Abstract
The adaptation to terrestrial life required the development of an organ capable of efficient air-blood gas exchange. To meet the metabolic load of cellular respiration, the mammalian respiratory system has evolved from a relatively simple structure, similar to the two-tube amphibian lung, to a highly complex tree-like system of branched epithelial airways connected to a vast network of gas exchanging units called alveoli. The development of such an elaborate organ in a relatively short time window is therefore an extraordinary feat and involves an intimate crosstalk between mesodermal and endodermal cell lineages. This review describes the molecular processes governing lung development with an emphasis on the current knowledge on the role of Wnt and Fgf signaling in lung epithelial differentiation.
Keywords: Fgf10, Wnt, Hippo, differentiation, epithelium, progenitor
The first morphological appearance of the mouse respiratory primordium occurs at embryonic day (E) 9.5 as two primary lung buds emerge from the ventral foregut (Fig. 1). These blind-ended hollow tubes consist of three cell layers: an inner endoderm-derived epithelial layer surrounded by loosely packed mesenchyme and a thin outer mesothelial layer (Rawlins, 2010). Reciprocal communication between these cell layers drives branching morphogenesis, which establishes the basic lobulation pattern. The lung develops asymmetrically and lobulation differs between mouse and human. In the mouse the right primary bud gives rise to four lobes (cranial, medial, caudal and accessory lobes), whereas the left bud forms only one lobe (Fig. 1). The human lung however consists of only three right lobes (superior, medial and inferior) and two left lobes (superior and inferior). Branching morphogenesis establishes the framework for future alveolar development.
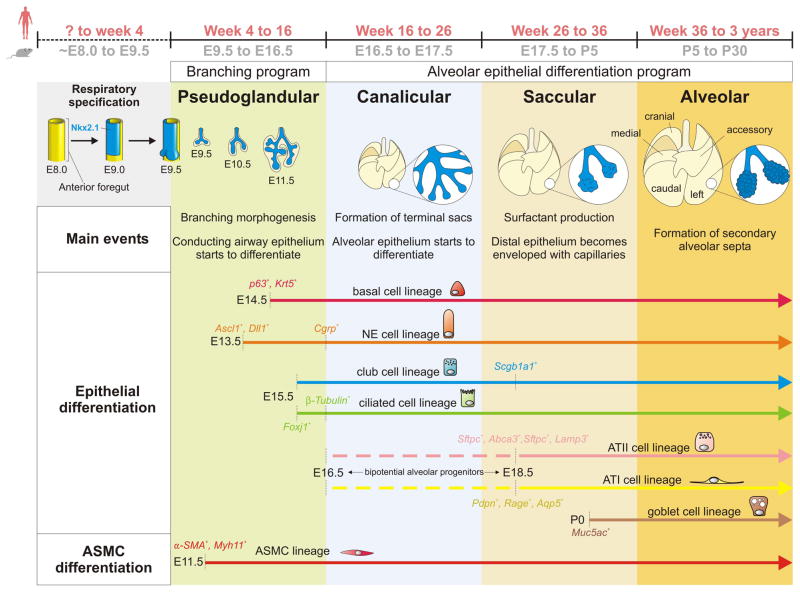
Fig. 1. An overview of lung development.
The respiratory system (trachea and lungs) originates from a population of endoderm progenitor cells located in the ventral region of the anterior foregut, which becomes specified towards respiratory fate (marked by Nkx2.1 expression, blue domain) at approximately embryonic day (E) 9 in mouse. Lung development initiates at E9.5 in mouse (~4 weeks in humans) with the outpocketing of two primary lung buds into the surrounding mesenchyme, followed by branching morphogenesis to generate the arborised respiratory tree. Historically and mostly based on histology, lung development was subdivided into four main stages: pseudoglandular, canalicular, saccular and alveolar stage. A more modern view of lung development involves two stages: the branching stage which corresponds to the pseudoglandular stage, and the alveolar differentiation stage. The conducting airway epithelium starts to differentiate during the branching stage, whereas alveolar epithelial differentiation initiates at ~E16.5 when the distal epithelium gives rise to bipotential alveolar progenitors (dashed lines). Goblet cells arise only after birth and are rare during normal homeostasis. Airway smooth muscle cell (ASMC) differentiation initiates early during the branching stage and is closely coordinated with the outgrowth of the epithelium. Note that the lung contains many more mesenchymal cell types, which are omitted from this figure and review. ATI, alveolar type I; NE, neuroendocrine.
Once the respiratory endoderm progenitors within the ventral side of the anterior foregut are specified to form the primary lung field, characterized by the expression of Nkx2.1 (NK2 homeobox 1), also called TTF-1 (thyroid transcription factor 1), two primary lung buds appear at around E9.5 in the mouse (~22 somite stage) and ~28 days in human. Lung development traditionally has been divided into four main stages: pseudoglandular, canalicular, saccular and alveolar (Fig. 1). However, a more modern view distinguishes between 2 main stages: 1) A branching stage which corresponds to the pseudoglandular stage, which starts at E9.5 and ends around E16.5, during which distal epithelial progenitors give rise to the conducting airway epithelium (Hashimoto et al., 2012; Chang et al., 2013; Rockich et al., 2013; Alanis et al., 2014) and 2) an alveolar differentiation stage which starts around E16.5 and slows down around P4 to conclude several weeks after birth, during which distal epithelial progenitors give rise to bipotential alveolar epithelial progenitors which then differentiate directly into alveolar type I (ATI) and ATII cells (Desai et al., 2014; Treutlein et al., 2014).
1. Overview of Wnt and Fgf pathways during lung development
The ability of one tissue to change the behavior of an adjacent tissue, also called induction, is a key process during organogenesis, including lung morphogenesis. Inductive interactions can be instructive or permissive. In instructive interactions the inducing cell initiates gene expression in the responding cell to specify it so that it can differentiate in a certain way. In permissive interactions however the responding cell is already specified and only needs the right environment to allow these traits to be expressed.
Endodermal-mesenchymal interactions are a recurrent theme throughout embryonic development. The concept that coordinated epithelial-mesenchymal interactions are vital to instruct lung morphogenesis has been demonstrated by a series of elegantly designed tissue transplant experiments (Shannon and Hyatt, 2004). A classic example is the study in which distal mouse lung mesenchyme is grafted on a portion of tracheal epithelium denuded from its own mesenchyme. In these recombinants, the tracheal epithelium branches in a pattern similar as the distal lung epithelium (Alescio and Cassini, 1962; Wessells, 1970). In a subsequent study, it was shown that tracheal epithelium induced by distal lung mesenchyme, expresses markers of distal lung epithelial progenitors such as Sftpc (surfactant protein C) (Shannon, 1994). These studies dramatically revealed the requirement for lung mesenchyme to initiate branching morphogenesis and direct epithelial cell fate.
A groundbreaking study by Taderera showed that epithelium isolated from an E12.5 lung disintegrated when cultured by itself on a 0.45 μm filter, but started to branch when mouse lung mesenchyme was placed on the opposite side (Taderera, 1967). This indicated that mesenchyme was able to induce the epithelium even though these two tissues were physically separated by a filter. He also observed that proper differentiation of lung mesenchyme (vasculature and smooth muscle) was dependent on the presence of epithelium, indicating that the induction of proper growth, patterning and differentiation occurs in both directions between endodermal epithelium and mesoderm-derived mesenchyme. Interestingly, epithelial induction was inhibited when it was positioned slightly to the edge of the opposing mesenchyme. Moreover, the epithelium never grew beyond the borders of the mesenchyme on the other side of the filter (Shannon and Hyatt, 2004). These pioneering observations indicated that a gradient of soluble factors have a limited range, which is most likely necessary to establish the different distal signaling centers in the developing lung. The diffusible factors necessary for this bidirectional interaction are called growth factors, and are able to induce different cellular outcomes. Several of these factors have been implicated in regulating lung morphogenesis, including fibroblast growth factors (FGFs), Wnts, bone morphogenetic proteins (BMPs) and Sonic Hedgehog (SHH).
1.1. FGF signaling in the developing lung
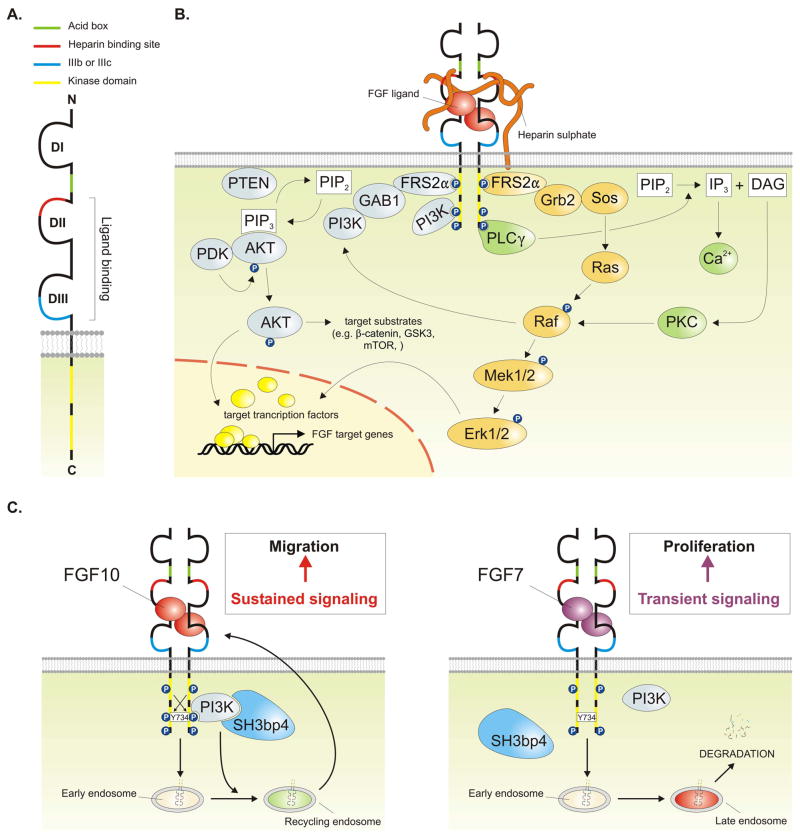
FGF ligands exert their wide array of biological effects by binding and activating the FGF receptor (FGFR) family of single pass transmembrane receptor tyrosine kinases (RTK) (Fig. 2A), which are encoded by four FGFR genes (Fgfr1-4) in vertebrates. The first half (N-terminal) of the FGFR’s extracellular D3 domain is encoded by exon 7 (also known as exon IIIa), whereas the second half is encoded by either exon 8 (also known as exon IIIb) or exon 9 (also known as exon IIIc) (Goetz and Mohammadi, 2013). Alternative splicing in the second half of the D3 domain of FGFR1-3 ultimately generates a total of seven FGFR isoforms that differ in their binding affinity towards FGF ligands (Johnson et al., 1991; Miki et al., 1992; Yayon et al., 1992; Orr-Urtreger et al., 1993; Yan et al., 1993; Chellaiah et al., 1994). Indeed, structural studies have shown that alternative splicing in the D3 domain of FGFR2 determines ligand-binding specificity by changing key residues that are important in forming hydrogen bonds with the ligand (Plotnikov et al., 2000; Yeh et al., 2003; Olsen et al., 2006). The alternative splicing event is regulated in a tissue-specific manner, with the FGFRIIIb and FGFRIIIc isoforms generally being expressed in epithelium and mesenchyme respectively (Orr-Urtreger et al., 1993). Epithelial FGFR isoforms preferentially bind FGF ligands that are expressed in mesenchymal tissue and vice versa (Zhang et al., 2006). This reciprocal tissue-specific expression of receptor isoforms and their ligands is fundamental to establish specific paracrine FGF signaling between epithelium and mesenchyme, which is crucial for orchestrating many developmental processes including lung morphogenesis. Binding of the FGF ligand in combination with heparan sulphate, which is required to stabilize the FGF/FGFR signal transduction complex (Schlessinger et al., 2000), induces receptor dimerization and transphosphorylation of specific tyrosine residues in the cytoplasmic kinase domains of the receptor. This facilitates the recruitment of several adaptor proteins, which are necessary for activating downstream FGF signaling cascades, including the MAPK (mitogen-activated protein kinase), PI3K/AKT (phosphatidylinositol-4,5-bisphosphate 3-kinase)/AKT, and the phospholipase C/Ca2+ pathways (Fig. 2B). FGF signaling is precisely regulated by negative feedback loop modulators, including members of the Sprouty (Spry) family of proteins that inhibit FGF-induced Ras-MAPK activation to varying degrees. Moreover, Spry-mediated modulation of RTK signaling is very flexible and cell- and context-dependent (Cabrita and Christofori, 2008).
Fig. 2. The FGF signaling pathway.
(A) The structure of the FGF receptor (FGFR). (B) FGFs signal through receptor tyrosine kinases, which have intrinsic protein tyrosine kinase activity in their cytosolic domains. Binding of dimeric FGFs causes receptor dimerization, which leads to a conformational shift in the receptor structure that activates the intracellular kinase domains and causes intermolecular transphosphorylation. These phosphorylated tyrosine residues then function as docking sites for adaptor proteins which themselves may be directly phosphorylated by FGFR, leading to the activation of multiple signal transduction pathways, including the PI3K-AKT pathway (blue), the Ras-Raf-Mek-Erk (MAPK) pathway (orange) and the phospholipase C/Ca2+ pathway (green). (C) Proposed model for the different epithelial responses to FGF10 (migration) versus FGF7 (proliferation) via FGFR2b. FGF10 specifically induces phosphorylation of Y734 of FGFR2b, which leads to the recruitment of PI3K and SH3bp4 resulting in receptor recycling and sustained signaling. Y734 does not get phosphorylated in response to FGF7 leading to receptor degradation and transient signaling. See main text for details.
FGF signaling is a critical component of the gene regulatory network regulating lung development, including maintenance of progenitor cell populations, epithelial and mesenchymal patterning and differentiation as well as branching morphogenesis. Several FGF ligands are expressed in the developing lung, including Fgf7, Fgf8, Fgf9, Fgf10, and Fgf18. Of these, only FGF10 is absolutely required for initial lung formation. Mice lacking Fgf10 or its main receptor Fgfr2b still develop a trachea but show complete lung agenesis (Min et al., 1998; Sekine et al., 1999; De Moerlooze et al., 2000; El Agha et al., 2012). Fgf10 is dynamically expressed in the submesothelial mesenchyme at sites where future lung buds will appear (Bellusci et al., 1997). It acts on the underlying Fgfr2b-expressing epithelium to induce bud outgrowth by eliciting a chemotactic/migration response (Bellusci et al., 1997; Park et al., 1998; Weaver et al., 2000; Hyatt et al., 2004) and keeps the distal epithelial progenitors in an undifferentiated state (Nyeng et al., 2008; Abler et al., 2009; Volckaert et al., 2013). Importantly, the Fgf10-expressing cells represent a pool of mesenchymal progenitors that give rise to airway and vascular smooth muscle cells as well as lipofibroblasts (Mailleux et al., 2005; El Agha et al., 2014).
Fgf7 expression can be detected in the developing lung starting at E14.5 throughout the lung mesenchyme surrounding lung endoderm (Mason et al., 1994; Finch et al., 1995). Inactivation of Fgf7 however does not affect lung morphogenesis (Guo et al., 1996), suggesting that other FGFs (e.g. FGF10) compensate for its loss. Epithelial-specific Fgf7 overexpression on the other hand increases lung size and epithelial proliferation and causes cystadenomatoid malformations (Tichelaar et al., 2000).
Both FGF10 and FGF7 act on the epithelium via FGFR2b, yet they elicit different cellular outcomes on isolated lung epithelial explants. Epithelial branching is enhanced in the presence of ubiquitous recombinant FGF10, whereas FGF7 induces proliferation resulting in epithelial hyperplasia and the formation of cyst-like structures (Shiratori et al., 1996; Zhou et al., 1996). This begs the question, how can FGF10 and FGF7 activate such different epithelial responses, even though they activate the same FGFR2b receptor? This conundrum has recently been elucidated by a study showing that FGF10 stimulates the phosphorylation of Y734 of FGFR2b, which facilitates the recruitment of PI3K followed by SH3 (SRC Homology 3) domain binding protein 4 (SH3bp4) (Fig. 2C). This complex, which forms upon FGF10 but not FGF7 stimulation, is the molecular key that stimulates FGFR2b internalization followed by recycling. As a result, FGF10 stimulation results in prolonged activation of the PI3K-AKT pathway, which results in increased cell migration and epithelial branching (Francavilla et al., 2013). FGF7 on the other hand promotes FGFR2b degradation, resulting in transient FGFR2b signaling and proliferation. Ectopic expression of a Y734F-mutated form of FGFR2b or Sh3bp4 knockdown in lung explants cultured in vitro alters the response to FGF10 from chemotactic (bud formation) to proliferative (cyst-like structure) (Francavilla et al., 2013). Interestingly, the PI3K-AKT pathway has been shown to directly activate β-catenin signaling during lung development as well as in the adult airway epithelium after injury (Volckaert et al., 2011; Volckaert et al., 2013) (see below). Therefore, the different epithelial outcomes in response to FGF10 versus FGF7 likely are caused by differences in downstream activation of β-catenin signaling.
In contrast to the mesenchymal localization of FGF10 and FGF7, Fgf9 is expressed in both the lung epithelium and mesothelium, the outermost layer of the lung until ~E12.5, after which it becomes restricted to the mesothelium (Colvin et al., 1999; del Moral et al., 2006). Fgf9−/− lungs show decreased mesenchymal proliferation and impaired epithelial branching resulting in perinatal death (Colvin et al., 2001; White et al., 2006). During lung development mesothelial-derived FGF9 signals to the submesothelial mesenchyme via FGFR1/2c (White et al., 2006), where it promotes proliferation in Fgf10-expressing cells and inhibits airway smooth muscle cell (ASMC) differentiation (Colvin et al., 2001; De Langhe et al., 2006; del Moral et al., 2006; Yi et al., 2009). In addition, epithelial-derived FGF9 regulates branching morphogenesis by autocrine activation of epithelial FGFRs or indirectly by regulating subepithelial mesenchymal FGFR signaling and their downstream targets (del Moral et al., 2006; White et al., 2006; Yin et al., 2008; Yin et al., 2011).
FGF8 and FGF18 are closely related ligands that are expressed in the developing lung epithelium, but the phenotype of Fgf18−/− lungs is quite different from that of Fgf8 hypomorphs. Fgf18 deficient mice have hypoplastic lungs with decreased cell proliferation and unaffected epithelial differentiation (Usui et al., 2004), whereas Fgf8 mutant lungs have a hyperplastic phenotype with distal epithelial and mesenchymal hyperproliferation, and disrupted epithelial differentiation (Yu et al., 2010). Moreover, Fgf18 overexpression during lung development results in ectopic cartilage formation (Whitsett et al., 2002).
1.2. Wnt/β-catenin and Hippo signaling in the developing lung
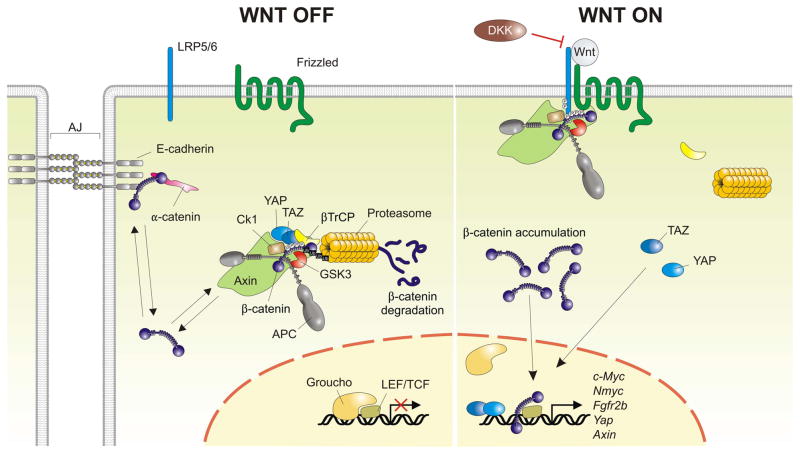
A vast and accumulating amount of data indicate that canonical Wnt signaling is a main regulator of lung development. In the absence of canonical Wnt signaling, the cytoplasmic levels of its downstream effector protein β-catenin are kept low by binding to the ‘destruction complex’, which consists of the central scaffolding protein Axin, APC (adenomatous polyposis coli), and the serine/threonine kinases GSK-3β (glycogen synthase kinase-3 beta) and CK1α (casein kinase-1 alpha) (Fig. 3). Captured β-catenin is sequentially phosphorylated on its N-terminus by CK1α (Ser45) and GSK-3β (Thr41, Ser37 and Ser33) (Liu et al., 2002). This phosphorylated motif is recognized by the ubiquitin E3 ligase β-TrCP (β-transducin repeat-containing protein), which ubiquitinates β-catenin and targets it for rapid degradation by the proteasome (Aberle et al., 1997; Kitagawa et al., 1999). Removal of β-catenin from the destruction complex through degradation by the proteasome, recycles the destruction complex for another round of β-catenin degradation (Li et al., 2012). Wnt ligands bind to a heterodimeric receptor complex, which consists of a Frizzled (Fzd) and a low density lipoprotein receptor-related protein (LRP) 5/6 protein. A hallmark of Wnt receptor activation is the phosphorylation of LRP5/6 (Tamai et al., 2004), which creates docking sites for the Axin-containing destruction complex (Mao et al., 2001; Tamai et al., 2004; Davidson et al., 2005; Zeng et al., 2005). As a consequence, the destruction complex is inhibited leading to the accumulation and nuclear translocation of β-catenin. How exactly the degradation complex is antagonized in response to Wnt signaling is still contentious (MacDonald et al., 2009). One model favors the hypothesis that Wnt signaling prevents β-catenin from binding to the destruction complex (Kim et al., 2013). However, a recent study showed that upon Wnt binding, the intact degradation complex is recruited to the activated receptor complex, where it is still able to capture and phosphorylate β-catenin but can no longer ubiquitinate it (Li et al., 2012) (Fig. 3). Therefore, dissociation of β-TrCP from the destruction complex represents a key step underlying the Wnt response (Li et al., 2012). Recruitment of β-TrCP depends on the incorporation of cytoplasmic YAP (Yes-associated protein) and TAZ (transcriptional co-activator with a PDZ-binding domain), collectively referred to as YAP/TAZ, which are also nuclear effectors of the Hippo pathway, in the destruction complex where they bind to Axin (Azzolin et al., 2014). In Wnt-stimulated cells, LRP dislodges YAP/TAZ from the destruction complex by displacing them from Axin, which renders captured β-catenin invisible to β-TrCP (Azzolin et al., 2014). The degradation complex quickly gets saturated with phosphorylated β-catenin, causing newly synthesized β-catenin to accumulate in the cytoplasm and translocate to the nucleus where it engages transcription factors mainly belonging to the LEF1/TCF (lymphoid enhancer-binding factor 1/T cell-specific transcription factor) family to activate the Wnt transcriptional program (Staal et al., 2002; Li et al., 2012).
Fig. 3. The canonical Wnt signaling pathway.
In the absence of Wnt ligands (Wnt OFF), cytosolic β-catenin that does not participate in adherens junction (AJ) stabilization is captured by the destruction complex, where it is first phosphorylated, and then ubiquitinated by recruiting β-TrCP followed by its proteasome-mediated degradation. According to the latest model, binding of Wnt ligands to their Lrp5/6-Frizzled receptor (Wnt ON) recruits the intact destruction complex to the activated receptor where it is still able to capture and phosphorylate β-catenin. Binding of Axin to activated LRP dislodges the Hippo co-transcriptonal effectors YAP/TAZ from the destruction complex, rendering β-catenin invisible to β-TrCP. As a result, β-catenin can no longer be ubiquitinated and newly synthesized β-catenin accumulates in the cytoplasm and translocates to the nucleus where it replaces the repressor Groucho and binds to LEF/TCF trancription factors to induce Wnt target genes. Note that once dislodged from the destruction complex, YAP/TAZ can translocate to the nucleus as well where they interact with TEAD transcription factors to initiate gene expression. YAP/TAZ/TEAD-mediated gene expression is therefore an integral part of the Wnt response. DKK proteins inhibit Wnt signaling by preventing the interaction of Wnt ligands with LRP5/6.
Wnt/β-catenin signaling is regulated at many levels, including by secreted inhibitory proteins that prevent Wnt ligands from binding to their receptor complex (Kawano and Kypta, 2003; Cruciat and Niehrs, 2013). The secreted Fzd-related proteins (sFRPs) and Wnt inhibitory factors (WIFs) antagonize Wnts by directly binding to them, which makes them unable to interact with Wnt receptors (Bovolenta et al., 2008). Members of the Dickkopf (DKK) family on the other hand antagonize Wnt signaling by directly binding to the ectodomains of the LRP5/6 co-receptor rendering them inaccessible to Wnt ligands by competing for the same binding site (Bao et al., 2012) (Fig. 3).
1.2.1. β-catenin mediates crosstalk between canonical Wnt signaling and cadherin-mediated cell adhesion
The β-catenin protein was originally identified as an integral structural component of cadherin-based adherens junctions (Ozawa et al., 1989) where it links the intracellular domain of cadherins to α-catenin, which in turn interacts with the actin cytoskeleton (Rimm et al., 1995; Drees et al., 2005; Yamada et al., 2005) (Fig. 3). However, the notion that this leads to a direct and stable linkage between cadherins and actin has been complicated by the finding that α-catenin binds to β-catenin and actin in a mutually exclusive manner (Yamada et al., 2005). Canonical Wnt signaling and cadherin-mediated cell adhesion compete for β-catenin. Overexpression of cadherins in Xenopus and Drosophila embryos reduces β-catenin’s availability to exert its nuclear function by sequestering it at cellular junctions and mimics phenotypes caused by inhibition of canonical Wnt signaling (Heasman et al., 1994; Sanson et al., 1996). Cadherin proteolysis can also affect Wnt signaling by stimulating the release of β-catenin, which is translocated to the nucleus to activate Wnt/β-catenin target genes. However, it seems that a compromised β-catenin degradation machinery is a prerequisite to activate β-catenin signaling upon the loss of cadherins (Kuphal and Behrens, 2006; Herzig et al., 2007; Heuberger and Birchmeier, 2010). In other words, released β-catenin upon protease-mediated loss of cadherins may function to enhance β-catenin signaling when Wnt signaling is already active. Phosphorylation events also regulate the switch between β-catenin signaling and cadherin-mediated cell adhesion. For example, carboxy-terminal phosphorylation of cadherins regulates interactions with β-catenin depending on the sites of phosphorylation (Lickert et al., 2000; Choi et al., 2006; Qi et al., 2006). In addition, phosporylation of β-catenin by the cytoplasmic tyrosine kinase Src and EGFR (epidermal growth factor receptor) on tyrosine residue Y654 reduces the ability of β-catenin to bind cadherin, which weakens cadherin-mediated adhesion and increases nuclear β-catenin (Roura et al., 1999; Rhee et al., 2007). Similarly, Y142 phosphorylation of β-catenin strengthens Wnt signaling by disrupting the interaction of β-catenin with α-catenin (Rosato et al., 1998; Piedra et al., 2003; Brembeck et al., 2004). This shows that phosphorylation-dependent release of β-catenin from the cadherin complex not only regulates cell-cell adhesion, but may also be an alternative mechanism to enhance/activate β-catenin signaling. In addition to directly phosphorylating β-catenin on Y654 and Y142 (Krejci et al., 2012), FGF signaling can also directly activate β-catenin signaling through the PI3K-AKT pathway, which acts to prevent β-catenin degradation by inhibiting GSK-3β, and by phosphorylating β-catenin on Ser552, which helps to drive β-catenin to the nucleus (Fang et al., 2007; He et al., 2007). Finally, many canonical Wnt target genes encode proteases that decrease cell-cell adhesion, such as matrix metalloproteinase (MMP) 7 (Crawford et al., 1999), and transcription factors that directly inhibit the E-cadherin gene promoter, such as Twist (Howe et al., 2003), Snail1 (Zhou et al., 2004; Bachelder et al., 2005) and Snail2 (Vallin et al., 2001; Conacci-Sorrell et al., 2003).
1.2.2. Crosstalk between Wnt and Hippo signaling
The evolutionary conserved Hippo pathway is gaining momentum as a critical regulator of organ size, tissue homeostasis and regeneration (Halder and Johnson, 2011; Zhao et al., 2011; Harvey et al., 2013; Lin et al., 2013; Yu and Guan, 2013). When active, the canonical Hippo pathway stimulates a kinase cascade consisting of the MST1/2 and LATS1/2 kinases, which ultimately phosphorylate the Hippo effector proteins YAP/TAZ, thereby promoting their cytoplasmic retention and degradation. Vice versa, inactive Hippo signaling drives YAP/TAZ to the nucleus where they interact with TEA domain (TEAD) transcription factors to regulate gene expression.
The biological responses mediated by nuclear YAP/TAZ are remarkably similar to those governed by Wnt/β-catenin, suggesting that the Hippo and Wnt signaling pathways functionally and genetically interact. One prime example of such crosstalk has already been briefly described above with the incorporation of cytoplasmic YAP/TAZ in the destruction complex, where they are essential for β-catenin as well as TAZ degradation by recruiting β-TrCP (Azzolin et al., 2012; Azzolin et al., 2014) (Fig. 3). Wnt activation leads to the dissociation of YAP/TAZ from the destruction complex, allowing them to enter the nucleus (Azzolin et al., 2014). In fact it was shown that β-catenin is only capable to translocate to the nucleus in cells which either feature nuclear YAP/TAZ or completely lack YAP/TAZ altogether (Azzolin et al., 2012; Azzolin et al., 2014). Therefore, YAP/TAZ/TEAD-mediated gene expression is an integral part of the Wnt transcriptional response. There is also evidence of Wnt/β-catenin activation as a result of nuclear YAP/TAZ in response to inactive Hippo signaling (Varelas et al., 2010; Heallen et al., 2011; Imajo et al., 2012). Indeed, driving YAP/TAZ to the nucleus diverts them away from the cytoplasmic β-catenin destruction complex suggesting that nuclear YAP/TAZ may activate β-catenin signaling perhaps even independently of Wnt ligands (Azzolin et al., 2014). In addition to the ability to inhibit β-catenin stabilization by recruiting β-TrCP to the destruction complex, cytoplasmic YAP/TAZ have also been shown to attenuate Wnt signaling by regulating CK1α-mediated phosporylation of dishevelled (Dvl) (Varelas et al., 2010; Imajo et al., 2012), a downstream mediator of both canonical and non-canonical Wnt signaling. However, the functional relevance of Dvl phosphorylation in the context of Wnt signal transduction is not well understood and there is evidence that Dvl phosphorylation occurs independently from the destruction complex (Gonzalez-Sancho et al., 2013; Azzolin et al., 2014). Interestingly, Dvl also plays a role in the nucleus where it promotes Wnt/β-catenin signaling by stabilizing the β-catenin/TCF complex (Itoh et al., 2005; Gan et al., 2008), a mechanism which is inhibited by cytoplasmic YAP by restricting the nuclear translocation of Dvl (Barry et al., 2013). Finally, Yap has also been shown to be a direct transcriptional target of β-catenin in colorectal cancer cells (Konsavage et al., 2012).
Adherens junctions form another cellular crossroad where Hippo and Wnt/β-catenin signaling meet. Similar to β-catenin, phosphorylated YAP interacts with α-catenin via 14-3-3 proteins and α-catenin deletion is sufficient to shift YAP to the nucleus and induce squamous cell carcinomas in the skin (Schlegelmilch et al., 2011; Silvis et al., 2011). Thus, the presence of these co-factors at cell-cell junctions represent an important aspect of their functional regulation and their influence on cell behavior. As such, partial epithelial-mesenchymal transition (EMT) as observed in cancer cells, which entails the loss of apicobasal cell polarity and destabilization of junctional complexes (Thiery et al., 2009; De Craene and Berx, 2013), is instrumental for the acquisition of stemness by nontransformed and tumor cells (Mani et al., 2008; Guo et al., 2012) and recent work has shown that this process is mainly mediated by inducing the nuclear translocation of TAZ (Cordenonsi et al., 2011). This suggests that destabilization of junctional complexes, which occurs during tumor progression and metastasis, may act to induce cell growth by dislodging β-catenin and YAP/TAZ from cell-cell junctions to increase Wnt and Hippo transcriptional activity.
1.2.3. Wnt/β-catenin signaling regulates respiratory specification and epithelial and mesenchymal differentiation during lung development
Numerous components of the Wnt signaling cascade are expressed in the developing lung and multiple reports have implicated several of these in the regulation of diverse aspects of lung morphogenesis. Of the nineteen Wnt genes present in the vertebrate genome, five have been reported to be expressed in the developing lung: Wnt2, Wnt2b, Wnt7b, Wnt5a, and Wnt11 (Lako et al., 1998; Li et al., 2002; Shu et al., 2002; Weidenfeld et al., 2002; De Langhe et al., 2005; Rajagopal et al., 2008; Goss et al., 2009). Wnt2 and Wnt2b expression is observed in the mesoderm surrounding the ventral anterior foregut from E9.0 to E10.5, at the time of lung specification and primary bud formation, and continue to be expressed at later stages in the developing lung mesenchyme with the highest expression distally (De Langhe et al., 2005; Goss et al., 2009). Loss of Wnt2 (Wnt2−/− null mice) results in severe lung hypoplasia, defects in ASMC development and reduced cell proliferation in both epithelial and mesenchymal compartments (Goss et al., 2009; Goss et al., 2011). Wnt2b−/− null mutants are viable without apparent abnormalities, but simultaneous ablation of both Wnt2 and Wnt2b (Wnt2−/−;Wnt2b−/−) leads to lung agenesis (Goss et al., 2009). Wnt7b is exclusively expressed in the ventral anterior foregut endoderm during the time of lung specification, and continues to be expressed by the airway epithelium throughout lung development, with the highest levels of Wnt7b located distally (Shu et al., 2002; Weidenfeld et al., 2002; De Langhe et al., 2005). Genetic inactivation of Wnt7b leads to a similar hypoplastic lung phenotype as Wnt2−/− mice, with defects in smooth muscle development (Shu et al., 2002; Rajagopal et al., 2008; Cohen et al., 2009) and the combined loss of Wnt2 and Wnt7b leads to more severe lung abnormalities, including a dramatic reduction in branching along with disrupted distal endoderm patterning (Miller et al., 2012). This suggests that Wnt2 and Wnt7b cooperate to regulate proximal-distal patterning and mesenchyme development in the lung. The non-canonical Wnt5a ligand is initially expressed at low levels in both mesenchyme and epithelium, but becomes restricted to the distal epithelium after E12.5 (Li et al., 2002; De Langhe et al., 2005). The absence of Wnt5a (Wnt5a−/−) results in increased mesenchymal proliferation, overexpansion of the distal epithelium and delayed lung maturation (Li et al., 2002). These defects could be due to increased β-catenin signaling as Wnt5a has been shown to antagonize the canonical Wnt pathway by promoting GSK-3β-independent β-catenin degradation (Topol et al., 2003). Fzd and LRP proteins are also expressed in tissue-specific patterns during lung development. The expression of Fzd8, Fzd10 and Lrp6 is restricted to the epithelium, Fzd1 and Fzd4 are expressed in the mesenchyme, whereas Fzd2, Fzd3, Fzd6, Fzd7 and Lrp5 expression can be found in both tissue compartments (De Langhe et al., 2005; Wang et al., 2005). These expression patterns suggest that Wnt signaling can be activated in a paracrine and/or autocrine manner in both epithelium and mesenchyme. The expression of endogenous Wnt modulators such as Dkk1, Dkk2, Dkk3, sFrp1, sFrp2 and sFrp4 is also detected in the developing epithelium and/or adjacent mesenchyme in specific spatio-temporal patterns (Tebar et al., 2001; De Langhe et al., 2005). Treatment of isolated foreguts with exogenous DKK1 inhibits primary lung bud formation (Chen et al., 2010). In addition treatment of E11.5 lung explants with DKK1 perturbs ASMC and vascular development, and results in severe branching defects, characterized by failed cleft formation and enlarged terminal buds (De Langhe et al., 2005; Mahoney et al., 2014), caused by decreased fibronectin deposition (De Langhe et al., 2005). In vivo Dkk1 overexpression inhibits the expression of distal epithelial markers while promoting the formation of proximal epithelium (Shu et al., 2005; Volckaert et al., 2013).
β-catenin as well as Tcf1, Lef1, Tcf3 and Tcf4 transcripts have been shown to localize in the developing endoderm and adjacent mesenchyme (Tebar et al., 2001). The vital functions of canonical (i.e. β-catenin-mediated) Wnt signaling during lung development have been uncovered by β-catenin knockout and stabilization studies. Early endoderm-specific deletion of Ctnnb1, the gene encoding β-catenin, leads to a complete failure to specify lung progenitors in the ventral foregut endoderm (Goss et al., 2009; Harris-Johnson et al., 2009) similar as observed in Wnt2/2b deficient mice (Goss et al., 2009). At later stages of lung development, epithelial-specific inactivation of Ctnnb1 using the Sftpc-rtTa;tet(O)Cre system results in respiratory failure due to severe lung abnormalities, including decreased branching and defective proximal–distal patterning in the lung, in part through the specific down-regulation of multiple critical target genes and pathways including N-myc, BMP4, and FGF signaling (Mucenski et al., 2003; Shu et al., 2005). Early endoderm-specific (Shh-Cre) stabilization of β-catenin, which lacks the region containing the GSK-3β-mediated phosphorylation sites encoded by exon 3 of Ctnnb1 (CtnnbΔ(ex3)), results in the reprogramming of esophagus and stomach endoderm to a lung endoderm progenitor fate (Goss et al., 2009; Harris-Johnson et al., 2009). The CtnnbΔ(ex3) allele has also been combined with other Cre driver lines to stabilize β-catenin in the developing epithelium at later stages of development. Nkx2.1-Cre driven β-catenin stabilization results in dilation of airways, inhibition of conducting airway epithelial cell type differentiation and the formation of polyp-like structures in the tracheobronchial epithelium (Li et al., 2009). In the adult lung, β-catenin stabilization in club cells using a Scgb1a1-Cre line perturbs epithelial cell differentiation, causes goblet cell hyperplasia, air space enlargement, and pulmonary tumors (Mucenski et al., 2005) and expands lung stem cell pools (Reynolds et al., 2008; Zhang et al., 2008). An Sftpc-Cre;CtnnbΔ(ex3) system was used to stabilize β-catenin in the presumptive bronchioles and distal tip endoderm, resulting in defective bronchiolar but not alveolar epithelial differentiation (Hashimoto et al., 2012). However, overexpression of a constitutively active β-catenin/LEF1 fusion protein using the same Sftpc-Cre driver blocks alveolar cell type-specific differentiation genes (Okubo and Hogan, 2004). In contrast to the effects of β-catenin stabilization prior to lung specification (Goss et al., 2009; Harris-Johnson et al., 2009) during which esophageal and stomach endoderm adopt a lung fate, overexpression of a constitutively active β-catenin/LEF1 fusion protein at later stages during lung development causes lung epithelial cells to acquire an intestinal fate (Okubo and Hogan, 2004), which suggests that the function of β-catenin in cell fate transition is context-dependent. An important difference between the CtnnbΔ(ex3) system and the β-catenin/LEF1 fusion protein is that the former requires endogenous co-expression of LEF/TCF factors to activate canonical Wnt signaling, whereas the latter does not. It is thus very well possible that the differential responses between these two strategies to stabilize β-catenin are due to the misexpression of the LEF1 transcription factor.
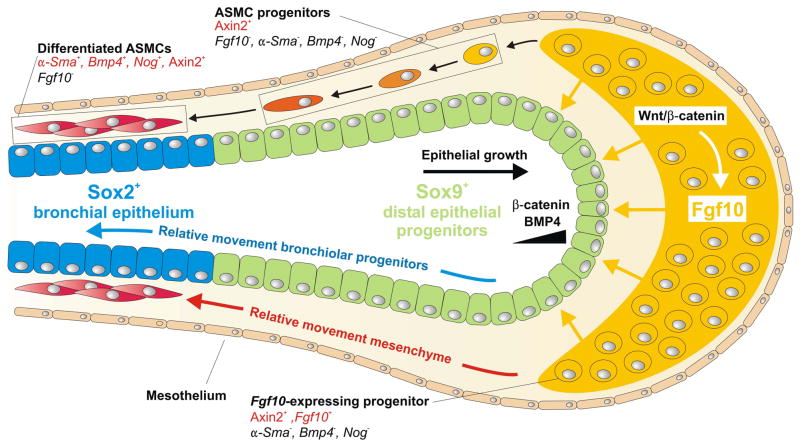
The role of canonical Wnt signaling in the developing lung mesenchyme has only recently been examined. Mesenchyme-specific β-catenin inactivation using a Dermo1-Cre line results in mesenchymal defects that are reminiscent of the Pitx1−/−;Pitx2−/− phenotype (Marcil et al., 2003), suggesting that mesenchymal β-catenin signaling relies on the PITX family of transcription factors (De Langhe et al., 2008). It was shown that mesenchymal β-catenin signaling regulates the formation and amplification of Fgf10-expressing ASMC progenitors and indirectly regulates the proper differentiation of angioblasts into mature endothelial cells (De Langhe et al., 2008). The finding that mesenchymal Wnt/β-catenin signaling is required for the expansion and development of ASMC precursors in the lung was confirmed by deleting β-catenin specifically in ASMC precursors as well as in mature ASMCs using an Sm22α-Cre line (Cohen et al., 2009). The authors further showed that this occurs through a Wnt/tenascin/platelet-derived growth factor receptor (Pdgfr) signaling axis, which is also highly upregulated in a mouse model of allergen-induced asthma as well as in human patients with pulmonary arterial hypertension (Cohen et al., 2009). More recent advanced lineage tracing approaches have further confirmed that all ASMCs are derived from distal lung mesenchyme and that mesenchymal Wnt signaling is required to instruct naive lung mesenchyme to adopt the ASMC progenitor fate (Kumar et al., 2014).
2. Role of Wnt and FGF signaling in the specification and initiation of the respiratory lineage
Soon after gastrulation (E7.5 in mice), the definitive endoderm folds to form the primitive gut tube. As the embryo develops, the actively moving gut tube endoderm becomes exposed to varying temporal and spatial mesenchyme-specific gene expression patterns. In response, the endoderm expresses transcription factors and key growth factors that act back on the mesenchyme. Indeed, a delicate endodermal-mesenchymal crosstalk, orchestrated by key signals including FGFs, Wnts, SHH and BMPs, is crucial to pattern the gut tube along the anterior-posterior (A-P) as well as ventral-dorsal (V-D) axes, and to specify the primary organ fields. The first specification step comprises the delineation of the primitive gut tube into a foregut, midgut and hindgut domain (Fig. 4A), each characterized by its own gene expression profile. At E7.5–E8.5, a A-P gradient of FGF4, BMP2 and Wnt ligands is produced by the mesoderm with low expression levels anteriorly and highest expression levels posteriorly, to promote hindgut and inhibit foregut fate, marked respectively by the expression of Cdx (caudal type homeobox) and Sox2/Hhex (hematopoietically-expressed homeobox protein) transcription factors (Zorn and Wells, 2009). Hox genes are expressed in both endoderm and mesoderm along the A-P axis and are also proposed to play an important role in foregut patterning (Ornitz and Yin, 2012), but their exact functions have not yet been elucidated. Once the delineation of the gut tube endoderm into foregut, midgut and hindgut has been established, a second specification step occurs in which these domains are further subdivided into organ-specific regions by the local endodermal-mesodermal signaling environment. Several organs originate from the foregut, including the lungs, trachea, esophagus, thyroid, stomach, liver, biliary system and pancreas (Fig. 4A). Endodermal progenitors in the anterior foregut that are destined to become the respiratory system and thyroid, express Nkx2.1 (Kimura et al., 1996), progenitors that give rise to the liver express Hex (Martinez Barbera et al., 2000), whereas prospective pancreatic and duodenal progenitors express Pdx1 (pancreatic and duodenal homeobox 1) (Offield et al., 1996). The midgut will form the small intestines and the hindgut will give rise to the colon (Fig. 4A).
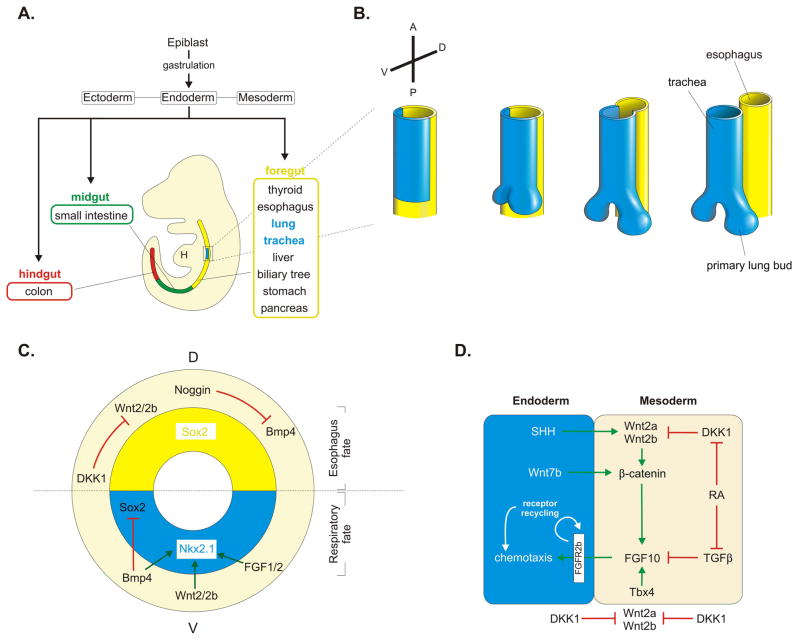
Fig. 4. Patterning of the developing endoderm and initiation of the respiratory system.
(A) An overview of endoderm formation in the developing mouse embryo. (B) Formation of the lungs and trachea from a group of Nkx2.1+ progenitor cells (blue domain) located in the ventral region of the anterior foregut. The single foregut tube anterior to the outgrowing primary lung buds eventually seperates into a ventral trachea connecting to the lung buds, and a dorsal esophagus, which is connected to the digestive system. (C) Ventral-dorsal cross-section of the developing single tube foregut showing the molecular players involved in ventral-dorsal patterning events, which instructs the dorsal endoderm to become esophagus (Sox2 domain, yellow) and the ventral endoderm to acquire respiratory fate (Nkx2.1 domain, blue). (D) Simplified model of the gene regulatory network underlying epithelial-mesenchymal crosstalk during primary lung bud outgrowth, with a special emphasis on the central role of FGF10 and its regulation.
Some of the signaling pathways that repress foregut fate initially, such as Wnt, FGF and BMP signaling, are crucial to establish respiratory fate later on (Fig. 4C). This seems like a paradox, but it demonstrates how important timing and location of these signals are for foregut endoderm patterning (Kadzik and Morrisey, 2012). It is intriguing how cells can change their response to the same cues in just a few hours. Growth factor signaling specifies the pulmonary domain in part through transcriptional activation of Nkx2.1 (Serls et al., 2005; Que et al., 2007; Goss et al., 2009; Harris-Johnson et al., 2009; Domyan et al., 2011), which is the earliest known marker of specified respiratory endoderm and is present in the prospective lung/tracheal region in the ventral foregut endoderm as early as E9.0 (Lazzaro et al., 1991; Minoo et al., 1999). However, Nkx2.1 is not exclusively expressed in the trachea and lung as it is also observed in the developing forebrain and thyroid (Lazzaro et al., 1991). At E9.5, two primary endodermal buds emerge from the ventral foregut which will form the lung. Shortly after formation of the lung primordium, the foregut just anterior of the developing lung buds starts to separate into a ventral trachea and a dorsal esophagus in a caudal to cranial direction (Fig. 4B).
Along the V-D axis the single anterior foregut is patterned by the expression of different transcription factor genes. Sox2 is initially expressed throughout the entire foregut, but is down-regulated ventrally in the prospective respiratory field at around E9.0, which is now marked by Nkx2.1 expression (Sherwood et al., 2009; Domyan and Sun, 2011) (Fig. 4C). Both transcription factors have important functions in V-D patterning of the anterior foregut, which is coupled to the specification and differentiation of the ventral trachea and dorsal esophagus. Deletion of Nkx2.1 causes ectopic Sox2 expression ventrally and results in an unseparated dorsalized foregut, specified as esophagus (Minoo et al., 1999; Que et al., 2007). Vice versa, loss of function of Sox2 expands Nkx2.1 expression dorsally and creates a ventralized foregut. This suggests that Sox2 and Nkx2.1 inhibit each other’s expression (Que et al., 2007). Whether this reciprocal regulation is direct or indirect is currently unknown. This phenotype in which the trachea and esophagus fail to seperate is frequently observed in mice deficient for growth factors implicated in anterior foregut patterning and resembles the human pathological disorder esophageal atresia with or without tracheoesophageal fistula (EA/TEF).
2.1. Mesenchymally expressed Wnt and Fgf ligands specify and establish the primary respiratory field in the anterior foregut
Dynamic morphogenic movements bring the developing anterior foregut in close proximity to the cardiac mesoderm before and during specification of hepatic and respiratory progenitors. Initially, the developing heart expresses low levels of Fgf2, which stimulates albumin (marker for liver fate) expression in the endoderm (Jung et al., 1999). Shortly after, coinciding with Nkx2.1 expression in the ventral foregut endoderm, Fgf2 expression becomes more robust and is joined by Fgf1 expression in the cardiac mesoderm (Jung et al., 1999). In vitro experiments on mouse foregut explants suggest that cardiac mesoderm-derived FGF1&2 play an important role in segregating a common pool of foregut endoderm cells into lung, liver or pancreatic fields in a dose-dependent manner, with highest FGF levels promoting Nkx2.1 expression and respiratory fate (Serls et al., 2005). In the absence of cardiac mesoderm or FGF1&2, the endoderm adopts a default pancreatic fate (Deutsch et al., 2001; Rossi et al., 2001; Serls et al., 2005). Thus, it seems that the intensity of total FGF ligand expression positively correlates with the sequential induction of liver and respiratory fate in the adjacent foregut endoderm. Studies in Xenopus confirmed that both mesoderm and FGF signaling are required to specify foregut organs, but suggest that it is the prolonged duration of FGF signaling that determines liver and lung fate, the latter of which requiring the longest duration of active FGF signaling and as a result becomes specified the latest of all foregut-derived organs (Shifley et al., 2012). This study also showed that FGF signaling acts mostly through the PI3K-AKT pathway to induce lung fate, which fits with a recent report that endoderm-specific inactivation of Mek1/2 or Erk1/2, which are crucial mediators of the MAPK arm of FGF signaling, does not affect lung specification (Boucherat et al., 2014). Fgf10 is strongly expressed in the ventral mesenchyme of the undivided foregut (Que et al., 2007). Treatment of mouse foregut explants with exogenous FGF10 results in an upregulation of Nkx2.1 and a downregulation of Sox2 expression and perturbs normal separation of the foregut tube (Que et al., 2007). However, although mice deficient for Fgf10 (Fgf10−/−) or its receptor Fgfr2b (Fgfr2b−/−) show complete lung agenesis, lung specification is not affected and foregut separation proceeds normally (Min et al., 1998). Other Fgfs expressed in the mesenchyme may compensate for the loss of Fgf10 (Serls et al., 2005).
Canonical Wnt/β-catenin signaling activity is dynamic in the early anterior foregut before and after separation into trachea and esophagus. At E9.5, Wnt/β-catenin signaling is active in the ventral foregut endoderm in response to Wnt2/2b secreted by the adjacent mesenchyme (Goss et al., 2009; Harris-Johnson et al., 2009; Jacobs et al., 2012) (Fig. 4C). Wnt2/2b−/− mutants or mice harboring an endoderm-specific (Shh-Cre) deletion of β-catenin (encoded by Ctnnb1) show complete lung agenesis and lack of Nkx2.1 expression, without affecting thyroid, liver and pancreas specification (Goss et al., 2009; Harris-Johnson et al., 2009). V-D patterning of the foregut tube is disrupted in these mutants, in which Sox2 expression is expanded into the ventral foregut endoderm at the expense of Nkx2.1 expression. This results in a failure to separate the foregut tube resulting in complete lung and trachea agenesis leaving behind a single foregut tube specified as esophagus (Goss et al., 2009; Harris-Johnson et al., 2009). Overexpression of the secreted Wnt inhibitor Dkk1, also prevents Nkx2.1 expression and abrogates formation of the primary respiratory field (Volckaert et al., 2013). Conversely, overexpression of a constitutively active β-catenin protein throughout the foregut endoderm results in ectopic Nkx2.1 expression and reprograms dorsal foregut endoderm (i.e. esophagus) and posterior foregut endoderm (i.e. anterior stomach) to a lung endoderm progenitor fate (Goss et al., 2009; Harris-Johnson et al., 2009). Altogether, these data indicate that Wnt signaling plays a crucial role during this patterning phase and is necessary and sufficient to specify the respiratory lineage. Expression of Tbx5, a member of the T-box transcription factor gene family, is first detected at E9.0 in the mesenchyme of the trachea and lung field, at a time when Nkx2.1 expression first starts to appear in the ventral foregut endoderm (Arora et al., 2012). Loss of Tbx5 leads to a unilateral loss in lung bud specification and a complete absence of tracheal specification. A severe reduction in Wnt2/2b expression is observed in these mutants, suggesting that Tbx5 lies upstream of these genes to control respiratory specification (Arora et al., 2012). How FGF and Wnt/β-catenin signaling cooperate to specify the primary lung field is not well understood. Work from our lab showed that FGF10 is not able to rescue lung specification in vivo in embryos in which Wnt signaling is inhibited by overexpressing Dkk1 (Volckaert et al., 2013). This indicates that, at the time of lung specification, FGF10 signaling cannot directly activate β-catenin in the absence of Wnt ligands, a mechanism that does occur later on during branching morphogenesis (Volckaert et al., 2013) and in the adult airway epithelium after injury (Volckaert et al., 2011). These findings were recently verified by similar experiments in the Xenopus embryo, in which activation of an inducible dominant active FGFR1 could not rescue Nkx2.1 expression and lung initiation in Wnt2/2b knockdowns (Rankin et al., 2014). Most likely, Wnt and FGF signaling are part of a feed-forward loop, similar to what has been reported in the distal lung mesenchyme (White et al., 2006; Yin et al., 2008; Yin et al., 2011), through which they control each other’s expression (see below).
BMP signaling has also been implicated in the patterning and specification of the anterior foregut endoderm (Que et al., 2006; Li et al., 2007; Li et al., 2008; Domyan et al., 2011). Between E8.5–E9.5, Bmp4 is expressed in the ventral mesenchyme of the developing foregut (Que et al., 2006; Li et al., 2008). By contrast, Noggin is expressed in the dorsal mesenchyme where it antagonizes Bmp4 expression to establish a gradient of BMP signaling in the embryonic foregut (Weaver et al., 1999; Que et al., 2006). Conditional ablation of Bmp4 by using the Foxg1-Cre line, which is active in foregut endoderm as early as E8.5 and in both endoderm and mesenchyme by E9.5, results in tracheal agenesis due to impaired epithelial and mesenchymal proliferation, without affecting initial respiratory specification (Li et al., 2008). Conditional deletion of the type I BMP receptor (BMPR) genes Bmpr1a and Bmpr1b in the foregut endoderm (Shhcre/+; Bmpr1afl/−;Bmpr1b−/− (hereafter Bmpr1a;b) results in tracheal agenesis due to a failure to maintain respiratory fate, which can not be rescued by constitutively activating Wnt signaling in foregut endoderm (Domyan et al., 2011). In these mutants Nkx2.1 expression is significantly decreased in the single foregut tube, which now acquires esophageal markers (p63, Sox2, Pax9) (Li et al., 2008; Domyan et al., 2011). Moreover, the ability of Wnt signaling to induce ectopic Nkx2.1 expression in posterior foregut endoderm (Goss et al., 2009; Harris-Johnson et al., 2009) requires intact BMP signaling (Domyan et al., 2011). The current model suggests that BMP and Wnt ligands signal cooperatively to regulate lung endoderm specification (Fig. 4C). Interestingly, inactivating Sox2 from the ventral endoderm of Bmpr1a;b mice rescues tracheal agenesis, indicating that signaling through BMPR1 and BMPR2 promotes respiratory fate through the inhibition of Sox2 (Domyan et al., 2011). Noggin-deficient mice (Nog−/−) show increased BMP signaling and about 70% of these mutants develop EA/TEF, which can be rescued by reducing Bmp4 expression by 50% (Que et al., 2006). This indicates that the dorsal foregut requires low levels of BMP signaling to acquire esophageal fate (Hines and Sun, 2014).
Shh is expressed in the ventral foregut endoderm (Litingtung et al., 1998). In Shh−/− mice the tracheoesophageal septum is not established and as a consequence trachea and esophagus do not separate from the foregut, which has a tracheal-like morphology (Litingtung et al., 1998). In mouse, the SHH signal is transduced by the three zinc-finger transcription factors Gli1,2 and 3, which are all strongly expressed in the splanchnic mesoderm during foregut development as well as in the lung mesenchyme during the pseudoglandular stage (Hui et al., 1994; Grindley et al., 1997). Gli2−/−;Gli3+/− mutants display EA/TEF similar to Shh−/− mice and ablation of both Gli2 and Gli3 genes (Gli2−/−;Gli3−/−) resulted in the complete absence of esophagus, lung and trachea (Motoyama et al., 1998). Interestingly, EA/TEF has also been observed in some patients with GLI3 mutations (Johnston et al., 2005).
Despite the progress that has been made to identify the factors responsible for respiratory specification and foregut patterning, very little is known about how these pathways interact and are integrated in a signaling network to regulate these processes. In addition, there are no markers available to distinguish between the lung and trachea lineages prior to the appearance of these structures. Surprisingly, although Nkx2.1 is the earliest known marker for the respiratory lineage, Nkx2.1−/− mice form primary lung buds, which fail to branch and lack any markers of epithelial differentiation (Minoo et al., 1999). This indicates that Nkx2.1 is not required for initial specification of the lung primordium and that additional unidentified factors underlie this process. In addition, because Nkx2.1 expression is not restricted to the respiratory lineage, this can pose a significant problem when trying to isolate a pure population of lung progenitor cells created from embryonic stem cells (ESCs) or induced pluripotent stem cells (iPS) cells. Therefore, there is a desperate need for additional early markers for respiratory progenitors.
2.2. Wnt ligands regulate mesenchymal Fgf10 expression which is required for primary lung bud formation in part by driving epithelial β-catenin signaling
The first morphological appearance of the lung primordium occurs at E9.5 (~25 somite stage) with the outpocketing of two buds from the ventral anterior foregut endoderm into the surrounding splanchnic mesoderm (Fig. 4B). Budding of the tracheal system in Drosophila is induced by branchless (an FGF homologue) expressed dynamically at prospective bud sites, which acts on breathless (an FGFR homologue) in the endoderm to initiate budding (Sutherland et al., 1996). Similarly, lung bud formation in mammals is crucially dependent on FGF signaling. Mice lacking Fgf10 (Fgf10−/−) or its receptor Fgfr2b (Fgfr2b−/−) show a wide range of morphological abnormalities including skeletal and craniofacial defects, as well as limb and lung agenesis (Min et al., 1998; Sekine et al., 1999; De Moerlooze et al., 2000). Unlike in Fgf10−/− mutants however, Fgfr2b−/− mice still form a rudimentary lung bud, which quickly undergoes apoptosis (De Moerlooze et al., 2000). This can be explained by the fact that FGF10 also binds to FGFR1b, albeit with a much lower affinity. In addition, grafting Fgf10-expressing distal mesenchyme isolated from E12 lungs on tracheal epithelium induces ectopic bud formation (Alescio and Cassini, 1962). The same effect is observed when placing a heparin-sepharose bead coated with recombinant FGF10 showing that FGF10 is sufficient to induce bud formation (Hyatt et al., 2004). The importance of mesenchymal FGF10 in initiating the lung primordium has spawned a great interest in the factors regulation its expression during this early stage of lung development.
Tbx4 is expressed in the foregut and lung mesenchyme of the developing chick and its domain overlaps with that of Fgf10. Misexpression of Tbx4 leads to ectopic Fgf10 expression and endodermal bud formation. Vice versa, misexpression of a dominant negative form of Tbx4 diminishes Fgf10 expression and prevents primary bud formation in one third of analyzed mutants (Sakiyama et al., 2003). In mouse, Tbx4 is expressed in the mesenchyme of the developing foregut at the time of lung bud formation (E9.5), but its deletion does not prevent initial specification and formation of lung and trachea primordia, although branching morphogenesis is affected later during development (Arora et al., 2012). Of note, the dominant negative Tbx4 repressor construct in the chick experiments uses the complete T-box domain, which is close to identical in Tbx4 and Tbx5. It is therefore plausible that the dominant negative Tbx4 also targets Tbx5, which has a crucial function in lung bud specification (see above).
Canonical Wnt signaling directly regulates Fgf10 expression in the developing heart and limb (Kawakami et al., 2001; Cohen et al., 2007). As described above, Wnt2 and Wnt2b are expressed in the mesoderm of the undivided anterior foregut from E9.0–E9.5, where they play a cooperative role in specifying lung endoderm progenitors, and continue to be expressed by the mesenchyme during branching morphogenesis (Yin et al., 2008; Goss et al., 2009; Yi et al., 2009). Loss of Wnt2 (Wnt2−/−) results in lung hypoplasia and a drastic downregulation in Fgf10 expression (Goss et al., 2009; Goss et al., 2011). Inhibition of Wnt signaling in foregut explants by either treating them with recombinant DKK1 or quercetin, a flavinoid known to disrupt the interaction between TCF and β-catenin in the nucleus, has a dose-dependent effect on primary lung bud formation. High doses completely prevent formation of the lung primordium and block Fgf10 expression in the presumptive lung field, whereas lower doses elicit formation of only one bud (Chen et al., 2010; Volckaert et al., 2013). On the other hand, activation of Wnt signaling in isolated foreguts leads to a significant increase in Fgf10 expression and induces ectopic bud formation (Chen et al., 2010).
Vitamin A deficiency has since long been associated with lung abnormalities, including lung agenesis, in rodents as well has humans (Wilson et al., 1953; Golzio et al., 2007; Gavrilova et al., 2009). Of note, hypovitaminosis A is the third most common nutritional deficiency in the world and is present in a large population of women of reproductive age in developing countries (West, 2002). Retinoic acid (RA), the biologically active form of vitamin A, is synthesized in the mesoderm (mesenchyme and mesothelium) of the anterior foregut around E8.5–E9.5 and analysis of embryos harboring a RARE-LacZ transgene shows widespread RA utilization in all cell layers of the foregut where the lung and trachea are forming (Malpel et al., 2000). Mice lacking the retinaldehyde dehydrogenase Raldh2 (also known as Aldh1a2), which has a prominent role in RA synthesis, show complete lung agenesis, although initial specification of lung progenitors is unaffected (Desai et al., 2006). Moreover, treatment of E8.5 embryos or isolated E8.5 foregut explants with the pan-RA receptor antagonist BMS493 also prevents lung bud formation, which was attributed to a failure to induce Fgf10 expression in the foregut mesenchyme of the prospective lung field (Desai et al., 2004). RA appears to regulate Fgf10 expression indirectly by inhibiting TGFβ (transforming growth factor beta) (Chen et al., 2007) and by allowing Wnt signaling through the local suppression of Dkk1 expression (Chen et al., 2010). Activating Wnt signaling in RA-deficient foreguts can only rescue the formation of one lung bud despite bilateral Fgf10 expression. However, simultaneously activating Wnt signaling and repressing TGFβ rescues bilateral lung bud formation in RA-deficient foreguts, suggesting that lung progenitors require different thresholds of FGF10 for proper formation of the right or left lung bud (Chen et al., 2010). As such, a model has been proposed in which endogenous RA balances Wnt and TGFβ signaling to control mesenchymal Fgf10 expression during initiation of the mouse lung primordium (Chen et al., 2010) (Fig. 4D).
3. Fgf10-KRAS/β-catenin signaling drives epithelial branching and prevents epithelial differentiation
The formation of a functional lung requires two fundamental developmental processes: branching morphogenesis, which builds the tree-like network of epithelial tubes, and epithelial differentiation to build the functional units (i.e. alveolar and conducting airway epithelium). Although both processes have been extensively studied individually, less is known about how they interact and cooperate to ensure that the functional units are correctly positioned and integrated in the branching design.
3.1. Epithelial branching generates the respiratory framework
Branching structures can be found throughout nature, from plants to river networks, from organs (e.g. lung, liver, kidney, mammary and salivary glands) to vasculature, from snowflakes to lightning bolts. Whereas branching in non-biological systems is solely controlled by the laws of physics, branching morphogenesis in organisms is driven by a combination of genetic regulatory factors and physical constraints.
3.1.1. Stereotypic branching morphogenesis sculptures the conducting airway network
The two emerging primary lung buds from the anterior foregut quickly elongate and undergo branching morphogenesis, which is accompanied by the impressive exponential growth of the lung during the pseudoglandular or branching stage. Given the elaborate branching network found in the lung, one might expect the process of branching morphogenesis to be highly complex. Yet, Metzger et al. have proposed a surprisingly simple model in which the bronchial tree is generated by a repeated use of three geometrically distinct local branching modes termed domain branching, planar bifurcation and orthogonal bifurcation, which occur in three different sequences (Metzger et al., 2008). In domain branching, daughter branches bud from the parent branch in rows (‘domains’) at different positions, much like the rows of bristles on a bottle brush. This branching mode generates the main secondary branches and establishes the lobulation pattern as well the general shape of each lobe. Both planar and orthogonal bifurcation modes on the other hand generate two daughter branches by splitting of the parent tip. Planar bifurcation mode is designated to branch splitting events when they occur in the same plane. When there is a ~90° rotation in the bifurcation plane relative to the following tip division, then the branching events are called orthogonal bifurcation (Metzger et al., 2008). Branching mode deployment varies between branch lineages, and a single parent branch can generate daughter branches through more than one mode. Most of the later generation branches are generated by planar or orthogonal bifurcation. The former is used for the thin edges of the lobes, whereas the latter forms the lobe surfaces and fills the interior.
3.1.2. FGF10 drives the branching program by inducing Sox9 expression
How is the information underlying the generation of the elaborate bronchial tree structure biologically encoded? In other words, how does the lung know where and when to form each branch during development? A major breakthrough in our understanding of the branching process came with the discovery that Fgf10 expression in the developing lung is localized to the distal submesothelial mesenchyme adjacent to future lung buds (Bellusci et al., 1997). The necessity of FGF10 for branching morphogenesis is dramatically illustrated by the complete absence of lungs in mice lacking Fgf10 (Fgf10−/−) or its primary receptor Fgfr2b (Fgfr2b−/−) (Min et al., 1998; Arman et al., 1999; Sekine et al., 1999; De Moerlooze et al., 2000). Because of the specific and dynamic Fgf10 expression pattern, it was thought for a very long time that directional lung bud outgrowth is dependent on the precisely localized expression of Fgf10. Supporting evidence for this came from pioneering explant experiments in which E12 distal Fgf10-expressing lung mesenchyme was grafted on the trachea, which normally does not undergo budding, where it was able to induce bud formation (Alescio and Cassini, 1962). This effect could be recapitulated when replacing distal mesenchyme with an FGF10-coated bead, indicating that FGF10 is sufficient to induce bud outgrowth (Shannon and Hyatt, 2004). However, isolated lung epithelium branches in MatrigelTM in the presence of ubiquitous recombinant FGF10, and Fgf10 becomes more broadly expressed throughout the mesenchyme from E13.5 onwards, when lung epithelium still continues to branch (Bellusci et al., 1997). We recently showed that ubiquitous Fgf10 overexpression on an Fgf10−/− background rescues lung formation with close to normal branching patterns (Volckaert et al., 2013). It thus seems that the effect of FGF10 on the epithelium is permissive rather that instructive. In other words, the branching information is already present in the epithelium, established by other currently unidentified molecular and physical mechanisms, and may only require FGF10 to manifest itself. These data support a model in which precisely localized Fgf10 expression in the distal mesenchyme is not necessary to direct branching morphogenesis but rather to promote the self-renewal of the distal epithelial progenitor population and to prevent their premature differentiation (Volckaert et al., 2013).
From this it is clear that although FGF10 is essential to drive branching morphogenesis, albeit in a permissive manner, other molecular and physical mechanisms must be in place to determine where, when and how many new branches are formed. For example, the PCP pathway, an arm of Wnt signaling controlling cytoskeletal organization, is gaining interest as a an important regulator of tube shape and branch point formation during early lung development. Subtle yet important branching defects have been reported in the absence of PCP components, such as scribbled, Celsr1 (cadherin EGF LAG seven-pass G-type receptor 1) or Vangl2 (Van Gogh-like-2) (Yates et al., 2010; Yates et al., 2013). Another recent study showed that Fzd2 is required for new branch point formation, branch extension as well as regulating epithelial tube dimensions. The authors demonstrated that Fzd2 mediates these processes independently of β-catenin by regulating Rho signaling and by the localization of phospho-myosin light chain 2 (Kadzik et al., 2014). Interestingly, this study further showed that Fzd2-deficient epithelium could not maintain proper branch morphology in the presence of focal FGF10 (Kadzik et al., 2014). Interactions between epithelial cells and the basement membrane (BM), which is a specialized sheet-like layer of extracellular matrix (ECM) composed primarily of laminin, collagen IV and fibronectin, are also thought to be crucial to direct branching morphogenesis. For example, fibronectin dynamically accumulates at branch bifurcations and its inhibition results in reduced branching and cleft formation (Sakai et al., 2003; De Langhe et al., 2005), suggesting that cell-ECM interactions are vital to instruct where new branches are formed. These interactions are mainly mediated by integrin receptors that translate mechanical information into biochemical signals. In light of this, loss of β1 integrin in the developing lung epithelium has been shown to block branching morphogenesis (Chen and Krasnow, 2012). The distal epithelial tips are also encapsulated by a BM, separating the distal epithelium from the mesenchyme and BM remodeling is assumed to occur to accommodate branching morphogenesis. As such, there is evidence that the BM at the distal tips becomes perforated by hundreds of microscopic holes, which locally results in a distensible mesh-like ECM network (Harunaga et al., 2014). This highly dynamic BM remodeling is established by protease and actomyosin activity (Harunaga et al., 2014). It will be of interest to unravel the mechanisms controlling the expression and localization of these proteins.
Sox9 expression is dynamic during the process of lung bud bifurcation, with the highest expression where two buds will form and lowest at the cleft (Rockich et al., 2013). Gain and loss of function experiments have attributed an important function for Sox9 in branching morphogenesis. Sox9 deletion or overexpression using a Shh-Cre driver both result in a smaller lung containing fewer airway branches with dilated cyst-like distal epithelial tips (Chang et al., 2013; Rockich et al., 2013). The observation that both gain and loss of Sox9 function result in a similar phenotype is most likely due to the limited possible outcomes when branching is perturbed. When an epithelial bud fails to bifurcate it will simply give rise to a larger cystic bud instead (Rockich et al., 2013). Interestingly, Shh-Cre;Sox9f/f lungs also have a higher level of Fgf10 expression in the distal mesenchyme (Chang et al., 2013). This indicates that in addition to the requirement of Sox9 in the epithelium, which functions downstream of epithelial FGFR2b/KRAS signaling (Chang et al., 2013), Sox9 also regulates mesenchymal Fgf10 expression in a non-cell autonomous way, most likely through the regulation of growth factor production involved in epithelial feedback mechanisms (Chang et al., 2013). Finally, Sox9 may also directly or indirectly regulate cytoskeletal organization, epithelial movement and deposition of ECM components to influence branching morphogenesis (Rockich et al., 2013). Sox2 on the other hand is expressed in non-branching epithelium and its overexpression in developing lung endoderm reduces branching (Gontan et al., 2008).
3.2. FGF10 maintains distal epithelial progenitor cells in the developing lung
Embryonic progenitor cells are at the heart of organ development. They self-renew and can give rise to multiple cell types. In the embryonic lung, a well characterized multipotent endodermal progenitor cell population resides in the distal tip of the developing lung buds. As the epithelial bud grows out, daughter cells assume more proximal positions, which allows them to differentiate into the multiple cell types that populate the airway epithelium. A delicate balance between progenitor cell self-renewal and differentiation must be maintained to allow for proper maturation of the lung epithelium. A complex and fine tuned network of signaling and transcription factors from both endoderm and mesoderm act together in a harmonious way to achieve this.
3.2.1. Multipotent Sox9+/Id2+ epithelial progenitor cells are located in the distal tip of the developing lung bud
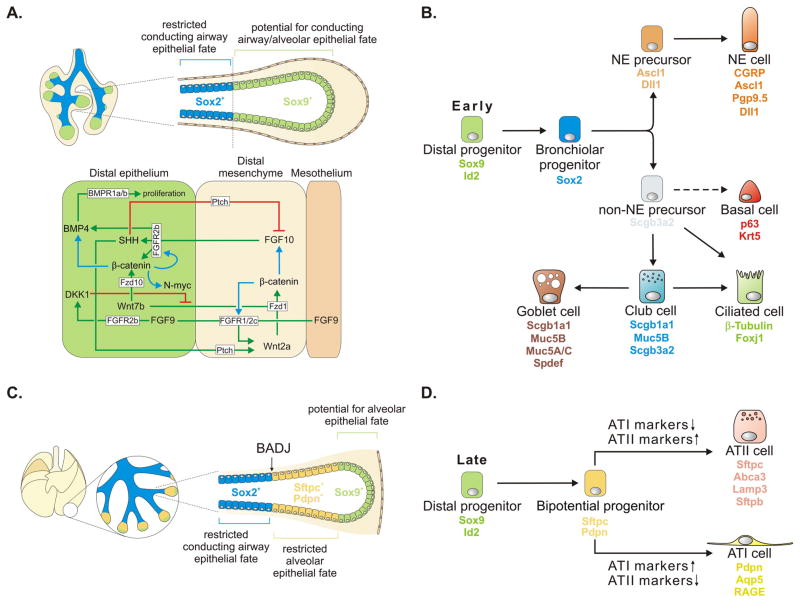
Lineage tracing experiments during branching morphogenesis of the developing pancreas have shown the presence of a distal population of multipotent epithelial progenitor cells (Zhou et al., 2007) (Fig. 5A). Similarly, in the developing lung, the distal epithelium has a high proliferation index (Okubo et al., 2005), is enriched for a wide array of transcription factors, including Sox9 (Perl et al., 2005; Chang et al., 2013; Rockich et al., 2013), Id2 (Inhibitor of DNA-binding 2) (Rawlins et al., 2009), Nmyc (Okubo et al., 2005), Etv (E26 transformation-specific (ETS) translocation variant) (Liu et al., 2003), Elf5 (E74-like factor 5) (Metzger et al., 2007), Foxp1 (forkhead box protein P1) and Foxp2 (Shu et al., 2007), and shows a high activity of the FGF, Wnt and BMP signaling pathways (Rawlins, 2008; Rawlins, 2010). The more proximal epithelial cells express Sox2, which is necessary for their differentiation into conducting airway epithelial cell types (Que et al., 2009; Tompkins et al., 2011). Lineage tracing using an Id2-CreERT2 knock-in mouse line to follow the fate of these distal epithelial progenitor cells at different stages during lung development (Rawlins et al., 2009), revealed that when labeled during the pseudoglandular or branching stage distal epithelial progenitors have the potential to give rise to all bronchiolar and alveolar epithelial cell types (Fig. 5A). However, when distal epithelial progenitors are labeled after E16.5 their fate has become restricted to the alveolar epithelial cell fate (Rawlins et al., 2009) (Fig. 5C). Interestingly, single Id2+ cells at E11.5 give rise to both bronchiolar and alveolar cell types, suggesting that the distal epithelium constitutes one homogenous population of self-renewing progenitor cells. The finding that the distal lung endoderm during early lung development is multipotent and highly plastic in its developmental potential, but becomes restricted at later stages was recently confirmed by a study utilizing the Sox9-CreER;RosamTmG lineage tracing system (Alanis et al., 2014). How this restriction in developmental fate is achieved is not known, but most likely involves temporal alterations in the expression of signaling molecules and transcription factors in both epithelial and mesenchymal compartments of the developing lung. In addition to being a marker for distal epithelial multipotent progenitors, Sox9 also functions in maintaining distal epithelial progenitors in an undifferentiated state. As such, Sox9 deletion leads to precocious conducting airway epithelial differentiation (Chang et al., 2013; Rockich et al., 2013), whereas Sox9 overexpression prevents the differentiation of distal epithelial progenitors (Rockich et al., 2013).
Fig. 5. Patterning and differentiation of the developing lung epithelium.
(A) (Upper part) Patterning of the developing lung epithelium during the branching stage (E9.5–E16.5). Shown is a schematic representiation of an E11.5 mouse lung. The distal tip of the developing lung buds contain a pool of multipotent Sox9+/Id2+ epithelial progenitor cells (green), which give rise to Sox2+ conducting airway epithelium (blue), but also have the potential to generate alveolar epithelium. (Lower part) Overview of the molecular players involved in the epithelial-mesenchymal crosstalk, which establishes and maintains a distal tip organizing center. FGF10 and Wnt signaling play a central role in this communication. Red arrows, negative regulation; green arrows, positive regulation; blue arrows, transcriptional activation. (B) Model showing the lineage relationships between epithelial progenitor cells and their differentiated progeny during early lung development. Note that basal cells can only be found in the cartilaginous (extrapulmonary) airways in the mouse. Basal cells most likely arise from Sox2+ progenitors but their origin has not been definitively established by lineage tracing experiments. The differentiated conducting airway epithelial cell types retain Sox2 expression throughout development and adult life (not shown). (C) Starting at around E16.5, the Sox9+ distal epithelial progenitor cells no longer give rise conducting airway epithelium, but instead become restricted to alveolar epithelial fate. This results in a stretch of Sox2−/Sox9− bipotential epithelial cells, expressing both ATI (Pdpn) and ATII (Sftpc) markers, located between the Sox2-expressing conducting airways and the leading edge of Sox9+ progenitor cells. At this time, the bronchioalveolar duct junction (BADJ) is being established. (D) Model showing the lineage relationships between distal epithelial progenitors and their progeny during alveolar epithelial differentiation. ATI, alveolar type I; ATII, alveolar type II. See main text for details.
3.2.2. FGF10-KRAS/β-catenin signaling maintains the distal epithelial progenitors by inducing Sox9 and suppressing Sox2 expression
The exact molecular mechanisms underlying the formation, maintenance and differentiation of the distal Sox9+/Id2+ progenitor cells are not well understood, but seem to involve a delicate epithelial-mesenchymal crosstalk. FGF, Wnt and BMP signaling are highly active in the distal epithelium and perturbing these pathways affects the balance between progenitor pool expansion through proliferation and reduction through differentiation. As such, a well maintained distal organizing center is essential for determining the ultimate size and morphology of the lung (Morrisey and Hogan, 2010) (Fig. 5A).
The effects of distal mesenchyme-derived FGF10 on the distal epithelium are mainly mediated by activating KRAS, directly activating β-catenin signaling (Volckaert et al., 2013), and inducing Bmp4 expression (Hyatt et al., 2004) (Fig. 5A). Fgf10 hypomorphs show a decrease in epithelial proliferation during the pseudoglandular stage, which is associated with reduced epithelial β-catenin signaling and decreased levels of p-Erk in the distal epithelium (Ramasamy et al., 2007). Moreover, conditional inactivation of FGF10/FGFR2b signaling after lung initiation inhibits distal fate as shown by the expansion of proximal Sox2-expressing epithelial cells at the expense of Sox9+ progenitor cells (Abler et al., 2009; Chang et al., 2013). Vice versa, overexpression of Fgf10 results in a distalized lung where the distal epithelial progenitors are arrested in an undifferentiated state which prevents them from differentiating into more proximal cell types (Nyeng et al., 2008; Volckaert et al., 2013). In addition, overexpressing a hyperactive Kras, a small GTPase downstream of RTK signaling (e.g. FGF) during lung development (Johnson et al., 1997; Tang et al., 2011), throughout the epithelium (Chang et al., 2013) or in Sox9-expressing epithelial progenitors (Alanis et al., 2014) results in overgrown branches with an expanded Sox9 expression domain. The transcription factors Etv4/5 and Elf5 are enriched in the distal endodermal buds and mediate some of the effects of epithelial FGF signaling (Liu et al., 2003; Metzger et al., 2007). Altogether, these data indicate that FGF10 signaling is a main regulator of the Sox9+ multipotent progenitor population in the distal endoderm by keeping them undifferentiated and stimulating their self-renewal.
Following its pivotal function in specifying lung endoderm and initial bud formation, β-catening signaling continues to be active in the developing epithelium downstream of FGF10 (Volckaert et al., 2013) with highest activity in the distal epithelial progenitors (De Langhe et al., 2005). Endogenous epithelial Wnt/β-catenin signaling promotes distal fate and inhibits differentiation (Mucenski et al., 2003; Shu et al., 2005), which is mediated primarily through its regulation of Fgfr2, Bmp4 and Nmyc expression in the distal epithelium (Shu et al., 2005). In line with this, epithelial-specific deletion of Nmyc also shifts the balance between progenitor cell proliferation versus differentiation towards the latter, whereas Nmyc overexpression in the epithelium expands the Sox9+ distal progenitor population and inhibits epithelial differentiation (Okubo et al., 2005). In addition, early deletion of Ctnnb1 in the Sox9+ distal epithelium (Sox9-CreER) results in a loss of Sox9 expression (Alanis et al., 2014), whereas ectopic activation of β-catenin signaling in the developing distal epithelium (Sftpc-Cre;Ctnnb1(ex3)flox) prevents airway epithelial differentiation by inhibiting Sox2 expression (Hashimoto et al., 2012). Bmp4 is a downstream target gene of both FGF10 (Hyatt et al., 2004) and β-catenin (Shu et al., 2005) signaling during lung development and has also been implicated in proximal-distal patterning of the developing lung (Bellusci et al., 1996; Weaver et al., 1999; Eblaghie et al., 2006; Wang et al., 2013). Inhibiting BMP signaling by deleting Bmpr1a (Eblaghie et al., 2006; Sun et al., 2008) or by overexpressing a dominant-negative (dn)Bmpr1b (Weaver et al., 1999), or the secreted antagonists Xnoggin (Weaver et al., 1999) or Gremlin (Lu et al., 2001) results in the expansion of Sox2 expression, while inhibiting distal fate. On the other hand, treatment of lung explants with exogenous BMP4 suppresses Sox2 expression and reduces the number of Sox2+ endodermal progenitors, while expanding Sox9+ progenitors (Wang et al., 2013). There is also evidence that Bmp4 is a direct target of the histone deacetylases 1 and 2 (Hdac1/2), which act to suppress Bmp4 expression in the proximal endoderm to allow for proper epithelial differentiation. Deletion of Hdac1/2 in the developing endoderm results in an expansion of Bmp4 expression throughout the airway epithelium instead of being confined to the distal tips, which results in a downregulation of Sox2 expression and an expansion of Sox9+ progenitors (Wang et al., 2013). However, Shh-Cre driven epithelial deletion of Bmp4 results in apparently normal Sox9/Sox2 domains (Alanis et al., 2014), suggesting the involvement of additional compensatory BMP ligands.
During early lung development, the Hippo effector YAP is localized in the nucleus of Sox9+ undifferentiated distal epithelial progenitors, but is cytoplasmic in proximal Sox2+ conducting airway epithelium (Mahoney et al., 2014), suggesting a role for nuclear YAP in maintaining distal epithelial progenitor status. Surprisingly, conditional epithelial inactivation of Yap during early lung development (Shh-Cre;Yapf/f) results in an increase in Sox9+ distal epithelial progenitors and a lack of differentiation of their progeny into Sox2+ conducting airway epithelial cells (Mahoney et al., 2014). The authors concluded that nuclear YAP cooperates with mesenchyme-derived TGF-β at the Sox2/Sox9 transition zone to promote Sox2 expression, but did not consider the possibility that cytoplasmic YAP as mentioned above could play an active role in repressing Sox9 expression by blocking β-catenin signaling (Hashimoto et al., 2012; Volckaert et al., 2013; Azzolin et al., 2014).
Finally, Notch is dynamically activated in the tips of lung epithelial buds (Post et al., 2000; Kong et al., 2004; Tsao et al., 2008) and pharmacological inhibition of Notch signaling in foregut or lung explants cultures results in an expansion of the distal epithelial progenitor pool (Tsao et al., 2008). Moreover, transgenic mice overexpressing a dominant active Notch3 in the distal lung epithelium show impaired differentiation of distal progenitor cells, which remain immature (Dang et al., 2003).
3.2.3. FGF10 blocks the differentiation of Sox2+ conducting airway epithelial cells along the ciliated cell lineage and promotes their differentiation along the basal cell lineage
The conducting airways of the adult mammalian respiratory system are home to an impressive repertoire of specialized epithelial cell types, including secretory cells (club and goblet cells), ciliated cells, basal cells and neuroendocrine (NE) cells. Knowledge on how these different epithelial cell types differentiate from the Sox9+ multipotent progenitor cells located in the distal tips of outgrowing buds is of major importance to understand many lung developmental defects, adult lung disorders as well as to be able to guide differentiation of ESCs or iPS cells for pharmacological studies and future cell replacement therapies.
As the lung epithelium grows out during the pseudoglandular or branching stage, the progeny of the multipotent distal epithelial progenitor cells assume more proximal positions along the outgrowing lung buds, become more restricted in their developmental potential and start to differentiate into the different cell types of the future bronchi and bronchioles including club, ciliated and NE cells. As these daughter cells exit the distal signaling center they downregulate the expression of Sox9, a charactistic marker of the distal epithelial progenitors, and start to express Sox2, which has been shown to be necessary and sufficient for conducting airway cell maturation (Gontan et al., 2008; Que et al., 2009; Tompkins et al., 2009; Tompkins et al., 2011). The ontogeny of basal cells, which in mice housed in a pathogen-free environment can only be found in the extrapulmonary airways, is more obscure. Although there is evidence that Sox9+ epithelial progenitors can give rise to basal cells under conditions of Fgf10 overexpression (Volckaert et al., 2013), it is likely that endogenous tracheal basal cells arise from an alternate source.
Basal cells, express keratin 5 (Krt5) and transformation-related protein 63 (Trp63 (or p63)) and in mice are enriched to the cartilagenous airways of the trachea and main stem bronchi, whereas in the human lung they can be found more distally. Basal cells are epithelial stem cells capable of long-term maintenance of the airway epithelium during normal homeostasis and contribute to epithelial regeneration after injury (for recent reviews see (Hogan et al., 2014; Kotton and Morrisey, 2014; Volckaert and De Langhe, 2014)). The transcription factor p63 is highly expressed in basal cells of stratified and pseudostratified epithelia throughout the body, including the skin epidermis, esophageal and tracheobronchial epithelium. The importance of p63 in basal stem cell specification and/or maintenance is highlighted by the complete absence of basal cells in p63 null mice in these epithelia, which form simple columnar epithelial structures instead (Mills et al., 1999; Yang et al., 1999; Daniely et al., 2004). This was confirmed by a recent study showing that conditionally ablation of p63 specifically in the adult basal cell population results in a simplification of the tracheal epithelium from a pseudostratified to simple columnar epithelium devoid of basal cells (Zhao et al., 2014), suggesting a critical role for p63 in basal stem cell maintenance. p63 has two isoforms that contain (TA) or lack (ΔN) a transactivator domain (Yang et al., 1998). ΔNp63 is the predominant isoform expressed in basal stem cells (Zhao et al., 2014) and its overexpression promotes epithelial stratification in the mouse lung (Romano et al., 2009), whereas the TAp63 isoform is dispensable for basal stem cell maintenance (Zhao et al., 2014). A functional and biochemical interaction between p63 and the Hippo transcriptional co-activator YAP to maintain adult airway basal stem cells (Zhao et al., 2014) was recently described.
Very little is known about the molecular processes underlying basal cell formation as well as the progenitor cells from which they are derived. Inhibition of Wnt signaling through overexpression of Dkk1 promotes basal cell differentiation along the lower conducting airways (Volckaert et al., 2013), likely by inhibiting Sox9 expression and boosting Sox2 expression which is known to regulate p63 expression (Hashimoto et al., 2012; Ochieng et al., 2014; Watanabe et al., 2014). This is in line with a recent report showing that β-catenin suppresses Sox2 expression (Hashimoto et al., 2012).
Furthermore, Fgf10 which besides the distal mesenchyme is also expressed in the trachea in between cartilage rings has been shown to be involved in basal cell differentiation and maintenance in the cartilagenous airways were basal cells normally reside. Fgf10−/− tracheas have fewer basal cells and basal cells are dramatically reduced in tracheas from mice overexpressing a dominant negative secreted receptor for FGF10 from E15.5 until E18.5. In addition, overexpression of Fgf10 directs the fate of Sox2+ lower conducting airway epithelial cells along the basal cell lineage while blocking differention along the ciliated cell lineage, suggesting that one reason why basal cells can only be found in the cartilagenous airways in wild-type mice during normal homeostasis is the presence of Fgf10 expression in the mesenchyme between the cartilaginous rings (Tiozzo et al., 2009; Sala et al., 2011), but not in the ASMCs that envelop the bronchi (Mailleux et al., 2005). Altogether these results indicate that FGF10 and Wnt signaling are important for basal cell development and/or maintenance (Volckaert et al., 2013). How exactly these pathways function and interact to initiate and/or maintain the basal cell program is still unknown.
The current model for conducting airway epithelial differentiation is that the first lineage decision among Sox2+ bronchiolar progenitors happens at around E13.5 when a distinction can be made between progenitor cells destined to become NE cells, marked by achaete-scute homolog 1 (Ascl1) (also called Mash1), and progenitors with non-NE fate, expressing Scgb3a2 (Fig. 5B). Shortly after, the non-NE cell population undergoes a second fate decision to commit to the ciliated cell (Foxj1+/β-tubulin+) or club cell, marked by the Scgb1a1 (secretoglobin family 1A member 1) (also called Clara cell-specific 10 kD protein (CC10) or Clara cell secretory protein (CCSP)) lineages. Ample evidence suggests that Notch signaling is involved in many lung epithelial cell fate decisions (Borges et al., 1997; Ito et al., 2000; Post et al., 2000; Shan et al., 2007; Guseh et al., 2009; Tsao et al., 2009; Morimoto et al., 2010; Guha et al., 2012; Morimoto et al., 2012; Zhang et al., 2013). However, a detailed description of the role of Notch signaling in conducting airway epithelial cell differentiation is beyond the scope of this current review.
3.2.4. The FGF10-KRAS-SOX9 driven branching program antagonizes the alveolar differentiation program
During the branching stage Sox9 positive distal epithelial progenitors give rise to the arborized network of Sox2+ conducting airway epithelium. However, starting around E15.5/E16.5 the multipotent Id2+/Sox9+ distal eptithelial progenitors no longer contribute to bronchial lineages but start to adopt a bipotential alveolar epithelial progenitor fate (Rawlins et al., 2009; Chang et al., 2013; Rockich et al., 2013; Volckaert et al., 2013; Alanis et al., 2014; Desai et al., 2014; Treutlein et al., 2014). These bipotential alveolar progenitors, expressing a subset of both ATI and ATII cell markers, will eventually differentiate into fully mature alveolar epithelial cells by sequentially turning off inappropriate cell markers and turning on cell type-specific late markers (Desai et al., 2014; Treutlein et al., 2014) (Fig. 5C, D). Mature alveoli are lined by two main epithelial cell types. Squamous ATI cells make up about 90% of the surface area and are located immediately adjacent to the capillaries, which allows for efficient oxygen and CO2 diffusion, whereas cuboidal ATII cells secrete surfactant to prevent alveolar collapse and have shown to be a bona fide long-term self-renewing stem cell population of the adult lung (Barkauskas et al., 2013; Desai et al., 2014). The sequential maturation of bipotential progenitors into ATI and ATII cells was recently mapped using a single-cell genomics approach (Treutlein et al., 2014). Similar to the differentiation of the bronchial epithelium, maturation of the alveolar epithelium occurs in a proximal to distal direction (Desai et al., 2014).
The FGF10-KRAS-SOX9 driven branching program antagonizes the alveolar differentiation program (Chang et al., 2013; Rockich et al., 2013; Volckaert et al., 2013; Alanis et al., 2014). Inactivation of Sox9 in the early lung epithelium induced precocious Sftpb expression in the distal lung buds as early as E11.5, indicating that Sox9, in addition to its pivotal role in branching morphogenesis and in defining the early distal lung epithelial progenitor program, suppresses premature activation of alveolar epithelial genes (Chang et al., 2013). However, other alveolar genes including Rage (receptor for advanced glycation end products) and Aqp1 (Aquaporin-1) are not precociously expressed in the absence of Sox9 (Chang et al., 2013), suggesting that other regulators are required for the full activation of the alveolar differentiation program. Conversely, overexpression of Fgf10 (Volckaert et al., 2013), a hyperactive Kras allele (Chang et al., 2013) or Sox9 (Rockich et al., 2013) from E15.5 onwards maintains the branching program and blocks the alveolar epithelial differentiation program. Interestingly, a nearly identical lung phenotype and block in alveolar epithelial differentiation was recently reported for Nkx2.1-Cre;Mst1/2f/f mice in which the Hippo kinases Mst1/2 are genetically inactivated from the respiratory epithelium during late lung development (Chung et al., 2013). In addition, knockout mice for Taz, one of the nuclear effectors of the Hippo pathway expressed in the distal epithelial progenitors during lung development (Park et al., 2004), display defects in alveolarization (Makita et al., 2008) that resemble those seen in human emphysema and bronchopulmonary dysplasia (BPD). Together, these findings suggest that nuclear YAP/TAZ prevent the differentiation of distal epithelial progenitors and that their cytoplasmic sequestration is required for alveolar epithelial differentiation to occur.
Temporal glucocorticoid signaling starting at E17.0 terminates the progression of the Sox2 domain and initiates the alveolar differentiation program in the epithelial progeny left behind by the progressing Sox9+ distal progenitors during late lung development (Alanis et al., 2014). Importantly, this model also provides insights into the formation of the bronchioalveolar duct junction (BADJ), the compartment boundary separating the conducting airway epithelium from alveolar epithelium.
4. Mesenchymal Wnt signaling regulates the amplification of Fgf10 expressing airway smooth muscle cell progenitors in the distal mesenchyme
The developing lung mesenchyme gives rise to a heterogeneous population of specialized cell types that play important roles during lung development and homeostasis in the adult, including airway and vascular smooth muscle cells, pericytes, endothelial cells, myofibroblasts and interstitial fibroblasts. Development of mesenchymal lineages closely follows that of the endoderm temporally and spatially through a complex network of signaling pathways involving the endoderm, mesenchyme and mesothelium. As such, paracrine signaling originating from the mesenchyme drives epithelial growth and differentiation as well as branching morphogenesis. Vice versa, epithelial and mesothelial-derived signals act on the mesenchyme to control its proliferation and differentiation. Here we will focus on the development of the ASMC lineage because of its important function as a niche for adult conducting airway epithelial stem cells (Hines et al., 2013; Volckaert et al., 2013). In the adult upper airways, smooth muscle can be found on the dorsal side of the trachea and the medial (inner) sides of the main bronchi opposite to the C-shaped cartilage rings. In the intrapulmonary mouse airways, the epithelium is devoid of cartilage and is completely enveloped by smooth muscle cells. ASMCs control the diameter of the airways by providing elasticity and constitute an epithelial stem cell niche. Defects in ASMC development can lead to chronic lung diseases including asthma.
4.1. Fgf10 expressing cells in the distal lung mesenchyme are airway smooth muscle cell progenitors
Lineage tracing experiments using an Fgf10LacZ transgenic, in which the LacZ gene is expressed by the Fgf10 promoter, or an Fgf10CreERT2 knock-in mouse, carrying a Cre-ERT2-IRES-YFP cassette under control of the Fgf10 promoter, have demonstrated that the Fgf10-expressing cells located in the submesothelial mesenchyme of the distal tips are progenitors for ASMCs during early lung development (Mailleux et al., 2005; El Agha et al., 2012; El Agha et al., 2014) (Fig. 6). Recent advanced lineage tracing approaches have further confirmed that all ASMCs are indeed derived from a pool of mesenchymal progenitors residing in the distal lung mesenchyme (Kumar et al., 2014). During the early branching stage these Fgf10-expressing mesenchymal progenitors also contribute to the formation of vascular smooth muscle cells and lipofibroblasts, whereas a second wave of Fgf10-expressing cells during the alveolar differentiation stage gives rise to mainly (lipo)fibroblasts (El Agha et al., 2014). Another population of smooth muscle cell progenitors has been identified near the lung hilum (Shan et al., 2008), where trachea and primary bronchi meet, and in the Wt1+ mesothelium (Dixit et al., 2013). Finally, a population of Wnt2+/Gli1+/Isl1+ multipotent cardiopulmonary mesoderm progenitors was recently identified that generates the majority of mesoderm-derived lineages in the lung and heart, including ASMCs, and coordinates heart and lung co-development (Peng et al., 2013).
Fig. 6. Model for the coordinated differentiation of conducting airway epithelium and airway smooth muscle.
A subset of Fgf10-expressing cells located in the distal submesothelial mesenchyme are progenitors for airway smooth muscle cells (ASMCs) and their amplification, as well as Fgf10 expression is dependent on mesenchymal Wnt/β-catenin signaling. FGF10 directly activates β-catenin in the distal epithelial progenitors, and induces Bmp4 and Sox9 expression, to maintain them and keep them from differentiating into Sox2+ conducting airway epithelium. As the epithelial tube grows towards the distal source of FGF10, progeny from the distal multipotent epithelial progenitors are left behind in the epithelial stalk and once they are out of FGF10’s reach they lose Sox9 expression, start expressing Sox2 and differentiate into conducting airway epithelium. At the same time, as ASMC progenitors passively acquire more proximal positions, they come in contact with epithelial-derived BMP4 and SHH causing them to stop expressing Fgf10 and ultimately differentiate into mature ASMCs that envelop the developing conducting airway epithelium.
4.2. FGF and Wnt signaling form the central regulatory axis in ASMC development
Development of the lung mesenchyme crucially depends on signals emanating from both epithelium and mesothelium. Histologically and molecularly, the lung mesenchyme can be subdivided into submesothelial and subepithelial domains (White et al., 2006). As discussed above, FGF9 is secreted by both the epithelium and mesothelium (Colvin et al., 1999; del Moral et al., 2006), and signals primarily via mesenchymal receptors FGFR1c/2c, although it also has the ability to signal to the epithelium in an autocrine manner (del Moral et al., 2006; White et al., 2006; Yin et al., 2008). Inactivation of Fgf9 throughout the mesothelium using a Dermo1-Cre driver dramatically reduces mesenchymal proliferation but has little effect on epithelial branching, whereas epithelial-derived FGF9 regulates branching morphogenesis in the absence of a mesenchymal phenotype (Yin et al., 2011). Mesenchymal FGF signaling is required for Wnt2a expression, which stabilizes mesenchymal β-catenin in an autocrine manner (Yin et al., 2008; Yi et al., 2009; Yin et al., 2011). Mesenchymal Wnt/β-catenin signaling in its turn is required to sustain the expression of FGFR1c/2c and responsiveness to FGF9 (De Langhe et al., 2008; Yin et al., 2008). Thus, mesenchymal FGF and Wnt/β-catenin signaling positively reinforce each other in a feed-forward signaling loop to regulate mesenchymal proliferation (Fig. 5A). Wnt7b, expressed by airway epithelium, also activates mesenchymal β-catenin signaling to regulate smooth muscle progenitors via a Wnt/tenascin/Pdgfr pathway (Shu et al., 2002; Rajagopal et al., 2008; Cohen et al., 2009).
Mesenchymal β-catenin signaling specifies the distal lung mesenchyme to adopt the ASMC progenitor fate (Kumar et al., 2014). However, mesenchymal FGF and β-catenin signaling signaling together regulate the amplification of Fgf10 expressing ASMC progenitors in the distal lung mesenchyme and inhibit their premature differentiation into the airway smooth muscle cell lineage (De Langhe et al., 2006; del Moral et al., 2006; De Langhe et al., 2008; Yi et al., 2009). Recently, the microRNA miR142-3p, was shown to be highly expressed in the developing lung mesenchyme where it promotes the amplifcation and prevents the premature differentiation of ASMC progenitors by downregulating mRNA levels of Apc a negative regulator of β-catenin (Carraro et al., 2014). Treatment of lung explants with DKK1 an inhibitor of Wnt signaling (De Langhe et al., 2005) or overexpression of Dkk1 during lung development also has been shown to impair ASMC development (Volckaert et al., 2013). However, at later stages Wnt signaling, which is active in developing ASMC progenitors (Shu et al., 2005), also stimulates ASMC differentiation in part by up-regulating the expression of Pdgfrs (Cohen et al., 2009; Goss et al., 2011).
Differentiation of the Fgf10 expressing ASMC progenitors is further driven by BMP4 secreted by the distal epithelium in response to FGF10 signaling (Lebeche et al., 1999; Weaver et al., 2000; Mailleux et al., 2005). The role of BMP4 signaling in ASMC differentiation is also substantiated by the expression of its downstream effector Smad1 in differentiated ASMCs during lung development (Chen et al., 2005). Paradoxically, the BMP antagonist Noggin is also expressed in ASMCs from E11.5 onwards (Weaver et al., 2003), suggesting that BMP signaling requires a tight control to regulate ASMC differentiation. Mesenchymal SHH signaling, which negatively regulates Fgf10 expression, is also important for the formation of airway and tracheal smooth muscle (Pepicelli et al., 1998; Li et al., 2004; Miller et al., 2004).
In the proximal cartilaginous airways, airway smooth muscle and cartilage are localized to complementary domains surrounding the epithelium. Smooth muscle cells are found at the dorsal side of the trachea and along the medial main bronchi, and develop in juxtaposition with the ventral/lateral cartilage cells. This spatial and temporal juxtaposition of cartilage and smooth muscle is required for the development of both tissues (Hines et al., 2013). Disrupting the formation of smooth muscle or cartilage results in a compensatory increase in the cell numbers of the remaining lineage, and in the main bronchi this corresponds with a circumferential expansion of the remaining cartilage or smooth muscle domain respectively (Hines et al., 2013). The molecular interactions underlying this communication between cartilage and smooth muscle cells remain unknown.
Conclusion
Much is to be gained from the investment in basic lung developmental research. Pathways that govern the differentiation of epithelial progenitors during development are often re-activated upon injury and regeneration. Therefore, a better understanding of how the different molecular pathway are integrated into gene regulatory networks to regulate progenitor cell populations during lung development will undoubtedly provide essential information about how the lung regenerates itself. However, the recapitulation of developmental pathways can become a curse when activated chronically or aberrantly, such as during tumorigenesis and in chronic lung diseases. While our understanding of lung development and its impact on lung regeneration has grown dramatically in recent years, more information is still needed regarding the capacity of lung epithelial progenitors to generate the various epithelial lineages of the adult lung. For example, very little is known about the progenitors that give rise to basal cells, which constitute the main adult lung stem cell population, as well as the developmental pathways involved in this process. Additional studies are also needed about the factors controlling the transition from the branching to alveolar differentiation program. Most preterm babies are born during the saccular stage of the alveolar differentiation stage (between week 26 and 36) when distal epithelial progenitors start to give rise to bipotential alveolar epithelial progenitors. In some extreme cases, at birth the lungs may have only developed until the end of the branching stage, when distal epithelial progenitors are still giving rise to conducting airway epithelium, but not alveolar epithelium. Unraveling the mechanisms involved in alveolar epithelial differentiation and septation may pave the way for new treatments to accelerate alveolar maturation, while minimizing ventilation-mediated damage to increase survival chances of preterm babies. Another important area of research is the controlled differentiation of lung epithelium from iPS cells, which holds great promise for its use in regenerative biology, diagnostics and personalized medicine. Thus far, the differentiation of human ESCs and iPS cells into pulmonary epithelium has been challenging. Several research groups have reported the successful differentiation into various lung epithelial cell types using a variety of protocols (Wang et al., 2007; Van Haute et al., 2009; Ameri et al., 2010; Green et al., 2011; Kadzik and Morrisey, 2012; Longmire et al., 2012; Mou et al., 2012; Ghaedi et al., 2013; Firth et al., 2014; Fox et al., 2014), which are based on sequentially exposing pluripotent stem cells to growth factors in a way that mimics the steps of lung endoderm development in vivo. However, the efficiency of this process is still subpar and usually generates a mixed population of epithelial cells. Therefore, a complete understanding of the molecular mechanisms that not only drive the specification of respiratory progenitors, but also their subsequent differentiation into the impressive array of specialized cell types that populate the lung epithelium will be critical to achieve this goal. An area of lung development that only recently started to gain attention is the study of the differentiation of the lung mesenchyme. This is very important as different mesenchymal populations make up different stem cell niches in the adult lung, which provide important signals in regards to stem cell activation and quiescence.
Acknowledgments
Grant support:
This work was supported by National Heart, Lung, and Blood Institute R01 HL092967 and the pulmonary fibrosis foundation Albert Rose established investigator award (to SDL.).
We apologize to those colleagues whose references have been omitted from this discussion, due to our focus on Wnt and Fgf signaling, we were unable to include all articles on this interesting and diverse subject matter.
Footnotes
The authors declare no conflict of interest
References
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abler LL, Mansour SL, Sun X. Conditional gene inactivation reveals roles for Fgf10 and Fgfr2 in establishing a normal pattern of epithelial branching in the mouse lung. Dev Dyn. 2009;238:1999–2013. doi: 10.1002/dvdy.22032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanis DM, Chang DR, Akiyama H, Krasnow MA, Chen J. Two nested developmental waves demarcate a compartment boundary in the mouse lung. Nat Commun. 2014;5:3923. doi: 10.1038/ncomms4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alescio T, Cassini A. Induction in vitro of tracheal buds by pulmonary mesenchyme grafted on tracheal epithelium. J Exp Zool. 1962;150:83–94. doi: 10.1002/jez.1401500202. [DOI] [PubMed] [Google Scholar]
- Ameri J, Stahlberg A, Pedersen J, Johansson JK, Johannesson MM, Artner I, Semb H. FGF2 specifies hESC-derived definitive endoderm into foregut/midgut cell lineages in a concentration-dependent manner. Stem Cells. 2010;28:45–56. doi: 10.1002/stem.249. [DOI] [PubMed] [Google Scholar]
- Arman E, Haffner-Krausz R, Gorivodsky M, Lonai P. Fgfr2 is required for limb outgrowth and lung-branching morphogenesis. Proc Natl Acad Sci U S A. 1999;96:11895–11899. doi: 10.1073/pnas.96.21.11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora R, Metzger RJ, Papaioannou VE. Multiple roles and interactions of Tbx4 and Tbx5 in development of the respiratory system. PLoS Genet. 2012;8:e1002866. doi: 10.1371/journal.pgen.1002866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, Fassina A, Cordenonsi M, Piccolo S. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, Cordenonsi M, Piccolo S. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151:1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Bachelder RE, Yoon SO, Franci C, de Herreros AG, Mercurio AM. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition. J Cell Biol. 2005;168:29–33. doi: 10.1083/jcb.200409067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Zheng JJ, Wu D. The structural basis of DKK-mediated inhibition of Wnt/LRP signaling. Sci Signal. 2012;5:pe22. doi: 10.1126/scisignal.2003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, Kuo CJ, Camargo FD. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–110. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–4878. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development. 1996;122:1693–1702. doi: 10.1242/dev.122.6.1693. [DOI] [PubMed] [Google Scholar]
- Borges M, Linnoila RI, van de Velde HJ, Chen H, Nelkin BD, Mabry M, Baylin SB, Ball DW. An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature. 1997;386:852–855. doi: 10.1038/386852a0. [DOI] [PubMed] [Google Scholar]
- Boucherat O, Nadeau V, Berube-Simard FA, Charron J, Jeannotte L. Crucial requirement of ERK/MAPK signaling in respiratory tract development. Development. 2014;141:3197–3211. doi: 10.1242/dev.110254. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121:737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9-2 in the switch between beta-catenin’s adhesive and transcriptional functions. Genes Dev. 2004;18:2225–2230. doi: 10.1101/gad.317604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrita MA, Christofori G. Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis. 2008;11:53–62. doi: 10.1007/s10456-008-9089-1. [DOI] [PubMed] [Google Scholar]
- Carraro G, Shrestha A, Rostkovius J, Contreras A, Chao CM, El Agha E, Mackenzie B, Dilai S, Guidolin D, Taketo MM, Gunther A, Kumar ME, Seeger W, De Langhe S, Barreto G, Bellusci S. miR-142-3p balances proliferation and differentiation of mesenchymal cells during lung development. Development. 2014;141:1272–1281. doi: 10.1242/dev.105908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DR, Martinez Alanis D, Miller RK, Ji H, Akiyama H, McCrea PD, Chen J. Lung epithelial branching program antagonizes alveolar differentiation. Proc Natl Acad Sci U S A. 2013;110:18042–18051. doi: 10.1073/pnas.1311760110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellaiah AT, McEwen DG, Werner S, Xu J, Ornitz DM. Fibroblast growth factor receptor (FGFR) 3. Alternative splicing in immunoglobulin-like domain III creates a receptor highly specific for acidic FGF/FGF-1. J Biol Chem. 1994;269:11620–11627. [PubMed] [Google Scholar]
- Chen C, Chen H, Sun J, Bringas P, Jr, Chen Y, Warburton D, Shi W. Smad1 expression and function during mouse embryonic lung branching morphogenesis. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1033–1039. doi: 10.1152/ajplung.00277.2004. [DOI] [PubMed] [Google Scholar]
- Chen F, Cao Y, Qian J, Shao F, Niederreither K, Cardoso WV. A retinoic acid-dependent network in the foregut controls formation of the mouse lung primordium. J Clin Invest. 2010;120:2040–2048. doi: 10.1172/JCI40253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Desai TJ, Qian J, Niederreither K, Lu J, Cardoso WV. Inhibition of Tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development. 2007;134:2969–2979. doi: 10.1242/dev.006221. [DOI] [PubMed] [Google Scholar]
- Chen J, Krasnow MA. Integrin Beta 1 suppresses multilayering of a simple epithelium. PLoS One. 2012;7:e52886. doi: 10.1371/journal.pone.0052886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Huber AH, Weis WI. Thermodynamics of beta-catenin-ligand interactions: the roles of the N- and C-terminal tails in modulating binding affinity. J Biol Chem. 2006;281:1027–1038. doi: 10.1074/jbc.M511338200. [DOI] [PubMed] [Google Scholar]
- Chung C, Kim T, Kim M, Song H, Kim TS, Seo E, Lee SH, Kim H, Kim SK, Yoo G, Lee DH, Hwang DS, Kinashi T, Kim JM, Lim DS. Hippo-Foxa2 signaling pathway plays a role in peripheral lung maturation and surfactant homeostasis. Proc Natl Acad Sci U S A. 2013;110:7732–7737. doi: 10.1073/pnas.1220603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ED, Ihida-Stansbury K, Lu MM, Panettieri RA, Jones PL, Morrisey EE. Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J Clin Invest. 2009;119:2538–2549. doi: 10.1172/JCI38079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, Morrisey EE. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J Clin Invest. 2007;117:1794–1804. doi: 10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin JS, Feldman B, Nadeau JH, Goldfarb M, Ornitz DM. Genomic organization and embryonic expression of the mouse fibroblast growth factor 9 gene. Dev Dyn. 1999;216:72–88. doi: 10.1002/(SICI)1097-0177(199909)216:1<72::AID-DVDY9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Colvin JS, White AC, Pratt SJ, Ornitz DM. Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator of lung mesenchyme. Development. 2001;128:2095–2106. doi: 10.1242/dev.128.11.2095. [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze’ev A. Autoregulation of E-cadherin expression by cadherin-cadherin interactions: the roles of beta-catenin signaling, Slug, and MAPK. J Cell Biol. 2003;163:847–857. doi: 10.1083/jcb.200308162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A, Daidone MG, Dupont S, Basso G, Bicciato S, Piccolo S. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, Polakis P, Matrisian LM. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene. 1999;18:2883–2891. doi: 10.1038/sj.onc.1202627. [DOI] [PubMed] [Google Scholar]
- Cruciat CM, Niehrs C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb Perspect Biol. 2013;5:a015081. doi: 10.1101/cshperspect.a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang TP, Eichenberger S, Gonzalez A, Olson S, Carbone DP. Constitutive activation of Notch3 inhibits terminal epithelial differentiation in lungs of transgenic mice. Oncogene. 2003;22:1988–1997. doi: 10.1038/sj.onc.1206230. [DOI] [PubMed] [Google Scholar]
- Daniely Y, Liao G, Dixon D, Linnoila RI, Lori A, Randell SH, Oren M, Jetten AM. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol. 2004;287:C171–181. doi: 10.1152/ajpcell.00226.2003. [DOI] [PubMed] [Google Scholar]
- Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- De Langhe SP, Carraro G, Tefft D, Li C, Xu X, Chai Y, Minoo P, Hajihosseini MK, Drouin J, Kaartinen V, Bellusci S. Formation and differentiation of multiple mesenchymal lineages during lung development is regulated by beta-catenin signaling. PLoS One. 2008;3:e1516. doi: 10.1371/journal.pone.0001516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Langhe SP, Carraro G, Warburton D, Hajihosseini MK, Bellusci S. Levels of mesenchymal FGFR2 signaling modulate smooth muscle progenitor cell commitment in the lung. Dev Biol. 2006;299:52–62. doi: 10.1016/j.ydbio.2006.07.001. [DOI] [PubMed] [Google Scholar]
- De Langhe SP, Sala FG, Del Moral PM, Fairbanks TJ, Yamada KM, Warburton D, Burns RC, Bellusci S. Dickkopf-1 (DKK1) reveals that fibronectin is a major target of Wnt signaling in branching morphogenesis of the mouse embryonic lung. Dev Biol. 2005;277:316–331. doi: 10.1016/j.ydbio.2004.09.023. [DOI] [PubMed] [Google Scholar]
- De Moerlooze L, Spencer-Dene B, Revest JM, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–492. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- del Moral PM, De Langhe SP, Sala FG, Veltmaat JM, Tefft D, Wang K, Warburton D, Bellusci S. Differential role of FGF9 on epithelium and mesenchyme in mouse embryonic lung. Dev Biol. 2006;293:77–89. doi: 10.1016/j.ydbio.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014 doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai TJ, Chen F, Lu J, Qian J, Niederreither K, Dolle P, Chambon P, Cardoso WV. Distinct roles for retinoic acid receptors alpha and beta in early lung morphogenesis. Dev Biol. 2006;291:12–24. doi: 10.1016/j.ydbio.2005.10.045. [DOI] [PubMed] [Google Scholar]
- Desai TJ, Malpel S, Flentke GR, Smith SM, Cardoso WV. Retinoic acid selectively regulates Fgf10 expression and maintains cell identity in the prospective lung field of the developing foregut. Dev Biol. 2004;273:402–415. doi: 10.1016/j.ydbio.2004.04.039. [DOI] [PubMed] [Google Scholar]
- Deutsch G, Jung J, Zheng M, Lora J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128:871–881. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- Dixit R, Ai X, Fine A. Derivation of lung mesenchymal lineages from the fetal mesothelium requires hedgehog signaling for mesothelial cell entry. Development. 2013;140:4398–4406. doi: 10.1242/dev.098079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domyan ET, Ferretti E, Throckmorton K, Mishina Y, Nicolis SK, Sun X. Signaling through BMP receptors promotes respiratory identity in the foregut via repression of Sox2. Development. 2011;138:971–981. doi: 10.1242/dev.053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domyan ET, Sun X. Patterning and plasticity in development of the respiratory lineage. Dev Dyn. 2011;240:477–485. doi: 10.1002/dvdy.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eblaghie MC, Reedy M, Oliver T, Mishina Y, Hogan BL. Evidence that autocrine signaling through Bmpr1a regulates the proliferation, survival and morphogenetic behavior of distal lung epithelial cells. Dev Biol. 2006;291:67–82. doi: 10.1016/j.ydbio.2005.12.006. [DOI] [PubMed] [Google Scholar]
- El Agha E, Al Alam D, Carraro G, MacKenzie B, Goth K, De Langhe SP, Voswinckel R, Hajihosseini MK, Rehan VK, Bellusci S. Characterization of a novel fibroblast growth factor 10 (Fgf10) knock-in mouse line to target mesenchymal progenitors during embryonic development. PLoS One. 2012;7:e38452. doi: 10.1371/journal.pone.0038452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Agha E, Herold S, Al Alam D, Quantius J, MacKenzie B, Carraro G, Moiseenko A, Chao CM, Minoo P, Seeger W, Bellusci S. Fgf10-positive cells represent a progenitor cell population during lung development and postnatally. Development. 2014;141:296–306. doi: 10.1242/dev.099747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch PW, Cunha GR, Rubin JS, Wong J, Ron D. Pattern of keratinocyte growth factor and keratinocyte growth factor receptor expression during mouse fetal development suggests a role in mediating morphogenetic mesenchymal-epithelial interactions. Dev Dyn. 1995;203:223–240. doi: 10.1002/aja.1002030210. [DOI] [PubMed] [Google Scholar]
- Firth AL, Dargitz CT, Qualls SJ, Menon T, Wright R, Singer O, Gage FH, Khanna A, Verma IM. Generation of multiciliated cells in functional airway epithelia from human induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2014;111:E1723–1730. doi: 10.1073/pnas.1403470111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E, Shojaie S, Wang J, Tseu I, Ackerley C, Bilodeau M, Post M. Three-dimensional culture and FGF signaling drive differentiation of murine pluripotent cells to distal lung epithelial cells. Stem Cells Dev. 2014 doi: 10.1089/scd.2014.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francavilla C, Rigbolt KT, Emdal KB, Carraro G, Vernet E, Bekker-Jensen DB, Streicher W, Wikstrom M, Sundstrom M, Bellusci S, Cavallaro U, Blagoev B, Olsen JV. Functional proteomics defines the molecular switch underlying FGF receptor trafficking and cellular outputs. Mol Cell. 2013;51:707–722. doi: 10.1016/j.molcel.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Gan XQ, Wang JY, Xi Y, Wu ZL, Li YP, Li L. Nuclear Dvl, c-Jun, beta-catenin, and TCF form a complex leading to stabilization of beta-catenin-TCF interaction. J Cell Biol. 2008;180:1087–1100. doi: 10.1083/jcb.200710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilova R, Babovic N, Lteif A, Eidem B, Kirmani S, Olson T, Babovic-Vuksanovic D. Vitamin A deficiency in an infant with PAGOD syndrome. Am J Med Genet A. 2009;149A:2241–2247. doi: 10.1002/ajmg.a.32998. [DOI] [PubMed] [Google Scholar]
- Ghaedi M, Calle EA, Mendez JJ, Gard AL, Balestrini J, Booth A, Bove PF, Gui L, White ES, Niklason LE. Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix. J Clin Invest. 2013;123:4950–4962. doi: 10.1172/JCI68793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz R, Mohammadi M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat Rev Mol Cell Biol. 2013;14:166–180. doi: 10.1038/nrm3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golzio C, Martinovic-Bouriel J, Thomas S, Mougou-Zrelli S, Grattagliano-Bessieres B, Bonniere M, Delahaye S, Munnich A, Encha-Razavi F, Lyonnet S, Vekemans M, Attie-Bitach T, Etchevers HC. Matthew-Wood syndrome is caused by truncating mutations in the retinol-binding protein receptor gene STRA6. Am J Hum Genet. 2007;80:1179–1187. doi: 10.1086/518177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gontan C, de Munck A, Vermeij M, Grosveld F, Tibboel D, Rottier R. Sox2 is important for two crucial processes in lung development: branching morphogenesis and epithelial cell differentiation. Dev Biol. 2008;317:296–309. doi: 10.1016/j.ydbio.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sancho JM, Greer YE, Abrahams CL, Takigawa Y, Baljinnyam B, Lee KH, Lee KS, Rubin JS, Brown AM. Functional consequences of Wnt-induced dishevelled 2 phosphorylation in canonical and noncanonical Wnt signaling. J Biol Chem. 2013;288:9428–9437. doi: 10.1074/jbc.M112.448480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss AM, Tian Y, Cheng L, Yang J, Zhou D, Cohen ED, Morrisey EE. Wnt2 signaling is necessary and sufficient to activate the airway smooth muscle program in the lung by regulating myocardin/Mrtf-B and Fgf10 expression. Dev Biol. 2011;356:541–552. doi: 10.1016/j.ydbio.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, Yamaguchi TP, Morrisey EE. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17:290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MD, Chen A, Nostro MC, d’Souza SL, Schaniel C, Lemischka IR, Gouon-Evans V, Keller G, Snoeck HW. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotechnol. 2011;29:267–272. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindley JC, Bellusci S, Perkins D, Hogan BL. Evidence for the involvement of the Gli gene family in embryonic mouse lung development. Dev Biol. 1997;188:337–348. doi: 10.1006/dbio.1997.8644. [DOI] [PubMed] [Google Scholar]
- Guha A, Vasconcelos M, Cai Y, Yoneda M, Hinds A, Qian J, Li G, Dickel L, Johnson JE, Kimura S, Guo J, McMahon J, McMahon AP, Cardoso WV. Neuroepithelial body microenvironment is a niche for a distinct subset of Clara-like precursors in the developing airways. Proc Natl Acad Sci U S A. 2012;109:12592–12597. doi: 10.1073/pnas.1204710109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Degenstein L, Fuchs E. Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev. 1996;10:165–175. doi: 10.1101/gad.10.2.165. [DOI] [PubMed] [Google Scholar]
- Guo W, Keckesova Z, Donaher JL, Shibue T, Tischler V, Reinhardt F, Itzkovitz S, Noske A, Zurrer-Hardi U, Bell G, Tam WL, Mani SA, van Oudenaarden A, Weinberg RA. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell. 2012;148:1015–1028. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA, Rajagopal J. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development. 2009;136:1751–1759. doi: 10.1242/dev.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Johnson KS, Domyan ET, Vezina CM, Sun X. beta-Catenin promotes respiratory progenitor identity in mouse foregut. Proc Natl Acad Sci U S A. 2009;106:16287–16292. doi: 10.1073/pnas.0902274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harunaga JS, Doyle AD, Yamada KM. Local and global dynamics of the basement membrane during branching morphogenesis require protease activity and actomyosin contractility. Dev Biol. 2014 doi: 10.1016/j.ydbio.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Chen H, Que J, Brockway BL, Drake JA, Snyder JC, Randell SH, Stripp BR. beta-Catenin-SOX2 signaling regulates the fate of developing airway epithelium. J Cell Sci. 2012;125:932–942. doi: 10.1242/jcs.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, Wiedemann LM, Barrett TA, Hood L, Wu H, Li L. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–198. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Herzig M, Savarese F, Novatchkova M, Semb H, Christofori G. Tumor progression induced by the loss of E-cadherin independent of beta-catenin/Tcf-mediated Wnt signaling. Oncogene. 2007;26:2290–2298. doi: 10.1038/sj.onc.1210029. [DOI] [PubMed] [Google Scholar]
- Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol. 2010;2:a002915. doi: 10.1101/cshperspect.a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines EA, Jones MK, Verheyden JM, Harvey JF, Sun X. Establishment of smooth muscle and cartilage juxtaposition in the developing mouse upper airways. Proc Natl Acad Sci U S A. 2013;110:19444–19449. doi: 10.1073/pnas.1313223110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines EA, Sun X. Tissue Crosstalk in Lung Development. J Cell Biochem. 2014 doi: 10.1002/jcb.24811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BL, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CC, Niklason L, Calle E, Le A, Randell SH, Rock J, Snitow M, Krummel M, Stripp BR, Vu T, White ES, Whitsett JA, Morrisey EE. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe LR, Watanabe O, Leonard J, Brown AM. Twist is up-regulated in response to Wnt1 and inhibits mouse mammary cell differentiation. Cancer Res. 2003;63:1906–1913. [PubMed] [Google Scholar]
- Hui CC, Slusarski D, Platt KA, Holmgren R, Joyner AL. Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm- and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev Biol. 1994;162:402–413. doi: 10.1006/dbio.1994.1097. [DOI] [PubMed] [Google Scholar]
- Hyatt BA, Shangguan X, Shannon JM. FGF-10 induces SP-C and Bmp4 and regulates proximal-distal patterning in embryonic tracheal epithelium. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1116–1126. doi: 10.1152/ajplung.00033.2004. [DOI] [PubMed] [Google Scholar]
- Imajo M, Miyatake K, Iimura A, Miyamoto A, Nishida E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/beta-catenin signalling. EMBO J. 2012;31:1109–1122. doi: 10.1038/emboj.2011.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Udaka N, Yazawa T, Okudela K, Hayashi H, Sudo T, Guillemot F, Kageyama R, Kitamura H. Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development. 2000;127:3913–3921. doi: 10.1242/dev.127.18.3913. [DOI] [PubMed] [Google Scholar]
- Itoh K, Brott BK, Bae GU, Ratcliffe MJ, Sokol SY. Nuclear localization is required for Dishevelled function in Wnt/beta-catenin signaling. J Biol. 2005;4:3. doi: 10.1186/jbiol20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs IJ, Ku WY, Que J. Genetic and cellular mechanisms regulating anterior foregut and esophageal development. Dev Biol. 2012;369:54–64. doi: 10.1016/j.ydbio.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, Lu J, Chen H, Werner S, Williams LT. The human fibroblast growth factor receptor genes: a common structural arrangement underlies the mechanisms for generating receptor forms that differ in their third immunoglobulin domain. Mol Cell Biol. 1991;11:4627–4634. doi: 10.1128/mcb.11.9.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L, Greenbaum D, Cichowski K, Mercer K, Murphy E, Schmitt E, Bronson RT, Umanoff H, Edelmann W, Kucherlapati R, Jacks T. K-ras is an essential gene in the mouse with partial functional overlap with N-ras. Genes Dev. 1997;11:2468–2481. doi: 10.1101/gad.11.19.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JJ, Olivos-Glander I, Killoran C, Elson E, Turner JT, Peters KF, Abbott MH, Aughton DJ, Aylsworth AS, Bamshad MJ, Booth C, Curry CJ, David A, Dinulos MB, Flannery DB, Fox MA, Graham JM, Grange DK, Guttmacher AE, Hannibal MC, Henn W, Hennekam RC, Holmes LB, Hoyme HE, Leppig KA, Lin AE, Macleod P, Manchester DK, Marcelis C, Mazzanti L, McCann E, McDonald MT, Mendelsohn NJ, Moeschler JB, Moghaddam B, Neri G, Newbury-Ecob R, Pagon RA, Phillips JA, Sadler LS, Stoler JM, Tilstra D, Walsh Vockley CM, Zackai EH, Zadeh TM, Brueton L, Black GC, Biesecker LG. Molecular and clinical analyses of Greig cephalopolysyndactyly and Pallister-Hall syndromes: robust phenotype prediction from the type and position of GLI3 mutations. Am J Hum Genet. 2005;76:609–622. doi: 10.1086/429346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- Kadzik RS, Cohen ED, Morley MP, Stewart KM, Lu MM, Morrisey EE. Wnt ligand/Frizzled 2 receptor signaling regulates tube shape and branch-point formation in the lung through control of epithelial cell shape. Proc Natl Acad Sci U S A. 2014;111:12444–12449. doi: 10.1073/pnas.1406639111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadzik RS, Morrisey EE. Directing lung endoderm differentiation in pluripotent stem cells. Cell Stem Cell. 2012;10:355–361. doi: 10.1016/j.stem.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, Capdevila J, Buscher D, Itoh T, Rodriguez Esteban C, Izpisua Belmonte JC. WNT signals control FGF-dependent limb initiation and AER induction in the chick embryo. Cell. 2001;104:891–900. doi: 10.1016/s0092-8674(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- Kim SE, Huang H, Zhao M, Zhang X, Zhang A, Semonov MV, MacDonald BT, Garcia Abreu J, Peng L, He X. Wnt stabilization of beta-catenin reveals principles for morphogen receptor-scaffold assemblies. Science. 2013;340:867–870. doi: 10.1126/science.1232389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Hatakeyama S, Shirane M, Matsumoto M, Ishida N, Hattori K, Nakamichi I, Kikuchi A, Nakayama K. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of beta-catenin. EMBO J. 1999;18:2401–2410. doi: 10.1093/emboj/18.9.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y, Glickman J, Subramaniam M, Shahsafaei A, Allamneni KP, Aster JC, Sklar J, Sunday ME. Functional diversity of notch family genes in fetal lung development. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1075–1083. doi: 10.1152/ajplung.00438.2002. [DOI] [PubMed] [Google Scholar]
- Konsavage WM, Jr, Kyler SL, Rennoll SA, Jin G, Yochum GS. Wnt/beta-catenin signaling regulates Yes-associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem. 2012;287:11730–11739. doi: 10.1074/jbc.M111.327767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotton DN, Morrisey EE. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat Med. 2014;20:822–832. doi: 10.1038/nm.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejci P, Aklian A, Kaucka M, Sevcikova E, Prochazkova J, Masek JK, Mikolka P, Pospisilova T, Spoustova T, Weis M, Paznekas WA, Wolf JH, Gutkind JS, Wilcox WR, Kozubik A, Jabs EW, Bryja V, Salazar L, Vesela I, Balek L. Receptor tyrosine kinases activate canonical WNT/beta-catenin signaling via MAP kinase/LRP6 pathway and direct beta-catenin phosphorylation. PLoS One. 2012;7:e35826. doi: 10.1371/journal.pone.0035826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar ME, Bogard PE, Espinoza FH, Menke DB, Kingsley DM, Krasnow MA. Mesenchymal cells. Defining a mesenchymal progenitor niche at single-cell resolution. Science. 2014;346:1258810. doi: 10.1126/science.1258810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuphal F, Behrens J. E-cadherin modulates Wnt-dependent transcription in colorectal cancer cells but does not alter Wnt-independent gene expression in fibroblasts. Exp Cell Res. 2006;312:457–467. doi: 10.1016/j.yexcr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Lako M, Strachan T, Bullen P, Wilson DI, Robson SC, Lindsay S. Isolation, characterisation and embryonic expression of WNT11, a gene which maps to 11q13.5 and has possible roles in the development of skeleton, kidney and lung. Gene. 1998;219:101–110. doi: 10.1016/s0378-1119(98)00393-x. [DOI] [PubMed] [Google Scholar]
- Lazzaro D, Price M, de Felice M, Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113:1093–1104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- Lebeche D, Malpel S, Cardoso WV. Fibroblast growth factor interactions in the developing lung. Mech Dev. 1999;86:125–136. doi: 10.1016/s0925-4773(99)00124-0. [DOI] [PubMed] [Google Scholar]
- Li C, Li A, Li M, Xing Y, Chen H, Hu L, Tiozzo C, Anderson S, Taketo MM, Minoo P. Stabilized beta-catenin in lung epithelial cells changes cell fate and leads to tracheal and bronchial polyposis. Dev Biol. 2009;334:97–108. doi: 10.1016/j.ydbio.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a participates in distal lung morphogenesis. Dev Biol. 2002;248:68–81. doi: 10.1006/dbio.2002.0729. [DOI] [PubMed] [Google Scholar]
- Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP, Mohammed S, Heck AJ, Maurice MM, Mahmoudi T, Clevers H. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Li Y, Gordon J, Manley NR, Litingtung Y, Chiang C. Bmp4 is required for tracheal formation: a novel mouse model for tracheal agenesis. Dev Biol. 2008;322:145–155. doi: 10.1016/j.ydbio.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Litingtung Y, Ten Dijke P, Chiang C. Aberrant Bmp signaling and notochord delamination in the pathogenesis of esophageal atresia. Dev Dyn. 2007;236:746–754. doi: 10.1002/dvdy.21075. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang H, Choi SC, Litingtung Y, Chiang C. Sonic hedgehog signaling regulates Gli3 processing, mesenchymal proliferation, and differentiation during mouse lung organogenesis. Dev Biol. 2004;270:214–231. doi: 10.1016/j.ydbio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Lickert H, Bauer A, Kemler R, Stappert J. Casein kinase II phosphorylation of E-cadherin increases E-cadherin/beta-catenin interaction and strengthens cell-cell adhesion. J Biol Chem. 2000;275:5090–5095. doi: 10.1074/jbc.275.7.5090. [DOI] [PubMed] [Google Scholar]
- Lin JI, Poon CL, Harvey KF. The Hippo size control pathway--ever expanding. Sci Signal. 2013;6:pe4. doi: 10.1126/scisignal.2003813. [DOI] [PubMed] [Google Scholar]
- Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jiang H, Crawford HC, Hogan BL. Role for ETS domain transcription factors Pea3/Erm in mouse lung development. Dev Biol. 2003;261:10–24. doi: 10.1016/s0012-1606(03)00359-2. [DOI] [PubMed] [Google Scholar]
- Longmire TA, Ikonomou L, Hawkins F, Christodoulou C, Cao Y, Jean JC, Kwok LW, Mou H, Rajagopal J, Shen SS, Dowton AA, Serra M, Weiss DJ, Green MD, Snoeck HW, Ramirez MI, Kotton DN. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10:398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MM, Yang H, Zhang L, Shu W, Blair DG, Morrisey EE. The bone morphogenic protein antagonist gremlin regulates proximal-distal patterning of the lung. Dev Dyn. 2001;222:667–680. doi: 10.1002/dvdy.1231. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney JE, Mori M, Szymaniak AD, Varelas X, Cardoso WV. The hippo pathway effector yap controls patterning and differentiation of airway epithelial progenitors. Dev Cell. 2014;30:137–150. doi: 10.1016/j.devcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux AA, Kelly R, Veltmaat JM, De Langhe SP, Zaffran S, Thiery JP, Bellusci S. Fgf10 expression identifies parabronchial smooth muscle cell progenitors and is required for their entry into the smooth muscle cell lineage. Development. 2005;132:2157–2166. doi: 10.1242/dev.01795. [DOI] [PubMed] [Google Scholar]
- Makita R, Uchijima Y, Nishiyama K, Amano T, Chen Q, Takeuchi T, Mitani A, Nagase T, Yatomi Y, Aburatani H, Nakagawa O, Small EV, Cobo-Stark P, Igarashi P, Murakami M, Tominaga J, Sato T, Asano T, Kurihara Y, Kurihara H. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol Renal Physiol. 2008;294:F542–553. doi: 10.1152/ajprenal.00201.2007. [DOI] [PubMed] [Google Scholar]
- Malpel S, Mendelsohn C, Cardoso WV. Regulation of retinoic acid signaling during lung morphogenesis. Development. 2000;127:3057–3067. doi: 10.1242/dev.127.14.3057. [DOI] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Wang J, Liu B, Pan W, Farr GH, 3rd, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7:801–809. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Marcil A, Dumontier E, Chamberland M, Camper SA, Drouin J. Pitx1 and Pitx2 are required for development of hindlimb buds. Development. 2003;130:45–55. doi: 10.1242/dev.00192. [DOI] [PubMed] [Google Scholar]
- Martinez Barbera JP, Clements M, Thomas P, Rodriguez T, Meloy D, Kioussis D, Beddington RS. The homeobox gene Hex is required in definitive endodermal tissues for normal forebrain, liver and thyroid formation. Development. 2000;127:2433–2445. doi: 10.1242/dev.127.11.2433. [DOI] [PubMed] [Google Scholar]
- Mason IJ, Fuller-Pace F, Smith R, Dickson C. FGF-7 (keratinocyte growth factor) expression during mouse development suggests roles in myogenesis, forebrain regionalisation and epithelial-mesenchymal interactions. Mech Dev. 1994;45:15–30. doi: 10.1016/0925-4773(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Metzger DE, Xu Y, Shannon JM. Elf5 is an epithelium-specific, fibroblast growth factor-sensitive transcription factor in the embryonic lung. Dev Dyn. 2007;236:1175–1192. doi: 10.1002/dvdy.21133. [DOI] [PubMed] [Google Scholar]
- Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature. 2008;453:745–750. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Bottaro DP, Fleming TP, Smith CL, Burgess WH, Chan AM, Aaronson SA. Determination of ligand-binding specificity by alternative splicing: two distinct growth factor receptors encoded by a single gene. Proc Natl Acad Sci U S A. 1992;89:246–250. doi: 10.1073/pnas.89.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LA, Wert SE, Clark JC, Xu Y, Perl AK, Whitsett JA. Role of Sonic hedgehog in patterning of tracheal-bronchial cartilage and the peripheral lung. Dev Dyn. 2004;231:57–71. doi: 10.1002/dvdy.20105. [DOI] [PubMed] [Google Scholar]
- Miller MF, Cohen ED, Baggs JE, Lu MM, Hogenesch JB, Morrisey EE. Wnt ligands signal in a cooperative manner to promote foregut organogenesis. Proc Natl Acad Sci U S A. 2012;109:15348–15353. doi: 10.1073/pnas.1201583109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- Min H, Danilenko DM, Scully SA, Bolon B, Ring BD, Tarpley JE, DeRose M, Simonet WS. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998;12:3156–3161. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoo P, Su G, Drum H, Bringas P, Kimura S. Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(−/−) mouse embryos. Dev Biol. 1999;209:60–71. doi: 10.1006/dbio.1999.9234. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Liu Z, Cheng HT, Winters N, Bader D, Kopan R. Canonical Notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara versus ciliated cell fate. J Cell Sci. 2010;123:213–224. doi: 10.1242/jcs.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto M, Nishinakamura R, Saga Y, Kopan R. Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development. 2012;139:4365–4373. doi: 10.1242/dev.083840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet. 1998;20:54–57. doi: 10.1038/1711. [DOI] [PubMed] [Google Scholar]
- Mou X, Wu Y, Cao H, Meng Q, Wang Q, Sun C, Hu S, Ma Y, Zhang H. Generation of disease-specific induced pluripotent stem cells from patients with different karyotypes of Down syndrome. Stem Cell Res Ther. 2012;3:14. doi: 10.1186/scrt105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski ML, Nation JM, Thitoff AR, Besnard V, Xu Y, Wert SE, Harada N, Taketo MM, Stahlman MT, Whitsett JA. Beta-catenin regulates differentiation of respiratory epithelial cells in vivo. Am J Physiol Lung Cell Mol Physiol. 2005;289:L971–979. doi: 10.1152/ajplung.00172.2005. [DOI] [PubMed] [Google Scholar]
- Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem. 2003;278:40231–40238. doi: 10.1074/jbc.M305892200. [DOI] [PubMed] [Google Scholar]
- Nyeng P, Norgaard GA, Kobberup S, Jensen J. FGF10 maintains distal lung bud epithelium and excessive signaling leads to progenitor state arrest, distalization, and goblet cell metaplasia. BMC Dev Biol. 2008;8:2. doi: 10.1186/1471-213X-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochieng JK, Schilders K, Kool H, Boerema-De Munck A, Buscop-Van Kempen M, Gontan C, Smits R, Grosveld FG, Wijnen RM, Tibboel D, Rottier RJ. Sox2 regulates the emergence of lung basal cells by directly activating the transcription of Trp63. Am J Respir Cell Mol Biol. 2014;51:311–322. doi: 10.1165/rcmb.2013-0419OC. [DOI] [PubMed] [Google Scholar]
- Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- Okubo T, Hogan BL. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J Biol. 2004;3:11. doi: 10.1186/jbiol3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T, Knoepfler PS, Eisenman RN, Hogan BL. Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development. 2005;132:1363–1374. doi: 10.1242/dev.01678. [DOI] [PubMed] [Google Scholar]
- Olsen SK, Li JY, Bromleigh C, Eliseenkova AV, Ibrahimi OA, Lao Z, Zhang F, Linhardt RJ, Joyner AL, Mohammadi M. Structural basis by which alternative splicing modulates the organizer activity of FGF8 in the brain. Genes Dev. 2006;20:185–198. doi: 10.1101/gad.1365406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Yin Y. Signaling networks regulating development of the lower respiratory tract. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Urtreger A, Bedford MT, Burakova T, Arman E, Zimmer Y, Yayon A, Givol D, Lonai P. Developmental localization of the splicing alternatives of fibroblast growth factor receptor-2 (FGFR2) Dev Biol. 1993;158:475–486. doi: 10.1006/dbio.1993.1205. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KS, Whitsett JA, Di Palma T, Hong JH, Yaffe MB, Zannini M. TAZ interacts with TTF-1 and regulates expression of surfactant protein-C. J Biol Chem. 2004;279:17384–17390. doi: 10.1074/jbc.M312569200. [DOI] [PubMed] [Google Scholar]
- Park WY, Miranda B, Lebeche D, Hashimoto G, Cardoso WV. FGF-10 is a chemotactic factor for distal epithelial buds during lung development. Dev Biol. 1998;201:125–134. doi: 10.1006/dbio.1998.8994. [DOI] [PubMed] [Google Scholar]
- Peng T, Tian Y, Boogerd CJ, Lu MM, Kadzik RS, Stewart KM, Evans SM, Morrisey EE. Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature. 2013;500:589–592. doi: 10.1038/nature12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8:1083–1086. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- Perl AK, Kist R, Shan Z, Scherer G, Whitsett JA. Normal lung development and function after Sox9 inactivation in the respiratory epithelium. Genesis. 2005;41:23–32. doi: 10.1002/gene.20093. [DOI] [PubMed] [Google Scholar]
- Piedra J, Miravet S, Castano J, Palmer HG, Heisterkamp N, Garcia de Herreros A, Dunach M. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin Interaction. Mol Cell Biol. 2003;23:2287–2297. doi: 10.1128/MCB.23.7.2287-2297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov AN, Hubbard SR, Schlessinger J, Mohammadi M. Crystal structures of two FGF-FGFR complexes reveal the determinants of ligand-receptor specificity. Cell. 2000;101:413–424. doi: 10.1016/s0092-8674(00)80851-x. [DOI] [PubMed] [Google Scholar]
- Post LC, Ternet M, Hogan BL. Notch/Delta expression in the developing mouse lung. Mech Dev. 2000;98:95–98. doi: 10.1016/s0925-4773(00)00432-9. [DOI] [PubMed] [Google Scholar]
- Qi J, Wang J, Romanyuk O, Siu CH. Involvement of Src family kinases in N-cadherin phosphorylation and beta-catenin dissociation during transendothelial migration of melanoma cells. Mol Biol Cell. 2006;17:1261–1272. doi: 10.1091/mbc.E05-10-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J, Choi M, Ziel JW, Klingensmith J, Hogan BL. Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation. 2006;74:422–437. doi: 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- Que J, Luo X, Schwartz RJ, Hogan BL. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136:1899–1907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, Taranova O, Pevny LH, Hogan BL. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134:2521–2531. doi: 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal J, Carroll TJ, Guseh JS, Bores SA, Blank LJ, Anderson WJ, Yu J, Zhou Q, McMahon AP, Melton DA. Wnt7b stimulates embryonic lung growth by coordinately increasing the replication of epithelium and mesenchyme. Development. 2008;135:1625–1634. doi: 10.1242/dev.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy SK, Mailleux AA, Gupte VV, Mata F, Sala FG, Veltmaat JM, Del Moral PM, De Langhe S, Parsa S, Kelly LK, Kelly R, Shia W, Keshet E, Minoo P, Warburton D, Bellusci S. Fgf10 dosage is critical for the amplification of epithelial cell progenitors and for the formation of multiple mesenchymal lineages during lung development. Dev Biol. 2007;307:237–247. doi: 10.1016/j.ydbio.2007.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin SA, Thi Tran H, Wlizla M, Mancini P, Shifley ET, Bloor SD, Han L, Vleminckx K, Wert SE, Zorn AM. A Molecular atlas of Xenopus respiratory system development. Dev Dyn. 2014 doi: 10.1002/dvdy.24180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL. Lung epithelial progenitor cells: lessons from development. Proc Am Thorac Soc. 2008;5:675–681. doi: 10.1513/pats.200801-006AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL. The building blocks of mammalian lung development. Dev Dyn. 2010;240:463–476. doi: 10.1002/dvdy.22482. [DOI] [PubMed] [Google Scholar]
- Rawlins EL, Clark CP, Xue Y, Hogan BL. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development. 2009;136:3741–3745. doi: 10.1242/dev.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SD, Zemke AC, Giangreco A, Brockway BL, Teisanu RM, Drake JA, Mariani T, Di PY, Taketo MM, Stripp BR. Conditional stabilization of beta-catenin expands the pool of lung stem cells. Stem Cells. 2008;26:1337–1346. doi: 10.1634/stemcells.2008-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee J, Buchan T, Zukerberg L, Lilien J, Balsamo J. Cables links Robo-bound Abl kinase to N-cadherin-bound beta-catenin to mediate Slit-induced modulation of adhesion and transcription. Nat Cell Biol. 2007;9:883–892. doi: 10.1038/ncb1614. [DOI] [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci U S A. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockich BE, Hrycaj SM, Shih HP, Nagy MS, Ferguson MA, Kopp JL, Sander M, Wellik DM, Spence JR. Sox9 plays multiple roles in the lung epithelium during branching morphogenesis. Proc Natl Acad Sci U S A. 2013;110:E4456–4464. doi: 10.1073/pnas.1311847110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano RA, Ortt K, Birkaya B, Smalley K, Sinha S. An active role of the DeltaN isoform of p63 in regulating basal keratin genes K5 and K14 and directing epidermal cell fate. PLoS One. 2009;4:e5623. doi: 10.1371/journal.pone.0005623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato R, Veltmaat JM, Groffen J, Heisterkamp N. Involvement of the tyrosine kinase fer in cell adhesion. Mol Cell Biol. 1998;18:5762–5770. doi: 10.1128/mcb.18.10.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36734–36740. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423:876–881. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- Sakiyama J, Yamagishi A, Kuroiwa A. Tbx4-Fgf10 system controls lung bud formation during chicken embryonic development. Development. 2003;130:1225–1234. doi: 10.1242/dev.00345. [DOI] [PubMed] [Google Scholar]
- Sala FG, Del Moral PM, Tiozzo C, Alam DA, Warburton D, Grikscheit T, Veltmaat JM, Bellusci S. FGF10 controls the patterning of the tracheal cartilage rings via Shh. Development. 2011;138:273–282. doi: 10.1242/dev.051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanson B, White P, Vincent JP. Uncoupling cadherin-based adhesion from wingless signalling in Drosophila. Nature. 1996;383:627–630. doi: 10.1038/383627a0. [DOI] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, Camargo FD. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J, Plotnikov AN, Ibrahimi OA, Eliseenkova AV, Yeh BK, Yayon A, Linhardt RJ, Mohammadi M. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol Cell. 2000;6:743–750. doi: 10.1016/s1097-2765(00)00073-3. [DOI] [PubMed] [Google Scholar]
- Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, Kato S. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21:138–141. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- Serls AE, Doherty S, Parvatiyar P, Wells JM, Deutsch GH. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development. 2005;132:35–47. doi: 10.1242/dev.01570. [DOI] [PubMed] [Google Scholar]
- Shan L, Aster JC, Sklar J, Sunday ME. Notch-1 regulates pulmonary neuroendocrine cell differentiation in cell lines and in transgenic mice. Am J Physiol Lung Cell Mol Physiol. 2007;292:L500–509. doi: 10.1152/ajplung.00052.2006. [DOI] [PubMed] [Google Scholar]
- Shan L, Subramaniam M, Emanuel RL, Degan S, Johnston P, Tefft D, Warburton D, Sunday ME. Centrifugal migration of mesenchymal cells in embryonic lung. Dev Dyn. 2008;237:750–757. doi: 10.1002/dvdy.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon JM. Induction of alveolar type II cell differentiation in fetal tracheal epithelium by grafted distal lung mesenchyme. Dev Biol. 1994;166:600–614. doi: 10.1006/dbio.1994.1340. [DOI] [PubMed] [Google Scholar]
- Shannon JM, Hyatt BA. Epithelial-mesenchymal interactions in the developing lung. Annu Rev Physiol. 2004;66:625–645. doi: 10.1146/annurev.physiol.66.032102.135749. [DOI] [PubMed] [Google Scholar]
- Sherwood RI, Chen TY, Melton DA. Transcriptional dynamics of endodermal organ formation. Dev Dyn. 2009;238:29–42. doi: 10.1002/dvdy.21810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifley ET, Kenny AP, Rankin SA, Zorn AM. Prolonged FGF signaling is necessary for lung and liver induction in Xenopus. BMC Dev Biol. 2012;12:27. doi: 10.1186/1471-213X-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori M, Oshika E, Ung LP, Singh G, Shinozuka H, Warburton D, Michalopoulos G, Katyal SL. Keratinocyte growth factor and embryonic rat lung morphogenesis. Am J Respir Cell Mol Biol. 1996;15:328–338. doi: 10.1165/ajrcmb.15.3.8810636. [DOI] [PubMed] [Google Scholar]
- Shu W, Guttentag S, Wang Z, Andl T, Ballard P, Lu MM, Piccolo S, Birchmeier W, Whitsett JA, Millar SE, Morrisey EE. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev Biol. 2005;283:226–239. doi: 10.1016/j.ydbio.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development. 2002;129:4831–4842. doi: 10.1242/dev.129.20.4831. [DOI] [PubMed] [Google Scholar]
- Shu W, Lu MM, Zhang Y, Tucker PW, Zhou D, Morrisey EE. Foxp2 and Foxp1 cooperatively regulate lung and esophagus development. Development. 2007;134:1991–2000. doi: 10.1242/dev.02846. [DOI] [PubMed] [Google Scholar]
- Silvis MR, Kreger BT, Lien WH, Klezovitch O, Rudakova GM, Camargo FD, Lantz DM, Seykora JT, Vasioukhin V. alpha-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal. 2011;4:ra33. doi: 10.1126/scisignal.2001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal FJ, Noort MvM, Strous GJ, Clevers HC. Wnt signals are transmitted through N-terminally dephosphorylated beta-catenin. EMBO Rep. 2002;3:63–68. doi: 10.1093/embo-reports/kvf002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Chen H, Chen C, Whitsett JA, Mishina Y, Bringas P, Jr, Ma JC, Warburton D, Shi W. Prenatal lung epithelial cell-specific abrogation of Alk3-bone morphogenetic protein signaling causes neonatal respiratory distress by disrupting distal airway formation. Am J Pathol. 2008;172:571–582. doi: 10.2353/ajpath.2008.070286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D, Samakovlis C, Krasnow MA. branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell. 1996;87:1091–1101. doi: 10.1016/s0092-8674(00)81803-6. [DOI] [PubMed] [Google Scholar]
- Taderera JV. Control of lung differentiation in vitro. Dev Biol. 1967;16:489–512. doi: 10.1016/0012-1606(67)90061-9. [DOI] [PubMed] [Google Scholar]
- Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- Tang N, Marshall WF, McMahon M, Metzger RJ, Martin GR. Control of mitotic spindle angle by the RAS-regulated ERK1/2 pathway determines lung tube shape. Science. 2011;333:342–345. doi: 10.1126/science.1204831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebar M, Destree O, de Vree WJ, Ten Have-Opbroek AA. Expression of Tcf/Lef and sFrp and localization of beta-catenin in the developing mouse lung. Mech Dev. 2001;109:437–440. doi: 10.1016/s0925-4773(01)00556-1. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Tichelaar JW, Lu W, Whitsett JA. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem. 2000;275:11858–11864. doi: 10.1074/jbc.275.16.11858. [DOI] [PubMed] [Google Scholar]
- Tiozzo C, De Langhe S, Carraro G, Alam DA, Nagy A, Wigfall C, Hajihosseini MK, Warburton D, Minoo P, Bellusci S. Fibroblast growth factor 10 plays a causative role in the tracheal cartilage defects in a mouse model of Apert syndrome. Pediatr Res. 2009;66:386–390. doi: 10.1203/PDR.0b013e3181b45580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins DH, Besnard V, Lange AW, Keiser AR, Wert SE, Bruno MD, Whitsett JA. Sox2 activates cell proliferation and differentiation in the respiratory epithelium. Am J Respir Cell Mol Biol. 2011;45:101–110. doi: 10.1165/rcmb.2010-0149OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins DH, Besnard V, Lange AW, Wert SE, Keiser AR, Smith AN, Lang R, Whitsett JA. Sox2 is required for maintenance and differentiation of bronchiolar Clara, ciliated, and goblet cells. PLoS One. 2009;4:e8248. doi: 10.1371/journal.pone.0008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein B, Brownfield DG, Wu AR, Neff NF, Mantalas GL, Espinoza FH, Desai TJ, Krasnow MA, Quake SR. Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature. 2014;509:371–375. doi: 10.1038/nature13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao PN, Chen F, Izvolsky KI, Walker J, Kukuruzinska MA, Lu J, Cardoso WV. Gamma-secretase activation of notch signaling regulates the balance of proximal and distal fates in progenitor cells of the developing lung. J Biol Chem. 2008;283:29532–29544. doi: 10.1074/jbc.M801565200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development. 2009;136:2297–2307. doi: 10.1242/dev.034884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui H, Shibayama M, Ohbayashi N, Konishi M, Takada S, Itoh N. Fgf18 is required for embryonic lung alveolar development. Biochem Biophys Res Commun. 2004;322:887–892. doi: 10.1016/j.bbrc.2004.07.198. [DOI] [PubMed] [Google Scholar]
- Vallin J, Thuret R, Giacomello E, Faraldo MM, Thiery JP, Broders F. Cloning and characterization of three Xenopus slug promoters reveal direct regulation by Lef/beta-catenin signaling. J Biol Chem. 2001;276:30350–30358. doi: 10.1074/jbc.M103167200. [DOI] [PubMed] [Google Scholar]
- Van Haute L, De Block G, Liebaers I, Sermon K, De Rycke M. Generation of lung epithelial-like tissue from human embryonic stem cells. Respir Res. 2009;10:105. doi: 10.1186/1465-9921-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA, Sakuma R, Pawson T, Hunziker W, McNeill H, Wrana JL, Attisano L. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010;18:579–591. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Volckaert T, Campbell A, Dill E, Li C, Minoo P, De Langhe S. Localized Fgf10 expression is not required for lung branching morphogenesis but prevents differentiation of epithelial progenitors. Development. 2013;140:3731–3742. doi: 10.1242/dev.096560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert T, De Langhe S. Lung epithelial stem cells and their niches: Fgf10 takes center stage. Fibrogenesis Tissue Repair. 2014;7:8. doi: 10.1186/1755-1536-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert T, Dill E, Campbell A, Tiozzo C, Majka S, Bellusci S, De Langhe SP. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J Clin Invest. 2011;121:4409–4419. doi: 10.1172/JCI58097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Haviland DL, Burns AR, Zsigmond E, Wetsel RA. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:4449–4454. doi: 10.1073/pnas.0700052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Tian Y, Morley MP, Lu MM, Demayo FJ, Olson EN, Morrisey EE. Development and regeneration of Sox2+ endoderm progenitors are regulated by a Hdac1/2-Bmp4/Rb1 regulatory pathway. Dev Cell. 2013;24:345–358. doi: 10.1016/j.devcel.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Shu W, Lu MM, Morrisey EE. Wnt7b activates canonical signaling in epithelial and vascular smooth muscle cells through interactions with Fzd1, Fzd10, and LRP5. Mol Cell Biol. 2005;25:5022–5030. doi: 10.1128/MCB.25.12.5022-5030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Ma Q, Peng S, Adelmant G, Swain D, Song W, Fox C, Francis JM, Pedamallu CS, DeLuca DS, Brooks AN, Wang S, Que J, Rustgi AK, Wong KK, Ligon KL, Liu XS, Marto JA, Meyerson M, Bass AJ. SOX2 and p63 colocalize at genetic loci in squamous cell carcinomas. J Clin Invest. 2014;124:1636–1645. doi: 10.1172/JCI71545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver M, Batts L, Hogan BL. Tissue interactions pattern the mesenchyme of the embryonic mouse lung. Dev Biol. 2003;258:169–184. doi: 10.1016/s0012-1606(03)00117-9. [DOI] [PubMed] [Google Scholar]
- Weaver M, Dunn NR, Hogan BL. Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development. 2000;127:2695–2704. doi: 10.1242/dev.127.12.2695. [DOI] [PubMed] [Google Scholar]
- Weaver M, Yingling JM, Dunn NR, Bellusci S, Hogan BL. Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development. 1999;126:4005–4015. doi: 10.1242/dev.126.18.4005. [DOI] [PubMed] [Google Scholar]
- Weidenfeld J, Shu W, Zhang L, Millar SE, Morrisey EE. The WNT7b promoter is regulated by TTF-1, GATA6, and Foxa2 in lung epithelium. J Biol Chem. 2002;277:21061–21070. doi: 10.1074/jbc.M111702200. [DOI] [PubMed] [Google Scholar]
- Wessells NK. Mammalian lung development: interactions in formation and morphogenesis of tracheal buds. J Exp Zool. 1970;175:455–466. doi: 10.1002/jez.1401750405. [DOI] [PubMed] [Google Scholar]
- West KP., Jr Extent of vitamin A deficiency among preschool children and women of reproductive age. J Nutr. 2002;132:2857S–2866S. doi: 10.1093/jn/132.9.2857S. [DOI] [PubMed] [Google Scholar]
- White AC, Xu J, Yin Y, Smith C, Schmid G, Ornitz DM. FGF9 and SHH signaling coordinate lung growth and development through regulation of distinct mesenchymal domains. Development. 2006;133:1507–1517. doi: 10.1242/dev.02313. [DOI] [PubMed] [Google Scholar]
- Whitsett JA, Clark JC, Picard L, Tichelaar JW, Wert SE, Itoh N, Perl AK, Stahlman MT. Fibroblast growth factor 18 influences proximal programming during lung morphogenesis. J Biol Chem. 2002;277:22743–22749. doi: 10.1074/jbc.M202253200. [DOI] [PubMed] [Google Scholar]
- Wilson JG, Roth CB, Warkany J. An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am J Anat. 1953;92:189–217. doi: 10.1002/aja.1000920202. [DOI] [PubMed] [Google Scholar]
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G, Fukabori Y, McBride G, Nikolaropolous S, McKeehan WL. Exon switching and activation of stromal and embryonic fibroblast growth factor (FGF)-FGF receptor genes in prostate epithelial cells accompany stromal independence and malignancy. Mol Cell Biol. 1993;13:4513–4522. doi: 10.1128/mcb.13.8.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, McKeon F. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- Yates LL, Schnatwinkel C, Hazelwood L, Chessum L, Paudyal A, Hilton H, Romero MR, Wilde J, Bogani D, Sanderson J, Formstone C, Murdoch JN, Niswander LA, Greenfield A, Dean CH. Scribble is required for normal epithelial cell-cell contacts and lumen morphogenesis in the mammalian lung. Dev Biol. 2013;373:267–280. doi: 10.1016/j.ydbio.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates LL, Schnatwinkel C, Murdoch JN, Bogani D, Formstone CJ, Townsend S, Greenfield A, Niswander LA, Dean CH. The PCP genes Celsr1 and Vangl2 are required for normal lung branching morphogenesis. Hum Mol Genet. 2010;19:2251–2267. doi: 10.1093/hmg/ddq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayon A, Zimmer Y, Shen GH, Avivi A, Yarden Y, Givol D. A confined variable region confers ligand specificity on fibroblast growth factor receptors: implications for the origin of the immunoglobulin fold. EMBO J. 1992;11:1885–1890. doi: 10.1002/j.1460-2075.1992.tb05240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh BK, Igarashi M, Eliseenkova AV, Plotnikov AN, Sher I, Ron D, Aaronson SA, Mohammadi M. Structural basis by which alternative splicing confers specificity in fibroblast growth factor receptors. Proc Natl Acad Sci U S A. 2003;100:2266–2271. doi: 10.1073/pnas.0436500100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L, Domyan ET, Lewandoski M, Sun X. Fibroblast growth factor 9 signaling inhibits airway smooth muscle differentiation in mouse lung. Dev Dyn. 2009;238:123–137. doi: 10.1002/dvdy.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Wang F, Ornitz DM. Mesothelial- and epithelial-derived FGF9 have distinct functions in the regulation of lung development. Development. 2011;138:3169–3177. doi: 10.1242/dev.065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, White AC, Huh SH, Hilton MJ, Kanazawa H, Long F, Ornitz DM. An FGF-WNT gene regulatory network controls lung mesenchyme development. Dev Biol. 2008;319:426–436. doi: 10.1016/j.ydbio.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Poe B, Schwarz M, Elliot SA, Albertine KH, Fenton S, Garg V, Moon AM. Fetal and postnatal lung defects reveal a novel and required role for Fgf8 in lung development. Dev Biol. 2010;347:92–108. doi: 10.1016/j.ydbio.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Loch AJ, Radtke F, Egan SE, Xu K. Jagged1 is the major regulator of Notch-dependent cell fate in proximal airways. Dev Dyn. 2013;242:678–686. doi: 10.1002/dvdy.23965. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Goss AM, Cohen ED, Kadzik R, Lepore JJ, Muthukumaraswamy K, Yang J, DeMayo FJ, Whitsett JA, Parmacek MS, Morrisey EE. A Gata6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nat Genet. 2008;40:862–870. doi: 10.1038/ng.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Fallon TR, Saladi SV, Pardo-Saganta A, Villoria J, Mou H, Vinarsky V, Gonzalez-Celeiro M, Nunna N, Hariri LP, Camargo F, Ellisen LW, Rajagopal J. Yap Tunes Airway Epithelial Size and Architecture by Regulating the Identity, Maintenance, and Self-Renewal of Stem Cells. Dev Cell. 2014 doi: 10.1016/j.devcel.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- Zhou L, Graeff RW, McCray PB, Jr, Simonet WS, Whitsett JA. Keratinocyte growth factor stimulates CFTR-independent fluid secretion in the fetal lung in vitro. Am J Physiol. 1996;271:L987–994. doi: 10.1152/ajplung.1996.271.6.L987. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell. 2007;13:103–114. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]