Abstract
Recent studies have reported that rats raised in an enriched condition (EC) have decreased dopamine transporter (DAT) function and expression in medial prefrontal cortex (mPFC), as well as increased d-amphetamine-induced glutamate release in nucleus accumbens compared to rats raised in an isolated condition (IC). In these previous studies, DAT function and expression were evaluated using mPFC pooled from four rats for each condition to obtain kinetic parameters due to sparse DAT expression in mPFC. In contrast, accumbal glutamate release was determined using individual rats. The current study extends the previous work and reports on the optimization of DAT and serotonin transporter (SERT) functional assays, as well as cell surface expression assays using both mPFC and orbital frontal cortex (OFC) from individual EC or IC rats. In addition, the effect of d-amphetamine on glutamate release in mPFC and OFC of EC and IC rats was determined using in vivo microdialysis. Results show that environmental enrichment decreased maximal transport velocity (Vmax) for [3H]dopamine uptake in mPFC, but increased Vmax for [3H]dopamine uptake in OFC. Corresponding changes in DAT cell surface expression were not found. In contrast, Vmax for [3H]serotonin uptake and cellular localization of SERT in mPFC and OFC were not different between EC and IC rats. Further, acute d-amphetamine (2 mg/kg, s.c.) increased extracellular glutamate concentrations in mPFC of EC rats only and in OFC of IC rats only. Overall, these results suggest that enrichment produces long-lasting alterations in mPFC and OFC DAT function via a trafficking-independent mechanism, as well as differential glutamate release in mPFC and OFC. Rearing-induced modulation of DAT function and glutamate release in prefrontal cortical subregions may contribute to the known protective effects of enrichment on drug abuse vulnerability.
Keywords: dopamine transporter, enrichment, glutamate, serotonin transporter, medial prefrontal cortex, orbitofrontal cortex
1. Introduction
Previous research reveals that both genetic and environmental factors contribute to individual differences in drug abuse vulnerability (Bardo et al., 2013). Preclinical models have been developed to evaluate pre-existing individual differences and to understand the neurobehavioral mechanisms that underlie drug abuse vulnerability. Enrichment and isolation housing conditions have been used to evaluate the role of environmental factors in drug abuse vulnerability. Rats reared in an enriched condition (EC) with social cohorts and novel objects exhibit decreased locomotor activity in an inescapable novel environment compared to those raised in an isolated condition (IC; Green et al., 2003; Zhu et al., 2004). EC rats exhibit decreased intravenous self-administration of low unit doses of d-amphetamine across repeated sessions compared to IC rats (Bardo et al., 2001). Also, environmental enrichment decreases impulsivity, which has been linked to drug abuse (Perry et al., 2005; Wood et al., 2006; Baler and Volkow, 2006; Perry et al., 2008). Taken together, environmental enrichment may protect against vulnerability to drug abuse.
The enrichment-induced reduction in vulnerability to abuse d-amphetamine and other drugs may be mediated, at least in part, by altered dopamine (DA), serotonin (5-HT) and glutamate function in brain regions implicated in reward (Zhu et al., 2004; Brenes et al., 2008; Rahman and Bardo, 2008, Bardo et al., 2013). EC rats exhibit an increased density of DA-immunoreactive fibers innervating striatum compared to IC rats (Wallace et al., 1992). In nucleus accumbens, EC rats have greater glucose utilization, as well as greater amphetamine-stimulated DA and glutamate release compared to IC rats (Bardo et al., 1999; Gonzalez-Lima et al., 1994; Rahman and Bardo, 2008). This suggests that enrichment may sensitize the mesolimbic reward system, perhaps leading to a compensatory decrease in the amount of drug that is self-administered by EC rats compared to IC rats.
Extracellular DA and 5-HT concentrations are regulated primarily by the DA transporter (DAT) and serotonin transporter (SERT), respectively. DAT and SERT are major targets for addictive drugs, which alter the function and trafficking of these transporters (Zahniser and Sorkin, 2009; Ramamoorthy et al., 2011). Environmental enrichment during development alters DAT function in a brain-region specific manner (Zhu et al., 2004). In mPFC, Vmax for [3H]DA uptake is decreased in EC rats compared to IC rats, with no differences between EC and IC rats in nucleus accumbens or striatum. Decreased DAT function in mPFC in EC rats is associated with a reduction in cell surface expression in EC compared with IC rats (Zhu et al., 2005). In these previous studies, DAT function and expression were evaluated using mPFC pooled from four rats to obtain kinetic parameters due to the sparse DAT expression in mPFC. Enrichment-induced alterations in DAT function have not been examined in other subregions of prefrontal cortex such as orbitofrontal cortex (OFC). OFC has been implicated in mediating impulsivity (Perry et al., 2011), which plays a role in drug abuse vulnerability. Moreover, EC rats exhibit less impulsivity relative to IC rats (Perry et al., 2008). Thus, assessment of enrichment-induced alterations in DAT function in OFC is important for elucidating the neural mechanisms underlying both the reduced impulsivity and reduced drug abuse vulnerability in EC compared to IC rats.
Recent evidence also indicates that the serotonergic system in mPFC and OFC is involved in impulsivity (Winstanley et al., 2010). Whether alterations in these cortical systems play a role in the protective effect of environmental enrichment on drug abuse vulnerability is not known. Impulsivity determined in the delay discounting task has been associated with changes in extracellular 5-HT concentrations in mPFC and extracellular dihydroxyphenylacetic acid (DOPAC) in OFC (Winstanley et al., 2006). However, relatively little is known about the effect of environmental enrichment on SERT function and cellular localization. In one study, the selective SERT inhibitor citalopram was ineffective in reversing aggressive behavior in isolated mice compared to group housed mice (Rilke et al., 2001). However, it remains to be determined if enrichment alters basal SERT function in either mPFC or OFC.
Although extracellular DA regulates the activity of dopaminoceptive glutamate neurons (Svenningsson et al., 2004), relatively little is known about enrichment-induced changes in glutamate systems. Compared to EC rats, IC rats have decreased glutamatergic tone mediated by mGluR2 receptors in mPFC (Melendez et al., 2004), suggesting a role of glutamatergic signaling in environment-induced effects. In addition, pretreatment with MK-801, a noncompetitive NMDA receptor antagonist, prevents amphetamine-induced increases in extracellular glutamate concentrations in nucleus accumbens of EC rats relative to IC rats (Rahman and Bardo, 2008). Although environment-induced alterations in glutamatergic activity in prefrontal cortex may play a role in enrichment-induced alterations in impulsivity (Melendez et al., 2004), the role of glutamate release and its regulation in response to psychostimulants, such as amphetamine, in the prefrontal cortex of EC and IC rats has not been investigated.
The current study investigated the effect of environmental enrichment on DAT and SERT function and expression in mPFC and OFC as potential mechanisms underlying the previously observed enrichment-induced protective effects on drug abuse vulnerability. In addition, basal and amphetamine-induced extracellular glutamate concentrations in mPFC and OFC were determined using in vivo microdialysis to determine whether glutamate transmission in these prefrontal regions is associated with the enrichment-induced protective effects.
2. Results
2.1 Effect of enrichment on kinetic parameters of [3H]DA uptake in mPFC and OFC
DAT function in mPFC and OFC from individual rats was determined using saturation analyses of [3H]DA uptake. Maximal [3H]DA uptake in mPFC was lower in EC rats than in IC rats (Vmax: 1.72 ± 0.06 and 2.87 ± 0.51 pmol/min/mg, respectively; t(15) = 2.39, p<0.05), with no change in Km (Fig 1). In contrast, maximal [3H]DA uptake in OFC was greater in EC rats than in IC rats (Vmax: 4.23 ± 0.43 and 2.68 ± 0.44 pmol/min/mg, respectively; t(17) = 2.49, p<0.05), with no change in Km (Fig 1). Thus, environmental enrichment produced opposite effects on [3H]DA uptake in the two prefrontal regions examined, with Vmax decreased by 40% in mPFC and increased by 55% in OFC of EC rats compared to IC rats.
Fig. 1. Environmental enrichment differentially alters maximal velocity (Vmax) of [3H]DA uptake in medial prefrontal cortex (mPFC) and orbitofrontal cortex (OFC).
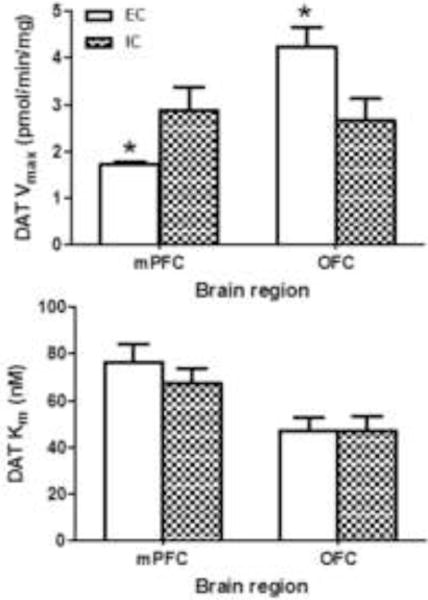
Data (mean ± S.E.M) for maximal velocity of [3H]DA uptake (Vmax, top panel) and affinity of DA for DAT (Km, bottom panel) are expressed as pmol/min/mg protein and nM, respectively. * p< 0.05, different from IC group. Both mPFC and OFC were obtained from individual rats in the EC and IC conditions. For the EC group, n = 8 for mPFC and n = 9 for OFC. For the IC group, n = 9 for mPFC and OFC.
2.2 Effect of enrichment on DAT surface expression in mPFC and OFC
To determine whether enrichment-induced changes in DAT function were associated with alterations in DAT subcellular localization, biotinylation and immunoblot assays were performed. DAT immunoreactivity was determined in total, non-biotinylated and biotinylated fractions of mPFC and OFC from individual EC and IC rats (Fig 2). DAT immunoreactive bands (~75 kDa) were detected in all three fractions of mPFC and OFC in both EC and IC rats; however, no differences between EC and IC rats in DAT protein levels (in all three fractions) in mPFC or OFC were found (Fig 2). Further, no differences were found between EC and IC rats in levels of control proteins (β-actin and PP2A).
Fig. 2. Environmental enrichment does not alter DAT immunoreactivity in total (I), non-biotinylated (II, intracellular) or biotinylated (III, cell surface) fractions from mPFC (left panel) or OFC (right panel).
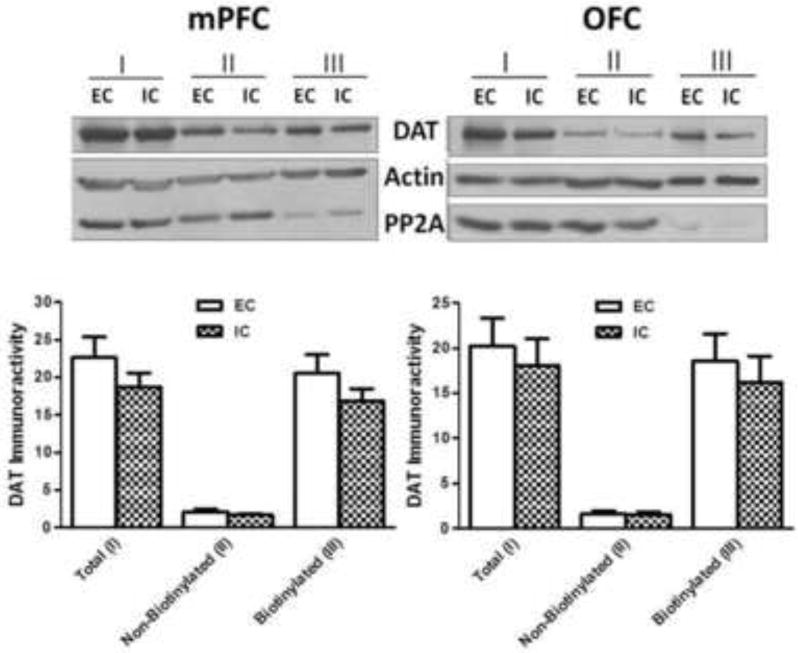
Subcellular fractions of mPFC and OFC from EC and IC rats were assayed using biotinylation and immunoblotting. Top panels show representative immunoblots of DAT expression from mPFC and OFC of EC and IC rats. Immunoreactive bands were within the linear range of detection. Bottom panels show mean ± SEM densitometry values for DAT immunoreactivity. β-Actin was used to monitor protein loading. Both mPFC and OFC were obtained from individual rats within the EC and IC conditions. For EC and IC groups, n = 8 rats for mPFC and OFC.
2.3 Effect of enrichment on kinetic parameters of [3H]5-HT uptake and on total SERT protein expression in mPFC and OFC
SERT function and total expression of immunoreactive SERT protein were determined in mPFC and OFC obtained from individual EC and IC rats. No differences were found between EC and IC rats for Vmax or Km of [3H]5-HT uptake in either mPFC or OFC (Fig 3). Additionally, no differences between EC and IC were found in SERT-immunoreactive bands (~70 kDa) or in the levels of β-actin in mPFC and OFC (Fig 4). Thus, enrichment did not alter SERT function or protein levels in mPFC or OFC.
Fig. 3. Environmental enrichment does not alter Vmax or Km values for [3H]5-HT uptake in mPFC or OFC obtained from EC and IC rats.
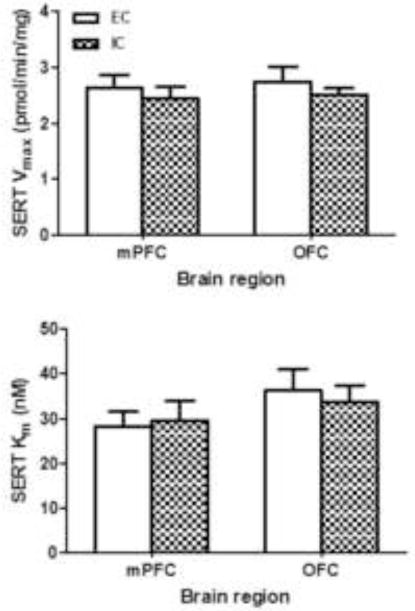
Data (mean ± S.E.M) for Vmax (top panel) and Km (bottom panel) are presented as pmol/min/mg protein and nM, respectively. Both mPFC and OFC were obtained from individual rats in the EC and IC conditions. For EC and IC groups, n = 9 rats for mPFC and OFC.
Fig 4. Environmental enrichment did not alter SERT immunoreactivity in mPFC or OFC from EC and IC rats.
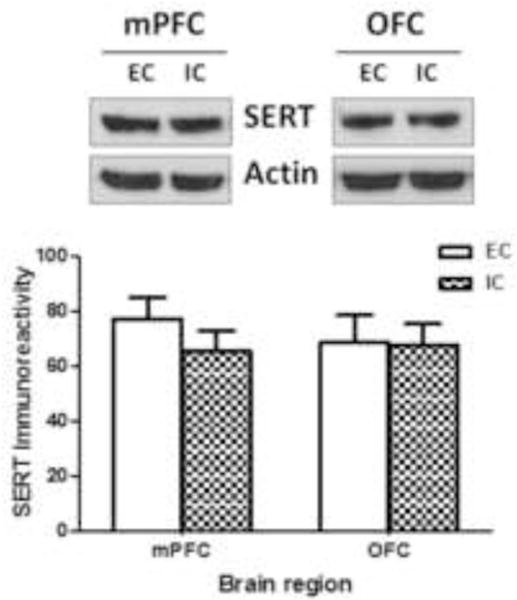
Top panel shows representative immunoblots of SERT expression in mPFC and OFC from EC and IC groups. Immunoreactive bands were within the linear range of detection. Bottom panel shows mean ± SEM of densitometry values for SERT immunoreactivity. β-Actin was used to monitor protein loading. Both mPFC and OFC were obtained from individual rats within the EC and IC conditions. For EC and IC groups, n = 8 rats for mPFC and OFC.
2.4 Effect of enrichment on d-amphetamine-induced extracellular glutamate concentration in mPFC and OFC
Fig. 5 illustrates approximate probe placements in mPFC and OFC of EC and IC rats plotted on representative sections from the rat brain atlas of Paxinos and Watson (1998). EC and IC rats had a similar distribution of probe placements across the medial-lateral extent of OFC. Overlapping probe placements are not shown in the figure for clarity of presentation.
Fig 5. Histological placements of microdialysis probes in mPFC and OFC.
Locations of microdialysis probe placements in mPFC and OFC as indicated by vertical lines. Numbers to the right indicate distance in mm from bregma according to Paxinos and Watson (1998). The active probe protruded 2 mm below the distal end of the guide cannulae for mPFC (top panels) and OFC (bottom panels). Probe placements for EC (left panels) and IC rats (right panels) are identified in two separate sections of both mPFC and OFC. Probe placements in EC and IC rats indicated by dark black and gray vertical lines, respectively.
There were no differences between EC and IC rats in basal extracellular glutamate concentration in either mPFC or OFC (mPFC, EC 169 ± 19.5 and IC 149 ± 46.7 pg/μl; OFC, EC 122 ± 33.4 and IC 123 ±19.7 pg/μl). Systemic injection of saline did not alter extracellular glutamate across 60 min of sampling in either mPFC or OFC of EC and IC rats compared to basal control. In contrast, d-amphetamine differentially increased extracellular glutamate concentrations in EC and IC rats. In mPFC, d-amphetamine transiently increased extracellular glutamate concentrations to a peak of ~97% compared to saline control levels in EC rats [F(8,40) = 2.32, p < 0.05; Fig. 6, top], whereas no significant change was observed in IC rats. In OFC, d-amphetamine transiently increased extracellular glutamate concentrations to a peak of ~122% compared to saline control levels in IC rats [F(8,32) = 2.75, p < 0.05; Fig. 6, bottom], whereas no significant change was observed in EC rats. Extracellular glutamate concentrations returned to saline control levels by 80–100 min after d-amphetamine injection.
Fig 6. Environmental enrichment modulated the effect of amphetamine on extracellular glutamate concentrations in mPFC and OFC.
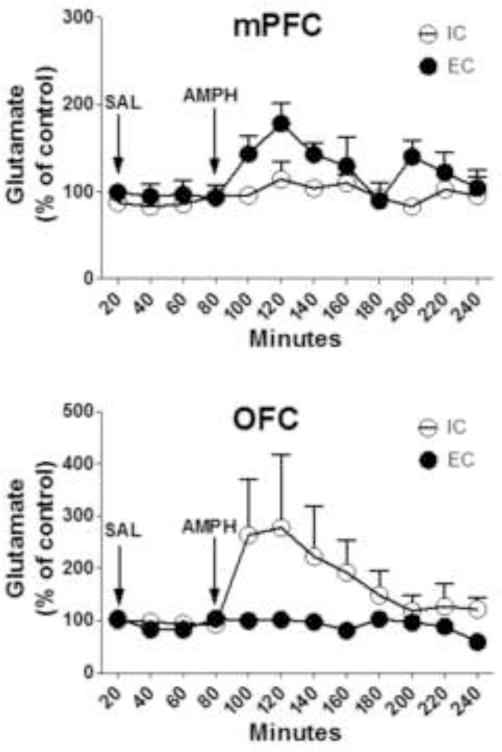
Top panel shows the time course of the effect of acute d-amphetamine (AMPH, 2 mg/kg, s.c.) on extracellular glutamate concentrations in mPFC of EC and IC rats. Bottom panel shows the time course of the effect of acute d-amphetamine (AMPH, 2 mg/kg, s.c.) on extracellular glutamate concentrations in OFC of EC and IC rats. After collection of basal samples, rats were injected with saline (SAL), as indicated by the left arrow, and then 60 min later, AMPH was injected, as indicated by the right arrow. Data are mean ± SEM percent change from control. For the EC group, n = 6 for mPFC and n = 4 for OFC. For the IC group, n = 5 for mPFC and OFC.
3. Discussion
The current study reports the effects of environmental enrichment during development on DAT and SERT function and cellular localization, as well as on extracellular glutamate concentrations in mPFC and OFC in response to acute d-amphetamine administration. Exposure to enrichment during development altered DAT function, but not DAT cellular localization, in mPFC and OFC. Specifically, EC rats exhibited a 40% decrease in maximal velocity of [3H]DA uptake in mPFC, but a 55% increase in OFC, compared to IC rats. Enrichment-induced alterations in DAT function in mPFC and OFC occurred through a trafficking-independent mechanism. In contrast, SERT function and total SERT protein expression did not differ between EC and IC rats in either brain region. Paralleling the differential effects of enrichment on DAT function, extracellular glutamate concentrations were increased by d-amphetamine in mPFC of EC rats only and increased in OFC of IC rats only. Taken together, the differential effects of rearing on DAT function and glutamate release in prefrontal cortical subregions may constitute neural mechanisms underlying the reported protective effects of enrichment on drug abuse vulnerability (Stairs and Bardo, 2009).
Enrichment-induced decreases in mPFC DAT function suggest that EC rats have higher extracellular DA concentrations and greater dopaminergic neurotransmission in this brain region relative to IC rats. The current results are consistent with our previous findings using mPFC pooled from several EC rats (Zhu et al., 2004) and suggest that the increased dopaminergic activity in mPFC may underlie the reduced locomotor activity in EC rats relative to IC rats. Support for this idea comes from a report showing that local administration of GBR 12909, a DAT inhibitor, into mPFC reduces locomotor activity in mice (Radcliffe and Erwin, 1996). In contrast, enrichment increased DAT function in OFC, suggesting lower extracellular DA concentrations in this brain region. Since OFC plays a critical role in impulsivity (Eagle et al., 2008; Schoenbaum et al., 1998, 2002; Mobini et al., 2002; Winstanley et al., 2004), the enrichment-induced increase in DAT function in OFC may explain why EC rats exhibit less impulse choice than IC rats using a delay discounting task (Perry et al., 2008). Further, DAT function in OFC in individual rats is negatively correlated with impulsive action determined using the cued go/no-go task (unpublished observations), suggesting that increased DAT function in OFC is associated with a reduction in both impulsive choice and impulsive action. Thus, the observed decrease in DAT function in mPFC in EC rats may be related to their decreased locomotor activity, whereas enrichment-induced increases in OFC DAT function may be related to their decreased impulsivity.
To determine potential mechanisms underlying the enrichment-induced alterations in DAT function in mPFC and OFC, biotinylation assays were conducted. However, no differences between EC and IC rats in DAT cellular localization were found. This finding contrasts with our previous results showing decreased mPFC DAT function to be associated with decreased mPFC DAT cell surface expression in EC compared to IC rats (Zhu et al., 2005). Explanations for the apparent discrepancies between our current and previously published results on DAT cellular localization in mPFC include two differences in methodology. In Zhu et al (2005), dissection of the mPFC included motor cortex, whereas this region was not included in the current dissection of mPFC. Thus, both observations may be correct in that somewhat different brain regions were evaluated. Furthermore, in our previously published study, surface DAT (biotinylated fraction) was estimated by subtracting the non-biotinylated fraction (intracellular DAT) from the total fraction. In the current study, DAT localization to the surface fraction was determined directly from the density of the DAT band in the biotinylated fraction. Quantification in the current study represents a more accurate measure of surface DAT expression.
In addition to DAT, effects of environmental enrichment on SERT function and expression in mPFC and OFC also were determined. Effects of environmental enrichment during development on SERT function and expression in mPFC and OFC were expected based on findings from previous studies. 5-HT levels in PFC were increased in EC rats relative to IC rats, although this may have been due to a higher turnover rate for this transmitter in IC rats (Brenes et al., 2008). However, no differences in SERT function or total protein levels between EC and IC were found in the current study.
In addition to DA and 5-HT, dopaminoceptive prefrontal pyramidal neurons may be a component of the impulsivity- and abuse-related neurocircuitry that is altered by environmental enrichment. In the current microdialysis experiments, basal extracellular glutamate concentrations were not different between EC and IC rats in either mPFC or OFC. These results are consistent with a previous study in which basal extracellular glutamate concentrations were not different in mPFC between EC and IC rats (Melendez et al., 2004). However, acute d-amphetamine administration increased extracellular glutamate in mPFC of EC rats, but not in IC rats, which extends previous results showing enhanced d-amphetamine-stimulated glutamate release in nucleus accumbens following enrichment (Rahman and Bardo, 2008). Taken together, these results suggest that enrichment may sensitize the mesocorticolimbic system to the effects of d-amphetamine on glutamate transmission. Since animals are thought to regulate their drug intake around some optimal reinforcement or satiation threshold (Lynch and Carroll, 2001; Tsilbulsky and Norman, 1999), perhaps a sensitized mesocorticolimbic reward system lowers the reinforcement or satiety threshold in EC rats, thus leading to reduced intake compared to IC rats.
The current microdialysis results also extend previous work by evaluating neurotransporter function and expression in OFC, a region implicated strongly in impulsivity (Schoenbaum et al., 1998; Eagle et al., 2008). In contrast to mPFC, acute d-amphetamine increased extracellular glutamate in OFC of IC rats, but not in EC rats. The augmented glutamate response to d-amphetamine following isolation may explain the reduction in impulsive choice observed in IC rats, but not EC rats, using a delay discounting task (Perry et al., 2008). Since d-amphetamine increases extracellular DA concentrations by reversing DAT function, this action may increase D1-receptor signaling on pyramidal neurons in prefrontal cortex (Gaspar et al., 1995; Santana et al., 2009). Increased D1-receptor signaling in prefrontal cortex may in turn affect downstream signaling in nucleus accumbens, with a subsequent increase in glutamate levels in both prefrontal cortex and nucleus accumbens. However, d-amphetamine also evokes the release of norepinephrine and 5-HT, which could be alternative candidates mediating the glutamate response. Taken together, these results suggest that differential effects of d-amphetamine on glutamate function in mPFC and OFC may be involved in the ability of enrichment to reduce abuse- and impulsivity-related behaviors.
3.1 Conclusions
In summary, enrichment decreased DAT function in mPFC and increased DAT function in OFC, and these functional changes occurred in a trafficking-independent manner. In contrast, no changes were found in SERT function or SERT protein expression. Consistent with a role for DA to regulate dopaminoceptive glutamate neurons, d-amphetamine increased extracellular glutamate in mPFC of EC rats only, while d-amphetamine increased extracellular glutamate in OFC of IC rats only. These enrichment-induced alterations in DAT function and extracellular glutamate concentrations in response to d-amphetamine may be important neuroadaptations underlying the differential risk for drug abuse among individuals with different environmental histories.
4. Experimental procedures
4.1 Materials
[3H]5-HT (5-[1,2-3H(N)-hydroxytryptamine creatinine sulfate; specific activity, 27.1 Ci/mmol) and [3H]DA (3,4-ethyl-2 [N-3H] dihydroxyphenylethylamine; specific activity, 31 Ci/mmol) were purchased from Perkin Elmer Life Sciences (Boston, MA). 5-Hydroxytryptamine creatinine sulfate (5-HT), dopamine HCl (DA), desipramine HCl, nomifensine maleate,1-(2-bis(4-fluorphenyl)-methoxy)-ethyl-4-(3-phenyl-propyl) piperazine HCl (GBR 12909), fluoxetine HCl, pargyline HCl, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), Tris [hydroxyl methyl] aminomethane hydrochloride (Tris-HCl), phenobarbital, catechol, L-ascorbic acid, d-amphetamine sulfate, D-glucose and β-mercaptoethanol were purchased from Sigma-Aldrich (St. Louis, MO). Paroxetine HCl was provided generously by Beecham Pharmaceuticals (Surrey, UK). Phosphate buffered saline (PBS), polyacrylamide, sodium dodecyl sulfate (SDS), Triton-X 100 buffer, Laemmli buffer and Tween-20 were purchased from Bio-Rad Laboratories (Hercules, CA). Immunopure immobilized monomeric avidin beads and sulfosuccinimidobiotin (sulfo-NHS-biotin) were purchased from Pierce Chemical (Rockford, IL). Immobilon-P transfer membranes (0.45 μm pore size) were purchased from Millipore Co. (Bedford, MA). Xylazine was purchased from Lloyd Laboratories Inc. (Metro Manila, Philippines). Ketamine was purchased from Putney Inc. (Portland, ME). Carprofen was purchased from Pfizer Animal Health (New York, NY). Complete protease inhibitor cocktail tablets (1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μM pepstatin, 250 μM phenylmethysulfonyl fluoride) were purchased from Roche Diagnostics (Indianapolis, IN). Primary antibodies recognizing rat DAT (C-20; goat polyclonal antibody) and protein phosphatase 2A (PP2A; sc-13601; mouse monoclonal antibody) and secondary antibodies including donkey anti-goat (sc-2020) and chicken anti-mouse (sc-2954), were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Primary antibody recognizing rat SERT (rabbit polyclonal) and secondary antibody donkey anti-rabbit were purchased from EMD Millipore (Billerica, MA) and GE Healthcare UK Limited (Buckinghamshire, UK), respectively. β-Actin (A 5441, mouse monoclonal antibody) was purchased from Sigma-Aldrich (St. Louis, MO). O-Phthalaldehyde (OPA) was purchased from Pickering Lab Inc., (Mountain View, CA). All other chemicals were purchased from Fisher Scientific Co. (Pittsburgh, PA).
4.2 Animals
Twenty-one day old male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) were housed in one of two rearing conditions (see below). Experiments were conducted during the light phase of the1 2:12 h light: dark cycle (lights on at 07:00 AM). Rats were cared for in accordance with the 2011 edition of the “Guide for the Care and Use of Laboratory Animals” (NIH), and procedures were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
4.3 Environmental conditions
Upon arrival, rats were assigned randomly to either the EC or IC group. EC rats were housed in a group of 8–12 rats per cage in a large metal cage (120 cm long × 60 cm wide × 45 cm high), with 14 hard plastic objects placed randomly in the cage. Each day, half of the objects were replaced with new plastic objects, and the remaining plastic objects were rearranged to enhance novelty. In contrast, IC rats were housed individually in wire mesh hanging cages (25 cm long × 18 cm wide × 17 cm high) with solid metal sides and walls, and were not handled. Rats were maintained in the respective home environments until 53–55 days of age when the neurochemical experiments were conducted.
4.4 [3H]DA and [3H]5-HT uptake assays
At 55 days of age, DAT and SERT function were assessed in both mPFC and OFC. Rats were killed by rapid decapitation and mPFC and OFC from each rat was dissected on an ice-cold dissection plate. One set of rats (n = 20, 10 EC and 10 IC rats) were used to determine kinetic parameters of DAT function in both mPFC and OFC, and another set of rats (n = 20, 10 EC and 10 IC rats) were used to determine the parameters for SERT function in both mPFC and OFC. Brain region was a within-subjects factor and environmental condition was a between-groups factor. Thus, DA or 5-HT uptake was assessed in both mPFC and OFC from an individual rat from each of the environmental conditions in each assay performed on a particular day.
DAT and SERT function were determined using saturation analysis of [3H]DA and [3H]5-HT uptake, respectively, to determine kinetic parameters of transporter function (Vmax, pmol/min/mg and Km, μM) using published methods (Norrholm et al., 2007; Marusich et al., 2011). mPFC and OFC were identified using the atlas of Paxinos and Watson (1998) and obtained by initially making a coronal cut in the frontal cortex at the anterior portion of the olfactory tubercles, removing the olfactory bulb, and making two parallel sagittal cuts ~1.2 mm on either side of the midline to obtain the mPFC. The remaining cortical tissues were placed adjacent to each other, divided equally using a transverse cut, and the ventral segments defined as OFC.
Each brain region from an individual rat was homogenized in 20 ml of ice-cold sucrose solution (0.32 M sucrose and 5 mM sodium bicarbonate, pH 7.4) with 16 passes of a Teflon pestle homogenizer. Homogenates were centrifuged at 2000g for 10 min at 4°C, and resulting supernatants were centrifuged at 20,000g for 15 min at 4°C. Resulting pellets were resuspended in 2.2 ml of ice-cold assay buffer (125 mM NaCl, 5 mM KCl, 1.5 mM MgSO4, 1.25 mM CaCl2, 1.5 mM KH2PO4, 10 mM glucose, 25 mM HEPES, 0.1 mM EDTA, 0.1 mM pargyline, and 0.1 mM L-ascorbic acid, saturated with 95% O2/5% CO2, pH 7.4) to obtain synaptosomal suspensions. Kinetic analysis of [3H]DA uptake by DAT was assessed in the presence of desipramine (5 nM) and paroxetine (5 nM) to inhibit norepinephrine transporter (NET) and SERT, respectively, isolating uptake to DAT (Marusich et al., 2011). [3H]5-HT uptake was determined using buffer containing desipramine (1 μM) and GBR 12909 (50 nM) to inhibit NET and DAT, respectively, isolating uptake to SERT (Bymaster et al., 2002; Zhou et al., 2002; Norrholm et al., 2007).
mPFC and OFC synaptosomes (40 and 50 μg protein/100 μl, respectively) were incubated in a metabolic shaker for 5 min at 34°C. Subsequently, 1 of 7 [3H]DA or [3H]5-HT concentrations (0.01–1 μM and 1–300 nM, respectively, in 25 μl) were added to assay tubes (total assay volume, 250 μl) and incubation continued for 5 min. Nomifensine (10 μM) and fluoxetine (10 μM) were used to determine nonspecific [3H]DA and [3H]5-HT uptake by DAT and SERT, respectively. Incubation was terminated by addition of 3 ml of ice-cold assay buffer. Samples were filtered immediately through Whatman GF/B glass fiber filters (presoaked with 1 mM pyrocatechol for 3 h) using a Brandel cell harvester (model MP-43RS; Brandel Inc., Gaithersburg, MD). Filters were washed 3 times with 3 ml of ice-cold buffer containing 1 mM pyrocatechol. Radioactivity was determined by liquid scintillation spectrometry (model B1600TR; PerkinElmer Life and Analytical Sciences). Protein concentrations were determined using bovine serum albumin as the standard (Bradford, 1976). Specific [3H]DA and [3H]5-HT uptake were calculated by subtracting nonspecific uptake from total uptake. Kinetic parameters (Vmax and Km) were determined using GraphPad Prism 4.0 (GraphPad Software Inc., San Diego, CA). When there was a lack of saturation of transmitter uptake, the data were excluded from analysis, because Vmax was not able to be determined. For [3H]DA uptake assays, 2 mPFC and 1 OFC in the EC group and 1 mPFC and 1 OFC in the IC group were not included in the analysis of the data. For [3H]5-HT uptake assays, 1 mPFC and 1 OFC in the EC group and 1 mPFC and 1 OFC in the IC group were not included in the analysis of the data.
4.5 DAT cellular localization
DAT cellular expression was determined using a biotinylation and Western blot assay using published methods (Somkuwar et al., 2013). Synaptosomes from mPFC (2500 μg protein/sample) and OFC (2500 μg protein/sample) of EC (n = 8) and IC (n = 8) rats were prepared as previously described. Synaptosomes were incubated for 1 h at 4°C with 500 μl of 1.5 mg/ml sulfo-NHS-biotin in PBS/Ca/Mg buffer (138 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 9.6 mM Na2HPO4, 1 mM MgCl2, 0.1 mM CaCl2, pH 7.3). After incubation, samples were centrifuged at 8,000g for 4 min at 4°C. To remove free sulfo-NHS-biotin, resulting pellets were resuspended and centrifuged (8,000g, 4 min, 4°C) 3 times with 1 ml of ice-cold 100 mM glycine in PBS/Ca/Mg buffer. Pellets were resuspended in 1 ml of ice-cold 100 mM glycine in PBS/Ca/Mg buffer and incubated with continual shaking for 30 min at 4°C. Subsequently, samples were centrifuged at 8,000g for 4 min at 4°C, and resulting pellets were resuspended in 1 ml ice-cold PBS/Ca/Mg buffer. Resuspension and centrifugation steps were repeated twice. Final pellets were lysed by sonication for 2–4 s in 300 μl Triton X-100 buffer (10 mM Tris, 150 mM NaCl, 1 mM EDTA, 1.0% Triton X-100, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μM pepstatin, 250 μM phenylmethysulfonyl fluoride, pH 7.4) followed by incubation and continual shaking for 20 min at 4°C. Lysates (150 μl) were centrifuged at 21,000g for 20 min at 4°C. Pellets were discarded, and 50 μl of the supernatants were stored at −20°C for determination of immunoreactive DAT in the total synaptosomal fraction. The remaining supernatants were incubated with continuous shaking in the presence of monomeric avidin beads in Triton-X100 buffer (50 μl/tube) for 1 h at room temperature. Subsequently, samples were centrifuged at 17,000g for 4 min at 4°C, and supernatants (150 μl; nonbiotinylated fraction) were stored at −20°C. Laemmli buffer (62.5 mM Tris-HCl, 20% glycerol, 2% SDS, 0.05% β-mercaptoethanol, and 0.05% bromophenol blue, pH 6.8, 1:1 ratio) was added to the total synaptosomal and non-biotinylated fractions before samples were stored at −20°C. Pellets from the previous centrifugation contained the avidin-adsorbed biotinylated proteins, which were resuspended in 1 ml of 1% Triton X-100 buffer and centrifuged (17,000g, 4 min, 4°C), and these steps were repeated twice. Final pellets were incubated with 50 μl of Laemmli buffer for 20 min at room temperature to elute the biotinylated cell-surface proteins. Following incubation, samples were centrifuged (17,000g, 4 min, 4°C) and 50 μl of the resulting supernatant (biotinylated fraction) was stored at −20°C until assay for cell-surface immunoreactive DAT.
To obtain immunoreactive DAT protein in total, non-biotinylated (intracellular) and biotinylated (cell-surface) fractions, samples were thawed and subjected to gel electrophoresis and Western blotting. Proteins were separated using 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) for 90 min at 140 V, and transferred to Immobilon-P transfer membranes (0.45 μm pore size; Millipore Co., Bedford, MA) in transfer buffer (50 mM Tris, 250 mM glycine, 3.5 mM SDS) using a semi-dry mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad Laboratories Ltd., Hercules, CA) for 1 h at 25 V. Transfer membranes containing DAT (75 kDa) and control proteins (β-actin: 42 kDa, PP2A: 34 kDa) were incubated overnight at 4°C with goat polyclonal DAT antibody (1:100 dilution in blocking buffer) and with mouse monoclonal β-actin and PP2A antibodies (1:10,000 and 1:1000 dilution in blocking buffer, respectively). Transfer membranes were washed 4 times with blocking buffer at room temperature. Transfer membranes containing DAT were incubated for 1 h at 22°C with donkey anti-goat (1:1000 dilutions in blocking buffer) and donkey anti-rabbit secondary (1:2000 dilutions in blocking buffer) antibodies, respectively. Transfer membranes containing β-actin and PP2A were incubated for 1 h at 22°C with chicken anti-mouse secondary antibody (1:5000 dilutions in blocking buffer). Chemiluminescence was detected by spraying the membranes with HyGLO™ chemiluminiscent (CL) horseradish peroxidase antibody detection reagent (Denville Scientific Inc., Metuchen, NJ) followed by development using hyblot CL autoradiography films (Denville Scientific Inc.). Multiple autoradiographs were obtained using different exposure times. Immunoreactive bands within the linear range of detection were quantified by densitometric scanning using Image J software (NIH, Bethesda, MD). Band density measurements, expressed as relative optical density, were used to determine amounts of DAT and control proteins in all fractions.
PP2A and β-actin (control proteins) monitored differences in the sample preparations. β-Actin was used to monitor protein loading between samples. PP2A is predominantly located in the intracellular compartment and was used to evaluate biotinylation efficiency. The membrane impermeant biotinylation reagent was expected to exhibit some biotinylation of the control proteins associated with the intracellular compartment due to the potential for broken membrane fragments and leaky synaptosomes.
4.6 Total SERT expression
To obtain immunoreactive total SERT protein in synaptosomes obtained from mPFC and OFC of EC and IC rats, samples were subjected to gel electrophoresis and Western blotting as described in methods section 4.5 except transfer membranes containing SERT protein (70 kDa) were incubated with rabbit polyclonal SERT antibody (1:500 dilution in blocking buffer). β-Actin was used to monitor protein loading between samples.
4.7 Glutamate microdialysis
Microdialysis surgeries were performed as previously described (Rahman et al., 2007) under aseptic conditions with anesthesia induced by xylazine (8 mg/kg, i.p.) and ketamine hydrochloride (60 mg/kg, i.p.). Rats were placed in a stereotaxic apparatus (Stoelting, Wood Dale, IL) and a microdialysis guide cannulae (MD-2251, 22 gauge; BAS, Indianapolis IN) was implanted unilaterally in mPFC or OFC according to the atlas of Paxinos and Watson (1998). Coordinates for mPFC were AP +2.7, ML +1.2, and DV −2.6 below the dura, and for OFC were AP +3.7, ML +2.4, and DV 3.4 below the dura. To minimize discomfort and pain, rats were given a non-opioid analgesic, carpofen (5 mg/kg, s.c), for 3 days post-surgery. Rats recovered for 3–4 days in their home cages following surgery.
Microdialysis experiments were performed in clear Plexiglas chambers (25 × 44 × 38 cm). Microdialysis probes (MD-2200, 2 mm active membrane, BAS) were inserted into guide cannulae, connected to polyethylene tubing (inner diameter = 0.12 mm, internal vol = 1.2 μl/100 mm length), and aligned through a wire set with a collar connector and swivel assembly. Artificial cerebrospinal fluid (ACSF; 145 mM NaCl, 2.7 mM KCl, 1.0 mM MgCl2, 1.2 mM CaCl2; pH adjusted to 7.4 with 2 mM sodium phosphate buffer; filtered through a 0.2 μm sterile filter) was perfused through the probe (flow rate = 1 μl/min) for 2 h prior to collection of the basal samples. After the equilibration period, 3 basal samples were collected into polyethylene microfuge tubes every 20 min for an additional 60 min. Five min after the last basal sample was collected, EC and IC rats randomly assigned to mPFC and OFC groups (n = 6 per group) were injected s.c. with 0.9% NaCl (saline), followed 60 min later by an injection of d-amphetamine (2 mg/kg; s.c; dose represents the sulfate salt weight) dissolved in saline. The d-amphetamine dose was chosen based on previous studies (Xue et al., 1996, Shoblock et al., 2003). Samples were collected at 20 min intervals for 160 min following d-amphetamine injection, frozen on dry ice, and stored at −70°C for quantification of glutamate.
Microdialysis samples were analyzed for glutamate using high performance liquid chromatography coupled with electrochemical detection (HPLC-ECD; ESA Inc., Chelmsford, MA) as previously described (Melendez et al., 2004). The HPLC system consisted of a solvent delivery unit, a Coulochem III electrochemical detector equipped with a 5014B analytical cell and 5020 guard cell. The guard cell was set at +600 mV, electrode 1 at −150 mV, and electrode 2 at +550 mV, with the gain at 1 μA. Precolumn derivatization of glutamate with OPA and 2-mercaptoethanol was performed using an ESA 542 autosampler. The mobile phase consisted of 100 mM Na2HPO4, 22% methanol and 3.5% acetonitrile; pH 6.75 adjusted with phosphoric acid (flow rate, 0.7 μl/min). Glutamate was separated using an analytical column (50 mm × 3 mm, Thermo Hypersil-Keystone, Bellefonte, PA). Glutamate concentration in the dialysis samples was quantified by comparing peak heights from samples to external standards using an ESA Chromatography data system (EZ Chrom Elite, Chelmsford, MA) and the detection limit for individual dialysate samples (27 μl) was approximately 1 ng.
4.8 Histology
At the end of the experiment, rats were anesthetized deeply with pentobarbital and the brain obtained and fixed for subsequent sectioning to determine microdialysis probe location. Probe placements were evaluated according to the atlas of Paxinos and Watson (1998). Cannulae were implanted into OFC of 12 rats, but data from only 11 rats with correct cannulae implants were included in statistical analyses. Cannulae were implanted into the mPFC of 12 rats, but data from only 9 rats with correct cannulae implants were included in statistical analyses.
4.9 Data analysis
For [3H]DA and [3H]5-HT uptake assays, data are presented as mean (± S.E.M.) and ‘n’ represents the number of independent experiments for EC and IC groups. Data were analyzed using GraphPad Prism 4.0 and differences between EC and IC groups in the kinetic parameters (Vmax and Km) were analyzed by unpaired Student’s t-tests. For Western blot analysis, densitometry values were obtained for each band representing total DAT, non-biotinylated DAT and biotinylated DAT, as well as for total SERT. Densitometry values were normalized to the respective β-actin band to control for variations in loaded protein. DAT densitometry values were corrected by multiplying the sum of the volumes in each fraction (50 μl total + 150 μl non-biotinylated fraction + 50 μl biotinylated fractions) divided by the specific fraction volume applied to the SDS-polyacrylamide gel (i.e., 40 μl for total fraction, 80 μl for non-biotinylated fraction and 20 μl for biotinylated fraction). The corrected value was divided by the ratio of the fraction volume to the synaptosomal volume (50:150 for total, and 100:150 for non-biotinylated and biotinylated fractions; Narayanaswami et al., 2013). To determine effects of rearing condition on DAT distribution, separate unpaired Student’s t-tests were conducted for total, intracellular and cell surface fractions to determine differences between EC and IC groups. Also, separate unpaired Student’s t-tests were used to determine effects of rearing condition on SERT in the total fraction.
For the microdialysis experiments, basal glutamate level for individual rats was defined as the average concentration of glutamate in the 3 samples prior to the saline injection. To determine if the saline injection altered glutamate levels, separate one-way repeated-measures ANOVAs were performed in mPFC and OFC of EC and IC rats comparing control basal levels to post-saline injection levels. To determine if d-amphetamine altered glutamate levels in the microdialysate, separate one-way repeated-measures ANOVAs were performed in mPFC and OFC of EC and IC rats comparing control saline levels to post-d-amphetamine levels. One-way ANOVAs were conducted using GraphPad Prism 4.0. For all experiments, statistical significance was determined as p<0.05.
Highlights.
Environmental enrichment decreased mPFC DAT function but increased OFC DAT function
Altered DAT function in mPFC and OFC of EC and IC rats is trafficking independent
Environmental enrichment did not alter SERT function in mPFC and OFC.
Amphetamine increased extracellular glutamate in mPFC of EC and OFC of IC rats.
Acknowledgments
This research was supported by NIH grants R01 DA12964, R00 DA033373 and UL1TR000117.
Abbreviations
- DAT
dopamine transporter
- SERT
serotonin transporter
- EC
enriched condition
- IC
isolated condition
- mPFC
medial prefrontal cortex
- OFC
orbitofrontal cortex
- Vmax
maximal transport velocity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends in Molecular Medicine. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology. 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacological Reviews. 2013;65:255–290. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Valone JM, Robinet PM, Shaw WB, Dwoskin LP. Environmental enrichment enhances the stimulant effect of intravenous amphetamine: search for a cellular mechanism in the nucleus accumbens. Psychobiology. 1999;27:292–299. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brenes JC, Rodriguez O, Fornaguera J. Differential effect of environment enrichment and social isolation on depressive-like behavior, spontaneous activity and serotonin and norepinephrine concentration in prefrontal cortex and ventral striatum. Pharmacology, Biochemistry, and Behavior. 2008;89:85–93. doi: 10.1016/j.pbb.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Zhang W, Carter PA, Shaw J, Chernet E, Phebus L, Wong DT, Perry KW. Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology. 2002;160:353–361. doi: 10.1007/s00213-001-0986-x. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Baunez C, Hutcheson DM, Lehmann O, Shah AP, Robbins TW. Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus. Cerebral Cortex. 2008;18:178–188. doi: 10.1093/cercor/bhm044. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Bloch B, Le Moine C. D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. The European Journal of Neuroscience. 1995;7:1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Ferchmin PA, Eterovic VA, Gonzalez-Lima EM. Metabolic activation of the brain of young rats after exposure to environmental complexity. Developmental Psychobiology. 1994;27:343–351. doi: 10.1002/dev.420270603. [DOI] [PubMed] [Google Scholar]
- Green TA, Cain ME, Thompson M, Bardo MT. Environmental enrichment decreases nicotine-induced hyperactivity in rats. Psychopharmacology. 2003;170:235–241. doi: 10.1007/s00213-003-1538-3. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Experimental and Clinical Psychopharmacology. 2001;9:131–143. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Darna M, Charnigo RJ, Dwoskin LP, Bardo MT. A multivariate assessment of individual differences in sensation seeking and impulsivity as predictors of amphetamine self-administration and prefrontal dopamine function in rats. Experimental and Clinical Psychopharmacology. 2011;19:275–84. doi: 10.1037/a0023897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Gregory ML, Bardo MT, Kalivas PW. Impoverished rearing environment alters metabotropic glutamate receptor expression and function in the prefrontal cortex. Neuropsychopharmacology. 2004;29:1980–1987. doi: 10.1038/sj.npp.1300507. [DOI] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2002;160:290–298. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- Narayanaswami V, Thompson AC, Cassis LA, Bardo MT, Dwoskin LP. Dietinduced obesity: dopamine transporter function, impulsivity and motivation. International Journal of Obesity. 2013;37:1095–1103. doi: 10.1038/ijo.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Horton DB, Dwoskin LP. The promiscuity of the dopamine transporter: implications for the kinetic analysis of [3H]serotonin uptake in rat hippocampal and striatal synaptosomes. Neuropharmacology. 2007;53:982–989. doi: 10.1016/j.neuropharm.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat Brain in Stereotaxic Coordinates. 6. Academic Press; London: 1998. [Google Scholar]
- Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, Huettl P, Dwoskin LP, Bardo MT. Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Research Reviews. 2011;65:124–149. doi: 10.1016/j.brainresrev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Perry JL, Stairs DJ, Bardo MT. Impulsive choice and environmental enrichment: effects of d-amphetamine and methylphenidate. Behavioural Brain Research. 2008;193:48–54. doi: 10.1016/j.bbr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliffe RA, Erwin VG. Alterations in locomotor activity after microinjections of GBR-12909, selective dopamine antagonists or neurotensin into the medial prefrontal cortex. The Journal of Pharmacology and Experimental Therapeutics. 1996;277:1467–1476. [PubMed] [Google Scholar]
- Rahman S, Bardo MT. Environmental enrichment increases amphetamine-induced glutamate neurotransmission in the nucleus accumbens: a neurochemical study. Brain Research. 2008;1197:40–46. doi: 10.1016/j.brainres.2007.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. The effects of a novel nicotinic receptor antagonist N,N-dodecane-1,12-diyl-bis-3-picolinium dibromide (bPiDDB) on acute and repeated nicotine-induced increases in extracellular dopamine in rat nucleus accumbens. Neuropharmacology. 2007;52:755–763. doi: 10.1016/j.neuropharm.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Shippenberg TS, Jayanthi LD. Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacology & Therapeutics. 2011;129:220–238. doi: 10.1016/j.pharmthera.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilke O, Will K, Jahkel M, Oehler J. Behavioral and neurochemical effects of anpirtoline and citalopram in isolated and group housed mice. Progress in Neuropsychopharmacology & Biological Psychiatry. 2001;25:1125–1144. doi: 10.1016/s0278-5846(01)00167-1. [DOI] [PubMed] [Google Scholar]
- Santana N, Mengod G, Artigas F. Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cerebral Cortex. 2009;19:849–860. doi: 10.1093/cercor/bhn134. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nature Neuroscience. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–90. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Sullivan EB, Maisonneuve IM, Glick IM. Neurochemical and behavioral differences between D-methamphetamine and D-amphetamine in rats. Psychopharmacology. 2003;165:359–369. doi: 10.1007/s00213-002-1288-7. [DOI] [PubMed] [Google Scholar]
- Somkuwar SS, Darna M, Kantak KM, Dwoskin LP. Adolescence methylphenidate treatment in a rodent model of attention deficit/hyperactivity disorder: dopamine transporter function and cellular distribution in adulthood. Biochemical Pharmacology. 2013;86:309–316. doi: 10.1016/j.bcp.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stairs DJ, Bardo MT. Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacology, Biochemistry, and Behavior. 2009;92:377–382. doi: 10.1016/j.pbb.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annual Review of Pharmacology and Toxicology. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Tsibulsky VL, Norman AB. Satiety threshold: a quantitative model of maintained cocaine self-administration. Brain Research. 1999;839:85–93. doi: 10.1016/s0006-8993(99)01717-5. [DOI] [PubMed] [Google Scholar]
- Wallace DM, Magnuson DJ, Gray TS. Organization of amygdaloid projections to brainstem dopaminergic, noradrenergic, and adrenergic cell groups in the rat. Brain Research Bulletin. 1992;28:447–454. doi: 10.1016/0361-9230(92)90046-z. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcoholism, Clinical and Experimental Research. 2010;34:1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. The Journal of Neuroscience: the official journal of the Society for Neuroscience. 2004;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Cardinal RN, Robbins TW. Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cerebral Cortex. 2006;16:106–114. doi: 10.1093/cercor/bhi088. [DOI] [PubMed] [Google Scholar]
- Wood DA, Siegel AK, Rebec GV. Environmental enrichment reduces impulsivity during appetitive conditioning. Physiology & Behavior. 2006;88:132–137. doi: 10.1016/j.physbeh.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Xue CJ, Ng JP, Li Y, Wolf ME. Acute and repeated systemic amphetamine administration: effects on extracellular glutamate, aspartate, and serine levels in rat ventral tegmental area and nucleus accumbens. Journal of Neurochemistry. 1996;67:352–363. doi: 10.1046/j.1471-4159.1996.67010352.x. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Sorkin A. Trafficking of dopamine transporters in psychostimulant actions. Seminars in Cell & Developmental Biology. 2009;20:411–417. doi: 10.1016/j.semcdb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Lesch KP, Murphy DL. Serotonin uptake into dopamine neurons via dopamine transporters: a compensatory alternative. Brain Research. 2002;942:109–119. doi: 10.1016/s0006-8993(02)02709-9. [DOI] [PubMed] [Google Scholar]
- Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. Journal of Neurochemistry. 2005;93:1434–1443. doi: 10.1111/j.1471-4159.2005.03130.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Green T, Bardo MT, Dwoskin LP. Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behavioural Brain Research. 2004;148:107–11. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]


