Abstract
Spinocerebellar Ataxia Type 1 (SCA1) is an incurable, dominantly inherited neurodegenerative disease of the cerebellum caused by a polyglutamine-repeat expansion in the protein ATXN1. While analysis of human autopsy material indicates significant glial pathology in SCA1, previous research has focused on characterizing neuronal dysfunction. In this study, we characterized astrocytic and microglial response in SCA1 using a comprehensive array of mouse models. We have discovered that astrocytes and microglia are activated very early in SCA1 pathogenesis even when mutant ATXN1 expression was limited to Purkinje neurons. Glial activation occurred in the absence of neuronal death, suggesting that glial activation results from signals emanating from dysfunctional neurons. Finally, in all different models examined glial activation closely correlated with disease progression, supporting the development of glial-based biomarkers to follow disease progression.
Keywords: Spinocerebellar ataxia type 1, SCA1, neurodegeneration, glia, astrocytes, microglia
Introduction
Spinocerebellar ataxia type 1 (SCA1) is an autosomal dominant neurodegenerative disorder that belongs to the family of trinucleotide repeat disorders, including Huntington's disease, Spinobulbar Muscular Atrophy (SBMA), Dentatorubral-pallidoluysian atrophy (DRPLA), and other Spinocerebellar Ataxias (SCA 2,3,6,7 and 17) (Orr and Zoghbi (2007). These disorders affect the nervous system, and are characterized by a CAG nucleotide expansion in the culprit gene that, in turn, produces a pathogenic polyglutamine expansion in the encoded protein. In SCA1, the expansion occurs in ATXN1 (ataxin-1), protein that is ubiquitously expressed in the brain; however SCA1 predominantly affects the cerebellum and brain stem. While it is still unclear what defines the susceptibility of neurons in certain brain regions to the toxic effects of mutant proteins, the response of cells surrounding the neurons may play a role. Previous studies focused on describing neuronal pathology in SCA1 due to their pronounced dysfunction and degeneration. Glial cells have been largely overlooked, despite reports of gliosis in autopsy samples of human patients (Genis et al., 1995; Gilman et al., 1996; Nino et al., 1980). Moreover, glial cells, astrocytes and microglia in particular, exert many complex functions crucial for brain homeostasis as evidenced by the observations that they (1) produce trophic factors to promote neuronal survival (2) help sculpt network connections by eliminating or promoting the maturation of synapses. In addition astrocytes (3) regulate blood flow and homeostatic balance of ions in the neurovascular niche (4) are the principal cells that reuptake released neurotransmitters, and (5) participate even more directly in neuronal signaling by releasing signaling chemicals —such as glutamate, ATP, GABA and D-Serine (Araque et al., 2014; Colangelo et al., 2014; Kettenmann H, 2013; Parkhurst CN, 2013).
In a whole range of neurological diseases such as Amyotrophic Lateral Sclerosis (ALS), Alzheimer's disease (AD), and Parkinson's disease(PD), astrocytes and microglia are activated undergoing morphological and functional changes, including release of pro-inflammatory cytokines, many of which could have a negative impact on neuronal survival (Glass et al., 2010). It is in this context that understanding glial activation has become an important avenue of study in neurology.
SCA1 is particularly well placed to shed light on this issue because of the existence of several robust mouse models. In addition to the transgenic and knock-in mouse models of mutant ATXN1 that closely recapitulate aspects of the human disease (Burright et al., 1995; Watase et al., 2002), there are also transgenic models that point to the crucial role of a phosphorylation site—serine 776— in pathogenesis. The most intriguing of these models are the ATXN1 [82Q]-A776 line, where replacing this serine with an alanine residue prevents toxicity of expanded ATXN1 (Emamian et al., 2003); and the ATXN1 [30Q]-D776 line, where replacing the same serine residue with a phosphomimetic aspartate residue causes features of SCA1 to occur even in the absence of a pathogenic polyglutamine tract (Duvick et al., 2010; Emamian et al., 2003). Finally, there are excellent conditional SCA1 models where mutant ATXN1 is under the control of a doxycycline (DOX) responsive promoter that can be turned on or off at will, thus influencing the disease phenotype (Ebner et al., 2013). Using these mouse models, we demonstrate that glial pathology in SCA1 closely correlates with disease onset and severity and that it is induced by neuronal dysfunction and not neuronal death.
Results
SCA1 knock-in mice have increased glial activation early in pathology
We began our study by examining glial activation in the SCA1 knock-in mice (Sca1154Q/2Q). In this mouse line— as would be the case in human patients— a single copy of the mutant version of ATXN1 with an expanded allele is expressed from the endogenous Atxn1 locus. These mice demonstrate both behavioral and pathological hallmark features of the disease: adult onset ataxia along with brainstem degeneration, accompanied by the typical Purkinje cell dendritic thinning and eventual loss of Purkinje neurons (Watase et al., 2002) (Cvetanovic et al., 2011).
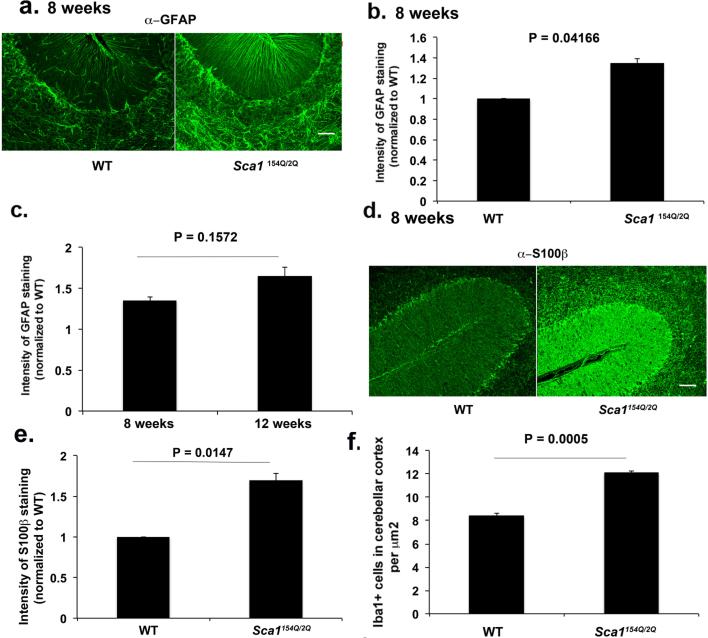
We reasoned that neuronal-glial interaction would be best studied by focusing on Bergmann glia (BG) a sub-type of cerebellar astrocytes that reside next to Purkinje neurons. Indeed, the radial processes of BG entwine the dendrites and synapses of Purkinje neurons to form one of most intimate neuron-astrocyte interactions in the mammalian central nervous system (Bellamy, 2006; Yamada et al., 2000). Since gliosis is characterized by hypertrophy of astrocytes accompanied by an increased expression of Glial Fibrillary Acidic Protein (GFAP), we performed immunofluorescence against GFAP (Pekny et al., 2014). We observed a significant increase in GFAP staining and hypertrophy of astrocytic processes in BG in Sca1154Q/2Q mice seen as early as 8 weeks. Note that this corresponds to the presymptomatic period, since in our hands these mice show the first subtle signs of ataxia no earlier than 12 weeks of age (Cvetanovic et al., 2011) (Figure 1 a-b, 135% over wild-type levels, n = 4 pairs of mice, Student's t test, P = 0.0041). GFAP staining shows trend for increase with disease progression (GFAP levels are 165% of wild type levels at 12 weeks and 135% of wild-type levels at 8 weeks, Figure 1c, n = 4 pairs of mice at each time point). We confirmed these findings by performing immunofluorescence for the calcium binding protein S100B, another astrocytic marker upregulated during gliosis (Sathe et al., 2012) (Figure 1 d-e, 165% over wild type levels at 8 weeks of age, n = 4 pairs of mice, Student's t test, P = 0.0147).
Figure 1. Glial activation is detectable early in Sca1154Q/2Q mice.
Cerebellar sections from 8 and 12 weeks old Sca1154Q/2Q mice and wild-type littermates were stained with GFAP, S100β and Iba1 antibodies. a and d Representative images of GFAP (a) and S100β (d) staining in cerebellar cortex. b, c and e Intensity of GFAP (a and c, N = 4) and S100β (e, N = 4) staining f Number of Iba1 positive (Iba1+) cells ( N= 5) in the cerebellar cortex. Student's t-test P values are shown with all the histograms. Error bars represent SEM. Scale bar = 90 μm
We then turned our attention to microglia, another glial sub-type that represent resident brain macrophages, and plays an important role in responding to brain injury (Wyss-Coray T, 2002). We quantified microglial response in the cerebellum by staining for the microglia specific ionized calcium-binding adapter molecule 1 (Iba1). We observed a significant increase in the number of the microglia in the cerebellar cortex of pre-symptomatic, 8 weeks old Sca1 154Q/2Q mice compared to their wild-type littermates (Figure 1f, 8.43 in wild-type vs 12.104 in Sca1 154Q/2Q mice, n = 5 pairs of mice, Student's t test, P = 0.0005). Thus, much like BG, microglia are activated early in the disease process in Sca1154Q/2Q mice.
As SCA1 is known to affect several other brain regions with varying degrees of neuronal pathology, we examined whether there is a correlation between spatial pattern of gliosis and regional vulnerability. We assessed glial activation in the cerebellar white matter that has milder pathology compared to cerebellar cortex, and the hippocampal dentate gyrus, with no reports of significant SCA1 pathology (Udo Rüba, 2013; Watase et al., 2002). We detected activated microglia and astrocytes in the cerebellar white matter but not in the hippocampus of 8 weeks old Sca1154Q/2Q mice (data not shown, n ≥ 3 pairs of mice. Student's t test for GFAP P = 0.0496 and P = 0.1582, and for Iba1 P = 0.0163 and P = 0.9322 for cerebellum and hippocampus respectively). Since the former is the region associated with neuronal pathology while the latter is not, this observation is consistent with the notion that glial activation could define the extent of neurodegeneration (Vinet J, 2012).
Glial activation occurs early in transgenic ATXN1[82Q] mice where mutant ATXN1 is expressed only in Purkinje neurons
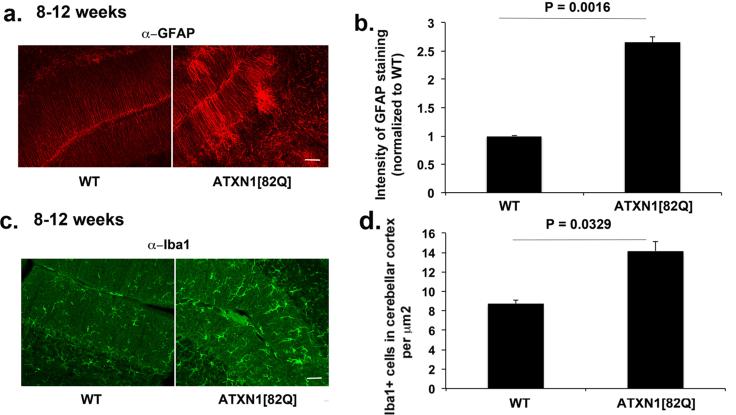
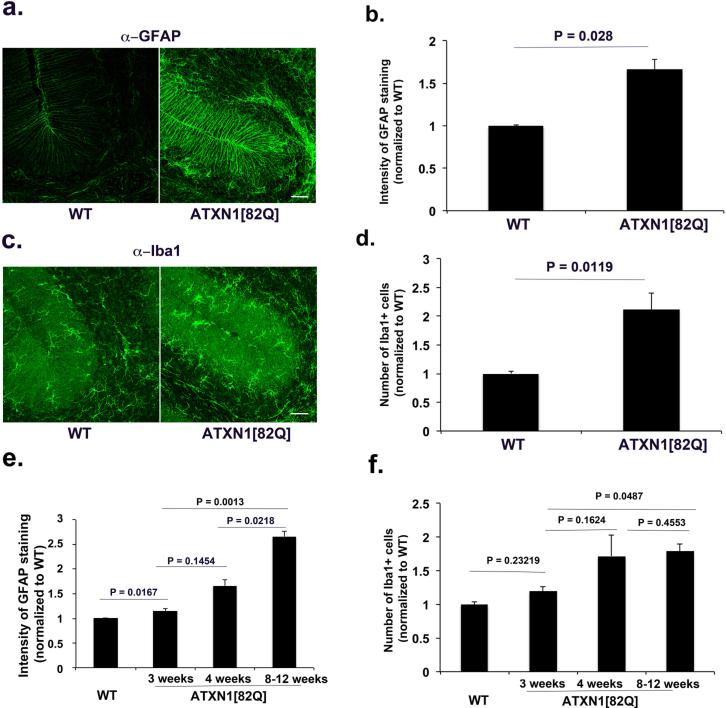
In Sca1154Q/2Q mice, the endogenous Atxn1 promoter drives mutant ATXN1 expression. Thus in these mice, mutant ATXN1 is expressed in all cells where wild type ATXN1 is expressed, including glial cells (Shiwaku et al., 2010). Glial activation could therefore be the consequence of mutant ATXN1 pathogenic effect within glia, similar to that seen in SCA7. Yet given the close interaction of neurons and glia in the cerebellum it is also conceivable that glial activation could occur solely in response to neuronal dysfunction or death. To test for this latter possibility, we examined glial activation in another model of SCA1: ATXN1[82Q] mice (also known as the BO5 line) (Burright et al., 1995). In this model, mutant ATXN1 contains 82 glutamine repeats and is expressed only in Purkinje neurons under the control of the Purkinje cell- specific Pcp2 promoter. Glial activation in this line, if it were to be seen, would suggest that in SCA1 glial ATXN1 expression is not required for their activation. As shown in Figure 2, we observed increased GFAP staining in BG of 8-12 weeks old mice (266 % over the GFAP levels in wild-type littermates, n = 6 for both genotypes, Student's t test, P = 0.0016, Figure 2 a-b). Similarly, staining with the Iba1 antibody also detected an increase in the number of microglia in the cerebellar cortex of 8-12 weeks old ATXN1[82Q] mice (160% over the Iba1+ cells in the wild-type littermates, n = 3, Student's t test, P = 0.0329, Figure 2 c-d). These data demonstrate that ATXN1[82Q] expression in neurons is sufficient to cause glial activation in SCA1. Moreover, given that there is a very limited loss of Purkinje neurons at this age (~8%;(Clark et al., 1997)), our results also suggest that it is neuronal dysfunction, and not necessarily neuronal death, that triggers glial activation. To confirm that neuronal death is not the triggering event, we tested for glial activation at four weeks of age, well before any neuronal loss occurs in ATXN1[82Q] mice that begins to occur by 12 weeks (Clark et al., 1997) and before the first signs of ataxia at 6 weeks of age (Duvick et al., 2010). As shown in Figure 3, we observed both Bergmann Glia (166 % of wild-type levels, n = 7, Student's t test, P = 0.0028, Figure 3 a-b) and microglial activation even at this early time point (177% of wild-type levels, n = 5, Student's t test, P = 0.0119, Figure 3 c-d). These results suggest that signals from dysfunctional Purkinje neurons cause glial activation early in SCA1.
Figure 2. Glial activation occurs in non-cell autonomous manner in transgenic ATXN1[82Q] mice.
Cerebellar sections from 8-12 weeks old ATXN1[82Q] mice and wild-type littermates were stained with GFAP and Iba1 antibodies. a and c Representative images of GFAP and Iba1 staining in cerebellar cortex. b and d Intensity of GFAP staining (b, N = 6) and number of Iba1+ cells (d, N = 3) in the cerebellar cortex. Student's t-test P values are shown with all the histograms. Error bars represent SEM. Scale bar = 45 μm.
Figure 3. Glial activation occurs early in transgenic ATXN1[82Q] mice before behavioral and cerebellar tissue pathology.
Cerebellar sections from four weeks old ATXN1[82Q] and wild-type littermates mice were stained with GFAP and Iba1 antibodies. a and c Representative images of GFAP (a) and Iba1 (c) staining in cerebellar cortex. b and e Intensity of GFAP staining in 4 week old mice (b, N = 7); e time course; d and f Number of Iba1 + cells (4 week old mice; N=5) f time course. Student's t-test P values are shown with all the histograms. Error bars represent SEM. Scale bar = 45 μm
To further examine the time-line of glial activation in response to transgene expression we performed immunofluorescence studies at three weeks, approximately one week after ATXN1 expression starts in ATXN1[82Q] mice (Ebner et al., 2013). We detected a statistically higher GFAP levels even at this early age that continued to increase with disease progression (Figure 3e, 115% of wild-type levels, n = 3 pairs, p= 0.0167 comparing 3 weeks old wild type and ATXN1[82Q] mice; P = 0.0013 comparing 3 weeks old to 8-12 weeks old ATXN1[82Q] mice). Microglial numbers in the cerebellar cortex of three weeks old ATXN1[82Q] mice showed a trend toward increased numbers (without reaching statistical significance) (Figure 3f, 119% of wild-type levels n = 4 pairs, P = 0.23219 compared to wild-type littermates). These experiments suggest that glial activation occurs in the narrow window of time between two and four weeks in ATXN1[82Q] mice.
Increase in the production of pro-inflammatory cytokines in the cerebella of SCA1 mice
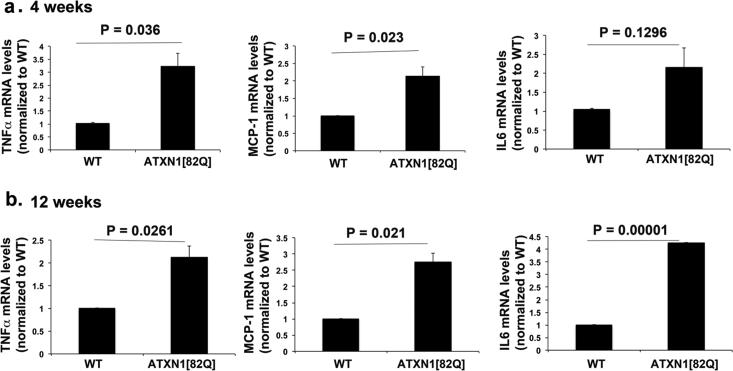
Activated astrocytes and microglia are capable of secreting numerous pro-inflammatory cytokines. Indeed, some of these inflammatory mediators such as Tumor Necrosis Factor Alpha (TNFα), Interleukin-6 (IL6) and monocyte chemoattractant protein-1 (MCP-1) have been associated with neurodegenerative diseases and may have harmful effects on neuron survival or function (Akassoglou et al., 1997; Campbell IL, 1993; Glass et al., 2010; Mosher and Wyss-Coray, 2014)(Bjorkqvist et al., 2008). Quantitative RT-PCR of cerebellar mRNA extracts from 12 weeks old transgenic ATXN1[82Q] mice showed increased levels of all three cytokines (specifically values were TNFα 2.1, MCP-1 2.76 and IL6 4.46 fold increase over wild-levels); increase in TNFα and MCP-1 levels was detectable even earlier, at four weeks of age (3.22 and 2.15 fold over wild-type levels for TNFα and MCP-1 respectively, while IL6 levels showed trend for increase) (n ≥ 5 pairs of mice of each genotype were used for each age, Student's t test P values are shown with each histogram, Figure 4 a-b). Similarly in 8 weeks old Sca1154Q/2Q mice RNA levels of TNFα and MCP-1 were significantly increased while IL6 levels showed trend for increase (data not shown, n ≥ 4 pairs, MCP1 2.28 fold increase over wild type levels, p = 0.002, IL6 2.03 fold increase over wild type levels, p = 0.071, and TNFα 2.3 fold increase over wild type levels, p = 0.0235).
Figure 4. SCA1 mice show increased levels of pro-inflammatory mediators TNFα, MCP-1 and IL6.
TNFα, MCP-1 and IL6 were quantified using RT qPCR of RNA isolated from the cerebella of 4 (a) and 12 (b) weeks old ATXN1[82Q] mice and their wild-type littermates. N ≥ 5 mice of each genotype were used for both ages. Error bars represent SEM. Student's t-test P values are shown with all the histograms.
Glial activation correlates to disease severity
Our data shows that neuronal dysfunction is sufficient to trigger glial activation. Early activation of astrocytes and microglia as well as its spatial pattern may suggest that glial activation plays a role in SCA1 pathology. This prompted us to correlate more closely the degree of neuronal dysfunction and disease severity with glial activation. For these experiments, we took advantage of the finding that a phosphorylation site, serine 776, is important for pathogenesis. If this site is mutated to an alanine, then transgenic mice that express an otherwise pathogenic polyglutamine tract —ATXN1[82Q]-S776A —fail to display the disease phenotype (Duvick et al., 2010; Emamian et al., 2003). On the other hand, mice generated with a phosphomimetic aspartate residue replacing serine 776 in ATXN1 with non-pathogenic number of glutamine repeats, ATXN1[30Q]-D776 mice display a robust ataxic phenotype starting from 6 weeks of age but do not progress to Purkinje cell loss (Duvick et al., 2010).
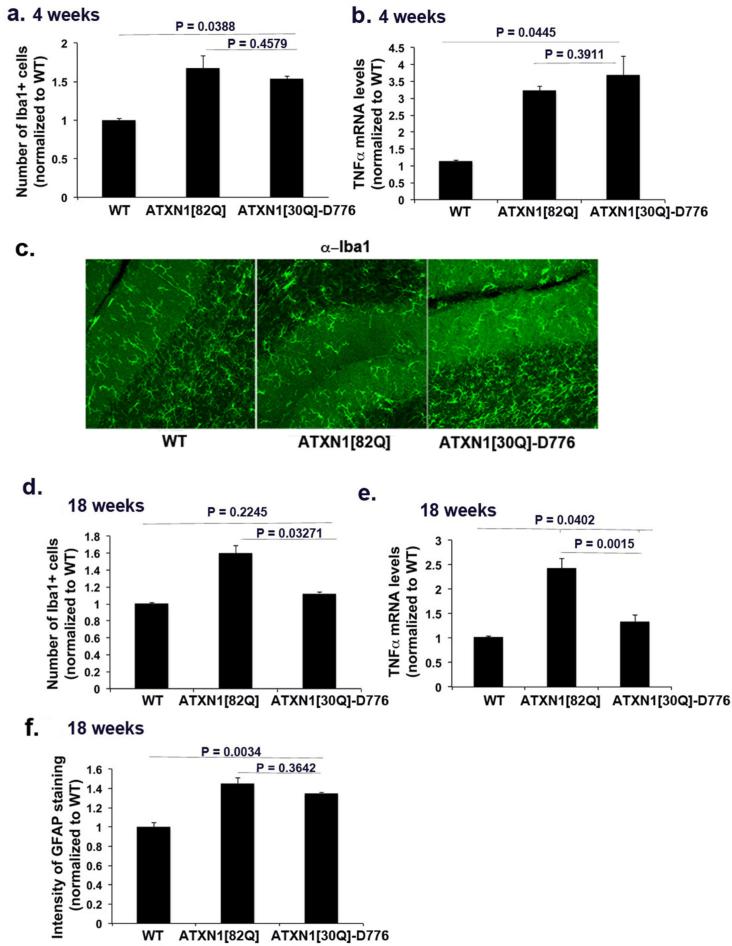
We found that expression of non-pathogenic ATXN1[84Q]-S776A in Purkinje neurons does not initiate glial activation or inflammation ( n= 4, Student's t test, P = 0.1387, P = 0.0562, P = 0.1354 for GFAP, Iba1 and TNFα respectively, data not shown). On the other hand, we observed a significant increase in microglial numbers, and an increase in the levels of TNFα in four week old ATXN1[30Q]-D776 mice (n ≥ 5, Student's t test, P = 0.0388, P = 0.0445 comparing ATXN1[30Q]-D776 to wild-type for Iba1 (179% of wild-type levels), and TNFα (350% of wild-type levels) respectively, Figure 5 a-c). These results further support the correlation between disease initiation, neuronal dysfunction and triggering of glial activation. Interestingly, in the older 18 weeks old ATXN1[30Q]-D776 mice, BG GFAP levels remain increased, but microglial numbers and secreted TNFα decrease ( n ≥ 5, Student's t test, P = 0.0327, P = 0.0015 and P = 0.3642, comparing ATXN1[30Q]-D776 to ATXN1[82Q] for Iba1 (112.5 % of wild-type), TNFα (115 % of wild-type) and GFAP (130% of wild-type) respectively Figure 5 d-f). Recall that in these mice, neuronal dysfunction does not proceed to Purkinje cell loss (Duvick et al., 2010). These data underscore the connection between microglial activation, inflammation, and neuronal death.
Figure 5. Microglial activation correlates with loss of Purkinje neurons in SCA1.
Cerebellar sections from 4 or 18 weeks old wild-type (WT), ATXN1[82Q], and ATXN1[30Q]-D776 mice were stained for Iba1 (c representative image) and GFAP. a and d Number of Iba1+ microglia in cerebellar cortex at 4 weeks (a) and 18 weeks (d). f GFAP intensity at 18 weeks. b and e Quantitative RT qPCR was used to determine relative levels of TNFα in 4 (b) or 18 weeks old (e) wild-type (WT), ATXN1[82Q], and ATXN1[30Q]-D776 cerebella. N ≥ 5 mice were used for all experiments. Student's t-test P values are shown with all the histograms. Error bars represent SEM.
Glial activation in reversible SCA1 mouse models
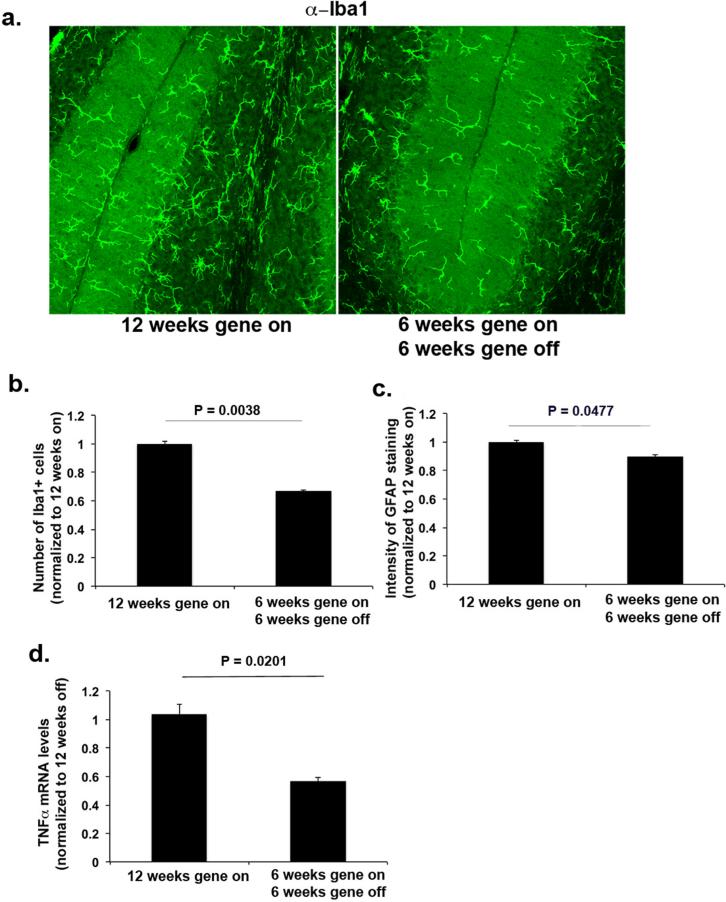
Recently, a conditional transgenic line cATXN1[30Q]-D776 was generated where the expression of the phosphomimetic pathogenic ATXN1 —ATXN1 [30Q] D776— can be turned off by administering the tetracycline derivative doxycycline (DOX) (Ebner et al., 2013). In cATXN1 [30Q]-D776 mice where the transgene was expressed for the first 6 weeks of life and turned off for another 6 weeks, a full recovery of motor behavior—as evidenced by their ability to stay on the accelerating rotarod—was attained (Ingram, unpublished data). Control mice were not administered doxycycline (12 weeks gene-on group) and expressed pathogenic ATXN1 until they were euthanized at 12 weeks of age. We tested whether this observed improvement in phenotype was associated with a decrease in glial activation. As shown in Figure 6, there was a statistically significant decrease in the number of Iba1+ microglia in the cerebellar cortex (66% of untreated), in the GFAP intensity (85% of untreated, and in the pro-inflammatory cytokine TNFα (58% of untreated) in 6 week gene on/6 week gene off cATXN1[30Q]-D776, compared to 12 week gene on cATXN1[30Q]-D776 mice that expressed pathogenic ATXN1 (Student's t test, P = 0.0038, P = 0.0477 and P = 0.0201, comparing treated (n=6) to untreated (n=3) mice, for Iba-1, GFAP and TNFα respectively, Figure 6 a-d). These data further emphasize the relationship between glial activation and disease pathogenesis, and suggest that chronic glial activation may be arrested or reversed.
Figure 6. Glial activation is reduced by modulating ATXN1 expression.
ATXN1 expression was turned off in the conditional cATXN1[30Q] -D776 mice at 6 weeks of age. Cerebellar sections from 12 weeks old untreated mice (N = 3, 12 weeks on –continuous expression of mutant ATXN1) or treated mice (N = 6, 6 weeks gene on, and 6 weeks gene off) were stained for Iba1 (a representative image) and GFAP. b Number of Iba1+ microglia in cerebellar cortex c GFAP intensity. d. mRNA levels of TNFα in the cerebella of untreated or DOX treated cATXN1[30Q]-D776 mice. Student's t-test P values are shown with all the histograms. Error bars represent SEM.
Discussion
Glial cells play an important role in maintaining neuronal homeostasis (Block et al., 2005). Astrocytes not only support the metabolic needs of neurons, but also help regulate the ionic milieu needed for optimal neuronal functioning and neurotransmitter levels, particularly in the synaptic clefts (Maragakis NJ, 2006). Microglia on the other hand play key roles in shaping brain connections and plasticity, in removing cellular debris and in modulating inflammatory responses (Block et al., 2005). The importance of glia to neuronal functioning is especially revealing in the studies in which glia pathology, such as caused by expression of mutant ataxin-7 in Bergmann glia only, causes neuronal pathology (Custer SK, 2006). Thus glial pathology or change in their function, if present, can contribute to neurodegenerative disease. Indeed glial activation has been described in almost every neurodegenerative disease including Alzheimer disease (Guillot-Sestier and Town, 2013), Parkinson's disease (L'Episcopo et al., 2013), amyotrophic lateral sclerosis (ALS) (Papadimitriou et al., 2010), frontotemporal dementia, multiple-system atrophy (Ahmed et al., 2012), prion disease (Riemer et al., 2009), Huntington disease (Shin et al., 2005), and Spinocerebellar Ataxia 7 (SCA7) (Furrer et al., 2011). However, a full understanding of the role of glial activation in neurodegeneration has been difficult to achieve.
The spectrum of mouse models developed for SCA1 makes it a particularly attractive disease to tackle important questions regarding glial activation. Although, Bergmann cell gliosis has been described in a transgenic ATXN1 model in previous study (Giovannoni R, 2007), a comprehensive study evaluating the spatio-temporal features of gliosis and their relationship to disease severity and progression has been lacking. In addressing this shortcoming, we have made several important observations: First, we demonstrate that glial activation occurs early in disease, well before the neuronal death or the onset of behavioral symptoms of ataxia. Second, we establish that glial activation occurs specifically in the most severely affected regions of the brain, indicating that glial activation correlates with disease pathology in spatio-temporal pattern and could thus play a role in defining specific neuronal vulnerability in this disease. Third, we demonstrate that mutant ATXN1 expression in microglia and astrocytes is not required for their activation. This finding is similar to the previous studies including the ones performed in the mouse models of Alzheimer's, Parkinson's disease and SCA7 that have also shown that neuronal dysfunction is sufficient to stimulate glial activation (Furrer et al., 2011 Zhang W 2005, Huang X 2012). However in other studies, including Huntington's disease (HD), ALS and a different SCA7 model, expression of mutant protein in astrocytes and/or microglia was shown to contribute to disease pathology (Boillee et al., 2006; Crotti A, 2014; Furrer et al., 2011; Yamanaka et al., 2008).
Fourth, we have mapped glial activation to a narrow temporal window and demonstrated that neuronal death is not required for glial activation in SCA1 (significant neuronal death has been described in 40 weeks-of –age and older Sca1154Q/2Q and ATXN1[82Q] mice (Watase K, 2002) (Duvick et al., 2010). We suspect that signals emanating from Purkinje neurons activate glia. Gliosis is controlled by a tightly orchestrated balance of activating signals, for example through Pattern recognition receptors (PRR), and inhibitory signals through Neuroimmune regulators (NIRs), such as CD200 and fractalkine. PRRs in particular have been shown to react to danger-associated molecular patterns (DAMPs) such as ATP, DNA and RNA molecules released from dying cells (Burda JE, 2014). However because glial activation occurs before the onset of neuronal death we believe that decreased signaling from the NIRs or unknown signals may play an important role in glial activation in SCA1.
Fifth, we show that the microglial activation correlates with neuronal loss. One can envisage a scenario where non-cell autonomous activation of glia rapidly exacerbates neuronal dysfunction that would then cause a further amplification of glial activation in a vicious, feed-forward cycle. Our studies suggest that pro-inflammatory cytokines released by activated microglia could be relevant, given that all the cytokines that we have studied here—TNFα, IL6 and MCP-1—are known to have neurotoxic effects (Glass CK, 2010). Elevated levels of cytokines and chemokines have been detected in Huntington's patients before the onset of disease (Bjorkqvist et al., 2008). Interestingly, overexpression of both IL6 and TNFα in mouse brains (using GFAP promoter) causes predominantly cerebellar phenotype with inflammation, degeneration and ataxia. (Akassoglou et al., 1997; Campbell et al., 1993). To complicate matters further, glia produce other factors such as BDNF that could have a beneficial effect in the context of neurodegeneration. Distinguishing those influences and their respective triggers is crucial for full understanding of the diseased CNS. Future studies that will manipulate different aspects of glial activation pharmacologically or genetically will point to their respective roles in disease pathology.
Lastly, we demonstrate that glial activation dynamically responds to neuronal dysfunction and can be arrested or reversed when mutant ATXN1 expression is prevented in neurons. Clearly, the most direct way to address the contribution of glial specific expression of mutant ataxin-1 to pathogenesis would be to express mutant ATXN1 only in glia and to test for pathogenicity. Alternatively one could delete the expression of ATXN1 in the glia while expressing it in neurons to test whether suppressing glial expression ameliorates the phenotype. Nonetheless our findings, taken together, suggest that glial-neuronal interactions are likely to be as complex in disease as they are in health. From a treatment perspective, we demonstrated that turning off the expression of toxic ATXN1 in neurons, after the initiation of disease reduces both microglial and astrocytic activation in the conditional mouse model. Interestingly in ATXN1[30Q]-D776 mice the decrease in microglial activation is not accompanied by a significant reduction in astrocytic activation. Important distinction between these models is that in the conditional mice there is complete reversal of the phenotype (both of neuronal dysfunction and loss) while in ATXN1[30Q]-D776 mice there is no cell death but neurons are still dysfunctional. One interpretation is that signals that maintain astrocytic vs microglial activation are differently affected in these mice, e.g., neuronal dysfunction in ATXN1[30Q]-D776 mice is sufficient to maintain astrocytic but not microglial activation. This could also suggest that astrocytes are more tuned to neuronal well-being than microglia.
Finally, there is a growing need for biomarkers for monitoring disease progression. Recent functional Magnetic Resonance Spectroscopy (MRS) findings have showed that increased levels of myo-inositol —a glial protein suggested to indicate gliosis- (Oz G, 2010; Oz et al., 2011) correlate well with disease progression. Our results provide further support for the presence of increasing glial activation in SCA1 and could serve as an impetus for developing glial based biomarkers, including myo-inositol (with MRS) and inflammatory mediators (cytokines and S100B providing that they are increased in blood serum as they are in the brain) that could serve as indicators of disease progression and severity.
Material and Methods
Mouse lines
The generation of all the mice in this study have been previously described (Burright et al., 1995; Duvick et al., 2010; Ebner et al., 2013; Emamian et al., 2003; Watase et al., 2002). The transgenic lines where relevant ATXN1 constructs are driven by the Purkinje cell specific (Pcp2) promoter were generated in a FVB/N background and include: (1) the ATXN1[82Q] line (Burright et al., 1995) (also called the B05 line) (2) the ATXN1[82Q]-A776 line (Emamian et al., 2003), (3) the ATXN1[30Q]-D776 line (Duvick et al., 2010), and (4) the conditional ATXN1 line [cATXN1 [30Q]-D776] (Ebner et al., 2013). The Sca1154Q/2Q knock-in line was generated by replacing the mouse ATXN1 with an extended polyglutamine tract in the ATXN1 locus. Originally generated in the C57BL/6J–129/SvEv mixed background (Watase et al., 2002), these mice have been backcrossed into a pure C57BL/6J background. Genotyping was performed by PCR on DNA derived from either tail or ear snips (Cvetanovic et al., 2011; Ebner et al., 2013). The cATXN1[30Q]-D776 mice were allowed to express the transgene for the first 6 weeks of their life. From that point onwards, mice were either treated with DOX (doxycycline) for six weeks (delivered in a sucrose solution) to stop the expression of the transgene (6 weeks on; 6 weeks off group); or were treated with sucrose solution alone to allow for the continued expression of the transgene (12 weeks on group). For further details see (Ebner et al., 2013).
Animal experimentation was approved by the relevant Institutional Animal Care and Use Committees (University of Minnesota, and Northwestern University), and was conducted in accordance with the National Institutes of Health's Principles of Laboratory Animal Care (86–23, revised 1985) and the American Physiological Society's Guiding Principles in the Use of Animals.
Immunohistochemistry
Mouse brains were fixed overnight in 4% paraformaldehyde, incubated in 30% sucrose and then sectioned by cryostat (Leica, CM 1850) into 40 μm sections. The sections were washed in cold Phosphate Buffered Saline (PBS) and permeabilized with PBST (1 % Triton X-100 in 1X PBS) for 10 min at room temperature. They were then processed for immunohistochemistry by incubation for 1 hr in blocking buffer (3% Normal Donkey Serum in PBST), followed by an overnight incubation at 4°C in blocking buffer containing the relevant primary antibody [anti-GFAP or S100B antibodies (Z0334 and Z0311 respectively, DAKO), and anti-Iba1 (019-19741, WAKO)]. Samples were then washed 3 to 5 times for 15 min in PBST and incubated overnight at 4°C with the relevant fluorescently labeled secondary antibody, before being mounted on slides with Vectashield mounting media containing DAPI (Vector Laboratories) for observation under the microscope (confocal UV LSM 500 Zeiss or Olympus FV1000) while blinded to the genotype. For statistical purposes, we studied at least six different Z-stacks of 25 μm from each mouse of the appropriate experimental genotype, and at each age examined (3, 4, 8-12 and 20 weeks) we have used at least n =3 littermates of each genotype. Briefly, we drew at least two rectangles per section in the cerebellar cortex and white matter or in the hippocampal dentate gyrus. The size of the rectangles varied from 4000-16,000 μm2 depending on the size of the available area in the image. For semi-quantitative measure of GFAP intensity, we used the “Measure” function in ImageJ to estimate the average intensity that was then normalized to the average intensity in the control group (wild-type mice). To quantify the number of microglia, we counted all the microglia present in the drawn area and divided that number with the surface area of rectangles to obtain the number of microglia per μm2. Once again this was normalized to the average number of microglia per μm2 in the control group (wild-type mice). All quantification was performed using ImageJ software. Data was analyzed using Student's t-test.
Quantitative RT-PCR
Quantitative reverse-transcriptase PCR (qRT-PCR) was performed as previously described (Cvetanovic et al., 2007)(Cvetanovic et al., 2012). The template used for qRT-PCR was cerebellar RNA isolated from experimental mice. Contaminating DNA was removed using the TURBO DNA free kit (Life technologies). 1μg of total RNA was subjected to first strand cDNA synthesis in duplicate (Superscript III First strand kit; Life technologies). Quantitative PCR was then performed on 1μl of cDNA employing IDT qPCR primers: TNFα : Mm.PT. 51.12575861, IL6: Mm.PT.5111799101.g; MCP-1: forward: 5’-CCA CTC ACC TGC TAC TCA-3’/reverse: 5’-TGG TGA TCC TCT TGT AGC TCT CC-3’; and18S RNA: forward: 5’- AGT CCC TGC CCT TTG TAC ACA-3’/ reverse: 5’-CGA TCC GAG GGC CTC ACT A-3’. All PCR reactions were performed in triplicate using Light Cycler 480 SYBR Green I Master Mix (Roche) and the Light Cycler 480II instrument (Roche). Data was analyzed using 2−ΔΔct method (Cvetanovic et al., 2007): RNA levels were normalized to 18SRNA levels, and values from wild type littermates were used as a reference. We have used n 3 mice per genotype at all ages examined (4, 8 and 12 weeks).
Highlights.
Glial activation occurs early and precedes neuronal death in SCA1 animal models.
Glial activation correlates with SCA1 pathology in spatio-temporal manner.
Mutant ATXN1 expression in glia is not required for their activation.
Microglial activation and inflammation correlates with neuronal loss in SCA1.
Glial activation arrests/reverses after ending of neuronal ATXN1[82Q] expression.
ACKNOWLEDGMENTS
This research was supported by startup funds for M.C. from the Institute for the Translational Neuroscience and Minnesota Medical Foundation, funding from the US National Institutes of Health training grant T3 (M.C.) with additional funding from the US National Institutes of Health (5RO1NSO045667 and 5R37NSO22920 to H. T. O. and 1R01 NS062051 and 1R01NS082351 to P. O.), the National Ataxia Foundation and the Brain Research Foundation (P.O.).
Abbreviations
- SCA1
Spinocerebellar Ataxia Type 1
- BG
Bergmann glia
- DOX
doxycycline
- ATXN1
ataxin-1
- GFAP
Glial Fibrilary Acidic Protein
- Iba1
ionized calcium-binding adapter molecule 1
- SEM
Standard Error of Mean
- TNFα
Tumor Necrosis Factor Alpha (TNFα)
- IL6
Interleukin-6
- MCP-1
monocyte chemoattractant protein-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Authors declare there is no conflict of interest.
References
- Ahmed Z, Asi YT, Sailer A, Lees AJ, Houlden H, Revesz T, Holton JL. The neuropathology, pathophysiology and genetics of multiple system atrophy. Neuropathol Appl Neurobiol. 2012;38:4–24. doi: 10.1111/j.1365-2990.2011.01234.x. [DOI] [PubMed] [Google Scholar]
- Akassoglou K, Probert L, Kontogeorgos G, Kollias G. Astrocyte-specific but not neuron-specific transmembrane TNF triggers inflammation and degeneration in the central nervous system of transgenic mice. J Immunol. 1997;158:438–445. [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron. 2014;81:728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy TC. Interactions between Purkinje neurones and Bergmann glia. Cerebellum. 2006;5:116–126. doi: 10.1080/14734220600724569. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist M, Wild EJ, Thiele J, Silvestroni A, Andre R, Lahiri N, Raibon E, Lee RV, Benn CL, Soulet D, et al. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington's disease. J Exp Med. 2008;205:1869–1877. doi: 10.1084/jem.20080178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block F, Dihne M, Loos M. Inflammation in areas of remote changes following focal brain lesion. Prog Neurobiol. 2005;75:342–365. doi: 10.1016/j.pneurobio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Bradford J, Shin JY, Roberts M, Wang CE, Sheng G, Li S, Li XJ. Mutant huntingtin in glial cells exacerbates neurological symptoms of Huntington disease mice. J Biol Chem. 2010;285:10653–10661. doi: 10.1074/jbc.M109.083287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda JE, S.M. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burright EN, Clark HB, Servadio A, Matilla T, Feddersen RM, Yunis WS, Duvick LA, Zoghbi HY, Orr HT. SCA1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell. 1995;82:937–948. doi: 10.1016/0092-8674(95)90273-2. [DOI] [PubMed] [Google Scholar]
- Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A. 1993;90:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IL, A.C., Masliah E, Kemper P, Inglis JD, Oldstone MB, Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A. 1993:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark HB, Burright EN, Yunis WS, Larson S, Wilcox C, Hartman B, Matilla A, Zoghbi HY, Orr HT. Purkinje cell expression of a mutant allele of SCA1 in transgenic mice leads to disparate effects on motor behaviors, followed by a progressive cerebellar dysfunction and histological alterations. J Neurosci. 1997;17:7385–7395. doi: 10.1523/JNEUROSCI.17-19-07385.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colangelo AM, Alberghina L, Papa M. Astrogliosis as a therapeutic target for neurodegenerative diseases. Neurosci Lett. 2014;565C:59–64. doi: 10.1016/j.neulet.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Crotti A,BC, Kerman BE, Gosselin D, Lagier-Tourenne C, Zuccato C, Cattaneo E, Gage FH, Cleveland DW, Glass CK. Mutant Huntingtin promotes autonomous microglia activation via myeloid lineage-determining factors. Nat Neurosci. 2014;17:513–521. doi: 10.1038/nn.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custer SK, G.G., Gill N, Rueb U, Libby RT, Schultz C, Guyenet SJ, Deller T, Westrum LE, Sopher BL, La Spada AR. Bergmann glia expression of polyglutamine-expanded ataxin-7 produces neurodegeneration by impairing glutamate transport. Nat Neurosci. 2006;9:1302–1311. doi: 10.1038/nn1750. [DOI] [PubMed] [Google Scholar]
- Cvetanovic M, Patel JM, Marti HH, Kini AR, Opal P. Vascular endothelial growth factor ameliorates the ataxic phenotype in a mouse model of spinocerebellar ataxia type 1. Nat Med. 2011;17:1445–1447. doi: 10.1038/nm.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvetanovic M, Rooney RJ, Garcia JJ, Toporovskaya N, Zoghbi HY, Opal P. The role of LANP and ataxin 1 in E4F-mediated transcriptional repression. EMBO Rep. 2007;8:671–677. doi: 10.1038/sj.embor.7400983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvick L, Barnes J, Ebner B, Agrawal S, Andresen M, Lim J, Giesler GJ, Zoghbi HY, Orr HT. SCA1-like disease in mice expressing wild-type ataxin-1 with a serine to aspartic acid replacement at residue 776. Neuron. 2010;67:929–935. doi: 10.1016/j.neuron.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner BA, Ingram MA, Barnes JA, Duvick LA, Frisch JL, Clark HB, Zoghbi HY, Ebner TJ, Orr HT. Purkinje cell ataxin-1 modulates climbing fiber synaptic input in developing and adult mouse cerebellum. J Neurosci. 2013;33:5806–5820. doi: 10.1523/JNEUROSCI.6311-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emamian ES, Kaytor MD, Duvick LA, Zu T, Tousey SK, Zoghbi HY, Clark HB, Orr HT. Serine 776 of ataxin-1 is critical for polyglutamine-induced disease in SCA1 transgenic mice. Neuron. 2003;38:375–387. doi: 10.1016/s0896-6273(03)00258-7. [DOI] [PubMed] [Google Scholar]
- Furrer SA, Mohanachandran MS, Waldherr SM, Chang C, Damian VA, Sopher BL, Garden GA, La Spada AR. Spinocerebellar ataxia type 7 cerebellar disease requires the coordinated action of mutant ataxin-7 in neurons and glia, and displays non-cell-autonomous bergmann glia degeneration. J Neurosci. 2011;31:16269–16278. doi: 10.1523/JNEUROSCI.4000-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genis D, Matilla T, Volpini V, Rosell J, Davalos A, Ferrer I, Molins A, Estivill X. Clinical, neuropathologic, and genetic studies of a large spinocerebellar ataxia type 1 (SCA1) kindred: (CAG)n expansion and early premonitory signs and symptoms. Neurology. 1995;45:24–30. doi: 10.1212/wnl.45.1.24. [DOI] [PubMed] [Google Scholar]
- Gilman S, Sima AA, Junck L, Kluin KJ, Koeppe RA, Lohman ME, Little R. Spinocerebellar ataxia type 1 with multiple system degeneration and glial cytoplasmic inclusions. Ann Neurol. 1996;39:241–255. doi: 10.1002/ana.410390214. [DOI] [PubMed] [Google Scholar]
- Giovannoni R, M.N., Rosaria Bianco M, Cavaliere C, Cirillo G, Lavitrano M, Papa M. Reactive astrocytosis and glial glutamate transporter clustering are early changes in a spinocerebellar ataxia type 1 transgenic mouse model. Neuron Glia Biol. 2007;3:335–351. doi: 10.1017/S1740925X08000185. [DOI] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, S.K., Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot-Sestier MV, Town T. Innate immunity in Alzheimer's disease: a complex affair. CNS Neurol Disord Drug Targets. 2013;12:593–607. doi: 10.2174/1871527311312050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, K.F., Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77:10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- L'Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Impagnatiello F, Pluchino S, Marchetti B. Aging-induced Nrf2-ARE pathway disruption in the subventricular zone drives neurogenic impairment in parkinsonian mice via PI3K-Wnt/beta-catenin dysregulation. J Neurosci. 2013;33:1462–1485. doi: 10.1523/JNEUROSCI.3206-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragakis NJ, R.J. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- Mosher KI, Wyss-Coray T. Microglial dysfunction in brain aging and Alzheimer's disease. Biochem Pharmacol. 2014;88:594–604. doi: 10.1016/j.bcp.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nino HE, Noreen HJ, Dubey DP, Resch JA, Namboodiri K, Elston RC, Yunis EJ. A family with hereditary ataxia: HLA typing. Neurology. 1980;30:12–20. doi: 10.1212/wnl.30.1.12. [DOI] [PubMed] [Google Scholar]
- Bugiani O. The many ways to frontotemporal degeneration and beyond. Neurol Sci. 2007;28:241–244. doi: 10.1007/s10072-007-0829-6. [DOI] [PubMed] [Google Scholar]
- Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- Oz G, H.D., Tkác I, Clark HB, Gross MD, Jiang H, Eberly LE, Bushara KO, Gomez CM. Neurochemical alterations in spinocerebellar ataxia type 1 and their correlations with clinical status. Mov Disord. 2010;25:1253–1261. doi: 10.1002/mds.23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz G, Vollmers ML, Nelson CD, Shanley R, Eberly LE, Orr HT, Clark HB. In vivo monitoring of recovery from neurodegeneration in conditional transgenic SCA1 mice. Exp Neurol. 2011;232:290–298. doi: 10.1016/j.expneurol.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimitriou D, Le Verche V, Jacquier A, Ikiz B, Przedborski S, Re DB. Inflammation in ALS and SMA: sorting out the good from the evil. Neurobiol Dis. 2010;37:493–502. doi: 10.1016/j.nbd.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst CN, Y.G., Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Wilhelmsson U, Pekna M. The dual role of astrocyte activation and reactive gliosis. Neurosci Lett. 2014;565C:30–38. doi: 10.1016/j.neulet.2013.12.071. [DOI] [PubMed] [Google Scholar]
- Riemer C, Gultner S, Heise I, Holtkamp N, Baier M. Neuroinflammation in prion diseases: concepts and targets for therapeutic intervention. CNS Neurol Disord Drug Targets. 2009;8:329–341. doi: 10.2174/187152709789542014. [DOI] [PubMed] [Google Scholar]
- Sathe K, Maetzler W, Lang JD, Mounsey RB, Fleckenstein C, Martin HL, Schulte C, Mustafa S, Synofzik M, Vukovic Z, et al. S100B is increased in Parkinson's disease and ablation protects against MPTP-induced toxicity through the RAGE and TNF-alpha pathway. Brain. 2012;135:3336–3347. doi: 10.1093/brain/aws250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JY, Fang ZH, Yu ZX, Wang CE, Li SH, Li XJ. Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J Cell Biol. 2005;171:1001–1012. doi: 10.1083/jcb.200508072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiwaku H, Yoshimura N, Tamura T, Sone M, Ogishima S, Watase K, Tagawa K, Okazawa H. Suppression of the novel ER protein Maxer by mutant ataxin-1 in Bergman glia contributes to non-cell-autonomous toxicity. Embo J. 2010;29:2446–2460. doi: 10.1038/emboj.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo Rüba B, Schölsc Ludger, Paulson Henry, Auburgere Georg, Kermerf Pawel, Jenh Joanna C., Seidela Kay, Korfa Horst-Werner, Dellerb Thomas. Clinical features, neurogenetics and neuropathology of the polyglutamine spinocerebellar ataxias type 1, 2, 3, 6 and 7. Progress in Neurobiology. 2013;104:38–66. doi: 10.1016/j.pneurobio.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Watase K, Weeber EJ, Xu B, Antalffy B, Yuva-Paylor L, Hashimoto K, Kano M, Atkinson R, Sun Y, Armstrong DL, et al. A Long CAG Repeat in the Mouse Sca1 Locus Replicates SCA1 Features and Reveals the Impact of Protein Solubility on Selective Neurodegeneration. Neuron. 2002;34:905–919. doi: 10.1016/s0896-6273(02)00733-x. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, M.L. Inflammation in neurodegenerative disease--a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Yamada K, Fukaya M, Shibata T, Kurihara H, Tanaka K, Inoue Y, Watanabe M. Dynamic transformation of Bergmann glial fibers proceeds in correlation with dendritic outgrowth and synapse formation of cerebellar Purkinje cells. J Comp Neurol. 2000;418:106–120. [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]