Abstract
Prostaglandins are a group of lipid signaling molecules involved in various physiological processes. In addition, prostaglandins have been implicated in the development and progression of diseases including cancer, cardiovascular disease, and arthritis. Prostaglandins exert their effects through the activation of specific G protein-coupled receptors (GPCRs). In this report, we examined the role of prostaglandin F2α receptor (FP) signaling as a regulator of chondrocyte differentiation. We found that FP expression was dramatically induced during the differentiation of chondrocytes and was up-regulated in cartilages. Forced expression of FP in ATDC5 chondrogenic cell line resulted in the increased expression of differentiation-related genes and increased synthesis of the extracellular matrix (ECM) regardless of the presence of insulin. Similarly, PGF2α treatment induced the expression of chondrogenic marker genes. In contrast, knockdown of endogenous FP expression suppressed the expression of chondrocyte marker genes and ECM synthesis. Organ culture of cartilage rudiments revealed that PGF2α induces chondrocyte hypertrophy. Additionally, FP overexpression increased the levels of Bmp-6, phospho-Smad1/5, and Bmpr1a, while knockdown of FP reduced expression of those genes. These results demonstrate that up-regulation of FP expression plays an important role in chondrocyte differentiation and modulates Bmp signaling.
Keywords: FP, Prostaglandin F2α, chondrocytes, Bmp
1. Introduction
Chondrogenic differentiation is a key process in endochondral ossification which occurs during the development, growth, and repair of the skeleton. During endochondral ossification, the condensed mesenchymal cells differentiate into chondrocytes to form the cartilage anlagen. Chondrocytes rapidly proliferate and synthesize cartilage extracellular matrix (ECM) such as collagen type II (Col2A1) and aggrecan (Acan1) [1, 2]. The chondrocytes then cease proliferation and enlarge by several folds, becoming hypertrophic chondrocytes. Hypertrophic chondrocytes are characterized by the expression of collagen type X (Col10A1) and matrix metalloproteinase 13 (Mmp13) [2–4]. Hypertrophic chondrocytes mineralize the surrounding matrix and secrete vascular endothelial growth factor (VEGF) for blood vessel formation. Eventually, these cells die by apoptosis and are replaced by osteoblasts that form trabecular bone. The differentiation of chondrocytes through the process of endochondral ossification is regulated by many growth factors and signaling molecules. One of the signaling molecules implicated in this process is prostaglandins.
Prostaglandins are a class of bioactive lipid signaling molecules, consisting of prostaglandin D2 (PGD2), prostaglandin E2 (PGE2), prostaglandin F2α (PGF2α), and prostacyclin (PGI2). They are generated from arachidonic acid by the sequential actions of cyclooxygenase (COX) and its respective prostaglandin synthases. Prostaglandins exert their effects through their interactions with specific cell surface receptors, which are G protein-coupled receptors (GPCRs). Prostaglandin receptors are classified on the basis of their response to a particular prostaglandin: PGD2 to DP, PGE2 to EP, PGF2α to FP, and PGI2 to IP. They are expressed in variable levels among different tissues and their expression is regulated by various physiological and pathological stimuli [5], providing an additional level of regulation in prostaglandin signaling. For example, LPS treatment highly induced the expressions of EP2 and EP4 receptors [6, 7] in macrophages and these inductions were suggested to be involved in the regulation of TNF-α [8]. In addition, the overexpression of the EP2 receptor in the epidermis of EP2 transgenic mice resulted in enhanced skin tumor formation, proliferation, and blood vessel formation [9].
Prostaglandins regulate cartilage metabolism and are implicated in the pathogenesis of osteoarthritis (OA) and rheumatoid arthritis (RA). In addition to PGE2, PGF2α is a major prostaglandin in the synovial fluid of joints [10]. However, only a few studies have suggested the role of PGF2α in cartilage metabolism and pathology while many studies investigating the effects of prostaglandins on chondrocyte physiology have focused on PGE2 signaling. PGF2α stimulated aggrecan synthesis in a rat chondrocyte cell line [11] and promoted the expression of Col2A1 mRNA in human articular chondrocytes [12]. Moreover, the levels of PGE2 and PGF2α are significantly increased in blood and synovial fluid samples from OA or RA patients [10]. The biological effect of PGF2α is mediated by FP. Upon binding to PGF2α, FP activates the Gq protein, which in turn increases the levels of inositol triphosphate (IP3)/diacylglycerol (DAG) [13]. FP expression has been recently reported in hypertrophic chondrocytes of growth plate cartilage [14], suggesting that FP signaling may be involved in chondrocyte hypertrophy. However, the expression and the role of FP in cartilage development and diseases have not been clearly determined.
In the present study, we investigated the role of FP signaling in chondrocyte differentiation. We found that FP expression is highly induced during chondrogenesis. We demonstrated that FP signaling stimulates and is required for chondrogenic differentiation. Moreover, our study suggests a possible cross-talk between FP and Bmp signaling pathways.
2. Materials and methods
2.1. Cell culture and treatment
ATDC5 cell line was purchased from Abgent (San Diego, CA) and was maintained in DMEM/Ham's F-12 medium (GIBCO®, Grand Island, NY) with 5% FBS (GIBCO®, Grand Island, NY), 10 µg/ml human transferrin (Sigma Chemical, St Louis, MO), 3 × 10−8 M sodium selenite (Sigma Chemical, St Louis, MO), 50 units/ml penicillin, and 50 mg/ml streptomycin (GIBCO®, Grand Island, NY). For induction of chondrogenic differentiation, the cells were plated in a 2 × 105 cells/60mm plate. The next day, 10 µg/ml bovine insulin (Sigma Chemical, St Louis, MO,) supplementation was started, as previously described [15]. The ATDC5 cells were treated with 100 nM PGF2α (Cayman chemical, Ann Arbor, MI) for the indicated days and the media with PGF2α was replaced every 2nd day. C3H10T1/2 cell line was purchased from American Type Culture Collection (ATCC, Manassas, VA) and maintained in DMEM medium (GIBCO®, Grand Island, NY) with 10% FBS (GIBCO®, Grand Island, NY), 50 units/ml penicillin and 50 mg/ml streptomycin (GIBCO®, Grand Island, NY) and micromass cultured with recombinant human Bmp2 (Sigma Chemical, St Louis, MO) for the indicated days [16].
2.2. Metatarsal organ culture and treatment
The metatarsal cartilage rudiments were isolated from the hind limb of the E15.5 stage C57BL/6 mouse fetus, and cultured in organ culture medium containing serum free DMEM with 50 μg/ml ascorbic acid, 300 μg/ml L-glutamine, 1 mM β -glycerophosphate, 0.2% BSA Cohn fraction V (Sigma Chemical, St Louis, MO), 50 units/ml penicillin, and 50 mg/ml streptomycin (GIBCO®, Grand Island, NY), as described previously [17]. The next day, the cartilage rudiments were treated with 100 nM PGF2α (Cayman chemical, Ann Arbor, MI) for 6 days. The media with PGF2α was replaced every 2nd day.
2.3. Establishment of stable and knockdown cell lines by lentiviral vector
The HEK 293T/17 cell was used as a packaging cell line and was transfected with lentiviral packaging vectors and a target vector. To generate a cell line which stably expressed FP, FP full length CDS sequence (NM_008966.3) was cloned from mouse cartilage cDNA by PCR amplification into pHAGE vector with a flag tag. To generate a FP knockdown cell line, target shRNA sequences of FP were designed by web-based shRNA sequence designing software (http://www.broadinstitute.org/rnai/public/seq/search). The sequences of FP shRNA oligomers are listed in table 3 of Supplementary information. The viral particles were harvested after 48hrs and were then incubated with ATDC5 host cell line culture with polybrene for next 48hrs. In shRNA lentiviral vector (pLBIP), the target sequence of FP was co-expressed with EGFP by an internal ribosome entry site (IRES) and an efficiency of lentiviral infection was assessed by EGFP fluorescence under microscope. The transfected cells were selected and maintained in selective antibiotic (puromycin, 2 µg/ml) containing media.
2.4. RNA extraction, semi-quantitative RT-PCR, and quantitative real-time PCR
Total RNA was extracted by using a RNeasy RNA extraction kit (QIAGEN, Valencia, CA) according to the manufacturer’s instructions. 2 µg of total RNA was used for the reverse transcription reaction using MMLV reverse transcriptase (Promega, Madison, WI). The resulting cDNA was diluted 1:3 in nuclease-free water and stored in aliquots at −80°C until used. For RT-PCR analysis of target genes, an initial amplification was done with a denaturation step at 95°C for 3 min, followed by denaturation at 95°C for 30 seconds, primer annealing at 58°C for 30 seconds, and primer extension at 72°C for 30 seconds. The primer sequences are provided in Supplementary table 1. Upon completion of the cycling steps, a final extension was performed at 72°C for 7 min. Quantitative real-time PCR analysis was conducted using a GoTaq® qPCR mixture (Promega, Madison, WI). Reactions were run in triplicate for three independent experiments. The mean of housekeeping gene β -Actin was used as an internal control to normalize the variability in expression levels. The primer sequences for real-time PCR analysis are provided in Supplemental table 2. Expression data were normalized to the mean of β -Actin to control the variability in expression levels and were analyzed using the 2−ΔΔCT method.
2.5. Protein extraction and Western blot analysis
Total protein was extracted with RIPA buffer [20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Sodium vanadate, 1 μg/ml leupeptin)] and was used for Western blot analysis. The 20 µg of extracted total proteins were separated in SDS-PAGE gel and transferred onto PVDF membrane (Millipore, Billerica, MA). Following incubation in blocking buffer (TBS with 5% nonfat dry milk and 0.1% Tween 20) for 1 hour at room temperature, the membrane was incubated at 4 °C overnight with various primary antibodies; Flag (#2368, Cell Signaling, Boston, MA), phospho-Smad1/5 (#9516, Cell Signaling, Boston, MA), phospho-Smad2 (#3101, Cell Signaling, Boston, MA), Smad1 (#9743, Cell Signaling, Boston, MA), Smad2 (#3103, Cell Signaling, Boston, MA), and Bmpr1a (#ABD51, Millipore, Billerica, MA). HRP-labeled secondary antibody was used according to the host species of the primary antibody. Western blots were developed using ECL substrate and exposed to x-ray film.
2.6. Alcian blue staining and measurement of staining intensity
The differentiated cell lines were fixed with Kahle’s fixative at room temperature for 10 min. After fixation, the cells were washed with PBS and stained with 1% alcian blue stain 8-GX at room temperature overnight. The cells were de-stained by washing with distilled water and observed under an Olympus CXX41 microscope (Olympus, Center Valley, PA). To measure the intensity of the alcian blue staining, the stained cells were incubated with 6 M guanidine solution for 30 min at room temperature on orbital shaker. Absorbance was detected by Synergy™ HT Multidetection Microplate Readers (BioTek™, Winooski, VT) at a wavelength of 630 nm.
2.7. Morphological analysis and immunohistochemistry of metatarsal cartilage rudiments
PGF2α - or vehicle- treated metatarsal cartilage rudiments were fixed overnight in 4% paraformaldehyde at 4 °C. Next day, the metatarsal cartilages were washed with 1x PBS for 2 min three times and then were embedded in paraffin. The sections were cut 5 µm thick and were used for morphological analysis by Hematoxylin and Eosin (H&E) staining by standard protocols. To measure the length of each cartilage zone, the images of samples were analyzed by ImageJ software [18]. For the immunohistochemical staining, the 5 µm thick sections from PGF2α - or vehicle- treated metatarsal rudiments were incubated with 3% H2O2 in methanol for 15 min at room temperature to deactivate endogenous peroxidase activity. The antigen retrieval was done by heat antigen retrieval in citrate buffer (10 mM sodium citrate buffer, pH 6.0) for 30 min. To prevent nonspecific binding, the sections were blocked with normal horse serum and incubated at room temperature for 30 min. The anti-Col10A1 (#234196, Calbiochem, San Diego, CA), Mmp13 (#AB8120, Millipore, Billerica, MA), and phospho-Smad1/5 (#9511, Cell Signaling, Boston, MA) primary antibodies were incubated at 4 °C, overnight. After three washings in 1x TBST (15 min each), the sections were incubated with secondary antibody (ImmPRESS anti-mouse Ig/anti-rabbit Ig, peroxidase Detection Kit, VECTOR, Burlingame, CA) at room temperature for 30 min and washed with 1x TBST three times (15 min each). The immunoreactivity was visualized by DAB peroxidase substrate (VECTOR, Burlingame, CA). For morphometric analysis, the stained sections were photographed under the Olympus BX51 microscope (Olympus, Center vally, PA). The image was acquired by an AxioCam MRc camera and analyzed by AxioVision AC Rel. 4.5 software (Carl Zeiss, Thornwood, NY).
2.8. Statistical analysis
p-values were calculated by unpaired Student's t-tests and one- or two-way analysis of variance (ANOVA) followed by the Bonferroni posttest. p <0.05 was considered significant and were denoted in the graph (*: p<0.05, **: p<0.01, and ***: p<0.001). Statistical analysis was performed using Prism software (Graphpad Software, La Jolla, CA). All experiments were repeated at least three times and the data were represented as represent the mean ± S.E.M of three independent experiments
3. Results
3.1. FP expression is highly induced during chondrocyte differentiation
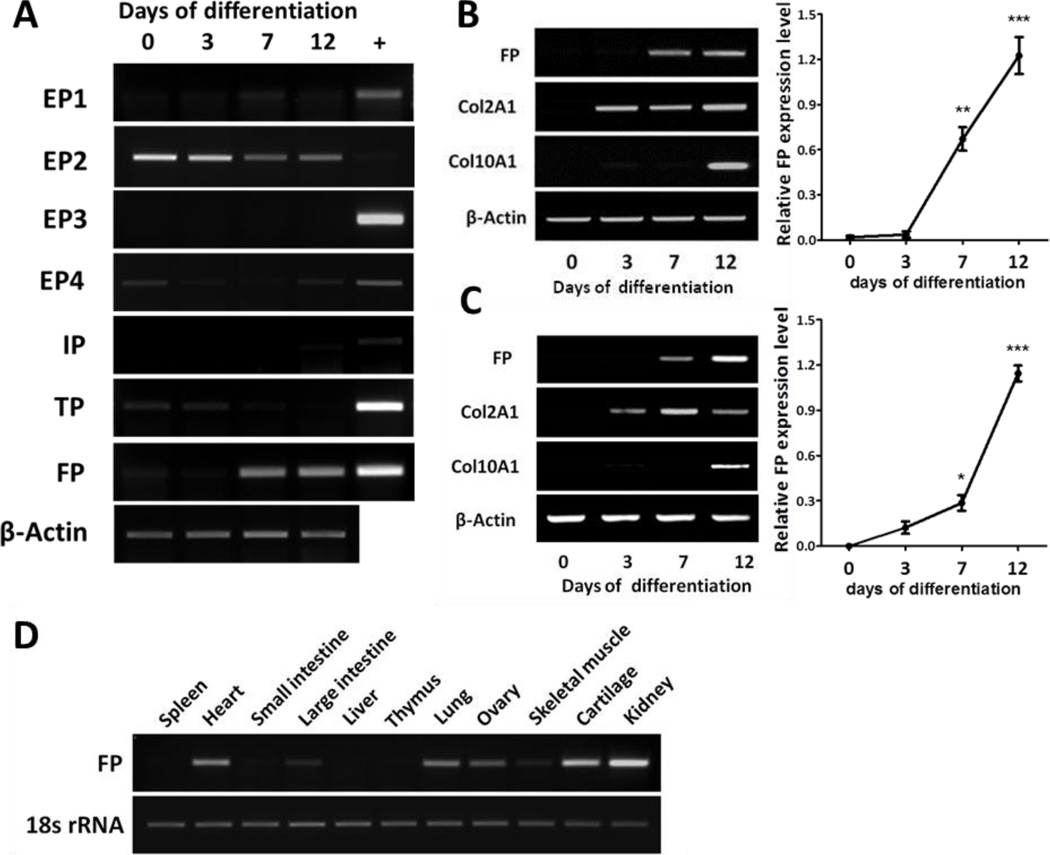
To investigate the role of prostaglandin signaling in chondrocyte differentiation, we assessed the expression levels of prostaglandin receptors during chondrogenic differentiation of the ATDC5 cell line. ATDC5 cells mimic multi-stages of chondrocyte differentiation from chondroporgenitors to fully differentiated hypertrophic chondrocytes. They proliferate and form cartilage nodules through cellular condensation when treated with insulin. These cells then differentiate into cartilage-matrix synthesizing chondrocytes and eventually are converted to pre-hypertrophic chondrocytes, followed by hypertrophy and mineralization [19]. Chondrocyte differentiation is associated with the sequential expressions of types II (Col2A1) and X (Col10A1) collagen mRNA, which are markers of cartilage matrix synthesis and hypertrophy, respectively [20] (Fig. 1B). As shown in Fig. 1A, the mRNA levels of EP1, EP3, and IP were almost undetectable whereas low levels of EP4 and TP mRNA were seen during chondrogenic differentiation of ATDC5 cells. EP2 mRNA was highly expressed in undifferentiated (day 0) and early differentiating chondrocytes (day 3), and then was gradually decreased at the later stages of differentiation (days 7 and 12) (Fig. 1A). In contrast, prostaglandin F2α receptor (FP) mRNA expression was very low at days 0 and 3, but was dramatically induced at days 7 and 12 of chondrogenic differentiation (Fig. 1A). As shown in Fig. 1B, induction of FP mRNA was preceded by the expression of early differentiation marker, Col2A1, and occurred before the expression of Col10A1, a hypertrophic chondrocyte marker. This suggests that FP may play a role in late chondrogenic differentiation. To determine whether the induction of FP is a specific phenomenon in ATDC5 cells, the expression of FP was examined in another cell line, C3H10T1/2, which is known to differentiate into chondrocytes with Bmp-2 supplement [16]. As shown in Fig. 1C, the expression of FP mRNA in C3H10T1/2 cell line was highly increased during chondrogenic differentiation, showing an identical pattern to that in ATDC5 cells. Because FP mRNA expression is highly increased in differentiating chondrocytes, we examined FP mRNA levels in cartilage along with other tissues. Consistent with the previous reports, FP was expressed in the ovary, kidney, heart, and lung [21, 22]. In addition, the cartilage exhibited high levels of FP mRNA (Fig. 1D). These results suggest that the signaling through FP may play an important role in chondrogenic differentiation in vivo and in vitro.
Figure 1. Prostaglandin F2α receptor (FP) mRNA is highly induced during chondrogenic differentiation of the ATDC5 cell line.
(A) Expression of prostaglandin receptors during chondrogenic differentiation of ATDC5 cell line. ATDC5 cells were induced to differentiate in the presence of 10 µg/ml insulin. RNA was isolated at the indicated time points and RT-PCR analysis was conducted. RNA from the mouse kidney and colon were used as a positive control (+). DP was not detected in any sample. (B) RT-PCR analysis of FP, Col2A1, Col10A1, and β-Actin during chondrogenic differentiation of ATDC5 cells (left panel). (n=3, **: p < 0.01, ***: p < 0.001) The level of FP mRNA at each time point was quantified and normalized to β -Actin mRNA using ImageJ software. The relative level of FP was plotted (right panel). (n=3, *: p < 0.05, ***: p < 0.001) (C) RT-PCR analysis of FP, Col2A1, Col10A1, and β -Actin during Bmp2-induced chondrogenic differentiation of C3H10T1/2 cell line (left panel). The expression level of FP at each time point was normalized to the β -Actin level and the relative level of FP was plotted (right panel). (D) RT-PCR analysis for FP expression in various tissues of 4 weeks old C57BL/6 mouse. 18s rRNA served as a loading control.
3.2. Stimulation of FP signaling promotes chondrocyte differentiation
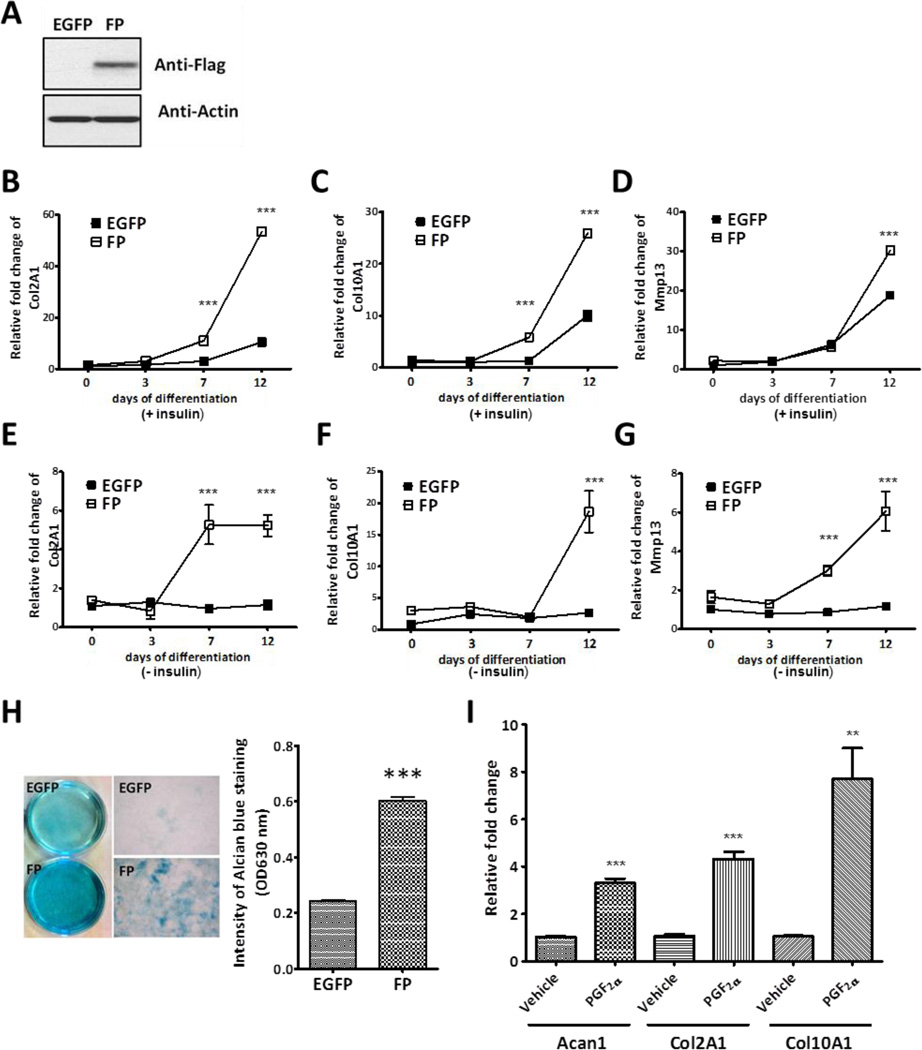
To investigate the functional significance of increased FP expression during chondrogenic differentiation, we generated an ATDC5 cell line that stably expressed FP. ATDC5 cells were infected with the FP-encoding lentivirus and selected in the presence of puromycin. Puromycin-resistant clones were pooled for further experiments. Western blot analysis showed that FLAG-tagged mouse FP was highly expressed in the infected cells (Fig. 2A). To determine the effect of increased FP expression on chondrocyte differentiation, mRNA levels of differentiation markers were assessed by quantitative real-time PCR. When the cells were induced to differentiate in the presence of insulin, Col2A1 mRNA expression at days 7 and 12 was significantly up-regulated in FP over-expressing ATDC5 cells compared to that in EGFP control cells (Fig. 2B). Likewise, expression of Col10A1 mRNA in FP over-expressing ATDC5 cells increased significantly at days 7 and 12 and the expression of Mmp13, another hypertrophy marker, was increased at day 12 compared to those in control (Fig, 2C and 2D), suggesting that an induction of FP may be associated with chondrogenic differentiation.
Figure 2. Stimulation of FP signaling promotes chondrocyte differentiation.
(A) Western blot analysis for FLAG-tagged mouse FP. Total lysates from control (EGFP) and FP over-expressing (FP) ATDC5 cells were probed for FLAG. The membrane was stripped and reprobed for β -Actin to confirm equal loading. (B, C, and D) The control and FP over-expressing ATDC5 cells were cultured in the presence of insulin and then the expression level of Col2A1 (B), Col10A1(C), and Mmp13 (D) at each time point was analyzed by quantitative real time PCR (qRT-PCR). The expression levels of marker genes were normalized to β -Actin. Data were expressed as fold changes over control (EGFP) cells at day 0. The data represent the mean ± S.E.M of three independent experiments. (***: p < 0.001) (E, F, and G) The control and FP over-expressing ATDC5 cell lines were cultured for the indicated time points without insulin supplementation. The expression levels of Col2A1 (E), Col10A1 (F), and Mmp13 (G) were analyzed by qRT-PCR. The expression levels of marker genes were normalized to β -Actin. Data were expressed as fold change over control (EGFP) cells at day 0. The data represent the mean ± S.E.M of three independent experiments. (***: p < 0.001) (H) Alcian blue staining of control and FP over-expressing ATDC5 cells at day 12 of culture without insulin supplement (left panel). The relative intensities of alcian blue staining in control and FP stable cell lines were measured by dissolving alcian blue stained cells with 6M guanidine solution. The optical density at 630nm was plotted (***: p < 0.001) (right panel). (I) Effects of PGF2a on the levels of chondrogenic marker. ATDC5 cells were treated with either vehicle or 100 nM PGF2a for 7 days. Expression of Acan1, Col2A1, and Col10A1 mRNAs were quantified by qRT-PCR. The expression levels of marker genes were normalized to β -Actin. Data were expressed as fold change over vehicle treated cells. The data represent the mean ± S.E.M. of three independent experiments. (**: p < 0.01, ***: p < 0.001)
As previously described [15], chondrogenic differentiation of ATDC5 cell line requires insulin. Stimulation with insulin resulted in the differentiation of ATDC5 cells into chondrocytes and the formation of cartilage nodules. Subsequently, these cells produce an extracellular matrix that can be stained with alcian blue. To corroborate the role of FP induction in chondrogenesis, we cultured control and FP over-expressing ATDC5 cells in the media without insulin and examined the expression of chondrogenic maker genes. Although less prominent than in the presence of insulin, FP over-expressing ATDC5 cells underwent chondrogenic differentiation even in the absence of insulin and mimicked the insulin-inducible expression of marker genes. As shown in Fig. 2E, the expression of Col2A1 mRNA at days 7 and 12 in FP over-expressing ATDC5 cells cultured in the absence of insulin was significantly increased relative to that in the control cells. Furthermore, the forced expression of FP resulted in a prominent increase in the level of Col10A1 and Mmp13 mRNA, suggesting a possible role of FP in chondrocyte hypertrophy (Fig. 2F and 2G). In addition to the marker analysis, an increased differentiation in FP over-expressing ATDC5 cells was confirmed by alcian blue staining. Control and FP over-expressing ATDC5 cell lines were cultured for 12 days in the absence of insulin and were stained with alcian blue. As shown in Fig. 2H (left panel), FP over-expressing cells formed alcian blue-positive cartilage nodules whereas control ATDC5 cells lacked the formation of cartilage nodules. The quantitative analysis of alcian blue staining revealed that the stain intensity in the FP over-expressing cells was 2.5 fold higher than that in the control cells (Fig. 2H, right graph). These results suggest that the induction of FP during chondrogenesis may promote and thus be required for chondrogenic differentiation. Furthermore, stimulation of FP signaling with its ligand, PGF2α, increased the expression of genes related to chondrocyte differentiation. As shown in Fig. 2I, treatment of ATDC5 cells with PGF2α highly induced the expression of Acan1, Col2A1, and Col10A1 mRNAs. Taken together, these results demonstrate that PGF2α signaling via FP stimulates chondrogenic differentiation.
3.3. Knockdown of endogenous FP expression suppresses chondrogenic differentiation
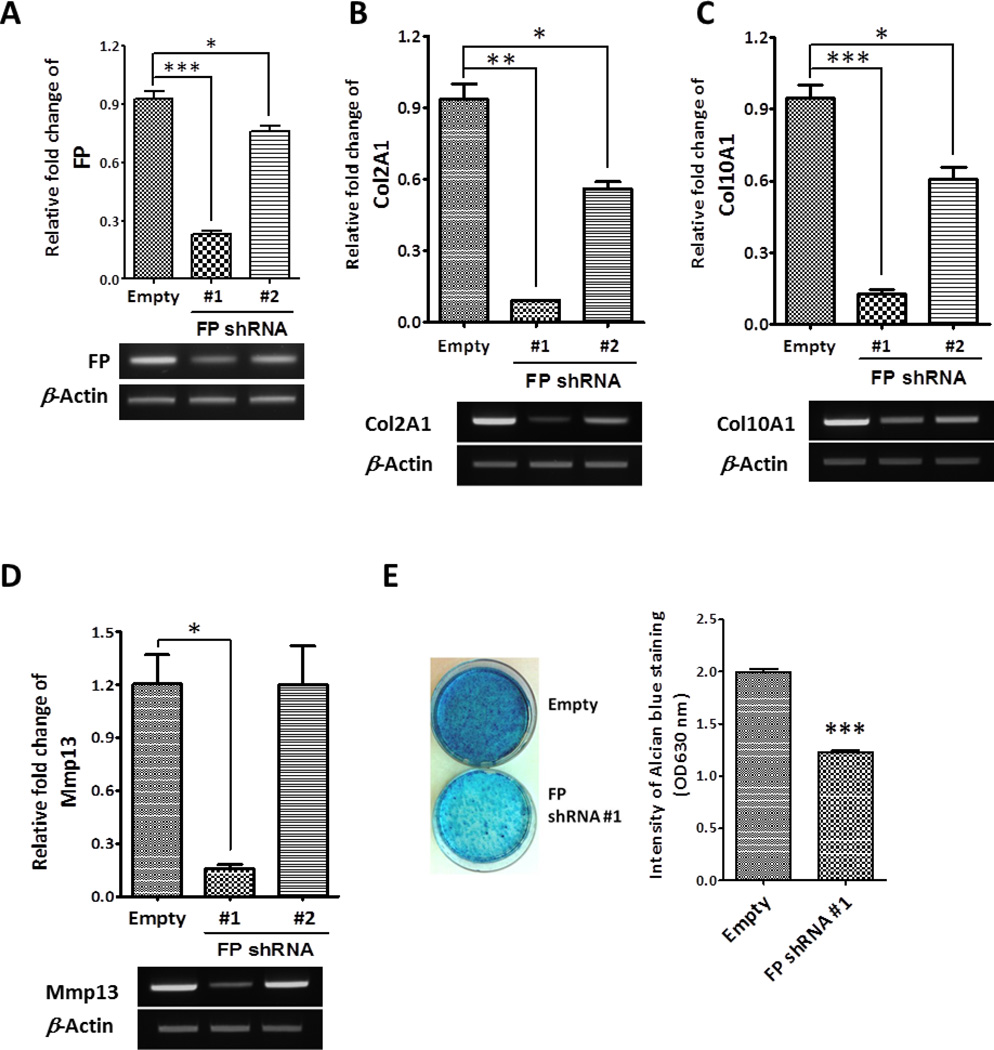
To examine the endogenous function of FP in chondrogenic differentiation of ATDC5 cell line, we generated stable FP knockdown ATDC5 cells using shRNA. We infected ATDC5 cells with the lentivirus expressing FP-specific shRNA. The resulting puromycin-resistant clones were pooled and induced to differentiate in the presence of insulin. Since FP expression was induced at day 7 and 12 during chondrogenic differentiation (Fig. 1A and B), we isolated RNAs from the empty vector-infected control and the FP shRNA-expressing ATDC5 cells at day 12 of differentiation. As shown in Fig. 3A, the quantitative real-time PCR analysis revealed that the expression of FP shRNA (FP shRNA #1) resulted in a 75% reduction of FP mRNA levels compared to that of the control cells. The reduction of FP by FP shRNA was confirmed by semi-quantitative RT-PCR (Fig.3A, lower panel). The suppression of FP induction by shRNA resulted in a 90% decrease in Col2A1 and Col10A1 mRNA expression compared to that of the control cell line (Fig. 3B and 3C), indicating that FP induction is required for chondrogenic differentiation. The expression level of Mmp13 was also highly suppressed in FP shRNA #1 cell line (Fig.3D). Similarly, the knockdown of FP caused a significant decrease in the intensity of alcian blue staining following differentiation for 15 days (Fig. 3E). To avoid potential off-target effects, FP expression was knocked down by another shRNA targeting in a different region of mouse FP (FP shRNA #2). Although knockdown efficiency of the second shRNA was lower compared to the first shRNA, the levels of Col2A1 and Col10A1 mRNA were significantly reduced in the FP shRNA #2 cells (Fig. 3B and 3C). These results suggest that the induction of FP expression during chondrogenic differentiation is required for efficient chondrogenesis.
Figure 3. Knockdown of endogenous FP expression suppresses chondrogenic differentiation.
(A) The relative FP expression in ATDC5 cells infected with the empty vector- or FP shRNA-encoding (FP shRNA #1 and #2) lentivirus at day 12 of insulin-induced chondrogenic differentiation was analyzed by qRT-PCR and normalized to β -Actin. The expression levels of FP and β -Actin in FP shRNA cell lines were also determined by RT-PCR (gel image under graph A). (B, C, and D) The mRNA levels of (B) Col2A1, (C) Col10A1, and (D) Mmp13 in FP knockdown cells (FP shRNA #1 and #2) were determined by qRT-PCR (upper panel). The relative gene expression was normalized to β -Actin. Data were expressed as fold change over the control cell line. The expression levels of Col2A1, Col10A1, and Mmp13 were also determined by RT-PCR (lower panel). (E) Alcian blue staining of control (empty) and FP knockdown (FP shRNA #1) ATDC5 cells cultured in the presence of 10 µg/ml insulin for 15 days. The intensities of alcian blue staining in control and FP knockdown cell lines at 630 nm was plotted (right panel). (*: p < 0.05, **: p < 0.01, ***: p < 0.001)
3.4. PGF2α enhances chondrocyte hypertrophy in metatarsal cartilage rudiments
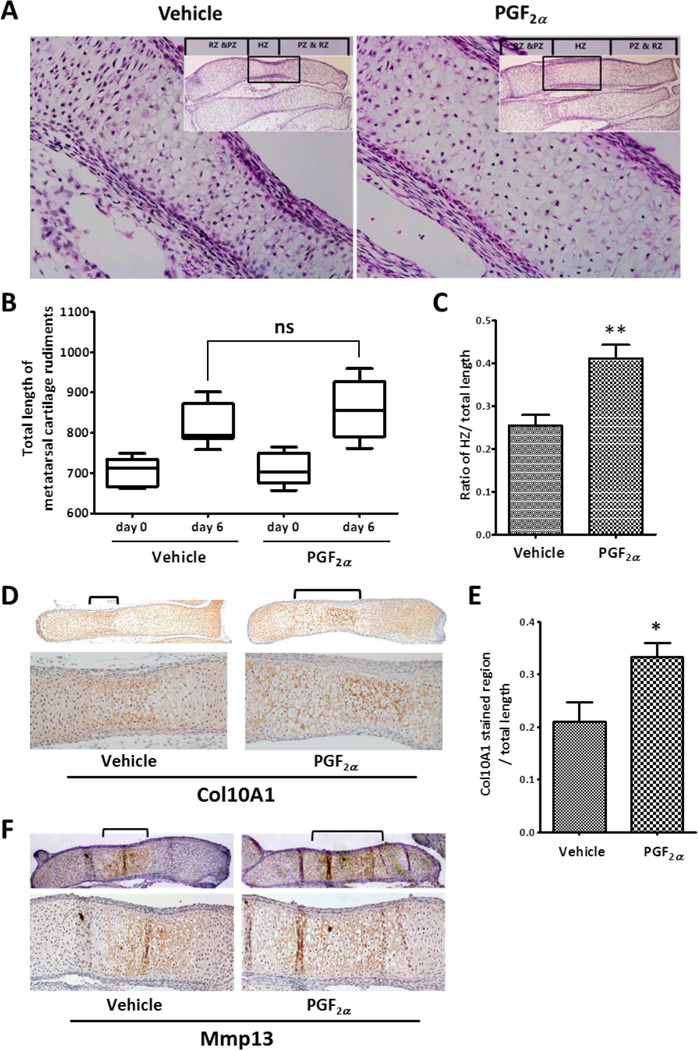
To further examine the effects of FP signaling on chondrogenic differentiation, mouse fetal metatarsal cartilage rudiments were isolated from the E15.5 fetus and were treated with 100 nM PGF2α for 6 days. Organ culture of the metatarsal cartilage rudiments allows for the study of biological processes in a three-dimensional structure in the context of native cell-cell and cell-extracellular matrix interactions [17, 23]. When E15.5 metatarsals, which are primarily composed of immature chondrocytes, were cultured for 6 days, they underwent longitudinal growth and development, showing a well-organized growth plate composed of proliferating (PZ), pre-hypertrophic (PHZ), and hypertrophic zones (HZ). As shown in Figure 4A, PGF2α -treated rudiments had extended HZ compared to that in vehicle-treated control while there was no detectable difference in the overall length between vehicle- and PGF2α -treated rudiments (Fig. 4B). Histomorphometric analysis showed that PGF2α treatment resulted in a 40% increase in the ratio of HZ to total length compared to that in the vehicle-treated control (Fig. 4C), suggesting that PGF2α signaling stimulates hypertrophic differentiation of chondrocytes in cartilage rudiments. To confirm the increase in the area of hypertrophic cartilage by PGF2α treatment, immunohistochemical analysis of Col10A1 was performed in the metatarsal cartilage sections. As shown in Fig. 4D, the Col10A1-stained region was more extended in PGF2α-treated cartilage rudiments compared to that in vehicle-treated cartilage. The quantitative analysis revealed that the PGF2α resulted in a 36 % increase in the length of Col10A1 expressing zone compared to control (Fig. 4E). In addition, PGF2a treatment increased the expression of Mmp13 in the cartilage rudiments (Fig. 4F). These results suggest that FP signaling may stimulate chondrocyte hypertrophy.
Figure 4. PGF2α enhances ex vivo differentiation of hypertrophic chondrocytes in metatarsal cartilage rudiments.
(A) H&E staining of vehicle (A, left panel) or PGF2α (A, right panel) treated metatarsal cartilage rudiments. The metatarsal cartilage rudiments were cultured in the presence of 100 nM PGF2a for 6 days. The resting and proliferating zone (RZ & PZ) and hypertrophic zone (HZ) were denoted in the image. The inset shows the lower magnification image of the rudiment. (B) The total length of the cartilage rudiment is plotted on the graph. There was no significant difference in total length between vehicle- and PGF2α-treated cartilages (n=5 per each treatment group, ns = no significance). (C) The ratio of the HZ to the total length of cartilage rudiment is presented on the graph. (n=5 per each treatment group, **: p < 0.01) (D) Immunohistochemical staining of Col10A1 in metatarsal cartilage rudiments treated with vehicle or 100 nM PGF2α for 6 days. Lower magnification images are shown in the upper panel. The lower panel represents a higher magnification image of the area marked with a bracket on the upper image. (E) The ratio of the Col10A1 stained region to the total area was presented on the graph. (n=5 per each treatment group, *: p < 0.05) (F) Immunohistochemical staining of Mmp13 in metatarsal cartilage rudiments treated with vehicle or 100 nM PGF2α for 6 days. Lower magnification images are shown in the upper panel. The lower panel represents a higher magnification image of the area marked with a bracket on the upper image
3.5. FP signaling up-regulates the expression of Bmp signaling components
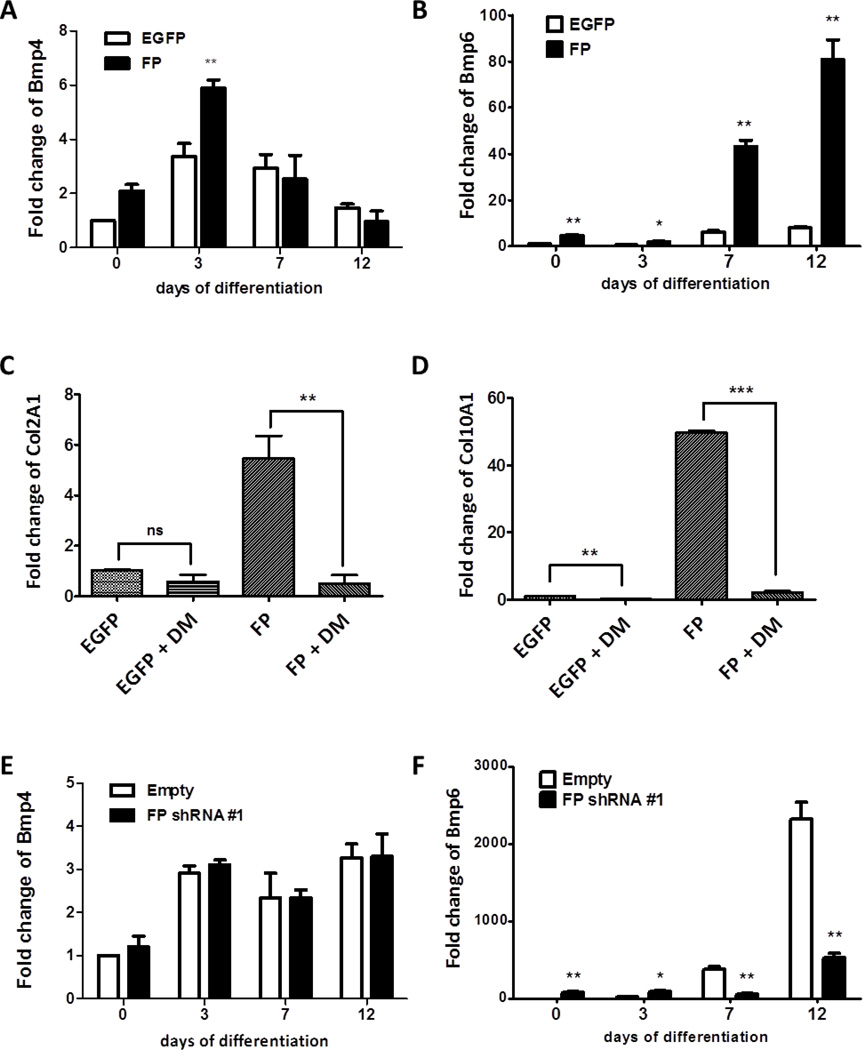
It has been shown that insulin-induced chondrogenic differentiation of ATDC5 cells requires Bmp autocrine signaling [24]. Tgf-β /Bmp signaling pathways are two major signaling pathways that control cartilage development and homeostasis [25–33]. Tgf-β /Bmp ligands bind to type II receptors which then recruit the corresponding type I serine/threonine kinase receptor. Upon ligand binding, Bmp type I receptors phosphorylate Smad1/5/8, whereas Tgf-β type I receptors activate Smad 2/3 [34]. It was demonstrated that exogenously added Bmp can induce the differentiation of ATDC5 cells in the absence of insulin [31]. Since stimulation of FP signaling induced the chondrogenic differentiation of ATDC5 cells even in the absence of insulin, we examined whether the stimulation of FP signaling is associated with the alterations in Tgf-β /Bmp signaling. To determine the effect of FP signaling on the levels of Tgf-β /Bmp ligands, control and FP over-expressing ATDC5 cells were cultured in the media without insulin and the quantitative real-time PCR analysis for Tgf-β /Bmp ligands was conducted. Consistent with the previous reports [35], the levels of Bmp-2 and Bmp-7 transcripts were undetectable (data not shown and Supplementary Figure) in both control and FP over-expressing ATDC5 cells. As shown in Fig. 5A, FP expression resulted in 2 fold increase in basal level of Bmp-4 expression compared to control cells. Although 1.5- to 2-fold increase of Bmp-4 mRNA levels at day 3 was detected in FP overexpressing cells, there were no significant differences in Bmp-4 mRNA levels between the control and the FP over-expressing cells at the later time points. However, the Bmp-6 gene exhibited a differential expression pattern between the two cell lines. As shown in Fig. 5B, FP expression resulted in a significant increase in Bmp-6 mRNA levels compared to those in the control. Furthermore, incubation with dorsomorphin, a selective inhibitor of Bmp signaling, suppressed FP-induced increase in the level of Col2A1 and Col10A1 mRNA (Fig. 5C and D), suggesting that FP may promote chondrogenic differentiation by modulating Bmp signaling. To define the role of endogenous FP on the synthesis of Bmp ligands, we cultured control and FP knockdown ATDC5 cells in the presence of insulin and determined the mRNA levels of Bmp ligands. Bmp-2 and Bmp-7 mRNAs were undetectable (Supplementary Figure) in control and FP knockdown cells, while Bmp-4 mRNA was constitutively expressed at all differentiation stages in both cell lines (Fig. 5E). However, knockdown of FP resulted in the alteration of Bmp-6 mRNA expression. In the control cell line, Bmp-6 mRNA was highly induced at days 7 and 12 (Fig. 5F), at which FP expression was observed (Fig. 1A and B). However, knockdown of FP resulted in a delay in Bmp-6 mRNA expression. Furthermore, the level of Bmp-6 mRNA was significantly reduced in FP knockdown cells compared to that of the control.
Figure 5. FP signaling regulates Bmp expression.
(A and B) qRT-PCR analysis of Bmp-4 (A) and Bmp-6 (B) during chondrogenic differentiation of control (EGFP) and FP over-expressing (FP) ATDC5 cells. The control and FP over-expressing ATDC5 cell lines were cultured for the indicated time without insulin supplementation. Data were expressed as fold changes over control (EGFP) cells at day 0.The data represent the mean ± S.E.M of three independent experiments and were analyzed by two-way ANOVA followed by the Bonferroni posttest. (*: p < 0.05, **: p < 0.01, ***: p < 0.001) (C and D) The control (EGFP) and FP over-expressing ATDC5 cell lines were cultured in insulin-free media in the absence or presence (2µM) of dorsomorphin for 7 days. Col2A1 (C) and Col10A1(D) mRNA levels were determined by qRT-PCR analysis. Data were expressed as fold changes over control (EGFP) cells at day 0. The data represent the mean ± S.E.M. of three independent experiments. The fold change of respective genes was compared by t-test and it was significantly different. (**: p < 0.01, ***: p < 0.001) (E and F) qRT-PCR analysis of Bmp-4 (E) and Bmp-6 (F) during insulin-induced chondrogenic differentiation of control (empty) and FP knock-down (FP shRNA #1) ATDC5 cell lines. The cells were induced to differentiate in the presence of 10 µg/ml insulin. Data were expressed as fold changes over control (empty) cells at day 0. The data represent the mean ± S.E.M of three independent experiments and were analyzed by two-way ANOVA followed by the Bonferroni posttest. (*: p < 0.05, **: p < 0.01)
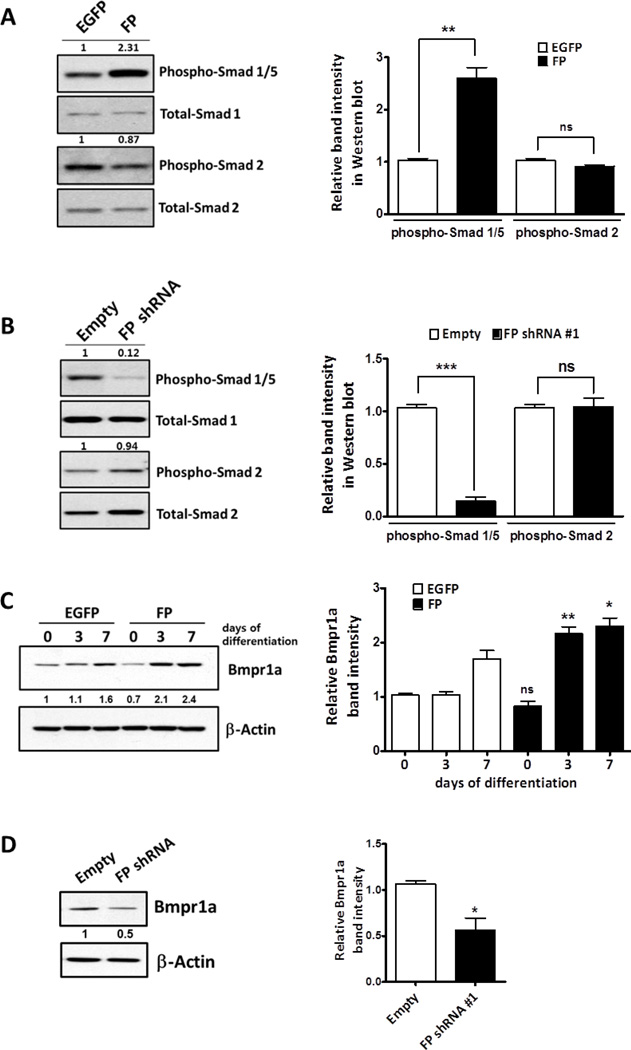
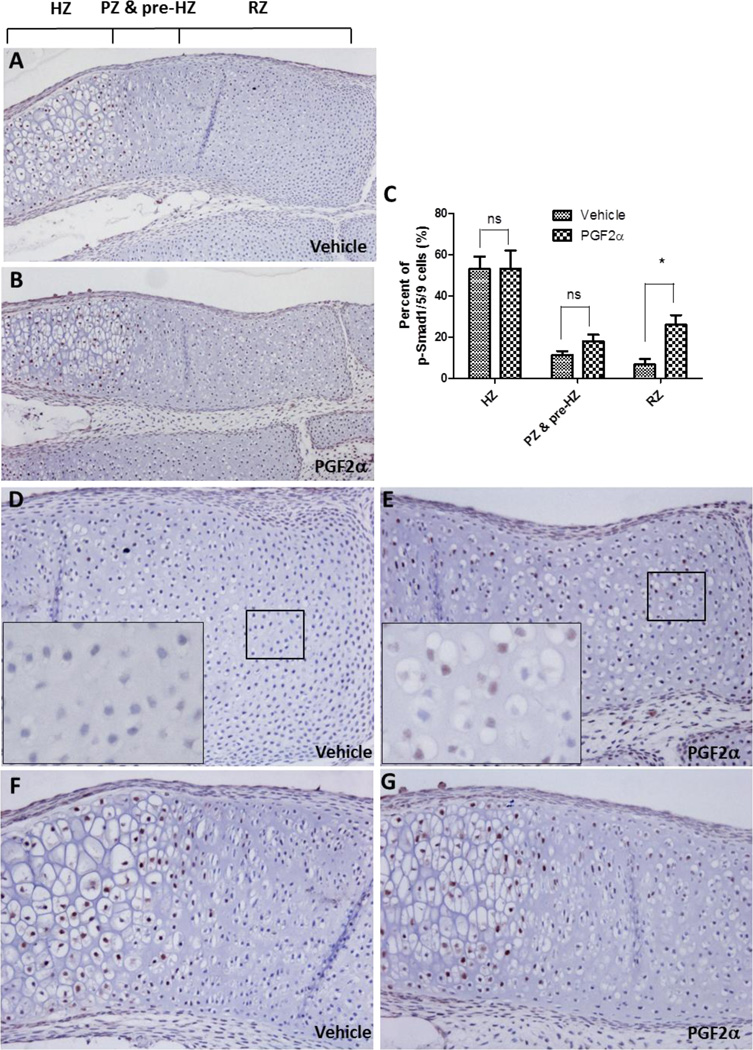
Next, we examined the phosphorylation status of Smads in control and FP over-expressing ATDC5 cell lines. As shown in Fig. 6A, the level of phospho-Smad1/5 was increased in FP over-expressing ATDC5 cells even in the absence of insulin while the total amount of Smad1 protein was not significantly altered, suggesting that FP signaling resulted in the activation of the Bmp pathway. Interestingly, the level of phospho-Smad2 was slightly decreased in the FP over-expressing cell line. We also determined the levels of phospho-Smads in FP knockdown cells at day 7 of insulin-induced chondrogenic differentiation, when FP mRNA expression begins to be observed (Fig. 1A and B). As shown in Fig. 6B, knockdown of endogenous FP induction resulted in decreased levels of phospho-Smad1/5 while it did not significantly affect phospho-Smad2 levels, suggesting that endogenous FP signaling is required for activation of the Bmp pathway. Smad1/5 proteins are phosphorylated by the heteromeric complexes of type II and type I Bmp receptors. It has been shown that Bmpr1a, one of the type I Bmp receptors, and Bmpr2, a type II Bmp receptor, are expressed in ATDC5 cells [35]. Since FP signaling appears to modulate phosphorylation of Smad1/5, we examined whether the levels of Bmp receptors are regulated by FP. As shown in Fig.6C, Western blot analysis revealed that Bmpr1a expression is increased in FP over-expressing ATDC5 cells compared to the control cells. However, knockdown of FP expression reduced the levels of Bmpr1a (Fig. 6D). Finally, to corroborate that PGF2α signaling regulates the Bmp pathway, we determined whether PGF2α treatment alters the expression of phospho-Smad1/5/9 in growth plates of cartilage rudiments. Consistent with the previous reports [25, 36], phosphorylated Smad1/5/9 were detected in pre-hypertrophic and hypertrophic chondrocytes of both the control and PGF2α-treated rudiments. However, incubation with PGF2α caused a significant increase in the number of phospho-Smad1/5/9 positive chondrocytes in the resting zone (RZ) while the chondrocytes in the RZ of control rudiments rarely expressed phospho-Smad1/5/9 (Fig. 7). Taken together, these results suggest that FP signaling is associated with the activation of the Bmp pathway.
Figure 6. FP signaling stimulates the expression of Bmp signaling components.
(A) Western blot analysis of phospho-Smad 1/5 and phospho-Smad2 in control (EGFP) and FP cell line. Control and FP over-expressing ATDC5 cells were cultured for 3 days in the absence of insulin and the total lysates were subjected to Western blot analysis. The membrane was stripped and reprobed for total Smad1 and Smad2. The level of phospho- Smads were quantified and normalized to total Smads using ImageJ software. The numbers on the Western blot represent quantification normalized to total Smads. The graph (right panel) shows fold changes of normalized phospho-Smad1/5 and phospho-Smad2 (compared with EGFP control). (n=3, **: p < 0.01) (B) Western blot analysis of phospho-Smad 1/5 and phospho-Smad2 in control and FP knockdown ATDC5 cell lines at day 7 of insulin-induced differentiation. The membrane was stripped and reprobed for Smad1 and Smad2. The numbers on the Western blot represent quantification normalized to total Smads. The graph (right panel) shows fold changes of normalized phospho-Smad1/5 and phospho-Smad2 (compared with control). (n=3, ***: p < 0.001) (C) The expression of Bmpr1a during chondrogenic differentiation of control and FP cell lines was analyzed by Western blot. The numbers shown on the Western blot represent relative Bmpr1a levels obtained by normalizing densities of each band to β -Actin bands from the same blot. The graph (right panel) shows densitometric analysis of Western blot of Bmpr1a protein. (n=3, *: p < 0.05, **: p < 0.01). (D) Western blot analysis of Bmpr1a in control and FP knockdown ATDC5 cell line at day 7 of differentiation. The numbers shown on the Western blot represent relative Bmpr1a levels obtained by normalizing densities of each band to β-Actin bands from the same blot. The graph (right panel) shows fold changes of normalized Bmpr1a (compared with control). (n=3, **: p < 0.05)
Figure 7. PGF2α increases levels of phosphorylated Smad1/5/9 in metatarsal cartilage.
(A and B) Immunohistochemical staining of phospho-Smad1/5/9 in metatarsal cartilage rudiments treated with vehicle (A) or 100nM PGF2α (B) for 6 days. The resting (RZ), proliferating zone and pre-hypertrophic (PZ & pre-HZ), and hypertrophic zone (HZ) were denoted in the image. (C) Graphical presentation of phospho-Smad1/5/9 positive cells. (n=3, *: p <0.05) (D and E) High magnification images of resting zone. Insets show higher magnification images of the areas indicated by boxes. (F and G) High magnification images of proliferating, pre-hypertrophic, and hypertrophic zones.
4. Discussion
In this article, we have shown that FP expression is highly induced during chondrocyte differentiation. We have defined the functional significance of FP induction during chondrogenesis. Our data demonstrated that FP expression stimulates and is required for efficient chondrocyte differentiation. In addition, our results suggest that FP signaling modulates Bmp signaling.
During the chondrogenic differentiation of ATDC5 cells, EP2 and FP mRNA levels are differentially regulated (Fig. 1A). EP2 expression was high at days 0 and 3 of chondrogenic differentiation and gradually decreased in the later stages of differentiation. This suggests that the down-regulation of EP2 signaling may be associated with the onset of differentiation in chondrocytes. In contrast, the basal level of FP was very low. However, FP expression was highly induced in the later stages of chondrogenesis, suggesting that sustained FP signaling may be required for chondrogenic differentiation. Our results also suggest that PGF2α signaling may be regulated by the levels of its receptor, FP, as well as by the PGF2α level. Consistent with this notion, the expression of FP mRNA has shown to be regulated by various stimuli. FP mRNA expression in mouse skin was increased immediately after the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) treatment, followed by a down-regulation at later time points [37]. In addition, administration of pregnant mare serum gonadotropin (PMSG) increased the ovarian FP mRNA level in mice [38].
Prostaglandin receptors have been implicated in chondrocyte metabolism. It has been shown that PGE2 receptors (EP1-4) are expressed in both growth plates and primary chondrocytes [39]. Stimulation of human articular chondrocytes with PGE2 through the EP2 receptor suppressed proteoglycan accumulation and synthesis [40]. However, the role of FP in chondrocyte metabolism has not been delineated. We demonstrated that knockdown of FP mRNA induction interfered with chondrogenic differentiation while forced expression of FP promotes differentiation, suggesting that induction of FP expression is required for chondrogenic differentiation. Further, our data suggests that FP signaling may be involved in chondrocyte hypertrophy. First, FP mRNA expression was observed before the expression of Col10A1, a marker of hypertrophic chondrocytes, while it was preceded by Col2A1 expression, suggesting that expression of Col10A1 may require FP. In addition, FP overexpression or PGF2α treatment resulted in greater increase of Col10A1 relative to Col2A1 mRNA. Furthermore, the incubation of metatarsal cartilage rudiments with PGF2α increased the length of the hypertrophic zone which expresses Col10A1 and Mmp13. Recently, expression of FP mRNA was reported in hypertrophic chondrocytes of growth plate cartilage [14], further suggesting a putative role of FP in hypertrophic differentiation of chondrocytes. We were not able to determine the expression of FP protein due to the lack of reliable, commercially available FP antibodies. Therefore, FP protein expression during chondrogenic differentiation or in cartilage remains to be determined. In addition, the reported phenotypes of the FP knockout mouse do not include any gross skeletal abnormalities [41]. This suggests the possible in vivo redundancy of prostanoid receptor signaling that controls chondrocyte differentiation. FP, EP1, and TP are coupled to Gq proteins and act through an increase in calcium and inositol triphosphate [42]. Therefore, it is possible that an increase in the expression or sensitivity of another prostanoid receptor may compensate for the lack of FP.
Several lines of reports have shown that prostaglandins regulate expression of Bmps. For example, PGE2 has up-regulated the expression of Bmp-7 mRNA and protein in vitro and in vivo, probably by post-transcriptional mechanisms [43]. In addition, PGE2 has induced the expression of Bmp-2 in human tendon stem cells [44]. We found that Bmp-6 mRNA expression was up-regulated by the stimulation of FP signaling but was suppressed by the inhibition of endogenous FP expression, suggesting that FP may be involved in the regulation of the Bmp-6 gene. Since FP signaling appears to be involved in chondrocyte hypertrophy, it may be possible that the effect of FP on chondrocyte differentiation is mediated via Bmp-6. In fact, exogenously added Bmp-6 has shown to facilitate the phenotypic conversion of proliferating ATDC5 cells to hypertrophic cells [45]. In addition, phospho-Smad1/5 levels were increased in FP over-expressing cells while its expression was decreased in differentiating FP knockdown cells. Moreover, incubation of cartilage rudiments with PGF2α increased the number of phospho-Smad1/5/9 positive cells. Given that Smad1 plays a critical role in chondrocyte hypertrophy [25], our results suggest that FP signaling regulates hypertrophic differentiation of chondrocytes by modulating Bmp signaling. In line with this notion, we found that an inhibition of Bmp signaling by dorsomorphin suppressed FP-induced chondrogenic differentiation and marker gene expression.
Our results also suggest the possible role of FP signaling in the pathogenesis of arthritis. Under normal conditions, chondrocytes in the articular cartilage remain in a resting state and maintain extracellular matrix integrity. However, some chondrocytes in OA-affected cartilages lose their phenotype and undergo similar differentiation programs as growth plate chondrocytes. As a result, affected articular chondrocytes differentiate into hypertrophic chondrocytes and undergo apoptosis, eventually leading to cartilage loss [46]. Increased expression of genes related to chondrocyte hypertrophy including Col10A1 and Mmp13 is frequently observed in OA-affected cartilages. We have shown that FP signaling promotes and is required for the expression of hypertrophic markers including Col10A1 and Mmp13. Additionally, we observed that FP signaling stimulated Mmp13 promoter activity (data not shown). Increased Mmp13 expression and activity in OA cartilage has been suggested to play a critical role in the pathogenesis of OA. Given the fact that PGF2α levels are elevated in OA-affected joint fluid [10], our findings suggest that increased FP signaling may accelerate hypertrophic chondrocyte differentiation in OA-affected joints, contributing to the development and/or progression of OA. Future study will define how FP signaling interacts with other signaling pathways involved in cartilage development and diseases.
In summary, our study uncovered a novel regulatory role of FP signaling on chondrocyte differentiation. Additionally, the findings in this study provide a plausible mechanism for chondrocytes to respond to various prostaglandins, which may allow us to develop more effective treatment strategies for skeletal diseases while minimizing side effects.
Supplementary Material
Highlights.
PGF2α receptor (FP) is highly induced during chondrogenic differentiation.
Forced expression of FP promotes chondrogenic differentiation.
PGF2α treatment enhances in vitro and ex vivo chondrogenic differentiation.
Knockdown of endogenous FP expression suppresses chondrogenic differentiation.
FP signaling regulates the expression of Bmp signaling components.
Acknowledgements
This work was supported by the National Institutes of Health [grant number 5R00ES016757-04].
References
- 1.Kravis D, Upholt WB. Quantitation of type II procollagen mRNA levels during chick limb cartilage differentiation. Dev Biol. 1985;108:164–172. doi: 10.1016/0012-1606(85)90018-1. [DOI] [PubMed] [Google Scholar]
- 2.O'Keefe RJ, Puzas JE, Loveys L, Hicks DG, Rosier RN. Analysis of type II and type X collagen synthesis in cultured growth plate chondrocytes by in situ hybridization: rapid induction of type X collagen in culture. J Bone Miner Res. 1994;9:1713–1722. doi: 10.1002/jbmr.5650091107. [DOI] [PubMed] [Google Scholar]
- 3.Wu CW, Tchetina EV, Mwale F, Hasty K, Pidoux I, Reiner A, Chen J, Van Wart HE, Poole AR. Proteolysis involving matrix metalloproteinase 13 (collagenase-3) is required for chondrocyte differentiation that is associated with matrix mineralization. J Bone Miner Res. 2002;17:639–651. doi: 10.1359/jbmr.2002.17.4.639. [DOI] [PubMed] [Google Scholar]
- 4.Mattot V, Raes MB, Henriet P, Eeckhout Y, Stehelin D, Vandenbunder B, Desbiens X. Expression of interstitial collagenase is restricted to skeletal tissue during mouse embryogenesis. J Cell Sci. 1995;108(Pt 2):529–535. doi: 10.1242/jcs.108.2.529. [DOI] [PubMed] [Google Scholar]
- 5.Sugimoto Y, Narumiya S. Prostaglandin E receptors. The Journal of biological chemistry. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 6.Ikegami R, Sugimoto Y, Segi E, Katsuyama M, Karahashi H, Amano F, Maruyama T, Yamane H, Tsuchiya S, Ichikawa A. The expression of prostaglandin E receptors EP2 and EP4 and their different regulation by lipopolysaccharide in C3H/HeN peritoneal macrophages. J Immunol. 2001;166:4689–4696. doi: 10.4049/jimmunol.166.7.4689. [DOI] [PubMed] [Google Scholar]
- 7.Hubbard NE, Lee S, Lim D, Erickson KL. Differential mRNA expression of prostaglandin receptor subtypes in macrophage activation. Prostaglandins Leukot Essent Fatty Acids. 2001;65:287–294. doi: 10.1054/plef.2001.0327. [DOI] [PubMed] [Google Scholar]
- 8.Katsuyama K, Kojima R, Yokoyama S, Yanai M, Sueda N, Sugita M, Momose K, Yamada H. N2733, 1-[3-(3-pyridyl)-acryloyl]-2-pyrrolidinone hydrochloride inhibits LPS-induced TNF-alpha production and improves survival in endotoxemic mice. Biosci Biotechnol Biochem. 1998;62:2177–2181. doi: 10.1271/bbb.62.2177. [DOI] [PubMed] [Google Scholar]
- 9.Sung YM, He G, Hwang DH, Fischer SM. Overexpression of the prostaglandin E2 receptor EP2 results in enhanced skin tumor development. Oncogene. 2006;25:5507–5516. doi: 10.1038/sj.onc.1209538. [DOI] [PubMed] [Google Scholar]
- 10.Basu S, Whiteman M, Mattey DL, Halliwell B. Raised levels of F(2)-isoprostanes and prostaglandin F(2alpha) in different rheumatic diseases. Ann Rheum Dis. 2001;60:627–631. doi: 10.1136/ard.60.6.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowe GN, Fu YH, McDougall S, Polendo R, Williams A, Benya PD, Hahn TJ. Effects of prostaglandins on deoxyribonucleic acid and aggrecan synthesis in the RCJ 3.1C5.18 chondrocyte cell line: role of second messengers. Endocrinology. 1996;137:2208–2216. doi: 10.1210/endo.137.6.8641167. [DOI] [PubMed] [Google Scholar]
- 12.Jakob M, Demarteau O, Suetterlin R, Heberer M, Martin I. Chondrogenesis of expanded adult human articular chondrocytes is enhanced by specific prostaglandins. Rheumatology (Oxford) 2004;43:852–857. doi: 10.1093/rheumatology/keh197. [DOI] [PubMed] [Google Scholar]
- 13.Milne SA, Jabbour HN. Prostaglandin (PG) F(2alpha) receptor expression and signaling in human endometrium: role of PGF(2alpha) in epithelial cell proliferation. J Clin Endocrinol Metab. 2003;88:1825–1832. doi: 10.1210/jc.2002-021368. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Middleton F, Horton JA, Reichel L, Farnum CE, Damron TA. Microarray analysis of proliferative and hypertrophic growth plate zones identifies differentiation markers and signal pathways. Bone. 2004;35:1273–1293. doi: 10.1016/j.bone.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Shukunami C, Shigeno C, Atsumi T, Ishizeki K, Suzuki F, Hiraki Y. Chondrogenic differentiation of clonal mouse embryonic cell line ATDC5 in vitro: differentiation-dependent gene expression of parathyroid hormone (PTH)/PTH-related peptide receptor. J Cell Biol. 1996;133:457–468. doi: 10.1083/jcb.133.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denker AE, Haas AR, Nicoll SB, Tuan RS. Chondrogenic differentiation of murine C3H10T1/2 multipotential mesenchymal cells: I. Stimulation by bone morphogenetic protein-2 in high-density micromass cultures. Differentiation. 1999;64:67–76. doi: 10.1046/j.1432-0436.1999.6420067.x. [DOI] [PubMed] [Google Scholar]
- 17.Sahni M, Ambrosetti DC, Mansukhani A, Gertner R, Levy D, Basilico C. FGF signaling inhibits chondrocyte proliferation and regulates bone development through the STAT-1 pathway. Genes & development. 1999;13:1361–1366. doi: 10.1101/gad.13.11.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Meth. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altaf FM, Hering TM, Kazmi NH, Yoo JU, Johnstone B. Ascorbate-enhanced chondrogenesis of ATDC5 cells. European cells & materials. 2006;12:64–69. doi: 10.22203/ecm.v012a08. discussion 69–70. [DOI] [PubMed] [Google Scholar]
- 20.Shukunami C, Ohta Y, Sakuda M, Hiraki Y. Sequential progression of the differentiation program by bone morphogenetic protein-2 in chondrogenic cell line ATDC5. Exp Cell Res. 1998;241:1–11. doi: 10.1006/excr.1998.4045. [DOI] [PubMed] [Google Scholar]
- 21.Saito O, Guan Y, Qi Z, Davis LS, Komhoff M, Sugimoto Y, Narumiya S, Breyer RM, Breyer MD. Expression of the prostaglandin F receptor (FP) gene along the mouse genitourinary tract. Am J Physiol Renal Physiol. 2003;284:F1164–F1170. doi: 10.1152/ajprenal.00441.2002. [DOI] [PubMed] [Google Scholar]
- 22.Sugimoto Y, Hasumoto K, Namba T, Irie A, Katsuyama M, Negishi M, Kakizuka A, Narumiya S, Ichikawa A. Cloning and expression of a cDNA for mouse prostaglandin F receptor. J Biol Chem. 1994;269:1356–1360. [PubMed] [Google Scholar]
- 23.Alvarez J, Sohn P, Zeng X, Doetschman T, Robbins DJ, Serra R. Tgfbeta2 mediates the effects of hedgehog on hypertrophic differentiation and PTHrP expression. Development. 2002;129:1913–1924. doi: 10.1242/dev.129.8.1913. [DOI] [PubMed] [Google Scholar]
- 24.Shukunami C, Akiyama H, Nakamura T, Hiraki Y. Requirement of autocrine signaling by bone morphogenetic protein-4 for chondrogenic differentiation of ATDC5 cells. FEBS letters. 2000;469:83–87. doi: 10.1016/s0014-5793(00)01251-5. [DOI] [PubMed] [Google Scholar]
- 25.Retting KN, Song B, Yoon BS, Lyons KM. Bmp canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development. 2009;136:1093–1104. doi: 10.1242/dev.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blaney Davidson EN, Remst DF, Vitters EL, van Beuningen HM, Blom AB, Goumans MJ, van den Berg WB, van der Kraan PM. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J Immunol. 2009;182:7937–7945. doi: 10.4049/jimmunol.0803991. [DOI] [PubMed] [Google Scholar]
- 27.Spagnoli A, O'Rear L, Chandler RL, Granero-Molto F, Mortlock DP, Gorska AE, Weis JA, Longobardi L, Chytil A, Shimer K, Moses HL. Tgf-beta signaling is essential for joint morphogenesis. J Cell Biol. 2007;177:1105–1117. doi: 10.1083/jcb.200611031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li TF, Darowish M, Zuscik MJ, Chen D, Schwarz EM, Rosier RN, Drissi H, O'Keefe RJ. Smad3-deficient chondrocytes have enhanced Bmp signaling and accelerated differentiation. J Bone Miner Res. 2006;21:4–16. doi: 10.1359/JBMR.050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson CM, Schwarz EM, Reynolds PR, Puzas JE, Rosier RN, O'Keefe RJ. Smad2 and 3 mediate transforming growth factor-beta1-induced inhibition of chondrocyte maturation. Endocrinology. 2000;141:4728–4735. doi: 10.1210/endo.141.12.7848. [DOI] [PubMed] [Google Scholar]
- 30.Spinella-Jaegle S, Roman-Roman S, Faucheu C, Dunn FW, Kawai S, Gallea S, Stiot V, Blanchet AM, Courtois B, Baron R, Rawadi G. Opposite effects of bone morphogenetic protein-2 and transforming growth factor-beta1 on osteoblast differentiation. Bone. 2001;29:323–330. doi: 10.1016/s8756-3282(01)00580-4. [DOI] [PubMed] [Google Scholar]
- 31.Keller B, Yang T, Chen Y, Munivez E, Bertin T, Zabel B, Lee B. Interaction of Tgfbeta and Bmp signaling pathways during chondrogenesis. PLoS One. 2011;6:e16421. doi: 10.1371/journal.pone.0016421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatakeyama Y, Tuan RS, Shum L. Distinct functions of Bmp4 and GDF5 in the regulation of chondrogenesis. J Cell Biochem. 2004;91:1204–1217. doi: 10.1002/jcb.20019. [DOI] [PubMed] [Google Scholar]
- 33.Mehlhorn AT, Niemeyer P, Kaschte K, Muller L, Finkenzeller G, Hartl D, Sudkamp NP, Schmal H. Differential effects of Bmp-2 and Tgf-beta1 on chondrogenic differentiation of adipose derived stem cells. Cell Prolif. 2007;40:809–823. doi: 10.1111/j.1365-2184.2007.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Y, Massague J. Mechanisms of Tgf-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 35.Akiyama H, Shukunami C, Nakamura T, Hiraki Y. Differential expressions of Bmp family genes during chondrogenic differentiation of mouse ATDC5 cells. Cell structure and function. 2000;25:195–204. doi: 10.1247/csf.25.195. [DOI] [PubMed] [Google Scholar]
- 36.Kluppel M, Wight TN, Chan C, Hinek A, Wrana JL. Maintenance of chondroitin sulfation balance by chondroitin-4-sulfotransferase 1 is required for chondrocyte development and growth factor signaling during cartilage morphogenesis. Development. 2005;132:3989–4003. doi: 10.1242/dev.01948. [DOI] [PubMed] [Google Scholar]
- 37.Muller K, Krieg P, Marks F, Furstenberger G. Expression of PGF(2alpha) receptor mRNA in normal, hyperplastic and neoplastic skin. Carcinogenesis. 2000;21:1063–1066. doi: 10.1093/carcin/21.5.1063. [DOI] [PubMed] [Google Scholar]
- 38.Sugatani J, Masu Y, Nishizawa M, Sakamoto K, Houtani T, Sugimoto T, Ito S. Hormonal regulation of prostaglandin F2 alpha receptor gene expression in mouse ovary. The American journal of physiology. 1996;271:E686–E693. doi: 10.1152/ajpendo.1996.271.4.E686. [DOI] [PubMed] [Google Scholar]
- 39.Brochhausen C, Neuland P, Kirkpatrick CJ, Nusing RM, Klaus G. Cyclooxygenases and prostaglandin E2 receptors in growth plate chondrocytes in vitro and in situ--prostaglandin E2 dependent proliferation of growth plate chondrocytes. Arthritis research & therapy. 2006;8:R78. doi: 10.1186/ar1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Ellman M, Muddasani P, Wang JH, Cs-Szabo G, van Wijnen AJ, Im HJ. Prostaglandin E2 and its cognate EP receptors control human adult articular cartilage homeostasis and are linked to the pathophysiology of osteoarthritis. Arthritis Rheum. 2009;60:513–523. doi: 10.1002/art.24258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugimoto Y, Yamasaki A, Segi E, Tsuboi K, Aze Y, Nishimura T, Oida H, Yoshida N, Tanaka T, Katsuyama M, Hasumoto K, Murata T, Hirata M, Ushikubi F, Negishi M, Ichikawa A, Narumiya S. Failure of parturition in mice lacking the prostaglandin F receptor. Science. 1997;277:681–683. doi: 10.1126/science.277.5326.681. [DOI] [PubMed] [Google Scholar]
- 42.Baxter GS, Clayton JK, Coleman RA, Marshall K, Sangha R, Senior J. Characterization of the prostanoid receptors mediating constriction and relaxation of human isolated uterine artery. British journal of pharmacology. 1995;116:1692–1696. doi: 10.1111/j.1476-5381.1995.tb16393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paralkar VM, Grasser WA, Mansolf AL, Baumann AP, Owen TA, Smock SL, Martinovic S, Borovecki F, Vukicevic S, Ke HZ, Thompson DD. Regulation of Bmp-7 expression by retinoic acid and prostaglandin E(2) J Cell Physiol. 2002;190:207–217. doi: 10.1002/jcp.10048. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Wang JH. Bmp-2 mediates PGE(2) -induced reduction of proliferation and osteogenic differentiation of human tendon stem cells. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2012;30:47–52. doi: 10.1002/jor.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ito H, Akiyama H, Shigeno C, Nakamura T. Bone morphogenetic protein-6 and parathyroid hormonerelated protein coordinately regulate the hypertrophic conversion in mouse clonal chondrogenic EC cells, ATDC5. Biochim Biophys Acta. 1999;1451:263–270. doi: 10.1016/s0167-4889(99)00100-7. [DOI] [PubMed] [Google Scholar]
- 46.Tchetina EV, Squires G, Poole AR. Increased type II collagen degradation and very early focal cartilage degeneration is associated with upregulation of chondrocyte differentiation related genes in early human articular cartilage lesions. J Rheumatol. 2005;32:876–886. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.