Abstract
Biomarker studies for early detection of acute kidney injury (AKI) have been limited by non-selective testing and uncertainties in using small changes in serum creatinine as a reference standard. Here we examine the ability of urine L-type fatty acid binding protein (L-FABP), neutrophil gelatinase-associated lipocalin (NGAL), Interleukin-18 (IL-18), and Kidney Injury Moledule-1 (KIM-1) to predict injury progression, dialysis, or death within 7 days in critically ill adults with early AKI. Of 152 patients with known baseline creatinine examined, 36 experienced the composite outcome. Urine L-FABP demonstrated an area under the receiver-operating characteristic curve (AUC-ROC) of 0.79 (95% confidence interval 0.70-0.86), which improved to 0.82 (95% confidence interval 0.75-0.90) when added to the clinical model (AUC-ROC of 0.74). Urine NGAL, IL-18, and KIM-1 had AUC-ROCs of 0.65, 0.64, and 0.62, respectively, but did not significantly improve discrimination of the clinical model. The category free net reclassification index improved with urine L-FABP [total net reclassification index for non-events 31.0%] and urine NGAL [total net reclassification index for events 33.3%]. However, only urine L-FABP significantly improved the integrated discriminative index. Thus, modest early changes in serum creatinine can help target biomarker measurement for determining prognosis with urine L-FABP providing independent and additive prognostic information when combined with clinical predictors.
Keywords: Biomarkers, Acute Kidney Injury, Prognosis
Introduction
Acute kidney injury (AKI) is a common complication of critical illness that strongly associates with morbidity, mortality, and long-term loss of kidney function.(1-3) Lack of effective treatments has prompted the development of novel biomarkers to provide complimentary information on the timing, nature, and prognosis of injury.(4, 5) To date, the evaluation of these markers has focused largely on the early detection of AKI.(6-11) Recent large cohort studies have reported only moderate performance,(12, 13) potentially reflecting uncertainties in using smaller changes in serum creatinine as a reference standard. In addition, non-selective testing within populations may also be limiting predictive performance.(14, 15)
We hypothesized that examining prediction of clinically meaningful endpoints in a high-risk population would enhance the value of a panel of novel injury biomarkers. We further hypothesized that leveraging early changes in serum creatinine to guide testing would improve predictive performance. To test these hypotheses, we examined the ability of uNGAL,(7) uL-FABP,(16) uIL-18,(17, 18) and uKIM-1(19, 20) to predict progression of injury, dialysis, and death within 7 days in a prospective cohort of critically ill adults with evidence of Kidney Disease Improving Global Outcomes (KDIGO) Stage 1 AKI at the time of biomarker measurement. We further examined the incremental ability of these biomarkers to predict outcomes beyond the available clinical predictors.
Results
Subject Characteristics
A total of 170 patients with a known outpatient baseline creatinine and Stage I AKI at the time of biomarker measurement were eligible for study. (Figure 1). Of these, 152 had complete biomarker data available. Thirty-six (24%) patients experienced the composite outcome or persistent doubling of serum creatinine (≥2 days), dialysis, or death within 7 days of biomarker measurement (Table 1). Eighteen patients experienced persistent doubling of creatinine (50%), 5 experienced dialysis (14%), and 13 experienced death (36%) as their first event. No statistically significant differences were noted in the distribution of age, gender, race, or ICU location. Patients experiencing the composite outcome had higher severity of illness, as assessed by Acute Physiology and Chronic Health Evaluation II (APACHE II) scores [median 32 (IQR: 28-37) versus 27 (IQR: 21-31), p<0.001] and a higher prevalence of sepsis at biomarker measurement (69% versus 47%, p=0.021) than patients who did not experience the composite outcome. Median baseline serum creatinine and eGFR for the cohort were 1.18 (IQR: 0.92-1.49) mg/dl and 64 (IQR: 46-81) ml/min/1.73 m2 respectively, with no significant difference between the two groups. Median serum creatinine and eGFR at the time of biomarker measurement (i.e. Stage 1 injury) were also not significantly different between patients experiencing and not experiencing the composite outcome.
Figure 1. Flow chart depicting selection of the study cohort.
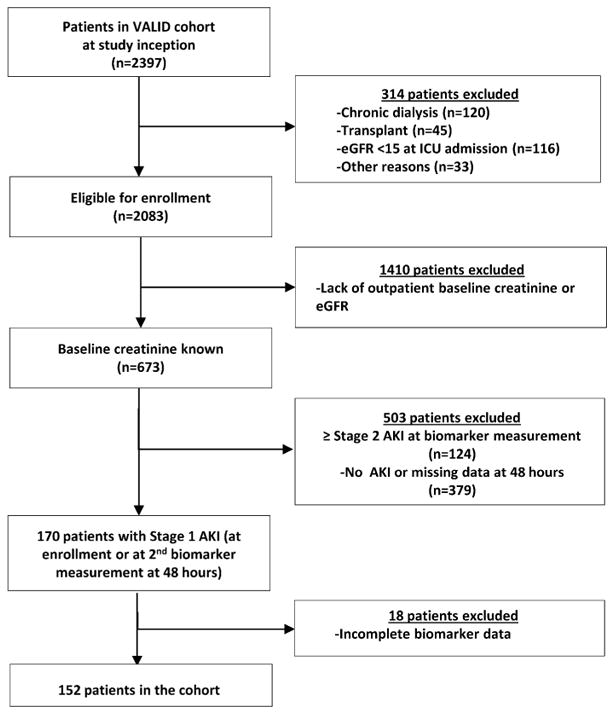
Table 1. Characteristics of the Study Subjects by Composite Outcome—Persistent Doubling of Serum Creatinine, Dialysis, or Death.
| Variable | Composite Outcome (N=36) | No Composite Outcome (N=116) | P Value |
|---|---|---|---|
|
| |||
| Age | 58(51-67) | 61(53-66) | 0.83 |
|
| |||
| Gender | |||
| Female | 15(42%) | 40(34%) | 0.43 |
| Male | 21(58%) | 76(66%) | |
|
| |||
| Ethnicity (%Non-White) | 3(8.3%) | 18(15.5%) | 0.28 |
|
| |||
| APACHE II Score | 32(28-37) | 27(21-31) | <0.001 |
|
| |||
| Baseline Sepsis | 25(69%) | 55(47%) | 0.021 |
|
| |||
| Patient Location | 0.48 | ||
|
| |||
| Surgical ICU | 10(28%) | 47(41%) | |
|
| |||
| Medical ICU | 24(67%) | 61(53%) | |
|
| |||
| Trauma ICU | 0(0%) | 1(1%) | |
|
| |||
| Cardiac ICU | 2(6%) | 7(6%) | |
|
| |||
| Baseline Creatinine (mg/dL) | 1.13(0.90-1.48) | 1.19(0.93-1.50) | 0.37 |
|
| |||
| Baseline eGFR (mL/min/1.73m2) | 62(44-79) | 64(46-82) | 0.96 |
|
| |||
| Creatinine at KDIGO Stage 1 AKI (mg/dL) (i.e. biomarker measurement) | 1.80(1.47-2.32) | 1.73(1.38-2.19) | 0.66 |
|
| |||
| Peak Serum Creatinine (7 days) | 2.56(1.81-3.21) | 1.70(1.32-2.31) | <0.001 |
|
| |||
| Distribution of First Composite Outcome Event within 7 days | |||
| Persistent Doubling of Serum Creatinine | 18(50%) | ||
| Dialysis | 5(14%) | ||
| Death | 13(36%) | ||
Continuous variables are reported as median (interquartile range). Nominal and categorical variables are listed as total number (percentage).
Biomarker Value Comparisons and Outcome Discrimination
Median biomarker values for all patients at the time of Stage I injury (per mg of creatinine) were 195 ng/mg (IQR: 39-668) for uNGAL, 141 ng/mg (IQR: 57-442) for uL-FABP, 313 pg/mg (IQR: 175-665) for uIL-18, and 5.8 ng/mg (IQR: 3.3-11.6) for uKIM-1. Differences in median biomarker values based on outcome status are shown in Figure 2 and Table 2. Median values for all biomarkers were higher for those who experienced the composite outcome compared to those who did not (all p values<0.05).
Figure 2. Box-plot of levels of urine neutrophil gelatinase–associated lipocalin (uNGAL), urine L-type fatty acid–binding protein (uL-FABP), urine interleukin-18 (uIL-18), and urine Kidney Injury Molecule 1 (uKIM-1) grouped by whether or not patients with KDIGO Stage I injury experienced the composite outcome of persistent doubling of baseline serum creatinine, dialysis, or death within 7 days of biomarker measurement.
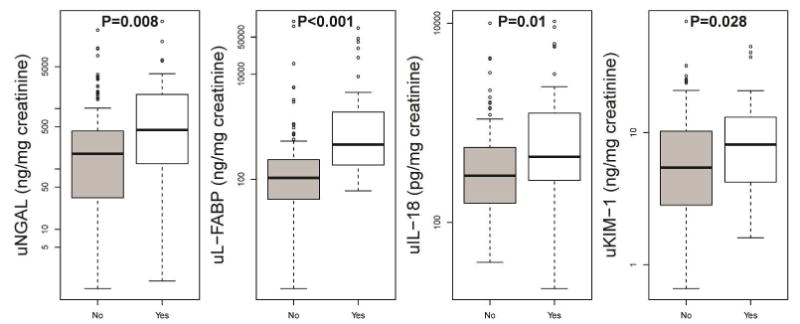
Levels are adjusted for urine creatinine values. P values <0.05 denote statistical significance.
Table 2. Biomarker Values Grouped According to Outcome Status.
| Biomarker | Composite Yes (N=36) | Composite No (N=116) | P Value | Death (N=13)* | Doubling Creatinine (N=18)* | Dialysis (N=5)* |
|---|---|---|---|---|---|---|
| uNGAL (ng/mg) | 445 (127-1706) | 181 (34-421) | 0.008 | 430 (131-1730) | 464 (123-1458) | 459 (151-930) |
| uL-FABP (ng/mg) | 459 (192-1558) | 107 (42-237) | <0.001 | 777 (92-4441) | 459 (273-1185) | 440 (305-1193) |
| uIL-18 (pg/mg) | 458 (281-1232) | 294 (156-566) | 0.01 | 793 (361-5835) | 398 (252-612) | 2130 (166-2307) |
| uKIM-1 (ng/mg) | 8.1 (4.2-12.8) | 5.4 (2.8-10.2) | 0.028 | 10.5 (6.7-15.3) | 4.7 (2.8-11.4) | 10.8 (9.4-12.4) |
| Urine creatinine (mg/ml) | 0.68 (0.47-0.84) | 0.76 (0.51-1.05) | 0.071 | 0.60 (0.32-0.80) | 0.7 (0.49-0.84) | 0.73 (0.71-0.84) |
Abbreviations: uNGAL, urine neutrophil gelatinase-associated lipocalin; uL-FABP, urine L-type fatty acid binding protein; uIL-18, urine Interleukin-18; uKIM-1, urine Kidney Injury Molecule-1.
Biomarker values are reported as median (interquartile range).
Categorized by first composite outcome event experienced.
Discrimination between patients developing and not developing the composite outcome within seven days is shown in Figure 3. The area under the receiver operating curve (AUC-ROC) values were 0.65 (95% CI: 0.54-0.74) for uNGAL, 0.79 (95% CI: 0.70-0.86) for uL-FABP, 0.64 (95% CI: 0.53-0.75) for uIL-18, 0.62 (95% CI: 0.53-0.72) for uKIM-1, and 0.81 (95%CI: 0.73-0.89) for all 4 biomarkers combined.
Figure 3. Area Under the Receiver Operating Curve (AUC-ROC) indicating performance for each biomarker in discriminating between KDIGO Stage 1 injury who experience and do not experience a persistent doubling of baseline creatinine, dialysis, or death within 7 days of biomarker measurement.
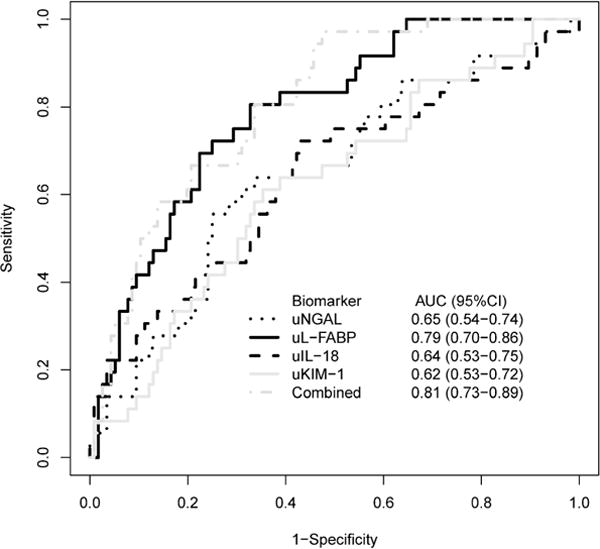
Area under the receiver-operating curves for (a) uNGAL (black dotted line), (b) uL-FABP (black solid line), (c) uIL-18 (black dashed line), (d) u-KIM-1(gray solid line), and (e) combined biomarkers (gray dashed line). 95% Confidence Intervals also shown.
Combined Risk Prediction of Biomarkers and the Clinical Model
AUC-ROC Analysis
We also tested the performance of a clinical model to discriminate between patients who did and did not experience the composite outcome. A priori-selected variables selected based on known associations with AKI included age, serum creatinine at the time of biomarker measurement (Stage 1 injury), APACHE II score, and the presence of sepsis. The AUC-ROC for the clinical model alone was 0.74 (95% CI: 0.68-0.84). To test the incremental improvement in discrimination when biomarker data were added to the clinical model, AUC-ROC values were constructed incorporating biomarker data with clinical data. The AUC-ROC of the clinical model improved to 0.75 (95% CI: 0.69-0.85) with the addition of uNGAL, 0.82 (95% CI: 0.75-0.90) with uL-FABP, 0.76 (95% CI: 0.69-0.85) with uIL-18, and 0.75 (95% CI: 0.69-0.85) with uKIM-1 (Figure 4). Only uL-FABP provided a statistically significant improvement in discrimination beyond that of the clinical model alone (p=0.013).
Figure 4. Area Under the Receiver Operating Curves (AUC-ROCs) combining the clinical model with the addition of each biomarker to measure discrimination between patients with KDIGO Stage 1 injury who experience and do not experience a persistent doubling of baseline creatinine, dialysis, or death within 7 days of biomarker measurement.
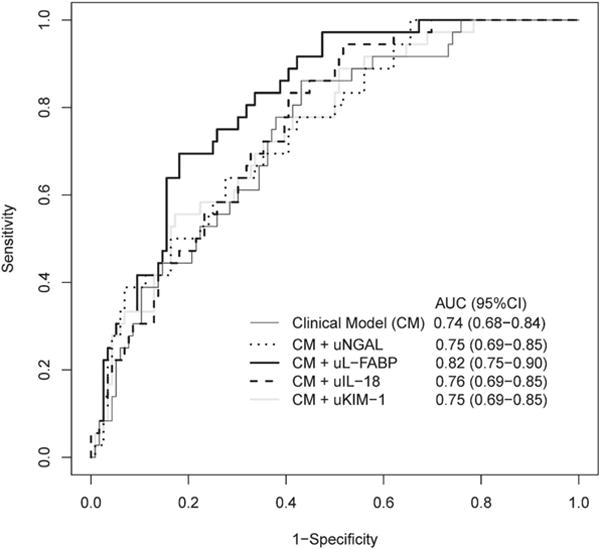
(a) clinical model alone (thin black solid line), (b) clinical model + uNGAL (black dotted line), (c) clinical model + uL-FABP (thick black solid line), (d) clinical model + uIL-18 (black dashed line), and (e) clinical model + uKIM-1 (gray solid line). 95% Confidence Intervals also shown.
Logistic Regression
Risk prediction was assessed using multivariable logistic regression, category free net reclassification index (cfNRI), and the integrated discrimination index (IDI) for the clinical model. The clinical model is shown in Table 3a and contains a priori-selected predictors including age, sepsis, serum creatinine at the time of biomarker measurement, and APACHE II score. Following multivariable adjustment, only APACHE II score remained predictive of the combined composite outcome [OR 2.9 per interquartile range (95%CI: 1.6-5.5), p<0.001]. We next assessed the ability of urine injury markers to provide incremental prognostic information (Table 3b). Among these, only uL-FABP remained predictive for the composite outcome after adjusting for other clinical factors [OR 2.3 per interquartile range (95%CI: 1.4-3.8), p=0.001].
Table 3a. Clinical Model for the Prediction of Persistent Doubling of Serum Creatinine, Dialysis, or Death.
| Variable | OR | 95% CI |
|---|---|---|
| Age | 0.9 | 0.6-1.4 |
| APACHE II Score | 2.9 | 1.6-5.5 |
| Serum Creatinine | 0.7 | 0.4-1.3 |
| Sepsis | 2.1 | 0.9-4.9 |
Abbreviations: OR, odds ratio; CI, confidence interval; APACHE II, Acute Physiology and Chronic Health Evaluation II. ORs are represented per interquartile range of the corresponding variable.
Table 3b. Combined Model Using both Clinical + Biomarker Model* for Prediction of Persistent Doubling of Serum Creatinine, Dialysis, or Death.
| Variable | OR | 95% CI |
|---|---|---|
| Clinical Model +: | ||
| uNGAL | 1.4 | 0.8-2.5 |
| uL-FABP | 2.3 | 1.4-3.8 |
| uIL-18 | 1.4 | 0.9-2.2 |
| uKIM-1 | 1.5 | 0.8-2.7 |
Abbreviations: OR, odds ratio; CI, confidence interval; uNGAL, urine neutrophil gelatinase-associated lipocalin; uL-FABP, urine L-type fatty acid binding protein; uIL-18, urine Interleukin-18; uKIM-1, urine Kidney Injury Molecule-1.
Biomarkers were log10 transformed. ORs are represented per interquartile range based on biomarker concentration.
Adjusted for age, APACHE II score, serum creatinine at KDIGO Stage I, and presence or absence of sepsis at baseline
Risk Reclassification
To further describe the ability of these markers to risk-stratify patients beyond our clinical risk prediction model, cfNRI and IDI values were calculated (Table 4). Category-free NRI (cfNRI) provides a measure of the direction of change in reclassification that a biomarker adds to the clinical model, with results reported as proportions.(21). Because it is possible for 100% of both events and nonevents to be reclassified, the maximum value of the total cfNRI (events + nonevents) is 200%.(22) In contrast, the IDI provides information on both the direction and magnitude of mean change in predicted probabilities for events and non-events when additional variables or biomarkers are added.(23) Rather than a proportion, total IDI is expressed as an absolute number whose maximum value is 2.
Table 4. Integrated Discrimination Index and Category Free Net Reclassification Index for Individual Biomarkers in Persistent Doubling of Creatinine Model.
| uNGAL | uL-FABP | uIL-18 | uKIM-1 | |
|---|---|---|---|---|
| IDI | ||||
| Events to higher risk | 0.0099 | 0.0680 | 0.0095 | 0.0111 |
| Non-Events to lower risk | 0.0031 | 0.0211 | 0.0030 | 0.0034 |
| Total | 0.0129 | 0.0891 | 0.0125 | 0.0145 |
| (95% CI) | (-0.0052-0.0310) | (0.0336-0.1445) | (-0.0118-0.0367) | (-0.0052-0.0343) |
| NRI | ||||
| %Events to higher risk | 66.7 | 55.6 | 58.3 | 63.9 |
| %Events to lower risk | 33.3 | 44.4 | 41.7 | 36.1 |
| %Non-Events to higher risk | 47.4 | 34.5 | 43.1 | 43.1 |
| %Non-Events to lower risk | 52.6 | 65.5 | 56.9 | 56.9 |
| Total NRI for events | 33.3 | 11.1 | 16.7 | 27.8 |
| (95% CI) | (0.7-66.0) | (-21.6-43.8) | (-16.0-49.3) | (-4.9-60.4) |
| Total NRI for non-events | 5.2 | 31.0 | 13.8 | 13.8 |
| (95% CI) | (-13.0-23.4) | (12.8-49.2) | (-4.4-32.0) | (-4.4-32.0) |
| Total cfNRI | 38.5 | 42.1 | 30.5 | 41.6 |
| (95% CI) | (1.1-75.9) | (4.8-79.5) | (-6.9-67.9) | (4.2-79.0) |
Urine L-FABP correctly reclassified 36 of 116 non-events to a lower level of risk [non-event NRI=31.0% (95%CI: 12.8-49.2)], while the correct reclassification of events to a higher level of risk was not statistically significant. Urine NGAL correctly reclassified 12 of 36 events to a higher level of risk [event NRI=33.3% (95%CI: 0.7-66.0)], while the correct reclassification of non-events to a lower level of risk was not statistically significant. Urine IL-18 and KIM-1 did not aid in risk reclassification. Of the biomarkers tested, only uL-FABP appropriately changed the mean probability of risk in patients both experiencing the composite outcome (higher) and those not experiencing the composite outcome (lower), resulting in a total IDI of 0.0891 (95% CI: 0.0336-0.1445).
Supplemental Analyses
Our composite outcome was comprised of persistent doubling of serum creatinine, dialysis, and death. We chose persistent doubling of baseline creatinine for the primary analysis to enrich for patients more likely to have parenchymal injury, rather than transient functional changes. Further, observational data has suggested that duration of injury is as important as severity in predicting outcomes following AKI.(24) In order to illustrate the effect of not requiring persistent changes in creatinine, we performed an additional analysis using the composite outcome of any doubling of serum creatinine (without requiring persistence), dialysis, and death. Using these criteria, an additional 12 patients were included (total n=48). In this combined analysis, the distribution of first outcome achieved included 35 patients with a doubling of serum creatinine (transient OR persistent), 3 patients experiencing dialysis, and 10 patients experiencing death, respectively. The AUC-ROC values for the prediction of this alternative composite outcome were 0.61 (95% CI 0.53-0.71) for uNGAL, 0.72 (95% CI 0.63-0.80) for uL-FABP, 0.60 (95% CI 0.51-0.69) for uIL-18, 0.58 (95% CI 0.50-0.67) for KIM-1, and 0.72 (95% CI: 0.65-0.82) for all 4 biomarkers combined, compared with AUC-ROC for clinical prediction model alone of 0.73 (95% CI 0.67-0.83). When combined with clinical predictors, the AUC-ROC were 0.74 (95% CI 0.68-0.84) for uNGAL, 0.77 (95% CI 0.71-0.85) for uL-FABP, 0.74 (95% CI 0.68-0.83) for uIL-18, and 0.74 (95% CI 0.69-0.83) for uKIM-1.
Discussion
We hypothesized that a panel of biomarkers would have prognostic value in critically ill adults with early evidence of AKI and add incremental prognostic information to known clinical risk factors. Our results demonstrate that uL-FABP has independent and combined value in combination with known clinical predictors to discriminate between patients with early AKI who develop proximately relevant outcomes and those who will not. Urine L-FABP also reclassified patients to a more appropriate level of risk using cfNRI and IDI. In contrast, uNGAL, uIL-18, and uKIM-1 exhibited modest discrimination for the composite outcome and did not significantly improve the predictive ability of the clinical model, or substantially improve reclassification.
To date, larger biomarker studies have suggested modest performance for early detection of AKI.(6, 12, 25) However, it is unclear whether this lack of performance reflects limitations of the markers themselves or inherent uncertainty in using modest changes in serum creatinine as a reference standard.(26, 27) Indeed, recent data suggest that such changes alone may not necessarily reflect significant parenchymal damage.(28) Determining the prognostic yield of novel biomarkers in predicting less ambiguous outcomes is therefore needed and has helped to establish the clinical utility of other injury markers (e.g.troponin-I).(29) We have previously demonstrated that uNGAL, uIL-18, and uL-FABP can modestly predict dialysis and death among unselected critically ill adults.(25, 30, 31) However, although critically ill patients are invariably among the highest risk for poor outcomes, the relatively low prevalence when measured in ‘all-comers’ may reduce predictive value.(32) Furthermore, non-selective biomarker measurement is not cost-effective and can inflate the likelihood of false positive results, which carries its own untoward effects.(33). We therefore used early changes in serum creatinine to target a higher risk population in whom clinical uncertainty often exists regarding evolving parenchymal injury, where novel ‘damage markers’ may provide clinically relevant information on prognosis.
Other studies have also recently begun to examine the prognostic value of these candidate markers.(34) Singer et al. evaluated uNGAL in hospitalized patients and demonstrated an AUC-ROC of 0.71 (95% CI 0.62 to 0.8) for the prediction of progression of AKI, dialysis, and death.(35) However, in contrast to our study, 78% of patients had already achieved KDIGO Stage 2 or 3 AKI, indicating many of these patients already had moderate to severe injury at the time of biomarker measurement. Similarly, Kashani et al. examined the ability of several biomarkers to predict progression to KDIGO Stage 2 or 3 AKI within 12 hours in critically ill ICU patients from the SAPPHIRE study.(36) Reported AUC-ROCs for uNGAL, uKIM-1, and uIL-18 were 0.72, 0.70, and 0.69, respectively. Notably, the clinical prediction model already showed good discrimination with an AUC-ROC of 0.81, suggesting that the risk for injury progression within such a short time frame may have already been clinically evident in many patients. Lastly, Doi et al. demonstrated that uL-FABP, uNGAL, and uIL-18 were able to effectively discriminate 14-day mortality in critically ill patients.(37) Our results extend upon these results by demonstrating prognostic value of these markers for additional renal-relevant outcomes where the risk for their development is less certain.
There are several potential reasons for the superior discriminative ability of uL-FABP we observed. It is possible that the peak concentration of uL-FABP following renal insult may occur later and/or be more sustained than other biomarkers in the setting of critical illness. Previous studies have demonstrated peak urinary concentrations of IL-18 and KIM-1 within 12 hours after discrete injury,(34) and variable timing of peak uNGAL.(9, 34) By selecting for a group of patients already having early creatinine-based evidence of AKI, it is possible that the window to obtain peak biomarker concentration differences was missed with these markers. However, Arthur et al. recently demonstrated that uIL-18, uKIM-1, and uNGAL are able to predict poor outcomes in patients with Stage I AKI following cardiac surgery, making this hypothesis less likely.(38) Our results complement these data by suggesting that some of these markers have prognostic value when measured in a similar fashion in a more heterogeneous critically ill population, where the timing of injury is less certain. It is also possible that uL-FABP in is more specific to certain types of renal injury than other biomarkers in a heterogeneous population of critically ill patients. For example, preclinical data suggest that uL-FABP may be particularly useful in detecting severe AKI during sepsis,(39) which was present in a substantial proportion of our cohort.
In this study, we used cfNRI and IDI to further describe the incremental improvement in discrimination (AUC-ROC) observed with the addition of biomarkers. However, there are limitations with the use of cfNRI and IDI and our results should be interpreted with caution. For example, cfNRI does not account for the magnitude of change in predicted risk, and small changes may not improve clinical decision-making. Although the total cfNRI for L-FABP was largely driven by reclassification of non-events to lower risk, the IDI for non-events was relatively modest. Additionally, although cfNRI of events using uNGAL was statistically significant, the IDI for events was not statistically significant. Further work to determine meaningful thresholds and identify subgroups of patients with the highest incremental improvement in predicted risk is necessary before these results can be translated to clinical practice.
Strengths of this study include a large, well-phenotyped, heterogeneous ICU population and the examination of harder clinical endpoints within a relatively proximate time frame. By including only patients with known baseline creatinine, we also reduced misclassification of early AKI status. There are also several limitations. Excluding patients with chronic underlying severe lung disease and those without known baseline serum creatinine may have reduced the generalizability of our results. However, as the goal of our study was to examine the prognostic performance of biomarkers in patients with early stage injury, it was necessary to reduce misclassification of AKI status. We also did not have hourly urine output data available, which may have reduced our ability to categorize early AKI. Additionally, we did not have serial biomarker measurements available within a given day, which limited determination of the kinetics of biomarkers in relation to early AKI. APACHE II scores were determined for all patients at the time of ICU enrollment, but were not calculated for subsequent hospital days. Lastly, although one of the elements of our composite outcome used changes in serum creatinine (i.e. persistent doubling) and does not eliminate the risk of misclassification, the definition used is arguably more specific for parenchymal injury and associated with clinically relevant downstream outcomes.(24) Our sensitivity analysis showed less robust performance using non-persistent creatinine doubling, suggesting that studies relying on achieving a threshold using a single serum creatinine value should be interpreted with caution, as this may still indicate a temporary functional change rather than true parenchymal injury. Our results need to be validated in larger multi-center cohorts of critically ill patients.
In summary, among critically ill patients with evidence of early AKI, uL-FABP provided independent and additive short-term prognostic information when combined with known clinical predictors. Urine NGAL, uKIM-1, and uIL-18 did not improve the ability to discriminate patients who went on to develop events from those who did not. Our results also demonstrate that rather than being used solely as a comparator, modest changes in creatinine may be helpful in targeting of biomarker measurement. Future multi-center studies are warranted to examine the effectiveness of using the combination of early changes in serum creatinine and tubular injury markers to provide prognostic information and identify appropriate patients for testing of interventions.
Materials and Methods
Patients
The study was performed in the previously described Validation of biomarkers for Acute Lung Injury Diagnosis (VALID) study cohort.(25, 30) In brief, VALID is a single-center, multi-ICU prospective cohort study with a total enrollment of 2397 patients whose primary objective is to discover and validate new and existing protein biomarkers to diagnose organ injury including, but not limited to, Acute Lung Injury and AKI. All adult (≥18 years of age) patients admitted to one of four ICUs (Medical, Cardiac, Surgical, Trauma) at Vanderbilt University Medical Center (VUMC) who were eligible were enrolled within 24 hours of ICU admission. Patients were excluded from the parent study if they had chronic lung disease requiring oxygen supplementation, pulmonary fibrosis, experienced a cardiac arrest prior to enrollment, had transfer orders written or anticipated within 4 hours, died or were discharged within 48 hours of ICU admission, were admitted for uncomplicated overdose, or were in the ICU for more than 3 days prior to enrollment.
For the current study, patients were required to have an outpatient serum creatinine available 7-365 days prior to hospital admission to calculate baseline serum creatinine (average), and only patients with early AKI at the time of biomarker measurement were included. Early AKI was captured using KDIGO criteria for Stage 1 injury, defined as an increase in creatinine of 0.3 mg/dL or 50% from baseline. Exclusion criteria included those with greater injury severity (i.e. KDIGO Stages 2 and 3) at the time of biomarker measurement, a history of renal transplant, chronic dialysis, nephrectomy, and eGFR < 15 ml/min/1.73 m2 at the time of ICU enrollment.
Clinical Data Collection
Demographic and physiological data were collected at the time of enrollment. APACHE Il and SAPS Il were calculated at the time of ICU admission.(40, 41) The presence of the systemic inflammatory response syndrome (SIRS), sepsis, or severe sepsis was determined on a daily basis according to the American College of Chest Physicians/Society of Critical Care Medicine Consensus definition.(42) Patients were followed prospectively until hospital discharge, with ICU and hospital length of stay, and hospital mortality recorded. The VALID database has been cross-referenced to the Social Security Death Index (SSDI) to allow for longitudinal determination of mortality. The Social Security Death Index is 88.2% sensitive for death for the general population.(43) Patients without a recorded death in the SSDI or medical record were considered alive at 7 days after study enrollment.
Biosample Collection and Definitions
Urine biomarker measurement occurred at two points: study enrollment and at 48 hours later. Serum creatinine was measured per clinical care for critically ill patients. For patients meeting KDIGO Stage 1 injury at both time points, the earlier of the two time points was chosen. Glomerular filtration rate was estimated by the abbreviated Modification of Diet and Renal Disease (MDRD) equation [GFR (mL/min/1.73 m2) = 186 × (SCr)-1.154 × (Age)-0.203 × (0.742 if female) × (1.210 if African-American)].(44) The primary outcome in this study was a composite outcome of persistent doubling of baseline serum creatinine (defined as ≥2 days), dialysis, or death within 7 days of biomarker measurement. The rationale for the 7-day time window was to enrich for the examination of outcomes more likely to be proximately related to the biomarker measurement.
Laboratory Data Collection
Urine samples were collected the morning of enrollment and 48 hours later from the proximal meter reservoir of the Foley catheter, immediately placed on ice, pipetted into 400 microliter aliquots and frozen at -80°C within 1 hour of collection. Urine biomarker levels were measured in urine using commercially available ELISA kits for NGAL (R&D Systems, Minneapolis, MN), L-FABP (CMIC Holdings, Tokyo, Japan), IL-18 (MBL Nagoya, Japan), KIM-1 (R&D Systems, Minneapolis, MN), and urine creatinine (R&D Systems, Minneapolis, MN). Samples were run in duplicate and lab personnel were blinded to the injury status of each patient. Each ELISA kit underwent an additional in-lab validation for measurement in human urine. In brief, recombinant protein standards at various concentrations were supplied by the ELISA manufacturer and spiked into normal control urine. After subtracting the concentration of the analyte of the unspiked control from the recovered values in the spiked samples, we determined that there was good correlation between the spiked and recovery concentrations within the standard curve for each analyte. The mean intra-assay coefficients of variation (CV) in our laboratory were 6.6% for uNGAL, 4.2% for uL-FABP, 3.4% for uIL-18, 3.6% for uKIM-1, and 3.6% for urine creatinine.
Statistical Analysis
Patient characteristics were described as medians with interquartile range [IQR] for continuous variables and compared using the Wilcoxon rank sum test. Categorical variables were expressed as proportions and compared using the Pearson χ2. Biomarker values were adjusted for volume status by dividing by the urine creatinine value. The ability of biomarkers to discriminate between patients experiencing the primary outcome within 7 days of biomarker measurement was determined using AUC-ROC curves providing sensitivity and specificity at different cutoff values to detect AKI. The combined effect of all 4 biomarkers was estimated with logistic regression modeling including the linear combination of all 4 biomarkers. The improvement in AUC-ROC with the addition of individual biomarkers to the clinical model was evaluated using nonparametric comparison of areas under correlated ROC curves.(45) To assess the independent predictive ability of the individual and combined biomarker measurements relative to a priori-specified predictors of AKI including age, APACHE II score, presence of sepsis, and serum creatinine at the time of biomarker measurement, multivariable logistic regression modeling was used and internally validated using bootstrap resampling. Adjusted effects of biomarkers were presented as odds ratios with 95% confidence intervals showing their contribution to the existing clinical predictors. The cfNRI and IDI were calculated to assess the incremental predictive ability of each biomarker when added to the clinical model.(21) The cfNRI was the sum of correct reclassification among patients with and without composite outcome. Among patients with the composite outcome, improved reclassification was the difference between the percentage of patients who were reclassified as being in a higher risk group and the percentage of patients who were reclassified in the lower risk group.(46) Similarly among patients without the composite outcome, improved reclassification was the difference between the percentage of patients who were reclassified as being in a lower risk group and the percentage of patients who were reclassified in the higher risk group. The IDI was used to quantify the actual change in calculated risk for each individual for those with and those without events.(47) The total IDI provides the difference in mean predicted probabilities, representing the amount by which the addition of a biomarker to a clinical model increases the separation of the mean predicted probabilities for events and non-events.(23) The statistical software package R version 2.15.0 (www.r-project.org) and SAS version 9 were used for analyses.
Acknowledgments
This study is supported by NIH Grant UO1 HL081332, R21HL112656-02 and K24 HL103836 from the National Heart, Lung and Blood Institute; K24 DK62849 from the National Institute of Diabetes, Digestive and Kidney Diseases; and Clinical Translational Science Award 1UL-1RR024975 from the National Center for Research Resources. EDS is supported by NIH Grant K23 DK088964-03 from the National Institute of Diabetes and Digestive and Kidney Diseases. SKP is supported by NIH Training Grant 5 T32 DK007569-24.
Footnotes
Disclosures: EDS has consulted for Alere, Inc. and Abbvie, Inc.
This work was previously presented in abstract form at the 19th Annual International Conference on Advances in Critical Care (CRRT 2014), San Diego, CA, March 2014, and at the National Kidney Foundation 9th Annual National Young Investigators Forum, Las Vegas, NV, April 2014.
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–70. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–8. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 3.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2011;81(5):442–8. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven't worked and what is on the horizon. Clin J Am Soc Nephrol. 2007;2(2):356–65. doi: 10.2215/CJN.03280906. [DOI] [PubMed] [Google Scholar]
- 5.Siew ED, Ware LB, Ikizler TA. Biological markers of acute kidney injury. J Am Soc Nephrol. 2011;22(5):810–20. doi: 10.1681/ASN.2010080796. [DOI] [PubMed] [Google Scholar]
- 6.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 7.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14(10):2534–43. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 8.Luo Q, Zhou F, Dong H, Wu L, Chai L, Lan K, et al. Implication of combined urinary biomarkers in early diagnosis of acute kidney injury following percutaneous coronary intervention. Clin Nephrol. 2013;79(2):85–92. doi: 10.5414/CN106852. [DOI] [PubMed] [Google Scholar]
- 9.Koyner JL, Vaidya VS, Bennett MR, Ma Q, Worcester E, Akhter SA, et al. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol. 2010;5(12):2154–65. doi: 10.2215/CJN.00740110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruz DN, de Cal M, Garzotto F, Perazella MA, Lentini P, Corradi V, et al. Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med. 2010;36(3):444–51. doi: 10.1007/s00134-009-1711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Geus HR, Bakker J, Lesaffre EM, le Noble JL. Neutrophil gelatinase-associated lipocalin at ICU admission predicts for acute kidney injury in adult patients. Am J Respir Crit Care Med. 2011;183(7):907–14. doi: 10.1164/rccm.200908-1214OC. [DOI] [PubMed] [Google Scholar]
- 12.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22(9):1748–57. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parikh CR, Devarajan P, Zappitelli M, Sint K, Thiessen-Philbrook H, Li S, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol. 2011;22(9):1737–47. doi: 10.1681/ASN.2010111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waikar SS, Betensky RA, Bonventre JV. Creatinine as the gold standard for kidney injury biomarker studies? Nephrol Dial Transplant. 2009;24(11):3263–5. doi: 10.1093/ndt/gfp428. [DOI] [PubMed] [Google Scholar]
- 15.Basu RK, Zappitelli M, Brunner L, Wang Y, Wong HR, Chawla LS, et al. Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int. 2014;85(3):659–67. doi: 10.1038/ki.2013.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto T, Noiri E, Ono Y, Doi K, Negishi K, Kamijo A, et al. Renal L-type fatty acid--binding protein in acute ischemic injury. J Am Soc Nephrol. 2007;18(11):2894–902. doi: 10.1681/ASN.2007010097. [DOI] [PubMed] [Google Scholar]
- 17.Melnikov VY, Ecder T, Fantuzzi G, Siegmund B, Lucia MS, Dinarello CA, et al. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest. 2001;107(9):1145–52. doi: 10.1172/JCI12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis. 2004;43(3):405–14. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 19.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273(7):4135–42. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 20.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–44. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 21.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–72. doi: 10.1002/sim.2929. discussion 207-12. [DOI] [PubMed] [Google Scholar]
- 22.Pickering JW, Endre ZH. New metrics for assessing diagnostic potential of candidate biomarkers. Clin J Am Soc Nephrol. 2012;7(8):1355–64. doi: 10.2215/CJN.09590911. [DOI] [PubMed] [Google Scholar]
- 23.Pencina MJ, D'Agostino RB, Pencina KM, Janssens AC, Greenland P. Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol. 2012;176(6):473–81. doi: 10.1093/aje/kws207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coca SG, King JT, Jr, Rosenthal RA, Perkal MF, Parikh CR. The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int. 2010;78(9):926–33. doi: 10.1038/ki.2010.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siew ED, Ware LB, Gebretsadik T, Shintani A, Moons KG, Wickersham N, et al. Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol. 2009;20(8):1823–32. doi: 10.1681/ASN.2008070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waikar SS, Betensky RA, Emerson SC, Bonventre JV. Imperfect gold standards for kidney injury biomarker evaluation. J Am Soc Nephrol. 2012;23(1):13–21. doi: 10.1681/ASN.2010111124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu RK, Hsu CY. We can diagnose AKI “early”. Clin J Am Soc Nephrol. 2012;7(11):1741–2. doi: 10.2215/CJN.09740912. [DOI] [PubMed] [Google Scholar]
- 28.Coca SG, Garg AX, Swaminathan M, Garwood S, Hong K, Thiessen-Philbrook H, et al. Preoperative angiotensin-converting enzyme inhibitors and angiotensin receptor blocker use and acute kidney injury in patients undergoing cardiac surgery. Nephrol Dial Transplant. 2013;28(11):2787–99. doi: 10.1093/ndt/gft405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antman EM, Tanasijevic MJ, Thompson B, Schactman M, McCabe CH, Cannon CP, et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996;335(18):1342–9. doi: 10.1056/NEJM199610313351802. [DOI] [PubMed] [Google Scholar]
- 30.Siew ED, Ware LB, Bian A, Shintani A, Eden SK, Wickersham N, et al. Distinct injury markers for the early detection and prognosis of incident acute kidney injury in critically ill adults with preserved kidney function. Kidney Int. 2013;84(4):786–94. doi: 10.1038/ki.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siew ED, Ikizler TA, Gebretsadik T, Shintani A, Wickersham N, Bossert F, et al. Elevated urinary IL-18 levels at the time of ICU admission predict adverse clinical outcomes. Clin J Am Soc Nephrol. 2010;5(8):1497–505. doi: 10.2215/CJN.09061209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brenner H, Gefeller O. Variation of sensitivity, specificity, likelihood ratios and predictive values with disease prevalence. Stat Med. 1997;16(9):981–91. doi: 10.1002/(sici)1097-0258(19970515)16:9<981::aid-sim510>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 33.Polanczyk CA, Lee TH, Cook EF, Walls R, Wybenga D, Printy-Klein G, et al. Cardiac troponin I as a predictor of major cardiac events in emergency department patients with acute chest pain. J Am Coll Cardiol. 1998;32(1):8–14. doi: 10.1016/s0735-1097(98)00176-4. [DOI] [PubMed] [Google Scholar]
- 34.Endre ZH, Pickering JW, Walker RJ, Devarajan P, Edelstein CL, Bonventre JV, et al. Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int. 2011;79(10):1119–30. doi: 10.1038/ki.2010.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singer E, Elger A, Elitok S, Kettritz R, Nickolas TL, Barasch J, et al. Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney Int. 2011;80(4):405–14. doi: 10.1038/ki.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17(1):R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doi K, Negishi K, Ishizu T, Katagiri D, Fujita T, Matsubara T, et al. Evaluation of new acute kidney injury biomarkers in a mixed intensive care unit. Crit Care Med. 2011;39(11):2464–9. doi: 10.1097/CCM.0b013e318225761a. [DOI] [PubMed] [Google Scholar]
- 38.Arthur JM, Hill EG, Alge JL, Lewis EC, Neely BA, Janech MG, et al. Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney Int. 2014;85(2):431–8. doi: 10.1038/ki.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doi K, Noiri E, Maeda-Mamiya R, Ishii T, Negishi K, Hamasaki Y, et al. Urinary L-type fatty acid-binding protein as a new biomarker of sepsis complicated with acute kidney injury. Crit Care Med. 2010;38(10):2037–42. doi: 10.1097/CCM.0b013e3181eedac0. [DOI] [PubMed] [Google Scholar]
- 40.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29. [PubMed] [Google Scholar]
- 41.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–63. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 42.Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101(6):1481–3. doi: 10.1378/chest.101.6.1481. [DOI] [PubMed] [Google Scholar]
- 43.Schisterman EF, Whitcomb BW. Use of the Social Security Administration Death Master File for ascertainment of mortality status. Population health metrics. 2004;2(1):2. doi: 10.1186/1478-7954-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 45.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. [PubMed] [Google Scholar]
- 46.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pencina MJ, D'Agostino RalphB, Vasan RamachandraS. Comments on ‘Integrated discrimination and net reclassification improvements-Practical advice’. Stat Med. 27(2):207–12. [Google Scholar]


