Abstract
Tubulin and microtubules are subject to a remarkable number of posttranslational modifications. Understanding the roles these modifications play in determining functions and properties of microtubules has presented a major challenge that is only now being met. Many of these modifications are found concurrently, leading to considerable diversity in cellular microtubules, which varies with development, differentiation, cell compartment and cell cycle. We now know that posttranslational modifications of tubulin affect not only the dynamics of the microtubules, but also their organization and interaction with other cellular components. Many early suggestions of how posttranslational modifications affect microtubules have been replaced with new ideas and even new modifications as our understanding of cellular microtubule diversity comes into focus.
Keywords: Tubulin, microtubule, detyrosination, acetylation, polyglutamylation, polyamination
Microtubule Diversity and Posttranslational Modifications
Forty years ago, the first tubulin posttranslational modification (PTM) was described: RNA-independent enzymatic incorporation of tyrosine [1]. A remarkable number of modifications to tubulin and microtubules (MTs) have since been identified (Table 1), including the most recent, polyamination [2]. Tubulin PTMs are found in all cells with MTs [3, 4] and they are particularly diverse in neurons [3-5], but many questions remain, such as the fraction of tubulins with a given modification, the distribution of modifications along a MT or between MTs, and the functional consequences of many modifications. Although regional differences in MT dynamics and stability are important for all cells [6], the significance of PTMs goes beyond MT dynamics.
Table 1.
Posttranslational Modifications of tubulin and microtubules.
| Modification | α/β | site | enzyme | references |
|---|---|---|---|---|
| Acetylation Deacetylation |
α
α |
Lys40 | TAT (MEC17) HDAC6, SIRT2, HDAC5 |
[58] [83, 89, 92] |
| Detyrosination Tyrosination |
α
α |
C-Terminal Tyr Add to C-terminal |
Unknown TTL |
[3] [10, 11, 17] |
| Δ2deglutamylation) | α | Removal of C-terminal Glu of detyrosinated MTs | CCP1, CCP2, CCP3, CCP4, CCP5, and CCP6 | [35, 37] |
| Glutamylation Deglutamylation of branch point Glu |
α/β α/β |
Various C-terminal Glu | TTLL4, 5, and 7 CCP5 & CCP1, CCP4, CCP6 |
[3, 4] [36, 37] |
| Polyglutamylation Deglutamylation to shorten polyGlu side chain |
α/β | Add γ-linked Glu to C-terminal | TTLL1, 6, 11 and 13 CCP1, CCP4, CCP6 |
[3, 4] [37] |
| Glycylation Polyglycylation Deglycylation |
α/β | Add γ-linked Gly to C-terminal Glu Addition to γ-linked Gly C-terminal |
TTLL3 and 8 TTLL10 Unknown |
[54, 55] [54] [3, 4] |
| Polyamination | α/β | β tubulins Q15, others | Transglutaminase | [2] |
| Glycososylation | α/β | various | Unknown | [4] |
| Glycation | α/β | various | Nonenzymatic | [118] |
| Palmitoylation | α | Cys376 | Unknown | [121] |
| Phosphorylation | α/β | β tubulin Ser172, others | Various | [4] |
| Sumoylation | α | multiple | Unknown | [136, 137] |
| Ubiquitylation | α | Multiple | Parkin | [131] |
Blue, modifications on tubulin in MTs; red, modifications restricted to soluble tubulin dimers; and black, modifications that are either undetermined or occur on both.
Tubulin may be modified as a soluble dimer or in a MT (Table 1), and some PTMs occur on both. The variable C-terminal domains of α-tubulin represent a hot spot for modification, while fewer modifications appear to associate with the C-termini of β-tubulin (Fig. 2). In addition, some modifications map to other regions of the tubulin dimer. Studies have revealed that MTs are modified in a heterogeneous manner, with PTMs being coextensive or concentrated in distinct domains on MTs, thereby adding an additional level of complexity. Indeed, assigning specific functions to a given modification in vivo is complicated by the heterogeneity of modifications found in the MT as well as the multiple modifications that may be present on the individual tubulin dimers themselves (Fig. 1).
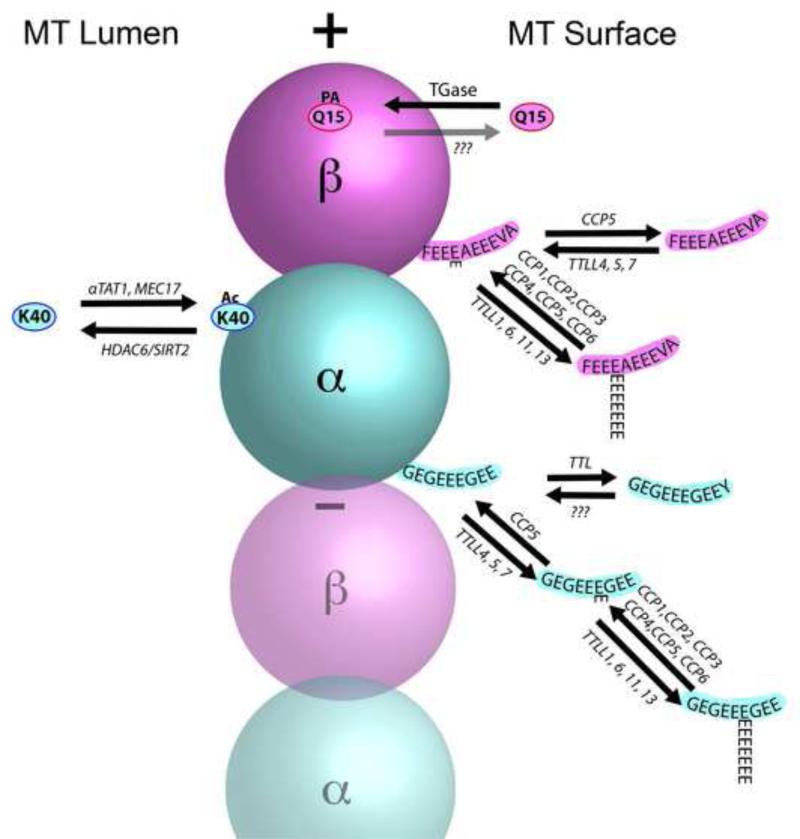
Figure 2. Pathways and sites for the major modifications of tubulin in a microtubule.
Acetylation is primarily found on K40 of α-tubulins, which is exposed in the lumen of the microtubule. αTAT1 and Mec17 are the major enzymes responsible for acetylation (Ac), and HDAC6 and SIRT2 are the enzymes involved in deacetylation. The C-terminus of α-tubulin is a hot spot for modification and various combinations are illustrated. Tubulin Tyrosine Ligase (TTL) is the enzyme responsible for tyrosination of tubulin dimers, but the enzyme that catalyzes detyrosination remains to be identified. In contrast, multiple enzymes are capable of removing the penultimate glutamate residue to create Δ2-tubulin, including all six Cytoplasmic Carboxypeptidases (CCP1-6). These same enzymes can also trim the length of polyglutamylated chains, however, only one enzyme has been shown to remove the final iso-peptide linked glutamate (CCP5). Detyrosination is restricted to α-tubulins, but glutamylation and polyglutamylation can also occur on various glutamates in the C-terminal domain of β-tubulins. Multiple related enzymes have been identified that can add the iso-peptide link for initiating polyglutamylation (Tubulin Tyrosine Ligase Like family members (TTLL4,5,7)). Other members of the same family (TTLL1, 6, 11, 13) can add glycine to the same set of residues in the C-terminal domain of tubulins (not shown), but this reaction is seen only in ciliary microtubules. Curiously, the enzyme responsible for extension of polyglycine chains (TTLL10) is not functional in humans and only monoglycine modifications are seen. Finally, polyamination (PA) is mediated by transglutaminases, particularly TG2 in the nervous system. One putative modification site for polyamination identified by Mass Spectrometry is the conserved Q15 of β-tubulins. However, several other sites on both α- and β-tubulin may also be subject to polyamination. Enzyme(s) responsible for removing polyamines have not been confirmed and the degree to which polyamination is reversible in vivo is uncertain.
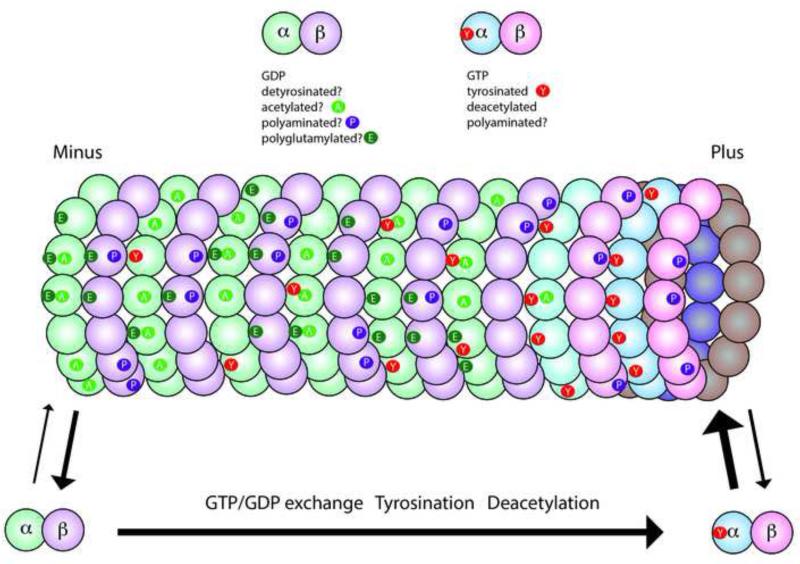
Figure 1. Microtubules and tubulins are subject to a variety of posttranslational modifications.
This diagram illustrates the major modifications in axonal microtubules. Tubulin dimers are GTP-rich (blue/pink shading) in the soluble pool and on the plus end of the microtubule, but polymeric tubulin gradually hydrolyzes GTP and becomes GDP-rich (green/purple shading). Some modifications are associated specifically with tubulin polymerized in microtubules including acetylation (A), detyrosination, and polyglutamylation (E), while others may occur only on soluble tubulin like tyrosination (Y), or on either soluble or polymerized tubulin like polyamination (P). Some tubulins will have a single modification, while others have multiple modifications and some have no modification. However, the exact distribution along a microtubule and the fraction of the tubulin modified is highly variable, even within a single cell. A few rules have emerged such as tyrosinated tubulin is enriched at the plus ends of growing microtubules, while acetylation and detyrosination are more likely to be seen in the middle or minus end of the microtubule.
In this review, we examine recent studies that have provided insights into the roles that PTMs play in specifying MT function. Given the diversity of PTMs in neurons and their importance in neuronal function, we emphasize PTMs in the nervous system while citing examples from other cell types, keeping in mind that PTMs may play different roles in different cellular contexts, such as cell division.
Detyrosination and Tyrosination of Tubulin
Most, but not all, α-tubulins contain a terminal tyrosine. Exceptions include human TUBA4A with a terminal glutamate and TUBA8 with a terminal phenylalanine [7]. Detyrosination was the first modification identified that preferentially affected MTs, rather than tubulin dimers [8]. The terminal tyrosine is removed by an unidentified cytosolic carboxypeptidase (CCP) to expose a glutamate, but surprisingly little is known about the regulation of this activity. The development of methods to prepare pure fractions of polymerizable tyrosinated and detyrosinated tubulin [9] may provide a basis for identifying this elusive carboxypeptidase.
In contrast, the tubulin tyrosine ligase (TTL) that rapidly tyrosinates detyrosinated tubulins released from MTs is well characterized [3, 4], completing a detyrosination/tyrosination cycle. Structural studies indicate that TTL has multiple interactions with both α- and β-tubulin in the dimer [10]. TTL binding to dimer interferes with polymerization and TTL overexpression inhibits MT polymerization in vivo [11]. Stathmin, a protein that interacts with tubulin dimers, and regulates MT polymerization [12] may also regulate TTL function. TTL and stathmin compete for binding on tubulin dimers, due either to partial overlap in binding sites or altered conformation of stathmin–tubulin complexes; and stathmin inhibits tyrosination of tubulin [13].
Both detyrosinated and tyrosinated tubulins [14-16] are distributed in patches along axonal MTs with enrichment of detyrosinated tubulin in proximal segments of axon shafts and enrichment of tyrosination at growth cones [15, 16]. This distribution is critical for development as revealed by the TTL-null mouse, which dies within 24h after birth with respiratory problems, ataxia, and profoundly altered neuronal morphologies [17]. Lethality may be associated with changes in neurite formation, axon guidance, and increased Rac1 signaling [18]. Detyrosination has been associated with MT longevity because many detyrosinated MTs turn over more slowly than tyrosinated MTs in vivo, but MTs enriched in detyrosinated tubulin are not intrinsically more stable [19]. Enrichment of detyrosinated tubulin in stable MTs reflects preferential action of the carboxypeptidase on MTs, creating composite MTs with newly formed plus ends rich in tyrosinated tubulin and older domains enriched in detyrosinated tubulin.
The differential interactions of proteins with tyrosinated and detyrosinated tubulins are well documented (Fig.4). The reciprocal reactions of detyrosination/tyrosination of tubulin help define distinct subcellular compartments and generate a scaffold for cellular signaling by regulating protein interactions with MTs [20, 21]. For example, although EB1 binds plus ends of MTs independent of tubulin tyrosination, CAP-Gly domain proteins such as CLIP170 or P150 Glued need both EB1 and tyrosinated tubulin to localize to MT plus ends [22]. MT tyrosination also affects the activity of the MT severing protein spastin, which preferentially cleaves detyrosinated MTs [23], despite the association of detyrosination with long-lived MTs.
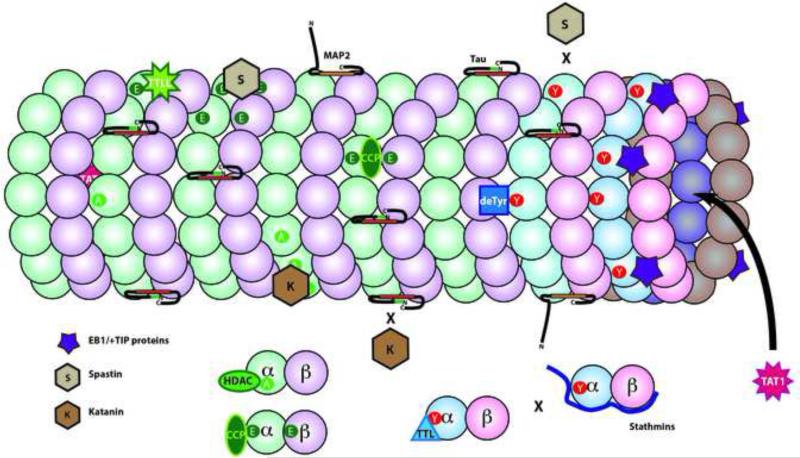
Figure 4. Microtubule Associated Proteins and Posttranslational Modifications of Tubulins.
One function of tubulin modifications is to affect the interaction of other proteins and enzymes. In complex with EB1, some +TIP binding proteins (purple 5 point star) bind selectively to tyrosinated tubulin. As a result, these +TIP proteins are enriched on the plus end of dynamic microtubules, which are rich in newly polymerized tyrosinated α-tubulin. Other proteins interact with particular tubulin isotypes like a subset of α-tubulins (deTyr for detyrosination) or to the C-termini of both α- and β-tubulins (TTLLs for polyglutamylation or polyglycylation). Tubulin acetylases (TAT1) are unique in binding to the interior of the microtubule lumen. Deacetylases (HDAC) or Tubulin Tyrosine Ligase (TTL) bind only to tubulin dimers. Stathmins bind to free tubulins as well, but partially overlap and compete with tubulin binding to TTL. The microtubule severing proteins, katanin (K) and spastin (S) are directed to particular microtubule domains by a combination of tubulin modifications and microtubule associated proteins. Katanin preferentially binds and severs acetylated domains of a microtubule, but katanin binding is inhibited by the presence of tau. In contrast, spastin preferentially binds and severs in polyglutamylated domains, but does not sever microtubules enriched in tyrosinated tubulin.
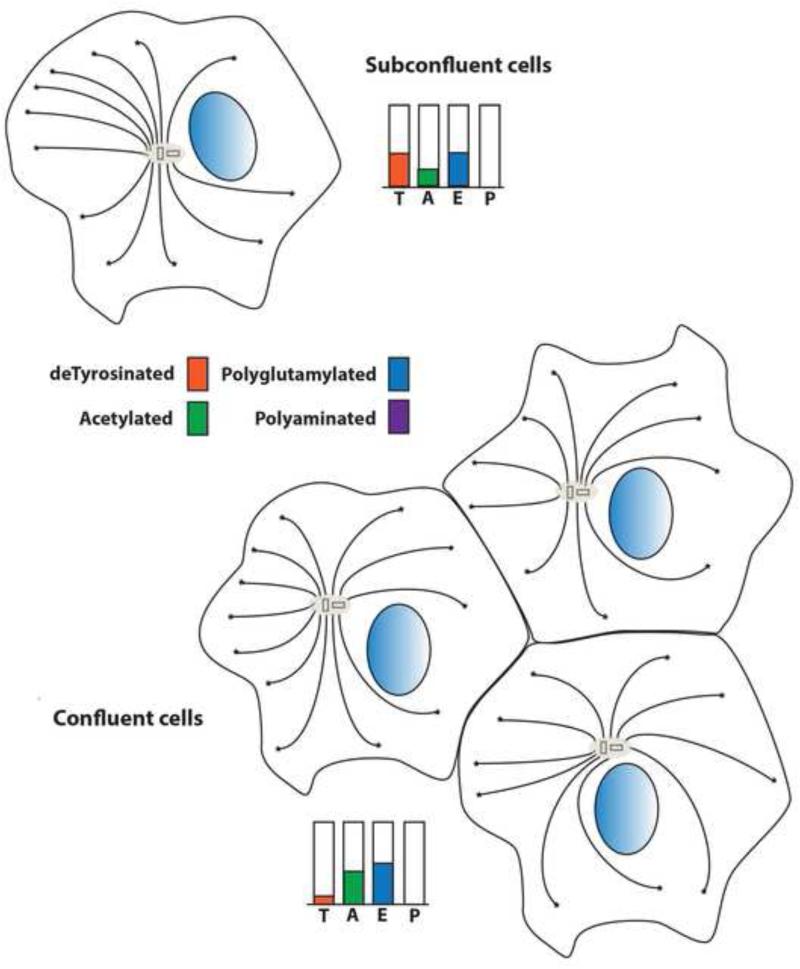
Detyrosination/tyrosination dependent changes in MT dynamics and interactions can occur in different cell types at various developmental stages. For example, detyrosination affects MT interactions with intermediate filaments [24] and microfilaments in vivo [18]. In addition, detyrosination is associated with differentiation and polarization of some cells [25, 26] (Fig. 3). In these studies, subconfluent cells have moderate levels of detyrosination, which increases with confluency and declines as confluent cells become polarized [25, 26]. Local reversibility of detyrosination generates tubulin and MTs with distinct properties more efficiently than protein synthesis and degradation. .
Figure 3. Posttranslational modifications of microtubules are dynamic and vary with cellular context.
In some epithelial cells in culture, the degree of cell confluency and polarity of the cells affects the distribution and organization of modified microtubules. At early stages, microtubules are anchored to the microtubule organizing center at their negative ends. In subconfluent cells, microtubules are relatively rich in detyrosinated tubulin, but poor in acetylated tubulins. In confluent cells, the microtubule organization becomes more symmetrical and detryrosinated microtubules are reduced as acetylation of tubulin increases. Polyglutamylation levels appear to decrease slightly between these two states. Polyamination is generally undetectable in non-neuronal cells and may be specific to neurons.
Tubulin detyrosination and tyrosination may play a role in motor protein function, although the effects may depend on the model and assays used. Kinesin-13 binds preferentially to tyrosinated plus ends and depolymerizes MTs both in vivo and in vitro [27, 28], suggesting that detyrosination would protect MTs. In addition, detyrosination enhanced nucleotide-free binding of kinesin-1 to MTs in vitro with a Kd of 29 nM (deTyr) and 81 nM (Tyr) [29], but the physiological significance of this observation is uncertain, because kinesin concentrations in neurons (500 nM) are an order of magnitude higher. In cultured neurons, some kinesins (i.e. kinesin-2 and -3) accumulate in both axons and dendrites, while others (kinesin-1) enter axons preferentially, raising the possibility that differences in MT tracks might explain differential targeting [30]. Increasing detyrosinated MT levels in neurons led to kinesin-1 accumulation in all neurites [30] and GFP-kinesin-1 preferentially associated with detyrosinated MTs and exhibited a slower rate of movement [31]. Similarly, mutations in a region proposed to be sensitive to tyrosination convert kinesin-1 from an axon-only to an all-neurite motor [30]. However, a subsequent study failed to find a direct correlation between detyrosinated MT content of a neurite and targeting of kinesin-1 [32]. Extensive analysis of in vitro motor interactions with modified tubulin C-terminals using chimeric yeast tubulins [33] found that detyrosination modestly decreased kinesin-1 processivity without affecting its velocity or dynein movement. In contrast, detyrosination increased both processivity and velocity of kinesin-2, suggesting that tyrosination effects on motor function are complex.
Δ2-Tubulin
Δ2-tubulin is a further modification of detyrosinated tubulin where the penultimate glutamate is also removed [5].All six selective CCPs (CCP1 [34], CCP2, CCP3 [35], CCP5 [36], CCP4 and CCP6 [37]) modify α-tubulin C-terminals by removing terminal glutamates or shortening polyglutamate chains (Table 1). Like detyrosination, Δ2-tubulin accumulates in long-lived MTs and is enriched in differentiated neurons as well as in centrioles and cilia [38]. Unlike detyrosination, Δ2-tubulin is a terminal modification whereby the removal of the penultimate glutamate produces tubulins that cannot be tyrosinated [39]. Removal of the penultimate glutamate affects availability of tubulin for modifications, such as tyrosination and polyglutamylation [4]. Although Δ2-tubulin may represent ≥35% of tubulin in the brain [38], the functional role of Δ2-MTs remains poorly understood.
Polyglutamylation and Polyglycylation of tubulin in MTs
Polyglutamylation is abundant in neurons [40] as well as in cilia and centrioles [41, 42]. Enzymes containing a domain related to TTL (i.e. TTL-like (TTLL) proteins) add amino acids to tubulins through an RNA-independent enzymatic reaction. Different TTLLs add glutamate or glycine to side chains initiated on one or more of the glutamates in the C-terminal domains of both α and β-tubulins [3, 4, 43, 44]. Some TTLL glutamylases (TTLL4, 5, and 7) add a single glutamate by forming a γ-linked isopeptide bond, while others (TTLL1, 6, 11 and 13) add glutamate side chains to the γ-linked glutamate through standard peptide linkages to form polyglutamate side chains [3]. Glutamates may be added differentially to specific tubulin isotypes or at different sites. For example, TTLL7 preferentially modifies β-tubulin, while TTLL5 and 6 prefer α-tubulin as a substrate[44]. Some TTLLs also modify other cytoplasmic and nuclear proteins [44].
The number of combinatorials for polyglutamylation with variable chain lengths and modifications on different glutamates in the C-terminal domain as well as on both α and β tubulin isotypes make it difficult to determine its functions. One common element is that the adaptor PGs1 is required to localize the polyglutamylase complex to MTs in cilia and neurons [45], and loss of PGs1 function in the ROSA22 mouse significantly reduces MT polyglutamylation [46]. This mouse is viable but exhibits abnormal sperm motility, behavioral changes, reduced body fat [47] and altered synaptic composition and function [46]. Polyglutamylation effects may depend on cellular context and associated proteins.
As with other tubulin PTMs, polyglutamylation may alter protein interactions with MTs. For example, it differentially affects MAP1 and MAP2 binding to tubulin [48] and stimulates spastin-mediated MT severing [49] (Fig. 4). In contrast, the effects of polyglutamylation on MT motors are less clear. One study reported that reducing tubulin polyglutamylation in the brain altered distribution of kinesin-3, but not kinesin-1 [46]. However, a second study reported that increases in MT polyglutamylation following inhibition of synaptic activity correlated with inhibition of kinesin-1, but not kinesin-3 mobility [50]. The connection with synaptic activity suggested that local changes in polyglutamylation might serve to target selected cargoes [50]. However, these finding are in contrast with studies using chimeric yeast tubulin C-terminal domains for assays of in vitro motility of kinesins and cytoplasmic dynein [33]. The addition of polyglutamate to the C-terminus of β3-tubulin affected various kinesins and dynein differentially: Chains of Glu10 reduced processivity but not velocity of kinesin-1, while Glu3 chains had no effect. Kinesin-2 also appeared unaffected by polyglutamylation, while dynein showed increased processivity. However, these polyglutamatylations were linked to tubulin by maleimide through an engineered Cys residue [33] rather than through the normal glutamate γ-carboxyl isopeptide bond. The structure and effects of these artificial constructs could differ from endogenous polyglutamylations.
CCP1 (Nna1) is upregulated during motor neuron development and regeneration suggesting a role in neurite growth [51] (Fig. 5). The pcd mouse, which results from a loss of function mutation in CCP1, exhibits degeneration of certain neurons (including Purkinje cells) and increased polyglutamylation of tubulin in adults [37]. When CCP1 was identified as the mutant gene, the neuropathology in pcd mice was presumed to result from decreased Δ2-tubulin, but Δ2-tubulin levels are normal in adult pcd mice and polyglutamylated tubulin continues to accumulate. Consistent with increased polyglutamylation causing neuropathology, suppressing TTLL1 protected Purkinje cells from degeneration in pcd mice [34, 37]. Other CCPs may compensate for loss of CCP1 in spared tissues [37], while severely affected neurons (Purkinje and mitral cells) express lower levels of other CCPs, showing the greatest differences in tubulin modification.
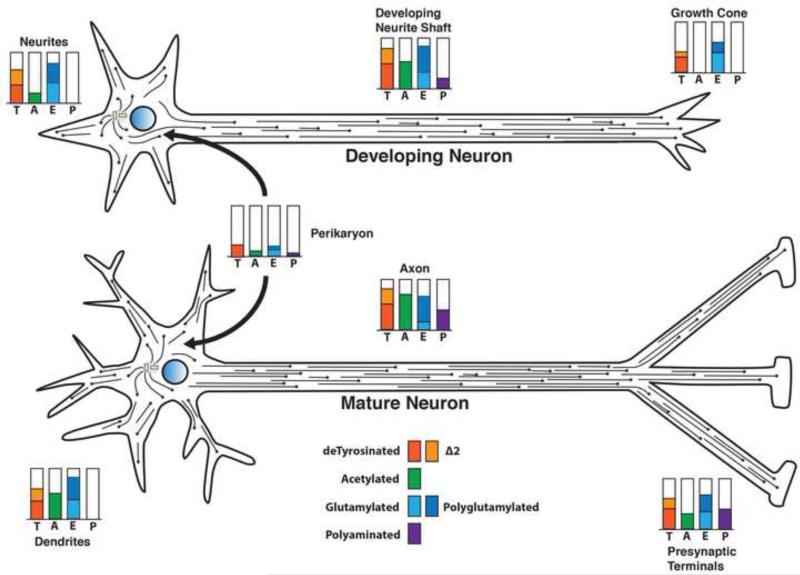
Figure 5. Posttranslational modifications of tubulin and microtubules vary in different regions of a neuron and change during neuronal differentiation.
Microtubules in neuronal axons and dendrites are not anchored at the microtubule organizing center. The microtubules vary in length but have both plus and minus ends free. In the developing neuron (upper), when the axon is extending but the dendrites have not yet differentiated, the microtubules in all neurites are plus end out. Modification levels for detyrosination (T), Δ2 tubulin (Δ2), glutamylation (E), and polyamination (P) in the neuronal perikaryon are relatively low. Acetylation, glutamylation and detyrosination are elevated in the growing axon, but all three are reduced in the growth cone. Little or no acetylation is seen in the microtubules of the growth cone, consistent with the presence of highly dynamic microtubules. The minor neurites at this stage are lower in acetylation, but relatively rich in detyrosinated and glutamylated/polyglutamylated microtubules. Polyamination may be detectable in the growing axon at a low level.
In a mature neuron (lower), as both dendrites and synaptic specializations form, tubulin modifications change both quantitatively and qualitatively. Dendritic microtubules exhibit mixed polarity as dendrites form, while axonal microtubules remain plus end out. Polyamination of axonal microtubules increases with maturation, but may be relatively low or absent from dendritic domains. Detyrosinated and Δ2 tubulin levels remain relatively high in differentiated dendrites and axons, while acetylation increases in both axon shafts and dendrites. Acetylation is also detectable in the distal axon and presynaptic regions, consistent with reduced numbers of highly dynamic microtubules in stable connections. During the differentiation and maturation of neurons, there are also changes in microtubule associated proteins in different neuronal subcellular domains. Adapted from Janke and Kneussel [5].
In contrast to polyglutamylation, polyglycylation is restricted to ciliary tubulins [42, 52] and may be important in ciliary assembly in some organisms [53]. Since glutamylation and glycylation target the same glutamates next to the C-terminus, a reciprocal pathway whereby glycylation modulates polyglutamylation may exist [4]. Both TTLL3 and 8 add a single glycine to glutamates in the C-terminal domain through a γ-linked isopeptide bond [53, 54], which is extended in polyglycylation by TTLL10 [55]. Since TTLL10 is inactive in humans, human sperm flagellar tubulins are monoglycylated [54], and the absence of long chain polyglycylation does not affect ciliary motility [56], so the functional significance of extended polyglycine chains is uncertain. However, a knockout of TTLL3 eliminates all tubulin glycylation in the colon, where TTLL3 is the only glycylase expressed [57]. This absence of glycylation led to reductions in primary cilia and enhanced carcinogenesis in the colon, suggesting that glycylation of tubulin may play a role in the maintenance of primary cilia and cell proliferation.
Acetylation and Deacetylation of Tubulins
Acetylation
Tubulin is the major cytoplasmic target for acetylation [58]. Unlike detyrosination and glutamylation, the canonical site for tubulin acetylation is α-tubulin Lys40 [59] located on the MT lumenal surface [60]. How the acetylase gains access to Lys40 has been unclear, but recent studies suggest that tubulin acetyltransferase (TAT) efficiently scans MTs bidirectionally within the lumen using surface diffusion, acetylating Lys40 stochastically [61]. This activity is not limited by constraints to lumenal diffusion, consistent with observations that rates of tubulin acetylation are similar for short and long MTs [62]; therefore, acetylation is not restricted to MT ends but is distributed along MTs. Ultrastructural studies also show acetylase binding to MT surfaces in vitro, independent of acetylation state [63].
Although several enzymes can acetylate tubulin, including ELP3, ARD1-NAT1 and GCN5 [58], the major tubulin acetylase is αTAT1/MEC17 [64-67]. Elimination of αTAT1/MEC17 activity effectively abolishes MT acetylation in various models, but regulation and substrate specificity of αTAT1 is poorly understood. The major substrate for αTAT1 is α-tubulin, but αTAT1 also auto-acetylates [68]. Eliminating αTAT1 auto-acetylation sites does not affect MT binding, but does reduce tubulin acetylation [68], suggesting it is regulated by auto-acetylation. αTAT1 is also enriched near active clathrin-mediated endocytosis, due to its interaction with clathrin assembly protein, AP2 [69]. Furthermore, αTAT1 associates with acetylated MTs in axons and cilia in vivo [65, 66]. Indeed, male mice lacking αTAT1 are less fertile due to altered sperm morphology and motility [65]; however, they do not exhibit obvious behavioral or neurological defects beyond subtle changes in brain architecture [70]. However, in mice without αTAT1 or acetylated MTs, the MTs were more resistant to nocodazole, suggesting increased MT stability [65]. MEC17 may also have other functions because the expression of enzyme-dead MEC17 restores touch sensitivity in the absence of tubulin acetylation [67].
Although Lys40 is highly conserved in most mammalian α-tubulins isotypes (an exception is human TUBA8), not all isotypes are acetylated to the same extent. In Physarum [71] and Trypanosoma [72], only TUBA3 is acetylated, while proteomic analysis of acetylation in human cells [73] suggests preferential acetylation of the widely expressed TUBA1 and TUBA4 [7]. Tubulin acetylation also varies in different cells and tissues [58], being lower in rapidly dividing cells than in postmitotic cells. However, some cells and organisms lack acetylated tubulin [58], indicating that tubulin acetylation is not essential for cell survival. For example, MEC12 is the only α-tubulin gene in C. elegans with a Lys40, but a MEC12 Lys40Gln mutant eliminates acetylation without affecting viability [67]. Additional acetylation sites on tubulin have also been identified in proteomic screens [73] and in studies of other acetyltransferases [74]. The prevalence and function of these sites are not well understood, but may affect MT polymerization [74].
Despite being a major PTM of tubulin, there is little consensus on the functions for tubulin acetylation [58]. The association of acetylation with long-lived MTs suggested a role in the stabilization of MTs. While this idea persists, direct tests showed no effect of acetylation on MT dynamics [3]. Acetylation is enriched in long-lived MTs, because soluble tubulin dimers are rapidly deacetylated and newly formed MTs are deficient in acetylation. The dependence of αTAT1 on lattice geometry of MTs and its slow catalytic rate effectively limit acetylation of dynamic MTs [61]. Further, both active and acetylase-dead forms of mouse αTAT1 can bind to MTs and destabilize them in vitro [68].
Acetylation may also affect MT structure. Wild-type MTs in C. elegans touch sensitive neurons have 15 protofilaments, and mutations in MEC17 or in MEC12 result in a reduced number of MTs in touch neurons, while the remaining MTs have a variable protofilament number [67, 75]. However, the effect of acetylation on MT protofilaments is not observed in other systems where acetylated MTs have the standard 13 protofilaments. Futhermore, cryo-electron microscopy failed to find structural differences between acetylated and deacetylated MTs [63].
MT acetylation affects protein interactions with MTs. Indeed, the binding of Hsp90 to MTs is enhanced by acetylation, and recruiting Hsp90 activates Akt and p53 [76]. Increasing MT acetylation can also induce IL10 (an anti-inflammatory cytokine) and activate p38 MAP kinase in macrophages [77]. Acetylated tubulin is the preferred site of action for the severing protein katanin [78] (Fig. 4) and overexpressing katanin produced MT cleavage in nonneuronal cells and somatodendritic regions of neurons [78]. In contrast, axonal MTs are less sensitive to katanin, because axonal tau protects acetylated MTs from katanin [79, 80]. The question of how katanin senses lumenal acetylation of MTs remains to be answered.
MT acetylation was also reported to enhance kinesin-based transport [81], because inhibition of tubulin deacetylase modestly enhanced AMP-PNP mediated kinesin binding to MTs and redistributed a putative kinesin cargo. However, acetylation and kinesin motility were not correlated in vivo [32], and in vitro analysis of kinesin velocity and run length found no effect of MT acetylation [82], leaving the role of acetylation on motor function unresolved.
Deacetylation
While acetylation preferentially occurs on tubulin in MTs, tubulin dimers are rapidly deacetylated when released from MTs [58]. Eukaryotic protein deacetylases comprise two families: 11 classical histone deacetylases (HDACs) [83] and 7 sirtuins [84]. Although HDAC6 is the primary α-tubulin deacetylase in non-dividing cells [85], it has other substrates that may not be related to its effects on tubulin, such as Hsp90, cortactin, β-catenin, and periredoxins. HDAC6 also interacts with ubiquitin independent of its acetylase activity [86], suggesting a role in responding to protein aggregates and other stressors [87]. Thus, manipulations of HDAC6 levels or activity may affect multiple pathways [83-85]. However, while nuclear HDAC knockouts are embryonic or perinatal lethal [83], HDAC6 null mice are viable and develop normally, albeit with hyperacetylated tubulin [88]. The mild phenotype of HDAC6 null mice suggests that compensatory pathways exist for HDAC6 functions. These may include HDACs that are normally nuclear, such as HDAC5, which is exported from the nucleus of sensory neurons after injury and transported into the axon where it can deacetylate tubulin [89, 90].
Nonclassical HDACs or sirtuins (SIRT) are NAD+-dependent deacetylases and ADP-Ribosyltransferases [84] that are structurally unrelated to classical HDACs [83]. SIRTs have multiple substrates [91], but only SIRT2 deacetylates α-tubulin [92]. SIRT2 is primarily cytoplasmic, but may be shuttled to the nucleus [93]. Although α-tubulins are a preferred cytoplasmic substrate, SIRT2 has nuclear substrates [84] and may play a role in cell cycle regulation by deacetylating histone-4 and/or α-tubulin [94].
While overexpressed SIRT2 and HDAC6 interact and co-immunoprecipitate, either can individually deacetylate α-tubulin [92]. Treating cells with siRNA for SIRT2 or HDAC6 increases tubulin acetylation, but HDAC6 silencing is more effective. SIRT2 deacetylase is regulated by cell cycle dependent kinases including Cdk1, Cdk2 and neuronal Cdk5 [95]. Since Cdk5 is a major neurofilament kinase [96], whose activity is high in neurons [97], SIRT2 deacetylation of tubulin in neurons may be limited due to phosphorylation-dependent inhibition by Cdk5 [98]. However, SIRT2-dependent acetylation of tubulin plays a key role in dividing cells and cancer [91]. Both SIRT2 knockout and SIRT2/HDAC6 double knockout mice are also viable and normal [98], despite tubulin hyperacetylation. The minimal phenotype is puzzling given that tubulin deacetylases have multiple substrates in addition to α-tubulins. A phenotype for these mice may emerge late in life or in response to specific stressors, but neither hypo- nor hyperacetylation of tubulin significantly disrupts cell or organismal function, therefore physiological roles of α-tubulin acetylation remain enigmatic.
Polyamination of tubulin in microtubules
MTs in mature axons are unusually stable, remaining intact after depolymerizing treatments such as cold, Ca2+ and antimitotic drugs [2, 99]. This tubulin fraction contains biochemically distinct tubulins significantly more basic than the sequence predicts [99], suggesting a novel posttranslational modification that adds positive charges. Polyamination, in which a transglutaminase (TG) covalently links a polyamine to intracellular proteins is such a modification.
TGs mainly catalyze two reactions on proteins in vivo: 1) Covalent cross-linking of peptides and proteins through γ-glutamyl-ε-lysyl bonds; 2) Polyamination, i.e. covalent formation of γ-glutamyl amine bonds to poly/di/monoamines such as putrescine, spermine and spermidine [2]. When polyamine levels are high, as in the nervous system, polyamination of glutamine predominates and is likely to be irreversible under physiological conditions. TG crosslinking activity is dependent on calcium and can be inhibited by free GTP/GDP [100]. Deamidation may occur in low pH conditions in vitro, where polyamines can be removed. The major cytoplasmic TG (TG2) may also function as a kinase [101], GTPase [100, 102] and protein disulfide isomerase [103].
Modification of proteins by TG2 may play a role in apoptosis [104]. TG2 may stabilize misfolded protein aggregates and promote inflammation in diseases such as Alzheimer's, Huntington's, Parkinson's and amyotrophic lateral sclerosis (ALS) [105, 106]. TG2 knockout mice are viable and grossly normal despite some abnormalities in insulin secretion, wound healing, autoimmune reaction and inflammation [107, 108].
Neuronal tubulin, polymerized MTs and taxol-stabilized MTs are all substrates for TG-catalyzed polyamination in vitro and this modification conferred resistance of MTs against cold and Ca2+ [2]. Inhibiting polyamine synthesis by difluoromethylornithine (DFMO) or genetically knocking out TG2 reduced MT stability in vivo [2]. Some TG activity and stable MTs remain in brain homogenates from TG2 null mice, suggesting that multiple TGs contribute to the stabilization of axonal MTs [2].
Polyaminated tubulin facilitated MT nucleation and polymerization in vitro, preventing MT dissociation, consistent with polyamination regulating MT dynamics [109]. TG protein levels and enzymatic activity increase in axons as neurons develop and mature, correlating with increased stable MTs. Inhibiting TG2 activity in culture reduces neurite outgrowth and maturation, while neurons in a demyelinating mouse model exhibit lower TG2 activity and decreased MT stability [109, 110].
Neuronal structure, axonal guidance, growth and retraction among other parameters require a balance between MT stability and plasticity. Both polyamines and TG levels correlate with MT stability during neuronal development and maturation. Questions remain about the distribution and functions of tubulin polyamination in neurons, but altered MT stability and plasticity via this pathway may affect many aspects of brain development, aging, regeneration and degeneration. Changes in tubulin polyamination may be neuroprotective when MT hyperstability helps maintain axonal structures, but may exacerbate pathology by reducing plasticity and altering synaptic function.
Antibodies against polyaminated tubulins based on identified polyamination sites along MTs [2] will facilitate studies of tubulin polyamination. Similarly, fluorescent TG-inhibitors [111] will help track TG2 distribution and activity at various stages of neurons, defining roles for TG2 in axonal health and disease. Combining these reagents with the TG2 knockout mouse [112] will further our understanding of tubulin polyamination.
Other Modifications of Tubulin
The tubulin PTMs discussed so far affect a large fraction of the tubulin/MTs in the brain and/or other tissues. However, a number of additional modifications have been reported, which are less abundant or less common. These PTMs may affect minor fractions of tubulin or MTs, may be restricted to specific cell types or subcellular compartments, or may be transient. Nonetheless, they appear to play an important role in the functional diversity of MTs.
Phosphorylation of tubulin
Phosphorylation is one of the most common posttranslational modifications. Although phosphorylation of microtubule-associated proteins receives the most attention, tubulins can also be phosphorylated. Phosphorylation of β-tubulin was initially reported in MTs of differentiated neuroblastoma cells [113]. Subsequently, α and β-tubulins were shown to be phosphorylated by serine/threonine kinases like Cdk1, CK2, and CamKII as well as tyrosine kinases like Jak2, Syk, Fes, and Src [4]. Most of these kinases phosphorylate tubulin in MTs [4], but the cell cycle kinase Cdk1 phosphorylates S172 on soluble β-tubulin and inhibits polymerization [114]. Tubulin phosphorylation may also be associated with MT interactions with membranes [4] or the regulation of polymerization [114, 115].
Glycosylation and Glycation of tubulin
Carbohydrates can be added to tubulin through either O-linked glycosylation [116, 117] or non-enzymatic glycation [118]. O-linked glycosylation often occurs on phosphorylation sites and may regulate protein-protein interactions and activity. Tubulin O-linked glycosylation appears heterogeneous and cell specific, but our knowledge regarding its cellular functions is limited. It is known that glycosylated tubulin can assemble into MTs and MTs near the nucleus are positive for a sialyloligosaccharide antibody [117]. The differentiation of a neuron-like cell line by retinoic acid alters O-GlcNAcylation of α and β-tubulins and inhibits tubulin polymerization [119]. Furthermore, glycosylation of TUBB3 may be associated with resistance to chemotherapeutic drugs [116].
Whereas O-linked glycosylation is a normal physiological event, non-enzymatic glycation is pathological and associated with diabetes [118]. Proteins are glycated when high levels of glucose associated with hyperglycemia react with amino acids [120]. Tubulin glycation in axons is significant in diabetes and may be a factor in diabetic neuropathy [118] as glycation may denature tubulin and lead to depolymerization.
Palmitoylation of Tubulin
Low levels of tubulin in membrane fractions are consistently observed [121], but poorly understood. Palmitoylation can affect protein interactions with membranes and subcellular trafficking [122]. While multiple mechanisms may mediate tubulin interactions with membranes, palmitate can be reversibly linked to α-tubulin Cys376 [123]. Palmitoylation of tubulin may affect membrane microviscosity or alter tubulin interaction with membrane proteins [121]; however, palmitoylated tubulin is not restricted to membranes. For example, C376S mutant α-tubulin affects organization of yeast astral MTs and nuclear positioning [123]. In addition, palmitoylated tubulin does not cycle efficiently, but can incorporate into MTs [124]. The antimitotic vinblastine inhibits tubulin palmitoylation [125], but it is not known whether this plays a role in the action of vinblastine on MT polymerization.
Ubiquitylation and Sumoylation of Tubulin
Ubiquitin and SUMO are small proteins added covalently to proteins by selective ligases [126, 127]. The addition of polymeric ubiquitin or SUMO is typically associated with proteasomal degradation, but adding a single ubiquitin or SUMO may affect protein interactions, enzyme activity, protein transport or localization [126, 128].
The ubiquitin E3 ligase parkin, which is mutated in some cases of familial Parkinson's disease [129, 130], binds and ubiquitylates tubulin dimers, increasing tubulin degradation or recycling [131]. Parkin and ubiquitinylated α-tubulin can accumulate in aggregates of Parkinson's disease and ALS [132, 133], which is enhanced by proteasome inhibition [134]. Ubiquitylation of α-tubulin, but not β-tubulin was essential for the disassembly and resorption of flagella in Chlamydomonas [135], but the responsible E3 ligase was not identified. In addition, sumoylated tubulin was identified in proteomic screens in yeast [136] and then mammalian cells [137], where TUBA1, TUBA6 and TUBB6 were modified. Sumoylation is associated with cellular functions that involve MTs such as intracellular trafficking and plasticity and may be altered in neurological diseases [126]. Thus, these modifications play a role in the degradation of tubulin, but may have additional effects that are less well understood.
Concluding remarks
Relating specific tubulin modifications to specific cellular functions remains a major challenge. Initial correlations often broke down with additional study due to the overlap of different modifications. Nonetheless, common themes are emerging and questions continue to arise (Outstanding questions box). First, the regulation of MT dynamics remains a key aspect of tubulin PTMs, but new mechanisms have been identified and new functions are emerging. Second, MTs are intrinsically heterogeneous. There are not only tyrosinated plus ends, but other PTMs may be concentrated in specific domains along the MT and these domains may overlap. Individual subunits within the MT may be unmodified or have multiple modifications. Third, PTMs affect the interaction of specific proteins with MTs, such as the association of some +TIP proteins with tyrosinated tubulin or the targeting of severing proteins by acetylation and polyglutamylation. As a result, PTMs may play a critical role in defining MTs as a cellular compartment for organizing signaling cascades within the cell. Fourth, the cellular contexts of PTMs remain critical for proper cell function, including enrichment of polyamination in axons and polyglycylation in cilia. MTs in these cellular domains require specific properties, such as the need for unusual stability in axonal domains that may extend a meter or more from the primary site of tubulin synthesis. Finally, tubulin PTMs are dynamic and must be regulated during development and differentiation and in response to injury and stress. Just as MTs can be dynamic or long-lived, both the distribution and levels of a given PTM may be changed in response to physiological and pathological cues.
Highlights.
Tubulin modifications contribute to functional diversity of MTs
PTMs affect MT dynamics, organization and interactions with other cellular components
Various PTMs overlap or localize to specific MT domains demonstrating heterogeneity
PTMs vary with time, cell type, subcellular compartment and physiological state
Outstanding Questions Box.
Many substantive questions remain about tubulin PTMs:
How can we reconcile in vitro and in vivo effects of tubulin PTMs?
Which PTMs regulate MT dynamics and which ones change as a consequence of altered dynamics?
What are the enzymes responsible for specific tubulin PTMs in vivo, i.e. what is the carboxypeptidase responsible for detyrosination and the removal of glycylation?
Why are there multiple enzymes to make the same modification and how do their physiological roles differ?
What is the role of modified MTs as scaffolds for localization of signaling complexes?
What are the implications of PTMs in neurodevelopment and neurological disorders?
Acknowledgements
Preparation for this manuscript was supported in part by grants from the National Institute of Neurological Disorders and Stroke (NS023868 and NS041170) to STB and from HHMI and the Grass Foundation (YS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barra HS, et al. A soluble preparation from rat brain that incorporates into its own proteins ( 14 C)arginine by a ribonuclease-sensitive system and ( 14 C)tyrosine by a ribonuclease-insensitive system. J Neurochem. 1973;20:97–108. doi: 10.1111/j.1471-4159.1973.tb12108.x. [DOI] [PubMed] [Google Scholar]
- 2.Song Y, et al. Transglutaminase and Polyamination of Tubulin: Posttranslational Modification for Stabilizing Axonal Microtubules. Neuron. 2013;78:108–123. doi: 10.1016/j.neuron.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12:773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- 4.Wloga D, Gaertig J. Post-translational modifications of microtubules. J Cell Sci. 2010;123:3447–3455. doi: 10.1242/jcs.063727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janke C, Kneussel M. Tubulin post-translational modifications: encoding functions on the neuronal microtubule cytoskeleton. Trends Neurosci. 2010;33:362–372. doi: 10.1016/j.tins.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- 7.Ludueña RF, Banerjee A. The Isotypes of Tubulin: Distribution and Functional Significance. In: Fojo AT, editor. Cancer Drug Discovery and Development: The Role of Microtubules in Cell Biology, Neurobiology, and Oncology. Humana Press; 2008. [Google Scholar]
- 8.Gundersen GG, et al. Postpolymerization detyrosination of alpha-tubulin: a mechanism for subcellular differentiation of microtubules. J Cell Biol. 1987;105:251–264. doi: 10.1083/jcb.105.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lafanechere L, Job D. Preparation of pure tyrosinated or detyrosinated tubulin isoforms. Methods Mol Biol. 2011;777:71–86. doi: 10.1007/978-1-61779-252-6_5. [DOI] [PubMed] [Google Scholar]
- 10.Prota AE, et al. Structural basis of tubulin tyrosination by tubulin tyrosine ligase. J Cell Biol. 2013;200:259–270. doi: 10.1083/jcb.201211017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szyk A, et al. Tubulin tyrosine ligase structure reveals adaptation of an ancient fold to bind and modify tubulin. Nature structural & molecular biology. 2011;18:1250–1258. doi: 10.1038/nsmb.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinmetz MO. Structure and thermodynamics of the tubulin-stathmin interaction. J Struct Biol. 2007;158:137–147. doi: 10.1016/j.jsb.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Szyk A, et al. Tubulin tyrosine ligase and stathmin compete for tubulin binding in vitro. J Mol Biol. 2013;425:2412–2414. doi: 10.1016/j.jmb.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baas PW, Black MM. Individual microtubules in the axon consist of domains that differ in both composition and stability. J Cell Biol. 1990;111:495–509. doi: 10.1083/jcb.111.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown A, et al. Composite microtubules of the axon: Quantitative analysis of tyrosinated and acetylated tubulin along individual axonal microtubules. J. Cell Sci. 1993;104:339–352. doi: 10.1242/jcs.104.2.339. [DOI] [PubMed] [Google Scholar]
- 16.Geuens G, et al. Ultrastructural colocalization of tyrosinated and detyrosinated alpha-tubulin in interphase and mitotic cells. J Cell Biol. 1986;103:1883–1893. doi: 10.1083/jcb.103.5.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erck C, et al. A vital role of tubulin-tyrosine-ligase for neuronal organization. Proc Natl Acad Sci U S A. 2005;102:7853–7858. doi: 10.1073/pnas.0409626102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcos S, et al. Tubulin tyrosination is required for the proper organization and pathfinding of the growth cone. PLoS One. 2009;4:e5405. doi: 10.1371/journal.pone.0005405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webster DR, et al. Detyrosination of alpha tubulin does not stabilize microtubules in vivo. J Cell Biol. 1990;111:113–122. doi: 10.1083/jcb.111.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galjart N. Plus-end-tracking proteins and their interactions at microtubule ends. Curr Biol. 2010;20:R528–537. doi: 10.1016/j.cub.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Jiang K, Akhmanova A. Microtubule tip-interacting proteins: a view from both ends. Curr Opin Cell Biol. 2011;23:94–101. doi: 10.1016/j.ceb.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Bieling P, et al. CLIP-170 tracks growing microtubule ends by dynamically recognizing composite EB1/tubulin-binding sites. J Cell Biol. 2008;183:1223–1233. doi: 10.1083/jcb.200809190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roll-Mecak A, Vale RD. Structural basis of microtubule severing by the hereditary spastic paraplegia protein spastin. Nature. 2008;451:363–367. doi: 10.1038/nature06482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreitzer G, et al. Detyrosination of tubulin regulates the interaction of intermediate filaments with microtubules in vivo via a kinesin-dependent mechanism. Molec. Biol. Cell. 1999;10:1105–1118. doi: 10.1091/mbc.10.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quinones GB, et al. The posttranslational modification of tubulin undergoes a switch from detyrosination to acetylation as epithelial cells become polarized. Mol Biol Cell. 2011;22:1045–1057. doi: 10.1091/mbc.E10-06-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zink S, et al. Tubulin detyrosination promotes monolayer formation and apical trafficking in epithelial cells. J Cell Sci. 2012;125:5998–6008. doi: 10.1242/jcs.109470. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh-Roy A, et al. Kinesin-13 and tubulin posttranslational modifications regulate microtubule growth in axon regeneration. Dev Cell. 2012;23:716–728. doi: 10.1016/j.devcel.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peris L, et al. Motor-dependent microtubule disassembly driven by tubulin tyrosination. J Cell Biol. 2009;185:1159–1166. doi: 10.1083/jcb.200902142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao G, Gundersen GG. Kinesin is a candidate for cross-bridging microtubules and intermediate filaments. J. Biol. Chem. 1998;273:9797–9803. doi: 10.1074/jbc.273.16.9797. [DOI] [PubMed] [Google Scholar]
- 30.Konishi Y, Setou M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat Neurosci. 2009;12:559–567. doi: 10.1038/nn.2314. [DOI] [PubMed] [Google Scholar]
- 31.Dunn S, et al. Differential trafficking of Kif5c on tyrosinated and detyrosinated microtubules in live cells. J Cell Sci. 2008;121:1085–1095. doi: 10.1242/jcs.026492. [DOI] [PubMed] [Google Scholar]
- 32.Hammond JW, et al. Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol Biol Cell. 2010;21:572–583. doi: 10.1091/mbc.E09-01-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sirajuddin M, et al. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat Cell Biol. 2014 doi: 10.1038/ncb2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berezniuk I, et al. Cytosolic carboxypeptidase 1 is involved in processing alpha- and beta-tubulin. J Biol Chem. 2012;287:6503–6517. doi: 10.1074/jbc.M111.309138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tort O, et al. The cytosolic carboxypeptidases CCP2 and CCP3 catalyze posttranslational removal of acidic amino acids. Mol Biol Cell. 2014;25:3017–3027. doi: 10.1091/mbc.E14-06-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berezniuk I, et al. Cytosolic carboxypeptidase 5 removes alpha- and gamma-linked glutamates from tubulin. J Biol Chem. 2013;288:30445–30453. doi: 10.1074/jbc.M113.497917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogowski K, et al. A family of protein-deglutamylating enzymes associated with neurodegeneration. Cell. 2010;143:564–578. doi: 10.1016/j.cell.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Paturle-Lafanechere L, et al. Accumulation of delta 2-tubulin, a major tubulin variant that cannot be tyrosinated, in neuronal tissues and in stable microtubule assemblies. J Cell Sci. 1994;107(Pt 6):1529–1543. doi: 10.1242/jcs.107.6.1529. [DOI] [PubMed] [Google Scholar]
- 39.Lafanechere L, Job D. The third tubulin pool. Neurochem Res. 2000;25:11–18. doi: 10.1023/a:1007575012904. [DOI] [PubMed] [Google Scholar]
- 40.Audebert S, et al. Developmental regulation of polyglutamylated alpha- and beta-tubulin in mouse brain neurons. J Cell Sci. 1994;107(Pt 8):2313–2322. doi: 10.1242/jcs.107.8.2313. [DOI] [PubMed] [Google Scholar]
- 41.Abal M, et al. Centrioles resist forces applied on centrosomes during G2/M transition. Biol Cell. 2005;97:425–434. doi: 10.1042/BC20040112. [DOI] [PubMed] [Google Scholar]
- 42.Gaertig J, Wloga D. Ciliary tubulin and its post-translational modifications. Curr Top Dev Biol. 2008;85:83–113. doi: 10.1016/S0070-2153(08)00804-1. [DOI] [PubMed] [Google Scholar]
- 43.Mukai M, et al. Recombinant mammalian tubulin polyglutamylase TTLL7 performs both initiation and elongation of polyglutamylation on beta-tubulin through a random sequential pathway. Biochemistry. 2009;48:1084–1093. doi: 10.1021/bi802047y. [DOI] [PubMed] [Google Scholar]
- 44.van Dijk J, et al. Polyglutamylation is a post-translational modification with a broad range of substrates. J Biol Chem. 2008;283:3915–3922. doi: 10.1074/jbc.M705813200. [DOI] [PubMed] [Google Scholar]
- 45.Regnard C, et al. Characterisation of PGs1, a subunit of a protein complex co-purifying with tubulin polyglutamylase. J Cell Sci. 2003;116:4181–4190. doi: 10.1242/jcs.00743. [DOI] [PubMed] [Google Scholar]
- 46.Ikegami K, et al. Loss of alpha-tubulin polyglutamylation in ROSA22 mice is associated with abnormal targeting of KIF1A and modulated synaptic function. Proc Natl Acad Sci U S A. 2007;104:3213–3218. doi: 10.1073/pnas.0611547104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell PK, et al. Mutation of a novel gene results in abnormal development of spermatid flagella, loss of intermale aggression and reduced body fat in mice. Genetics. 2002;162:307–320. doi: 10.1093/genetics/162.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonnet C, et al. Differential binding regulation of microtubule-associated proteins MAP1A, MAP1B, and MAP2 by tubulin polyglutamylation. J Biol Chem. 2001;276:12839–12848. doi: 10.1074/jbc.M011380200. [DOI] [PubMed] [Google Scholar]
- 49.Lacroix B, et al. Tubulin polyglutamylation stimulates spastin-mediated microtubule severing. J Cell Biol. 2010 doi: 10.1083/jcb.201001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maas C, et al. Synaptic activation modifies microtubules underlying transport of postsynaptic cargo. Proc Natl Acad Sci U S A. 2009;106:8731–8736. doi: 10.1073/pnas.0812391106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris A, et al. Regenerating motor neurons express Nna1, a novel ATP/GTP-binding protein related to zinc carboxypeptidases. Mol Cell Neurosci. 2000;16:578–596. doi: 10.1006/mcne.2000.0900. [DOI] [PubMed] [Google Scholar]
- 52.Sloboda RD. Posttranslational protein modifications in cilia and flagella. Methods Cell Biol. 2009;94:347–363. doi: 10.1016/S0091-679X(08)94018-8. [DOI] [PubMed] [Google Scholar]
- 53.Wloga D, et al. TTLL3 Is a tubulin glycine ligase that regulates the assembly of cilia. Dev Cell. 2009;16:867–876. doi: 10.1016/j.devcel.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 54.Rogowski K, et al. Evolutionary divergence of enzymatic mechanisms for posttranslational polyglycylation. Cell. 2009;137:1076–1087. doi: 10.1016/j.cell.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 55.Ikegami K, Setou M. TTLL10 can perform tubulin glycylation when co-expressed with TTLL8. FEBS Lett. 2009;583:1957–1963. doi: 10.1016/j.febslet.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 56.Dossou SJ, et al. Mammalian cilia function is independent of the polymeric state of tubulin glycylation. Cell Motil Cytoskeleton. 2007;64:847–855. doi: 10.1002/cm.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rocha C, et al. Tubulin glycylases are required for primary cilia, control of cell proliferation and tumor development in colon. EMBO J. 2014 doi: 10.15252/embj.201488466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perdiz D, et al. The ins and outs of tubulin acetylation: more than just a post-translational modification? Cell Signal. 2011;23:763–771. doi: 10.1016/j.cellsig.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 59.L'Hernault SW, Rosenbaum JL. Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry. 1985;24:473–478. doi: 10.1021/bi00323a034. [DOI] [PubMed] [Google Scholar]
- 60.Soppina V, et al. Luminal localization of alpha-tubulin K40 acetylation by cryo-EM analysis of fab-labeled microtubules. PLoS One. 2012;7:e48204. doi: 10.1371/journal.pone.0048204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szyk A, et al. Molecular basis for age-dependent microtubule acetylation by tubulin acetyltransferase. Cell. 2014;157:1405–1415. doi: 10.1016/j.cell.2014.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maruta H, et al. The acetylation of alpha-tubulin and its relationship to the assembly and disassembly of microtubules. J. Cell. Biol. 1986;103:571–579. doi: 10.1083/jcb.103.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Howes SC, et al. Effects of tubulin acetylation and tubulin acetyltransferase binding on microtubule structure. Mol Biol Cell. 2014;25:257–266. doi: 10.1091/mbc.E13-07-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akella JS, et al. MEC-17 is an alpha-tubulin acetyltransferase. Nature. 2010;467:218–222. doi: 10.1038/nature09324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kalebic N, et al. alphaTAT1 is the major alpha-tubulin acetyltransferase in mice. Nature communications. 2013;4:1962. doi: 10.1038/ncomms2962. [DOI] [PubMed] [Google Scholar]
- 66.Shida T, et al. The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc Natl Acad Sci U S A. 2010;107:21517–21522. doi: 10.1073/pnas.1013728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Topalidou I, et al. Genetically separable functions of the MEC-17 tubulin acetyltransferase affect microtubule organization. Curr Biol. 2012;22:1057–1065. doi: 10.1016/j.cub.2012.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalebic N, et al. Tubulin acetyltransferase alphaTAT1 destabilizes microtubules independently of its acetylation activity. Mol Cell Biol. 2013;33:1114–1123. doi: 10.1128/MCB.01044-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Montagnac G, et al. alphaTAT1 catalyses microtubule acetylation at clathrin-coated pits. Nature. 2013;502:567–570. doi: 10.1038/nature12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim GW, et al. Mice lacking alpha-tubulin acetyltransferase 1 are viable but display alpha-tubulin acetylation deficiency and dentate gyrus distortion. J Biol Chem. 2013;288:20334–20350. doi: 10.1074/jbc.M113.464792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sasse R, et al. Acetylated alpha-tubulin in Physarum: immunological characterization of the isotype and its usage in particular microtubular organelles. J Cell Biol. 1987;104:41–49. doi: 10.1083/jcb.104.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Souto-Padron T, et al. Acetylated alpha-tubulin in Trypanosoma cruzi: immunocytochemical localization. Memorias do Instituto Oswaldo Cruz. 1993;88:517–528. doi: 10.1590/s0074-02761993000400004. [DOI] [PubMed] [Google Scholar]
- 73.Choudhary C, et al. Lysine acetylation targets protein complexes and co- regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 74.Chu CW, et al. A novel acetylation of beta-tubulin by San modulates microtubule polymerization via down-regulating tubulin incorporation. Mol Biol Cell. 2011;22:448–456. doi: 10.1091/mbc.E10-03-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cueva JG, et al. Posttranslational acetylation of alpha-tubulin constrains protofilament number in native microtubules. Curr Biol. 2012;22:1066–1074. doi: 10.1016/j.cub.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Giustiniani J, et al. Tubulin acetylation favors Hsp90 recruitment to microtubules and stimulates the signaling function of the Hsp90 clients Akt/PKB and p53. Cell Signal. 2009;21:529–539. doi: 10.1016/j.cellsig.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 77.Wang B, et al. Microtubule acetylation amplifies p38 kinase signalling and anti-inflammatory IL-10 production. Nature communications. 2014;5:3479. doi: 10.1038/ncomms4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sudo H, Baas PW. Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts. J Neurosci. 2010;30:7215–7226. doi: 10.1523/JNEUROSCI.0048-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qiang L, et al. Tau protects microtubules in the axon from severing by katanin. J Neurosci. 2006;26:3120–3129. doi: 10.1523/JNEUROSCI.5392-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu W, et al. The Microtubule-severing Proteins Spastin and Katanin Participate Differently in the Formation of Axonal Branches. Mol Biol Cell. 2008 doi: 10.1091/mbc.E07-09-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reed NA, et al. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol. 2006;16:2166–2172. doi: 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 82.Walter WJ, et al. Tubulin acetylation alone does not affect kinesin-1 velocity and run length in vitro. PLoS One. 2012;7:e42218. doi: 10.1371/journal.pone.0042218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Witt O, et al. HDAC family: What are the cancer relevant targets? Cancer Lett. 2009;277:8–21. doi: 10.1016/j.canlet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 84.Yao YL, Yang WM. Beyond histone and deacetylase: an overview of cytoplasmic histone deacetylases and their nonhistone substrates. J Biomed Biotechnol. 2011;2011:146493. doi: 10.1155/2011/146493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang PH, et al. HDAC6: Physiological function and its selective inhibitors for cancer treatment. Drug discoveries & therapeutics. 2013;7:233–242. doi: 10.5582/ddt.2013.v7.6.233. [DOI] [PubMed] [Google Scholar]
- 86.Boyault C, et al. HDAC6-p97/VCP controlled polyubiquitin chain turnover. EMBO J. 2006;25:3357–3366. doi: 10.1038/sj.emboj.7601210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boyault C, et al. HDAC6, at the crossroads between cytoskeleton and cell signaling by acetylation and ubiquitination. Oncogene. 2007;26:5468–5476. doi: 10.1038/sj.onc.1210614. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Y, et al. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol Cell Biol. 2008;28:1688–1701. doi: 10.1128/MCB.01154-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cho Y, Cavalli V. HDAC5 is a novel injury-regulated tubulin deacetylase controlling axon regeneration. EMBO J. 2012;31:3063–3078. doi: 10.1038/emboj.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cho Y, et al. Injury-induced HDAC5 nuclear export is essential for axon regeneration. Cell. 2013;155:894–908. doi: 10.1016/j.cell.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stunkel W, Campbell RM. Sirtuin 1 (SIRT1): the misunderstood HDAC. J Biomol Screen. 2011;16:1153–1169. doi: 10.1177/1087057111422103. [DOI] [PubMed] [Google Scholar]
- 92.North BJ, et al. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 93.North BJ, Verdin E. Interphase nucleo-cytoplasmic shuttling and localization of SIRT2 during mitosis. PLoS One. 2007;2:e784. doi: 10.1371/journal.pone.0000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Inoue T, et al. The molecular biology of mammalian SIRT proteins: SIRT2 in cell cycle regulation. Cell Cycle. 2007;6:1011–1018. doi: 10.4161/cc.6.9.4219. [DOI] [PubMed] [Google Scholar]
- 95.Pandithage R, et al. The regulation of SIRT2 function by cyclin- dependent kinases affects cell motility. J Cell Biol. 2008;180:915–929. doi: 10.1083/jcb.200707126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shetty VK, et al. Neuronal cyclin-dependent kinase-5 phosphorylation sites in neurofilament protein (NF-H) are dephosphorylated by protein phosphatase 2A. J. Neurochem. 1995;64:2681–2690. doi: 10.1046/j.1471-4159.1995.64062681.x. [DOI] [PubMed] [Google Scholar]
- 97.Dhariwala FA, Rajadhyaksha MS. An unusual member of the Cdk family: Cdk5. Cell Mol Neurobiol. 2008;28:351–369. doi: 10.1007/s10571-007-9242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bobrowska A, et al. SIRT2 ablation has no effect on tubulin acetylation in brain, cholesterol biosynthesis or the progression of Huntington's disease phenotypes in vivo. PLoS One. 2012;7:e34805. doi: 10.1371/journal.pone.0034805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brady ST, et al. Axonal tubulin and axonal microtubules: Biochemical evidence for cold stability. J. Cell Biol. 1984;99:1716–1724. doi: 10.1083/jcb.99.5.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fesus L, Piacentini M. Transglutaminase 2: an enigmatic enzyme with diverse functions. Trends Biochem Sci. 2002;27:534–539. doi: 10.1016/s0968-0004(02)02182-5. [DOI] [PubMed] [Google Scholar]
- 101.Mishra S, Murphy LJ. Tissue transglutaminase has intrinsic kinase activity: identification of transglutaminase 2 as an insulin-like growth factor-binding protein-3 kinase. J Biol Chem. 2004;279:23863–23868. doi: 10.1074/jbc.M311919200. [DOI] [PubMed] [Google Scholar]
- 102.Kiraly R, et al. Protein transamidation by transglutaminase 2 in cells: a disputed Ca2+-dependent action of a multifunctional protein. FEBS J. 2011;278:4717–4739. doi: 10.1111/j.1742-4658.2011.08345.x. [DOI] [PubMed] [Google Scholar]
- 103.Hasegawa G, et al. A novel function of tissue-type transglutaminase: protein disulphide isomerase. Biochem J. 2003;373:793–803. doi: 10.1042/BJ20021084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Facchiano F, et al. The role of transglutaminase-2 and its substrates in human diseases. Front Biosci. 2006;11:1758–1773. doi: 10.2741/1921. [DOI] [PubMed] [Google Scholar]
- 105.Oono M, et al. Transglutaminase 2 accelerates neuroinflammation in amyotrophic lateral sclerosis through interaction with misfolded superoxide dismutase 1. J Neurochem. 2014;128:403–418. doi: 10.1111/jnc.12441. [DOI] [PubMed] [Google Scholar]
- 106.Ricotta M, et al. Physio-pathological roles of transglutaminase catalyzed reactions. World journal of biological chemistry. 2010;1:181–187. doi: 10.4331/wjbc.v1.i5.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Deasey S, et al. Tissue-specific responses to loss of transglutaminase 2. Amino Acids. 2011 doi: 10.1007/s00726-011-1183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iismaa SE, et al. Transglutaminases and disease: lessons from genetically engineered mouse models and inherited disorders. Physiological reviews. 2009;89:991–1023. doi: 10.1152/physrev.00044.2008. [DOI] [PubMed] [Google Scholar]
- 109.Song Y. Stabilization of Neuronal Microtubules by Polyamines and Transglutaminase: Its Roles in Brain Function. Vol. 199. Dept of Anatomy and Cell Biology, University of Illinois at Chicago College of Medicine; 2010. [Google Scholar]
- 110.Kirkpatrick LL, et al. Changes in microtubule stability and density in myelin-deficient shiverer mouse CNS axons. J Neurosci. 2001;21:2288–2297. doi: 10.1523/JNEUROSCI.21-07-02288.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chabot N, et al. Fluorescent probes of tissue transglutaminase reveal its association with arterial stiffening. Chem Biol. 2010;17:1143–1150. doi: 10.1016/j.chembiol.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 112.Sarang Z, et al. Some lessons from the tissue transglutaminase knockout mouse. Amino Acids. 2009;36:625–631. doi: 10.1007/s00726-008-0130-x. [DOI] [PubMed] [Google Scholar]
- 113.Gard DL, Kirschner M. A polymer dependent increase in phosphorylation of ß-tubulin accompanies differentiation of a mouse neuroblastoma cell line. J. Cell Biol. 1985;100:764–774. doi: 10.1083/jcb.100.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fourest-Lieuvin A, et al. Microtubule regulation in mitosis: tubulin phosphorylation by the cyclin-dependent kinase Cdk1. Mol Biol Cell. 2006;17:1041–1050. doi: 10.1091/mbc.E05-07-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Laurent CE, et al. The human c-Fes tyrosine kinase binds tubulin and microtubules through separate domains and promotes microtubule assembly. Mol Cell Biol. 2004;24:9351–9358. doi: 10.1128/MCB.24.21.9351-9358.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cicchillitti L, et al. Proteomic characterization of cytoskeletal and mitochondrial class III beta-tubulin. Mol Cancer Ther. 2008;7:2070–2079. doi: 10.1158/1535-7163.MCT-07-2370. [DOI] [PubMed] [Google Scholar]
- 117.Hino M, et al. Glycosylation of the alpha and beta tubulin by sialyloligosaccharides. Zoological science. 2003;20:709–715. doi: 10.2108/zsj.20.709. [DOI] [PubMed] [Google Scholar]
- 118.McLean WG. The role of axonal cytoskeleton in diabetic neuropathy. Neurochem Res. 1997;22:951–956. doi: 10.1023/a:1022466624223. [DOI] [PubMed] [Google Scholar]
- 119.Ji S, et al. O-GlcNAcylation of tubulin inhibits its polymerization. Amino Acids. 2011;40:809–818. doi: 10.1007/s00726-010-0698-9. [DOI] [PubMed] [Google Scholar]
- 120.Miyazawa T, et al. Lipid glycation and protein glycation in diabetes and atherosclerosis. Amino Acids. 2012;42:1163–1170. doi: 10.1007/s00726-010-0772-3. [DOI] [PubMed] [Google Scholar]
- 121.Wolff J. Plasma membrane tubulin. Biochim Biophys Acta. 2009;1788:1415–1433. doi: 10.1016/j.bbamem.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 122.Fukata Y, Fukata M. Protein palmitoylation in neuronal development and synaptic plasticity. Nat Rev Neurosci. 2010;11:161–175. doi: 10.1038/nrn2788. [DOI] [PubMed] [Google Scholar]
- 123.Caron JM, et al. Single site alpha-tubulin mutation affects astral microtubules and nuclear positioning during anaphase in Saccharomyces cerevisiae: possible role for palmitoylation of alpha-tubulin. Mol Biol Cell. 2001;12:2672–2687. doi: 10.1091/mbc.12.9.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wolff J, et al. Autopalmitoylation of tubulin. Protein Sci. 2000;9:1357–1364. doi: 10.1110/ps.9.7.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Caron JM, Herwood M. Vinblastine, a chemotherapeutic drug, inhibits palmitoylation of tubulin in human leukemic lymphocytes. Chemotherapy. 2007;53:51–58. doi: 10.1159/000098419. [DOI] [PubMed] [Google Scholar]
- 126.Flotho A, Melchior F. Sumoylation: a regulatory protein modification in health and disease. Annu Rev Biochem. 2013;82:357–385. doi: 10.1146/annurev-biochem-061909-093311. [DOI] [PubMed] [Google Scholar]
- 127.Varshavsky A. The ubiquitin system, an immense realm. Annu Rev Biochem. 2012;81:167–176. doi: 10.1146/annurev-biochem-051910-094049. [DOI] [PubMed] [Google Scholar]
- 128.Komander D, Rape M. The ubiquitin code. Annu Rev Biochem. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 129.Cookson MR, Bandmann O. Parkinson's disease: insights from pathways. Hum Mol Genet. 2010;19:R21–27. doi: 10.1093/hmg/ddq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hardy J, et al. The genetics of Parkinson's syndromes: a critical review. Curr Opin Genet Dev. 2009;19:254–265. doi: 10.1016/j.gde.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 131.Ren Y, et al. Parkin binds to alpha/beta tubulin and increases their ubiquitination and degradation. J Neurosci. 2003;23:3316–3324. doi: 10.1523/JNEUROSCI.23-08-03316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ardley HC, et al. Inhibition of proteasomal activity causes inclusion formation in neuronal and non-neuronal cells overexpressing Parkin. Mol Biol Cell. 2003;14:4541–4556. doi: 10.1091/mbc.E03-02-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Muqit MM, et al. Parkin is recruited into aggresomes in a stress-specific manner: over-expression of parkin reduces aggresome formation but can be dissociated from parkin's effect on neuronal survival. Hum Mol Genet. 2004;13:117–135. doi: 10.1093/hmg/ddh012. [DOI] [PubMed] [Google Scholar]
- 134.Hyun DH, et al. Proteasomal inhibition causes the formation of protein aggregates containing a wide range of proteins, including nitrated proteins. J Neurochem. 2003;86:363–373. doi: 10.1046/j.1471-4159.2003.01841.x. [DOI] [PubMed] [Google Scholar]
- 135.Huang K, et al. The ubiquitin conjugation system is involved in the disassembly of cilia and flagella. J Cell Biol. 2009;186:601–613. doi: 10.1083/jcb.200903066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Panse VG, et al. A proteome-wide approach identifies sumoylated substrate proteins in yeast. J Biol Chem. 2004;279:41346–41351. doi: 10.1074/jbc.M407950200. [DOI] [PubMed] [Google Scholar]
- 137.Rosas-Acosta G, et al. A universal strategy for proteomic studies of SUMO and other ubiquitin-like modifiers. Mol Cell Proteomics. 2005;4:56–72. doi: 10.1074/mcp.M400149-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]