Abstract
Since its discovery in 1994, cellular functions for the scaffold protein IQGAP1 have expanded immensely. Over 100 unique IQGAP1 interacting proteins have been identified, implicating IQGAP1 as a critical integrator of cellular signaling pathways. Initial research established functions for IQGAP1 in cell-cell adhesion, cell migration, and cell signaling. Recent studies have revealed additional IQGAP1 binding partners, expanding the biological roles of IQGAP1. These include crosstalk between signaling cascades, regulation of nuclear function, and Wnt pathway potentiation. Investigation of the IQGAP2 and IQGAP3 homologues demonstrate unique functions, some of which differ from those of IQGAP1. Summarized here are recent observations that enhance our understanding of IQGAP proteins in integrating diverse signaling pathways.
Keywords: IQGAP1, IQGAP2, IQGAP3, scaffold, signaling
IQGAPs regulate cellular functions
Since the discovery of IQGAP1 20 years ago [1], over 100 interacting proteins and diverse functions have been identified. IQGAP proteins are expressed in eukaryotes, from Saccharomyces cerevisiae to humans. Mammals express three isoforms: IQGAP1, IQGAP2 and IQGAP3 (Box 1) that have similar domain composition (Box 2), but divergent functions, tissue expression and subcellular localization [1–3]. IQGAPs regulate diverse biological processes, and several reviews have covered IQGAP1 functions in the cytoskeleton [4, 5], cell-cell adhesion [6], Ca2+ and small G-protein signaling [4], protein trafficking [7], neoplasia [8, 9], and microbial pathogenesis [10]. The multiple domains in IQGAPs mediate protein-protein interactions with an array of binding partners that regulate a myriad of signaling pathways (Table 1 lists selected interactors). IQGAP1 coordinates communication between binding partners through a number of different mechanisms, including serving as a scaffold.
Box 1. Functions of IQGAP isoforms.
IQGAP1, which is ubiquitously expressed, is the best-characterized IQGAP isoform. Considerably less is known about IQGAP2 and, especially, IQGAP3. Salient functions of IQGAP2 and IQGAP3 are summarized below, and are incorporated in the main text where protein interactors and/or functions analogous to those for IQGAP1 have been evaluated.
IQGAP2 acts as a tumor suppressor
IQGAP2, which is expressed predominantly in the liver, was first described in 1996 [2]. Despite their 62% sequence identity, IQGAP1 is an oncogene, while IQGAP2 is a tumor suppressor [8, 9]. Decreased expression of IQGAP2 was observed in human hepatocellular [92–94], prostate [54] and gastric [95] carcinomas. In hepatocellular carcinoma, IQGAP1 and IQGAP2 are reciprocally altered, with increased IQGAP1 and decreased IQGAP2 [94]. Interestingly, IQGAP2 knockout mice develop hepatocellular carcinoma, but IQGAP1 and IQGAP2 double knockout mice have normal survival [89], suggesting opposing functions for IQGAP1 and IQGAP2. IQGAP2 also participates in metabolism, as IQGAP2 null mice have impaired uptake of long-chain fatty acids and enhanced insulin sensitivity [96]. The mechanisms that mediate the opposing effects of IQGAP1 and IQGAP2 are unknown.
IQGAP3 regulates cell proliferation and motility
Although identified in 2007 [3], IQGAP3 has received little attention. Analogous to IQGAP1, IQGAP3 promotes proliferation of liver [97] and mammary [47] epithelial cells. IQGAP3 expression also correlates with enhanced migration, invasion and proliferation of lung cancer cells [48]. Both IQGAP1 and IQGAP3 promote extracellular signal-regulated kinase (ERK) activation. However IQGAP3 interacts only with ERK1 [48], whereas IQGAP1 interacts with ERK1 and ERK2 [13, 14]. IQGAP1 [87] and IQGAP3 [3] both regulate the formation of membrane extensions during neurite outgrowth. Moreover, depletion of IQGAP1 [86] or IQGAP3 [98] attenuated the accumulation of adenomatous polyposis coli (APC) at the leading edge of migrating Vero cells or PC12 extensions, respectively. Additional studies are needed to elucidate the binding partners and biological roles of IQGAP3.
Box 2. Unique properties of IQGAP domains.
IQGAP1, IQGAP2, and IQGAP3 contain several domains, namely a calponin homology domain (CHD), WW domain, IQ region, Ras GTPase-activating protein-related domain (GRD), and RasGAP_C-terminus (RGCT). This domain composition is not identical in all organisms (e.g., Iqg1p in Saccharomyces cerevisiae lacks the WW domain) and the number of IQ motifs varies among IQGAPs [70].
While almost all the domains present in the IQGAPs are found in numerous other proteins, they have unique properties in IQGAPs. These include both the molecules that bind to the domains and the mechanisms of interaction. The association of F-actin with the CHD is a function conserved with several other actin-binding proteins. However, while most actin-binding proteins bind F-actin via tandem CHDs, IQGAP1 forms a high affinity interaction with F-actin through a single CHD [99, 100]. Additionally, the IQGAP1 CHD associates with calmodulin and Ca2+ [12]. Ca2+ association is not common for CHDs. While the IQ domain in IQGAP1 binds to typical IQ binding partners, such as calmodulin [12, 101] and S100 [102, 103] proteins, the interaction of calmodulin with the IQ motifs of IQGAP1 [104] differs from its interaction with the IQ motifs in unconventional myosins. Moreover, the IQGAP1 IQ domain forms unexpected interactions with EGFR [39], MEK [14], Rap1 [105], and other proteins [106]. The IQ motifs in IQGAP1 also mediate the formation of IQGAP1 dimers or oligomers [90], which adds further complexity to IQGAP1 signaling. Collectively, these data suggest that the IQ motifs in IQGAP1 are different to those in other proteins. WW domains usually bind to proline-rich regions in the binding partner. By contrast, the IQGAP1 WW domain binds to proteins lacking a proline-rich region, like ERK1/2 [13, 14]. The GRD illustrates other unique properties of IQGAPs. Though similar in sequence to GAPs, the IQGAP GRD has an arginine replaced by threonine at a conserved catalytic residue found in GAPs [107]. This is a key feature to bind to and stabilize small G-proteins in their GTP bound form. In addition, analysis with a panel of mutant Cdc42 and Rac1 constructs revealed that the residues involved in the complexes formed with IQGAP1 differ from those formed with other effector proteins and GAPs [108]. The RGCT is a region unique to IQGAPs and binds diverse targets. Additionally, regions of IQGAPs outside the recognized domains interact with binding partners. For example, ShcA binds to IQGAP1 between the CHD and WW domain. While ShcA usually has a high affinity interaction with tyrosine phosphorylated sites in NPxpY sequences through its PTB domain, unphosphorylated IQGAP1 binds outside of the phosphotyrosine binding pocket found in the PTB [41]. Furthermore, other molecules can bind to the extreme C-terminus of IQGAPs. For example, IQGAP1 and IQGAP2 contain an atypical phosphoinositide binding domain in this region that mediates their association with PtdInsP3 [35]. This PtdInsP3 binding region in IQGAP1 and IQGAP2 differs from all previously identified PtdInsP3 binding regions. The unique binding properties of the IQGAPs facilitate the formation of multiple signaling complexes to regulate diverse cellular processes.
Table I.
Selected IQGAP interacting proteinsa.
| Interactor | Proposed Function(s)b | Reference |
|---|---|---|
| IQGAP1, IQGAP2 & IQGAP3 | ||
| Calmodulin | Regulates IQGAP1 interactions with other proteinsc | IQGAP1 [12, 101, 109] IQGAP2 [2] IQGAP3g [110] |
| Cdc42 | Stabilizes active Cdc42-GTPd Promotes cell motilityc |
IQGAP1 [23, 109, 111, 112] IQGAP2 [2] IQGAP3 [3] |
| F-actin | Promotes actin polymerizationc | IQGAP1 [12, 111, 113–115] IQGAP2 [116] IQGAP3 [3] |
| Rac1 | Stabilizes active Rac1-GTPd Promotes cell motilityc |
IQGAP1 [111] IQGAP2 [2] IQGAP3 [3] |
| IQGAP1 and IQGAP2 | ||
| β-catenin | IQGAP1: Modulates cell-cell adhesion; enhances β-catenin transcriptional activity IQGAP2: Modulates Wnt/ β-catenin signaling |
IQGAP1 [65, 71] IQGAP2 [89] |
| IQGAP1 and IQGAP3 | ||
| ERK1 | Modulates ERK1 activation | IQGAP1 [14] IQGAP3 [48] |
| Ras | IQGAP1: Promotes the interaction between K-Ras and B-Raf IQGAP3: Modulates H-Ras/ERK signaling |
IQGAP1 [44] IQGAP3 [47] |
| IQGAP1 interactors | ||
| RTKs | ||
| EGFRe | Modulates EGFR activation | [39] |
| FGFR1 | Bridges FGFR1 to N-WASP-Arp2/3 complex | [117] |
| HER2 | Regulates HER2 levels and activity | [51] |
| PDGFβR | Modulates focal adhesion assembly | [18] |
| VEGFR2 | Cell migration and proliferation, vascular repair and maintenance, angiogenesis | [50, 118] |
| Receptor serine/threonine kinase | ||
| TGFβR2 | Regulates TGFβR2 degradation and signaling | [119] |
| GPCRs | ||
| CXCR2 | Unknown | [120] |
| GPR161 | Regulates cell migration and proliferation | [62] |
| KISS1R | Connects KISS1R to EGFR activation | [121] |
| LPA1 | Regulates cell migration and invasion | [61] |
| MAPK components | ||
| B-Raf | Regulates MAPK activation | [45, 46] |
| C-Raf | Regulates MAPK activation | [122, 123] |
| ERK2 | Modulates ERK2 activation | [13] |
| MEK1 | Regulates MAPK activation | [14] |
| MEK2 | Regulates MAPK activation | [14] |
| Lipids and their regulators | ||
| Akt | Regulates Akt activation | [50, 51, 122, 124] |
| DGKζe | Phagocytosis by macrophages | [125] |
| Protein 4.1R | Modulates cell migration | [126] |
| Ezring | Unknown | [127] |
| mTorc1 | Modulates cell growth | [52, 124] |
| PtdIns4,5P2 | Promotes actin polymerization and branching | [34] |
| PtdInsP3f | Unknown | [35, 128]g |
| PIPKIγ | Recruits IQGAP1 to leading edge membrane | [34] |
| Scaffolds | ||
| AKAP220 | IQGAP1: Integrates Ca2+ and cAMP signals; regulates cell migration IQGAP2: Recruits active Rac1 to promote membrane ruffling. |
IQGAP1 [27] IQGAP2 [26] |
| β-arrestin2 | Forms complex with IQGAP1 and LPA1 or GPR161 to regulate cell migration | [61, 62] |
| p14-MP1 | Regulates focal adhesion maturation | [21] |
| ShcA | May link RTK to cytoskeleton | [41] |
| Small GTPases and their regulators | ||
| FGD6 | Regulates podosome formation | [129] |
| Rab27a | Regulates endocytosis of insulin secretory membranes | [31] |
| RacGAP1 | Regulates cell migration and invasion | [24] |
| Ran | Regulates β-catenin transcriptional function | [67] |
| Rap1 | Regulates Rap1 activation | [105] |
| RhoA/C | Modulates RhoA/C activation; regulates cell proliferation and migration | [130] |
| Tiam1 | Unknown | [131] |
| Wnt signaling molecules | ||
| Dvl | Facilitates nuclear import of Dvl/β-catenin complex and modulates Wnt signaling | [67, 68] |
| LGR4e,f | Required for potentiation of β-catenin signaling by RSPO | [66] |
| MCAM | Required for WRAMP structure assembly; bridges MCAM to cytoskeleton | [30] |
| Nuclear proteins | ||
| ERα | Modulates ERα transcriptional function | [73] |
| ERα | Unknown | [73] |
| Importin-β5 | Modulates nuclear import of the IQGAP1/β-catenin/Dvl complex and transactivation of Wnt target genes | [67] |
| NFAT1 | Regulates nuclear translocation and function | [75] |
| NRONf | Forms RNA-scaffold complex to regulate NFAT1 | [75] |
| PCNAh | Unknown. | [72] |
| RNase L | Required for ECyd-induced JNK phosphorylation and apoptosis | [132] |
| RPA2h | Unknown | [72] |
| TrkA | Unknown | [133] |
| mRNA regulation | ||
| SMG-9 | Unknown | [84] |
| Staufen | Unknown | [81] |
| Adhesion associated proteins | ||
| CD13 | Unknown | [134] |
| Integrin β3 | Regulates pulmonary vascular permeability | [135] |
| Filamin-A | Regulates directional cell migration | [25] |
| Nephrin | Regulates podocyte migration and barrier functions | [136] |
| N-cadherin | Links N-cadherin to ERK1/2 signaling during fear memory formation | [137] |
| Podocin | Regulates podocyte migration and barrier functions | [136] |
| VASP | Unknown | [138] |
| Kinases and phosphatases | ||
| Aurora A | Stabilizes Aurora A | [139] |
| PTPμ | Mediates neurite outgrowth | [140] |
| Neuronal proteins | ||
| NR2A | Contributes to NMDAR trafficking and ERK signaling | [141] |
| NR2B | Contributes to NMDAR trafficking and ERK signaling | [141] |
| PSD-95 | Contributes to NMDAR trafficking and ERK signaling | [141] |
Interactors were selected to illustrate the diverse cellular processes regulated by IQGAPs. Several additional putative interactors have been identified by mass spectrometry analysis [127, 142–144]; interactions without further validation were excluded.
Refers to proposed functional consequences of the interaction with IQGAPs
No published evidence for this function for IQGAP2 or IQGAP3
Function demonstrated for IQGAP1 and IQGAP2, but not IQGAP3
Also interacts with IQGAP3; function undetermined
Also interacts with IQGAP2; function undetermined
Binding to full-length protein was not examined
Interaction identified via co-localization only.
Scaffold proteins can assemble pathway components to regulate signaling [11]. A scaffolding function for IQGAP1 was first proposed when it was observed to link Ca2+/calmodulin and Cdc42 signaling [12]. Perhaps the best characterized example of IQGAP1 scaffold function is in the mitogen-activated protein kinase (MAPK) pathway (see Glossary) [13, 14]. IQGAP1 scaffolds several components of the MAPK signaling pathway to facilitate diverse cellular functions [13, 14]. Moreover, recent evidence demonstrates interactions between scaffolds that may modulate signaling. IQGAP1 interacts with other MAPK scaffolds, such as MP1 and β-arrestin, which permits communication between complexes and may provide precise control of signaling (Figure 1A). Although the biological roles remain to be established, interactions between IQGAP1 and other scaffolds have the potential to influence a variety of pathways. Scaffold-scaffold interactions may mediate signaling from discrete subcellular locations in response to specific stimuli. For example, activation of protein kinase C (PKC) promotes MAPK signaling along the cytoskeleton in a caveolin-1- and IQGAP1-dependent pathway [15]. Caveolin-1 scaffolds upstream signaling components, while IQGAP1 assembles downstream components to link MAPK signaling to the cytoskeleton [15] (Figure 1Bi). Alternatively, individual scaffolds may compete for binding to common signaling components. By sequestering specific proteins, the scaffold can ensure that a particular stimulus activates the appropriate pathway or negatively regulates signaling (Figure 1Bii). Thus, interactions between IQGAPs and other scaffolds exert meticulous control of cellular responses to distinct stimuli. Here we focus on emerging roles for IQGAPs in vertebrates, with an emphasis on IQGAP1 as an integrator of cell signaling.
Figure 1. Models of IQGAP1 interactions with scaffolds.
(A) (i) Activation of cell surface receptors leads to downstream signaling. Interactions between IQGAP1 and several signaling components have been identified, including cell surface receptors (e.g., EGFR) [39], the adaptor ShcA [41], and the small GTPase Ras [44]. IQGAP1 scaffolds the Raf [45, 46], MEK [13] and ERK kinases [13, 14] to promote ERK activation. Interactions of IQGAP1 with the MAPK scaffolds β-arrestin2 [61, 62] (ii) and MP-1 [21] (iii) affect recruitment of IQGAP1 to the leading edge of migrating cells and focal adhesion maturation, respectively. (B) (i) Activation of PKC by phorbol esters initiates signaling upstream of IQGAP1. PKC activates Ras on caveolin-1 which communicates with Raf, MEK and ERK on IQGAP1 that links ERK activation with the actin-cytoskeleton [15]. Scaffold-scaffold interactions allow for the formation of complete signaling complexes. (ii) Alternatively, competition for common signaling components between scaffolds may inhibit signaling. (iii) IQGAP1 interacts with AKAP220 in a complex that integrates Ca2+ and cAMP signaling. In the presence of Ca2+, IQGAP1 binds to AKAP220 and associates with microtubules through CLASP2. In unstimulated cells, GSK3β phosphorylates CLASP2, preventing its interaction with IQGAP1. Synthesis of cAMP activates PKA, which inhibits GSK3β by phosphorylation. Additionally, PP1 phosphatase dephosphorylates CLASP2, promoting the IQGAP1-CLASP2 interaction [26]. Abbreviations: βArr2, β-arrestin2; CaM, calmodulin; DAG, diacylglycerol; EGFR, epidermal growth factor receptor; FA, focal adhesion; GSK3β, glycogen synthase kinase 3β; GPCR, G-protein coupled receptor; Kin1, kinesin-1; LE, late endosome; MT, microtubule; PI3K, phosphatidylinositol-3-kinase; PKA, protein kinase A; PKC, protein kinase C; PP1, protein tyrosine phosphatase-1; PtdIns3,4,5P3, phosphatidylinositol 3, 4, 5 trisphosphate.
IQGAP1 regulates cell migration
Through its ability to regulate the cytoskeleton and integrate cellular signaling pathways, IQGAP1 is a well-established component of cell migration (reviewed in [16]). Nevertheless, recent evidence has identified additional complexity in the molecular mechanisms by which IQGAP1 modulates migration. Adherent cells form focal adhesions through integrins that attach to the extracellular matrix (ECM), linking it to the cytoskeleton [17]. IQGAP1 participates in focal adhesion assembly, maturation and turnover, which are required for proper cell motility. For example, platelet-derived growth factor (PDGF) promotes the formation of a complex containing IQGAP1, PDGF receptor β (PDGFRβ), and the focal adhesion proteins paxillin and vinculin [18]. This study suggests that IQGAP1 is necessary for focal adhesion assembly at the leading edge of vascular smooth muscle cells.
During migration, focal adhesions are assembled and disassembled in a coordinated fashion. MP1 plays a role in focal adhesion dynamics and activates ERK1 by scaffolding MEK1 and ERK1 [19, 20]. This complex is bound through the p14 protein to late endosome trafficking compartments [20]. Recently, MP1 and p14 were found to traffic along microtubules toward focal adhesions during focal adhesion maturation [21]. Interestingly, siRNA knockdown of p14 or MP1 resulted in defects in migration with elongated focal adhesions that accumulate IQGAP1, indicating impaired focal adhesion dynamics (Figure 1Aiii) [21]. Whether MAPK signaling from IQGAP1 or MP1 is affected by this interaction was not investigated. Potentially, MP1 and IQGAP1 regulate the localization and function of one another in focal adhesions and/or MAPK signaling from endosomes.
Signaling from integrins also impacts actin dynamics. At the leading edge of migrating cells, actin polymerization drives protrusion, which is controlled by small GTPases like Rac1 and Cdc42 [22]. In migrating cells, IQGAP1 localizes at the leading edge and promotes cell migration in a Rac1- and Cdc42-dependent manner [23]. Furthermore, IQGAP1 forms a complex with filamin-A, an actin cross-linking protein, and activated β1 integrin to recruit RacGAP1, which inactivates Rac1 and activates RhoA GTPase [24, 25]. Decreased expression of IQGAP1, filamin-A or RacGAP1 results in uncontrolled membrane protrusion and impaired directional cell migration [25]. Thus, IQGAP1 regulates cell motility at the leading edge by coordinating focal adhesion and cytoskeletal dynamics.
IQGAPs also influence cell motility through interactions with AKAP220 [26, 27]. AKAP (A-kinase anchoring protein) scaffold proteins are targeted to distinct cellular compartments where they assemble signaling complexes with protein kinase A (PKA) and other kinases, phosphatases and proteins to integrate [Ca2+]i (intracellular free Ca2+ concentrations) and other signaling pathways [28]. IQGAP1 and IQGAP2 were each documented to associate with AKAP220 [26, 27], but the functional sequelae differ. A complex comprising IQGAP1, AKAP220, PKA and glycogen synthase kinase 3β (GSK-3β) is present in cells, which integrates Ca2+ and cAMP signals [27]. Increasing [Ca2+]i enhances the interaction of IQGAP1 with both AKAP220 and CLASP2, a microtubule binding protein. GSK-3β phosphorylation of CLASP2 inhibits its binding to IQGAP1. However, cAMP activates PKA, which phosphorylates GSK-3β, inhibiting its kinase activity. Protein phosphatase 1 (PP1) may mediate dephosphorylation of CLASP2, thereby promoting CLASP2 interaction with IQGAP1 [27]. AKAP220 anchors IQGAP1 to the leading edge of migrating cells, where this complex enhances CLASP2-IQGAP1 interaction with microtubules, promoting cell migration (Figure 1Biii) [27]. The functional interaction of AKAP220 with IQGAP2 is different. In a complex with AKAP220, PKA phosphorylates IQGAP2 at T716, which enhances Rac1 binding and promotes membrane ruffling [26]. PKA catalyzes IQGAP2 phosphorylation in vitro, whereas IQGAP1 is not a substrate for this kinase. While IQGAP1 and IQGAP2 interact with AKAP220, each has a unique mechanism to control cell motility. A mass spectrometry screen suggests IQGAP3 may bind AKAP220 [27], but the data are preliminary and require confirmation. It is possible that other members of the AKAP family could have functional interactions with IQGAPs.
At the trailing edge of the cell, focal adhesions are disassembled and motor proteins pull the cell along the cytoskeleton [22]. Roles for IQGAP1 at the trailing edge are now being defined. In some cell lines, such as B16F10 mouse melanoma, IQGAP1 is present in regions of the cell undergoing retraction, distinct from the leading edge or focal adhesions [29]. By contrast, IQGAP2 localizes to protruding edges [29], suggesting distinct roles for the isoforms in cell motility. The secreted signaling protein Wnt5a promotes the formation of the Wnt-receptor-actin-myosin-polarity (WRAMP) structure, which coordinates retraction at the trailing edge. WRAMP assembly requires an interaction between IQGAP1 and the transmembrane protein melanoma cell adhesion molecule (MCAM) in melanoma cells [30]. Once assembled, WRAMP coordinates several processes that promote membrane retraction, including Ca2+ release from the endoplasmic reticulum at the rear of the cell. Ca2+ both activates the protease calpain, which cleaves proteins at the trailing edge, and facilitates actomyosin contraction, leading to membrane retraction [30]. These findings indicate that IQGAP1 operates at both the leading and trailing edge of migrating cells.
IQGAP1 in endocytosis
Analysis of the insulin secretory pathway revealed a role for IQGAP1 in endocytosis [31]. In pancreatic β-cells, glucose stimulates fusion of insulin secretory vesicles with the plasma membrane. Following insulin release, the secretory membrane is endocytosed, which requires Rab27a-GDP and coronin-3, an actin regulatory protein. A complex of IQGAP1 and Cdc42 recruits Rab27a and coronin-3 to endocytic sites on the plasma membrane [31]. Depletion of IQGAP1 or inhibition of the IQGAP1-Rab27a interaction reduces clathrin-dependent endocytosis. Thus, IQGAP1 scaffolds Cdc42, Rab27a and coronin-3 to control vesicle tethering and endocytosis in β-cells.
IQGAPs and phosphoinositides
The membrane lipid phosphatidylinositol (PtdIns) can be phosphorylated on its d-myo-inositol head group at the 3, 4 or 5 hydroxyl to generate phosphatidylinositol monophosphates (PtdInsP), bisphosphates (PtdInsP2) and trisphosphate (PtdInsP3) signaling molecules. These PtdInsPn molecules bind to proteins containing phosphoinositide binding domains to regulate their subcellular localization or induce conformational changes to mediate functions [32]. At the plasma membrane, phosphorylation of PtdIns4P by type I phosphatidylinositol-4-phosphate-5-kinase (PIPKI) generates high levels of PtdIns4,5P2. PtdIns4,5P2 can directly bind to effector molecules, be phosphorylated by phosphatidylinositol-3-phosphate-kinase (PI3K) to generate the signaling lipid, PtdInsP3, or cleaved by phospholipase C (PLC) to form the second messengers DAG and IP3 [32]. PtdInsP3 can then activate phosphoinositide-dependent-kinase-1 (Pdk1) and Akt to promote proliferation and survival [33]. Beyond its participation in Akt activation downstream of phosphoinositides (see RTK signaling), IQGAP1 also binds directly to phosphoinositides.
IQGAP1 was identified in a screen for PIPKIγ interacting proteins [34]. In addition to directly binding PIPKIγ through its IQ motifs, IQGAP1 interacts with the lipid product, PtdIns4,5P2, through a polybasic motif in the RGCT. Together these interactions recruit IQGAP1 to the plasma membrane. PtdIns4,5P2 binding to IQGAP1 enhances actin polymerization and branching through N-Wiskott-Aldrich syndrome protein (N-WASP)-Actin related protein 2/3 (Arp 2/3) complex in vitro [34]. Thus, PtdIns4,5P2 modulates both IQGAP1 localization and its ability to regulate the cytoskeleton. Additionally, IQGAP1 and IQGAP2, but not IQGAP3, bind to PtdInsP3 through an atypical-phosphoinositide binding motif near their extreme C-termini [35], which could provide another signal to recruit IQGAP1 and IQGAP2 to membranes. Both the PtdIns4,5P2 and PtdInsP3 binding sites are located near the C-terminus, but it is unknown whether the two lipids compete for binding to IQGAP1 or whether both lipids can bind simultaneously.
IQGAP1 modulates cellular signaling
Extracellular molecules, such as growth factors and cytokines, are detected by specific receptors (e.g., receptor tyrosine kinases (RTKs) and G-protein coupled receptors (GPCRs) [36, 37]) that transduce the information into cellular responses. IQGAP1 modulates signaling in response to a variety of inputs by regulating numerous receptors (see [38] for a discussion of early publications) (Table 1). Several additional receptors have been identified as IQGAP binding partners.
RTK signaling
The epidermal growth factor receptor (EGFR) cascade is a classic example of RTK signaling (Figure 1Ai). EGF binding to EGFR activates its intrinsic tyrosine kinase, leading to receptor autophosphorylation which triggers signaling cascades, such as MAPK and PI3K-Akt pathways. IQGAP1 binds directly to EGFR and modulates EGFR activation [39]. Loss of IQGAP1 attenuates EGF-stimulated EGFR phosphorylation, which is rescued by wild-type IQGAP1. EGFR stimulates PKCα, which phosphorylates IQGAP1 on S1443 [39]. Importantly, a phosphomimetic IQGAP1 mutant construct (Ser1443 is replaced with Glu) enhances EGFR phosphorylation. These findings indicate that interdependent regulation of IQGAP1 and EGFR is necessary for EGF to fully activate the receptor.
Adaptor proteins with phosphotyrosine binding (PTB) or Src homology 2 (SH2) domains, such as ShcA and Grb2 [40], recognize tyrosine phosphorylated proteins, including active EGFR [36] (Figure 1Ai). The PTB of ShcA binds IQGAP1, but surprisingly this interaction is constitutive and does not require tyrosine phosphorylation of IQGAP1 [41]. EGF stimulation recruits ShcA and IQGAP1 to membrane ruffles, which is necessary for lamellipodium formation. Although the structure of a fragment of IQGAP1 bound to ShcA PTB has been solved [41], the biological role of the interaction and a potential link to MAPK signaling remain to be established.
To propagate signaling, Grb2 binds to and activates son-of-sevenless (Sos), which promotes activation of the GTPase Ras (Figure 1Ai). Active Ras stimulates the sequential phosphorylation from Raf kinase to MAPK-ERK kinase (MEK) to extracellular signal-regulated kinase (ERK). Active ERK then phosphorylates substrates in the cytoplasm and nucleus to affect biological processes. Signal transduction from the receptor at the plasma membrane to downstream MAPK components requires coordination among signaling proteins. IQGAP1 associates with several MAPK proteins, including EGFR [42, 43], K-Ras [44], Raf [45, 46], MEK [14] and ERK [13, 14] (Figure 1Ai) (Table 1), providing a scaffold which promotes EGF-induced MAPK signaling [13, 45]. IQGAP3, which interacts with H-Ras [47] and ERK1 [48], also augments EGF-stimulated MAPK signaling.
In addition to MAPK, several RTKs also signal through PI3K, which produces PtdInsP3 that activates Akt [49]. Knockdown of IQGAP1 abrogates Akt activation by vascular endothelial growth factor receptor 2 (VEGFR2) [50] or human epidermal growth factor receptor 2 (HER2) [51]. Increased IQGAP1 expression promotes Akt signaling in hepatocellular carcinoma [52], but has no effect on Akt signaling in thyroid cancer [53]. IQGAP2 overexpression inhibits Akt activation in prostate cancer cells [54]. By contrast, neither overexpression nor knockdown of IQGAP3 in HeLa and A549 lung adenocarcinoma cell lines, respectively, alters Akt activation [48]. Interestingly, phosphatase and tensin homolog (PTEN), which terminates Akt signaling by dephosphorylating PtdInsP3 [49], co-immunoprecipitates with IQGAP1 [55]. Although the function of this interaction has not been identified, it could serve as an additional point of regulation of Akt. While IQGAP1 associates with several components of the Akt pathway, additional studies are required to ascertain whether IQGAPs scaffold Akt signaling.
IQGAP1 can influence cytoskeletal dynamics downstream of RTKs without a direct interaction with the receptor itself. Hepatocyte growth factor (HGF) activates the RTK, c-MET, which regulates the cytoskeleton and cell-cell contacts [56]. HGF stimulation strengthens barrier function in some cell types, e.g., endothelial cells [57]. siRNA knockdown of IQGAP1 or Rac1 attenuates HGF-induced increase in endothelial barrier strength [58]. HGF induced the formation of a Rac-1 dependent complex containing IQGAP1, EB1 and cortactin. Cortactin promotes actin polymerization, and the complex is necessary for cortical actin remodeling and peripheral microtubule growth that are required for strengthening barrier functions [58]. These findings indicate that IQGAP1 functions as a scaffold between actin and microtubules to promote enhanced barrier functions downstream of HGF.
In some tissues, HGF promotes disassembly of cell-cell adhesions and cell migration [56]. For example, HGF induces colony escape of DU145 prostate cancer and HT29 colon cancer cells [59]. This effect is mediated by a complex between IQGAP1, E-cadherin and PAK6 [59], a kinase that is often overexpressed in cancers [60]. IQGAP1 promotes activation of PAK6, which then phosphorylates β-catenin, a component of cell-cell junctions, to mediate junction disassembly [59]. Thus, RTKs and IQGAP1 are critical regulators of the cytoskeleton for cell-cell contacts and migration.
β-arrestins and GPCR signaling
Most commonly, activated GPCRs signal through their associated heterotrimeric G-proteins (Gα and Gβ/γ), altering cellular concentrations of second messengers, including cyclic AMP (cAMP), diacylglycerol (DAG) and inositol trisphosphate (IP3) [37]. Alternatively, β-arrestins associate with the cytoplasmic tails of GPCRs to serve as scaffolds for signaling pathways, such as MAPK, independent of G-proteins [37]. Recent studies identified multiprotein complexes comprising IQGAP1, β-arrestin2 and the GPCRs, lysophosphatidic acid receptor 1 (LPA1) [61] or GPR161 [62]. Each receptor independently forms a complex with β-arrestin2 and IQGAP1 that promotes cell motility [61, 62], or proliferation [62]. The consequences of these interactions on signaling through IQGAP1 are poorly characterized. β-arrestin2 also scaffolds Raf, MEK and ERK [37] (Figure 1Aii). Whether IQGAP1 and β-arrestin share common signaling components remains to be determined. The interactions between the scaffolds may permit signaling crosstalk to conceivably enhance or inhibit β-arrestin function (Figure 1Bii).
Wnt signaling
Secreted Wnt glycoproteins bind to transmembrane receptors, activating intracellular signaling cascades that lead to cell proliferation, migration, differentiation, and polarity [63]. There are two main pathways, the canonical pathway, which requires β-catenin, and the non-canonical pathways, which include the planar cell polarity (PCP) pathway and the Wnt/Ca2+ pathway [64] (Figure 2). Interestingly, IQGAP1 enhances signaling through both canonical and non-canonical Wnt pathways [65–68].
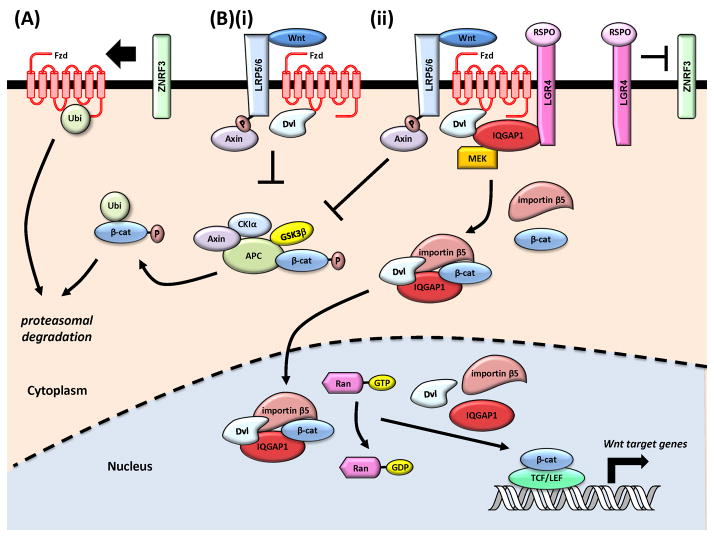
Figure 2. Models of IQGAP1 enhancement of Wnt signaling.
A, B Canonical Wnt pathway; C, D Noncanonical Wnt pathway. (A) In the resting state, nuclear translocation and activation of β-catenin is inhibited by the APC destruction complex, which sequesters β-catenin in the cytoplasm and leads to its ubiquitination and degradation by the proteasome. The Fzd receptor is also targeted for degradation by ZNRF3 ubiquitin ligase [64]. (B) (i) IQGAP1 regulates β-catenin in canonical Wnt signaling. Wnt binds to the Fzd-LRP5/6 receptor complex, recruits Dvl, inactivates the APC destruction complex, and β-catenin is stabilized and translocates to the nucleus [64]. (ii) Although not required for canonical Wnt signaling, RSPO augments this pathway [64]. Wnt/RSPO co-stimulation activates LGR4 and clears ZNRF3 from the cytoplasm, which increases the amount of active Fzd in the Wnt-Fzd/RSPO-LGR4/IQGAP1 “supercomplex” [66]. This enhances β-catenin/IQGAP1/Dvl complex formation and facilitates the nuclear import of β-catenin in an importin-β5/Ran dependent manner [67, 68]. Ran-GTP hydrolysis releases β-catenin, which interacts with TCF/LEF transcription factors. (C) The noncanonical planar cell polarity pathway regulates oriented cell movement for polarization. Wnt/RSPO increases the association of IQGAP1 with the actin polymerization proteins mDia1 and N-WASP and activates the small GTPases Rho, Rac1, and Cdc42 to promote cell polarity [66]. This may occur through association of IQGAP1 with Dvl, a reported activator of Rho GTPases. Abbreviations: APC, adenomatous polyposis coli; β-cat, β-catenin; CK1, casein kinase 1; Dvl, Dishevelled; DYRK, dual-specificity tyrosine-regulated kinase; FAK, focal adhesion kinase; Fzd, Frizzled; GSK3β, glycogen synthase kinaseβ; LRP5/6, Frizzled-low density lipoprotein receptor-related proteins; mDia1, Diaphanous-related formin-1; N-WASP, Wiskott–Aldrich Syndrome protein; PCP, planar cell polarity; P, phosphorylation.
β-catenin is both a key signaling molecule in the canonical Wnt pathway [64] and an integral structural component in cell-cell adhesion [69]. In resting epithelial cells, β-catenin is primarily localized at cell-cell junctions. Accumulation of cytoplasmic and nuclear β-catenin is repressed by the adenomatous polyposis coli (APC) destruction complex, which phosphorylates β-catenin to mark it for ubiquitination and degradation (Figure 2A). Wnt binding to Frizzled (Fzd) and the co-receptor lipoprotein receptor-related proteins 5/6 (LRP5/6) recruits Dishevelled (Dvl), destabilizing the APC destruction complex (Figure 2Bi). Stabilized β-catenin translocates into the nucleus to target TCF/LEF transcription factors and activate Wnt target genes [64].
IQGAP1 influences β-catenin function in several ways. Binding of IQGAP1 to β-catenin regulates cell-cell adhesion and actin polymerization [70, 71]. We documented that IQGAP1 also regulates β-catenin in canonical Wnt signaling [65] (Figure 2Bii). In human colon carcinoma cells, overexpression of IQGAP1 enhances β-catenin nuclear accumulation and transcriptional co-activation. Investigation into the mechanism revealed that overexpression of IQGAP1 slows the turnover of soluble, but not total, β-catenin. These results suggest that IQGAP1 sequesters β-catenin in the cytoplasm, protects it from degradation, and increases β-catenin nuclear import.
Expanding upon these initial studies, IQGAP1 may enhance canonical Wnt signaling by acting as a shuttling protein that binds to Wnt pathway components and facilitates their nuclear translocation [67, 68] (Figure 2Bii). In Wnt stimulated Xenopus embryos, IQGAP1 interacts with both β-catenin and Dvl to form a complex [67, 68]. Importin-β5 and Ran stimulate the nuclear import of the IQGAP1/β-catenin/Dvl complex and transactivation of Wnt target genes [67]. However, it has not been established whether this mechanism operates in mammalian cells. Nonetheless, these findings suggest that IQGAP1 could enhance the canonical nuclear function of β-catenin by sequestering it from degradation and by acting as a scaffold for β-catenin and Dvl to enhance their nuclear transport.
IQGAP1 also promotes canonical Wnt signaling by acting in concert with R-spondins (RSPOs) [66]. RSPOs are secreted growth factors that bind to leucine-rich repeat-containing G-protein coupled receptors (LGRs) and have been reported to enhance Wnt signaling [64]. IQGAP1 interacts with LGR4 to mediate RSPO1-induced Wnt/β-catenin target gene expression and LRP6 phosphorylation [66]. Furthermore, in mammalian cells overexpressing both IQGAP1 and LGR4, a complex containing LGR4, Dvl, LRP6, and MEK1/2 co-immunoprecipitates with IQGAP1, suggesting that MEK1/2 recruitment by IQGAP1 may phosphorylate and activate LRP6, which destabilizes the APC destruction complex, allowing β-catenin to enter the nucleus (Figure 2Bii).
The two non-canonical Wnt pathways are the planar cell polarity (PCP) and Wnt/Ca2+ pathways [64]. Instead of β-catenin, they use diverse Wnt receptors, co-receptors, and other downstream effectors to mediate their response. Stimulation of the PCP pathway activates the small GTPases Rac and Rho, reorganizing the cytoskeleton for cell polarization, and IQGAP1 is known to participate in the PCP pathway [66]. Wnt and RSPO increase the association of IQGAP1 with proteins involved in actin polymerization and focal adhesion, namely mDia1, N-WASP, focal adhesion kinase (FAK), and paxillin [66] (Figure 2C). Consistent with its established role in regulating the actin cytoskeleton [5, 70], IQGAP1 coordinates actin dynamics and focal adhesion assembly in the non-canonical PCP pathway. It is not known whether IQGAP1 participates in the Wnt/Ca2+ pathway.
Nuclear function: IQGAP impacts transcription
While primarily cytoplasmic, accumulating evidence reveals the participation of IQGAP1 in nuclear function. IQGAP1 interacts with several nuclear proteins (Table 1) and accumulates in the nucleus at the G1/S phase of the cell cycle [72]. Recent evidence reveals that IQGAP1 modulates transcription.
IQGAP1 interacts with and influences the function of a variety of transcription factors, including estrogen receptor α (ERα) [73], nuclear factor erythroid 2 related factor 2 (Nrf2) [74], and nuclear factor of activated T-cells 1 (NFAT1) [75]. ERα is a steroid hormone receptor that dimerizes upon binding estrogen and translocates into the nucleus where it acts as a transcription factor. IQGAP1 binds directly to ERα and is required for normal ERα transcriptional function [73] (Figure 3A). Analogous to its effect on β-catenin [65], IQGAP1 directly interacts with Nrf2 and enhances its stability [74] (Figure 3B). Further investigation indicates that IQGAP1 modulates Nrf2 activation through the MEK-ERK pathway, suggesting that IQGAP1 may scaffold MAPK and Nrf2 signaling [76]. In addition, the isoform IQGAP2 has been implicated in transcriptional activation [77]. The cytokine TNFα triggers a signaling cascade, activating the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [78]. Mass spectrometry identified multiple proteins implicated in this pathway, including IQGAP2 [77]. Importantly, knockdown of IQGAP2 impaired the TNFα stimulated NF-κB pathway. While the role of IQGAP2 in transcription remains to be determined, IQGAP1 appears to regulate selected transcription factors.
Figure 3. Model of IQGAP1 and the nucleus.
A) ERα is steroid hormone receptor that binds estrogen and translocates to the nucleus where it acts as a transcription factor. IQGAP1 binds to ERα and enhances its transcriptional function [73]. This mechanism may involve the assembly of ERα with co-regulators, alteration of ERα post-translational modifications, and/or modulation of ERα conformational dynamics. Activation of ERα by E2 attenuates ERα binding to IQGAP1. Binding of ERα co-regulators to IQGAP1 is speculative; this has not been documented. B) Ca2+ enhances IQGAP1 binding to Nrf2, stimulating the nuclear translocation of Nrf2 and activation of HO-1 stress response [74]. C) IQGAP1 forms a large RNA-scaffold complex with NRON to repress NFAT1 activation by recruiting the kinases CK1, DYRK, and GSK3β [75]. When cells are stimulated, the increased [Ca2+]i activates calmodulin, which activates calcineurin. Calcineurin dephosphorylates NFAT1, inducing its translocation to the nucleus where it upregulates genes involved in the immune response. The dashed arrow indicates putative signaling. Abbreviations: CK1, casein kinase 1; CaM, calmodulin; CN, calcineurin; DYRK, dual-specificity tyrosine-regulated kinases; E2, estrogen; ERα, estrogen receptor α; ERα co-R, estrogen receptor co-regulators; GSK3β, glycogen synthase kinase 3 β; HO-1, heme oxygenase; NFAT1, nuclear factor of activated T cells 1; Nrf2, nuclear factor erythroid 2-related factor 2; P, phosphorylation.
Interestingly, IQGAP1 can also repress transcription [75]. Upon Ca2+ stimulation, the Ca2+/calmodulin-dependent phosphatase calcineurin dephosphorylates the transcription factor NFAT1, inducing its translocation to the nucleus where it promotes the transcription of multiple cytokines [79]. When phosphorylated by NFAT1 kinases (casein kinase 1 (CK1), dual-specificity tyrosine-regulated kinase (DYRK), and glycogen synthase kinase (GSK3)), NFAT1 is confined to the cytoplasm, rendering it inactive. The long intergeneic non-coding RNA (lincRNA) NRON also represses the nuclear import of NFAT1, presumably by sequestering its nuclear transport proteins [80]. However, a more complicated role has been suggested in which NRON forms a large cytoplasmic RNA-protein scaffold complex with NFAT1, IQGAP proteins (IQGAP1 and IQGAP2), calmodulin, and three NFAT1 kinases (Figure 3C) [75]. In the resting state, this complex sequesters inactive NFAT1 in the cytoplasm and blocks calcineurin access. Upon stimulation, calcineurin dephosphorylates NFAT1, which dissociates from IQGAP1 and NFAT1 kinases, and translocates into the nucleus (Figure 3C). Consistent with this model, knockdown of NRON and IQGAP1 enhances nuclear import of NFAT1, and both NRON-depleted T cells and T cells from IQGAP1-deficient mice exhibit increased production of NFAT1-dependent cytokines [75]. Collectively, these data identify an IQGAP1-lincRNA complex that negatively regulates gene transcription.
IQGAPs interact with mRNA decay machinery
In addition to regulating transcription in the nucleus, gene expression is also controlled through the cytoplasmic localization and degradation of mRNA transcripts. IQGAP1 interacts with Staufen [81], an RNA-binding protein that regulates mRNA localization and decay [82]. Purified Staufen complexes contain IQGAP1, Cdc42, and Rac1 [81]. These proteins control cytoskeletal structure, supporting a model in which the Staufen/IQGAP1 complex may regulate the localization of mRNAs. Although IQGAP1 has not been reported to participate in Staufen-mediated mRNA decay, IQGAP1 has been implicated in nonsense-mediated mRNA decay (NMD). The NMD complex eliminates mRNA transcripts that contain premature stop codons, which prevents the translation of gain-of-function or dominant-negative proteins [83]. Through binding to SMG-9, a subunit required for NMD complex formation, IQGAP1 prevents formation of the NMD complex [84]. IQGAP3 was identified by mass spectrometry in a complex with ROD1 (regulator of differentiation 1) [85], a protein that participates in NMD, but it is not known whether IQGAP3 influences NMD. Taken together, these studies suggest that IQGAP1 controls gene expression not only through transcriptional activation and repression, but also possibly through mRNA decay.
Mitotic spindle orientation
IQGAP1 participates in microtubule dynamics that occur during cell polarization [86]. Evidence reveals that IQGAP1 localizes to the basolateral membrane of epithelial cells to correctly orient the mitotic spindle [42]. Interestingly, EGFR is required for this localization of IQGAP1 as well as for the polarized anchoring of microtubules during epithelial cell division and to control spindle orientation. Disrupting the basolateral localization of either IQGAP1 or EGFR results in misorientation of the mitotic spindle and defects in kidney lumen formation [42]. Thus, IQGAP1 interactions with cell surface receptors regulate tissue morphology.
Concluding remarks
IQGAP proteins scaffold a plethora of molecules to control cellular processes. Modular IQGAP domains associate with components of several signaling pathways and regulate signal propagation. IQGAPs have the ability to bind multiple proteins in a single pathway and interact with constituents of distinct pathways, such as MAPK and small GTPases. Through these diverse interactions, IQGAPs facilitate crosstalk between signaling cascades to coordinate cellular activities. While numerous IQGAP-associated proteins have been identified, the regulated assembly of these complexes has not been closely examined. Many factors may determine which IQGAP complexes are formed, including binding affinity, subcellular localization, cell type and regulation by stimuli (e.g. Ca2+/calmodulin regulation of IQGAP1 binding to its targets, or phosphorylation of IQGAP1 [87] and IQGAP2 [27]). These elements may contribute to the formation of complexes necessary for specific signaling modules.
Possible redundancy among IQGAP1, IQGAP2, and IQGAP3 remains poorly characterized. The IQGAP isoforms have similar domain compositions. Therefore, there is potential for functional redundancy among IQGAPs for some pathways. Alternatively, distinct regions of each isoform may permit the formation of unique isoform-specific multiprotein complexes. IQGAP1 and IQGAP2 knockout mice remain viable, but have increased incidence of late-onset gastric hyperplasia [88] and hepatocellular carcinoma [89] respectively. The generation of IQGAP3-null or triple IQGAP-null mice (lack all three IQGAP isoforms) has not been reported, but would broaden our comprehension of IQGAP redundancy. Additionally, hetero- and homo-oligomers of different IQGAP isoforms may further modulate processes. For example, oligomerization of IQGAP1 regulates Cdc42 activation [90].
Deregulation of IQGAPs may contribute to a variety of diseases, ranging from microbial pathogenesis to cancer. Since IQGAPs are implicated in multiple cellular processes, small molecule inhibitors that block interactions of specific signaling molecules with IQGAPs have the potential to precisely target selected signaling cascades that cause disease. The development of B-Raf driven tumors in mice was prevented by a peptide that inhibits the IQGAP1-ERK1 interaction [91]. This initial work suggests that further elucidation of the dynamics of IQGAP signaling networks will uncover biology that may be translated to clinical settings, including the design of effective pharmacologic agents with broad therapeutic implications.
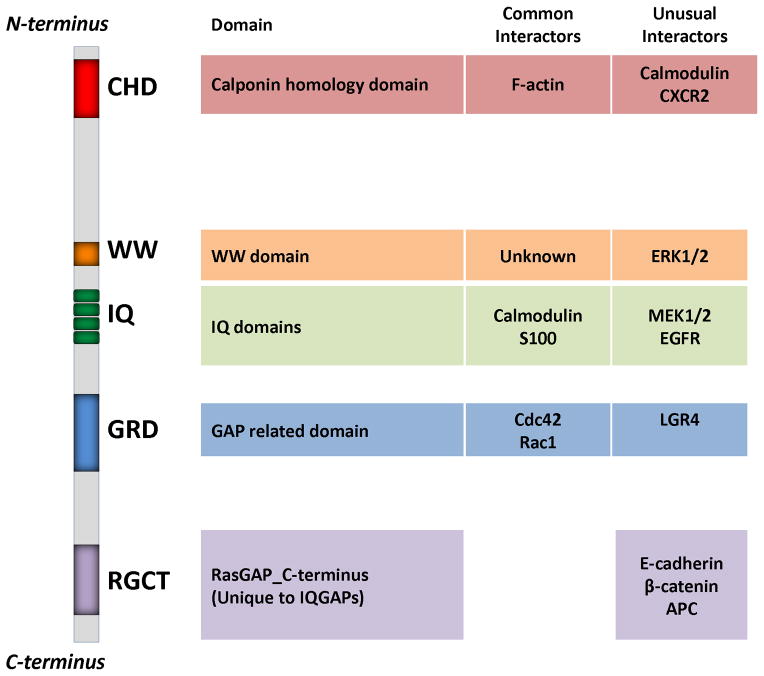
Figure I. IQGAP1 domain structure.
Mammalian IQGAPs contain several domains that mediate protein-protein interactions. Though many of these domains are found in other proteins, the binding partners for IQGAPs are often unique. The five domains in IQGAP1 are the calponin homology domain (CHD), WW domain, IQ domain (which contains four tandem IQ motifs), GAP related domain (GRD), and the RasGAP_C-terminus (RGCT) [38, 106]. Each domain has diverse interacting partners. Common interactors are binding proteins expected to interact with the specific domain. Unusual interactors are unexpected binding partners for the type of domain. The RGCT represents a domain known only in IQGAPs, therefore, all interactors for this region can be considered unique to IQGAPs.
Highlights.
IQGAP proteins scaffold diverse signaling molecules.
IQGAPs mediate crosstalk between signaling pathways.
IQGAP1 regulates nuclear processes, including transcription.
Acknowledgments
We apologize to the authors whose primary work was omitted due to space restrictions. This work was supported by the Intramural Research Program of the National Institutes of Health.
Glossary
- AKAP
A-kinase anchoring proteins, a large family proteins that are targeted to specific cellular locations, and form signaling complexes that contain protein kinase A, and other signaling molecules
- Akt
A kinase activated by PtdInsP3 binding and Akt phosphorylation. Akt phosphorylates substrates to regulate cellular processes, including cell survival and proliferation
- APC
Ademoatous polyposis coli. Forms a destruction complex with Axin, casein kinase 1 (CK1), dual-specificity tyrosine-regulated kinase (DYRK), and glycogen synthase kinase 3β (GSK3β). The APC destruction complex phosphorylates β-catenin to mark it for ubiquitination and degradation, thereby inhibiting canonical Wnt signaling. APC can also coordinate actin and microtubule dynamics during cell migration through interactions with β-catenin and E-cadherin
- CHD
Calponin homology domain. A domain that interacts with F-actin
- Dvl
Dishevelled, a cytoplasmic protein that binds to Frizzled (Fzd) upon Wnt-Fzd stimulation. Dvl inhibits GSK3β activity and allows for the nuclear translocation of β-catenin and activation of canonical Wnt signaling
- Focal adhesions
Complexes of proteins that attach to extracellular matrix proteins and interact with the cytoskeleton
- GAP
GTPase activating protein. Proteins that terminate signaling by binding to GTP-bound small GTPases, enhancing their intrinsic GTPase activity, which hydrolyzes GTP to GDP
- GEF
Guanine nucleotide exchange factor. Protein that catalyzes the release of GDP from inactive small GTPases. This enables GTP, which is in excess in the cell, to bind to the GTPase
- GPCR
G-protein coupled receptors are a family of 7-transmembrane receptors involved in signal transduction from a variety of inputs. Typically, GPCRs are associated with heterotrimeric Gα and Gβ/Gγ G-proteins. Ligand binding results in Gα regulation of enzymes to alter concentrations of second messengers, such as cAMP, IP3, DAG, and Ca2+
- GRD
GAP related domain. A domain similar to GAPs, but has no GAP activity in IQGAPs
- IQ
Sequences containing Iso/Leu and Gln residues that often are found in multiple motifs within a protein. IQ motifs are important for mediating interactions with Ca2+-binding proteins, including calmodulin and S100 family proteins
- MAPK
Mitogen activated protein kinase pathway involves sequential activation of multiple kinases to activate ERK, which regulates processes like cell motility and proliferation
- Microtubule plus-end binding protein
Proteins that bind to the growing (plus) end of microtubules, connecting them to subcellular locations necessary for processes like migration and cytokinesis. Examples include EB1 and CLASP2
- Non-protein coding RNA (ncRNA)
lack open reading frames, yet have important roles in the control of gene expression, includes NRON
- N-WASP
Neuronal Wiskott-Aldrich syndrome protein, binds Arp2/3 complex to promote actin branching
- Ras
Family of small GTPases that are activated downstream of receptors to regulate activity of kinases/other signaling proteins in signaling pathways
- RGCT
RasGAP_C-terminus domain that is unique to IQGAP proteins
- RTK
Receptor tyrosine kinase, a family of proteins that mediate signal transduction from extracellular signaling molecules to initiate intracellular signaling pathways. Ligand activation of RTK usually results in receptor dimerization and autophosphorylation, with subsequent phosphorylation of substrates on tyrosine residues, and assembly of signaling adaptors on phosphorylated tyrosines
- Small GTPases
Family of signaling molecules. In their active GTP bound form they can interact with membranes and other proteins to regulate function, they possess intrinsic GTPase activity to inactivate signaling by catalyzing hydrolysis of GTP to GDP
- Transcription factors
DNA-binding proteins that target specific regulatory sequences to control gene expression
- WRAMP
Wnt-receptor-actin-myosin-polarity is a structure that assembles on the endoplasmic reticulum near the midpoint of the trailing edge of migrating cells. It assembles multiple proteins required for cell retraction to promote cleavage of proteins by calpain and retraction by myosin along actin filaments
- WW
Tryptophan containing domain that in most proteins interacts with proline rich regions
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weissbach L, et al. Identification of a human rasGAP-related protein containing calmodulin-binding motifs. J Biol Chem. 1994;269:20517–20521. [PubMed] [Google Scholar]
- 2.Brill S, et al. The Ras GTPase-activating-protein-related human protein IQGAP2 harbors a potential actin binding domain and interacts with calmodulin and Rho family GTPases. Mol Cell Biol. 1996;16:4869–4878. doi: 10.1128/mcb.16.9.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S, et al. IQGAP3, a novel effector of Rac1 and Cdc42, regulates neurite outgrowth. J Cell Sci. 2007;120:567–577. doi: 10.1242/jcs.03356. [DOI] [PubMed] [Google Scholar]
- 4.Briggs MW, Sacks DB. IQGAP1 as signal integrator: Ca2+, calmodulin, Cdc42 and the cytoskeleton. FEBS Lett. 2003;542:7–11. doi: 10.1016/s0014-5793(03)00333-8. [DOI] [PubMed] [Google Scholar]
- 5.Mateer SC, et al. IQGAPs: integrators of the cytoskeleton, cell adhesion machinery, and signaling networks. Cell Motil Cytoskeleton. 2003;55:147–155. doi: 10.1002/cm.10118. [DOI] [PubMed] [Google Scholar]
- 6.Kaibuchi K, et al. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- 7.Osman M. An emerging role for IQGAP1 in regulating protein traffic. Scientific World Journal. 2010;10:944–953. doi: 10.1100/tsw.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson M, et al. IQGAP1 regulation and roles in cancer. Cell Signal. 2009;21:1471–1478. doi: 10.1016/j.cellsig.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 9.White CD, et al. IQGAPs in cancer: a family of scaffold proteins underlying tumorigenesis. FEBS Lett. 2009;583:1817–1824. doi: 10.1016/j.febslet.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim H, et al. IQGAP1 in microbial pathogenesis: Targeting the actin cytoskeleton. FEBS Lett. 2011;585:723–729. doi: 10.1016/j.febslet.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown MD, Sacks DB. Protein scaffolds in MAP kinase signalling. Cell Signal. 2009;21:462–469. doi: 10.1016/j.cellsig.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho YD, et al. IQGAP1 integrates Ca2+/calmodulin and Cdc42 signaling. J Biol Chem. 1999;274:464–470. doi: 10.1074/jbc.274.1.464. [DOI] [PubMed] [Google Scholar]
- 13.Roy M, et al. IQGAP1 binds ERK2 and modulates its activity. J Biol Chem. 2004;279:17329–17337. doi: 10.1074/jbc.M308405200. [DOI] [PubMed] [Google Scholar]
- 14.Roy M, et al. IQGAP1 is a scaffold for mitogen-activated protein kinase signaling. Mol Cell Biol. 2005;25:7940–7952. doi: 10.1128/MCB.25.18.7940-7952.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vetterkind S, et al. Hierarchical scaffolding of an ERK1/2 activation pathway. Cell Commun Signal. 2013;11:65. doi: 10.1186/1478-811X-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noritake J, et al. IQGAP1: a key regulator of adhesion and migration. J Cell Sci. 2005;118:2085–2092. doi: 10.1242/jcs.02379. [DOI] [PubMed] [Google Scholar]
- 17.Wehrle-Haller B. Assembly and disassembly of cell matrix adhesions. Curr Opin Cell Biol. 2012;24:569–581. doi: 10.1016/j.ceb.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Kohno T, et al. IQGAP1 links PDGF receptor-beta signal to focal adhesions involved in vascular smooth muscle cell migration: role in neointimal formation after vascular injury. Am J Physiol Cell Physiol. 2013;305:C591–600. doi: 10.1152/ajpcell.00011.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaeffer HJ, et al. MP1: a MEK binding partner that enhances enzymatic activation of the MAP kinase cascade. Science. 1998;281:1668–1671. doi: 10.1126/science.281.5383.1668. [DOI] [PubMed] [Google Scholar]
- 20.Wunderlich W, et al. A novel 14-kilodalton protein interacts with the mitogen-activated protein kinase scaffold mp1 on a late endosomal/lysosomal compartment. J Cell Biol. 2001;152:765–776. doi: 10.1083/jcb.152.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiefermeier N, et al. The late endosomal p14-MP1 (LAMTOR2/3) complex regulates focal adhesion dynamics during cell migration. J Cell Biol. 2014;205:525–540. doi: 10.1083/jcb.201310043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ridley AJ, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 23.Mataraza JM, et al. IQGAP1 promotes cell motility and invasion. J Biol Chem. 2003;278:41237–41245. doi: 10.1074/jbc.M304838200. [DOI] [PubMed] [Google Scholar]
- 24.Jacquemet G, et al. RCP-driven alpha5beta1 recycling suppresses Rac and promotes RhoA activity via the RacGAP1-IQGAP1 complex. J Cell Biol. 2013;202:917–935. doi: 10.1083/jcb.201302041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacquemet G, et al. Rac1 is deactivated at integrin activation sites through an IQGAP1-filamin-A-RacGAP1 pathway. J Cell Sci. 2013;126:4121–4135. doi: 10.1242/jcs.121988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logue JS, et al. Anchored protein kinase A recruitment of active Rac GTPase. J Biol Chem. 2011;286:22113–22121. doi: 10.1074/jbc.M111.232660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Logue JS, et al. AKAP220 protein organizes signaling elements that impact cell migration. J Biol Chem. 2011;286:39269–39281. doi: 10.1074/jbc.M111.277756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esseltine JL, Scott JD. AKAP signaling complexes: pointing towards the next generation of therapeutic targets? Trends Pharmacol Sci. 2013;34:648–655. doi: 10.1016/j.tips.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foroutannejad S, et al. A novel role for IQGAP1 protein in cell motility through cell retraction. Biochem Biophys Res Commun. 2014;448:39–44. doi: 10.1016/j.bbrc.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witze ES, et al. Wnt5a directs polarized calcium gradients by recruiting cortical endoplasmic reticulum to the cell trailing edge. Dev Cell. 2013;26:645–657. doi: 10.1016/j.devcel.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimura T, et al. Activated Cdc42-bound IQGAP1 determines the cellular endocytic site. Mol Cell Biol. 2013;33:4834–4843. doi: 10.1128/MCB.00895-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heck JN, et al. A conspicuous connection: structure defines function for the phosphatidylinositol-phosphate kinase family. Crit Rev Biochem Mol Biol. 2007;42:15–39. doi: 10.1080/10409230601162752. [DOI] [PubMed] [Google Scholar]
- 33.Hennessy BT, et al. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 34.Choi S, et al. IQGAP1 is a novel phosphatidylinositol 4,5 bisphosphate effector in regulation of directional cell migration. EMBO J. 2013;32:2617–2630. doi: 10.1038/emboj.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dixon MJ, et al. IQGAP proteins reveal an atypical phosphoinositide (aPI) binding domain with a pseudo C2 domain fold. J Biol Chem. 2012;287:22483–22496. doi: 10.1074/jbc.M112.352773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hupfeld CJ, Olefsky JM. Regulation of receptor tyrosine kinase signaling by GRKs and beta-arrestins. Annu Rev Physiol. 2007;69:561–577. doi: 10.1146/annurev.physiol.69.022405.154626. [DOI] [PubMed] [Google Scholar]
- 37.Shukla AK, et al. Emerging paradigms of beta-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci. 2011;36:457–469. doi: 10.1016/j.tibs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown MD, Sacks DB. IQGAP1 in cellular signaling: bridging the GAP. Trends Cell Biol. 2006;16:242–249. doi: 10.1016/j.tcb.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 39.McNulty DE, et al. MAPK scaffold IQGAP1 binds the EGF receptor and modulates its activation. J Biol Chem. 2011;286:15010–15021. doi: 10.1074/jbc.M111.227694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott JD, Pawson T. Cell signaling in space and time: where proteins come together and when they’re apart. Science. 2009;326:1220–1224. doi: 10.1126/science.1175668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith MJ, et al. The PTB domain of ShcA couples receptor activation to the cytoskeletal regulator IQGAP1. EMBO J. 2010;29:884–896. doi: 10.1038/emboj.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banon-Rodriguez I, et al. EGFR controls IQGAP basolateral membrane localization and mitotic spindle orientation during epithelial morphogenesis. EMBO J. 2014;33:129–145. doi: 10.1002/embj.201385946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foerster S, et al. Characterization of the EGFR interactome reveals associated protein complex networks and intracellular receptor dynamics. Proteomics. 2013;13:3131–3144. doi: 10.1002/pmic.201300154. [DOI] [PubMed] [Google Scholar]
- 44.Matsunaga H, et al. IQGAP1 selectively interacts with K-Ras but not with H-Ras and modulates K-Ras function. Biochem Biophys Res Commun. 2014;444:360–364. doi: 10.1016/j.bbrc.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 45.Ren JG, et al. IQGAP1 modulates activation of B-Raf. Proc Natl Acad Sci U S A. 2007;104:10465–10469. doi: 10.1073/pnas.0611308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ren JG, et al. IQGAP1 integrates Ca2+/calmodulin and B-Raf signaling. J Biol Chem. 2008;283:22972–22982. doi: 10.1074/jbc.M804626200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nojima H, et al. IQGAP3 regulates cell proliferation through the Ras/ERK signalling cascade. Nat Cell Biol. 2008;10:971–978. doi: 10.1038/ncb1757. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y, et al. IQGAP3 promotes EGFR-ERK signaling and the growth and metastasis of lung cancer cells. PLoS One. 2014;9:e97578. doi: 10.1371/journal.pone.0097578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song MS, et al. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 50.Yamaoka-Tojo M, et al. IQGAP1, a novel vascular endothelial growth factor receptor binding protein, is involved in reactive oxygen species--dependent endothelial migration and proliferation. Circ Res. 2004;95:276–283. doi: 10.1161/01.RES.0000136522.58649.60. [DOI] [PubMed] [Google Scholar]
- 51.White CD, et al. IQGAP1 protein binds human epidermal growth factor receptor 2 (HER2) and modulates trastuzumab resistance. J Biol Chem. 2011;286:29734–29747. doi: 10.1074/jbc.M111.220939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen F, et al. IQGAP1 is overexpressed in hepatocellular carcinoma and promotes cell proliferation by Akt activation. Exp Mol Med. 2010;42:477–483. doi: 10.3858/emm.2010.42.7.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Z, et al. IQGAP1 plays an important role in the invasiveness of thyroid cancer. Clin Cancer Res. 2010;16:6009–6018. doi: 10.1158/1078-0432.CCR-10-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie Y, et al. IQGAP2, A candidate tumour suppressor of prostate tumorigenesis. Biochim Biophys Acta. 2012;1822:875–884. doi: 10.1016/j.bbadis.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 55.Gunaratne J, et al. Protein interactions of phosphatase and tensin homologue (PTEN) and its cancer-associated G20E mutant compared by using stable isotope labeling by amino acids in cell culture-based parallel affinity purification. J Biol Chem. 2011;286:18093–18103. doi: 10.1074/jbc.M111.221184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trusolino L, et al. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 57.Liu F, et al. Hepatocyte growth factor enhances endothelial cell barrier function and cortical cytoskeletal rearrangement: potential role of glycogen synthase kinase-3beta. FASEB J. 2002;16:950–962. doi: 10.1096/fj.01-0870com. [DOI] [PubMed] [Google Scholar]
- 58.Tian Y, et al. IQGAP1 regulates endothelial barrier function via EB1 -cortactin crosstalk. Mol Cell Biol. 2014;34:3546–3558. doi: 10.1128/MCB.00248-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fram S, et al. A PAK6-IQGAP1 complex promotes disassembly of cell-cell adhesions. Cell Mol Life Sci. 2014;71:2759–2773. doi: 10.1007/s00018-013-1528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wells CM, Jones GE. The emerging importance of group II PAKs. Biochem J. 2010;425:465–473. doi: 10.1042/BJ20091173. [DOI] [PubMed] [Google Scholar]
- 61.Alemayehu M, et al. beta-Arrestin2 regulates lysophosphatidic acid-induced human breast tumor cell migration and invasion via Rap1 and IQGAP1. PLoS One. 2013;8:e56174. doi: 10.1371/journal.pone.0056174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feigin ME, et al. G-protein-coupled receptor GPR161 is overexpressed in breast cancer and is a promoter of cell proliferation and invasion. Proc Natl Acad Sci U S A. 2014;111:4191–4196. doi: 10.1073/pnas.1320239111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 64.Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 65.Briggs MW, et al. IQGAP1-mediated stimulation of transcriptional co-activation by beta-catenin is modulated by calmodulin. J Biol Chem. 2002;277:7453–7465. doi: 10.1074/jbc.M104315200. [DOI] [PubMed] [Google Scholar]
- 66.Carmon KS, et al. RSPO-LGR4 functions via IQGAP1 to potentiate Wnt signaling. Proc Natl Acad Sci U S A. 2014;111:E1221–1229. doi: 10.1073/pnas.1323106111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goto T, et al. IQGAP1 protein regulates nuclear localization of beta-catenin via importin-beta5 protein in Wnt signaling. J Biol Chem. 2013;288:36351–36360. doi: 10.1074/jbc.M113.520528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goto T, et al. IQGAP1 functions as a modulator of dishevelled nuclear localization in Wnt signaling. PLoS One. 2013;8:e60865. doi: 10.1371/journal.pone.0060865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol. 2010;2:a002915. doi: 10.1101/cshperspect.a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Briggs MW, Sacks DB. IQGAP proteins are integral components of cytoskeletal regulation. EMBO reports. 2003;4:571–574. doi: 10.1038/sj.embor.embor867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuroda S, et al. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin- mediated cell-cell adhesion. Science. 1998;281:832–835. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- 72.Johnson M, et al. IQGAP1 translocates to the nucleus in early S-phase and contributes to cell cycle progression after DNA replication arrest. Int J Biochem Cell Biol. 2011;43:65–73. doi: 10.1016/j.biocel.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 73.Erdemir HH, et al. IQGAP1 binds to estrogen receptor-alpha and modulates its function. J Biol Chem. 2014;289:9100–9112. doi: 10.1074/jbc.M114.553511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim JH, et al. Identification and functional studies of a new Nrf2 partner IQGAP1: a critical role in the stability and transactivation of Nrf2. Antioxid Redox Signal. 2013;19:89–101. doi: 10.1089/ars.2012.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma S, et al. Dephosphorylation of the nuclear factor of activated T cells (NFAT) transcription factor is regulated by an RNA-protein scaffold complex. Proc Natl Acad Sci U S A. 2011;108:11381–11386. doi: 10.1073/pnas.1019711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheung KL, et al. The Ras GTPase-activating-like protein IQGAP1 mediates Nrf2 protein activation via the mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) kinase (MEK)-ERK pathway. J Biol Chem. 2013;288:22378–22386. doi: 10.1074/jbc.M112.444182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bouwmeester T, et al. A physical and functional map of the human TNF-alpha/NF-kappa B signal transduction pathway. Nat Cell Biol. 2004;6:97–105. doi: 10.1038/ncb1086. [DOI] [PubMed] [Google Scholar]
- 78.Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Muller MR, Rao A. NFAT, immunity and cancer: a transcription factor comes of age. Nat Rev Immunol. 2010;10:645–656. doi: 10.1038/nri2818. [DOI] [PubMed] [Google Scholar]
- 80.Willingham AT, et al. A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science. 2005;309:1570–1573. doi: 10.1126/science.1115901. [DOI] [PubMed] [Google Scholar]
- 81.Villace P, et al. The composition of Staufen-containing RNA granules from human cells indicates their role in the regulated transport and translation of messenger RNAs. Nucleic Acids Res. 2004;32:2411–2420. doi: 10.1093/nar/gkh552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meyer EL, Gavis ER. Staufen does double duty. Nat Struct Mol Biol. 2005;12:291–292. doi: 10.1038/nsmb0405-291. [DOI] [PubMed] [Google Scholar]
- 83.Popp MW, Maquat LE. Organizing principles of mammalian nonsense-mediated mRNA decay. Annu Rev Genet. 2013;47:139–165. doi: 10.1146/annurev-genet-111212-133424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takeda S, et al. Role of a tyrosine phosphorylation of SMG-9 in binding of SMG-9 to IQGAP and the NMD complex. Biochem Biophys Res Commun. 2011;410:29–33. doi: 10.1016/j.bbrc.2011.05.099. [DOI] [PubMed] [Google Scholar]
- 85.Brazao TF, et al. A new function of ROD1 in nonsense-mediated mRNA decay. FEBS Lett. 2012;586:1101–1110. doi: 10.1016/j.febslet.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 86.Watanabe T, et al. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell. 2004;7:871–883. doi: 10.1016/j.devcel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 87.Li Z, et al. IQGAP1 promotes neurite outgrowth in a phosphorylation-dependent manner. J Biol Chem. 2005;280:13871–13878. doi: 10.1074/jbc.M413482200. [DOI] [PubMed] [Google Scholar]
- 88.Li S, et al. Gastric hyperplasia in mice lacking the putative Cdc42 effector IQGAP1. Mol Cell Biol. 2000;20:697–701. doi: 10.1128/mcb.20.2.697-701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmidt VA, et al. Development of hepatocellular carcinoma in Iqgap2-deficient mice is IQGAP1 dependent. Mol Cell Biol. 2008;28:1489–1502. doi: 10.1128/MCB.01090-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ren JG, et al. Self-association of IQGAP1: Characterization and functional sequelae. J Biol Chem. 2005;280:34548–34557. doi: 10.1074/jbc.M507321200. [DOI] [PubMed] [Google Scholar]
- 91.Jameson KL, et al. IQGAP1 scaffold-kinase interaction blockade selectively targets RAS-MAP kinase-driven tumors. Nat Med. 2013;19:626–630. doi: 10.1038/nm.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gnatenko DV, et al. Transcript profiling identifies iqgap2(−/−) mouse as a model for advanced human hepatocellular carcinoma. PLoS One. 2013;8:e71826. doi: 10.1371/journal.pone.0071826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun Y, et al. Quantitative proteomic signature of liver cancer cells: tissue transglutaminase 2 could be a novel protein candidate of human hepatocellular carcinoma. J Proteome Res. 2008;7:3847–3859. doi: 10.1021/pr800153s. [DOI] [PubMed] [Google Scholar]
- 94.White CD, et al. IQGAP1 and IQGAP2 are reciprocally altered in hepatocellular carcinoma. BMC Gastroenterol. 2010;10:125. doi: 10.1186/1471-230X-10-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jin SH, et al. IQGAP2 inactivation through aberrant promoter methylation and promotion of invasion in gastric cancer cells. Int J Cancer. 2008;122:1040–1046. doi: 10.1002/ijc.23181. [DOI] [PubMed] [Google Scholar]
- 96.Chiariello CS, et al. Ablation of Iqgap2 protects from diet-induced hepatic steatosis due to impaired fatty acid uptake. Regul Pept. 2011;173:36–46. doi: 10.1016/j.regpep.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kunimoto K, et al. Involvement of IQGAP3, a regulator of Ras/ERK-related cascade, in hepatocyte proliferation in mouse liver regeneration and development. J Cell Physiol. 2009;220:621–631. doi: 10.1002/jcp.21798. [DOI] [PubMed] [Google Scholar]
- 98.Caro-Gonzalez HY, et al. Mitogen-activated protein kinase (MAPK/ERK) regulates adenomatous polyposis coli during growth-factor-induced cell extension. J Cell Sci. 2012;125:1247–1258. doi: 10.1242/jcs.095166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mateer SC, et al. Actin filament binding by a monomeric IQGAP1 fragment with a single calponin homology domain. Cell Motil Cytoskeleton. 2004;58:231–241. doi: 10.1002/cm.20013. [DOI] [PubMed] [Google Scholar]
- 100.Umemoto R, et al. NMR structure of the calponin homology domain of human IQGAP1 and its implications for the actin recognition mode. J Biomol NMR. 2010;48:59–64. doi: 10.1007/s10858-010-9434-8. [DOI] [PubMed] [Google Scholar]
- 101.Joyal JL, et al. Calmodulin modulates the interaction between IQGAP1 and Cdc42. Identification of IQGAP1 by nanoelectrospray tandem mass spectrometry. J Biol Chem. 1997;272:15419–15425. doi: 10.1074/jbc.272.24.15419. [DOI] [PubMed] [Google Scholar]
- 102.Heil A, et al. S100P is a novel interaction partner and regulator of IQGAP1. J Biol Chem. 2011;286:7227–7238. doi: 10.1074/jbc.M110.135095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pathmanathan S, et al. IQ motif selectivity in human IQGAP1: binding of myosin essential light chain and S100B. Mol Cell Biochem. 2008;318:43–51. doi: 10.1007/s11010-008-9855-9. [DOI] [PubMed] [Google Scholar]
- 104.Li Z, Sacks DB. Elucidation of the interaction of calmodulin with the IQ motifs of IQGAP1. J Biol Chem. 2003;278:4347–4352. doi: 10.1074/jbc.M208579200. [DOI] [PubMed] [Google Scholar]
- 105.Jeong HW, et al. IQGAP1 binds Rap1 and modulates its activity. J Biol Chem. 2007;282:20752–20762. doi: 10.1074/jbc.M700487200. [DOI] [PubMed] [Google Scholar]
- 106.White CD, et al. IQGAP1 and its binding proteins control diverse biological functions. Cell Signal. 2012;24:826–834. doi: 10.1016/j.cellsig.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kurella VB, et al. Crystal structure of the GTPase-activating protein-related domain from IQGAP1. J Biol Chem. 2009;284:14857–14865. doi: 10.1074/jbc.M808974200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Owen D, et al. The IQGAP1-Rac1 and IQGAP1-Cdc42 Interactions: interfaces differ between the complexes. J Biol Chem. 2008;283:1692–1704. doi: 10.1074/jbc.M707257200. [DOI] [PubMed] [Google Scholar]
- 109.Hart MJ, et al. IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. EMBO J. 1996;15:2997–3005. [PMC free article] [PubMed] [Google Scholar]
- 110.Atcheson E, et al. IQ-motif selectivity in human IQGAP2 and IQGAP3: binding of calmodulin and myosin essential light chain. Biosci Rep. 2011;31:371–379. doi: 10.1042/BSR20100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kuroda S, et al. Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. J Biol Chem. 1996;271:23363–23367. doi: 10.1074/jbc.271.38.23363. [DOI] [PubMed] [Google Scholar]
- 112.Swart-Mataraza JM, et al. IQGAP1 is a component of Cdc42 signaling to the cytoskeleton. J Biol Chem. 2002;277:24753–24763. doi: 10.1074/jbc.M111165200. [DOI] [PubMed] [Google Scholar]
- 113.Erickson JW, et al. Identification of an actin cytoskeletal complex that includes IQGAP and the Cdc42 GTPase. J Biol Chem. 1997;272:24443–24447. doi: 10.1074/jbc.272.39.24443. [DOI] [PubMed] [Google Scholar]
- 114.Fukata M, et al. Regulation of cross-linking of actin filament by IQGAP1, a target for Cdc42. J Biol Chem. 1997;272:29579–29583. doi: 10.1074/jbc.272.47.29579. [DOI] [PubMed] [Google Scholar]
- 115.Mateer SC, et al. The mechanism for regulation of the F-actin binding activity of IQGAP1 by calcium/calmodulin. J Biol Chem. 2002;277:12324–12333. doi: 10.1074/jbc.M109535200. [DOI] [PubMed] [Google Scholar]
- 116.Schmidt VA, et al. IQGAP2 functions as a GTP-dependent effector protein in thrombin-induced platelet cytoskeletal reorganization. Blood. 2003;101:3021–3028. doi: 10.1182/blood-2002-09-2807. [DOI] [PubMed] [Google Scholar]
- 117.Bensenor LB, et al. IQGAP1 regulates cell motility by linking growth factor signaling to actin assembly. J Cell Sci. 2007;120:658–669. doi: 10.1242/jcs.03376. [DOI] [PubMed] [Google Scholar]
- 118.Yamaoka-Tojo M, et al. IQGAP1 mediates VE-cadherin-based cell-cell contacts and VEGF signaling at adherence junctions linked to angiogenesis. Arterioscler Thromb Vasc Biol. 2006;26:1991–1997. doi: 10.1161/01.ATV.0000231524.14873.e7. [DOI] [PubMed] [Google Scholar]
- 119.Liu C, et al. IQGAP1 suppresses T RII-mediated myofibroblastic activation and metastatic growth in liver. J Clin Invest. 2013;123:1138–1156. doi: 10.1172/JCI63836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Neel NF, et al. IQGAP1 is a novel CXCR2-interacting protein and essential component of the “chemosynapse”. PLoS ONE. 2011;6:e23813. doi: 10.1371/journal.pone.0023813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cvetkovic D, et al. KISS1R induces invasiveness of estrogen receptor-negative human mammary epithelial and breast cancer cells. Endocrinology. 2013;154:1999–2014. doi: 10.1210/en.2012-2164. [DOI] [PubMed] [Google Scholar]
- 122.Sbroggio M, et al. ERK1/2 activation in heart is controlled by melusin, focal adhesion kinase and the scaffold protein IQGAP1. J Cell Sci. 2011;124:3515–3524. doi: 10.1242/jcs.091140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sbroggio M, et al. IQGAP1 regulates ERK1/2 and AKT signalling in the heart and sustains functional remodelling upon pressure overload. Cardiovasc Res. 2011;91:456–464. doi: 10.1093/cvr/cvr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tekletsadik YK, et al. A conserved role of IQGAP1 in regulating TOR complex 1. J Cell Sci. 2012;125:2041–2052. doi: 10.1242/jcs.098947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Okada M, et al. DGKzeta is involved in LPS-activated phagocytosis through IQGAP1/Rac1 pathway. Biochem Biophys Res Commun. 2012;420:479–484. doi: 10.1016/j.bbrc.2012.03.057. [DOI] [PubMed] [Google Scholar]
- 126.Ruiz-Saenz A, et al. Protein 4.1R regulates cell migration and IQGAP1 recruitment to the leading edge. J Cell Sci. 2011;124:2529–2538. doi: 10.1242/jcs.083634. [DOI] [PubMed] [Google Scholar]
- 127.Liu J, et al. Conserved sequence repeats of IQGAP1 mediate binding to Ezrin. J Proteome Res. 2013;13:1156–1166. doi: 10.1021/pr400787p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dixon MJ, et al. A screen for novel phosphoinositide 3-kinase effector proteins. Mol Cell Proteomics. 2011;10:M110 003178. doi: 10.1074/mcp.M110.003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Steenblock C, et al. The Cdc42 Guanine Nucleotide Exchange Factor FGD6 Coordinates Cell Polarity and Endosomal Membrane Recycling in Osteoclasts. J Biol Chem. 2014;289:18347–18359. doi: 10.1074/jbc.M113.504894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Casteel DE, et al. Rho isoform-specific interaction with IQGAP1 promotes breast cancer cell proliferation and migration. J Biol Chem. 2012;287:38367–38378. doi: 10.1074/jbc.M112.377499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Usatyuk PV, et al. Phospholipase D-mediated activation of IQGAP1 through Rac1 regulates hyperoxia-induced p47phox translocation and reactive oxygen species generation in lung endothelial cells. J Biol Chem. 2009;284:15339–15352. doi: 10.1074/jbc.M109.005439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sato A, et al. Association of RNase L with a Ras GTPase-activating-like protein IQGAP1 in mediating the apoptosis of a human cancer cell-line. FEBS J. 2010;277:4464–4473. doi: 10.1111/j.1742-4658.2010.07833.x. [DOI] [PubMed] [Google Scholar]
- 133.Com E, et al. Nerve growth factor receptor TrkA signaling in breast cancer cells involves Ku70 to prevent apoptosis. Mol Cell Proteomics. 2007;6:1842–1854. doi: 10.1074/mcp.M700119-MCP200. [DOI] [PubMed] [Google Scholar]
- 134.Subramani J, et al. Tyrosine phosphorylation of CD13 regulates inflammatory cell-cell adhesion and monocyte trafficking. J Immunol. 2013;191:3905–3912. doi: 10.4049/jimmunol.1301348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bhattacharya M, et al. IQGAP1 is necessary for pulmonary vascular barrier protection in murine acute lung injury and pneumonia. Am J Physiol Lung Cell Mol Physiol. 303:L12–19. doi: 10.1152/ajplung.00375.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rigothier C, et al. IQGAP1 interacts with components of the slit diaphragm complex in podocytes and is involved in podocyte migration and permeability in vitro. PLoS ONE. 2012;7:e37695. doi: 10.1371/journal.pone.0037695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schrick C, et al. N-cadherin regulates cytoskeletally associated IQGAP1/ERK signaling and memory formation. Neuron. 2007;55:786–798. doi: 10.1016/j.neuron.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Routray C, et al. Protein kinase G signaling disrupts Rac1-dependent focal adhesion assembly in liver specific pericytes. Am J Physiol Cell Physiol. 2011;301:C66–74. doi: 10.1152/ajpcell.00038.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yin N, et al. IQGAP1 interacts with Aurora-A and enhances its stability and its role in cancer. Biochem Biophys Res Commun. 2012;421:64–69. doi: 10.1016/j.bbrc.2012.03.112. [DOI] [PubMed] [Google Scholar]
- 140.Phillips-Mason PJ, et al. The receptor protein-tyrosine phosphatase PTPmu interacts with IQGAP1. J Biol Chem. 2006;281:4903–4910. doi: 10.1074/jbc.M506414200. [DOI] [PubMed] [Google Scholar]
- 141.Gao C, et al. IQGAP1 regulates NR2A signaling, spine density, and cognitive processes. J Neurosci. 2011;31:8533–8542. doi: 10.1523/JNEUROSCI.1300-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ebmeier CC, Taatjes DJ. Activator-Mediator binding regulates Mediator-cofactor interactions. Proc Natl Acad Sci U S A. 2010;107:11283–11288. doi: 10.1073/pnas.0914215107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jacquemet G, Humphries MJ. IQGAP1 is a key node within the small GTPase network. Small GTPases. 2013;4:199–207. doi: 10.4161/sgtp.27451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li J, et al. Identification and characterization of nardilysin as a novel dimethyl H3K4-binding protein involved in transcriptional regulation. J Biol Chem. 2012;287:10089–10098. doi: 10.1074/jbc.M111.313965. [DOI] [PMC free article] [PubMed] [Google Scholar]