-lymphotropic virus (HTLV-1) infected T-cells and Epstein-Barr virus (EBV)-associated lymphoma cell lines in 1995 [18,19]. This initial evidence was confirmed more recently by the recognition of recurrent genetic alterations in JAKs and STATs in patients with diverse malignancies [20]. It is now well established that JAK2 mutations are frequently associated with BCR-ABL1-negative MPN, which include polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). The remarkable presence of the JAK2V617F mutation in the majority of patients with MPN paved the way towards the development of the first ATP-competitive inhibitor of JAK1 and JAK2, ruxolitinib, which was licensed in for patients with intermediate- or high–risk myelofibrosis (MF) in USA in late 2011 and Europe in early 2013[21,22]. It is of interest that JAK-STAT pathway deregulation has been noted in almost all MPN patients, independently of the presence or absence of JAK2V617F mutation. The observation that this drug was able to accord substantial symptomatic relief and a reduction of splenomegaly in most patients with advanced MF, but without a major alteration of the candidate molecular correlates, such as a reduction in the JAK2 mutant allele burden, suggested that its principal action was on the dysregulated JAK-STAT pathway. Dysregulation of the JAK-STAT pathway has now been described in lymphoid and myeloid malignancies [23].
In addition to direct activation of JAK/STAT signaling and the resulting transcriptional responses in malignant cells, several “non-canonical” roles for JAKs and STATs in the pathogenesis of hematological neoplasms have been described. JAK2 plays a novel role in the nucleus, mediating phosphorylation of histone H3 and altering epigenetic regulation of MYC [24]. Serine phosphorylation at positions S725 and S779 is required for hematologic transformation by a constitutively active STAT5 mutant [25], while other studies have identified a role for mitochondrial STAT3 in RAS-mediated transformation [26].
Lymphoid neoplasms
Considerable progress has been made in unraveling the molecular pathogenesis related to the constitutively activated JAK/STAT pathway in lymphoid neoplasms of both B-cell and T-cell lineages [27,28]. The JAK/STAT pathway and various cytokines which bind to the receptors IL-2, IL-4, IL-7, IL-9, L-15 and IL-21, all of which harbor the common γ chain, play a principal role in the regulation of B, T and NK cells.
The importance of a dysregulated JAK/STAT signaling was recognized initially in primary mediastinal B-cell lymphoma (PMBL) and classical Hodgkin lymphoma (cHL), two subtypes of lymphomas that are histologically distinct but share a common genetic basis [29,30]. The molecular signatures of these lymphomas is driven by the recurrent amplicon involving JAK2 on chromosome 9 and also the JMJD2C gene, which is involved in lymphomagenesis. JMJD2C appears to cooperate with JAK2 in activating genes, possibly including RANBP6, epigenetically (Figure 2). Furthermore, cell line models of PMBL and cHL are inhibited by JAK inhibitors, lending support for the candidacy of these agents in the clinical treatment of these lymphomas [31].
Figure 2.
JAK2 and JMJDC2 cooperate in the epigenetic deregulation of oncogenes. Amplification of 9p24 increases the copy number of both JAK2 and JMJD2C, both of which modify histone H3 tails and impede the recruitment of the heterochromatin protein HP1a. The resulting active chromatin structure facilitates the expression of MYC, and perhaps JAK2, JMJD2C, and IL4R. Adapted with permission from [31].
The underlying genetics of these lymphomas, and indeed of several other subtypes, where the importance of JAK/STAT signaling is recognized, remains rather poorly understood. Recently, further progress have been made with the description of loss-of-function mutations in the suppressor of cytokine signaling-1 (SOCS1) and deletions in the tumor suppressor PTPN2, resulting in activation of JAK/STAT signaling in PMBL and cHL [32,33]. Other functionally important candidate JAK2 fusion proteins include SEC31A-JAK2, associated with t(4;9)(q21;p24), recognized in cHL and activated B-cell-like (ABC) molecular subtype of diffuse large B-cell lymphoma (DLBCL) [34,35]. This mutant fusion protein acts a constitutively activated tyrosine kinase and is inhibited by JAK inhibitors. The molecular signature characterized by constitutive activation of the NF-κB pathway in the ABC-DLBCL, a high level of expression of STAT3, IL-6 and IL-10 promoting cell proliferation and survival, has been validated by several investigators (Figure 3) [36-38]. ABC-DLBCL cell lines exhibiting high levels of STAT3 were also found to be inhibited by a JAK inhibitor and there was synergy when used concomitantly with a NF-κB inhibitor, suggesting the potential to target these unique molecular abnormalities therapeutically (Figure 4) [36]. Furthermore, a phase I study of an oral JAK2/FLT3 inhibitor, pacritinib (SB1518), in patients with chronic lymphoid neoplasms, including relapsed/refractory cHL, follicular lymphoma and DLBL, has suggested some clinical benefits [39,40]}.
Figure 3.

A subset of ABC-DLBCL shows activation of STAT3 and IL-6 and IL-10 expression. A. Expression of STAT3 target genes and ABC DLBCL signature genes in STAT3-high ABC DLBCL, STAT3-low ABC DLBCL, and GCB DLBCL. B. STAT3 mRNA, IL-6 mRNA, and IL-10 mRNA levels (mean ± SD) among STAT3-high ABC DLBCL, STAT3-low ABC DLBCL, and GCB DLBCL. C. Immunostaining with a p-STAT3 (Tyr705)-specific antibody showing positive staining in ABC DLBCL (left panel). Adapted with permission from [36].
Figure 4.
Molecular cross-talk of NF-κB and JAK/STAT pathways in ABC-DLBCL. Adapted with permission from [36].
An autocrine cytokine signaling loop initiated by a mutation (L256P) in MYD88, an adaptor protein in toll-like receptor (TLR) and interleukin-1 receptor signaling, which results in constitutive activation of JAKs and NF-κB has been demonstrated in patients with various subtypes of lymphomas [41]. The principal cytokines involved in this loop appear to be IL-6, IL-10 and IL-13. The MYD88 L265P mutation has been found in patients with ABC-DLBL, primary central nervous system lymphoma, primary cutaneous lymphoma, marginal zone B-cell lymphomas (MZBL) and Waldenström’s macroglobulinemia (WM) with increasing frequency [42-44]. Treon and colleagues have noted that MYD88 L256P induces phosphorylation of Bruton’s tyrosine kinase (BTK), while the inhibition of BTK or IRAK-1 and -4 kinase activity induces apoptosis of WM cells [45]. An on-going multicenter trial of ibrutinib, an irreversible small molecule inhibitor of BTK, has demonstrated significant response rates in patients with relapsed/refractory ABC-DLBCL, but interesting, no activity of note in patients with germinal-center B-cell-like (GCB) subtype of DLBCL [46]. Another area of interest and unmet clinical need is peripheral T-cell lymphoma. In cutaneous T-cell lymphoma, constitutive activation of STAT3 and STAT5 in the malignant cells has been demonstrated [47]. In anaplastic large cell lymphoma, activation of STAT3 by the NPM1-ALK fusion tyrosine kinase is essential for lymphomagenesis [48], whereas the role of STAT5 activation is less clear, as NPM1-ALK and STAT5A reciprocally repress one another, implying a tumor suppressor role for STAT5A [49].
The JAK-STAT pathway has also been implicated in precursor lymphoid (lymphoblastic) leukemias. T-cell acute lymphoblastic leukemia (T-ALL) was the first human malignancy to be associated with an aberration in a JAK gene with the discovery of the TEL-JAK2 fusion protein [50]. The TEL-JAK2 fusion protein constitutively activates STAT1, STAT5, PI3K, RAS/ERK, p38, and NF-κB signaling pathways. Several similar fusion proteins, including the pericentriolar material (PCM1)-JAK2 fusion protein, sometimes associated with a t(8;9) translocation, PAX5-JAK2, and RPN1-JAK2 have been described in ALL [51-53]. A common theme of the fusion partners of JAK2 is a domain that mediates self-association or oligomerization, leading to constitutive activation of the kinase.
In addition to JAK2 fusions, point mutations in JAK family genes have been described in the lymphoblastic leukemias. Mutations in JAK1 and JAK3 have been reported in about 18% of T-ALLs and 3% of B-ALL [54-56]. These mutations frequently occur within the JAK pseudokinase domain, in some cases at the residue homologous to V617 in JAK2 (e.g., JAK1V658F), causing constitutive activation and making them candidates for JAK inhibitor therapy [57]. Somatically acquired mutations at the JAK2R683 position have been described in childhood Down’s syndrome (DS)-associated B-cell ALL, and mutations in JAK1 in patients with ABC-DLBL, T-ALL, adult T-cell leukemia/lymphoma, (ATLL) and NK-cell lymphoma [58]. A unique mutation in the JAK2 gene in a DS patient with precursor B-cell ALL, known as ΔIREED mutation, which involves the deletion of five amino-acid residues from position 682 to 686 in the pseudokinase domain of JAK2, has also been described [59]. Mutations involving SSBP2 in t(5;9) and others involving the catalytic tyrosine kinase domain of JAK2 have been identified in B-lineage ALL in patients with or without DS [60,61]. Finally, RNAi screening of T-ALL cell lines recently revealed a dependence on TYK2 and downstream pSTAT1-mediated BCL2 expression for proliferation and survival of T-ALL patient samples, either via novel activating mutations in TYK2 (~25%) or through IL-10 signaling [62]. While these results validate TYK2 as a novel target for therapy in T-ALL, further studies of larger patient cohorts and the development of specific TYK2 inhibitors will be necessary to move this into the clinic.
Additional support for the role of JAK/STAT in B-lymphoid leukemogenesis comes from the identification of two cytokine receptors, the cytokine receptor-like factor-2 (CRLF2), formerly known as thymic stromal lymphopoietin receptor (TSLPR) and IL7RA, which binds JAK2 in lymphoid cells [63-68]. Expression of CRLF2 has been found in approximately one third of all children with DS who develop B-ALL, and in some cases, T-ALL. Many of these patients also harbor JAK2 mutations, leading to the hypothesis that the CRLF2 receptor functions as a scaffold for constitutive signaling by the mutant JAK2 [69]. Mullighan and colleagues also observed that rearrangement of CRLF2 can be associated with alterations of IKZF1 and is more common in children of Hispanic ethnicity [70]. Genomic analysis of high-risk childhood B-ALL with IKZF1 mutations has defined a “Ph-like” gene expression profile similar to BCR-ABL1-expressing B-ALL. While about half of these cases harbor JAK2 mutations and CRLF2 overexpression, others have rearrangements involving multiple TK genes, including ABL1, JAK2, PDGFRB, and EPOR [71], with evidence of dysregulated TK signaling including STAT5 activation.
Here, it should be noted that there are significant differences in the signaling abnormalities induced by fusion proteins involving JAK2 and other TKs, which are generally strongly activated kinases inducing high levels of cytosolic tyrosyl phosphorylation, and signaling by JAK point mutants, which may require scaffolding receptors and generally induce low levels of phosphorylation that can be further augmented by cytokines binding to these receptors [11].
Progress has also been made in characterizing the molecular underpinnings, in particular JAK3 mutations and the relationship to the JAK/STAT pathway, in the rare childhood early T-cell precursor (ETP)-ALL and natural killer/T-cell lymphoma (NKTCL), both of which carry a grave prognosis. A high frequency of activating mutations in genes regulating cytokine receptor and RAS signaling, similar to those seen in myeloid malignancies, have been observed in patients with ETP-ALL [72]. In patients with NKTCL, somatic activating mutations in JAK3 (A572V and A573V) have been reported [73,74]. These JAK3 mutations were noted to be involved in cytokine-independent JAK/STAT constitutive activation leading to increased cell growth, and cell lines exposed to a novel pan-JAK inhibitor, CP-690550, showed a dose-dependent reduction of phosphorylated STAT5, reduced cell viability, and increased apoptosis. It would therefore be reasonable to consider JAK inhibitors to inhibit cytokine receptor and JAK signaling in these candidate disorders with an unmet need.
Plasma cell neoplasms
Multiple myeloma (MM) relies heavily on its microenvironment for survival and the interaction between MM cells and bone marrow stromal cells (BMSC) is critical to the tumor growth as well as survival. This interaction with the tumor microenvironment results in increased bone resorption, suppression of bone formation, and increased angiogenesis, all leading to enhanced tumor growth and drug resistance. MM clonal cells and BMSC interact by both direct cell-cell contact and paracrine signaling using cytokines, leading to increased cytokine secretion both by the MM cells BMSC, in particular VEGF, IL6 and IGF (Figure 5). The increased cytokine levels lead to an up-regulation of signaling pathways within myeloma cells that ultimately results in increased transcription of proliferation related genes. Relevant cytokine-induced signaling pathways include NF-κB, JAK/STAT3, PI3K/AKT, and RAS/MEK/MAPK pathway. The JAK/STAT pathway is stimulated by cytokines, especially IL-6. High levels of constitutively active STAT3 have been reported in plasma cells and BMSCs from MM patients, causing induction of anti-apoptotic proteins MCL-1 and BCL-XL [75].
Figure 5.
JAK/STAT pathways in multiple myeloma. Binding of MM cells to bone marrow stroma cells (BMSC) activated MM cell proliferation, survival, drug resistance, and migration via adhesion- and cytokine-mediated pathways. Binding of MM cells to BMSCs increases secretion of multiple cytokines (IL-6, IGF-1, VEGF, SDF-1) from BMSCs and MM cells, with subsequent activation of several signaling pathways, including ERK, JAK/STAT3, and PI3K/AKT, and their downstream targets including cytokines (IL-6, IGF-1, VEGF) and anti-apoptotic proteins (Bcl-xL, IAPs, MCL-1). Adhesion-mediated activation of NF-B up-regulates several; adhesion molecules (ICAM-1, VCAM-1) on both MM cells and BMSCs, further enhancing adhesion of MM cells to BMSCs. Adapted with permission from Hideshima et al., Blood 2004;104:607-618.
IL-6, secreted primarily by BMSC, is the primary cytokine necessary for maturation of plasma cells. Even in the absence of activated T-cells, IL-6 is able to stimulate immunoglobulin G (IgG) secretion in non-dividing B cells, suggesting IL-6 is sufficient for terminal maturation of plasma cells [76]. Oversecretion of IL-6 in transgenic mice causes polyclonal plasmacytosis, resulting in a 120- to 400-fold increase in IgG1 and plasma cell infiltration of the reticuloendothelial system, lungs, and kidneys [77]. As in animal models, IL-6 plays a role in plasma cell dyscrasias in humans. Patients with higher International Staging System (ISS) or with plasma cell leukemia have higher IL-6 levels that correlate with shorter survival [78]. Thirty-five percent and 100% of patients with MM and plasma cell leukemia, respectively, have elevated levels of IL-6 [79].
IL-6 activation of STAT3 via JAK was first demonstrated with in vitro studies on U266 cell lines, a human autocrine IL-6 dependent MM cell line. This cell line has constitutive STAT3 activation and overexpression of BCL-XL, an anti-apoptotic protein. Treatment with an IL-6 superantagonist reduces STAT3 dimerization, downstream signaling, and cell survival [80]. Similar finding were observed in clinical samples of patients with MM, in which inhibition of constitutively activated STAT3 reduced cell viability [81]. This data suggest that JAK is an important mediator for STAT3 activation and IL-6 dependent cell survival in plasma cell neoplasms.
STAT3 has four major targets relevant to MM: MCL-1 (myeloid cell leukemia-1), VEGF, BCL-3, and microRNA-21 (miR-21). MCL-1 belongs to the BCL-2 family and prevents oligomerization of BAK or BAX and subsequent cytochrome C release and apoptosis. In several MM cell lines as well as patient cells, knockdown of MCL-1 results in increased apoptosis [82]. Reduction of MCL-1 levels with the tyrphostin JAK2 inhibitor AG490 also reduced survival [83], although it should be noted that this inhibitor is relatively non-specific. VEGF secretion by MM cells lead to increased IL-6 production by the BMSC and activates a positive paracrine feedback loop between MM and BMSC cells, resulting in upregulation of the MCL-1 and reduction of apoptosis [84]. VEGF also promotes angiogenesis, which is a risk factor for progression from monoclonal gammopathy of unknown significance (MGUS) to MM and a prognostic marker in MM [85]. STAT3 also targets BCL-3, an IκB family protein, which is particularly interesting since this intersects the NF-κB pathway. Gene expression profiles of newly diagnosed MM patients show that higher levels of BCL-3 correlate with shorter progression free and overall survival [86]. Overexpression of BCL-3 increases apoptosis, but its underexpression does not affect viability [87]. The last major target is miR-21, an oncogenic miRNA that is upregulated in many malignancies [88]. The binding site of STAT3 on miR-21 is conserved across vertebrates, suggesting a critical role for STAT3 [89]. Knock down of STAT3 results in decreased levels of miR-21 and cell viability. Thus, JAK/STAT3 and its downstream targets play a central role mediating IL-6 dependent myeloma cell survival, and inhibition of this pathway could be a rational therapeutic strategy in select patients with MM.
Multiple points of this pathway have been targeted in pre-clinical data, including JAK kinases and STAT3 inhibition. INCB000020 is a pan-JAK inhibitor that decreases cell viability via dose-dependent inhibition of STAT3 phosphorylation in both the IL-6-dependent MM cell line INA-6 and in primary myeloma cells [90]. Increasing concentrations of INCB20 resulted in decreased STAT3 phosphorylation and reduction of mean tumor volume in a xenograft tumor model, confirming successful inhibition of JAK and consequent tumor suppression. INCB16562, a JAK1/2 inhibitor, can inhibit a dose-dependent phosphorylation of STAT3 and cell growth in INA-6 and in primary myeloma cells [91]. While it has only a modest effect on cell growth as a single agent, INCB16562 works synergistically with bortezomib and melphalan in cell culture and in a xenograft model. Ruxolitinib, a JAK inhibitor, can inhibit myeloma growth in IL-6 dependent cell lines, but did not affect BMSC or IL-6 production [92]. These findings were confirmed on a myeloma cell isolated from IL-6-dependent plasma cell leukemia. Other JAK2 inhibitors such as TG101209 induce dose and time dependent inhibition of cell cycle progression and induction of apoptosis in a variety of MM cell lines [93]. Evaluation of TG101209 against U266 cell line and primary patient cells, which have a mix of CD45 positive and negative cells, demonstrated more profound cytotoxicity on the CD45+ population relative to the CD45- cells. In the preclinical studies, significant synergy was observed when the JAK2 inhibitor was combined with bortezomib. Thus, inhibition of JAK kinases may have a future role, but would likely be limited to IL-6 dependent myeloma, and then only in combination with other chemotherapies.
STAT3, a second potential target in multiple myeloma, is particularly intriguing since STAT3 is not required for normal cells survival [94]. Nifuroxazide, an oral nitrofuran antibiotic, was identified as an inhibitor STAT3, JAk2, and TYK2 in a library of compounds. In IL-6-dependent cell lines overexpressing of phosphorylated STAT3, it decreased MCL-1 expression and myeloma cell viability, even in the presence of BMSC. Like JAK inhibitors, combination with other agents improved its cytotoxicity. A second agent, FLLL32, derived from a natural compound curcumin, has improved bioavailability and it may have therapeutic potential [95]. In IL-6-dependent cell lines, FLLL32 downregulated phosphorylation of STAT3, its downstream targets including Bcl-2, and induced apoptosis. Nifuroxazide and FLLL32 provide proof of concept of STAT3 inhibition as a therapeutic target in myeloma.
In summary, IL-6 can stimulate proliferation and survival of MM cells via the JAK/STAT pathway. Inhibition of JAK and STAT3 has shown promise in pre-clinical studies, especially when used concurrently with other active agents. While clinical investigation is warranted, the initial cohort should be comprised of IL-6-dependent myeloma.
Myeloid neoplasms
Though mutations of the JAK family members in patients with MPN has been known since 2005, the initial interest in the JAK/STAT pathway in myeloid neoplasms stemmed following the seminal identification of the TEL-JAK2 fusion protein in T-ALL in 1997 and the recognition of the role of STAT5 in maintaining myeloid stem cells [50,96-98]. Many efforts have since established the importance of a deregulated JAK/STAT pathway, found in ~20-50% of patients with acute myeloid leukemias (AML) [99,100]. Most of these efforts were driven by array-based technologies and candidate gene sequencing, which are now been supported with exome and whole-genome sequencing. Numerous mutations have now been described in modifiers of the JAK-STAT pathway including mutations in MPL, CBL, LNK, and have been reviewed elsewhere [101-105]. Several fusion proteins, such as BCR-JAK2, RPN1-JAK2, PCM1-JAK2 have now been described in patients with AML. Multiple JAK2 point mutations, in particular JAK2V617F but also others such as JAK2T875N, have been noted in about 5% of de novo AML and secondary AML arising from a prior MPN [106-108]. It is of interest that JAK mutations may influence prognosis in AML, in particular CBF-AML [109].
Somatic mutations in JAK1 gene involving the pseudokinase domain have also been described in about 2% of AML cases, and might well be important in leukemogenesis [110]. Mutations involving JAK3 appear to be rare in AML, with the exception of acute megakaryoblastic leukemia (AMKL; AML-M7). Walters and colleagues have identified several nonrecurrent JAK3 mutations, in particular JAK3A572V and JAK3V722I, both of which constitutively activate the JAK3 protein [111]. Several other JAK3 mutations, such as JAK3P132T, have also been described, but their significance is not known [59,112]. An interstitial deletion within chromosome 17 resulting in a STAT5b-RARα fusion protein has also been described in a patient with acute promyelocytic leukemia (APL) [113].
AMLs without mutations in JAKs or STATs may nonetheless be dependent on JAK/STAT signaling. About a third of AMLs are characterized by mutations in the receptor TK FLT3, through internal tandem duplications or kinase domain point mutations [114]. Dysregulated FLT3 signaling activates STAT5 in the leukemic cells [115], although there are conflicting data whether FLT3 mutations are acquired early or later in the genesis of AML [116]. Other efforts have demonstrated the dependence of AMLs associated with transcription factor fusion proteins, including MOZ-TIF2 [97] and AML1-ETO [117-119], on JAK-STAT signaling. These observations further support the role of an enhanced JAK/STAT signaling in de novo AML and the candidacy of JAK inhibitors as potential treatment. A related study of combined array and promoter occupancy (ChIP-chip) analyses demonstrated that the t(8;21) fusion protein AML1-ETO and its alternatively spliced variant AML1-ETO9a (AE9a) enhance JAK/STAT pathway signaling via downregulation of CD45 [120]. These investigators have now published a report confirming the successful inhibition of t(8;21) AML cell lines by ruxolitinib and SAR302503 (TG101209) [121].
A recent phase 2 study of ruxolitinib in relapsed/refractory AML, including post-MPN AML, showed a modest activity as a monotherapy [122]. Ruxolitinib, like other JAK inhibitors in current clinical trials, is not selective for cells with mutated JAK2. It also now established that there is no correlation between the presence of the JAK2V617F mutation and clinical response to JAK inhibition [21,22]. At the same time, in the largest clinical trial to date of ruxolitinib in refractory non-MPN leukemias, all patients who experienced significant responses to ruxolitinib had AML antecedent from a preceding MPN [122]. These data are suggestive of a potential utility of JAK inhibitors in AML transformed from MPNs. The lack of more significant efficacy of ruxolitinib monotherapy in acute leukemia patients is suggestive of a lack of absolute dependence on JAK1/2 signaling in these patients, a lack of sufficient pathway inhibition, and/or the activation of parallel mitogenic pathways despite effective JAK inhibition. Hence, future efforts should assess the notion of targeting JAK/STAT signaling in combination with other candidate drugs, such as dasatinib, hypomethylating agents and others [123].
Interest in the JAK/STAT pathway in patients with chronic myeloid neoplasms, in particular the MPNs, came into prominence following the seminal discovery of the JAK2V617F mutation in 2005 [5-8]. This mutation was noted in the great majority of patients with PV and ~50% of patients with ET or PMF. The JAK2V617F mutation is also found at a much lower frequency in other chronic myeloid malignancies. In “non-classical” MPN, such as systemic mastocytosis, it is detectable in about 6% of patients. In patients with myelodysplastic/myeloproliferative overlap syndromes, it affects about 60% of those with refractory anemia with ringed sideroblasts associated with thrombocytosis (RARS-T) (60%), 20% with atypical CML and 6% with chronic myelomonocytic leukemia [124-127]. A related mutation in exon 12 of JAK2 or MPL is often found in patients with MPN who do not harbor the JAK2V617F mutation. Recent studies have confirmed the enormous complexity of the molecular biology of MPN and related disorders and the precise relationship between the JAK mutations, the JAK/STAT, the disease phenotype and the prognosis [128]. Additionally it is of interest that, irrespective of the JAK2V617F status, patients with PV tend to have high phospho-STAT3 and phospho-STAT5 expression, patients with ET exhibit high phospho-STAT 3 only, whereas patients with PMF have low expression of both activated STATs [129]. Another study of JAK2V617F heterozygous hematopoietic colonies from MPN patients found that differential interferon signaling coupled to STAT1 activation was characteristic of an ET-like phenotype, while down-regulation of pSTAT1 correlated with PV-like disease, suggesting the balance between STAT5 and STAT1 activation was critical [130]. The clinical impact of such observations on disease phenotype and indeed on treatment is unclear at present. Regardless, there is considerable interest in developing JAK inhibitors for the treatment of these disorders. Indeed the first in class drug, ruxolitinib, was licensed in 2011 for patients with myelofibrosis, which comprises of PMF as well as post-PV and post-ET MF [21,22]. The drug was found to be effective, irrespective of the presence or absence of the JAK2V617F mutation, in ameliorating symptoms, reducing splenomegaly and according a survival benefit [131].
Interest in the JAK/STAT pathway in patients with CML began in 1996 following the observation that BCR-ABL1 expression in CML cell lines results in the activation of JAK2 [132,133]. More recently, the relationship between these two genes has attracted additional attention as a potential mechanism to overcome clinical resistance to the tyrosine kinase inhibitors (TKIs), such as imatinib mesylate, in patients with CML [134]. JAK2 appears to be activated by BCR-ABL1 in a kinase-independent fashion, leading support to the notion of combining JAK inhibitors with BCR-ABL1 TKIs. Furthermore, a role between the serine/threonine phosphatase (PP2A) pathway for CML to transform into blast phase, and JAK2 was suggested in 2005, and in vitro studies suggest that inhibition of JAK2 in BCR-ABL1 positive cells might overcome imatinib-induced resistance [135,136]. More recent work confirms the potential usefulness of targeting BCR-ABL-1 and JAK2 simultaneously through inhibition of an AHI-1-BCR-ABL1-JAK2 complex and thereby overcoming imatinib resistance [137].
In contrast, Hantschel and colleagues have shown in murine models that JAK2 is not required for induction of CML-like MPN by BCR-ABL1 [138], but did not specifically assess a role for JAK2 in CML stem cells. Of interest, these investigators did confirm previous observations from Sherr and co-workers of a role for JAK2 in lymphoid transformation and leukemogenesis by BCR-ABL1 [139]. Hantschel et al. further observed that BCR-ABL1-induced JAK2 activation is not required for STAT5 phosphorylation and activation in leukemic cells, and that BCR-ABL1 directly phosphorylates STAT5 on Tyr694 [138]. In BCR-ABL1-transformed cells, STAT5 overexpression decreases the sensitivity to TKIs, whereas CML patients who progress on imatinib show increased STAT5 expression [140]. These observations suggest that targeting STAT5 with agents such as pimozide, a neuroleptic drug, might be a strategy to overcome TKI resistance [141,142]. Clearly, further studies are required to elucidate the precise role of the JAK/STAT/BCR-ABL1 axis in CML. In the interim, clinical trials assessing the potential benefits of JAK inhibitors in patients with CML responding suboptimally to TKIs are already in progress [143]. In the MPN subtype of chronic neutrophilic leukemia/atypical CML, a BCR-ABL1-negative neoplasm, activating mutations in CSF3R (G-CSF receptor) gene are found in >50% of patients, involving distinct truncation and membrane-proximal mutations [127]. The latter mutation class is associated with JAK2 activation, and a patient with such a CSF3R mutation responded clinically to ruxolitinib therapy [127].
Conclusions and some thoughts for the future
The past two decades have clearly witnessed significant advances in our understanding of the JAK/STAT pathways but many challenges remain in assessing the clinical impact and treatment [144,145]. Arguably, the pivotal therapeutic efforts so far have been the introduction of JAK inhibitors for the treatment of a subset of patient with MPN. It is now clear that these agents have shown durable clinical efficacy, safety and potential survival benefits. It is presently not clear if ruxolitinib or the multiple selective JAK2 inhibitors in clinical trials will alter the natural historyof MF [21], nor is their precise effect on the JAK/STAT pathway understood. Ruxolitinib has a profound inhibitory action on a variety of cytokines, which might impact the deregulated JAK/STAT pathway. It is of interest that the efficacy of these drugs in MF does not depend on the presence or absence of the JAK2V617F mutation. Therefore, it appears reasonable to explore JAK inhibitors in all hematological neoplasms for which there is evidence of a deregulated JAK/STAT pathway.
What can we expect from these efforts? It is apparent that the extraordinary response of CML to TKI monotherapy is likely to be the exception rather than the rule, and we can anticipate that any clinical responses to inhibition of the JAK/STAT pathway in other hematologic malignancies will be less robust, even when these malignant cells exhibit activated JAK/STAT signaling. Indeed, this has already been observed in MF, where the effects of JAK2 inhibition on cytopenia, marrow fibrosis, and JAK2V617F allele burden have been modest [146]. It will be important to arrive at a molecular understanding of the limited effect of JAK inhibition on the malignant clone in MPN, and to determine if this can be overcome by novel classes of TKIs [147]. For lymphoma, myeloma, and acute leukemia, it is clear that the future of JAK/STAT targeted therapy is in combination with other regimens, including cytotoxic agents. Again, there is precedent for this from Ph+ B-ALL, where ABL1 TKIs have been successfully incorporated into combination chemotherapy regimens, with potential benefit in terms of rates of remission and overall survival [148]. Melding JAK inhibitors with cytotoxic agents may be complicated by the tendency of the former drugs to cause thrombocytopenia, which may necessitate changes in dosage schedule or support with thrombopoietic agents. As we unravel the underlying molecular complexities of this pathway further, we can anticipate identification of new therapeutic targets, which could lead to the use of future candidate targeted therapies in sequence or in combination with the current JAK inhibitors.
Acknowledgements
The authors wish to thank Dr. Alpa Parmar of Alpine Oncology Foundation (Switzerland) who helped to organize the JAK-STAT in hematological malignancies roundtable, Incyte Corporation (USA) for the unrestricted educational support and Dr. Nicholas Sarlis (USA) for his help and insights. This work was supported in part by NIH grant HL089747 to R.A.V.
Footnotes
Authorship contributions
T.I.M., R.A.V. and W.H.W. designed the outline strategy. T.I.M. wrote the preliminary version of the paper. All authors participated in writing significant sections of the paper.
Conflict of interest Disclosures
O. Abdel-Wahab: No conflicts of interest
J-J. Kiladjian: Advisory boards: Novartis, Sanofi, Shire, AOP Orphan; Research grants: Celgene, Novartis.
S. Kumar: No conflicts of interest
T. Mughal: Speakers’ bureau: Bristol-Myers Squibb; Consultancy: Incyte and Novartis
S. Rosen: No conflicts of interest
G. Saaulius: No conflicts of interest
R. Van Etten: Consultancy: Bristol-Myers Squibb, TEVA Pharmaceuticals; Research support: TEVA Pharmaceuticals.
A. Wiestner: No conflicts of interest
W. Wilson: No conflicts of interest
References
- [1].O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368:161–170. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- [3].Mughal T, Goldman JM. Chronic Myeloid Leukemia. In: Warrell DA, Cox TM, Firth JD, editors. Oxford Textbook of Medicine. 5th edition Oxford University Press; Univesity of Oxford, U.K.: 2010. [Google Scholar]
- [4].Stark GR, Darnell JE., Jr. The JAK-STAT pathway at twenty. Immunity. 2012;36:503–514. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- [6].Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- [7].Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- [8].Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- [9].Ihle JN. The Janus protein tyrosine kinase family and its role in cytokine signaling. Adv Immunol. 1995;60:1–35. doi: 10.1016/s0065-2776(08)60582-9. [DOI] [PubMed] [Google Scholar]
- [10].Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- [11].Vainchenker W, Constantinescu SN. JAK/STAT signaling in hematological malignancies. Oncogene. 2013;32:2601–2613. doi: 10.1038/onc.2012.347. [DOI] [PubMed] [Google Scholar]
- [12].Darnell JE., Jr. Transcription factors as targets for cancer therapy. Nat Rev Cancer. 2002;2:740–749. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- [13].Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol. 2012;30:1005–1014. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Levy DE, Darnell JE., Jr. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- [15].Cho JH, Gregersen PK. Genomics and the multifactorial nature of human autoimmune disease. N Engl J Med. 2011;365:1612–1623. doi: 10.1056/NEJMra1100030. [DOI] [PubMed] [Google Scholar]
- [16].Darnell JE, Jr., Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- [17].Yu CL, Meyer DJ, Campbell GS, et al. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science. 1995;269:81–83. doi: 10.1126/science.7541555. [DOI] [PubMed] [Google Scholar]
- [18].Migone TS, Lin JX, Cereseto A, et al. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science. 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- [19].Weber-Nordt RM, Egen C, Wehinger J, et al. Constitutive activation of STAT proteins in primary lymphoid and myeloid leukemia cells and in Epstein-Barr virus (EBV)-related lymphoma cell lines. Blood. 1996;88:809–816. [PubMed] [Google Scholar]
- [20].Bromberg JF, Wrzeszczynska MH, Devgan G, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- [21].Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- [23].Chen E, Staudt LM, Green AR. Janus kinase deregulation in leukemia and lymphoma. Immunity. 2012;36:529–541. doi: 10.1016/j.immuni.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dawson MA, Bannister AJ, Gottgens B, et al. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature. 2009;461:819–822. doi: 10.1038/nature08448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Friedbichler K, Kerenyi MA, Kovacic B, et al. Stat5a serine 725 and 779 phosphorylation is a prerequisite for hematopoietic transformation. Blood. 2010;116:1548–1558. doi: 10.1182/blood-2009-12-258913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gough DJ, Corlett A, Schlessinger K, Wegrzyn J, Larner AC, Levy DE. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science. 2009;324:1713–1716. doi: 10.1126/science.1171721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Scott LM. Lymphoid malignancies: Another face to the Janus kinases. Blood Rev. 2013;27:63–70. doi: 10.1016/j.blre.2012.12.004. [DOI] [PubMed] [Google Scholar]
- [28].Mullally A, Ebert BL. Janus reveals another face: the biologic rationale for targeting Janus kinase 2 in lymphoma. J Clin Oncol. 2012;30:4168–4170. doi: 10.1200/JCO.2012.44.0347. [DOI] [PubMed] [Google Scholar]
- [29].Joos S, Kupper M, Ohl S, et al. Genomic imbalances including amplification of the tyrosine kinase gene JAK2 in CD30+ Hodgkin cells. Cancer Res. 2000;60:549–552. [PubMed] [Google Scholar]
- [30].Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198:851–862. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rui L, Emre NC, Kruhlak MJ, et al. Cooperative epigenetic modulation by cancer amplicon genes. Cancer Cell. 2010;18:590–605. doi: 10.1016/j.ccr.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Weniger MA, Melzner I, Menz CK, et al. Mutations of the tumor suppressor gene SOCS-1 in classical Hodgkin lymphoma are frequent and associated with nuclear phospho-STAT5 accumulation. Oncogene. 2006;25:2679–2684. doi: 10.1038/sj.onc.1209151. [DOI] [PubMed] [Google Scholar]
- [33].Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Van Roosbroeck K, Cox L, Tousseyn T, et al. JAK2 rearrangements, including the novel SEC31A-JAK2 fusion, are recurrent in classical Hodgkin lymphoma. Blood. 2011;117:4056–4064. doi: 10.1182/blood-2010-06-291310. [DOI] [PubMed] [Google Scholar]
- [35].Lohr JG, Stojanov P, Lawrence MS, et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc Natl Acad Sci USA. 2012;109:3879–3884. doi: 10.1073/pnas.1121343109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lam LT, Wright G, Davis RE, et al. Cooperative signaling through the signal transducer and activator of transcription 3 and nuclear factor-{kappa}B pathways in subtypes of diffuse large B-cell lymphoma. Blood. 2008;111:3701–3713. doi: 10.1182/blood-2007-09-111948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ding BB, Yu JJ, Yu RY, et al. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood. 2008;111:1515–1523. doi: 10.1182/blood-2007-04-087734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gupta M, Han JJ, Stenson M, et al. Elevated serum IL-10 levels in diffuse large B-cell lymphoma: a mechanism of aberrant JAK2 activation. Blood. 2012;119:2844–2853. doi: 10.1182/blood-2011-10-388538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Younes A, Romaguera J, Fanale M, et al. Phase I study of a novel oral Janus kinase 2 inhibitor, SB1518, in patients with relapsed lymphoma: evidence of clinical and biologic activity in multiple lymphoma subtypes. J Clin Oncol. 2012;30:4161–4167. doi: 10.1200/JCO.2012.42.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Derenzini E, Younes A. Targeting the JAK-STAT pathway in lymphoma: a focus on pacritinib. Expert Opin Investig Drugs. 2013;22:775–785. doi: 10.1517/13543784.2013.775244. [DOI] [PubMed] [Google Scholar]
- [41].Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Li ZM, Rinaldi A, Cavalli A, et al. MYD88 somatic mutations in MALT lymphomas. Br J Haematol. 2012;158:662–664. doi: 10.1111/j.1365-2141.2012.09176.x. [DOI] [PubMed] [Google Scholar]
- [43].Montesinos-Rongen M, Godlewska E, Brunn A, Wiestler OD, Siebert R, Deckert M. Activating L265P mutations of the MYD88 gene are common in primary central nervous system lymphoma. Acta Neuropathol. 2011;122:791–792. doi: 10.1007/s00401-011-0891-2. [DOI] [PubMed] [Google Scholar]
- [44].Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenstrom’s macroglobulinemia. N Engl J Med. 2012;367:826–833. doi: 10.1056/NEJMoa1200710. [DOI] [PubMed] [Google Scholar]
- [45].Yang G, Zhou Y, Liu X, et al. A mutation in MYD88 (L265P) supports the survival of lymphoplasmacytic cells by activation of Bruton tyrosine kinase in Waldenstrom macroglobulinemia. Blood. 2013;122:1222–1232. doi: 10.1182/blood-2012-12-475111. [DOI] [PubMed] [Google Scholar]
- [46].Wilson WH, Gerecitano JF, Goy A, et al. The Bruton’s Tyrosine Kinase (BTK) Inhibitor, Ibrutinib (PCI-32765), Has Preferential Activity in the ABC Subtype of Relapsed/Refractory De Novo Diffuse Large B-Cell Lymphoma (DLBCL): Interim Results of a Multicenter, Open-Label, Phase 2 Study. Blood. 2012;120:686a. [Google Scholar]
- [47].Zhang Q, Nowak I, Vonderheid EC, et al. Activation of Jak/STAT proteins involved in signal transduction pathway mediated by receptor for interleukin 2 in malignant T lymphocytes derived from cutaneous anaplastic large T-cell lymphoma and Sezary syndrome. Proc Natl Acad Sci USA. 1996;93:9148–9153. doi: 10.1073/pnas.93.17.9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chiarle R, Simmons WJ, Cai H, et al. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat Med. 2005;11:623–629. doi: 10.1038/nm1249. [DOI] [PubMed] [Google Scholar]
- [49].Zhang Q, Wang HY, Liu X, Wasik MA. STAT5A is epigenetically silenced by the tyrosine kinase NPM1-ALK and acts as a tumor suppressor by reciprocally inhibiting NPM1-ALK expression. Nat Med. 2007;13:1341–1348. doi: 10.1038/nm1659. [DOI] [PubMed] [Google Scholar]
- [50].Lacronique V, Boureux A, Valle VD, et al. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997;278:1309–1312. doi: 10.1126/science.278.5341.1309. [DOI] [PubMed] [Google Scholar]
- [51].Reiter A, Walz C, Watmore A, et al. The t(8;9)(p22;p24) is a recurrent abnormality in chronic and acute leukemia that fuses PCM1 to JAK2. Cancer Res. 2005;65:2662–2667. doi: 10.1158/0008-5472.CAN-04-4263. [DOI] [PubMed] [Google Scholar]
- [52].Nebral K, Denk D, Attarbaschi A, et al. Incidence and diversity of PAX5 fusion genes in childhood acute lymphoblastic leukemia. Leukemia. 2009;23:134–143. doi: 10.1038/leu.2008.306. [DOI] [PubMed] [Google Scholar]
- [53].Patnaik MM, Knudson RA, Gangat N, et al. Chromosome 9p24 abnormalities: prevalence, description of novel JAK2 translocations, JAK2V617F mutation analysis and clinicopathologic correlates. Eur J Haematol. 2010;84:518–524. doi: 10.1111/j.1600-0609.2010.01428.x. [DOI] [PubMed] [Google Scholar]
- [54].Jeong EG, Kim MS, Nam HK, et al. Somatic mutations of JAK1 and JAK3 in acute leukemias and solid cancers. Clin Cancer Res. 2008;14:3716–3721. doi: 10.1158/1078-0432.CCR-07-4839. [DOI] [PubMed] [Google Scholar]
- [55].Flex E, Petrangeli V, Stella L, et al. Somatically acquired JAK1 mutations in adult acute lymphoblastic leukemia. J Exp Med. 2008;205:751–758. doi: 10.1084/jem.20072182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Asnafi V, Le Noir S, Lhermitte L, et al. JAK1 mutations are not frequent events in adult T-ALL: a GRAALL study. Br J Haematol. 2010;148:178–179. doi: 10.1111/j.1365-2141.2009.07912.x. [DOI] [PubMed] [Google Scholar]
- [57].Staerk J, Kallin A, Demoulin JB, Vainchenker W, Constantinescu SN. JAK1 and Tyk2 activation by the homologous polycythemia vera JAK2 V617F mutation: cross-talk with IGF1 receptor. J Biol Chem. 2005;280:41893–41899. doi: 10.1074/jbc.C500358200. [DOI] [PubMed] [Google Scholar]
- [58].Kearney L, Gonzalez De Castro D, Yeung J, et al. Specific JAK2 mutation (JAK2R683) and multiple gene deletions in Down syndrome acute lymphoblastic leukemia. Blood. 2009;113:646–648. doi: 10.1182/blood-2008-08-170928. [DOI] [PubMed] [Google Scholar]
- [59].Malinge S, Ben-Abdelali R, Settegrana C, et al. Novel activating JAK2 mutation in a patient with Down syndrome and B-cell precursor acute lymphoblastic leukemia. Blood. 2007;109:2202–2204. doi: 10.1182/blood-2006-09-045963. [DOI] [PubMed] [Google Scholar]
- [60].Mullighan CG, Zhang J, Harvey RC, et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc Natl Acad Sci USA. 2009;106:9414–9418. doi: 10.1073/pnas.0811761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Poitras JL, Dal Cin P, Aster JC, Deangelo DJ, Morton CC. Novel SSBP2-JAK2 fusion gene resulting from a t(5;9)(q14.1;p24.1) in pre-B acute lymphocytic leukemia. Genes Chromosomes Cancer. 2008;47:884–889. doi: 10.1002/gcc.20585. [DOI] [PubMed] [Google Scholar]
- [62].Sanda T, Tyner JW, Gutierrez A, et al. TYK2-STAT1-BCL2 pathway dependence in T-cell acute lymphoblastic leukemia. Cancer Discov. 2013;3:564–577. doi: 10.1158/2159-8290.CD-12-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bercovich D, Ganmore I, Scott LM, et al. Mutations of JAK2 in acute lymphoblastic leukaemias associated with Down’s syndrome. Lancet. 2008;372:1484–1492. doi: 10.1016/S0140-6736(08)61341-0. [DOI] [PubMed] [Google Scholar]
- [64].Russell LJ, Capasso M, Vater I, et al. Deregulated expression of cytokine receptor gene, CRLF2, is involved in lymphoid transformation in B-cell precursor acute lymphoblastic leukemia. Blood. 2009;114:2688–2698. doi: 10.1182/blood-2009-03-208397. [DOI] [PubMed] [Google Scholar]
- [65].Mullighan CG, Collins-Underwood JR, Phillips LA, et al. Rearrangement of CRLF2 in B-progenitor- and Down syndrome-associated acute lymphoblastic leukemia. Nat Genet. 2009;41:1243–1246. doi: 10.1038/ng.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hertzberg L, Vendramini E, Ganmore I, et al. Down syndrome acute lymphoblastic leukemia, a highly heterogeneous disease in which aberrant expression of CRLF2 is associated with mutated JAK2: a report from the International BFM Study Group. Blood. 2010;115:1006–1017. doi: 10.1182/blood-2009-08-235408. [DOI] [PubMed] [Google Scholar]
- [67].Yoda A, Yoda Y, Chiaretti S, et al. Functional screening identifies CRLF2 in precursor B-cell acute lymphoblastic leukemia. Proc Natl Acad Sci USA. 2010;107:252–257. doi: 10.1073/pnas.0911726107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Shochat C, Tal N, Bandapalli OR, et al. Gain-of-function mutations in interleukin-7 receptor-alpha (IL7R) in childhood acute lymphoblastic leukemias. J Exp Med. 2011;208:901–908. doi: 10.1084/jem.20110580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zenatti PP, Ribeiro D, Li W, et al. Oncogenic IL7R gain-of-function mutations in childhood T-cell acute lymphoblastic leukemia. Nat Genet. 2011;43:932–939. doi: 10.1038/ng.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Harvey RC, Mullighan CG, Chen IM, et al. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood. 2010;115:5312–5321. doi: 10.1182/blood-2009-09-245944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Roberts KG, Morin RD, Zhang J, et al. Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Cancer Cell. 2012;22:153–166. doi: 10.1016/j.ccr.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zhang J, Ding L, Holmfeldt L, et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481:157–163. doi: 10.1038/nature10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Elliott NE, Cleveland SM, Grann V, Janik J, Waldmann TA, Dave UP. FERM domain mutations induce gain of function in JAK3 in adult T-cell leukemia/lymphoma. Blood. 2011;118:3911–3921. doi: 10.1182/blood-2010-12-319467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Koo GC, Tan SY, Tang T, et al. Janus kinase 3-activating mutations identified in natural killer/T-cell lymphoma. Cancer Discov. 2012;2:591–597. doi: 10.1158/2159-8290.CD-12-0028. [DOI] [PubMed] [Google Scholar]
- [75].Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- [76].Vernino L, McAnally LM, Ramberg J, Lipsky PE. Generation of nondividing high rate Ig-secreting plasma cells in cultures of human B cells stimulated with anti-CD3-activated T cells. J Immunol. 1992;148:404–410. [PubMed] [Google Scholar]
- [77].Suematsu S, Matsuda T, Aozasa K, et al. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci USA. 1989;86:7547–7551. doi: 10.1073/pnas.86.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ludwig H, Nachbaur DM, Fritz E, Krainer M, Huber H. Interleukin-6 is a prognostic factor in multiple myeloma. Blood. 1991;77:2794–2795. [PubMed] [Google Scholar]
- [79].Bataille R, Jourdan M, Zhang XG, Klein B. Serum levels of interleukin 6, a potent myeloma cell growth factor, as a reflect of disease severity in plasma cell dyscrasias. J Clin Invest. 1989;84:2008–2011. doi: 10.1172/JCI114392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Catlett-Falcone R, Landowski TH, Oshiro MM, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- [81].Bharti AC, Shishodia S, Reuben JM, et al. Nuclear factor-kappaB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood. 2004;103:3175–3184. doi: 10.1182/blood-2003-06-2151. [DOI] [PubMed] [Google Scholar]
- [82].Derenne S, Monia B, Dean NM, et al. Antisense strategy shows that Mcl-1 rather than Bcl-2 or Bcl-x(L) is an essential survival protein of human myeloma cells. Blood. 2002;100:194–199. doi: 10.1182/blood.v100.1.194. [DOI] [PubMed] [Google Scholar]
- [83].Puthier D, Bataille R, Amiot M. IL-6 up-regulates mcl-1 in human myeloma cells through JAK / STAT rather than ras / MAP kinase pathway. Eur J Immunol. 1999;29:3945–3950. doi: 10.1002/(SICI)1521-4141(199912)29:12<3945::AID-IMMU3945>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- [84].Le Gouill S, Podar K, Amiot M, et al. VEGF induces Mcl-1 up-regulation and protects multiple myeloma cells against apoptosis. Blood. 2004;104:2886–2892. doi: 10.1182/blood-2004-05-1760. [DOI] [PubMed] [Google Scholar]
- [85].Vacca A, Ribatti D, Roncali L, et al. Bone marrow angiogenesis and progression in multiple myeloma. Br J Haematol. 1994;87:503–508. doi: 10.1111/j.1365-2141.1994.tb08304.x. [DOI] [PubMed] [Google Scholar]
- [86].Brenne AT, Fagerli UM, Shaughnessy JD, Jr., et al. High expression of BCL3 in human myeloma cells is associated with increased proliferation and inferior prognosis. Eur J Haematol. 2009;82:354–363. doi: 10.1111/j.1600-0609.2009.01225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Brocke-Heidrich K, Ge B, Cvijic H, et al. BCL3 is induced by IL-6 via Stat3 binding to intronic enhancer HS4 and represses its own transcription. Oncogene. 2006;25:7297–7304. doi: 10.1038/sj.onc.1209711. [DOI] [PubMed] [Google Scholar]
- [88].Pan X, Wang ZX, Wang R. MicroRNA-21: a novel therapeutic target in human cancer. Cancer Biol Ther. 2010;10:1224–1232. doi: 10.4161/cbt.10.12.14252. [DOI] [PubMed] [Google Scholar]
- [89].Loffler D, Brocke-Heidrich K, Pfeifer G, et al. Interleukin-6 dependent survival of multiple myeloma cells involves the Stat3-mediated induction of microRNA-21 through a highly conserved enhancer. Blood. 2007;110:1330–1333. doi: 10.1182/blood-2007-03-081133. [DOI] [PubMed] [Google Scholar]
- [90].Burger R, Le Gouill S, Tai YT, et al. Janus kinase inhibitor INCB20 has antiproliferative and apoptotic effects on human myeloma cells in vitro and in vivo. Mol Cancer Ther. 2009;8:26–35. doi: 10.1158/1535-7163.MCT-08-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Li J, Favata M, Kelley JA, et al. INCB16562, a JAK1/2 selective inhibitor, is efficacious against multiple myeloma cells and reverses the protective effects of cytokine and stromal cell support. Neoplasia. 2010;12:28–38. doi: 10.1593/neo.91192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Burger R, Bugdahn T, Peipp M, Günther A, Gramatzki M. Preclinical activity of the JAK1/2 inhibitor ruxolitinib on malignant plasma cell growth and survival. J Clin Oncol. 2012;30 abstr e18565. [Google Scholar]
- [93].Ramakrishnan V, Kimlinger T, Haug J, et al. TG101209, a novel JAK2 inhibitor, has significant in vitro activity in multiple myeloma and displays preferential cytotoxicity for CD45+ myeloma cells. Am J Hematol. 2010;85:675–686. doi: 10.1002/ajh.21785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Nelson EA, Walker SR, Kepich A, et al. Nifuroxazide inhibits survival of multiple myeloma cells by directly inhibiting STAT3. Blood. 2008;112:5095–5102. doi: 10.1182/blood-2007-12-129718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Lin L, Deangelis S, Foust E, et al. A novel small molecule inhibits STAT3 phosphorylation and DNA binding activity and exhibits potent growth suppressive activity in human cancer cells. Mol Cancer. 2010;9:217. doi: 10.1186/1476-4598-9-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ilaria RL, Hawley RG, Van Etten RA. Dominant negative mutants implicate STAT5 in myeloid cell proliferation and neutrophil differentiation. Blood. 1999;93:4154–4166. [PubMed] [Google Scholar]
- [97].Tam WF, Hahnel PS, Schuler A, et al. STAT5 is crucial to maintain leukemic stem cells in acute myelogenous leukemias induced by MOZ-TIF2. Cancer Res. 2013;73:373–384. doi: 10.1158/0008-5472.CAN-12-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Steensma DP, McClure RF, Karp JE, et al. JAK2 V617F is a rare finding in de novo acute myeloid leukemia, but STAT3 activation is common and remains unexplained. Leukemia. 2006;20:971–978. doi: 10.1038/sj.leu.2404206. [DOI] [PubMed] [Google Scholar]
- [99].Lee HJ, Daver N, Kantarjian HM, Verstovsek S, Ravandi F. The role of JAK pathway dysregulation in the pathogenesis and treatment of acute myeloid leukemia. Clin Cancer Res. 2013;19:327–335. doi: 10.1158/1078-0432.CCR-12-2087. [DOI] [PubMed] [Google Scholar]
- [100].Ghoshal Gupta S, Baumann H, Wetzler M. Epigenetic regulation of signal transducer and activator of transcription 3 in acute myeloid leukemia. Leuk Res. 2008;32:1005–1014. doi: 10.1016/j.leukres.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Abdel-Wahab O, Pardanani A, Bernard OA, et al. Unraveling the genetic underpinnings of myeloproliferative neoplasms and understanding their effect on disease course and response to therapy: Proceedings from the 6th international post-ASH symposium. Am J Hematol. 2012;87:562–568. doi: 10.1002/ajh.23169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Pikman Y, Lee BH, Mercher T, et al. MPLW515L Is a Novel Somatic Activating Mutation in Myelofibrosis with Myeloid Metaplasia. PLoS Med. 2006;3:e270. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Grand FH, Hidalgo-Curtis CE, Ernst T, et al. Frequent CBL mutations associated with 11q acquired uniparental disomy in myeloproliferative neoplasms. Blood. 2009;113:6182–6192. doi: 10.1182/blood-2008-12-194548. [DOI] [PubMed] [Google Scholar]
- [104].Oh ST, Simonds EF, Jones C, et al. Novel mutations in the inhibitory adaptor protein LNK drive JAK-STAT signaling in patients with myeloproliferative neoplasms. Blood. 2010;116:988–992. doi: 10.1182/blood-2010-02-270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Delhommeau F, Dupont S, Della Valle V, et al. Mutation in TET2 in myeloid cancers. N Engl J Med. 2009;360:2289–2301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- [106].Steensma DP, Dewald GW, Lasho TL, et al. The JAK2 V617F activating tyrosine kinase mutation is an infrequent event in both “atypical” myeloproliferative disorders and myelodysplastic syndromes. Blood. 2005;106:1207–1209. doi: 10.1182/blood-2005-03-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356:459–468. doi: 10.1056/NEJMoa065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Schnittger S, Bacher U, Kern W, Haferlach C, Haferlach T. JAK2 seems to be a typical cooperating mutation in therapy-related t(8;21)/ AML1-ETO-positive AML. Leukemia. 2007;21:183–184. doi: 10.1038/sj.leu.2404465. [DOI] [PubMed] [Google Scholar]
- [109].Illmer T, Schaich M, Ehninger G, Thiede C. Tyrosine kinase mutations of JAK2 are rare events in AML but influence prognosis of patients with CBF-leukemias. Haematologica. 2007;92:137–138. doi: 10.3324/haematol.10489. [DOI] [PubMed] [Google Scholar]
- [110].Xiang Z, Zhao Y, Mitaksov V, et al. Identification of somatic JAK1 mutations in patients with acute myeloid leukemia. Blood. 2008;111:4809–4812. doi: 10.1182/blood-2007-05-090308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Walters DK, Mercher T, Gu TL, et al. Activating alleles of JAK3 in acute megakaryoblastic leukemia. Cancer Cell. 2006;10:65–75. doi: 10.1016/j.ccr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- [112].Constantinescu SN, Girardot M, Pecquet C. Mining for JAK-STAT mutations in cancer. Trends Biochem Sci. 2008;33:122–131. doi: 10.1016/j.tibs.2007.12.002. [DOI] [PubMed] [Google Scholar]
- [113].Maurer AB, Wichmann C, Gross A, et al. The Stat5-RARalpha fusion protein represses transcription and differentiation through interaction with a corepressor complex. Blood. 2002;99:2647–2652. doi: 10.1182/blood.v99.8.2647. [DOI] [PubMed] [Google Scholar]
- [114].Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Na Rev Cancer. 2003;3:650–665. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- [115].Choudhary C, Brandts C, Schwable J, et al. Activation mechanisms of STAT5 by oncogenic Flt3-ITD. Blood. 2007;110:370–374. doi: 10.1182/blood-2006-05-024018. [DOI] [PubMed] [Google Scholar]
- [116].Levis M, Murphy KM, Pham R, et al. Internal tandem duplications of the FLT3 gene are present in leukemia stem cells. Blood. 2005;106:673–680. doi: 10.1182/blood-2004-05-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Crispino JD. JAKing up AML1-ETO. Blood. 2012;120:703–704. doi: 10.1182/blood-2012-05-413104. [DOI] [PubMed] [Google Scholar]
- [118].Chou FS, Griesinger A, Wunderlich M, et al. The thrombopoietin/MPL/Bcl-xL pathway is essential for survival and self-renewal in human preleukemia induced by AML1-ETO. Blood. 2012;120:709–719. doi: 10.1182/blood-2012-01-403212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Pulikkan JA, Madera D, Xue L, et al. Thrombopoietin/MPL participates in initiating and maintaining RUNX1-ETO acute myeloid leukemia via PI3K/AKT signaling. Blood. 2012;120:868–879. doi: 10.1182/blood-2012-03-414649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Lo MC, Peterson LF, Yan M, et al. Combined gene expression and DNA occupancy profiling identifies potential therapeutic targets of t(8;21) AML. Blood. 2012;120:1473–1484. doi: 10.1182/blood-2011-12-395335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Lo MC, Peterson LF, Yan M, et al. JAK inhibitors suppress t(8;21) fusion protein-induced leukemia. Leukemia. 2013 doi: 10.1038/leu.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Eghtedar A, Verstovsek S, Estrov Z, et al. Phase 2 study of the JAK kinase inhibitor ruxolitinib in patients with refractory leukemias, including postmyeloproliferative neoplasm acute myeloid leukemia. Blood. 2012;119:4614–4618. doi: 10.1182/blood-2011-12-400051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Weisberg E, Liu Q, Nelson E, et al. Using combination therapy to override stromal-mediated chemoresistance in mutant FLT3-positive AML: synergism between FLT3 inhibitors, dasatinib/multi-targeted inhibitors and JAK inhibitors. Leukemia. 2012;26:2233–2244. doi: 10.1038/leu.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Szpurka H, Tiu R, Murugesan G, et al. Refractory anemia with ringed sideroblasts associated with marked thrombocytosis (RARS-T), another myeloproliferative condition characterized by JAK2 V617F mutation. Blood. 2006;108:2173–2181. doi: 10.1182/blood-2006-02-005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Jelinek J, Oki Y, Gharibyan V, et al. JAK2 mutation 1849G>T is rare in acute leukemias but can be found in CMML, Philadelphia chromosome-negative CML, and megakaryocytic leukemia. Blood. 2005;106:3370–3373. doi: 10.1182/blood-2005-05-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Jones AV, Kreil S, Zoi K, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106:2162–2168. doi: 10.1182/blood-2005-03-1320. [DOI] [PubMed] [Google Scholar]
- [127].Maxson JE, Gotlib J, Pollyea DA, et al. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N Engl J Med. 2013;368:1781–1790. doi: 10.1056/NEJMoa1214514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Godfrey AL, Green AR. The biology of myeloproliferative neoplasms. EHA Education Program. 2013;7:267–276. [Google Scholar]
- [129].Teofili L, Martini M, Cenci T, et al. Different STAT-3 and STAT-5 phosphorylation discriminates among Ph-negative chronic myeloproliferative diseases and is independent of the V617F JAK-2 mutation. Blood. 2007;110:354–359. doi: 10.1182/blood-2007-01-069237. [DOI] [PubMed] [Google Scholar]
- [130].Chen E, Beer PA, Godfrey AL, et al. Distinct clinical phenotypes associated with JAK2V617F reflect differential STAT1 signaling. Cancer Cell. 2010;18:524–535. doi: 10.1016/j.ccr.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Mesa RA, Kiladjian JJ, Verstovsek S, et al. Comparison of placebo and best available therapy for the treatment of myelofibrosis in the phase 3 COMFORT studies. Haematologica. 2013 doi: 10.3324/haematol.2013.087650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Walz C, Cross NC, Van Etten RA, Reiter A. Comparison of mutated ABL1 and JAK2 as oncogenes and drug targets in myeloproliferative disorders. Leukemia. 2008;22:1320–1334. doi: 10.1038/leu.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Ilaria RL, Van Etten RA. P210 and P190BCR/ABL induce the tyrosine phosphorylation and DNA binding activity of multiple specific STAT family members. J Biol Chem. 1996;271:31704–31710. doi: 10.1074/jbc.271.49.31704. [DOI] [PubMed] [Google Scholar]
- [134].Samanta A, Perazzona B, Chakraborty S, et al. Janus kinase 2 regulates Bcr-Abl signaling in chronic myeloid leukemia. Leukemia. 2011;25:463–472. doi: 10.1038/leu.2010.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Neviani P, Santhanam R, Trotta R, et al. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell. 2005;8:355–368. doi: 10.1016/j.ccr.2005.10.015. [DOI] [PubMed] [Google Scholar]
- [136].Samanta AK, Chakraborty SN, Wang Y, et al. Jak2 inhibition deactivates Lyn kinase through the SET-PP2A-SHP1 pathway, causing apoptosis in drug-resistant cells from chronic myelogenous leukemia patients. Oncogene. 2009;28:1669–1681. doi: 10.1038/onc.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Chen M, Gallipoli P, Degeer D, et al. Targeting Primitive Chronic Myeloid Leukemia Cells by Effective Inhibition of a New AHI-1-BCR-ABL-JAK2 Complex. J Natl Cancer Inst. 2013;105:405–423. doi: 10.1093/jnci/djt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Hantschel O, Warsch W, Eckelhart E, et al. BCR-ABL uncouples canonical JAK2-STAT5 signaling in chronic myeloid leukemia. Nat Chem Biol. 2012;8:285–293. doi: 10.1038/nchembio.775. [DOI] [PubMed] [Google Scholar]
- [139].Williams RT, Roussel MF, Sherr CJ. Arf gene loss enhances oncogenicity and limits imatinib response in mouse models of Bcr-Abl-induced acute lymphoblastic leukemia. Proc Natl Acad Sci USA. 2006;103:6688–66893. doi: 10.1073/pnas.0602030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Warsch W, Kollmann K, Eckelhart E, et al. High STAT5 levels mediate imatinib resistance and indicate disease progression in chronic myeloid leukemia. Blood. 2011;117:3409–3420. doi: 10.1182/blood-2009-10-248211. [DOI] [PubMed] [Google Scholar]
- [141].Nelson EA, Walker SR, Weisberg E, et al. The STAT5 inhibitor pimozide decreases survival of chronic myelogenous leukemia cells resistant to kinase inhibitors. Blood. 2011;117:3421–3429. doi: 10.1182/blood-2009-11-255232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Fabbro D. BCR-ABL signaling: A new STATus in CML. Nat Chem Biol. 2012;8:228–229. doi: 10.1038/nchembio.900. [DOI] [PubMed] [Google Scholar]
- [143].Nair RR, Tolentino JH, Argilagos RF, Zhang L, Pinilla-Ibarz J, Hazlehurst LA. Potentiation of Nilotinib-mediated cell death in the context of the bone marrow microenvironment requires a promiscuous JAK inhibitor in CML. Leuk Res. 2012;36:756–763. doi: 10.1016/j.leukres.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Barrio S, Gallardo M, Arenas A, et al. Inhibition of related JAK/STAT pathways with molecular targeted drugs shows strong synergy with ruxolitinib in chronic myeloproliferative neoplasm. Br J Haematol. 2013;161:667–676. doi: 10.1111/bjh.12308. [DOI] [PubMed] [Google Scholar]
- [145].Quintas-Cardama A, Verstovsek S. Molecular pathways: Jak/STAT pathway: mutations, inhibitors, and resistance. Clin Cancer Res. 2013;19:1933–1940. doi: 10.1158/1078-0432.CCR-12-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Tefferi A. Challenges facing JAK inhibitor therapy for myeloproliferative neoplasms. N Engl J Med. 2012;366:844–846. doi: 10.1056/NEJMe1115119. [DOI] [PubMed] [Google Scholar]
- [147].Koppikar P, Bhagwat N, Kilpivaara O, et al. Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature. 2012;489:155–159. doi: 10.1038/nature11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Ravandi F, O’Brien S, Thomas D, et al. First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Blood. 2010;116:2070–2077. doi: 10.1182/blood-2009-12-261586. [DOI] [PMC free article] [PubMed] [Google Scholar]