Abstract
Parkinson’s disease (PD) is associated with motor and non-motor rigidity symptoms (e.g., cognitive and personality). The question is raised as to whether rigidity in PD also extends to perception, and if so, whether perceptual, cognitive, and personality rigidities are correlated. Bistable stimuli were presented to 28 non-demented individuals with PD and 26 normal control adults (NC). Necker cube perception and binocular rivalry were examined during passive viewing, and the Necker cube was additionally used for two volitional-control conditions: Hold one percept in front, and Switch between the two percepts. Relative to passive viewing, PD were significantly less able than NC to reduce dominance durations in the Switch condition, indicating perceptual rigidity. Tests of cognitive flexibility and a personality questionnaire were administered to explore the association with perceptual rigidity. Cognitive flexibility was not correlated with perceptual rigidity for either group. Personality (novelty seeking) correlated with dominance durations on Necker passive viewing for PD but not NC. The results indicate the presence in mild-moderate PD of perceptual rigidity and suggest shared neural substrates with novelty seeking, but functional divergence from those supporting cognitive flexibility. The possibility is raised that perceptual rigidity may be a harbinger of cognitive inflexibility later in the disease course.
Keywords: bistable perception, volitional control, cognitive flexibility, novelty-seeking, personality, attention, binocular rivalry
1. Introduction
Parkinson’s disease (PD) has primarily been conceptualized as a movement disorder, as it is characterized by tremor, motor rigidity (e.g., axial rigidity, decreased arm swing and stride length, lack of spontaneous eye movement), bradykinesia, and postural instability. As an example of the increasing emphasis on non-motor as well as motor symptoms (reviewed in Cronin-Golomb, 2013), PD has been described as producing “rigidity” across motor, cognitive, and personality domains (Cools, van den Bercken, Horstink, van Spaendonck, & Berger, 1984; Cools, Barker, Sahakian, & Robbins, 2001; personality changes reviewed in McNamara, 2011). Specifically, those with PD are unable to shift their current behaviors to consider the best response consistent with environmental demands. An empirical question is whether this shifting deficit in PD also extends to perception. Bistable stimuli are useful tools to investigate how the perceptual system selects a particular interpretation to be represented in awareness and provide the opportunity to examine whether PD is associated with perceptual rigidity; that is, the inability or slowness to consider both perceptual interpretations while observing a bistable stimulus.
Under certain conditions, the visual system cannot reach one particular perceptual interpretation and vacillates between two competing, equally possible percepts. Leopold and Logothetis (1999) proposed an “environment explanation” theory purporting that perceptual reversals during ambiguous perception are the necessary consequences of a generalized high-level exploratory mechanism that directs attention in a way that forces low-level perceptual systems to periodically refresh. Due to the ambiguity of the stimulus, the visual scene requires continual exploration, and reversals in perceptual interpretation consistently occur. This selection theory suggests that attention-related frontal-parietal areas are responsible for initiating perceptual alternations by sending top-down signals to guide activity in visual cortex toward one representation or the other. Fronto-parietal activation during bistable perception has been supported by imaging studies using ambiguous figures and binocular rivalry paradigms (Amir, 2007; Blake & Logothetis, 2002; Britz, Landis, & Michel, 2009; Knapen, Brascamp, Pearson, van ee, Blake, 2011; Sterzer & Kleinschmidt, 2007; Weilnhammer, Ludwig, Hesselmann, Sterzer, 2013; Wilcke, O’Shea, & Watts, 2009). PD affects the cortico-striato-thalamocortical circuitry including connections to frontal and parietal cortices (for reviews see Christopher & Strafella, 2013; Tinaz, Courtney & Stern, 2011), raising the question of whether there is perceptual rigidity in PD; that is, how individuals with this disorder perceive bistable figures and experience binocular rivalry.
Researchers have explored the implications of compromised frontal functioning on perceptual ambiguity in other clinical groups, including patients with frontal-lobe damage and schizophrenia (McBain, Norton, Kim, & Chen, 2011; Ricci & Blundo, 1990; Windmann, Wehrmann, Calabrese & Gunturkun, 2006). Studies describing the role of the frontal lobes in bistable perception found that patients reported significantly fewer reversals when passively viewing a bistable figure when compared to a healthy control group (Yacorzynski & Davis, 1945) and compared to patients with posterior (parietal) lobe damage (Ricci & Blundo, 1990). McBain and colleagues (2010) reported that individuals with schizophrenia, a psychiatric disorder with known frontal-lobe compromise, were unable to hold one particular face of the Necker cube compared to a matched control group. These studies suggest that poor performance on bistable perception might be considered a frontal sign.
Windmann and colleagues (2006) expanded earlier findings with patients with frontal-lobe damage by using multiple bistable visual stimuli, including the Necker cube, and two volitional-control conditions in addition to passive viewing: Hold one percept as long as possible, and Switch between the two percepts as quickly as possible. The investigators predicted group differences if the prefrontal cortex subserved the stabilization of a dominant percept (Hold) and if it subserved the selection among competing inputs and the promotion of perceptual alternations consistent with goals (Switch). They found that compared to a healthy control group, the patients’ passive viewing and ability to hold a percept were not impaired, but they were less able to intentionally switch between percepts. Windmann and colleagues suggested that the patients’ impairment in the Switch condition could have resulted from a reduced ability to intentionally “let go” of the dominant pattern, which was hypothesized to be a consequence of set-shifting deficits (i.e., cognitive inflexibility). In a study of PD that required a decision on whether an image was monostable and bistable, the subgroup of individuals who made monostable-bistable distinction errors performed significantly more poorly on a measure of attentional set-shifting, the Trail Making Test (B-A), than the subgroup who performed the bistable-image assessment in the normal range (Shine, Halliday, Carlos, Naismith & Lewis, 2012), suggesting that in PD, there may be difficulties in switching between percepts as well as in cognitive switching. A recent imaging study has implicated frontal and parietal hubs of the dorsal attentional network in the ability of those with PD to successfully perform this same behavioral task (Shine et al., 2014). In conjunction with known dysfunction of fronto-parietal attentional networks in PD, the results of these studies together suggest that the perception of bistable images may be compromised in this neurological disorder.
The aims of the present study were to assess whether rigidity extends to perception in non-demented individuals with PD and whether this perceptual rigidity (if it exists) is associated with other rigidity symptoms in PD—specifically, in cognition and personality. Performance of individuals with PD and healthy age-matched adults was compared under three conditions using two bistable stimuli. Necker cube perception and binocular rivalry were examined during passive viewing, and the Necker cube was additionally used for the two volitional control conditions: Hold and Switch. The main hypothesis was that relative to a control group, those with PD would show a reduced ability to volitionally switch between the two possible percepts; that is, they would demonstrate rigidity by holding any one percept for longer than would a normal control group.
2. Materials and Methods
2.1. Participants
The study included 28 participants with idiopathic PD and 26 age-and education-matched normal control adults (NC). Participants with PD were recruited from the Parkinson’s Disease Clinic at the Boston Medical Center, the Michael J. Fox Foundation Trial Finder, and through local PD support groups. The NC group was recruited from local PD support groups, the Fox Trial Finder, and the community. Potential participants were interviewed about their medical history to rule out confounding diagnoses such as stroke, head injury, and serious medical illness (e.g., diabetes). No participant had undergone surgery affecting the thalamus, basal ganglia, or other brain regions. As part of a larger parent study on perception, cognition, and gait in PD, all participants underwent detailed neuro-ophthalmological examination at the New England Eye Institute in Boston. None of the participants was found on exam or by history to have any ocular illnesses or abnormalities that would have influenced performance on the visually-presented measures of interest. Participant characteristics are provided in Table 1.
Table 1.
Participant characteristics for individuals with Parkinson’s disease (PD) and matched normal control adults (NC).
| PD n = 28 15F,13M |
NC n = 26 14F,12M |
t-test | p-value | partial η2 | 95% CI lower upper | |
|---|---|---|---|---|---|---|
| Age, years | 64.2 (6.4) | 64.4 (7.7) | t(52) = .13 | .90 | ||
| Education, years | 17.5 (2.1) | 16.9 (2.4) | t(52) = 1.0 | .32 | ||
| MMSE | 28.8 (.74) | 28.8 (1.0) | t(51) = .10 | .92 | ||
| Near Acuity, logMAR [Snellen] | .07 (.23) [20/24] | .03 (.11) [20/22] | t(51) = .79 | .44 | ||
| BDI-II | 5.5 (3.6) | 2.1 (3.0) | t(46) = 3.5 | .001 | .21 | 1.42 5.28 |
| GDS | 4.9 (2.9) | 3.4 (3.2) | t(52) = 1.3 | .21 | ||
| BAI | 5.1 (2.7) | 1.5 (1.7) | t(46) = 5.4 | .001 | .39 | 2.28 4.96 |
| WTAR | 46.0 (5.3) | 44.4 (5.2) | t(51) = 1.1 | .28 | ||
| Disease Duration, years | 5.4 (4.0) | ----------- | ||||
| UPDRS Total | 29.5 (9.9) | ----------- | ||||
| Hoehn & Yahr Stage | 2.0 [1–3] | ----------- | ||||
| LED (mg/day) | 221.7 (213.6) | ----------- |
All values are reported as means (standard deviations [SD]) except Hoehn & Yahr (H&Y) motor stage, which is reported as median (range). MMSE: Mini-Mental State Examination; BDI-II: Beck Depression Inventory-II; BAI: Beck Anxiety Inventory; WTAR: Wechsler Test of Adult Reading; UPDRS: Unified Parkinson’s Disease Rating Scale; LED: Levodopa Equivalent Dose
Groups were matched for age, education, and ratio of women to men. All participants were non-demented as indexed by their scores on the modified Mini-Mental State Examination (mMMSE, Stern, Sano, Paulson, & Mayeux, 1987; cut-off score converted to standard MMSE of 27). The two groups significantly differed in their depressive and anxiety symptoms as measured by the Beck Depression Inventory-2nd Edition (BDI-II) (Beck, Steer, Ball, & Ranieri, 1996), the Geriatric Depression Inventory (GDS) (Yesavage et al., 1982) and the Beck Anxiety Inventory (BAI) (Beck, Epstein, Brown & Steer, 1988). PD participants were staged according to the Hoehn & Yahr scale of motor disability (Hoehn & Yahr, 1967). Disease severity was determined with the use of the Unified Parkinson’s Disease Rating Scale (UPDRS, Fahn & Elton, 1987). All PD participants were taking medication for their parkinsonian symptoms and at the time of testing were in their “on” period (Levodopa equivalent dosage [LED] mean: 474 [298] mg/day).
2.2. Materials and procedures
2.2.1 Necker cube
A right-face forward-down Necker cube was presented on a white background in the center of a 21-inch LCD monitor (Figure 1; width = 8° of visual angle). A fixation cross was presented in the center of the cube. Observers were instructed to maintain fixation throughout each 60-s trial and to avoid eye movements. We tracked eye movements with an Applied Science Laboratories (ASL) eye tracking system. The model D6 camera array was placed underneath the stimulus monitor and used infrared light to discern the pupil and corneal reflection. The reflections at these two points were constantly monitored through EyeTrac software and remote head tracking software and hardware. The camera had a sampling rate of 60 Hz, and the system used an ASL EYE-TRAC 6 Control unit (system accuracy is 0.5° of visual angle, and resolution is 0.25°). We were unable to collect reliable eye tracking data from all participants for reasons including bumpy sclera, or small pupils or eyes; 17 PD and 15 NC provided reliable data. Participants with eye-tracking data did not significantly differ in demographic characteristics or performance on the Necker cube experiments from those participants who did not have (reliable) eye-tracking data.
Figure 1.
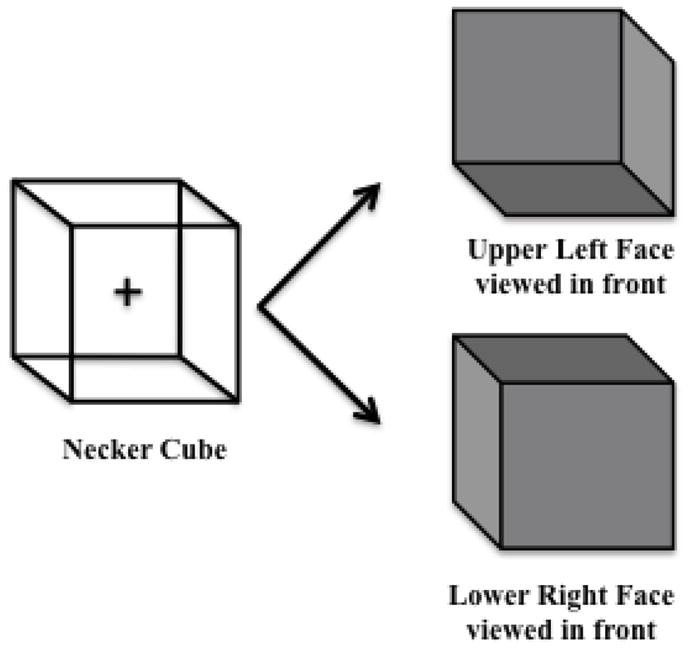
Necker cube stimulus and the two possible percepts: the upper left cube perspective (upper left face is perceived in the front) and lower right cube perspective (lower right face is perceived in the front).
2.2.2. Binocular Rivalry
We investigated whether the hypothesized perceptual rigidity in PD is reflected in the perception of low-level ambiguous stimuli, in addition to high-level ambiguous figures such as the Necker cube. Binocular rivalry has been routinely used to explore how automatic, stimulus-driven information dictates what the brain reports as conscious perception (Blake, 2001; Blake & Logothetis, 2002; Leopold & Logothetis, 1996; Meng & Tong, 2004). It is produced by simultaneously presenting two dissimilar images, one to each eye. Each image competes for perceptual dominance: one image dominates for a few seconds to later be suppressed while the other image dominates for a few seconds. In the present study, sine-wave luminance gratings were presented to both eyes using a mirror stereoscope (Figure 2). The two oblique gratings, one oriented at 45° and the other at 135°, were counterbalanced for presentation to each eye. The contrast and spatial frequency of the gratings were set at 30% and 1.6 cycles/degrees of visual angle, respectively, and the luminance of the background was 2.5 cd/m2. Each grating was surrounded by a circular frame (0.04 cd/m2 luminance). A small black dot (22.2 min arc both in width and height) was presented at the center of each grating and the observer was instructed to maintain steady fixation upon it during each trial. The circular frame and fixation dot were used to maintain stable vergence. These stimuli were chosen from ones that had been piloted with healthy younger and older adults to ensure the right combination of physical parameters (e.g., size, luminance, line orientation) that minimized piecemeal/mixed rivalry that would result in perceiving a blend of the two stimuli at the same time (Blake, O’Shea, & Mueller, 1992; Kang, 2009) and maximized PD participants’ ability to perceive the gratings. A chin rest was used to maintain head stability at a viewing distance of 62 cm. Because the stimuli were not particularly bright and were viewed in a dimly lit room, all participants were adapted to the lighting conditions of the room for 5–10 minutes before beginning the experimental trials. The use of the mirror stereoscope to elicit binocular rivalry precluded the recording of eye movements during this experiment, which is a limitation of this methodology as noted by Carmel, Arcaro, Kastner, & Hasson (2010).
Figure 2.
Binocular rivalry stimulus. When each eye is presented with a different stimulus, instead of perceiving a fusion of the two, the observer reports that one percept dominates for a period of time, while the other stimulus is suppressed. Then, the other stimulus slowly emerges creating a mixed percept in which the participant perceives a fusion of the two stimuli, until it reaches complete dominance. This rivalry continues as long as the stimuli are presented to the participant.
For the Necker cube, all participants were tested under a passive viewing condition and two volitional-control conditions (Hold and Switch). The binocular rivalry experiment (passive viewing only) was conducted in a separate session with a subset of 16 PD and 16 NC who completed the Necker cube experiment. Like for the Necker cube experiment, for the binocular rivalry experiment the PD and NC groups were matched for age, years of education, and ratio of women to men. PD included 9 women and 7 men; mean age: 65.8 years (5.3); mean education: 17.4 years (2.2). NC included 9 women and 7 men; mean age: 63.9 years (8.3) mean education: 16.6 years (2.5).
2.2.3. Necker cube, procedure
After providing informed consent, participants received a comprehensive interview to collect historical and demographic information and were screened in regard to inclusion and exclusion criteria. After screening, participants completed mood assessments (e.g., BDI-II, BAI and GDS). In order to minimize fatigue from the perceptual experiments, these were administered in short sessions alternating with the neuropsychological assessments (described below).
Participants were initially presented with two 3-D models of a cube and asked if they had seen these types of cubes before. The experimenter then explained that the same cube could have different interpretations depending on the viewing angle if the person were to rotate it. After viewing the 3-D models, participants were presented with a 2-D graphic of an ambiguous Necker cube on an 11″ × 8 ½″ piece of paper and asked whether they could perceive the two possible cube interpretations. Once the participant reported both percepts, the experimenter showed another 2-D graphic with three cubes: (1) an ambiguous Necker cube in the middle, (2) an unambiguous Necker cube denoting the right cube interpretation on the right (right face perceived to be in front), and (3) an unambiguous Necker cube denoting the left cube interpretation on the left (left face perceived to be in front). Participants were instructed, with the help of these drawings, to report aloud “right” every time the cube in the middle resembled the unambiguous cube on the right, and to say “left” every time the cube in the middle resembled the unambiguous cube on the left, all while maintaining fixation on a cross placed in the middle of the ambiguous Necker cube.
The perceptual experiment began with five 60-second learning trials to ensure reliable reporting of perceptual alternations. For the first two practice trials, one graphic demonstrating the right cube interpretation and one graphic representing the left cube interpretation were placed on either side of the computer monitor to ensure reliable reporting of reversals. The graphics were then removed for the last three practice trials. Data were collected during all five practice trials for eye movements and behavioral responses of reversals.
Following the practice trials, participants were introduced to the Passive condition. Here they were instructed to “just look at the cube passively without trying to force any of the percepts.” The order of the two volitional conditions was counterbalanced across participants. In the Hold condition, participants were instructed to “attempt to hold the lower right cube in front as long as possible” for three 60s trials, and “attempt to hold the upper left cube in front as long as possible” for the last three 60s trials, all while reporting switches. In the Switch condition they were to “attempt to speed up between the two cube percepts as fast as possible” (Figure 2). Participants continuously monitored their perceptual state and reported perceptual reversals aloud (e.g., “right” for lower right cube or “left” for upper left cube) and the examiner pressed the respective key of the computer to record the response. The examiner pressed the keys in order to eliminate potential effects of PD motor rigidity, tremor and slowness of movement on motor response. Each Passive and Switch condition was presented for five 60-second trials and the Hold condition was presented for six (three “Hold right” and three “Hold left”) trials of the same duration.
2.2.4. Binocular rivalry, procedure
Participants were initially presented with a 2-D graphic of a mirror stereoscope and two gratings on an 11″ × 8 ½″ piece of paper and were told that the mirror stereoscope isolates what each eye sees; by the use of the four mirrors and a rectangular board dividing the monitor, the left eye sees the image presented on the left of the monitor, while the right eye sees the image presented on the right side of the monitor. Participants were told that with the arrangement of the two external mirrors, they were going to see only one image with alternating lines going either right or left. They were then presented with another 2-D graphic with an oblique grating oriented at 45° and an otherwise identical oblique grating oriented at 135°. With the help of these drawings, participants were instructed to report aloud “right” every time they perceived the 45° grating, to say “left” every time they perceived the 135° grating, and to say “mixed” every time they perceived a mixed percept or piecemeal rivalry of any kind, all while maintaining fixation.
Before the experiment began, we presented the testing gratings with the contrast set to 100% to maximize the participants’ ability to report perceptual alternations. Contrast was then set to 30% for all experimental trials. There were two 30-second learning trials to ensure reliable reporting. Data were collected during the practice trials for behavioral responses of reversals. Following the practice trials, participants were introduced to the Passive condition. The instructions were similar to the ones used with the Necker cube experiment. The experiment consisted of four 45-seconds trials during which participants continuously monitored their perceptual state and reported the switches aloud, while the experimenter pressed one of three keys for each verbal response (e.g., right, left and mixed).
2.2.5. Eye tracking and calibration
Before the Necker cube practice trial began, the experimenter determined the participant’s eye dominance to begin eye-tracking calibration in the dark. The dominant eye was used for the calibration and eye tracking throughout the experiments. Calibration consisted of presenting nine fixation points, one at a time, while instructing the participant to fixate on the points. For the calibration to be efficient, the experimenter needed to locate both the pupil and cornea reflections from the eye. After the nine fixation points, the participants were then instructed to fixate in three centered points, one at a time. These three points served to calculate the standard deviation of participants’ eye movements during their fixation. Once calibration was effective, practice trials began.
2.2.6. Neuropsychological (cognitive) assessment
Participants were administered several neuropsychological tests in order to gauge the overall cognitive level of the PD group relative to NC, and further to examine whether perceptual rigidity in PD was associated with cognitive rigidity. Domains that were assessed included inhibition and attentional control (Stroop Color Word Test; Golden, 1978; Stroop, 1935), letter fluency and semantic fluency, with semantic including a switch condition (fruit-furniture) (D-KEFS Verbal Fluency; Delis, Kaplan, & Kramer, 2001; Delis, Kramer, Kaplan, & Holdnack, 2004), nonverbal figural fluency (Ruff Figural Fluency Test; Ruff, Light & Evans, 1987), attention, working memory, and cognitive flexibility (Trail Making Tests A and B; Tombaugh, 2004), abstract reasoning and the ability to shift cognitive strategies in response to changing environmental contingencies (Wisconsin Card Sorting Test – 64 Computer Version; WCST-64; Kongs, Thompson, Iverson & Heaton, 2000). The key measures of cognitive inflexibility were the Stroop interference score, switch fluency, Trail Making Test B-A, and the perseverative errors score of the Wisconsin Card Sorting Test.
2.2.7. Personality assessment
Participants were administered the Temperament and Character Inventory (TCI) in order to examine the association between several aspects of personality, including low novelty-seeking (conceptualized in the literature as personality rigidity), and perceptual rigidity as measured by their performance in the Necker cube and binocular rivalry experiments. The TCI has 240 items designed to identify the intensity of and relations between the seven basic personality dimensions of temperament and character. The four temperament dimensions measured are Novelty Seeking (NS), Harm Avoidance (HA), Reward Dependence (RD), and Persistence (PS). The three character dimensions measured are Self-Directedness (SD), Cooperativeness (CO), and Self-Transcendence (ST) (Cloninger, Svrakic & Przybeck, 1993). In the present study, we report on the temperament dimensions only; there were no group differences for the character dimensions (p>.05 for each comparison).
2.2.8. Statistical analysis
Dominance durations were analyzed for each participant, namely the average time in seconds spent perceiving either the left or right cube. Outlier trials were identified across participants in each group. Dominance durations above or below two standard deviations from the group mean in each condition (Necker cube: Passive, Hold, and Switch; Binocular rivalry: Passive) were eliminated from the analysis. For the Necker cube experiments, one individual with PD was able to perform under the Passive condition only, and another PD was unable to perform the Switch condition. Out of the remaining PDs, 1.3% of the data were eliminated (1 out of 78 dominance durations; each mean consisting of 5–6 trials). For NC, 6.7% of the data were eliminated (5 out of 75 dominance durations; each mean consisting of 5–6 trials). For binocular rivalry, 6.3% of the Passive data for each group were eliminated (3 out of 48; each mean consisting of 4 trials). For each group, the absolute dominance durations for the Necker cube experiment were then normalized to the Passive condition by dividing the mean dominance duration of the Hold and Switch conditions by the mean dominance duration of the Passive condition. By normalizing the data to Passive, it is possible to compare how participants increased or decreased their dominance durations in the Hold and Switch conditions relative to their performance in the Passive condition. For both PD and NC, 4.0% of their normalized durations were eliminated (2 out of 50). The average number of Switches in the Necker cube experiment (e.g., switch rate) was also calculated for each participant. For PD, 2.6% of switches were eliminated (2 out of 76). For NC, 6.7% of switches were eliminated (5 out 75).
For binocular rivalry, perceptual transitions were subdivided into two classes following Robertson, Kravitz, Freyberg, Baron-Cohen & Baker’s (2013) study on autism: Switches (shift from left to right, or right to left, with or without an intermediate mixed percept) and reversals (reversion to the previous percept – left or right – after a mixed percept). No outliers were identified for the perceptual transitions during binocular rivalry in either group. Mixed-model ANOVAs with group as the between subject factor and condition (dominance durations and switch rate) as the within subject factor were used to determine significant group differences between PD and NC. Planned independent groups t tests were performed to compare the effect of group (NC, PD) on both dominance durations and number of switches to further explore group differences.
Our main hypothesis was that relative to NC, PD would show a reduced ability to switch between the two possible percepts of the Necker cube. We applied a one-tailed test on the main effect of group for this individual planned comparison of the normalized data. Two-tailed tests were used for all other analyses.
Pearson correlations were used with the neuropsychological (cognitive), personality and eye movement data to examine the association between these and performance (dominance durations) for the Passive, Hold and Switch conditions. We also correlated performance with medication level (LED) and disease severity (UPDRS) in PD, and we examined whether there was an effect of eye dominance on performance for either group.
3. Results
3.1. Necker cube and binocular rivalry: Absolute dominance durations
3.1.1. Passive viewing: Necker cube
PD showed comparable dominance durations to NC during Passive viewing. PD reported a perceptual alternation every 5.4s on average (standard deviation [SD] 1.6s), and for NC it was every 5.4s (2.4s) (t[48] = .02, p = 1.0).
3.1.2. Passive viewing: Binocular rivalry
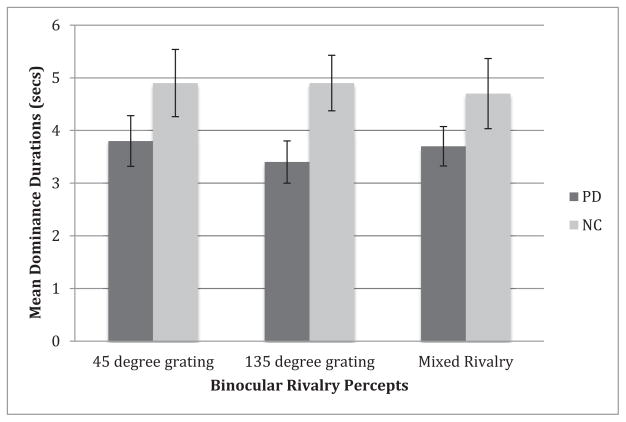
Absolute mean dominance durations for passive viewing during binocular rivalry are presented in Figure 3. On average, PD patients perceived the right grating (45°) for 3.5s (1.3s), the left grating (135°) for 3.1s (1.1s) and mixed percepts/piecemeal rivalry for 3.6s (1.4s); NC perceived the right grating (45°) for 4.9s (2.3s), the left (135°) for 4.9s (1.9s), and mixed for 4.7s (2.4s). A mixed design ANOVA with two groups and three conditions revealed a significant main effect of group (F[1, 24] = 6.72, p < .02, partial η2 = .22), with PD having shorter dominance durations than NC. There was no significant main effect of condition (F(1.51, 36.31) = 0.18, p = .78). The interaction between group and condition was also non-significant (F[1.51, 36.31] = 0.45, p = .60).
Figure 3.
Absolute dominance durations for binocular rivalry. Mean dominance durations for each percept (left grating [135 degrees], right grating [45 degrees] and mixed percepts) during passive viewing for individuals with Parkinson’s disease (PD) and normal control adults (NC). The groups significantly differed in their dominance durations, PD reporting shorter dominance durations for each of the three percepts. Error bars indicate the standard error of the mean.
3.1.3. Hold and Switch viewing: Necker cube
During Hold, PD showed an absolute dominance duration (M = 8.3s [3.0s]) that was comparable to that of NC (M = 8.7s [4.0s]). During Switch viewing, the PD mean dominance duration was 4.4s (2.0s), and for NC it was 3.3s (1.7s). A mixed design ANOVA with two levels of group (PD-NC) and two levels of volitional-control condition (Hold, and Switch) revealed a significant main effect of condition (F[1, 43] = 82.10, p <.002, partial η2 = .66), no main effect of group (F[1, 43] = .21, p = .66), and no interaction (F[1,43] = 2.31, p = .14).
Planned dependent groups t-tests were conducted to determine whether each group was able to increase (Hold) or decrease (Switch) their dominance durations compared to their performance during Passive viewing. The ability to do so was significant in each case. The changes relative to performance under Passive viewing were significant for both groups (PD - Hold: t [24] = 4.21, p < .000, partial η2 = .43, 95% CI [1.40, 4.10]; Switch: t[24] = 2.48, p <.021, partial η2 = ..20, 95% CI .15, 1.68; NC - Hold: t[21] = 5.39, p < .000, partial η2 = .58, 95% CI [2.37, 5.35]; Switch: t[21] = 4.06, p < .001, partial η2 = .44, 95% CI [1.00, 3.10]).
3.2. Relative dominance durations: Volitional control conditions normalized to passive viewing
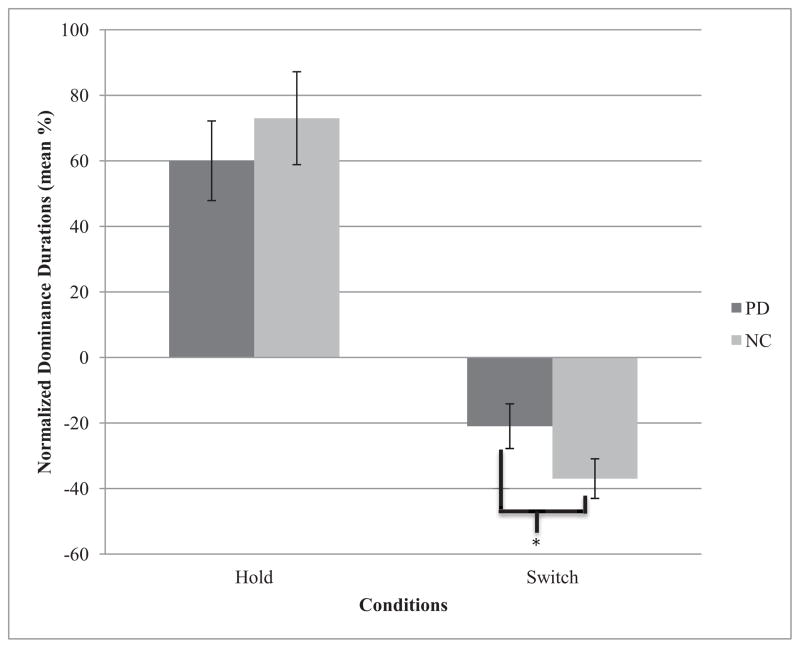
For each participant, data were normalized to the Passive condition and volitional modulation was calculated as (DX – DP)/DP*100, where DX is the normalized mean dominance duration of one of the volitional control conditions (Hold or Switch) and DP is the mean dominance duration of the Passive condition. Normalized dominance durations for Hold and Switch conditions are presented in Figure 4. Compared to passive viewing, PD increased their dominance duration by 60% (58%) during the Hold condition, whereas NC showed an increase of 74% (68%). In the Switch condition, PD decreased their dominance durations by 21% (32%) compared to the passive viewing, and NC by 37% (29%). A mixed design ANOVA, with group as a between-subject factor and condition as a within-subject factor, revealed a significant main effect of condition (F[1,43] = 94.12, p <.002, partial η2 = .69), a non-significant main effect of group (F[1,43] = .01, p = .94), and no group by condition interaction (F[1, 43] = 2.10, p = .16). Planned comparisons revealed that individuals with PD were not less able than NC to increase their dominance duration during the Hold condition (t [47] = 1.39, p = .18, but were significantly less able than NC to decrease their dominance durations during the Switch condition (t[45] = 1.97, p < .03, partial η2 = .08, 95% CI [.004, .37]) (one-tailed test for Switch planned comparison, as per directional hypothesis).
Figure 4.
Normalized mean dominance durations of individuals with Parkinson’s disease (PD) and normal control adults (NC) under the Hold and Switch conditions, Necker cube. PD were significantly less able than NC to decrease their dominance duration in the Switch condition, both relative to their performance during passive viewing. Error bars indicate the standard error of the mean.
3.3. Eye movements: deviation from fixation point
To assess the possible influence of eye movements on performance for those participants for whom eye movement data were reliable (17 PD, 15 NC), we calculated their ability to maintain fixation as the mean deviation from the fixation cross (in degrees of visual angle) for each experimental condition. Each participant had three mean deviation scores for horizontal eye positions and three mean deviations scores for vertical eye positions, with the three scores corresponding to the Passive, Hold and Switch conditions (absolute dominance durations). Positive values indicate eye movements to the right of center and above center, and negative values indicate left of center and below center, reported as means (standard deviations). For horizontal eye positions, PD moved their eyes 1.17° (1.08) left of center during the Passive condition, while NC moved their eyes 0.65° (.44) left of center. For the Hold condition, PD moved their eyes 0.53° (.71) left of center and NC 0.64° (.69). For the Switch condition, PD moved their eyes left of center by 0.24° (.90) and NC by 0.66° (.79). For vertical eye positions, PD moved their eyes 0.84° (1.60) above center during the Passive condition, while NC moved their eyes 0.11° (1.29) above center. For the Hold condition, PD moved their eyes 0.87° (1.42) above center and NC 0.67° (.61). For the Switch condition, PD moved their eyes above center by 0.51° (1.66) and NC by 0.92° (.80).
A mixed design ANOVA with three levels for horizontal eye movements (Passive, Hold and Switch conditions) and two groups (PD, NC) found no main effect of group (F[1,26] =.01, p = .91). The main effect of horizontal eye movement deviation from center (F[1.69, 44] = 5.25, p < .01, partial η2 = .17) and the interaction (F[1.69, 44] = 4.39, p < .02, partial η2 = .14) were significant. Both groups showed a tendency to move their eyes left of center. Planned independent groups t tests indicated a trend in the horizontal eye movements during passive viewing only, with PD, on average, moving their eyes more towards the left than NC (t[22.86] = 1.97, p = .06). A second mixed design ANOVA with three levels for vertical eye movements and two groups found no main effects (group, F[1,22] = .15, p = .70; condition, F[1.49, 32.67] = .81, p = .42) or interaction (F[1.49, 32.67] = 2.68, p = .10).
We also evaluated whether eye movements, specifically the deviation away from the fixation cross, impacted participants’ performance during the Passive, Hold and Switch conditions of the Necker cube. We found no significant correlations between horizontal eye movements and performance by PD (Passive: r = −.17; p = .53; Switch: r = −.23, p = .37) or NC (Passive: r = −.46, p = .10; Hold: r = −.41, p = .14; Switch: r = −.43, p = .13), with one PD trend for Hold (r = .49; p = .08). There were also no significant correlations between vertical eye movement and performance in either group (PD [Passive: r = .20, p = .47; Hold: r = −.16, p = .71; Switch: r = .02, p = .93]; NC [Passive: r = −.30, p = .33; Hold: r = .15, p = .64; Switch: r = −.06, p = .85]).
3.4. Association of perceptual performance with eye dominance and with indices of disease severity
Eye dominance data were available for 26/28 PD (17 right-eye dominant, 9 left) and 21/26 NC (13 right-eye dominant, 8 left). Participants with right eye dominance did not significantly differ from those with left eye dominance for either group for dominance durations on any condition of the Necker and binocular rivalry tasks (all t < 1.8, all p > .096).
We correlated scores on the indices of PD severity (UPDRS Total scores, LED) with dominance durations for Necker cube (3 conditions, alpha of .05/3 = .017) and binocular rivalry (3 conditions, alpha of .05/3 = .0167). There were no significant correlations for any condition (Table 2).
Table 2.
Pearson correlations between dominance durations by condition and indices of disease severity in the group with Parkinson’s disease. No correlations were significant (p=.017).
| UPDRS: r | UPDRS: p | LED: r | LED: p | |
|---|---|---|---|---|
| Necker: Passive | .14 | .49 | −.12 | .57 |
| Necker: Normalized Hold | −.09 | .68 | −.01 | .97 |
| Necker: Normalized Switch | −.19 | .39 | .37 | .09 |
| Binocular Rivalry (passive): right grating | −.17 | .55 | −.20 | .51 |
| Binocular Rivalry (passive): left grating | .17 | .55 | −.09 | .76 |
| Binocular Rivalry (passive): mixed grating | −.39 | .17 | −.48 | .10 |
UPDRS: Unified Parkinson’s Disease Rating Scale, total score; LED: Levodopa Equivalent Dose
3.5. Neuropsychological (cognitive) assessment: Association between dominance durations and cognitive performance
NC outperformed PD on the Stroop Test: Word, Color, and Color-Word; Semantic Fluency (D-KEFS – Animals); Trail Making Test – A; WCST Total Correct Score; and WCST Perseverative Responses. No group differences were found on Stroop Interference; Letter Fluency (D-KEFS - FAS); Switch Fluency (D KEFS – Switch Condition: fruit-furniture); Ruff Figural Fluency (unique designs); Trail Making Test – B; Trails B minus A (scores on B corrected by scores on A); or WCST Perseverative Errors (Table 3).
Table 3.
Cognitive performance of individuals with Parkinson’s disease (PD) and matched normal control adults (NC).
| Test | PD (n) | NC (n) | PD Mean (SD) | NC Mean (SD) | t-test | p-value | partial η2 | 95% CI lower upper |
|---|---|---|---|---|---|---|---|---|
| Stroop Word – Total Correct | 28 | 24 | 92.7 (14.1) | 101.1 (12.9) | t(50) = 2.2 | .03 | .09 | .83 15.98 |
| Stroop Color – Total Correct | 26 | 24 | 65.5 (8.1) | 71.9 (8.2) | t(48) = 2.8 | .008 | .14 | 1.78 11.05 |
| Stroop Color-Word – Total Correct | 26 | 24 | 37.5 (7.8) | 42.7 (8.5) | t(48) = 2.2 | .03 | .09 | .53 9.82 |
| Stroop Interference | 26 | 23 | .03(5.6) | .95 (7.1) | t(47) = .51 | .61 | ||
| Letter Fluency (FAS) – Total words | 27 | 25 | 43.7 (10.1) | 48.8 (13.5) | t(50) = 1.5 | .13 | ||
| Semantic Fluency (Animals) – Total words | 27 | 24 | 20.8 (5.0) | 24.2 (4.8) | t(49) = 2.5 | .02 | .11 | .66 6.2 |
| Switch Semantic Fluency – # Correct Alternations | 27 | 23 | 12.7 (2.8) | 14.4 (2.3) | t(48) = 2.0 | .052 | ||
| Ruff Figural Fluency – Total Unique Designs | 26 | 20 | 89.3(14.0) | 82.0 (16.7) | t(44) = 1.6 | .11 | ||
| Trails A time (sec) | 27 | 24 | 29.2 (6.3) | 25.6 (6.2) | t(49) = 2.0 | .047 | .08 | .06 7.10 |
| Trails B time (sec) | 27 | 22 | 63.6 (18.0) | 55.2 (15.3) | t(47) = 1.7 | .09 | ||
| Trails B-A (sec) | 26 | 21 | 33.9 (15.5) | 25.8 (12.9) | t(45) = 1.9 | .06 | ||
| WCST Total Correct Responses | 27 | 25 | 48.1 (7.1) | 52.4 (4.5) | t(50) = 2.6 | .01 | .12 | .98 7.68 |
| WCST Total Perseverative Responses | 27 | 25 | 7.0 (2.8) | 5.6 (2.2) | t(50) = 2.1 | .046 | .08 | .03 2.85 |
| WCST Total Perseverative Errors | 26 | 25 | 6.4 (2.1) | 5.5 (2.1) | t(49) = 1.6 | .11 |
Notes: Stroop conditions were 45 sec each. Fluency conditions were 60 sec each. Letter, Semantic and Switch Fluency Subtests are from the D-KEFS (Delis-Kaplan Executive Function Scale); WCST: Wisconsin Card Sorting Test. All values represent raw, non-standardized values, mean (SD).
We examined whether there was an association between perceptual performance on the Necker cube (absolute dominance durations in Passive viewing or normalized dominance durations under Hold and Switch conditions) and cognitive performance. We also conducted correlations between binocular rivalry results (passive viewing) and cognitive performance. Because we examined several tests of cognition, a conservative alpha of .01 was used to assess significance in each case. There were no significant correlations between perceptual performance (Necker cube, absolute passive and normalized hold/switch; binocular rivalry, absolute passive) and performance on any cognitive test for either the PD or NC group (PD Necker cube, all r < .35, all p > .012; NC Necker cube, all r < .38, all p > .08; PD binocular rivalry, all r < .46, all p >.10; NC binocular rivalry, all r < .59, all p > .06). There were trends in the PD group between durations in the normalized Hold Condition and performance on the Stroop Test (Stroop Word: r = −.49, p = .02; Stroop Color: r = −.53, p = .012) but not for Stroop Interference, which is the condition of principal interest in regard to cognitive flexibility (r = −.18, p = .40).
Besides Stroop Interference, the other three most direct measures of cognitive flexibility were switch fluency, Trails B-A, and Wisconsin Card Sorting perseverative errors. We examined the correlations of performance on these four tests with each other and found no significant results for PD or NC (one trend toward a correlation of Stroop Interference and switch fluency for PD, r = .35, p = .087); hence, we used an alpha of .05 to examine the correlations of performance on these cognitive tests with performance on the perceptual measures. There were no significant correlations, and just one trend toward a correlation of performance of Trails B-A with absolute dominance durations in the passive condition for PD (Table 4).
Table 4.
Pearson correlations of Necker perceptual performance and performance on select neuropsychological tests of cognitive flexibility in Parkinson’s disease (PD) and matched normal control adults (NC). Numbers are r values (p values; alpha of .05). No correlations were significant.
| PD | NC | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Test | Absolute Passive | Normalized Hold | Normalized Switch | Absolute Passive | Normalized Hold | Normalized Switch |
| Stroop Interference | .12 (.56) | −.18 (.40) | −.26 (.25) | .10 (.66) | −.12 (.57) | −.16 (.49) |
| Trails B-A | −.35 (.09) | .04 (.85) | .28 (.21) | .15 (.54) | .08 (.72) | −.38 (.11) |
| Switch Fluency (Accuracy) | .14 (.50) | −.23 (.29) | −.28 (.19) | −.08 (.74) | −.20 (.37) | .25 (.27) |
| WCST Perseverative Errors | −.05 (.81) | .19 (.40) | .35 (.11) | −.23 (.30) | −.10 (.63) | .23 (.30) |
WCST: Wisconsin Card Sorting Test
3.6. Personality: Association between dominance durations and personality traits
PD and NC did not significantly differ on any of the temperament dimensions: Novelty Seeking (PD mean: 15.7 [5.7], NC mean = 15.7 [6.0]; t[48] = .00, p = .99); Harm Avoidance (PD mean: 13.6 [5.0], NC mean = 11.7 [7.3]; t[38.16] = 1.04, p = .30); Reward Dependence (PD mean = 16.2 [3.9], NC mean = 15.1 [4.1], t[47] = .90, p = .37); Persistence (PD mean = 5.5 [2.3], NC mean = 4.8 [2.2], t[48] = 1.04, p = 31). The personality dimensions were not correlated with each other for PD or NC (all r < .35, all p > .07) with the exception of Harm Avoidance and Persistence in the NC group (r= .46, p= .025).
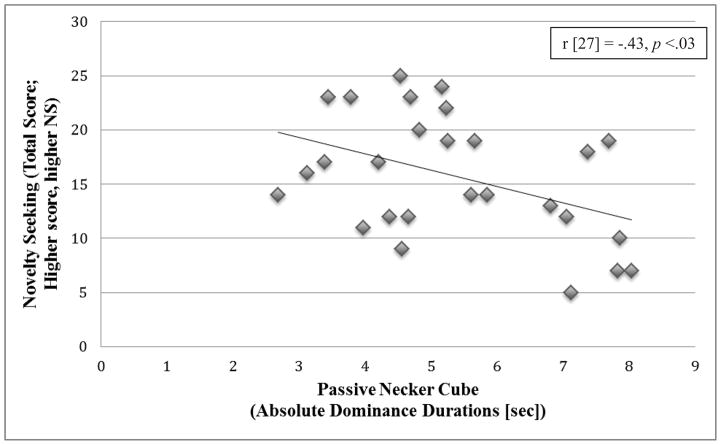
We examined whether personality was related to bistable perception. Under the Passive viewing condition (Necker cube), absolute dominance durations correlated with the score on Novelty Seeking in the PD group (r [27] = −.43, p < .03), but not in the NC group (r [21] = −.28, p = .22); that is, a lower tendency to seek novelty (more personality rigidity) was associated with increased dominance durations in PD (Figure 5). We used Meng’s Z test to compare the size of the correlations between absolute dominance durations and Novelty Seeking under passive viewing and found that the difference between these correlations was not significant (Z = .55, p > .05). For normalized Hold and Switch conditions, there was one trend in PD between normalized Hold and Novelty Seeking (r = − .37, p = .08); those PD reporting lower novelty seeking tended to hold one percept of the cube. No other correlations were significant (all r < .15, p > .37). All correlations used an alpha of .05, as the personality dimensions were not correlated except for one as noted above for NC; neither Harm Avoidance nor Persistence correlated with dominance durations in this group even at alpha of .05.
Figure 5.
Relation of novelty seeking (TCI subscale total score) to absolute dominance durations while passively viewing the Necker cube, PD group. Higher dominance durations during passive viewing correlated with lower novelty seeking personality traits.
For binocular rivalry, there was no correlation between dominance durations for either the left or right percept and novelty seeking; there was a positive correlation only for (relatively uninformative) mixed percepts, in the PD group only (PD, r [15] = .55, p = .03; NC, r [15] = .14, p = .60). The difference between the size of these correlations was not significant (Z = 1.61, p > .05). Correlations for other personality temperaments were not significant for either group (r < .29, p > .14).
3.7. Association between cognition and novelty seeking
We examined whether novelty seeking, which was correlated with perceptual flexibility as described above, was also related to cognition. There were no significant correlations between novelty seeking and performance on any cognitive test for either group (all r < .36, all p > .10).
4. Discussion
The present study examined whether rigidity in PD, usually described as motoric, also extends to perception, and the possible association between this hypothesized perceptual rigidity and other non-motor rigidities (e.g., cognitive and personality). Supporting our main hypothesis that individuals with PD would exhibit perceptual rigidity, they were significantly less able than NC to shorten dominance durations under the Switch volitional-control condition of the Necker cube experiment, relative to passive viewing. The PD group showed a different pattern of performance under the passive conditions eliciting higher-order processing (Necker cube; similar to NC) and lower-order, stimulus-driven processing (binocular rivalry; reduced dominance durations in PD relative to NC). The results did not support an association in PD between perceptual rigidity and cognitive inflexibility, but did support an association between perceptual rigidity and personality, specifically low novelty-seeking.
4.1. Passive viewing of bistable images: Necker cube (higher-order) vs. binocular rivalry (lower-order)
During passive viewing of the Necker cube, PD showed comparable dominance durations to NC. For the binocular rivalry experiment, PD perceived all three perceptual images (left and right gratings and mixed percepts) for shorter durations than did NC. Our lack of group differences during passive viewing with the Necker cube, and shorter dominance durations during binocular rivalry, suggest that mild-moderate PD differentially affects the neural substrates of higher-level (Necker cube) and lower-level processing of bistable stimuli (orthogonal gratings used in the binocular rivalry experiment) (Meng & Tong, 2004). Tong (2001) reviewed psychophysical and neuropsychological studies comparing the neural substrates associated with binocular rivalry and pattern competition (e.g., Necker cube) and concluded that V1 (“monocular visual cortex”) plays an important role in the selection of one particular percept during binocular rivalry. In regard to our findings, one possibility is that V1 is disinhibited as a result of higher-order cortical (fronto-parietal) dysfunction in PD. Without V1 inhibition by other cortical or subcortical areas (Varela & Singer 1987; Wilson, Blake & Lee 2001), the switch rate could be taken over by V1, resulting in reduced dominance durations per percept.
The dynamics of bistable perception at this level can be shaped by neurotransmitter modulation. A study by van Loon, Knapen, Scholte, St. John-Saaltink, Donnre and Lamme (2013) demonstrated an association of higher GABA concentrations in visual cortex (measured with magnetic resonance spectroscopy, MRS) with slower dynamics on binocular rivalry, motion-induced blindness, and structure-from-motion (increased dominance durations and reduced number of switches) in healthy young adults; further, use of lorazepam (to stimulate GABAA receptors) slowed the dynamics. Of importance, van Loon et al. also examined the dorsolateral prefrontal cortex (DLPFC) because of its known role in bistable perception in healthy adults and found no association of GABA concentration in DLPFC with perceptual dynamics. There is much evidence for abnormalities in multiple neurotransmitter systems including GABA in PD (e.g., Huot, Johnston, Koprich, Fox, & Brotchie, 2013); of most relevance here is a report of an increase in GABA levels (measured by ultra-high field MRS) in the putamen and pons (but not in DLPFC) in mild-moderate PD (Emir, Tuite, & Öz, 2012)—the same severity level as assessed in our study. The study by Emir et al. found no correlation of GABA levels and disease severity, presumably because of the restricted range of severity, mirrored in our study by the lack of correlation of perceptual rigidity with disease severity. These studies together provide evidence for heightened GABA levels in PD and higher GABA levels in certain areas (visual cortex, at least) being associated with what we describe as perceptual rigidity (reduced number of switches under bistable perception); further, the DLPFC, though it has a role in bistable perception, is not a site of GABA concentrations that are associated with perceptual flexibility. Because we found speeding rather than slowing of perceptual dynamics for binocular rivalry in particular, it is unlikely that GABA concentration is high in visual cortex at least in the mild to moderate stages of the disease. To our knowledge, GABA levels in occipital cortex in PD have not been investigated.
Though there has been little work to date on visual cortex and PD, it may be relevant to note that cortical thinning has been observed in the occipital lobe in individuals with PD who were similar in disease stage to those we describe here (Tinaz, Courtney & Stern, 2011). A related conceptualization is that whereas viewing of bistable figures may require top-down attentional control, viewing under conditions of binocular rivalry may be more stimulus driven. Specifically, there is evidence from PD that top-down control is impaired but stimulus-driven processing may be enhanced (Cools, Rogers, Barker, & Robbins, 2010; Sawada et al., 2012).
4.2. Perceptual rigidity in PD relative to NC, revealed through performance on Switch condition
Based on clinical studies suggesting the impact of compromised frontal and parietal brain areas on bistable perception (McBain et al., 2011; Rucci & Blundo, 1990; Windmann et al., 2006) as well as studies of healthy adults that implicate similar areas (Knapen et al., 2011; Sterzer & Kleinschmidt, 2007), we hypothesized that individuals with PD, relative to control adults, would show compromised ability to reduce dominance durations in the Switch condition of the Necker cube experiment. The data supported this hypothesis, pointing to the potential importance of the fronto-parietal-striatal attentional network for this function in PD. A neuroimaging study with healthy young adults has suggested that the basal ganglia, in particular the ventral striatum and pallidum, may modulate the activity in the prefrontal cortex when a person is confronted with novel stimuli that require switches in attention (van Schouwenburg, den Ouden, & Cools, 2010). In another study, de Graaf, de Jong, van Ee, & Sack (2011) used repetitive transcranial magnetic stimulation (rTMS) to cause virtual lesions in frontal and parietal regions during the passive viewing of a bistable structure-from-motion stimulus and found that rTMS in the DLPFC impacted the ability of healthy young adults to decrease their dominance durations of a bistable stimulus during the Switch condition (did not assess Hold). Relevant to PD in particular are the results of a recent imaging study that found that those with a higher number of errors on a task assessing the ability to distinguish monostable from bistable images showed less activation of frontal and parietal hubs of the dorsal attentional network, which is proposed to include the DLPFC, posterior parietal cortex, frontal eye fields, and corpus striatum; worse performance on the task correlated with the degree of decreased activation in a number of these hubs including DLPFC, superior parietal lobule, and frontal eye fields (Shine et al., 2014).
Researchers are beginning to distinguish the neural substrates for spontaneous (passive) vs. controlled viewing of ambiguous stimuli such as the Necker cube (Hold and Switch conditions), arguing that performance on these perceptual tasks is supported by different brain mechanisms. For example, in the study cited above, de Graaf and colleagues (2011) found that rTMS to DLPFC impacted the Switch condition, but rTMS to either frontal or parietal regions had no effect on passive viewing (note, there was no Hold condition in this experiment). Frässle, Sommer, Jansen, Naber, & Einhäuser (2014) used a combination of fMRI with measures of optokinetic nystagmus and pupil size to objectively and continuously map perceptual alternations during binocular rivalry (with both static and dynamic gratings) in order to assess neural activity while controlling for the confounding effects of verbal responses. They found that activation in frontal areas (e.g., middle frontal gyrus bilaterally) was absent when young adult observers passively viewed bistable stimuli without reporting perceptual alternations, whereas occipital and parietal areas remained active. These investigators suggested that frontal activation during binocular rivalry might be associated with introspection and verbally reporting a particular percept when passively viewing bistable stimuli. Future studies in PD and normal aging should employ imaging techniques to investigate whether frontal, parietal, and occipital brain areas are activated or deactivated during passive viewing under conditions of the Necker cube and binocular rivalry.
4.3. Personality (novelty seeking), cognition, and perceptual rigidity in PD
Previous studies have found that individuals with PD report lower novelty-seeking and higher harm-avoidance tendencies than healthy control adults (e.g., Fujii, Harada, Ohkoshi, Hayashi, Yoshizawa, 2000; Jacobs, Heberlein, Vieregge, & Vieregge, 2001; McNamara, Durso, & Harris, 2008; Menza, Golbe, Cody, & Forman, 1993; Tomer & Aharon-Perez, 2004). In our study, although PD and NC did not significantly differ in regard to any of the TCI personality temperaments, we found a significant association between lower novelty seeking and longer dominance durations during spontaneous (passive) viewing (PD group) for the bistable Necker cube, and a trend in the same direction for PD for the normalized Hold condition. For binocular rivalry, for which we found shorter dominance durations for PD than for NC, there was a significant association in PD between lower novelty seeking and shorter dominance durations, though only for mixed percepts. In PD, lower novelty seeking has been associated with reduced [18F] dopa update in the caudate (Menza, Mark, Burn, & Brooks, 1995; Tomer & Aharon-Peretz, 2004). In healthy adults, lower novelty seeking has been associated with less grey matter volume in frontal and posterior cingulate regions (Gardini, Cloninger, & Venneri, 2009).
Recently, novelty seeking in healthy adults was found to be positively correlated with fiber connectivity from the medial and lateral orbitofrontal cortex and amygdala to the striatum, but not from the DLPFC and posterior cingulate/retrosplenial cortex to the striatum (Lei et al., 2014). This pattern of connectivity may explain why we do not find significant correlations between novelty seeking and cognitive flexibility, as the DLPFC has been implicated in PD deficits on a number of such cognitive tasks (e.g., Sawada et al., 2012). Further, in healthy adults, increased novelty-seeking has been associated with higher volumes of caudate and globus pallidus (Laricchiuta, Musella, Rossi, Centonze, 2014) and with more activation of the substantia nigra/ventral tegmental area in response to novel cues even in the absence of reward (Krebs, Schott, & Duzel, 2009). In regard to the subcortical pathways including amygdala, it may be useful to consider a new conceptualization of individuals with PD as being “blind to blindsight”, meaning that whereas they preserve conscious vision, they show dysfunction of the phylogenetically older retino-colliculo-thalamo-amygdala and the retino-geniculo-extrastriate pathways, with attendant visual disorders including fragmentation of gaze shifts, slowness of saccades, and prolonged fixation time (Diederich, Stebbins, Schiltz, & Goetz, 2014), all potentially relevant to the ability to hold and switch percepts of bistable images.
Although novelty seeking correlated significantly with absolute dominance durations for PD, but not NC, under passive viewing of the Necker cube, and under the mixed percepts condition of binocular rivalry, the difference between the PD and NC correlations was not significant in any comparison. These results indicate that the association between novelty seeking and perception is not unique to either group. Rather, novelty seeking and the perception of bistable images are related in healthy adults as well as in PD, presumably because the tendency to switch between aspects of a bistable image depends upon, or at least reflects, a more general tendency to favor novelty over sameness. Hence, we surmise that at least some of the same structures that have been associated with novelty seeking (e.g., caudate, medial and lateral orbitofrontal cortex, amygdala, and substantia nigra/ventral tegmental area) are also important for perceptual flexibility.
By contrast, we found no evidence of an association between novelty seeking and performance on standard tests of cognitive flexibility including Stroop Interference, switch fluency, Trail Making Test B-A, and Wisconsin Card Sorting Test -perseverative errors. Similarly, Volpato and colleagues (2009) found no relation in PD between novelty seeking and executive function, including cognitive flexibility, whereas they did find significant correlations between cognitive flexibility and other aspects of personality (performance on Tower of London with Emotional Stability; alternating fluencies with Openness to Experience). These investigators suggested that changes in cognition and in some aspects of personality in PD may both be expressions of frontostriatal dysfunction, but that novelty seeking is not among these aspects of personality. Koerts and colleagues (2013) likewise found no association between novelty seeking and cognitive function in PD, including many of the same tests of cognition used in our study.
Because there is substantial evidence for cognitive flexibility being associated with prefrontal-mediated top-down attentional control, including a reported correlation between cognitive-attentional shifting (Trail Making Test) and the ability to distinguish bistable from monostable images in PD (Shine et al., 2012), it is incumbent on us to interpret the lack of correlation between this aspect of cognition and perceptual flexibility as assessed in the present study. As described above, though frontal areas including DLPFC have been found to be important to understanding perceptual reversals, there also appears to be a role for the parietal and occipital lobes, especially for more stimulus-driven processing (as in binocular rivalry), and for multiple areas in regard to novelty seeking (e.g., caudate, medial and lateral orbitofrontal cortex, amygdala, and substantia nigra/ventral tegmental area). Though non-overlapping brain areas may explain the lack of correlation of cognitive and perceptual flexibility, another possibility is differences in the relative sensitivity of perceptual and cognitive tasks to mild-moderate PD, or differences in the timing of the emergence of rigidities in these domains. PD is often associated with dysexecutive syndrome, but samples of individuals in mild to moderate stages of the disease may perform normally on many standard tests of cognitive flexibility, as we report here. What we may be seeing in the present study is the manifestation of perceptual rigidity (and reduced novelty seeking) early in the disease course, before the emergence of deficits in cognitive flexibility. That is, rather than perceptual rigidity (or novelty seeking) being unrelated to cognitive rigidity, the former may be a harbinger of the latter.
4.4. Limitations of the study
This study was subject to limitations. First, having the examiner record the participants’ verbal reports of perceptual state is a source of variability in the reaction time data. This design was dictated by the need to accommodate the motoric limitations of individuals with PD in regard to response modality; it may be argued that using a motor response would have introduced more variability than did our current design. Another potential limitation was that we did not provide the option (via key press) for the participant to view the Necker cube and choose neither face of the cube as their percept; that is, to allow reporting of a flat image. Sometimes participants reported one particular cube percept when in fact they were seeing a flat image of the cube. These instances could have introduced noise to the dominance duration data by increasing some of the cube durations. In regard to the lack of correlation of perceptual and cognitive measures, our assessment of cognitive flexibility was restricted to those included in a simultaneous larger study, though it should be noted that those we used were widely used, standard measures. It is possible that our perceptual measures (Necker cube and binocular rivalry) were more sensitive to perceptual abnormalities than the neuropsychological measures were to cognitive impairment in this high-functioning sample.
4.5. Conclusions
The results of the present study indicate that perceptual rigidity occurs in mild-to-moderate PD, and that it is not directly associated with performance on certain tests of cognitive flexibility. Perceptual rigidity is, however, associated with personality, specifically with novelty seeking, suggesting that common mechanisms may give rise to both novelty seeking and the ability to explore the perceptually ambiguous world.
In the future, it would be valuable to examine both perceptual and cognitive flexibility across a wide range of stages of PD, and to examine individuals longitudinally, to see if (and when) correlations emerge between cognitive rigidity and deficits in the volitional control of bistable perception. Further, it would be informative to evaluate how individuals with PD who have impulse-control disorders perceive bistable figures. This type of patient displays enhanced novelty-seeking and therefore may not show perceptual rigidity. Finally, we recently reported that use of appropriate low-level visual cues enhanced the ability of observers with PD to increase dominance durations during the Hold condition of a Necker cube task (which, as shown here, they perform normally) but not to decrease dominance durations during the Switch task (on which, as shown here, they exhibit impairments) (Díaz-Santos, Cao, Mauro, Yazdanbakhsh, Neargarder, & Cronin-Golomb, in press). Because individuals with PD may exhibit visual abnormalities (e.g., Armstrong, 2011; Laudate, Neargarder, & Cronin-Golomb, 2013), assessing those with a wider range of visual dysfunction than experienced by the participants in our studies might reveal that higher-order and lower-order processes of volitional control rely differentially on the integrity of visual abilities.
Highlights.
We assessed perceptual rigidity in Parkinson’s disease (PD) and normal control adults (NC).
With bistable stimuli, PD were less able than NC to switch percepts.
Perceptual rigidity correlated with low novelty-seeking but not with cognitive inflexibility.
Novelty seeking and perceptual flexibility may share neural substrates.
Perceptual rigidity may be a harbinger of cognitive inflexibility later in PD.
Acknowledgments
This research was supported in part by grants from the National Institute of Neurological Disorders and Stroke, including a Ruth L. Kirschstein National Research Service Award (F31 NS074892) to MDS, and RO1 NS067128 to ACG; a Clara Mayo Foundation Fellowship from the Department of Psychological and Brain Sciences at Boston University (to MDS); the National Science Foundation (NSF SBE-0354378) to AY; and the Office of Naval Research (ONR N00014-11-1-0535) to AY. These funding agencies did not have any involvement in the study design, data collection, analysis and interpretation, in writing the manuscript, or in the decision to submit the article for publication.
We would like to thank all of the individuals who participated in this study. Our recruitment efforts were supported, with our gratitude, by Marie Saint-Hilaire, M.D., and Cathi Thomas, R.N., M.S.N., of Boston Medical Center Neurology Associates, by Boston area Parkinson’s disease support groups, and the Fox Trial Finder. We thank Laura Pistorino for her assistance on screening and scheduling participants; Robert Salazar, Samantha Mauro, Lindsay Clark, Michael Horgan, and Tracy Abacherli for test administration and technical assistance; Mark O’Donoghue, O.D., for the eye examinations; and Laura Grande, Ph.D., for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mirella Díaz-Santos, Email: mdsantos@bu.edu.
Bo Cao, Email: ffcloud.tsao@gmail.com.
Arash Yazdanbakhsh, Email: yazdan@bu.edu.
Daniel J. Norton, Email: djn@bu.edu.
Sandy Neargarder, Email: sneargarder@bridgew.edu.
References
- Amir R. Selective biasing of a specific bistable-figure percept involves fMRI signal changes in frontostriatal circuits: A step towards unlocking the neural correlates of top-down control and self-regulation. American Journal of Clinical Hypnosis. 2007;50:137–156. doi: 10.1080/00029157.2007.10401611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RA. Visual symptoms in Parkinson’s disease. Parkinson’s disease. 2011;2011:1–9. doi: 10.4061/2011/908306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Blake R. A primer on binocular rivalry, including current controversies. Brain and Mind. 2001;2:5–38. [Google Scholar]
- Blake R, Logothetis NK. Visual competition. Nature Reviews Neuroscience. 2002;3:13–21. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- Blake R, O’Shea RP, Mueller TJ. Spatial zones of binocular rivalry in central and peripheral vision. Visual Neuroscience. 1992;8:469–478. doi: 10.1017/s0952523800004971. [DOI] [PubMed] [Google Scholar]
- Britz J, Landis T, Michel CM. Right parietal brain activity precedes perceptual alternation of bistable stimuli. Cerebral Cortex. 2009;19:55–65. doi: 10.1093/cercor/bhn056. [DOI] [PubMed] [Google Scholar]
- Carmel D, Arcaro M, Kastner S, Hasson U. How to create and use binocular rivalry. Journal of Visualized Experiments. 2010;45:1–8. doi: 10.3791/2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher L, Strafella AP. Neuroimaging of brain changes associated with cognitive impairment in Parkinson’s disease. Journal of Neuropsychology. 2013;7:225–240. doi: 10.1111/jnp.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Archives of General Psychiatry. 1993;50:975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Mechanisms of cognitive set flexibility in Parkinson’s disease. Brain. 2001;124:2503–2512. doi: 10.1093/brain/124.12.2503. [DOI] [PubMed] [Google Scholar]
- Cools R, Rogers R, Barker RA, Robbins TW. Top-down attentional control in Parkinson’s disease: Salient considerations. Journal of Cognitive Neuroscience. 2010;22:848–59. doi: 10.1162/jocn.2009.21227. [DOI] [PubMed] [Google Scholar]
- Cools AR, van den Bercken JH, Horstink MW, van Spaendonck KP, Berger HJ. Cognitive and motor shifting aptitude disorder in Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 1984;47:443–453. doi: 10.1136/jnnp.47.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin-Golomb A. Emergence of nonmotor symptoms as the focus of research and treatment of Parkinson’s disease: Introduction to the special section on nonmotor dysfunctions in Parkinson’s disease. Behavioral Neuroscience. 2013;127:135–138. doi: 10.1037/a0032142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf TA, van Ee R, Goebel R, de Jong MC, Sack AT. On the functional relevance of frontal cortex for passive and voluntarily controlled bistable vision. Cerebral Cortex. 2011;10:2322–2331. doi: 10.1093/cercor/bhr015. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Manual for the Delis-Kaplan Executive System (D-KEFS) San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: An update. Journal of the International Neuropsychological Society. 2004;10:301–303. doi: 10.1017/S1355617704102191. [DOI] [PubMed] [Google Scholar]
- Díaz-Santos M, Cao B, Mauro SA, Yazdanbakhsh A, Neargarder S, Cronin-Golomb A. The effect of visual cues on the resolution of perceptual ambiguity in Parkinson’s disease and normal aging. Journal of the International Neuropsychological Society. doi: 10.1017/S1355617715000065. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederich NJ, Stebbins G, Schiltz C, Goetz CG. Are patients with Parkinson’s disease blind to blindsight? Brain. 2014;137:1838–1849. doi: 10.1093/brain/awu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emir UE, Tuite PJ, Öz G. Elevated pontine and putamenal GABA levels in mild-moderate Parkinson disease detected by 7 tesla proton MRS. PLoS One 2012. 2012;7(1):e30918. doi: 10.1371/journal.pone.0030918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S, Elton R . Members of the UPDRS Development Committee. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Mardsen CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson’s Disease. Vol. 2. Florham Park, NJ: MacMillan Health Care Information; 1987. pp. 153–163.pp. 293–304. [Google Scholar]
- Frässle S, Sommer J, Jansen A, Naber M, Einhäuser W. Binocular rivalry: Frontal activity relates to introspection and action but not to perception. Journal of Neuroscience. 2014;34:1738–1747. doi: 10.1523/JNEUROSCI.4403-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii C, Harada S, Ohkoshi N, Hayashi A, Yoshizawa K. Cross-cultural traits for personality of patients with Parkinson’s disease in Japan. American Journal of Medical Genetics. 2000;7:1–3. [PubMed] [Google Scholar]
- Gardini S, Cloninger CR, Venneri A. Individual differences in personality traits reflect structural variance in specific brain regions. Brain Research Bulletin. 2009;79:265–270. doi: 10.1016/j.brainresbull.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Golden CJ. Stroop Color and Word Test. Wood Dale, IL: Stoelting Co; 1978. [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: Onset, progression, and mortality. Neurology. 1967;1(7):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Huot P, Johnston TH, Koprich JB, Fox SH, Brotchie JM. The pharmacology of L-DOPA-induced dyskinesia in Parkinson’s disease. Pharmacology Review. 2013;65:171–222. doi: 10.1124/pr.111.005678. [DOI] [PubMed] [Google Scholar]
- Jacobs H, Heberlein I, Vieregge A, Vieregge P. Personality traits in young patients with Parkinson’s disease. Acta Neurological Scandinavica. 2001;103:82–87. doi: 10.1034/j.1600-0404.2001.103002082.x. [DOI] [PubMed] [Google Scholar]
- Kang MS. Size matters: A study of binocular rivalry dynamics. Journal of Vision. 2009;9:1–11. doi: 10.1167/9.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerts J, Tucha L, Leenders KL, Tucha O. Neuropsychological and emotional correlates of personality traits in Parkinson’s disease. Behavioral Neurology. 2013;27:567–74. doi: 10.3233/BEN-129017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapen T, Brascamp J, Pearson J, van Ee R, Blake R. The role of frontal and parietal brain areas in bistable perception. Journal of Neuroscience. 2011;31:10293–10301. doi: 10.1523/JNEUROSCI.1727-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test – 64 Card Version: Professional Manual. Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- Krebs RM, Schott BH, Düzel E. Personality traits are differentially associated with patterns of reward and novelty processing in the human substantia nigra/ventral tegmental area. Biological Psychiatry. 2009;65:103–10. doi: 10.1016/j.biopsych.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Laricchiuta D, Musella A, Rossi S, Centonze D. Behavioral and electrophysiological effects of endocannabinoid and dopaminergic systems on salient stimuli. Frontiers in Behavioral Neuroscience. 2014;8:1–11. doi: 10.3389/fnbeh.2014.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudate TM, Neargarder S, Cronin-Golomb A. Line bisection in Parkinson’s disease: Investigation of contributions of visual field, retinal vision and scanning patterns to visuospatial function. Behavioral Neuroscience. 2013;127:151–163. doi: 10.1037/a0031618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Chen C, Xue F, He Q, Chen C, Liu Q, Moyzis RK, Xue G, Cao Z, Li J, Li H, Zhu B, Liu Y, Hsu AS, Li J, Dong Q. Fiber connectivity between the striatum and cortical and subcortical regions is associated with temperaments in Chinese males. Neuroimage. 2014;89:226–34. doi: 10.1016/j.neuroimage.2013.04.043. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Logothetis NK. Activity changes in early visual cortex reflect monkeys’ percepts during binocular rivalry. Nature. 1996;379:549–553. doi: 10.1038/379549a0. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Logothetis NK. Multistable phenomena: Changing views in perception. Trends in Cognitive Sciences. 1999;3:254–263. doi: 10.1016/s1364-6613(99)01332-7. [DOI] [PubMed] [Google Scholar]
- McBain R, Norton DJ, Kim J, Chen Y. Reduced cognitive control of a visually bistable image in schizophrenia. Journal of the International Neuropsychological Society. 2011;17:551. doi: 10.1017/S1355617711000245. [DOI] [PubMed] [Google Scholar]
- McNamara P. The Cognitive Neuropsychiatry of Parkinson’s Disease. Cambridge, MA: The Massachusetts Institute of Technology Press; 2011. The agentic self and personality changes in Parkinson’s disease; pp. 87–109. [Google Scholar]
- McNamara P, Durso R, Harris E. Alterations of the sense of self and personality in Parkinson’s disease. International Journal of Geriatric Psychiatry. 2008;23:79–84. doi: 10.1002/gps.1845. [DOI] [PubMed] [Google Scholar]
- Meenan JP, Miller LA. Perceptual flexibility after frontal or temporal lobectomy. Neuropsychologia. 1994;32:1145–1149. doi: 10.1016/0028-3932(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Meng M, Tong F. Can attention selectively bias bistable perception? Differences between binocular rivalry and ambiguous figures. Journal of Vision. 2004;4:539–551. doi: 10.1167/4.7.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menza MA, Forman NE, Goldstein HS, Golbe LI. Parkinson’s disease, personality, and dopamine. Journal of Neuropsychiatry and Clinical Neurosciences. 1990;2:282–287. doi: 10.1176/jnp.2.3.282. [DOI] [PubMed] [Google Scholar]
- Norman JF, Norman HF, Pattison K, Taylor MJ, Goforth KE. Aging and the depth of binocular rivalry suppression. Psychology and Aging. 2007;22:625–631. doi: 10.1037/0882-7974.22.3.625. [DOI] [PubMed] [Google Scholar]
- Ricci C, Blundo C. Perception of ambiguous figures after focal brain lesions. Neuropsychologia. 1990;28:1163–1173. doi: 10.1016/0028-3932(90)90052-p. [DOI] [PubMed] [Google Scholar]
- Robertson CE, Kravitz DJ, Freyberg J, Baron-Cohen S, Baker CI. Slower rate of binocular rivalry in autism. Journal of Neuroscience. 2013;33:16983–16991. doi: 10.1523/JNEUROSCI.0448-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff R, Light R, Evans R. The Ruff figural fluency test: A normatic study with adults. Developmental Neuropsychology. 1987;3:37–52. [Google Scholar]
- Sawada Y, Nishio Y, Suzuki K, Hirayama K, Takeda A, Hosokai Y, Ishioka T, Itoyama Y, Takahashi S, Fukuda H, Mori E. Attentional set-shifting deficit in Parkinson’s disease is associated with prefrontal dysfunction: An FDG-PET study. PLoS One 2012. 2012;7:e38498. doi: 10.1371/journal.pone.0038498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine JM, Halliday GH, Carlos M, Naismith SL, Lewis SJG. Investigating visual misperceptions in Parkinson’s disease: A novel behavioral paradigm. Movement Disorders. 2012;27:500–505. doi: 10.1002/mds.24900. [DOI] [PubMed] [Google Scholar]
- Shine JM, Halliday GM, Gilat M, Matar E, Bolitho SJ, Carlos M, Naismith SL, Lewis SJG. The role of dysfunctional attentional control networks in visual misperceptions in Parkinson’s disease. Human Brain Mapping. 2014;35:2206–2219. doi: 10.1002/hbm.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Sano M, Paulson J, Mayeux R. Modified Mini-Mental State Examination: Validity and reliability [abstract] Neurology. 1987;37(Suppl 1):179. [Google Scholar]
- Sterzer P, Kleinschmidt A. A neural basis for inference in perceptual ambiguity. Proceedings of the National Academy of Sciences. 2007;104(1):323–328. doi: 10.1073/pnas.0609006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinaz S, Courtney MG, Stern CE. Focal cortical and subcortical atrophy in early Parkinson’s disease. Movement Disorders. 2011;26:436–441. doi: 10.1002/mds.23453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong F. Competing theories of binocular rivalry: A possible resolution. Brain and Mind. 2001;2:55–83. [Google Scholar]
- Tombaugh TN. Trail Making Test A and B: Normative data stratified by age and education. Archives of Clinical Neuropsychology. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Tomer R, Aharon-Peretz J. Novelty seeking and harm avoidance in Parkinson’s disease: effects of asymmetric dopamine deficiency. Journal of Neurology, Neurosurgery, and Psychiatry. 2004;75(7):972–975. doi: 10.1136/jnnp.2003.024885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukai K, Ando H, Kuze J. Binocular rivalry alternation rate declines with age. Perceptual and Motor Skills. 2003;97:393–397. doi: 10.2466/pms.2003.97.2.393. [DOI] [PubMed] [Google Scholar]
- van Loon AM, Knapen T, Scholte HS, St John-Saaltink E, Donner TH, Lamme VA. GABA shapes the dynamics of bistable perception. Current Biology. 2013;23:823–827. doi: 10.1016/j.cub.2013.03.067. [DOI] [PubMed] [Google Scholar]
- van Schouwenburg MR, den Ouden HEM, Cools R. The human basal ganglia modulate frontal-posterior connectivity during attention shifting. Journal of Neuroscience. 2010;30:9910–9918. doi: 10.1523/JNEUROSCI.1111-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela FJ, Singer W. Neuronal dynamics in the visual corticothalamic pathway revealed through binocular rivalry. Experimental Brain Research. 1987;66:10–20. doi: 10.1007/BF00236196. [DOI] [PubMed] [Google Scholar]
- Volpato C, Signorini M, Meneghello F, Semenza C. Cognitive and personality features in Parkinson disease: 2 sides of the same coin? Cognitive and Behavioral Neurology. 2009;22:258–63. doi: 10.1097/WNN.0b013e3181c12c63. [DOI] [PubMed] [Google Scholar]
- Wang M, Aeteaga D, He BJ. Brain mechanisms for simple and bistable perception. Proceedings of the National Academy of Sciences. 2013;110:E3350–E3359. doi: 10.1073/pnas.1221945110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilnhammer VA, Ludwig K, Hesselmann G, Sterzer P. Frontoparietal cortex mediates perceptual transitions in bistable perception. Journal of Neuroscience. 2013;33:16009–16015. doi: 10.1523/JNEUROSCI.1418-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcke JC, O’Shea RP, Watts R. Frontoparietal activity and its structural connectivity in binocular rivalry. Brain Research. 2009;1305:96–107. doi: 10.1016/j.brainres.2009.09.080. [DOI] [PubMed] [Google Scholar]
- Wilson HR, Blake R, Lee SH. Dynamics of travelling waves in visual perception. Nature. 2001;412:907–910. doi: 10.1038/35091066. [DOI] [PubMed] [Google Scholar]
- Windmann S, Wehrmann M, Calabrese P, Gunturkun O. Role of the prefrontal cortex in attentional control over bistable vision. Journal of Cognitive Neuroscience. 2006;18:456–471. doi: 10.1162/089892906775990570. [DOI] [PubMed] [Google Scholar]
- Yacorzynski GK, Davis L. An experimental study of the functions of the frontal lobes in man. Psychosomatic Medicine. 1945;7:97–107. [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1982;17 doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]