Abstract
Brain 5-HT deficiency has long been implicated in psychiatric disease, but the effects of 5-HT deficiency on stress susceptibility remain largely unknown. Early life stress (ELS) has been suggested to contribute to adult psychopathology, but efforts to study the long-term consequences of ELS have been limited by a lack of appropriate preclinical models. Here, we evaluated the effects of 5-HT deficiency on several long-term cellular, molecular, and behavioral responses of mice to a new model of ELS that combines early-life maternal separation (MS) of pups and postpartum learned helplessness (LH) training in dams. Our data demonstrate that this paradigm (LH/MS) induces depressive-like behavior and impairs pup retrieval in dams. In addition, we show that brain 5-HT deficiency exacerbates anxiety-like behavior induced by LH/MS and blunts the effects of LH/MS on acoustic startle responses in adult offspring. Although the mechanisms underlying these effects remain unclear, following LH/MS, 5-HT-deficient animals had significantly less mRNA expression of the mineralocorticoid receptor in the amygdala than wild-type animals. In addition, 5-HT-deficient mice exhibited reduced mRNA levels of the 5-HT2a receptor and p11 in the hippocampus regardless of stress. LH/MS decreased the number of doublecortin+ immature neurons in the hippocampus in both wild-type (WT) and 5-HT-deficient animals. Our data emphasize the importance of complex interactions between genetic factors and early life experience in mediating long-term changes in emotional behavior. These findings may have important implications for our understanding of the combinatorial roles of 5-HT deficiency, ELS, and postpartum depression on the development of neuropsychiatric disorders.
1.1. Introduction
Brain 5-HT deficiency has been hypothesized to play a role in a wide range of psychiatric diseases, including major depression (Jacobsen et al., 2012a) and anxiety disorders (Bell and Nutt, 1998). Consistent with a potential role of brain 5-HT deficiency in psychiatric disease, mutations in tryptophan hydroxylase 2 (Tph2), the rate-limiting enzyme in brain 5-HT synthesis, have been identified in patients with depression (Zill et al., 2004, Zhang et al., 2005) and anxiety (Kim et al., 2009). Despite the identification of these mutations, the precise impact of brain 5-HT deficiency on the development of depression- and anxiety-like behavior remains unknown. Recent theories regarding the etiology of mental illness suggest that genetic mutations, such as those leading to dysfunctional 5-HT signaling, contribute to psychiatric disease risk through complex interactions with other genetic and environmental risk factors, such as exposure to stress during critical developmental periods (Burmeister et al., 2008, Schmidt, 2011, Homberg, 2012, Daskalakis et al., 2013). Here, we examined the effects of brain 5-HT deficiency on the long-term responses of mice to a new early life stress (ELS) paradigm that we developed. To model brain 5-HT deficiency, we utilized mice that express the murine equivalent of a rare loss-of-function mutation (R441H) in Tph2, which was initially identified in a cohort of patients with major depression (Zhang et al., 2005). These Tph2(R439H) knock-in animals (HO-Tph2KI mice) have been reported to exhibit 60–80% reductions in brain 5-HT (Beaulieu et al., 2008, Jacobsen et al., 2012b) and have been shown to exhibit behavioral disinhibition following exposure to repeated maternal separation (MS) (Sachs et al., 2013b).
Maternal psychosocial experience and a history of ELS are both thought to contribute to the development of psychiatric illnesses (Heim and Nemeroff, 2001, Bale et al., 2010), but the genetic factors that regulate susceptibility to ELS remain largely unknown. Postpartum depression, which affects as many as 7% of new mothers (Gavin et al., 2005), can have a profound effect on early life experience, in part by leading to impaired mother-infant interactions (Carter et al., 2001). Children with mothers suffering from postpartum depression exhibit increased rates of psychiatric illness and social and cognitive dysfunction (Beck, 1998). However, the cellular and molecular mechanisms underlying the negative consequences of ELS remain largely unknown.
Relatively few rodent models of postpartum depression have been reported (Maguire and Mody, 2008, Carini et al., 2013), and existing ELS models are highly variable. Indeed, multiple studies have reported significant long-term effects of repeated MS in rodents (Huot et al., 2001, Romeo et al., 2003, Plotsky et al., 2005, Murgatroyd et al., 2009), whereas other studies have found minimal or no phenotypes following MS (Millstein and Holmes, 2007, Parfitt et al., 2007). Novel paradigms combining MS with early weaning (Carlyle et al., 2012) or unpredictable mild maternal stress (Franklin et al., 2011) appear to be more robust, but additional models are warranted. Here, we report a new ELS model that combines MS with learned helplessness (LH) training in postpartum dams.
We have evaluated the effects of brain 5-HT deficiency on several long-term cellular, molecular, and behavioral responses of mice to this new LH/MS paradigm. As a potential cellular response to stress, we have investigated adult hippocampal neurogenesis, which is known to be influenced by stress (Gould et al., 1997) and 5-HT deficiency (Sachs et al., 2013a), and which has been suggested to play a role in buffering stress responses (Snyder et al., 2011). We also examined gene expression changes following LH/MS in two brain regions that have been implicated in stress responses, the hippocampus (Snyder et al., 2011) and the amygdala (Hariri et al., 2002).
2. Materials and Methods
2.1. Mice
Tph2R439H knock-in mice on a mixed background (c57BL6/J – 129S6/SvEv) were used for all experiments (Beaulieu et al., 2008). All experiments were conducted with an approved protocol from the Duke University Institutional Animal Care and Use Committee.
2.2. Learned Helplessness/Maternal Separation Procedure
HET-Tph2R439H knock-in breeders were used to generate WT, HET, and HO-Tph2KI pups. Half of the litters were subjected to 11 days of repeated maternal separation/isolation (LH/MS) starting at PND2 (LH/MS pups), while the other half of the litters (the control group) were raised under standard rearing conditions (except for measurements of USVs). For most studies, only the WT and HO-Tph2KI animals were analyzed. For the LH/MS procedure, pups were removed from their dams and placed into individual plastic containers on heating pads set to 30°C for 3 hr a day from PND2-PND12. During this time, LH/MS dams were subjected to learned helplessness training in which they received a series of 360 inescapable electric foot-shocks (2 s duration, 0.15 mA; 10 s variable inter-trial interval) over the course of 1 hr (Fukui et al., 2007). After each training session, dams were removed from the chambers and placed into standard mouse cages with no bedding for the remainder of the 3 hr separation period. Control dams remained in their home cages with their pups during this time.
On the final day of separation (PND12), LH/MS dams were evaluated in the learned helplessness test to determine if they had acquired depressive-like responses. Control dams were also evaluated in this test when their pups reached PND12. Learned helplessness testing was performed essentially as described previously (Fukui et al., 2007) with 40 trials of escape training and a 30–90 sec inter-trial interval. Each trial consisted of exposure to an escapable 0.15 mA foot shock. Each session began with the door between the chambers closed for a 5 min acclimatization period. For the first two test trials, the door opened immediately upon the onset of the foot-shock. For the remaining 38 trials, the door opened 2 s following the onset of the foot-shock. Foot-shocks were terminated when a mouse escaped to the non-shocked compartment or when it failed to escape within 20 s. The number of escape failures and the latency to each escape were determined by MedAssociates software.
2.3. Ultrasonic vocalizations (USVs)
For the LH/MS group, USVs were recorded over 5 min from each LH/MS pup immediately upon separation from the dam on PNDs 2, 4, 6, 8 and 10 using an ultrasonic monitor (Avisoft Bioacoustics, Glienicke, Germany). During USV measurements, pups were placed in individual plastic containers and remained at room temperature. Genotyping had not yet been performed on the pups during this time period, so USVs were measured from all three genotypes (WT, HET, and HO-Tph2KI animals). Each animal was individually marked on PND2 to facilitate its identification throughout the course of USV measurements. Control pups of the same postnatal ages were removed from their nests and USVs were recorded. After USV recordings, control pups were returned individually to the corner of the cage furthest from their nest, and the latencies for the dam to retrieve the pups to the nest were recorded. For LH/MS animals, following USV recording, pups were transferred (in individual containers) onto a heating pad for the duration of the separation period. At the end of the 3 hr separation period, dams were returned to their home cage, pups were placed in the home cage in the corner farthest from the nest, and pup retrieval latency was recorded.
2.4. Early behavioral phenotyping
Postnatal Approach Tests: For the postnatal approach tests, all dams were provided two extra nestlets (5 × 5 × 0.5cm; Ancare, Belmore, NY) the night prior to testing to ensure that sufficient nesting material was available for testing the following day. Intact home cages were transferred into the test room at the onset of the light cycle, and testing commenced 2 hr after onset of the light cycle. The order of testing was randomized by litter condition and genotype; and the investigators conducting the approach tests were blind to the experimental condition and genotypes of the pups.
To begin behavioral testing, the top of each cage was opened, and the resident dam was allowed to move at least one body length away from the nest, at which point the temperature of the nest was measured using an Extech IR200 Non-contact IR Thermometer (Extech Instruments/Instrumart, South Burlington, VT), which was held between 8–10 cm from the nest surface. The dam was then removed from the home cage and placed into a holding cage. Pups were collected as a single group with part of the nesting materials and were placed into an open bottom cylinder (internal diameter of 8 cm × 5 cm high) on a heating pad maintained at 34–36°C, a temperature range known to mimic the nest temperature in mice and reduce ultrasonic vocalizations (Okon, 1970). To ensure that the heating pad provided enough heat to prevent distress calling, an ultrasonic monitor (Avisoft Bioacoustics, Glienicke, Germany) was placed 16 cm above the holding area. A second cylinder with nesting material from the home cage was also placed on the heating pad to hold pups after testing.
Each pup was tested individually in a clean test cage (29 × 18 × 12 cm) placed on a heating pad at nest temperature. One side of the cage was designated the target zone, and it contained either a target HET-Tph2KI pup of the same age and sex in a wire mesh cage or the remaining half of the nesting materials from the home cage. The test pup was placed on the opposite side of the cage, and the time it took the pup to reach the target zone was recorded. For experiments using the nesting material as the target, the latency until the pup made contact with and began to enter the nest was measured. For experiments using a caged littermate, the test pup had to make contact with the target cage and remain alongside the cage for at least 5 sec. All pups were first examined for their ability to approach approximately 1.5–1.75 grams of nesting material from the home cage, placed into a slightly flattened circle 4–5 cm in diameter. Upon completion of the test, each pup was placed into the second holding cylinder on the heating pad. Once all pups had been examined for attraction to the home nesting material, they were then assessed a second time for approach to the HET-Tph2KI conspecific. A clean test cage was used for each test for each pup; cages were not reused. Upon completion of testing, all pups were returned to the home cage with all nesting materials, and the dam was then placed back into the cage with the appropriate litter. The latency of the pups to approach and make contact with the target during each test was recorded in seconds. Pups that failed to approach or make contact with the target within the 2 min test period had latencies recorded of 120 sec. The time spent immobile (in sec) during testing was also scored.
2.5. Light-Dark Emergence Test
The apparatus consisted of a mouse shuttle box with two chambers (20 × 16 × 21 cm/chamber) separated by an automated sliding door (MedAssociates, St. Albans, VT). One chamber was illuminated with a 170 mA light (with house lights, total illumination was ~600 lux), whereas the other was enclosed by a black cloth. Mice were placed on the darkened side; 5 s later the door to the adjoining chamber was opened and mice were given free access to the entire apparatus for 5 min. The latency to first enter the lighted side, the time spent on each side, and the activity of each animal was determined by MedAssociates software.
2.6. Elevated Zero Maze
This apparatus and procedure have been described (Fukui et al., 2007). Briefly, the mouse was placed into a closed quadrant and allowed to freely investigate the maze for 5 min. Subsequently, videos were analyzed with the Observer program (Noldus, Sterling, VA) and were scored for the percent time spent in the open areas.
2.7. Acoustic Startle Responses
To measure acoustic startle responses to auditory stimuli of varying intensity, mice were individually placed in a small Plexiglas holding tube in a mouse startle chamber (Med-Associates, St. Albans, VT). Following 5 min of acclimatization, mice were exposed to 42 trials of white noise stimuli of varying intensities (i.e., 80, 90, 100, 105, 110, 115, and 120 dB). Each of the seven stimulus intensities was presented six times per test, and the intensities were randomized across test trials. Trials were separated by an average inter-trial interval of 30 sec (range 15–45 sec). The peak startle response recorded as mAmp displacement within 70 msec from the onset of the 40 msec startle stimulus. The average response to each startle intensity for each animal was used in analyses of the data.
2.8. Social Interaction Test
Mice were placed in a 60 cm by 40 cm arena containing two small wire enclosures. One enclosure (i.e., the social target) contained a single sex-matched, ten-week-old C3HeJ mouse. The other enclosure (i.e., the non-social target) was empty. Animals were videotaped from above, and the amount of time each animal spent with the social target and non-social target was measured using Ethovision (Version 9.0; Noldus Information Technology, Leesburg, VA).
2.9. Immunohistochemistry
BrdU labeling and immunohistochemistry (IHC) for BrdU and DCX were performed essentially as described (Sachs et al., 2013a, Sachs et al., 2013b, Sachs et al., 2014). Briefly, mice were injected with BrdU three times (i.p., 100 mg/kg, once each at 24, 16 and 4 hr prior to euthanasia with ketamine/xylazine) and perfused with 10% neutral buffered formalin (NBF). Brains were removed, post-fixed in NBF overnight, cryopreserved in 30% sucrose in PBS for 48 hr, embedded in optimal cutting temperature compound (OCT; VWR International, Suwanee, GA), and cut into 25 μm sections using a cryostat. Alternate sections throughout the hippocampus were collected and mounted onto glass slides, and a total of 60 sections from each brain were collected. Every tenth section collected was processed for IHC, and both hemispheres were examined.
Sections were dehydrated in two consecutive 5 min washes in xylenes substitute (Sigma, St. Louis, MO, USA) then rehydrated through a series of washes in ethanol (EtOH): 100% EtOH for 10 min, 95% EtOH for 3 min, 85% EtOH for 3 min, then 70% EtOH for 3 min. Slides were washed in PBS for 5 min and then submerged in sodium citrate antigen retrieval buffer (10 mM sodium citrate and 10 mM citric acid, pH 6.0) and boiled in a microwave for 20 min. Sections were placed at 4°C for 20 min to cool and rinsed once in PBS for 5 min. Sections being processed for BrdU immunohistochemistry were then incubated for 30 min in 2 N hydrochloric acid in PBS and washed three times in PBS (5 min each). For DCX stainings, this hydrochloric acid step was omitted. All sections were then blocked in 5% bovine serum albumin (BSA) in PBS containing 0.1% Triton (PBS-t) for 1 hr. After three 5-min washes in PBS, sections were incubated overnight in primary antibody diluted in 1% BSA in PBS-t. A rat anti-BrdU primary antibody (OBT0030G, 1:500 dilution; Accurate Chemical Corporation, Westbury, NY) or a rabbit anti-DCX antibody (1:200 dilution; Abcam, Cambridge, MA) was used. Sections were washed three times in PBS and then incubated for at least 90 min at room temperature with Alexafluor 568-conjugated anti-rat or Alexafluor 488-conjugated anti-rabbit secondary antibodies (1:500 dilution; Life Technologies, Carlsbad, CA) in 5% BSA in PBS-t. Sections were then washed three times in PBS and coverslipped using SlowFade Gold Antifade Reagent containing the nuclear stain, DAPI (Life Technologies, Carlsbad, CA). Non-overlapping images of the entire subgranular zone were taken on a fluorescence microscope (Zeiss) by an individual blinded to the genotype and history of stress exposure of the animals. The number of BrdU+ or DCX+ cells was counted by a blinded observer. Every tenth section that was collected was processed for BrdU IHC, and both hemispheres were examined. The number of BrdU+ cells counted was then multiplied by ten to obtain the value for each mouse. The same IHC procedure was performed for confocal microscopy, except that nuclei were visualized using TOTO-3 staining, and SlowFade Gold coverslipping medium without DAPI was used.
2.10. Real time PCR
Brains were isolated, and bilateral punches (1 mm in diameter and 1 mm thickness) from the amygdala were obtained and snap frozen in liquid nitrogen. RNA was isolated using Trizol in combination with RNeasy minikits (Qiagen). Reverse transcription was performed using the iScript cDNA synthesis kit according to the manufacturer’s instructions. Real time PCR was performed using SYBR Select Master Mix (Applied Biosystems, Austin, TX). Primer sequences are as follows: GAPDH : Forward –5′-CAT GTT CCA GTA TGA CTC CACTC - 3′; Reverse – 5′-GGC CTC ACC CCA TTT GAT GT – 3′; CRH: Forward - 5′-CCT CAG CCG GTT CTG ATC C - 3′; Reverse – 5′-GCG GAA AAA GTT AGC CGC AG – 3′; 5-HT1a: Forward – 5′-GCG GTC ACC GAT CTC ATG G- 3′; Reverse – 5′-CAG TAC CTG TCT AGC GCG AT – 3′; 5-HT2a: Forward – 5′-CAC TTG CCA TAG CTG ATA TGC T -3′; Reverse – 5′-GGT AAA TCC AGA CGG CAC AGA – 3′; GR: Forward – 5′-AGC TCC CCC TGG TAG AGA C – 3′; Reverse – 5′-GGT GAA GAC GCA GAA ACC TTG – 3′; MR: Forward – 5′-GAA AGG CGC TGG AGT CAA GT - 3′; Reverse – 5′-TGT TCG GAG TAG CAC CGG AA – 3′; p11: Forward – 5′-TGG AAA CCA TGA TGC TTA CGT T – 3′; Reverse – 5′-GAA GCC CAC TTT GCC ATC TC – 3′; SGK1: Forward 5′-TCC GCC AAG TCC CTC TCA ACA AAT – 3′; Reverse – 5′ – TGC CTA GCC AGA AGA ACC TTT CCA – 3′; SERT: Forward – 5′ – TAT CCA ATG GGT ACT CCG CAG – 3′; Reverse – 5′ – CCG TTC CCC TTG GTG AAT CT – 3′.
2.11. Statistical Analysis
Data were analyzed using t-tests or two-way ANOVAs with Tukey’s or Bonferroni’s post hoc tests, with repeated measures where appropriate. Statistical analyses were performed using JMP (SAS, Cary, NC) or SPSS (SPSS Inc., Chicago, IL) software.
3. Results
3.1. Effects of LH/MS on dams
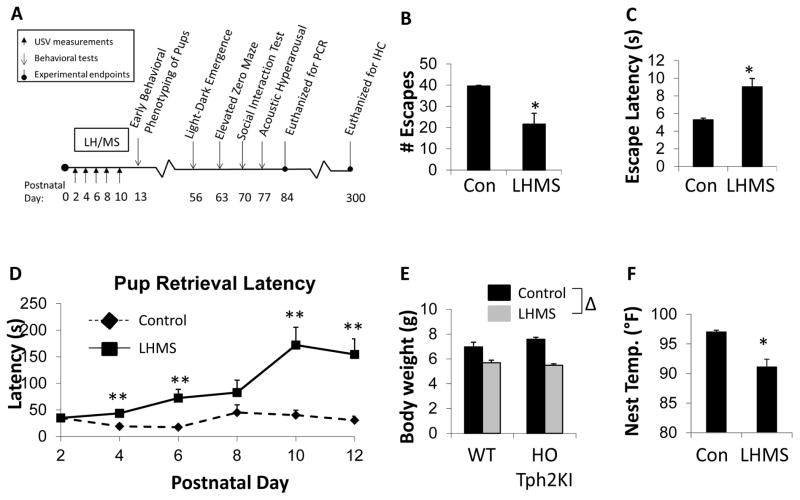
LH training, as a part of the LH/MS procedure (Figure 1A), results in the development of a “depression-like” state in heterozygous (HET) Tph2KI dams, as evidenced by the reduced numbers of escapes (p = 0.0074 by t-test; Figure 1B) and the increased escape latencies (p = 0.0036 by t-test; Figure 1C) that LH/MS-exposed dams exhibit in response to escapable foot-shocks when compared to control dams. In addition to their “depression-like” phenotype, LH/MS dams also display significantly longer latencies to retrieve their pups to the nest when compared to control dams [F(1,69) = 8.9, p = 0.004 by RMANOVA, Figure 1D]. Bonferroni’s post hoc comparisons confirmed that the effects of LH/MS on pup retrieval were significant on postnatal days (PNDs) 4, 6, 10 and 12 (p < 0.05), thus demonstrating that postpartum stress can impair maternal behavior in mice (Figure 1D). Consistent with this observation, the pups from LH/MS-exposed litters had significantly reduced body weights on PND 13 when compared to pups from control litters [main effect of stress by two-way ANOVA, F(1, 39) = 48.4, p < 0.0001; Figure 1E]. Similarly, the temperature of the nests of LH/MS-exposed dams was significantly reduced compared to that observed for control litters (p < 0.0001 by t-test; Figure 1F).
Figure 1.
Effects of LH/MS on dams and pups. (A) A schematic depicting the LH/MS paradigm and a timeline of the experiments performed. (B) The number of successful escapes in the learned helplessness paradigm in control and LH/MS-exposed heterozygous (HET)-Tph2KI dams. (C) The average escape latency in control and LH/MS-exposed HET-Tph2KI dams. (D) The average pup retrieval latency over PND 2–12 in control and LH/MS-exposed HET-Tph2KI dams. (E) The average body weights of control and LH/MS-exposed WT and HO-Tph2KI pups at PND13. (F) The nest temperature of control and LH/MS-exposed HET-Tph2KI dams. N = 7–8 per group for A – C. N = 7–13 per group in D and E. * indicates p < 0.05 from the control by t-test. ** indicates p < 0.05 by repeated measures ANOVA with Bonferroni’s. Δ indicates significant main effect of LH/MS versus control exposure by two-way ANOVA, p < 0.05.
3.2. Effects of LH/MS on offspring behavior
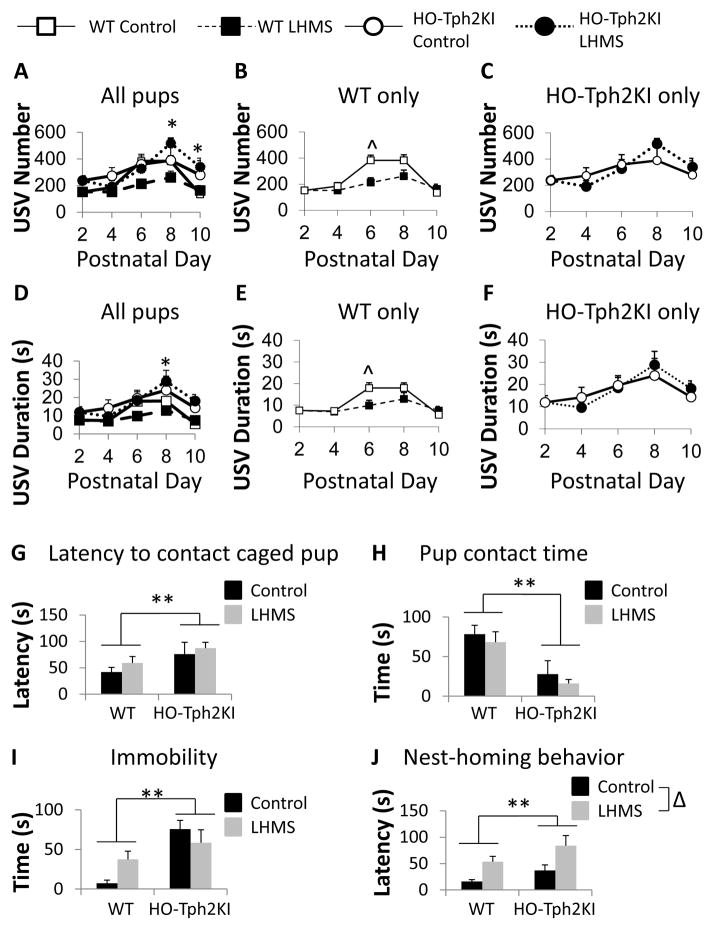
To evaluate the effects of 5-HT deficiency on the behavioral responses of pups to ELS, we compared ultrasonic vocalizations (USVs) in control and LH/MS-exposed WT and HO-Tph2KI pups every other day between PND2 and PND10. USVs are elicited by pups in response to MS and are considered to be an early indicator of emotional responses (Gardner, 1985, Takahashi et al., 2009). As expected, the number of USV calls elicited by pups was significantly impacted by the age of the pup [main effect of day by RMANOVA: F(4, 76) = 25.592, p < 0.001], but significant two-way [day by stress: F(4, 76) = 3.397, p = 0.01] and three-way interactions [day by stress by genotype: F(8, 72) = 2.676, p = 0.007] were also observed. Post-hoc analyses revealed that LH/MS-exposed HO-Tph2KI pups emit significantly more USVs than LH/MS-exposed WT pups on PNDs 8 and 10 (p = 0.014 and p = 0.036 by Bonferroni’s, respectively; Figure 2A–C), suggesting that these animals exhibit aberrant arousal responses to MS. HET-Tph2KI pups showed intermediate levels of USV responses when compared to WT and HO-Tph2KI animals at all time-points (data not shown). When the within-genotype effects of LH/MS were examined, LH/MS was shown to significantly reduce the number of USVs elicited by WT mice on PND 6 (p = 0.006 by Bonferroni’s; Figure 2A, B), but LH/MS did not significantly modify USV number at any time point in HO-Tph2KI pups (Figure 2A, C).
Figure 2.
The effects of LH/MS on ultrasonic vocalizations and behavior in WT and HO-Tph2KI pups. (A) The number of ultrasonic vocalizations (USVs) elicited by WT and HO-Tph2KI pups in response to once daily bouts of maternal separation over PNDs 2–10. (B) The effects of LH/MS on the number of USVs in WT pups alone. (C) The effects of LH/MS on the number of USVs in HO-Tph2KI pups alone. (D) The mean duration of USVs elicited by WT and HO-Tph2KI pups in response to repeated once daily bouts of maternal separation. (E) The effects of LH/MS on call duration in WT pups. (F) The effects of LH/MS on call duration in HO-Tph2KI pups. (G) Latency to contact a caged age-matched HET-Tph2KI pup. (H) Average pup contact time. (I) Time spent immobile during the pup interaction test. (J) Latency of pups to return to the nest. N = 5–18 per group. * indicates p < 0.05 comparing WT-LH/MS versus HO-LH/MS; ^ indicates p < 0.05 comparing WT-Control versus WT-LH/MS by Bonferroni’s. ** indicates significant main effect of genotype (p < 0.05) by two-way ANOVA; Δ indicates significant main effect of LH/MS (p < 0.05) by two-way ANOVA.
Similar relationships were observed when USV duration was analyzed (Figure 2D–F). USV call duration was also significantly affected by age [F(4, 76) = 21.521, p < 0.001], but significant two-way [day by stress: F(4, 76) = 3.559, p = 0.007] and three-way interactions [day by stress by genotype: F(8, 72) = 2.657, p = 0.008] were again observed. LH/MS-exposed HO-Tph2KI pups emitted a significantly longer total duration of USV calling than LH/MS-exposed WT pups on PNDs 8 and 10 (p = 0.011 and p = 0.024 by Bonferroni’s, respectively; Figure 2D–F). Within-genotype analysis revealed that LH/MS reduced the duration of USV calling in WT pups on PND 6 (p = 0.037 by Bonferroni’s; Figure 2D, E), but LH/MS did not significantly modify USV duration at any time-point in HO-Tph2KI pups (Figure 2D, F).
To further examine the effects of LH/MS and 5-HT deficiency on pup behavior, we analyzed homing and social behaviors in pups on PND 13. For social behavior, the latency to interact with a sex-matched HET-Tph2KI target littermate in a novel environment was increased in HO-Tph2KI pups compared to WT pups, regardless of LH/MS [main effect of genotype by two-way ANOVA, F(1, 38) = 6.14, p = 0.018; Figure 2G]. In addition, HO-Tph2KI pups also spent significantly less time with this social target than did WT pups [main effect of genotype by two-way ANOVA, F(1, 38) = 16.38, p = 0.0002; Figure 2H], suggesting that 5-HT deficiency can impair social behaviors even early in life. Exposure to LH/MS did not significantly affect either the latency to interact or the total social interaction time in either genotype. During this early life social interaction test, HO-Tph2KI pups exhibited a significant increase in time spent immobile in this novel environment compared to WT pups [main effect of genotype by two-way ANOVA, F(1, 38) = 17.83, p = 0.0001; Figure 2I]. A significant genotype by stress interaction was also observed [F(1, 38) = 8.17, p = 0.007], and Tukey’s post hoc analysis revealed that LH/MS led to a significant increase in immobility behavior in WT mice (p = 0.031), but not in HO-Tph2KI animals (p = 0.54). However, it is possible that a ceiling effect precluded any additional immobility in HO-Tph2KI pups. The latency of pups to reach nesting material in a novel environment was also measured, and HO-Tph2KI pups were shown to exhibit an overall increase in latency to reach the nesting material [main effect of genotype by two-way ANOVA, F(1,39) = 5.09, p = 0.03; Figure 2J]. LH/MS increased nest approach latency in both genotypes [main effect of stress by two-way ANOVA, F(1,39) = 13.81, p = 0.0006; Figure 2J].
3.3. Long-term behavioral consequences of LH/MS in adult offspring
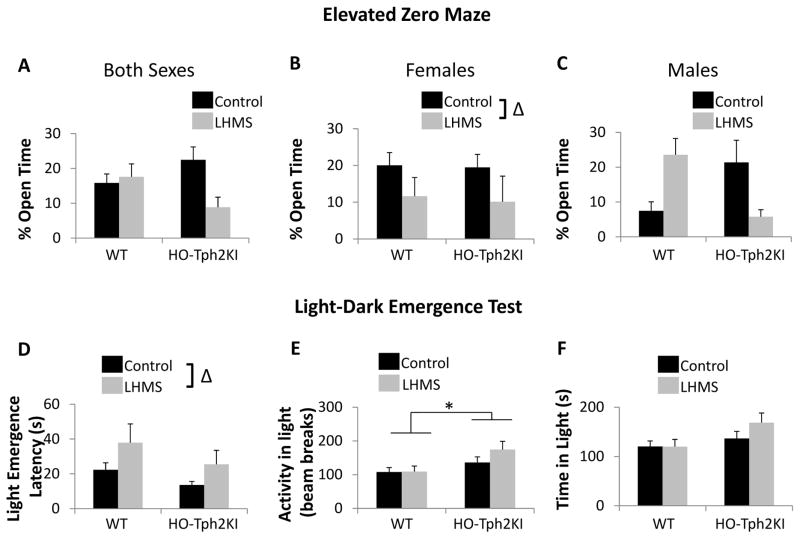
The long-lasting nature of the behavioral disturbances induced by LH/MS was next tested by examining anxiety-like behaviors in eight-week-old mice. When the amount of time that adult animals spent in the open arms of the elevated zero maze (EZM) was examined, a significant three-way interaction was observed [genotype by stress by sex interaction by three-way ANOVA, F(1, 87) = 5.52, p = 0.021; Figure 3A]. A significant two-way genotype by stress interaction was also observed [F(1, 87) = 6.23, p = 0.015; Figure 3A], but Tukey’s post hoc tests did not reveal any significant differences between the groups when the sexes were examined together. When the sexes were analyzed individually, LH/MS led to a significant increase in anxiety-like behavior in females of both genotypes [main effect of stress by two-way ANOVA, F(1,44) = 4.26, p = 0.045; Figure 3B]. In males, no significant main effects of LH/MS or genotype were observed. However, LH/MS led to opposing effects in WT and HO-Tph2KI animals, and a significant genotype by stress interaction was observed [F(1, 43) = 10.49, p = 0.0023; Figure 3C]. Specifically, LH/MS led to a significant decrease in anxiety-like behavior in WT males (p = 0.027), while leading to a trend toward increased anxiety-like behavior in HO-Tph2KI males (Figure 3C). Consequently, LH/MS-exposed HO-Tph2KI males exhibited increased anxiety-like behavior when compared to LH/MS-exposed WT males (p = 0.043), but no significant genotype differences were observed in control males.
Figure 3.
Anxiety-like behaviors in adult offspring. (A) Percent time in the open arms of the elevated zero maze for males and females combined. (B) Percent open arm time for females only. (C) Percent open arm time for males only. (D) Latency to emerge into the lighted chamber in the light-dark emergence test. (E) Activity in the lighted chamber. (F) Time spent in the lighted chamber. N = 13–35 per group for A. N = 5–21 per group for B. N = 8–14 per group for C. N = 25–42 for D–F. * indicates significant main effect of genotype (p < 0.05) by two-way ANOVA; Δ indicates significant main effect of LH/MS (p < 0.05) by two-way ANOVA.
In the light-dark emergence test, LH/MS significantly increased the latency to enter the lighted chamber [F(1,123) = 4.55, p = 0.035; Figure 3D], but no significant interactions were observed. LH/MS did not affect total activity or the activity in the lighted chamber in either genotype; however, activity in the lighted chamber was increased in HO-Tph2KI mice regardless of stress history [F(1,123) = 7.57 p = 0.0068; Figure 3E]. HO-Tph2KI mice also displayed a trend towards increased time spent in the lighted chamber, but this effect did not quite reach statistical significance (p = 0.059; Figure 3F). No significant effects of sex, stress, or genotype were observed on activity or time spent in the dark chamber.
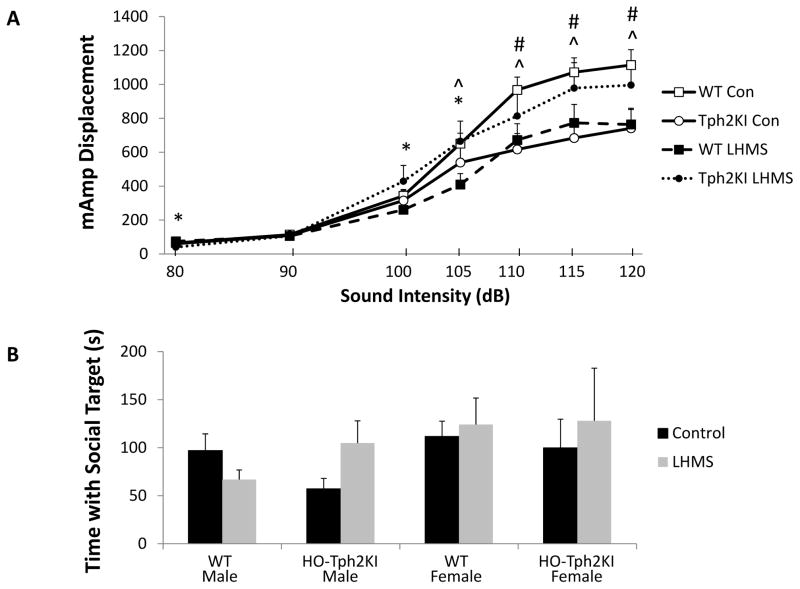
The effects of LH/MS, sex, and 5-HT deficiency were also examined in the acoustic hyper-arousal paradigm. Although no significant main effects of genotype, sex, or 5-HT deficiency were observed, a repeated measures ANOVA for startle responses revealed a significant three-way genotype by stress by startle intensity interaction [F(6, 91) = 5.99, p < 0.001, Figure 4A]. A significant genotype by stress interaction was also observed [F(1,95) = 7.26, p = 0.0083; Figure 4A]. In control animals, no genotype differences were observed in response to the 80, 90, 100 or 105 dB stimuli, but control HO-Tph2KI mice exhibited blunted startle responses to the 110, 115 and 120 dB stimuli (p = 0.007, p = 0.007 and p = 0.009, respectively, by Bonferroni’s). In LH/MS-exposed animals, HO-Tph2KI mice exhibited a reduced startle magnitude to the 80 dB stimulus (p = 0.007 by Bonferroni’s), but they displayed increased startle responses to the 100 dB (p = 0.034) and 105 dB (p = 0.038) stimuli when compared to WT mice. An investigation of the effects of LH/MS within genotype revealed that LH/MS in WT animals led to a significant blunting of the magnitude of startle responses to the 105 dB (p = 0.015), 110 dB (p = 0.015), 115 dB (p = 0.025) and 120 dB (p = 0.008) stimuli. In contrast, Bonferroni’s post-hoc tests revealed that LH/MS did not lead to any significant changes in startle responses in HO-Tph2KI animals.
Figure 4.
The effects of LH/MS on acoustic hyper-arousal and social behavior. (A) Startle responses of control and LH/MS-exposed WT and HO-Tph2KI mice across startle intensities. (B) Social affiliation time in male and female WT and HO-Tph2KI mice with an 8 week old, sex-matched animal. N = 15–34 per group for A and N = 15–29 per group for B. Significant genotype by stress interactions were observed by repeated measures two-way ANOVA (for panel A) and by two-way ANOVA (for panel B) (p < 0.05). A significant three-way genotype by stress by startle intensity interaction was also observed in A by repeated measures three-way ANOVA (p < 0.05). * denotes p < 0.05 for WT-LH/MS vs. HO-Tph2KI-LH/MS comparison, ^ denotes p < 0.05 for WT-Control vs. WT-LH/MS comparison, and # denotes p < 0.05 for WT-Control vs. HO-Tph2KI-Control comparison by Bonferroni’s.
3.4. Social behavior
When social interaction was examined in the social affiliation test, female mice spent more time interacting with a novel young female mouse than males spent interacting with a young male mouse [F(1,84) = 4.98, p = 0.028; Figure 4B]. LH/MS led to no overall effects on social interaction, but when males and females were analyzed independently, LH/MS was shown to differentially affect male WT and HO-Tph2KI mice [genotype by stress interaction: F(1,44) = 6.76, p = 0.013; Figure 4B]. Specifically, LH/MS led to a ~30% decrease in social interaction in male WT animals, but it enhanced social interaction by ~80% in HO-Tph2KI males (p > 0.05 by Tukey’s). In contrast, LH/MS had no significant effects on social interaction behavior in females (Figure 4B).
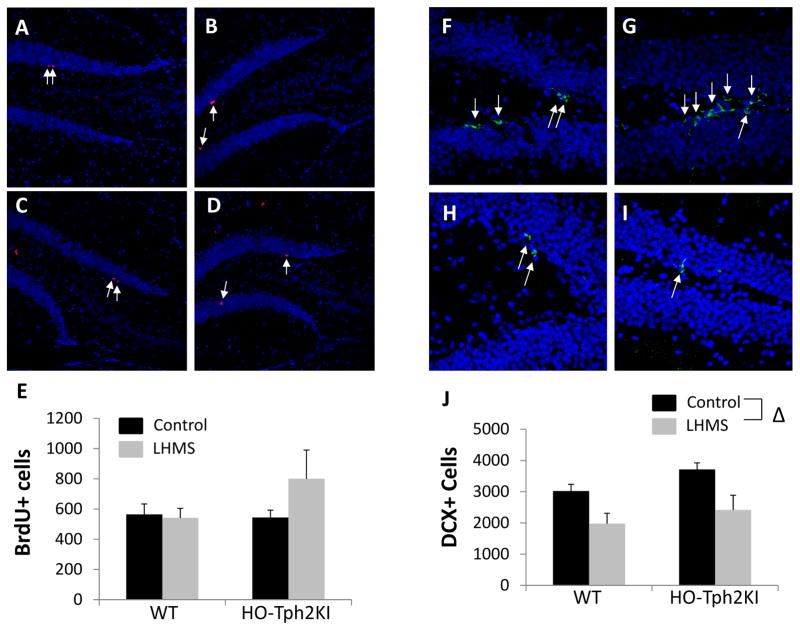
3.5. Effects of LH/MS on hippocampal cell proliferation and neurogenesis
To evaluate the potential cellular correlates of the behavioral changes induced by LH/MS, we performed immunohistochemistry for BrdU and doublecortin (DCX) to monitor adult neurogenesis in WT and HO-Tph2KI animals. No significant effects of sex, genotype, or LH/MS were observed on cell proliferation in the hippocampus of ten-month-old mice (Figure 5A–E). However, LH/MS led to a significant reduction in the number of DCX+ immature neurons in the hippocampus of both genotypes [F(1, 41) = 13.51, p = 0.0007; Figure 5F–J]. The number of DCX+ cells was higher in HO-Tph2KI mice than in WT mice under both control and LH/MS-exposed conditions, but this effect did not quite reach statistical significance (p = 0.056).
Figure 5.
The effects of LH/MS on cell proliferation and doublecortin expression in the hippocampus. Representative images depicting BrdU immunoreactivity from the hippocampus of WT-Control (A), HO-Tph2KI-Control (B), WT-LH/MS (C), and HO-Tph2KI-LH/MS (D) mice. The results of quantification are shown in (E). Representative maximum-intensity-projection images of confocal z-stacks depicting DCX immunoreactivity from the hippocampus of WT-Control (F), HO-Tph2KI-Control (G), WT-LH/MS (H), and HO-Tph2KI-LH/MS (I) mice. The results of quantification are shown in (J). Δ indicates a significant main effect of stress by two-way ANOVA (p < 0.001). N = 6–15 per group.
3.6. Effects of LH/MS on gene expression in the hippocampus and amygdala
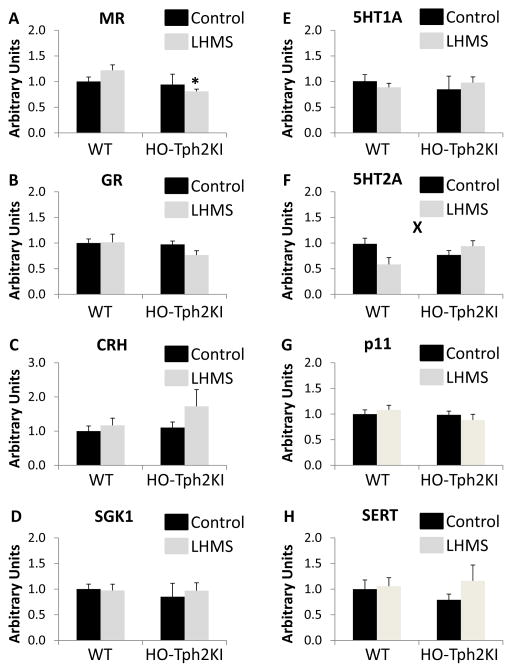
Real-time PCR was used to evaluate the mRNA expression levels of multiple genes involved in stress- and 5-HT-related signaling pathways in the hippocampus and amygdala of WT and HO-Tph2KI mice following LH/MS. No significant group differences in the expression of the mineralocorticoid receptor (MR, Figure 6A) or the glucocorticoid receptor (GR, Figure 6B) were observed in the hippocampus, although a trend towards decreased MR expression in HO-Tph2KI animals was observed [F(1,20) = 3.43, p = 0.0787, Figure 6A]. Similarly, no significant group differences were observed in the mRNA levels of corticotropin-releasing hormone in the hippocampus (Figure 6C). A trend towards decreased mRNA levels of 5-HT1A was observed in HO-Tph2KI mice [F(1,20) = 3.47, p = 0.0771, Figure 6D], but the results did not achieve statistical significance. However, significant reductions in the hippocampal mRNA levels of the 5-HT2A receptor [F(1, 20) = 6.388, p = 0.02, Figure 6E] and p11 (i.e., S100A10) [F(1,20) = 9.465, p = 0.006, Figure 6F], a known regulator of 5-HT receptor function (Svenningsson et al., 2006), were observed in HO-Tph2KI animals compared to WT controls. In contrast, no significant differences in the mRNA expression of the serotonin transporter (SERT) were observed (Figure 6G).
Figure 6.
The effects of LH/MS on gene expression in the hippocampus and amygdala. Quantification of real-time PCR data for MR (A), GR (B), CRH (C), 5-HT1a (D), 5-HT2a (E), p11 (F), and SERT (G) in the hippocampus. Quantification of real-time PCR data for MR (H), GR (I), CRH (J), 5-HT1a (K), 5-HT2a (L), p11 (M), and SERT (N) in the amygdala. N = 6–9 per group. * indicates p < 0.05 by Tukey’s post-hoc test compared to WT LH/MS. ** denotes significant main effect of genotype and ‘X’ denotes significant genotype by LH/MS interaction by two-way ANOVA (p < 0.05).
In the amygdala, which is also thought to play a key role in anxiety-related behaviors (Lalumiere, 2014), our results revealed that HO-Tph2KI mice exhibit an overall reduction in the levels of the MR [main effect of genotype, F(1,26) = 5.41, p = 0.028, Figure 6H]. Although no genotype by stress interaction was observed, post-hoc analyses suggested that this difference was more pronounced in LH/MS-exposed animals (p = 0.038 by Tukey’s) than in controls (p = 0.97). No significant group differences were observed in the expression of GR, but LH/MS-exposed HO-Tph2KI animals expressed approximately 25% less GR in the amygdala than LH/MS-exposed WT animals (Figure 6I). HO-Tph2KI mice had approximately 50% more corticotropin-releasing hormone (CRH) mRNA in the amygdala than WT mice following LH/MS, but this effect did not reach statistical significance (Figure 6J). Similarly, no significant differences in 5-HT1a receptor mRNA expression were observed (Figure 6K). However, a significant genotype by stress interaction was observed for 5-HT2a receptor levels in the amygdala [F(1, 26) = 7.26, p = 0.012, Figure 6L]. LH/MS decreased mRNA expression of 5-HT2a by ~40% in WT animals, but it led to a ~20% increase in HO-Tph2KI animals. No significant differences in the expression of p11 (Figure 6M) or the serotonin transporter (SERT, Figure 6N) were observed in the amygdala. Similarly, no significant genotype or LH/MS-related alterations in serum and glucocorticoid-regulated kinase 1 (SGK1) mRNA levels in the hippocampus or amygdala were observed (not shown).
4. Discussion
The current study demonstrates that the LH/MS paradigm is sufficient to induce significant long-term cellular, molecular, and behavioral changes in mice, and that the precise nature of these stress responses is influenced both by 5-HT deficiency and by sex. However, the magnitude of several of the observed effects was small, suggesting that further refinement of ELS paradigms may be required to successfully model severe ELS.
Our data demonstrate that the LH/MS paradigm leads to increased anxiety-like behavior in both WT and HO-Tph2KI mice. This result is distinct from the findings of our previous study examining standard MS alone (Sachs et al., 2013b), in which HO-Tph2KI mice were shown to exhibit a decrease in anxiety-like behavior following MS, while WT mice exhibited mild anxiogenesis (Sachs et al., 2013b). It should be noted that in our previous study (Sachs et al., 2013b), pups remained in contact with their littermates during the separation period, whereas the pups in the current study were housed individually during the 3 hour MS period. In addition, the pups in the current study were handled much more extensively for USV recordings and early behavioral testing than were pups from our previous study. Prior reports have shown that the conditions encountered by pups during MS (i.e., remaining together in the home cage vs. being individually housed) can influence long-term the behavioral responses to MS (Daskalakis et al., 2014), a fact that likely contributes to some of the variability in phenotypes reported using MS paradigms.
Our results here may be informative for the three-hit hypothesis of vulnerability and resilience, which posits that exposure to low levels of stress early in life can promote either adaptive responses or maladaptive responses to subsequent stressors depending on genomic or other biological factors (Daskalakis et al., 2013). Our data reveal that male WT animals displayed an adaptive, anxiolytic-like response in the EPM following LH/MS, but animals harboring either of two putative risk factors of psychiatric disease (5-HT deficiency and/or female sex) displayed increased anxiety-like behavior following the same LH/MS exposure. Importantly, this was not observed for all behavioral tests we examined (i.e., WT and HO-Tph2KI exhibited similar anxiogenesis following LH/MS in the light-dark emergence test), a fact that could reflect differences in the level of stress induced by a given behavioral paradigm.
Our data demonstrate that LH/MS led to a significant reduction in USVs in WT mice, whereas USVs in HO-Tph2KI pups were not significantly affected by LH/MS. Eliciting USVs has been suggested to serve as a coping mechanism by which animals modulate their levels of stress (Levine and Wiener, 1988), and thus the fact that LH/MS did not significantly modify USV responses in 5-HT-deficient animals might reflect their failure to engage in appropriate coping behavior. Interestingly, acoustic startle responses in adult animals exhibited a similar pattern as USV responses in pups. Specifically, startle magnitude was only blunted by LH/MS in WT mice, not in HO-Tph2KI animals. These findings may reflect a predictive adaptive response of WT animals. This adaptive response to LH/MS may be particularly behaviorally relevant in males, which exhibit anxiolytic-like behavior in the EZM following LH/MS, whereas females and 5-HT-deficient animals display increased anxiety following LH/MS.
The overall decrease in anxiety-like behavior observed in HO-Tph2KI mice in the light-dark emergence test is consistent with several prior studies that have associated 5-HT deficiency with anxiolysis (Fernandez and Gaspar, 2011). However, this finding is inconsistent with the initial report of increased anxiety-like behavior in HO-Tph2KI animals (Beaulieu et al., 2008). Although the reason for this discrepancy is unknown, it is possible that differences in experimental conditions or rearing procedures contributed to this effect.
The importance of adult hippocampal neurogenesis in the pathophysiology of affective disorders and preclinical models of depression and anxiety-like behavior remains controversial (Eisch et al., 2008). Our current results demonstrate that LH/MS significantly reduces the number of DCX+ immature neurons in the hippocampus without affecting hippocampal cell proliferation, which may indicate that LH/MS impacts adult neurogenesis by affecting neuronal survival. This finding is consistent with a prior report that stress impairs the survival, but not the proliferation, of newborn neurons (Thomas et al., 2007). However, additional research is required to fully elucidate the effects of 5-HT deficiency and LH/MS on adult hippocampal neurogenesis.
Prior work has shown that patients with major depression exhibit reduced MR mRNA expression in the hippocampus when compared to non-depressed controls (Klok et al., 2011). Clinical studies have also revealed that the MR plays an important role in moderating the effects of ELS on amygdala reactivity (Bogdan et al., 2012) and that the MR function is required for stress-induced changes in the activity of dorsal striatal and hippocampal memory systems (Schwabe et al., 2013), findings that suggest that MR dysfunction could have important implications for psychiatric disease. Preclinical work also supports a link between MR function and depression- and anxiety-like behavior. One published study demonstrated that overexpression of MR in the forebrain of mice leads to decreased anxiety-like behavior (Rozeboom et al., 2007), and MR antagonism has been shown to reduce corticosterone-induced depression like behavior in mice (Wu et al., 2013), although several other studies have reported minimal effects of manipulating MR expression on anxiety-like behavior (Berger et al., 2006, Harris et al., 2013). Our current results revealed a slight trend towards reduced MR mRNA in HO-Tph2KI mice in the hippocampus and a significant reduction in MR mRNA in the amygdala of HO-Tph2KI mice compared to WT animals. Our finding of reduced MR expression in the amygdala of LH/MS-exposed HO-Tph2KI mice compared to LH/MS-exposed WT animals provides further evidence for the inverse relationship between MR function/expression and anxiety-like behavior. The current study was not sufficiently powered to identify any sex differences in MR expression, but it will be important to examine sex differences in MR expression as a potential mechanism contributing to the behavioral sex differences observed in the current study (i.e., in the EZM).
Prior studies have also highlighted a potential role of the 5-HT2a receptor in the development of psychiatric disease. For example, low levels of 5-HT2a have been associated with post-traumatic stress disorder (Mellman et al., 2009), whereas increased 5-HT2a binding has been reported in the post-mortem brains of patients with borderline personality disorder (Soloff et al., 2007) and increased 5-HT2a mRNA has been reported in the amygdala of suicide victims (Anisman et al., 2008). Our data indicate that levels of 5-HT could be a major factor in determining whether stress increases or decreases the expression of 5-HT2a in the amygdala, an effect that could have an important impact on the ultimate behavioral consequences of stress. In addition, our results demonstrate that Tph2KI animals exhibit reduced 5-HT2a and p11 mRNA expression in the hippocampus, but the expression of these genes was not significantly influenced by LH/MS. Interestingly, prior research has reported that suicide victims exhibit reduced levels of p11 mRNA (but not 5-HT2a) expression in the hippocampus (Anisman et al., 2008).
The primary purpose of the current study was to determine whether brain 5-HT deficiency exacerbates the long-term effects of early life stress in mice. Consequently, several other potentially interesting questions were not examined and therefore, our study had several limitations. For example, based on our data, it is not possible to determine the relative contribution of the LH vs. the MS component of the stress paradigm to any of the behavioral phenotypes observed in the offspring. In addition, as we did not measure grooming or licking behavior, but only pup retrieval, the extent to which this LH/MS paradigm led to impairments in maternal behaviors is not completely resolved. In addition, the LH/MS model requires extensive handling of pups, especially if USVs are to be measured. The extent to which this extensive pup handling contributed to any of the phenotypes observed remains unclear. It is likely that further refinements to this paradigm, including lengthening and/or randomizing the duration of MS and incorporating additional relevant stressors could further improve our ability to model ELS.
In addition, although we report sex differences in a subset of the behavioral responses to LH/MS, we did not examine the effects of estrous cycle, a fact that represents another limitation of the current work. Indeed, estrous cycle is known to have significant effects on anxiety-like behavior in mice (Maguire et al., 2005, Walf et al., 2009, Bath et al., 2012). It is possible that some additional phenotypes would have been revealed had we controlled for estrous cycle, but future research will be required to evaluate this possibility. Regarding our analysis of hippocampal neurogenesis, we only investigated DCX expression and cell proliferation; additional studies of cell survival and/or differentiation could provide further insight into the effects of LH/MS on neurogenesis. Finally, we only evaluated gene expression changes in two brain regions and only examined a handful of potentially interesting targets. Future research investigating other stress-related pathways in the amygdala, hippocampus, and other brain regions could also reveal useful information regarding the mechanisms whereby LH/MS, 5-HT deficiency, and sex influence behavior.
5. Conclusion
Taken together, our data indicate that the LH/MS procedure is sufficient to impair pup-retrieval behavior and induce depressive-like behaviors in dams, and it leads to long-term cellular, molecular, and behavioral alterations in offspring. Furthermore, we have shown that 5-HT deficiency impacts anxiety-like behaviors and arousal responses throughout the lifespan of mice exposed to LH/MS. Although our present data do not conclusively elucidate the cellular and molecular mechanisms underlying the differential stress susceptibility observed here, it appears that specific molecular adaptations in both stress- (i.e., MR) and 5-HT- (i.e., 5-HT2a) related signaling pathways within the amygdala could play an important role. In contrast, our data do not provide support for a key role for differences in hippocampal neurogenesis in mediating the effects of 5-HT deficiency on stress susceptibility. Overall, our findings highlight the importance of reducing the incidence and impact of stress during the postpartum period and during early postnatal life, and they suggest that 5-HT deficiency may influence the development of psychiatric disease by regulating responses to ELS.
Highlights.
We report the development of a new animal model of early life and post-partum stress.
This new early life stress model leads to increased anxiety-like behavior and impaired hippocampal neurogenesis in offspring.
Brain serotonin deficiency significantly impacts the behavioral responses of mice to early life stress.
Acknowledgments
This work was supported in part by grants from the National Institute of Mental Health (MH79201 and MH60451) to MGC. BDS has been the recipient of a Minority Supplement award (MH79201-03S1) and an NRSA postdoctoral fellowship (F32- MH093092) from the National Institute of Mental Health.
Role of the Funding Sources
This work was supported in part by grants from the National Institutes of Health (MH79201 and MH60451) to MGC. These funding sources played no other roles in design of the study; the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Conflict of Interest
MGC has received compensation from Lundbeck as a member of their Psychopharmacology Advisory Board and is a consultant for Omeros Corp. MGC also owns stock in Acadia Pharmaceutical. MGC has also received compensation in the form of honoraria for lecturing at various academic institutions. None of the above presents any conflicts of interest with the results described in the present paper. All other authors have no disclosures or conflicts of interest.
Contributors
BDS and RMR designed and performed experiments, analyzed data and drafted the manuscript. HLT and AI performed experiments.
WCW and MGC designed experiments, analyzed data and drafted the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anisman H, Du L, Palkovits M, Faludi G, Kovacs GG, Szontagh-Kishazi P, Merali Z, Poulter MO. Serotonin receptor subtype and p11 mRNA expression in stress-relevant brain regions of suicide and control subjects. Journal of Psychiatry and Neuroscience. 2008;33:131–141. [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, Reyes TM, Simerly RB, Susser ES, Nestler EJ. Early life programming and neurodevelopmental disorders. Biological psychiatry. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Chuang J, Spencer-Segal JL, Amso D, Altemus M, McEwen BS, Lee FS. Variant brain-derived neurotrophic factor (Valine66Methionine) polymorphism contributes to developmental and estrous stage-specific expression of anxiety-like behavior in female mice. Biological psychiatry. 2012;72:499–504. doi: 10.1016/j.biopsych.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, Gainetdinov RR, Caron MG. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CT. The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs. 1998;12:12–20. doi: 10.1016/s0883-9417(98)80004-6. [DOI] [PubMed] [Google Scholar]
- Bell CJ, Nutt DJ. Serotonin and panic. The British journal of psychiatry : the journal of mental science. 1998;172:465–471. doi: 10.1192/bjp.172.6.465. [DOI] [PubMed] [Google Scholar]
- Berger S, Wolfer DP, Selbach O, Alter H, Erdmann G, Reichardt HM, Chepkova AN, Welzl H, Haas HL, Lipp HP, Schutz G. Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:195–200. doi: 10.1073/pnas.0503878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Williamson DE, Hariri AR. Mineralocorticoid receptor Iso/Val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. The American journal of psychiatry. 2012;169:515–522. doi: 10.1176/appi.ajp.2011.11060855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister M, McInnis MG, Zollner S. Psychiatric genetics: progress amid controversy. Nat Rev Genet. 2008;9:527–540. doi: 10.1038/nrg2381. [DOI] [PubMed] [Google Scholar]
- Carini LM, Murgatroyd CA, Nephew BC. Using chronic social stress to model postpartum depression in lactating rodents. J Vis Exp. 2013:e50324. doi: 10.3791/50324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyle BC, Duque A, Kitchen RR, Bordner KA, Coman D, Doolittle E, Papademetris X, Hyder F, Taylor JR, Simen AA. Maternal separation with early weaning: a rodent model providing novel insights into neglect associated developmental deficits. Development and psychopathology. 2012;24:1401–1416. doi: 10.1017/S095457941200079X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AS, Garrity-Rokous FE, Chazan-Cohen R, Little C, Briggs-Gowan MJ. Maternal depression and comorbidity: predicting early parenting, attachment security, and toddler social-emotional problems and competencies. J Am Acad Child Adolesc Psychiatry. 2001;40:18–26. doi: 10.1097/00004583-200101000-00012. [DOI] [PubMed] [Google Scholar]
- Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, de Kloet ER. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology. 2013;38:1858–1873. doi: 10.1016/j.psyneuen.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis NP, Diamantopoulou A, Claessens SE, Remmers E, Tjalve M, Oitzl MS, Champagne DL, de Kloet ER. Early experience of a novel-environment in isolation primes a fearful phenotype characterized by persistent amygdala activation. Psychoneuroendocrinology. 2014;39:39–57. doi: 10.1016/j.psyneuen.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Cameron HA, Encinas JM, Meltzer LA, Ming GL, Overstreet-Wadiche LS. Adult neurogenesis, mental health, and mental illness: hope or hype? The Journal of Neuroscience. 2008;28:11785–11791. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SP, Gaspar P. Investigating anxiety and depressive-like phenotypes in genetic mouse models of serotonin depletion. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.08.049. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Linder N, Russig H, Thony B, Mansuy IM. Influence of early stress on social abilities and serotonergic functions across generations in mice. PloS one. 2011;6:e21842. doi: 10.1371/journal.pone.0021842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui M, Rodriguiz RM, Zhou J, Jiang SX, Phillips LE, Caron MG, Wetsel WC. Vmat2 heterozygous mutant mice display a depressive-like phenotype. The Journal of Neuroscience. 2007;27:10520–10529. doi: 10.1523/JNEUROSCI.4388-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner CR. Distress vocalization in rat pups. A simple screening method for anxiolytic drugs. J Pharmacol Methods. 1985;14:181–187. doi: 10.1016/0160-5402(85)90031-2. [DOI] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106:1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. The Journal of Neuroscience. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Harris AP, Holmes MC, de Kloet ER, Chapman KE, Seckl JR. Mineralocorticoid and glucocorticoid receptor balance in control of HPA axis and behaviour. Psychoneuroendocrinology. 2013;38:648–658. doi: 10.1016/j.psyneuen.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Homberg JR. The stress-coping (mis)match hypothesis for nature x nurture interactions. Brain research. 2012;1432:114–121. doi: 10.1016/j.brainres.2011.11.037. [DOI] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology. 2001;158:366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Jacobsen JP, Medvedev IO, Caron MG. The 5-HT deficiency theory of depression: perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2Arg439His knockin mouse. Philos Trans R Soc Lond B Biol Sci. 2012a;367:2444–2459. doi: 10.1098/rstb.2012.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JP, Siesser WB, Sachs BD, Peterson S, Cools MJ, Setola V, Folgering JH, Flik G, Caron MG. Deficient serotonin neurotransmission and depression-like serotonin biomarker alterations in tryptophan hydroxylase 2 (Tph2) loss-of-function mice. Molecular psychiatry. 2012b;17:694–704. doi: 10.1038/mp.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Lee HJ, Yang JC, Hwang JA, Yoon HK. A tryptophan hydroxylase 2 gene polymorphism is associated with panic disorder. Behav Genet. 2009;39:170–175. doi: 10.1007/s10519-008-9254-8. [DOI] [PubMed] [Google Scholar]
- Klok MD, Alt SR, Irurzun Lafitte AJ, Turner JD, Lakke EA, Huitinga I, Muller CP, Zitman FG, de Kloet ER, Derijk RH. Decreased expression of mineralocorticoid receptor mRNA and its splice variants in postmortem brain regions of patients with major depressive disorder. Journal of psychiatric research. 2011;45:871–878. doi: 10.1016/j.jpsychires.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Lalumiere RT. Optogenetic dissection of amygdala functioning. Front Behav Neurosci. 2014;8:107. doi: 10.3389/fnbeh.2014.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S, Wiener SG. Psychoendocrine aspects of mother-infant relationships in nonhuman primates. Psychoneuroendocrinology. 1988;13:143–154. doi: 10.1016/0306-4530(88)90011-x. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nature neuroscience. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Alim T, Brown DD, Gorodetsky E, Buzas B, Lawson WB, Goldman D, Charney DS. Serotonin polymorphisms and posttraumatic stress disorder in a trauma exposed African American population. Depress Anxiety. 2009;26:993–997. doi: 10.1002/da.20627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neuroscience and Biobehavioral Reviews. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OF, Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nature neuroscience. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Okon EE. The effect of environmental temperature on the production of ultrasounds by isolated non-handled albino mouse pups. Journal of Zoology, London. 1970;162:71–83. [Google Scholar]
- Parfitt DB, Walton JR, Corriveau EA, Helmreich DL. Early life stress effects on adult stress-induced corticosterone secretion and anxiety-like behavior in the C57BL/6 mouse are not as robust as initially thought. Hormones and Behavior. 2007;52:417–426. doi: 10.1016/j.yhbeh.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG. Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Hormones and Behavior. 2003;43:561–567. doi: 10.1016/s0018-506x(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Rozeboom AM, Akil H, Seasholtz AF. Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4688–4693. doi: 10.1073/pnas.0606067104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs BD, Jacobsen JP, Thomas TL, Siesser WB, Roberts WL, Caron MG. The effects of congenital brain serotonin deficiency on responses to chronic fluoxetine. Translational psychiatry. 2013a;3:e291. doi: 10.1038/tp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs BD, Ni JR, Caron MG. Sex differences in response to chronic mild stress and congenital serotonin deficiency. Psychoneuroendocrinology. 2014;40:123–129. doi: 10.1016/j.psyneuen.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs BD, Rodriguiz RM, Siesser WB, Kenan A, Royer EL, Jacobsen JP, Wetsel WC, Caron MG. The effects of brain serotonin deficiency on behavioural disinhibition and anxiety-like behaviour following mild early life stress. The international journal of neuropsychopharmacology. 2013b:1–14. doi: 10.1017/S1461145713000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MV. Animal models for depression and the mismatch hypothesis of disease. Psychoneuroendocrinology. 2011;36:330–338. doi: 10.1016/j.psyneuen.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Tegenthoff M, Hoffken O, Wolf OT. Mineralocorticoid receptor blockade prevents stress-induced modulation of multiple memory systems in the human brain. Biological psychiatry. 2013;74:801–808. doi: 10.1016/j.biopsych.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011 doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, Price JC, Meltzer CC, Fabio A, Frank GK, Kaye WH. 5HT2A receptor binding is increased in borderline personality disorder. Biological psychiatry. 2007;62:580–587. doi: 10.1016/j.biopsych.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, Vaugeois JM, Nomikos GG, Greengard P. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Yap JJ, Bohager DZ, Faccidomo S, Clayton T, Cook JM, Miczek KA. Glutamatergic and GABAergic modulations of ultrasonic vocalizations during maternal separation distress in mouse pups. Psychopharmacology. 2009;204:61–71. doi: 10.1007/s00213-008-1437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RM, Hotsenpiller G, Peterson DA. Acute psychosocial stress reduces cell survival in adult hippocampal neurogenesis without altering proliferation. Journal of Neuroscience. 2007;27:2734–2743. doi: 10.1523/JNEUROSCI.3849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce C, Manley K, Frye CA. Proestrous compared to diestrous wildtype, but not estrogen receptor beta knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behavioural brain research. 2009;196:254–260. doi: 10.1016/j.bbr.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TC, Chen HT, Chang HY, Yang CY, Hsiao MC, Cheng ML, Chen JC. Mineralocorticoid receptor antagonist spironolactone prevents chronic corticosterone induced depression-like behavior. Psychoneuroendocrinology. 2013;38:871–883. doi: 10.1016/j.psyneuen.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gainetdinov RR, Beaulieu JM, Sotnikova TD, Burch LH, Williams RB, Schwartz DA, Krishnan KR, Caron MG. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Zill P, Baghai TC, Zwanzger P, Schule C, Eser D, Rupprecht R, Moller HJ, Bondy B, Ackenheil M. SNP and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene provide evidence for association with major depression. Mol Psychiatry. 2004;9:1030–1036. doi: 10.1038/sj.mp.4001525. [DOI] [PubMed] [Google Scholar]