Abstract
In human societies, cultural norms arise when behaviours are transmitted with high-fidelity social learning through social networks1. However a paucity of experimental studies has meant that there is no comparable understanding of the process by which socially transmitted behaviours may spread and persist in animal populations2,3. Here, we introduce alternative novel foraging techniques into replicated wild sub-populations of great tits (Parus major), and employ automated tracking to map the diffusion, establishment and long-term persistence of seeded behaviours. We further use social network analysis to examine social factors influencing diffusion dynamics. From just two trained birds in each sub-population, information spread rapidly through social network ties to reach an average of 75% of individuals, with 508 knowledgeable individuals performing 58,975 solutions. Sub-populations were heavily biased towards the technique originally introduced, resulting in established local arbitrary traditions that were stable over two generations, despite high population turnover. Finally, we demonstrate a strong effect of social conformity, with individuals disproportionately adopting the most frequent local variant when first learning, but then also continuing to favour social over personal information by matching their technique to the majority variant. Cultural conformity is thought to be a key factor in the evolution of complex culture in humans4-7. In providing the first experimental demonstration of conformity in a wild non-primate, and of cultural norms in foraging techniques in any wild animal, our results suggest a much wider evolutionary occurrence of such apparently complex cultural behaviour.
Social learning, where animals learn from others, can enable novel behaviours to spread between individuals to create group-level behaviours, termed cultural traditions6,8,9. Social transmission occurs between interacting individuals; hence group dynamics and population structure will determine the spread and persistence of traditions2,3,9-11. Additionally, individuals may use social learning strategically to maximize its adaptive value, with consequences for when, how, and what traditions establish4,12. However while the capacity for social learning has been described in many phylogenetically diverse taxa13 and detailed in comprehensive laboratory studies13-15, we have little knowledge of the social dynamics associated with such learning in natural systems. Experimentally quantifying cultural transmission in wild populations remains difficult, with limitations associated with isolating and training individuals5, tracking the spread of information across large numbers of animals14, and eliminating alternative explanations such as individual trial and error learning8,14.
Early observational studies of tits provide one of the most widely cited examples of animal innovation and culture, when British birds famously began to pierce the foil caps of milk bottles to steal cream16-18. More generally, great tits (Parus major) are known for being highly innovative, opportunistic foragers19, and for using social information in a wide range of contexts20. This, coupled with their fission-fusion social structure21, makes them excellent models for a large-scale empirical investigation of the social processes associated with cultural transmission. Here, we used a novel system incorporating automated data collection and passive integrated transponder (PIT) tags, together with recently developed methods in social network analysis, to investigate the spread, establishment and persistence of experimentally seeded traditions in wild great tits.
We first developed an automated puzzle-box baited with live mealworms (Fig. 1a) and performed a cultural diffusion experiment based on the two-action and control design14, but where treatment groups were exposed to a demonstrator trained on one of two distinct but equivalent actions. Two resident males were caught from each of eight sub-populations were exposed to one of three training regimes in captivity. In the first condition (‘control’, three replicates), neither individual was given any training. In the second condition (‘option A’, two replicates), both individuals were trained to access food from the puzzle-box by using their bill to push the blue side of the sliding door to the right. Finally, in the third condition (‘option B’, three replicates), the birds were trained to solve the puzzle-box by pushing the red side of the sliding door to the left (Supplementary Video 1). After 4 days of training, all birds were released back into the wild and 3 puzzle-boxes, with both options available, were installed 250m apart in each sub-population (Extended Data Fig. 1). We then automatically monitored individual visits to, and solutions of, these puzzle-boxes (‘solves’), over short term (20 days exposure over 4 weeks) and long term (5 days of exposure, 9 months later) time scales.
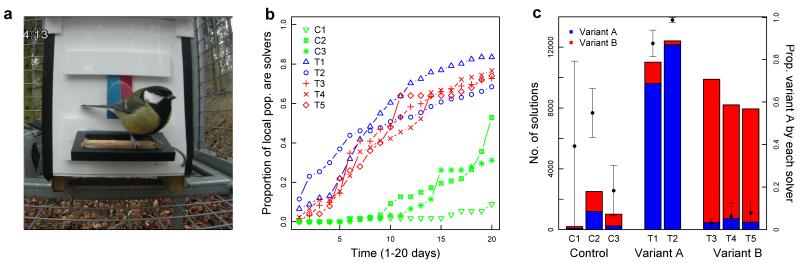
Fig 1. Cultural diffusion experiment.
a, Puzzle-box where birds can slide the door either way (from left, option A; or right, option B) to access a reward. Puzzle-box records identity, visit duration and solution choice, and resets after each visit. b, Diffusion curves for treatment sub-populations with demonstrators (T1-5; n=91, 130, 132, 90, 50) and control sub-populations without demonstrators (C1-3; n=56, 87, 61). c, Total number of solutions of each option in each replicate; x-axis indicates demonstrated option. Points show mean proportion of option A performed by individuals with 95% CI; y-axis on right. No. solvers=5, 46, 19 (control); 76, 89 (A); 96, 69, 37 (B).
In the five sub-populations seeded with trained demonstrators, knowledge of the novel puzzle spread rapidly over 20 days of exposure (Fig. 1b). An average of 75% (68%-83%; n=37-96) of each local population solved at least once (local population size assessed by independent visitation data at feeders, see SI). The diffusion of this behaviour was clearly sigmoidal (sigmoid vs. linear fit: ΔAIC ranging from 15.31-54.17), except in one replicate (T5; ΔAIC = 0.13). By contrast, many fewer individuals solved in control sub-populations (n=5-54, 9%-53%; Fig. 1b), where uptake initially relied on individual innovation. Latency to first solve, excluding the demonstrator, was significantly longer in control areas than in treatment areas (Welch two sample t-test: t(6) = −16.1, P < 0.01; Fig. 1b), and the total number of solutions was significantly lower (t(6) = 4.6, P = 0.02; Fig. 1c). There was a striking difference between replicates seeded with alternative solving techniques. Learning was heavily biased towards the technique originally demonstrated in all treatment sub-populations (t(8) = 9.7, P < 0.01, Fig. 1c), while no consistent side bias was observed between control sub-populations (t(4) = −0.03, P = 0.97, Fig. 1c).
We collected social networks for each sub-population independently of the social learning experiment, with 10 days’ sampling at a grid of sunflower-seed feeders equipped to log visitation data (Extended Data Fig. 2a-b). Co-occurrences were detected using a Gaussian mixture model to isolate clusters of visits in the spatio-temporal data streams22, with repeated foraging associations forming social networks (Extended Data Fig. 2b-c). Social networks for all replicates were significantly non-random, even at the most local scale (T1-5: p<0.001), and network-based diffusion analysis (NBDA) was used to quantify the extent to which these social ties predicted the acquisition of behaviour23. Pooling replicates, a network diffusion model including social transmission was overwhelmingly supported over asocial learning: ΔAIC = 1520.7; individual learning rate was estimated to increase by a factor of 12.0 per unit of association with knowledgeable individuals (Extended Data Fig. 3). An effect of age and sex was also supported, with juveniles and males having a faster learning rate (table 1). These results support a dominant effect of social learning on the emergence of this novel behaviour, and show additionally that the diffusion of innovation was influenced by fine-scale patterns of social interactions (Supplementary Video 3).
Table 1. Network-based diffusion analysis.
Summed Akaike weights ωi and delta Akaike values for network-based diffusion models, with maximum-likelihood parameter estimates of social transmission for five treatment replicates. Estimates and effect sizes are presented for individual-level variables (b). Diffusion analyses use a continuous time of acquisition model with a constant baseline learning rate (λ0), allowing for differing social transmission rates in each replicate.
| Transmission Model | ΔAIC (top model) | Σ ω i | S.T. Parameter Est. | 95%CI | |
|---|---|---|---|---|---|
| Social – multiplicative | 0 | 0.99 | 12.0 | 8.8-16.0 | |
| T1 | 22.4 | 11.8-30.2 | |||
| T2 | 12.2 | 8.2-17.1 | |||
| T3 | 7.3 | 2.9-14.3 | |||
| T4 | 29.8 | 10.9-42.6 | |||
| T5 | 13.4 | 8.3-20.02 | |||
| Social – additive | 33.7 | 0.01 | - | - | |
| Asocial | 1520.7 | 0 | (constrained to 0) | ||
| (b) Individual-level variable | Estimate | Effect Size | |||
| Age (Juv/Ad) | 0 | 0.99 | −0.18 | 0.70 | |
| Sex (F/M) | 0 | 0.97 | 0.10 | 1.22 | |
| Natal Origin (Res/Imm) | 3.9 | 0.13 | 0.07 | 1.16 | |
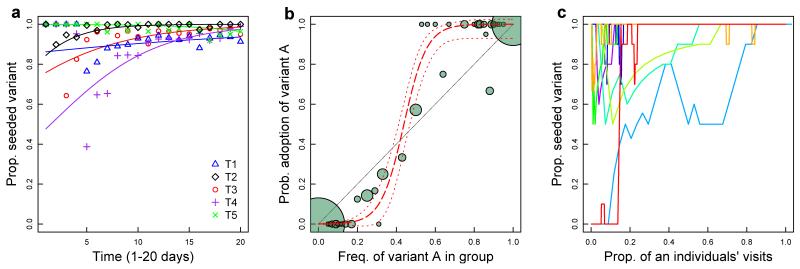
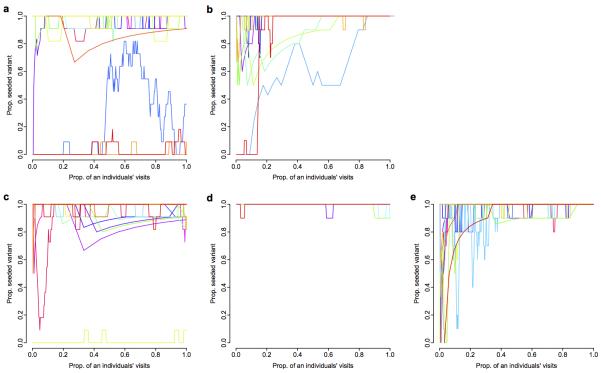
In all experimental replicates, the equally difficult and equally rewarded alternative solution was performed by at least one individual within the first six days of exposure (median day 4). However, in contrast with most previous studies where discovery of an alternative solution led to progressive erosion in use of the seeded variant2,5,24, we observed a pronounced strengthening of traditions over the rest of the experiment. To analyse this change in behaviour over time, we used a generalised estimating equation model (GEE)2 where the dependent variable was the proportion of solutions as seeded technique on each day of data collection, and explanatory variables were individual and replicate. Combining replicates, there was strong evidence that the preference for the arbitrary tradition increased over time (coefficient ± SE = 0.13±0.02, P < 0.001), with an estimated 14% increase in bias per day (95%CI = 8%-18%, Fig. 2a). This is consistent with a conformist transmission bias, where individuals preferentially adopt the more commonly practiced variant when solving the puzzle-box5,7,25,26. More conclusive evidence for such positive frequency-dependent copying25 was observed when only the first solutions for each individual was considered, with birds disproportionately likely to initially adopt the majority variant of their group (sigmoid vs. linear fit: ΔAIC 38.34; Fig. 2b).
Fig. 2. Evidence for social conformity.
a, Proportion of solutions as seeded technique in each replicate significantly increases over time. Points are proportion as seeded technique on each day; lines are GEE model fit. b, Comparison of frequency of option A in previous group with an individual’s first learnt option. Node size represents number of individuals (n=1-147). Black line shows expectation under unbiased copying, red lines show model fit with 95% CI. c, Solution trajectories from individuals that used both possible options in T2 replicate (n=10). Lines are running proportions of seeded technique for each individual over last 10 visits.
Individuals thus preferentially learnt the most common option when first learning (conformist transmission; Fig. 2b). Yet, remarkably, they also continued to prioritise social over personal information, matching their behaviour to the common variant even after experiencing an equally rewarding alternative. We analysed trajectories for those individuals (n=78) that used both options. The majority of these individuals (85%) retained a preference for the seeded variant (n=66, e.g. see Fig. 2c, Extended Data Fig. 4). Three birds had a strong preference for the uncommon variant and 8 birds switched from the alternative variant to the common variant, but no birds made the reciprocal switch; only 1 individual had no significant preference. A subset of birds that dispersed between experimental replicates (n=40, 24 between years) provided additional evidence. Of 27 birds that moved between replicates with the same seeded tradition, 26 (96%) retained their preference for the common variant. In contrast, of 14 individuals that moved between replicates with different seeded traditions, 10 (71%) changed their behaviour to match the common variant in the new location, while only 3 retained their initial preference (χ2(1) = 21.6, P < 0.001).
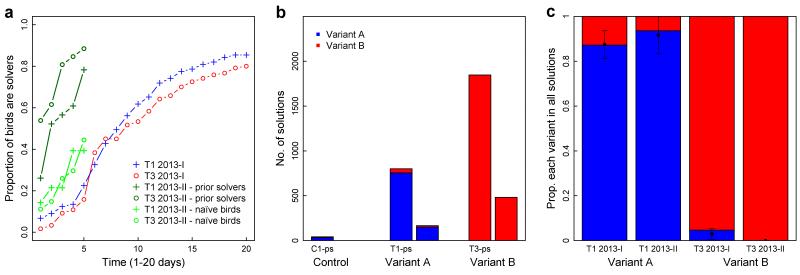
Seeded arbitrary traditions thus formed and persisted in each sub-population (Fig. 2). To investigate the long-term stability of these traditions, we re-installed the puzzle boxes in one replicate of each condition over five days in the following winter for T1, T3 and C1. Substantial turnover in the population had occurred owing to high mortality rates typical of this species27; on average just 40% of each sub-population were individuals that had been present the previous year. No additional demonstrators were trained and no individual had contact with the device in the intervening months. In the control sub-population, all solves (n=42) were performed by just three individuals, all of which had also solved the previous year. However in the two experimental sub-populations, knowledge of the puzzle-box emerged even faster than it had the preceding year, both among prior solvers and birds inexperienced in the task; in T1, 29 individuals solved 967 times, in T3, 35 individuals solved 2329 times (Fig. 3a, Fig. S4). Results suggested a strong initial effect of memory followed by a very rapid oblique transmission facilitated by the greater number of demonstrators: on the first day of exposure, 60% (T1) and 82% (T3) of ‘solvers’ were birds that solved in the initial experiment, outweighing their representation in the general population (36% in T1, 46% in T3). Sub-populations also retained their original technique, with solutions heavily biased towards the option seeded in the original experiment (Fig. 3b). Intriguingly, amongst birds that had occurred in both years, the within-individual bias towards the seeded variant had increased (LMM: t(83)= 2.80, P < 0.01; Fig. 3c), resulting in arbitrary traditions that were retained and strengthened.
Fig. 3. Local traditions persist between years.
a, Diffusion curves for initial (T1 2013-I/T3 2013-I; 1-20dy) and second exposure (T1 2013-II/T3 2013-II; 1-5dy). Uptake rate in second exposure is much higher for prior solvers (T1 2013-II/T3 2013-II; pop. sizes=23, 26), but also higher for naïve birds (T1 2013-II/T3 2013-II; pop. sizes=28, 27). b, Number of solutions as option A/B. In T1 one circuit board failed, so data are from 2/3 devices. Bars are split into prior solvers (ps) and naïve birds (nb). c, Proportion option A/B in initial and second exposure; x-axis indicates initially demonstrated option. Points show mean proportion option A performed by individuals with 95% CI.
In summary, we show that wild great tits use social learning to acquire novel behaviours, and that foraging techniques introduced by very few individuals (here just two in each replicate) can spread rapidly to the majority of the population, forming stable arbitrary traditions. Both social networks ties and individual characteristics determined the transmission of these foraging techniques23. Secondly, introduced arbitrary traditions were stable over both short and long-term periods, becoming increasingly entrenched over two generations. This stability appeared to be a result of informational conformity, with individuals matching their behaviour to the most common variant when first learning, and then continuously updating their personal information. Conformity has long been considered a central component of human culture25,26,28, but experimental evidence for its occurrence in wild animals has been limited to a study of food preferences in vervet monkeys5. We provide the first experimental demonstration for conformist transmission and cultural norms in foraging techniques in any wild animal. Our study argues against the previous view that such behaviour is restricted to the primate lineage26,28-30, and call for a re-thinking of the evolution and ecology of cultural conformity.
Materials and Methods
Study Population and Area
The study was conducted in a wintering population of tits in Wytham Woods, U.K. (51°46’N, 01°20’W; Extended Data Fig. 1). 1018 nest-boxes suitable for great tits are installed at this site, with the vast majority of great tits breeding in boxes. Individuals are trapped as nestlings and breeding adults at nest-boxes and fitted with both a British Trust for Ornithology metal leg ring and a plastic leg ring containing a uniquely identifiable passive integrated transponder (PIT) tag (IB Technology, Aylesbury, U.K.). There is a further mist-netting effort over autumn and winter to tag individuals immigrating into the population, and we estimate that over 90% of individuals were PIT-tagged at the time of the study21. In this population, great tits form loose fission-fusion flocks of unrelated individuals in autumn and winter. Flocks congregate at patchy food sources, and can be observed at bird feeders fitted with PIT-tag detecting antennae21,31. Experiments were conducted in eight sub-populations within Wytham Woods that had relatively little short-term between-area movement of individuals (Extended Data Fig. 1).
Puzzle-box Design
The experimental apparatus consisted of an opaque plastic box with a perch positioned in front of a door that could be slid to either side with the bill to gain access to a feeder concealed behind. Video observations suggested that all great tits used their bill to move the door. The left side of the door was colored blue and the right side red, with a raised front section on the door to allow an easier grip. The concealed feeder contained approximately 500 live mealworms and was refilled up to twice daily. Mealworms are a highly preferred food for great tits (Extended Data Fig. 5), and as live mealworms were used, solvers typically extracted one worm and then carried it away from the puzzle-box to kill and eat it (confirmed with video observations); Supplementary Video 1-2. Each puzzle-box was surrounded by a 1×1m cage with a 5×5cm mesh that gave unlimited access to small birds, but prevented access by large non-target species such as corvids or squirrels. A freely accessible bird feeder filled with peanut granules was also provided in the cage, at approximately 1m from the puzzle-box. Peanut granules are a much less preferred food source (Extended Data Fig. 5). Each peanut feeder had two access points fitted with RFID antenna and data-logging hardware. This feeder was used to attract the original demonstrator to the location, and to record the identity of individuals that did not contact the puzzle-box.
All puzzle-boxes contained a printed circuit board (PCB) and motor, and were powered by a 12V sealed battery. The perch also functioned as an RFID antenna that registered the visit duration (time to nearest second) and identity of the visiting individual. A “solve” was recorded if the door was opened during an individual visit to the device, with the side direction also noted. If a solution occurred without an accompanying identified individual, this was recorded as “unidentified solve”. One second after the solving bird departed the door reset back to the middle. If further individuals visited before this happened, then a “scrounge” was recorded, as they were assumed to have taken food from the open door (confirmed from video observations). The door reset immediately after two individuals were registered scrounging, preventing more than two possible scrounging events per solve (Supplementary Video 2).
Experimental Procedure
Two males were captured from each sub-population (11 adults, 5 juveniles) to act as demonstrators, either by removal from roosting boxes on Sunday night, or by mist-netting at a sunflower-seed feeder on Monday morning. They were transferred to individual cages in indoor captive facilities, and over four days each pair of birds was subjected to one of three training regimes using step-wise shaping, either: (i) given no training and left in the cage with ab lib food (control); (ii) trained to solve the novel puzzle-box by pushing the blue side of the door to the right (option B); or (iii) trained to solve the novel puzzle-box by pushing the red side of the door to the left (option A). With the exception of ‘control’ areas, which were clustered in the south of the woodland to avoid cross-contamination, sub-populations were randomly assigned to a training regime, with both demonstrators from a single sub-population trained on the same technique. During training, the demonstrators were initially exposed to an open puzzle-box baited with mealworms, which was then gradually closed over the course of four days until the subjects were reliably re-opening it. The other side of the door was fixed during training. On Friday morning the birds were released back at the site of capture in each respective sub-population; puzzle-boxes at which both options were available and equally rewarding were installed at three sites 250m apart on the following Sunday night (Extended Data Fig. 1). These puzzle-boxes were run over a four-week period at each site, continuously operating from Monday to Friday and then removed on Saturday and Sunday, for a total of 20 days of data collection.
Four replicates were conducted in the first year of data collection (December 2012-February 2013; C1-2, T1, T3). At three of these replicates (C1, T1, T3) puzzle-boxes were simultaneously re-installed at the same locations for 5 days of further data collection in December 2013. No additional demonstrators were trained, and no individual had contact with the puzzle-box in the 9 months between the two data collection periods. This second exposure aimed to test the long-term stability of social learning at the sub-population level. They were run prior to the second year of data-collection for the cultural diffusion experiment in order to exclude the possibility that dispersing individuals from new replicates could be re-introducing the novel behaviour. An additional four replicates were then conducted from December 2013 - February 2014 in new sub-populations, using the same initial protocol (C3, T2, T4, T5).
Data Analysis
The local population size for each replicate was defined as comprising all individuals in a replicate that had been recorded at least once at either: (i) the puzzle-box, (ii) the nearby peanut feeder, or (iii) the nearest network-logger feeders (operated Saturday-Sunday, see below), during the experimental period (i.e. from the weekend following the release of the demonstrators, to the weekend after the 20th day of operation of the puzzle-boxes). When three replicates were compared with the ‘persistence’ trial in the following year, the local population was defined just as (i) all individuals observed at the puzzle-box or (ii) nearby peanut feeder, so that areas were comparable.
To analyse the results of the initial experiment we first compared control replicates and treatment replicates, using Welch two-sided t-tests, and by fitting linear and sigmoidal models to the data, with the best model ascertained by difference in AIC values32. If individuals were using social information when learning about the puzzle-box, then we expected that there would be a difference between areas seeded with a trained demonstrator (treatment) and those without (control). Replicates were thus compared in terms of latency to first solve (seconds from beginning of the experimental period, excluding demonstrator), and the total number of solutions. Secondly, we compared the total number of solutions in the two different experimental treatments. Here if a more complex form of social learning than local enhancement to the feeding site was occurring, then we expected a consistent bias towards the seeded variant in the different treatments14.
To analyse the change in individual and population preferences for option A or B over time, we used a generalised estimating equation model (GEE)2 where the dependent variable was the proportion of solutions using the seeded technique on each day of data collection, and the explanatory variables were the individuals and replicate, weighted by the overall number of solutions per day. The seeded technique (A/B) was initially also included as an explanatory variable, but was not significant (coefficient ± SE = 0.13±0.22, P = 0.55). Three individual variables were included in a GEE model; sex, age and natal origin. Sex was determined at capture using plumage coloration, age was either determined from breeding records or plumage coloration, and individuals were classed as ‘immigrants’ if they had dispersed into the study site, and ‘locally-born’ if they had been ringed as a nestling in the study site27. Only age was significant (coefficient ± SE = −0.92±0.20, P < 0.001), and was included in the final model (sex: coefficient ± SE = 0.38±0.22, P = 0.08; natal origin: coefficient ± SE = −0.38±0.22, P = 0.08).
If population-level conformity was partly the result of a conformist transmission bias at first acquisition we would expect a sigmoidal relationship between population-level frequency of the variant and adoption probability, with adoption of the majority variant disproportionately more likely than its absolute frequency. By contrast, copying the last individual observed, or random copying, should yield a linear relationship25,26, with probability of adopting option A/B roughly equal to its proportion in the overall population. To investigate this, we isolated all individuals’ first observed solutions in all experimental replicates, and compared the option choice to the proportion of all previous solves as option A observed in the individual’s group at that site. Group length was set at 245 sec, which was the average group length observed using Gaussian mixture models on temporal patterns of flocking (see below) at network-logging sunflower feeders. Both linear and sigmoidal models were then fitted to the data, with the best model ascertained by difference in AIC values32.
We further examined the subset of individuals that moved between sub-populations (n=40). This subset included all individuals recorded in more than one experimental replicate, whether within the season (n=16), or between seasons (n=24). No individual was observed in more than two replicates, and this analysis did not include individuals in the ‘persistence trial’. A preference for option A/B at each location was defined as more than 75% of all solves for either option A/B in that replicate. Finally, in order to analyse the change in within-individual bias towards option A/B between the initial experiment and the second-year ‘persistence trials’, we used a general linear model where the dependent variable was the number of solves as the seeded variant over the total number of solves for each individual observed in both years. Explanatory variables were treatment type and year, with individual identity as a random effect.
Network Data Collection and Analysis
Sunflower bird-feeding stations were deployed at 65 locations around Wytham woods on an approximate 250×250m square grid, as part of long-term research into social-network structure in tits (see21,22). Each station had two access points, each fitted with RFID antennae and data logging hardware. Feeding stations automatically opened from dawn to dusk on Saturday and Sunday, scanning for PIT-tags every 16th of a second. This study used the data from the eight nearest locations to each set of puzzle-boxes, for 10 dates within and surrounding the cultural diffusion experiment (the standard logging protocol runs from September-February in Wytham Woods21).
Great tits were detecting visiting feeding stations and individually identified by their PIT-tags. We then applied a Gaussian mixture model to the spatiotemporal data stream to detect distinct clusters of visits. This method locates high-density periods of feeding activity, isolating flocks of feeding birds without imposing artificial assumptions about group boundaries22,33. A gambit of the group approach34 was used with a simple-ratio index to calculate social associations, where individual association strengths (network edges) were scaled between 0 (never observed foraging together in the same group) to 1 (always observed in the same group, never observed apart). While a single co-occurrence may not be meaningful, our automated data collection method resulted in thousands of repeated group sampling events, allowing social ties between individuals to be built up from multiple observations of co-occurrences over time and across spatial locations. Networks contained 123 (T1), 137 (T2), 154 (T3), 95 (T4) and 110 (T5) nodes; average edge strength was 0.09 (T1), 0.05 (T2), 0.08 (T3), 0.07 (T4) and 0.07 (T5). To test whether networks contained significantly preferred and avoided relationships, we ran permutation tests on the grouping data, controlling for group size and the number of observations, restricting swaps within days and sites35,36. We tested whether observed patterns of associations were non-random by comparing the coefficient of variance in the observed network to the coefficient of variance in the randomised networks35. Social networks for all replicates significantly differed from random, even at local scales (T1: P<0.0001; T2: P=0.0005; T3: P<0.0001; T4: P=0.0002; T5: P=0.0002)
Finally, we used network-based approaches to ask whether the behaviour was socially transmitted through foraging associations. Network-based diffusion analysis (NBDA) tests for social learning by assuming that if social transmission is occurring, then the spread of trait acquisition should follow patterns of relationships between individuals, with transmission rate linearly proportional to association strength23,37,38. We used NBDA R code v.1.238, with the time of each individual’s first solution (seconds since the beginning of the experiment) entered into the continuous time of acquisition analysis function. Individuals that solved, but that did not appear in the social network (i.e. had not been recorded in the standardised weekend logging) were excluded from the analysis. The effects of three individual level variables were also incorporated into the analysis: sex, age, and natal origin. All combinations of NBDA provided in the NBDA R code v1.238 were run with social transmission rate allowed to vary for each replicate. An AIC model averaging approach was used to find the best-supported model38.
Extended Data
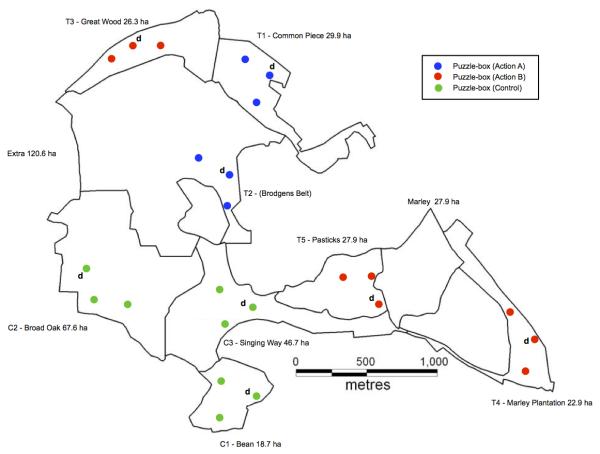
Extended Data Fig. 1. Wytham Woods (51°46’N, 01°20’W), showing the location of replicates and puzzle-boxes.
Total area of Wytham Woods is 385ha; location and size of the separate woodland areas within this are labeled on the map. Green points indicate puzzle-box locations for three ‘control’ replicates C1-3: Broad Oak, Bean, Singing Way. Blue points indicate location of puzzle-boxes for two ‘option A’ replicates T1-2: Common Piece, Brogdens Belt. Red points indicate location of puzzle-boxes for three ‘option B’ replicates T3-5: Great Wood, Pasticks, Marley Plantation. (d) indicates locations where trained demonstrators were caught from and released to.
Extended Data Fig. 2. Social network data collection.
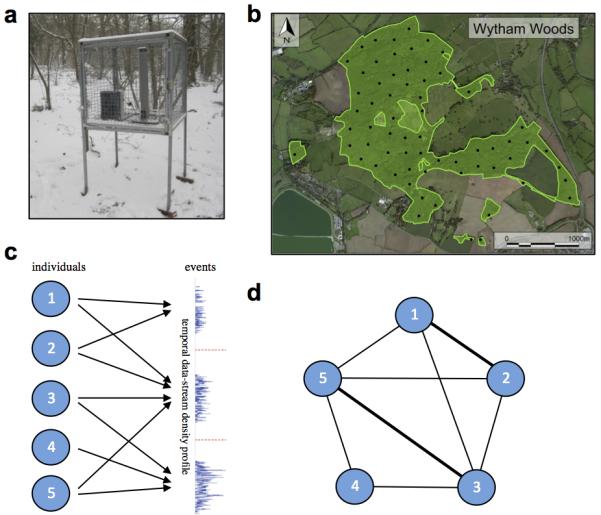
a, Feeding station (shut), with sunflower-feeder, RFID antennae, and data-logging hardware. Cage is to restrict access to small passerines only. b, Map of study area showing placement of 65 feeding stations. Stations are approximately 250m apart and open simultaneously dawn-dusk on Saturday and Sunday over winter. c, Grouping events are inferred from the temporal data stream gained from feeding stations, with individuals assigned to grouping events in a bipartite network. d, Repeated co-occurrences are used to create social networks (adapted from Psorakis et al. (2012)).
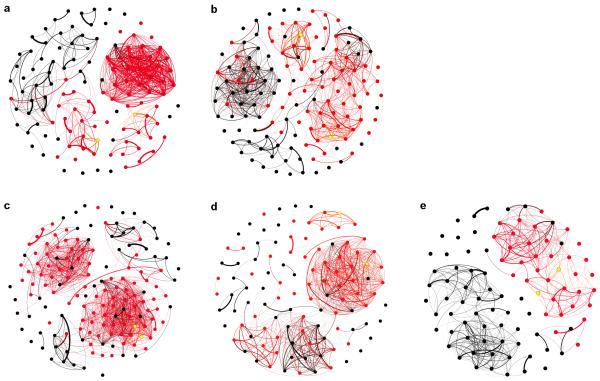
Extended Data Fig. 3. Social Networks showing diffusion of innovation.
Red nodes are individuals that acquired the novel behaviour after 20 days of exposure, black nodes are naïve individuals and yellow nodes are trained demonstrators. Networks are heavily thresholded to only show links above the average edge strength for each replicate (T1-5: 0.09, 0.05, 0.08 0.07, 0.07). a, Social network for T1 replicate (n=123). b, Network for T2 replicate (n=137). c, Network for T3 replicate (n=154). d, Network for T4 replicate (n=95). e, Network for T5 replicate (n=110).
Extended Data Fig. 4. Individual trajectories (option A/B) for each replicate.
Only individuals that performed both options are included, and Individuals that moved between replicates are excluded. Lines are running proportions of seeded variant for each individual over its last 10 visits. a, T1 (option A), n=30; b, T2 (option A), n=10; c, T3 (option B), n=19; d, T4 (option B), n=4; e, T5 (option B), n=15.
Extended Data Fig. 5. Food preferences trials.
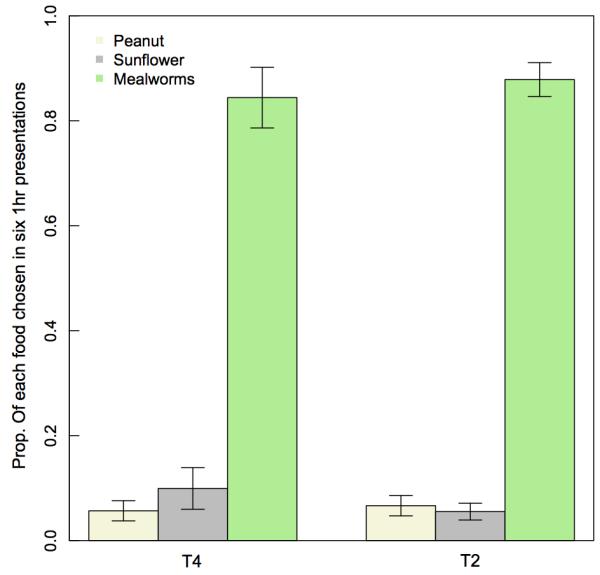
Birds were presented with a freely available mix of 40 mealworms, peanut granules and sunflower seeds for 1 hr on 2 days over 1 week at 6 sites (3 sites in T4 and T2). Trials were conducted 2 weeks after the end of the main experiment, in March 2014. Food choice was identified from video camera footage, and the trial was halted when all of one prey item was taken. Only great tits were included, but birds could not be individually identified. Birds clearly preferred the live mealworms to either peanut granules or sunflower seeds.
Supplementary Material
Acknowledgments
This project was supported by grants from BBSRC (BB/L006081/1) and ERC (AdG 250164) to BCS, who was also supported by a visiting professorship at Uppsala University. LMA was also supported by an Australian Postgraduate Award and AT by a BBSRC David Phillips Fellowship (BB/H021817/1). Work was subject to review by the Department of Zoology ethical committee, University of Oxford, and carried out under Natural England licence 20123075 and 20131205. The EGI social networks group, Stephen Lang and Keith McMahon provided assistance in the field, and Martin Whitaker (Stickman Technologies Inc. Southampton, U.K.) produced electronic components for the puzzle-boxes.
References
- 1.Rogers EM. Diffusion of Innovations. 4th edn The Free Press; 1995. [Google Scholar]
- 2.Claidiere N, Messer EJE, Hoppitt W, Whiten A. Diffusion dynamics of socially learned foraging techniques in squirrel monkeys. Curr Biol. 2013;23:1251–1255. doi: 10.1016/j.cub.2013.05.036. doi:10.1016/J.Cub.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 3.Cantor M, Whitehead H. The interplay between social networks and culture: theoretically and among whales and dolphins. Philos T R Soc B. 2013;368 doi: 10.1098/rstb.2012.0340. doi:10.1098/Rstb.2012.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rendell L, et al. Cognitive culture: theoretical and empirical insights into social learning strategies. Trends Cogn Sci. 2011;15:68–76. doi: 10.1016/j.tics.2010.12.002. doi:10.1016/J.Tics.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 5.van de Waal E, Borgeaud C, Whiten A. Potent social learning and conformity shape a wild primate’s foraging decisions. Science. 2013;340:483–485. doi: 10.1126/science.1232769. doi:10.1126/science.123769. [DOI] [PubMed] [Google Scholar]
- 6.Whiten A, Hinde RA, Laland KN, Stringer CB. Culture evolves. Philos T R Soc B. 2011;366:938–948. doi: 10.1098/rstb.2010.0372. doi:10.1098/rstb.2010.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whiten A, Horner V, de waal FB. Conformity to cultural norms of tool use in chimpanzees. Nature. 2005;437:737–740. doi: 10.1038/nature04047. doi:10.1038/nature04047. [DOI] [PubMed] [Google Scholar]
- 8.Laland KN, Janik VM. The animal cultures debate. Trends Ecol Evol. 2006;21:542–547. doi: 10.1016/j.tree.2006.06.005. doi:10.1016/J.Tree.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Warner RR. Traditionality of mating-site preferences in a coral reef fish. Nature. 1988;335:719–721. doi:10.1038/335719a0. [Google Scholar]
- 10.Coussi-Korbel S, Fragaszy DM. On the relation between social dynamics and social learning. Anim Behav. 1995;50:1441–1453. doi:10.1016/0003-3472(95)80001-8. [Google Scholar]
- 11.Dean LG, Kendal RL, Schapiro SJ, Thierry B, Laland KN. Identification of the social and cognitive processes underlying human cumulative culture. Science. 2012;335:1114–1118. doi: 10.1126/science.1213969. doi:10.1126/Science.1213969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laland K. Social learning strategies. Learn Behav. 2004;32:4–14. doi: 10.3758/bf03196002. doi:10.3758/BF03196002. [DOI] [PubMed] [Google Scholar]
- 13.Galef BG, Laland KN. Social learning in animals: empirical studies and theoretical models. Bioscience. 2005;55:489–499. doi:10.1641/0006-3568. [Google Scholar]
- 14.Whiten A, Mesoudi A. Establishing an experimental science of culture: animal social diffusion experiments. Philos T R Soc B. 2008;363:3477–3488. doi: 10.1098/rstb.2008.0134. doi:10.1098/Rstb.2008.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galef BG. In: Oxford Handbook of Comparative Cognition. Zentall TR, Wasserman E, editors. Oxford University Press; 2012. pp. 803–818. Ch. 40. [Google Scholar]
- 16.Fisher JB, Hinde RA. The opening of milk bottles by birds. British Birds. 1949;42:347–357. [Google Scholar]
- 17.Sherry DF, Galef BG. Cultural transmission without imitation - milk bottle opening by birds. Anim Behav. 1984;32:937–938. [Google Scholar]
- 18.Aplin LM, Sheldon B, Morand-Ferron J. Milk-bottles revisited: social learning and individual variation in the blue tit (Cyanistes caeruleus) Anim Behav. 2013;85:1225–1232. doi:10.1016/j.anbehav.2013.03.009. [Google Scholar]
- 19.Morand-Ferron J, Cole EF, Rawles JEC, Quinn JL. Who are the innovators? A field experiment with 2 passerine species. Behav Ecol. 2011;22:1241–1248. doi:10.1093/Beheco/Arr120. [Google Scholar]
- 20.Slagsvold T, Wiebe KL. Social learning in birds and its role in shaping a foraging niche. Philos T R Soc B. 2011;366:969–977. doi: 10.1098/rstb.2010.0343. doi:10.1098/Rstb.2010.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aplin LM, et al. Individual personalities predict social behaviour in wild networks of great tits (Parus major) Ecol Lett. 2013;16:1365–1372. doi: 10.1111/ele.12181. doi:10.1111/ele.12181. [DOI] [PubMed] [Google Scholar]
- 22.Psorakis I, Roberts SJ, Rezek I, Sheldon BC. Inferring social network structure in ecological systems from spatio-temporal data streams. J R Soc Interface. 2012;9:3055–3066. doi: 10.1098/rsif.2012.0223. doi:10.1098/rsif.2012.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen J, Weinrich MT, Hoppitt W, Rendell L. Network-based diffusion analysis reveals cultural transmission of lobtail feeding in humpback whales. Science. 2013;340:485–488. doi: 10.1126/science.1231976. doi:10.1126/science.1231976. [DOI] [PubMed] [Google Scholar]
- 24.Thornton A, Malapert A. The rise and fall of an arbitrary tradition: an experiment with wild meerkats. P Roy Soc B-Biol Sci. 2009;276:1269–1276. doi: 10.1098/rspb.2008.1794. doi:10.1098/Rspb.2008.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan TJH, Laland K. The biological bases of conformity. Front Neurosci. 2012;6:1–7. doi: 10.3389/fnins.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Leeuwen EJC, Haun DBM. Conformity in nonhuman primates: fad or fact? Evol Hum Behav. 2013;34:1–7. doi:10.1016/J.Evolhumbehav.2012.07.005. [Google Scholar]
- 27.Bouwhuis S, Choquet R, Sheldon BC, Verhulst S. The forms and fitness cost of senescence: age-specific recapture, survival, reproduction, and reproductive value in a wild bird population. Am Nat. 2012;179:E15–E27. doi: 10.1086/663194. doi:10.1086/663194. [DOI] [PubMed] [Google Scholar]
- 28.Haun DBM, Rekers Y, Tomasello M. Majority-Biased Transmission in Chimpanzees and Human Children, but Not Orangutans. Curr Biol. 2012;22:727–731. doi: 10.1016/j.cub.2012.03.006. doi:10.1016/J.Cub.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 29.de waal FB. Animal conformists. Science. 2013;340:437–438. doi: 10.1126/science.1237521. doi:10.1126/science.1237521. [DOI] [PubMed] [Google Scholar]
- 30.van Schaik CP. Animal culture: chimpanzee conformity? Curr Biol. 2012;22:R402–R404. doi: 10.1016/j.cub.2012.04.001. doi:10.1016/j.cub.2012.04.001. [DOI] [PubMed] [Google Scholar]
Materials and Methods – References
- 31.Aplin LM, Farine DR, Morand-Ferron J, Sheldon BC. Social networks predict patch discovery in a wild population of songbirds. P Roy Soc B-Biol Sci. 2012;279:4199–4205. doi: 10.1098/rspb.2012.1591. doi:10.1098/rspb.2012.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach. 2nd edn Springer; 2002. [Google Scholar]
- 33.Farine DR. Animal social network inference and permutations for ecologist in R using asnipe. Methods Ecol Evol. 2013;4:1187–1194. doi:10.1111/2041-210X.12121. [Google Scholar]
- 34.Franks DW, Ruxton GD, James R. Sampling animal association networks with the gambit of the group. Behav Ecol Sociobiol. 2010;64:493–503. doi:10.1007/S00265-009-0865-8. [Google Scholar]
- 35.Whitehead H. Analyzing animal societies: quantative methods for vertebrate social analysis. The University of Chicago Press; 2008. [Google Scholar]
- 36.Bejder L, Fletcher D, Brager S. A method for testing association patterns of social animals. Anim Behav. 1998;56:719–725. doi: 10.1006/anbe.1998.0802. doi: 10.1006/Anbe.1998.0802. [DOI] [PubMed] [Google Scholar]
- 37.Franz M, Nunn CL. Network-based diffusion analysis: a new method for detecting social learning. P Roy Soc B-Biol Sci. 2009;276:1829–1836. doi: 10.1098/rspb.2008.1824. doi:10.1098/Rspb.2008.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoppitt W, Boogert NJ, Laland KN. Detecting social transmission in networks. J Theor Biol. 2010;263:544–555. doi: 10.1016/j.jtbi.2010.01.004. doi:10.1016/J.Jtbi.2010.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.