Abstract
Background
Clinical guidelines recommend annual follow-up of most patients with monoclonal gammopathy of undetermined significance (MGUS), but evidence supporting this practice is lacking. We performed a population-based study to examine patterns of disease presentation and outcomes of patients with multiple myeloma, Waldenström macroglobulinemia, and lymphoplasmacytic lymphoma (monoclonal gammopathy–associated malignancies) comparing those with or without a previous MGUS follow-up.
Methods
Patients with monoclonal gammopathy-associated malignancy from 1994 through 2007 were identified using the Surveillance, Epidemiology, and End Results–Medicare linked database and divided into 2 cohorts: Follow-up (MGUS follow-up preceding diagnosis) and No Follow-up (no such follow-up). We compared outcomes including rates of major complications at cancer diagnosis (acute kidney injury, cord compression, dialysis use, fracture, and hypercalcemia) and survival using propensity score adjustment and Cox-proportional hazard models. All statistical tests were 2-sided.
Results
Of the 17 457 study patients, 6% had MGUS follow-up. After multivariable modeling, Follow-up patients had significantly fewer major complications at diagnosis (odds ratio=0.68; 95% confidence interval [CI]=0.57 to 0.80) and better disease-specific (median 38 months vs 29 months, P < .001; hazard ratio [HR]=0.85; 95% CI= 0.76 to 0.94) and overall (median 23 months vs 19 months, P < .001; HR=0.87; 95% CI=0.80 to 0.95) survivals.
Conclusions
Patients with MGUS follow-up preceding the diagnosis of a monoclonal gammopathy–associated malignancy may experience fewer major complications and have longer survival than those not followed. Future studies replicating our findings in the non-Medicare population and determining the optimal schedule and cost effectiveness of MGUS follow-up are warranted.
Keywords: complications, outcomes, multiple myeloma, Waldenström macroglobulinemia, lymphoplasmacytic lymphoma
Introduction
Monoclonal gammopathy of undetermined significance (MGUS) is the incidental finding of a monoclonal protein in the blood or urine in individuals without an associated hematologic malignancy. A prevalence study among the predominantly White residents of Olmsted County, Minnesota, found MGUS in 3% of individuals over age 50 years.1 Although studies reflecting the ethnic diversity in the United States have not been done, it is known that MGUS and multiple myeloma are 2 to 3 times more common in Blacks.2 At least 3.6 million people in the United States may have MGUS.3 MGUS is a potentially premalignant condition with a continuous risk of progression to lymphoplasmacytic malignancies of approximately 1% per year.4 However, if competing causes of death are taken into account, the risk of progression is only 0.4% per year.5 Approximately 90% of patients with MGUS will never develop lymphoplasmacytic malignancies, and because no therapy is presently available to prevent its transformation among those who are destined to do so, screening for MGUS in the general population is not currently recommended.6
The biological significance of MGUS is now established: virtually all multiple myeloma cases are preceded by MGUS.7 There is currently no consensus on what is necessary to make MGUS clinically significant. Nevertheless, we believe a reasonable goal is to show whether patients with lymphoplasmacytic malignancies with a preceding MGUS diagnosis and being followed on a regular basis have better outcomes with longer survival, lesser complications, or better quality of life than those who are not followed.3 Recent clinical guidelines recommend long-term annual follow-up of most MGUS patients.8 However, evidence supporting the utility of this practice is lacking, including whether it is associated with improved disease-related outcomes. Moreover, MGUS follow-up may be associated with potential harms, including the psychological burden of cancer anticipation, substantial costs to society, and exacerbation of the projected shortage of physicians in the United States.3 Therefore, we performed a population-based study to examine patterns of disease presentation and outcomes of patients with multiple myeloma, Waldenström macroglobulinemia, and lymphoplasmacytic lymphoma (the 3 most common malignancies associated with MGUS, henceforth collectively referred to as monoclonal gammopathy–associated malignancies) and compared those with or without a previous MGUS follow-up.
Methods
Study Design
We performed a retrospective cohort study using the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked registry. The data came from the SEER program of 17 distinct cancer registries that collect clinical, demographic, and cause-of-death information for persons with cancer and the subsequent linkage to Medicare claims for covered health care services from the time of a person’s Medicare eligibility until death.9 SEER-Medicare data through December 31, 2009, were available and used in the analyses.
Patient Selection
We included incident cases of multiple myeloma (ICD-O: 9731-9734), Waldenström macroglobulinemia (ICD-O: 9671), and lymphoplasmacytic lymphoma (ICD-O: 9761) diagnosed from 1994-2007 (N = 28,879) who were 67 years and older to allow for at least 15 months of MGUS follow-up before diagnosis of an associated malignancy. We excluded patients with the following characteristics: prior diagnosis of an associated malignancy (n = 6), diagnosis at autopsy or on death certificate only (n = 824), prior invasive cancer within the last 5 years (n = 1788), unknown date of death (n = 51), unknown month of diagnosis (n = 892), incomplete Medicare Parts A and B coverage or with health maintenance organization (HMO) enrollment 15 months before through 3 months after diagnosis of an associated malignancy (n = 7711), and those who had dialysis more than 3 months before diagnosis of an associated malignancy (n = 150). Patients enrolled in HMO (Medicare Advantage) plans were excluded because their billing claims are not submitted to Medicare for reimbursement. Patients who were on chronic dialysis prior to diagnosis of an associated malignancy likely had end-stage renal disease caused by other conditions.
Patient Characteristics and Definitions
Sociodemographic characteristics captured were age, sex, race, ethnicity, type of residence at time of cancer diagnosis based on Area Health Resource File classification, and median household income measured at the census tract level.10 A comorbidity score was calculated based on the modified Charlson Index using diagnostic claims in the 12 months preceding diagnosis of an associated malignancy.11
We identified patients as having MGUS with follow-up if they had at least 1 MGUS diagnosis claim (ICD-9: 273.1) in the interval between 4 and 15 months prior to diagnosis of an associated malignancy (the Follow-up cohort). Previous studies have used 6- to 24-month cutoffs to exclude an undiagnosed or subclinical associated malignancy.7,12,13 We did not include patients whose only MGUS follow-up was either more than 15 months or fewer than 4 months prior to diagnosis of an associated malignancy in the Follow-up cohort because they were either much less likely to benefit from MGUS follow-up (follow-up more than 15 months before diagnosis) or were most likely to have an undiagnosed associated malignancy (follow-up fewer than 4 months before diagnosis). Those without MGUS follow-up as described comprised the No Follow-up cohort.
To determine major disease-presenting complications, we searched for presence of acute kidney injury (AKI), cord compression, dialysis use, hypercalcemia, and pathologic or compression fracture using diagnosis and procedure claims reported within 3 months of diagnosis of an associated malignancy. A major complication was considered to have occurred if there were 1 or more inpatient diagnosis claims, 2 or more outpatient diagnosis claims, or 1 or more procedure claims, whether inpatient or outpatient.14 We did not include anemia as a complication because this is a clinical sign common to many diseases in elderly people. Patients with diagnosis of an associated malignancy who had no complication or treatment claims and who had not died from the malignancy were considered to have smoldering malignancy. Patients not meeting this definition were considered to have active malignancy. A detailed description of the codes used and the sources of the claims are provided in Supplemental Tables 1-5.
Statistical Analysis
To determine the proportion of patients with MGUS follow-up in the interval between 4 and 15 months prior to diagnosis of an associated malignancy, we included cases diagnosed from 1994 through 2007. For analyses of major complication rates and survival, cases diagnosed from 1994 through 2005 were included. We did not include cases diagnosed from 2006 onwards in the complication and survival analyses because we did not have access to data on the use of oral chemotherapy agents when Medicare Part D went into effect. Smoldering malignancy is more frequently diagnosed among patients with MGUS follow-up,15 so we excluded those patients in the analyses of complications at diagnosis. For patients with smoldering malignancy at diagnosis, subsequent initiation of treatment or development of complication indicated progression into active malignancy. To minimize lead-time bias, overall survival (OS) and disease-specific survival (DSS) were measured from the time a patient was determined to have active malignancy. Specific survival data were obtained from the SEER registry with follow-up through 2009.
Patient characteristics were summarized using descriptive statistics, and differences between groups were assessed using χ2 tests for categorical variables and t tests for continuous variables. The changes in the proportion of MGUS follow-up and smoldering malignancy over time were modeled with a Cochran-Armitage trend test. We analyzed OS and DSS using the Kaplan-Meier method, and the log-rank test was used to compare these survival distributions between cohorts at the univariable level. We studied the prognostic effect of the various sociodemographic and clinical variables using multivariable Cox proportional hazards models. All tests of statistical significance were 2-sided, and P < .05 was considered statistically significant.
For comparison with traditional multivariable survival models, propensity score models were built as follows: (1) Propensity to have MGUS follow-up was scored via logistic regression using the available covariables, that is, year of cancer diagnosis, type of cancer diagnosis, comorbidity score, race, SEER registry, age at diagnosis, median annual household income, residence at time of diagnosis, sex, and ethnicity; (2) Propensity score histograms were visually inspected for similarity between the cohorts; (3) Mean and standard deviations were compared via t tests and variance test between the cohorts to assess uniformity of scores; (4) Individual χ2 tests and Cochran-Mantel-Haenszel tests were used to compare the distribution of covariables between the cohorts per propensity score quintile; and (5) While adjusting for propensity score quintiles, multivariable Cox proportional hazards models were built using the propensity score as an independent predictor along with the indication for MGUS follow-up.
Results
Patient Characteristics
We included 17,457 patients with diagnosis of an associated malignancy from 1994 through 2005 who were 67 years of age or older. The patients had a median age of 77 years (range 67-104 years), and 50.6% were women. The majority had multiple myeloma (89.7%) and were White (80.9%), non-Hispanic (94.9%), residing in either large metropolitan (58.2%) or metropolitan (26.8%) areas, with a median annual household income of $25,001 to $65,000 (69.5%). Most had low comorbidity scores of 0 to 1 (85.2%). Demographic and clinical data are provided in Table 1.
Table 1.
Sociodemographic and clinical characteristics of patients at time of cancer diagnosisa
| Characteristic | All (N = 17,457) |
Cohort
|
P | |
|---|---|---|---|---|
| No Follow-up (n = 16,416) |
Follow-up (n = 1041) |
|||
| Age group, y | .256 | |||
| 67-75 | 7175 (41.1) | 6825 (41.6) | 350 (33.6) | |
| 76-84 | 7388 (42.3) | 6868 (41.8) | 520 (49.9) | |
| ≥ 85 | 2894 (16.6) | 2723 (16.6) | 171 (16.4) | |
| Men | 8620 (49.4) | 8050 (49.0) | 570 (54.8) | < .001 |
| Race | .219 | |||
| White | 14,152 (80.9) | 13,284 (81.0) | 868 (83.4) | |
| Black | 2270 (13.1) | 2142 (13.1) | 128 (12.3) | |
| Asian | 382 (2.2) | 364 (2.2) | 18 (1.7) | |
| Hispanic | 326 (1.9) | 313 (1.9) | 13 (1.3) | |
| American Indian/Alaska native | 53 (0.3) | ____b | ____b | |
| Other | 240 (1.4) | 230 (1.4) | 10 (1.0) | |
| Unknown | 34 (0.2) | ____b | ____b | |
| Ethnicity | .113 | |||
| Hispanic | 887 (5.1) | 845 (5.1) | 42 (4.0) | |
| Non-Hispanic | 16,570 (94.9) | 15,571 (94.9) | 999 (96.0) | |
| Residence at time of diagnosis | .624 | |||
| Large metropolitan | 10,153 (58.2) | 9557 (58.2) | 596 (57.3) | |
| Metropolitan | 4677 (26.8) | 4380 (26.7) | 297 (28.5) | |
| Urban | 972 (5.6) | 916 (5.6) | 56 (5.4) | |
| Less urban | 1362 (7.8) | 1283 (7.8) | 79 (7.6) | |
| Rural | ____b | ____b | ____b | |
| Unknown | ____b | ____b | ____b | |
| Median annual household income | .688 | |||
| ≤ $25,000 | 1579 (9.1) | 1483 (9.0) | 96 (9.2) | |
| $25,001-$45,000 | 7008 (40.1) | 6601 (40.2) | 407 (39.1) | |
| $45,001-$65,000 | 5131 (29.4) | 4836 (29.5) | 295 (28.3) | |
| $65,001-$85,000 | 2265 (13.0) | 2122 (12.9) | 143 (13.7) | |
| > $85,000 | 1364 (7.8) | ____b | ____b | |
| Not available | 110 (0.6) | ____b | ____b | |
| Comorbidity score | .036 | |||
| 0 | 12,054 (69.1) | 11,375 (69.3) | 679 (65.2) | |
| 1 | 2817 (16.1) | 2636 (16.1) | 181 (17.4) | |
| 2 | 1348 (7.7) | 1256 (7.6) | 92 (8.8) | |
| ≥3 | 1238 (7.1) | 1149 (7.0) | 89 (8.6) | |
| SEER registry | .001 | |||
| Atlanta | 638 (3.7) | 605 (3.7) | 33 (3.2) | |
| Connecticut | 1508 (8.6) | 1407 (8.6) | 101 (9.7) | |
| Detroit | 2012 (11.5) | 1888 (11.5) | 124 (11.9) | |
| Greater California | 2033 (11.7) | 1915 (11.7) | 118 (11.3) | |
| Hawaii | 216 (1.2) | ____b | ____b | |
| Iowa | 1621 (9.3) | 1520 (9.3) | 101 (9.7) | |
| Kentucky | 964 (5.5) | 912 (5.6) | 52 (5.0) | |
| Los Angeles | 1613 (9.2) | 1542 (9.4) | 71 (6.8) | |
| Louisiana | 970 (5.6) | 914 (5.6) | 56 (5.4) | |
| New Jersey | 2142 (12.3) | 2011 (12.3) | 131 (12.6) | |
| New Mexico | 476 (2.7) | 452 (2.8) | 24 (2.3) | |
| Rural Georgia | 49 (0.3) | ____b | ____b | |
| San Francisco | 785 (4.5) | 745 (4.5) | 40 (3.8) | |
| San Jose | 498 (2.8) | 477 (2.9) | 21 (2.0) | |
| Seattle | 1340 (7.7) | 1219 (7.4) | 121 (11.6) | |
| Utah | 592 (3.4) | 560 (3.4) | 32 (3.1) | |
| Cancer type | < .001 | |||
| Lymphoplasmacytic lymphoma | 747 (4.3) | 701 (4.3) | 46 (4.4) | |
| Multiple myeloma | 15,664 (89.7) | 14,769 (90.0) | 895 (86.0) | |
| Waldenström macroglobulinemia | 1046 (6.0) | 946 (5.8) | 100 (9.6) | |
| Year of cancer diagnosis | < .001 | |||
| 1994-1999 | 4744 (27.2) | 4551 (27.7) | 193 (18.5) | |
| 2000-2007 | 12,713 (72.8) | 11,865 (72.3) | 848 (81.5) | |
Abbreviation: SEER = Surveillance, Epidemiology, and End Results.
All statistical tests were 2-sided. Data are presented as number of patients (%).
Numbers are suppressed since cell size is less than 11, per SEER-Medicare data reporting requirements.
MGUS Follow-up and Smoldering Monoclonal Gammopathy–Associated Malignancy Rates
Overall, 6.0% (n = 1041) of the patients had at least 1 MGUS follow-up in the interval from 4 to 15 months before malignancy diagnosis. The MGUS follow-up rate increased over time from 2.6% in 1994 to 6.9% in 2007 (trend test, P < .001). Compared with the No Follow-up cohort, the Follow-up cohort had a higher proportion of women and of Waldenström macroglobulinemia/lymphoplasmacytic lymphoma diagnoses (Table 1). At diagnosis, malignancy was considered smoldering in 15.2% of the patients. The proportion of patients with smoldering malignancy did not change over time (trend test, P = .49). Patients with smoldering malignancy were more likely than those with active malignancy to have MGUS follow-up between 4 and 15 months prior to diagnosis (9.1% vs 5.1%; P < .001).
Major Complications at Monoclonal Gammopathy–Associated Malignancy Diagnosis
Among all patients with active malignancy, 57.9% had at least 1 major complication, including AKI (23.9%), cord compression (7.4%), dialysis requirement (7.5%), fracture (32.3%), and hypercalcemia (18.8%) (Fig. 1). The proportion of patients with any major complication increased over time from 56.0% in 1994 to 63.6% in 2005 (trend test, P < .001). Unadjusted complication rates at diagnosis were significantly lower in the Follow-up cohort than in the No Follow-up cohort except for dialysis requirement and cord compression (Fig. 1). The presence of MGUS follow-up was an independent predictor of lower rates of complications at malignancy diagnosis (P < .001, odds ratio [OR] = 0.67; 95% CI = 0.57 to 0.80) (Table 2).
Figure 1.
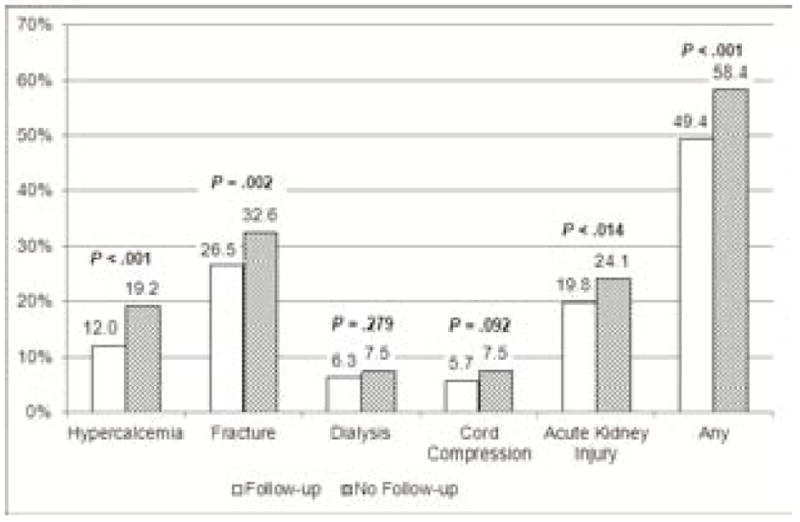
Complication Rates at Time of Cancer Diagnosis by Follow-Up and No Follow-Up Cohorts
Table 2.
Variables associated with risk of major complications at time of cancer diagnosisa
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
|
| ||||
| OR (95% CI) | P | OR (95% CI) | P | |
| Age group, y | ||||
| 67-75 | Reference | |||
| 76-84 | 1.02 (0.94-1.11) | .599 | Not significant | |
| ≥ 85 | 1.11 (0.99-1.24) | .058 | ||
| Sex | ||||
| Men | Reference | Reference | ||
| Women | 1.20 (1.12-1.29) | < .001 | 1.22 (1.13-1.31) | < .001 |
| Race | ||||
| White | Reference | |||
| Black | 1.18 (1.08-1.29) | < .001 | ||
| Other | 0.92 (0.78-1.08) | .307 | Not significant | |
| Unknown | 0.23 (0.14-0.38) | < .001 | ||
| Ethnicity | ||||
| Non-Hispanic | Reference | Not significant | ||
| Hispanic | 1.19 (1.00-1.40) | .046 | ||
| Residence | ||||
| Large metropolitan | Reference | |||
| Metropolitan | 1.01 (0.93-1.10) | .845 | ||
| Urban | 0.98 (0.83-1.15) | .787 | Not significant | |
| Less urban | 1.05 (0.92-1.21) | .464 | ||
| Rural | 0.94 (0.71-1.23) | .636 | ||
| Median annual household income | ||||
| ≤ $25,000 | Reference | |||
| $25,001-$45,000 | 0.83 (0.73-0.95) | .006 | ||
| $45,001-$65,000 | 0.81 (0.71-0.93) | .003 | ||
| $65,001-$85,000 | 0.79 (0.68-0.93) | .004 | Not Significant | |
| > $85,000 | 0.76 (0.64-0.91) | .003 | ||
| Not available | 0.63 (0.40-1.00) | .047 | ||
| Comorbidity score | ||||
| 0 | Reference | Reference | ||
| 1 | 1.45 (1.31-1.61) | < .001 | 1.40 (1.26-1.56) | < .001 |
| 2 | 1.68 (1.45-1.94) | < .001 | 1.69 (1.46-1.96) | < .001 |
| ≥3 | 2.48 (2.10-2.92) | < .001 | 2.43 (2.08-2.88) | < .001 |
| Cancer type | ||||
| Multiple myeloma | Reference | Reference | ||
| Lymphoplasmacytic lymphoma | 0.19 (0.15-0.24) | < .001 | 0.19 (0.15-0.24) | < .001 |
| Waldenström macroglobulinemia | 0.26 (0.22-0.31) | < .001 | 0.27 (0.23-0.33) | < .001 |
| Year of diagnosis | ||||
| 1994-1999 | Reference | Reference | ||
| 2000-2005 | 1.25 (1.16-1.35) | < .001 | 1.20 (1.10-1.31) | < .001 |
| SEER site | ||||
| California | Reference | Reference | ||
| Atlanta | 0.98 (0.81-1.19) | .837 | 0.91 (0.75-1.11) | .372 |
| Connecticut | 1.01 (0.88-1.16) | .938 | 1.04 (0.90-1.20) | .638 |
| Detroit | 1.12 (0.99-1.27) | .071 | 1.10 (0.96-1.25) | .170 |
| Hawaii | 0.70 (0.50-0.99) | .042 | 0.79 (0.56-1.12) | .189 |
| Iowa | 0.81 (0.71-0.92) | .002 | 0.84 (0.73-0.96) | .011 |
| Kentucky | 1.30 (1.09-1.55) | .003 | 1.10 (0.92-1.32) | .299 |
| Louisiana | 1.28 (1.08-1.52) | .006 | 1.08 (0.90-1.30) | .410 |
| New Jersey | 1.16 (1.02-1.32) | .027 | 1.05 (0.91-1.20) | .521 |
| New Mexico | 0.91 (0.73-1.15) | .436 | 0.93 (0.74-1.18) | .560 |
| Rural Georgia | 1.09 (0.58-2.05) | .800 | 0.94 (0.50-1.80) | .861 |
| Seattle | 0.68 (0.59-0.78) | < .001 | 0.73 (0.63-0.85) | < .001 |
| Utah | 0.98 (0.79-1.20) | .819 | 0.95 (0.77-1.17) | .617 |
| MGUS follow-up | ||||
| No | Reference | Reference | ||
| Yes | 0.70 (0.59-0.82) | < .001 | 0.67 (0.57-0.80) | < .001 |
Abbreviations: OR = odds ratio; CI = confidence interval; SEER = Surveillance, Epidemiology, and End Results; MGUS = monoclonal gammopathy of undetermined significance.
All statistical tests were 2-sided.
Survival
Patients in the Follow-up cohort had a significantly longer OS than those in the No Follow-up cohort (P < .001) with a median of 23 months versus 19 months (Fig. 2). The OS advantage remained even after adjusting for other pertinent variables (hazard ratio [HR] = 0.87; 95% CI = 0.80 to 0.95) (Table 3). Similar findings were observed for DSS in terms of survival time (P < .001) with a median of 38 vs 29 months (adjusted HR = 0.85; 95% CI = 0.76 to 0.94) (Fig. 2). The results were similar when we compared the propensity score–adjusted cohorts (P < .001, OS HR = 0.86, 95% CI = 0.79 to 0.93; P < .001, DSS HR = 0.84, 95% CI = 0.76 to 0.93). There was a slight difference in the mean propensity score and variance between the cohorts (propensity score of 6.3 vs 5.4 and variance of 2.5 vs. 2.1 for Follow-up and No Follow-up cohorts, respectively; P < .001 for both comparisons). The histograms appeared similar by visual inspection. Individual χ2 and Cochran-Mantel-Haenszel tests comparing covariables showed significant differences only for ethnicity in the third quintile (4.0% vs. 8.1% Hispanic in the No Follow-up and Follow-up cohorts, respectively; P = .010).
Figure 2.
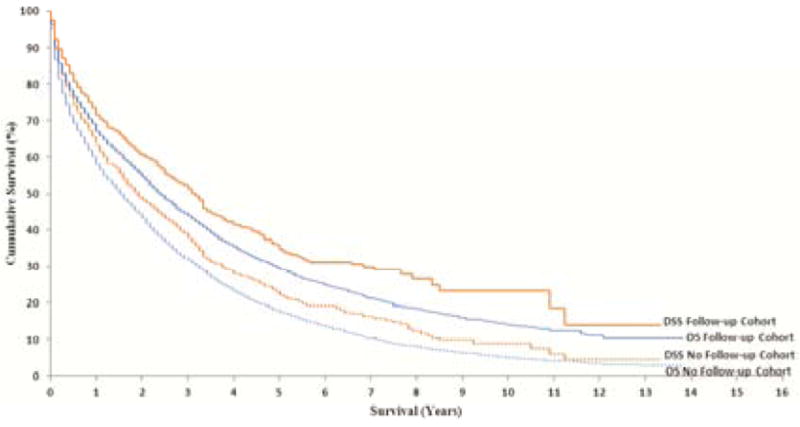
Survival Rates by Follow-up and No Follow-up Cohorts. OS = overall survival; DSS = disease-specific survival.
Table 3.
Variables associated with risk of all-cause mortalitya
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
|
| ||||
| Median, mo (95% CI) | P | HR (95% CI) | P | |
| Age group, y | < .001 | |||
| 67-75 | 28 (26-29) | Reference | ||
| 76-84 | 18 (17-19) | 1.36 (1.30-1.41) | < .001 | |
| ≥ 85 | 7 (6-8) | 2.32 (2.20-2.46) | < .001 | |
| Sex | ||||
| Men | 18 (17-19) | Reference | ||
| Women | 19 (18-20) | .049 | 0.91 (0.88-0.94) | < .001 |
| Race | ||||
| White | 19 (45-48) | Reference | ||
| Black | 17 (16-19) | < .001 | 0.93 (0.87-0.99) | .026 |
| Other | 23 (20-26) | 0.97 (0.86-1.09) | .605 | |
| Unknown | 125 (55-125) | 0.28 (0.18-0.45) | < .001 | |
| Ethnicity | .436 | Not significant | ||
| Non-Hispanic | 19 (18-19) | |||
| Hispanic | 18 (16-21) | |||
| Residence | .018 | Not significant | ||
| Large metropolitan | 19 (18-20) | |||
| Metropolitan | 20 (18-21) | |||
| Urban | 19 (17-22) | |||
| Less urban | 17 (14-19) | |||
| Rural | 16 (11-20) | |||
| Median annual household income | ||||
| ≤ $25,000 | 16 (14-17) | Reference | ||
| $25,001-$45,000 | 17 (16-18) | 0.96 (0.90-1.03) | .259 | |
| $45,001-$65,000 | 20 (19-22) | < .001 | 0.87 (0.80-0.93) | < .001 |
| $65,001-$85,000 | 21 (19-23) | 0.85 (0.78-0.93) | < .001 | |
| > $85,000 | 25 (22-28) | 0.77 (0.70-0.86) | < .001 | |
| Not available | 20 (12-24) | 0.94 (0.75-1.17) | .552 | |
| Comorbidity score | ||||
| 0 | 23 (22-24) | Reference | ||
| 1 | 15 (13-17) | < .001 | 1.21 (1.15-1.27) | < .001 |
| 2 | 10 (8-11) | 1.50 (1.40-1.61) | < .001 | |
| ≥3 | 7 (6-8) | 1.85 (1.71-1.99) | < .001 | |
| Cancer type | ||||
| Multiple myeloma | 17 (17-18) | Reference | ||
| Lymphoplasmacytic lymphoma | 41 (34-49) | < .001 | 0.49 (0.44-0.54) | < .001 |
| Waldenström macroglobulinemia | 42 (38-48) | 0.54 (0.49-0.59) | < .001 | |
| Year of diagnosis | ||||
| 1994-1999 | 19 (18-20) | Reference | ||
| 2000-2005 | 19 (18-20) | .006 | 0.94 (0.90-0.98) | .003 |
| SEER site | ||||
| California | 18 (17-19) | Reference | ||
| Atlanta | 14 (13-18) | 1.11 (1.01-1.23) | .037 | |
| Connecticut | 21 (18-24) | 0.96 (0.90-1.04) | .319 | |
| Detroit | 17 (15-18) | 1.04 (0.98-1.11) | .233 | |
| Hawaii | 26 (18-31) | 0.93 (0.77-1.12) | .429 | |
| Iowa | 20 (18-22) | 0.93 (0.86-0.99) | .034 | |
| Kentucky | 15 (13-19) | < .001 | 1.01 (0.92-1.11) | .786 |
| Louisiana | 18 (14-21) | 0.95 (0.87-1.05) | .325 | |
| New Jersey | 19 (17-21) | 0.97 (0.90-1.04) | .319 | |
| New Mexico | 20 (17-25) | 0.94 (0.84-1.07) | .350 | |
| Rural Georgia | 12 (7-17) | 1.15 (0.84-1.58) | .382 | |
| Seattle | 24 (21-26) | 0.89 (0.83-0.97) | .004 | |
| Utah | 17 (13-23) | 1.03 (0.93-1.14) | .626 | |
| MGUS follow-up | ||||
| No | 19 (18-19) | Reference | ||
| Yes | 23 (20-27) | < .001 | 0.87 (0.80-0.95) | < .001 |
Abbreviations: CI = confidence interval; HR = hazard ratio; SEER = Surveillance, Epidemiology, and End Results; MGUS = monoclonal gammopathy of undetermined significance.
All statistical tests were two-sided.
Discussion
It is estimated that approximately 25,000 new cases of monoclonal gammopathy–associated malignancy will be diagnosed in 2013, and nearly half of this number of patients will die of the disease.16 Although monoclonal gammopathy–associated malignancies account for only about 1% of all cancers, the economic costs of their treatment are perhaps among the highest.17 In addition to better therapy, clinically meaningful strategies for prevention and early detection of monoclonal gammopathy–associated malignancies are urgently needed. Our study shows that approximately 6% of patients with monoclonal gammopathy–associated malignancies in the United States age 67 years or older had MGUS follow-up between 4 and 15 months prior to diagnosis (the Follow-up cohort). Even after excluding those with smoldering disease, patients with MGUS follow-up may have a third fewer major complications at cancer diagnosis and may have longer OS and DSS compared with those without follow-up. Therefore, our findings may provide support for the practice of MGUS follow-up.
The prevalence of clinically diagnosed MGUS before diagnosis of an associated malignancy is unknown because population-based data are not available. Single-institution studies have reported varying rates of 20%, 13%, and 3% in cohorts from Mayo Clinic,18 Medical University of Vienna,19 and University of Arkansas,20 respectively. Our MGUS prevalence of 6% is an underestimation because we included only patients with MGUS follow-up between 4 and 15 months before a diagnosis of an associated malignancy. Our study provides population-based estimates of major complication rates encountered at the time of malignancy diagnosis: AKI (1 in 4), cord compression (1 in 14), need for dialysis (1 in 13), pathologic/compression fracture (1 in 3), hypercalcemia (1 in 5), and any of the above (1 in 2). We show that MGUS follow-up preceding the development of active malignancy is associated with a third fewer complications at cancer diagnosis. This is important because patients with smoldering malignancy were excluded in our complications analyses to minimize surveillance bias favoring the Follow-up cohort.15 Although it is intuitive that early detection of a cancer will reduce morbidities at the time of diagnosis, this has never been shown previously in monoclonal gammopathy–associated malignancies.
Prior retrospective studies comparing the survival of patients with and without MGUS follow-up prior to diagnosis of an associated malignancy were limited by small cohort sizes,12,13,19-21 single-institution experiences,12,18-21 and lead-time bias.13,18,20 Most of the studies (predominantly in multiple myeloma) showed no difference in survival. Our cohort had the largest number of patients with MGUS follow-up prior to diagnosis of an associated malignancy reported in the literature. It was also a population-based study covering a wide geo-socio-demographic range in the United States. For patients who had smoldering malignancy at diagnosis, the survival time was calculated from the time the disease became active (initiation of treatment or development of complications) in order to minimize lead-time bias in favor of the Follow-up cohort. Nonetheless, our study showed an approximately 13% lower overall mortality in the Follow-up cohort, suggesting the need to further explore these differences. The median OS of 29 to 38 months in our study cohorts are shorter than currently expected because we included only Medicare patients, and over half of the patients were diagnosed before the era of novel agents (1994-2003).
Although treatment is not recommended for MGUS, patients who have high-risk disease may have a nearly 80% risk of progression to an associated malignancy at 15 years. However, this group comprises only about 1% of the MGUS population.22 Studies are ongoing to determine how to prevent or delay the progression of MGUS to malignancy.23 Our study finding of reduced complications at diagnosis for the entire MGUS cohort strongly supports the importance of continued work to determine whether there are additional intermediate-risk patients, and to determine the optimal follow-up for all. Our analysis did not address whether close follow-up of kidney function, calcium levels, and other potential markers of progression for MGUS by a primary care physician can provide similar outcomes at less expense than specialist care; future studies should address this question, as well as whether intermediate-risk patients who should be followed by specialists can be identified.
In our study, patients with Waldenström macroglobulinemia and lymphoplasmacytic lymphoma enjoyed longer survival and had lower cancer complications rates than those with multiple myeloma (Tables 2 and 3). The survival findings are consistent with population data even when non-Medicare patients are included.24 The difference in complication rates is also expected since 3 of the 5 complications of interest in our study (cord compression, fracture, and hypercalcemia) are uncommonly seen in Waldenström macroglobulinemia and lymphoplasmacytic lymphoma. It is notable that patients from the Seattle and Iowa SEER registries had better clinical outcomes than those from other registries. The reasons for these findings cannot be determined from our data. A recently published study showed higher rates of upfront high-dose chemotherapy and autologous stem cell transplantation in these areas, which may be a potential explanation.25
Our study has several limitations. This was not a prospective trial comparing 2 groups of MGUS patients followed long-term for development of monoclonal gammopathy–associated malignancy with random assignment to have, or not to have, MGUS follow-up—the ideal study design to determine the value of MGUS follow-up. However, we are unaware of such a study being conducted, and it is unlikely that such a study will be performed because it would require a very large MGUS population and take decades to complete. We cannot determine from our study whether MGUS follow-up tests, such as laboratory tests and radiographs, were responsible for improved outcomes in the Follow-up cohort, or if having a prior MGUS diagnosis in itself provided the benefit. The mere presence of an MGUS diagnosis in the medical history may heighten a care provider’s awareness for monoclonal gammopathy-associated malignancies when appropriate signs or symptoms arise. To control for potential MGUS follow-up selection bias, we used propensity score matching, which closely balanced the patient characteristics in the 2 cohorts. This should limit the possibility that MGUS patients are followed more carefully by their medical teams in all aspects of their health, although perhaps not eliminate it. Because our study included only Medicare patients, further investigation is needed in the younger population. However, the applicability of our findings should be broad because the median ages at diagnoses of an associated malignancy and MGUS are approximately 69 and 72 years, respectively.1,9 Incorrect diagnosis of MGUS (“rule out” diagnosis) in our cohort is extremely unlikely because we now know that if routine screening were practiced, virtually all cases of monoclonal gammopathy–associated malignancy would be preceded by MGUS.
Conclusion
Our study provides new evidence that may support the clinical significance of MGUS follow-up. Based on our results, we recommend that similar studies be performed using other large databases and in the non-Medicare population. If these findings are replicated, then determining the optimal schedule of follow-up among MGUS patients and the cost effectiveness of such a strategy will be imperative.
Supplementary Material
Supplemental Table 1. Medicare files and types of claims used in the study
Supplemental Table 2. Codes used in complication claims
Supplemental Table 3. Codes used in chemotherapy claims
Supplemental Table 4. Codes used in stem cell transplant claims
Supplemental Table 5. Codes used in radiation claims
Clinical Practice Points.
Despite absence of evidence, indefinite follow-up of MGUS patients is practiced. The assumption is that if malignant transformation occurs, the cancer will be diagnosed earlier and treated sooner.
Our study provides initial data that Medicare patients being followed for MGUS may develop less serious complications and liver longer.
Similar studies in non-Medicare patients should be performed and cost-effectiveness of MGUS follow-up determined.
Acknowledgments
This work was supported by the National Cancer Institute at the National Institutes of Health (CA152374 to R.S.G.). The funder had no involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Conflict of Interest
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354:1362–9. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 2.Landgren O, Weiss BM. Patterns of monoclonal gammopathy of undetermined significance and multiple myeloma in various ethnic/racial groups: support for genetic factors in pathogenesis. Leukemia. 2009;23:1691–7. doi: 10.1038/leu.2009.134. [DOI] [PubMed] [Google Scholar]
- 3.Go RS, Doyle LM. A monoclonal gammopathy in search of clinical significance: 57 years later. Blood. 2009;114:2355–6. doi: 10.1182/blood-2009-06-229120. [DOI] [PubMed] [Google Scholar]
- 4.Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–9. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 5.Rajkumar SV, Kyle RA, Therneau TM, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106:812–7. doi: 10.1182/blood-2005-03-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyle RA, Rajkumar SV. Monoclonal gammopathy of undetermined significance. Br J Haematol. 2006;134:573–89. doi: 10.1111/j.1365-2141.2006.06235.x. [DOI] [PubMed] [Google Scholar]
- 7.Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–7. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyle RA, Durie BG, Rajkumar SV, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121–7. doi: 10.1038/leu.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.SEER-Medicare Linked Database. National Cancer Institute Web site; [May 15, 2014]. http://healthservices.cancer.gov/seermedicare/ [Google Scholar]
- 10.Area Health Resource File (AHRF) Rockville, MD: US Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Professions; 2012-2013. [Google Scholar]
- 11.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 12.Pineda-Roman M, Bolejack V, Arzoumanian V, et al. Complete response in myeloma extends survival without, but not with history of prior monoclonal gammopathy of undetermined significance or smouldering disease. Br J Haematol. 2007;136:393–9. doi: 10.1111/j.1365-2141.2006.06441.x. [DOI] [PubMed] [Google Scholar]
- 13.Gregersen H, Sorensen HT, Engebjerg MC, Jensen P, Severinsen MT, Norgaard M. Survival of cancer patients with prior monoclonal gammopathy of undetermined significance. Eur J Intern Med. 2010;21:564–8. doi: 10.1016/j.ejim.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Chronic Conditions Data Warehouse (CCW) [May 15, 2014]; https://www.ccwdata.org/web/guest/home. Updated 2013.
- 15.Bianchi G, Kyle RA, Colby CL, et al. Impact of optimal follow-up of monoclonal gammopathy of undetermined significance on early diagnosis and prevention of myeloma-related complications. Blood. 2010;116:2019–25. doi: 10.1182/blood-2010-04-277566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 17.Cook R. Economic and clinical impact of multiple myeloma to managed care. J Manag Care Pharm. 2008;14(7 Suppl):19–25. doi: 10.18553/jmcp.2008.14.S7-A.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann H, Ackermann J, Odelga V, et al. Cytogenetic patterns in multiple myeloma after a phase of preceding MGUS. Eur J Clin Invest. 2008;38:53–60. doi: 10.1111/j.1365-2362.2007.01903.x. [DOI] [PubMed] [Google Scholar]
- 20.Patriarca F, Fanin R, Silvestri F, et al. Clinical features and outcome of multiple myeloma arising from the transformation of a monoclonal gammapathy of undetermined significance. Leuk Lymphoma. 1999;34:591–6. doi: 10.3109/10428199909058488. [DOI] [PubMed] [Google Scholar]
- 21.Kumar SK, Dingli D, Lacy MQ, et al. Outcome after autologous stem cell transplantation for multiple myeloma in patients with preceding plasma cell disorders. Br J Haematol. 2008;141:205–11. doi: 10.1111/j.1365-2141.2008.07069.x. [DOI] [PubMed] [Google Scholar]
- 22.Katzmann JA, Clark R, Kyle RA, et al. Suppression of uninvolved immunoglobulins defined by heavy/light chain pair suppression is a risk factor for progression of MGUS. Leukemia. 2013;27:208–12. doi: 10.1038/leu.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajkumar SV. Preventive strategies in monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. Am J Hematol. 2012;87:453–4. doi: 10.1002/ajh.23204. [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute Web site; [August 27, 2014]. Surveillance, Epidemiology, and End Results Program. http://seer.cancer.gov/ [Google Scholar]
- 25.Al-Hamadani M, Hashmi SK, Go RS. Use of autologous stem cell transplantation as initial therapy in multiple myeloma and the impact of socio-geo-demographic factors in the era of novel agents. Am J Hematol. 2014;89:825–30. doi: 10.1002/ajh.23753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Medicare files and types of claims used in the study
Supplemental Table 2. Codes used in complication claims
Supplemental Table 3. Codes used in chemotherapy claims
Supplemental Table 4. Codes used in stem cell transplant claims
Supplemental Table 5. Codes used in radiation claims


