Abstract
For social interactions to be successful, individuals must establish shared mental representations that allow them to reach a common understanding and “get on the same page”. We refer to this process as social coordination. While examples of social coordination are ubiquitous in daily life, relatively little is known about the neuroanatomic basis of this complex behavior. This is particularly true in a language context, as previous studies have used overly complex paradigms to study this. Although traditional views of language processing and the recent interactive-alignment account of conversation focus on peri-Sylvian regions, our model of social coordination predicts prefrontal involvement. To test this hypothesis, we examine the neural basis of social coordination during conversational exchanges in non-aphasic patients with behavioral variant frontotemporal degeneration (bvFTD). bvFTD patients show impairments in executive function and social comportment due to disease in frontal and anterior temporal regions. To investigate social coordination in bvFTD, we developed a novel language-based task that assesses patients’ ability to convey an object’s description to a conversational partner. Experimental conditions manipulated the amount of information shared by the participant and the conversational partner, and the associated working memory demands. Our results indicate that, although patients did not have difficulty identifying the features of the objects, they did produce descriptions that included insufficient or inappropriate adjectives and thus struggled to communicate effectively. Impaired performance was related to gray matter atrophy particularly in medial prefrontal and orbitofrontal cortices. Our findings suggest an important role for non-language brain areas that belong to a large-scale neurocognitive network for social coordination.
Keywords: frontotemporal degeneration, prefrontal cortex, language, discourse, social cognition, perspective-taking
1. Introduction
As humans, we navigate a complex world of social interactions, from negotiating with colleagues at work to gossiping with friends over coffee. For these interactions to be successful, individuals must establish shared mental representations to mediate common understanding. Behavioral game theory, rooted in principles of rational decision-making and strategy, refers to this process as social coordination (Clark, 2011). While examples of social coordination dominate our daily lives, surprisingly little is known about the neural mechanisms supporting this complex behavior. This is particularly true within the domain of language, which is the most common way in which we exchange information. In this study, we examine the neural basis for social coordination by studying semi-structured conversational exchanges in patients with behavioral variant of frontotemporal degeneration (bvFTD).
Traditional views of language suggest the core processing regions reside in left hemisphere peri-Sylvian cortex. Based primarily on studies of segmental language, this classic model fails to account for the complexities of real-world communication. Indeed, some recent models of language processing suggest prefrontal cortex and areas associated with cognitive control and social cognition are needed to supplement core language processing regions (Cooke et al., 2006; Novais-Santos et al., 2007; Ferstl et al., 2008; Troiani et al., 2008; Hagoort, 2014).
We examine this possibility here and investigate the neural basis for social coordination during conversation by studying patients with behavioral variant frontotemporal degeneration (bvFTD). bvFTD is a rare neurodegenerative disease characterized by executive and social limitations due to progressive atrophy in frontal and temporal regions (Rascovsky et al., 2011). Patients with bvFTD demonstrate relatively preserved language, although higher-order narrative deficits have been reported (Ash et al., 2006; Cosentino et al., 2006; Farag et al., 2010). Because segmental language function is largely spared and patients are considered non-aphasic, these narrative deficits are often attributed to executive and social difficulties. A recent study using a single-word task also demonstrated impaired social coordination in bvFTD (McMillan et al., 2012). In this study, bvFTD patients differed from healthy controls in providing responses (e.g. a boy’s name) that “others just like themselves” might provide. It remains unknown whether deficits in social coordination also contribute to difficulty with conversational discourse.
Here, we investigate social coordination using a novel, language-based task that involves describing a single object to a conversational partner. Much of the previous work examining perspective-taking during language use has employed complex narratives, many illustrating false beliefs or social faux pas. These studies consistently demonstrate a deficit in bvFTD (Gregory et al., 2002; Kipps and Hodges, 2006; Lough et al., 2006; Torralva et al., 2007, 2009; Fernandez-Duque et al., 2009; Kipps et al., 2009; Freedman et al., 2013). These narrative-based measures, however, require patients to track complex activities that involve multiple actors and extend over time. Since executive and working memory limitations have been documented in bvFTD (Kramer et al., 2003; Libon et al., 2007), the results of these demanding studies are controversial and potentially confounded (Henry et al., 2014). For instance, some studies have suggested that the results of these traditional, story-based, theory of mind tasks may reflect deficits related to task demands and executive functioning, rather than mentalizing or social cognition per se (Fernandez-Duque et al., 2009; Le Bouc et al., 2012). Such a relationship between executive function and theory of mind in bvFTD remains a source of contention, however, with a number of studies reporting that the two deficits are dissociable and independent. For example, Torralva et al. (2007) report a deficit in theory of mind in bvFTD patients that is consistent across the Reading the Mind in the Eyes and faux pas tasks but independent of a general deficit in decision making. Similarly, Freedman et al. (2013) found significant deficits in second-order false belief performance that persisted when controlling for deficits in executive function. In the latter study, the authors also demonstrated that the patient deficit was specific: no deficits in visual perspective-taking were observed.
Beyond the potential confounds related to executive function, the existing theory of mind tasks are also limited in their ecological validity. These comprehension-based tasks only ask patients to be passive observers; they do not require subjects to play an active role in the experimental situation and use their understanding of a conversational partner’s perspective.
To our knowledge, this study is the first to examine social coordination in a natural, semi-structured discourse context. Furthermore, we manipulate two aspects of coordination. The first is perspective-taking, or the ability to adopt another’s point of view. We examine perspective-taking by assessing the patient’s sensitivity to the amount of information available to the conversational partner. Second, we examine the resource demands associated with tracking the multiple elements of a conversation. We independently examine the effect of resource demands by manipulating the number of objects sharing perceptual features and competing with the target object described by the patient.
Previous work in bvFTD has related both narrative (Ash et al., 2006; Farag et al., 2010) and social (Eslinger et al., 2007; Kipps et al., 2009; Mendez and Shapira, 2009; Grossman et al., 2010; Couto et al., 2013) deficits to prefrontal disease. fMRI studies of non-verbal coordination in healthy adults also implicate prefrontal regions (Kuo et al., 2009; Yoshida et al., 2010). Accordingly, our model of coordination predicts essential roles of medial prefrontal (mPFC), dorsolateral prefrontal (dlPFC), and orbitofrontal cortices (OFC), areas associated with mentalizing/perspective-taking, working memory, and decision-making, respectively (Amodio and Frith, 2006; Wallis, 2007; Badre, 2008). Therefore, in the context of the current experiment, we predict a priori that impaired behavioral performance on the social coordination task in bvFTD will be related to reduce gray matter density in these regions.
The interactive-alignment account provides an alternative, although not necessarily mutually exclusive, hypothesis (Pickering and Garrod, 2004). According to this perspective, effective interpersonal communication results from alignment at multiple levels of linguistic representation, including lexical selection and syntactic construction. Citing evidence that speakers and listeners both activate peri-Sylvian regions and show correlated brain activity during communication, Menenti et al. (2012) propose co-activation of the language network as a mechanism for conversational alignment. Relatedly, simulation theory suggests that social interactions are supported by mirror neuron activity in premotor areas (including Broca’s area) (Gallese, 2007). The present investigation may help clarify the relative contributions of social processing dependent upon prefrontal regions (coordination) versus linguistic priming dependent upon peri-Sylvian language-specific regions (interactive-alignment) and simulation dependent upon premotor regions (mirror neurons) to communication.
2. Methods
2.1 Participants
Participants included twelve patients with bvFTD who were demographically-comparable with fourteen healthy seniors in terms of age, education, and gender. Demographic and clinical characteristics are summarized in Table 1. Patients with bvFTD were diagnosed by board-certified neurologists (M.G., D.J.I) using a consensus procedure and published criteria (Rascovsky et al., 2011). Alternative causes of cognitive difficulty due to other neurodegenerative conditions such as Alzheimer’s disease, hydrocephalus, stroke or head trauma were excluded by clinical exam, neuroimaging, CSF, and blood tests. As summarized in Table 1, severity of overall cognitive impairment was assessed in patients using the Mini-Mental State Examination. On average, patients were not in the demented range (mean MMSE=25.75, SD=3.47), and individual scores all fell in the range of minimal to mild impairment (range: 18–29). To test specificity and ensure that any observed deficits in social coordination were not the result of linguistic deficits, patients with bvFTD also completed a short battery of language tests. These tests included an abbreviated version of the Boston Naming Test (Kaplan, Goodglass, and Weintraub, 1983), a test of lexical retrieval in which participants name 30 black and white line drawings of objects that are graded in difficulty; the language section of the Philadelphia Brief Assessment of Cognition (PBAC), which yields a composite measure based on a broad spectrum of language skills including lexical retrieval, semantic knowledge, conversational fluency, reading, writing, and repetition (Avants et al., 2014; Libon et al., 2011); and the Pyramid and Palm Trees test (Howard and Patterson, 1982), a test of semantic access in which participants must identify the word or picture that is associated with the presented target. Finally, patient caregivers were administered the Neuropsychiatric Inventory (Cummings et al., 1994), which is a commonly used tool assessing the severity and frequency of neuropsychiatric symptoms (including apathy/indifference) in patients with dementia.
Table 1.
Mean (±SEM) demographic and neuropsychological data for behavioral variant frontotemporal degeneration and control groups.
| Patients (N=12) |
Healthy Seniors (N=14) |
|
|---|---|---|
| Age (years)1 | 65.3 (1.7) | 70.2 (2.02) |
| Education (years)1 | 15.75 (0.84) | 15.54 (0.70) |
| Gender (N male)1 | 9 | 6 |
| Mini-Mental State Examination (max = 30)2 | 25.83 (1.05) | – |
| Boston Naming Test (max = 30)3 | 24.27 (0.96) | – |
| Language Scale, Philadelphia Brief Assessment of Cognition (max = 19)4 | 16.01 (0.29) | – |
| Pyramids and Palm Trees (max = 104)5 | 96.27 (2.04) | – |
NOTES
bvFTD patients and healthy seniors did not significantly differ in terms of age [t(24) = 1.82; p = 0.08], education level [t(23) = 0.12, p = 0.85 ], or gender [χ2=2.74, p = 0.13].
Mini Mental State Examination: Overall measure of cognitive impairment.
Boston Naming Test: Picture naming task that assesses word retrieval and semantic impairment.
Language Scale, Philadelphia Brief Assessment of Cognition: Composite measure of overall language functioning.
Pyramids and Palm Trees: Semantic association task.
Informed consent was obtained from all participants according to a protocol approved by the Institutional Review Board at the University of Pennsylvania.
2.2 Discourse Social Coordination Task
2.2.1 Stimuli
Participants were presented with two-scene stories illustrating the movement of a target toy animal. In each story, the target animal was moved from the floor to a shelf (i.e. a three by four grid) of competing objects variably sharing color, size, and pattern features with the target object (see Figure 1). Participants were asked to describe the scene with sufficient detail so a conversational partner (an avatar visible behind the shelf) could correctly identify the moving animal.
Figure 1.
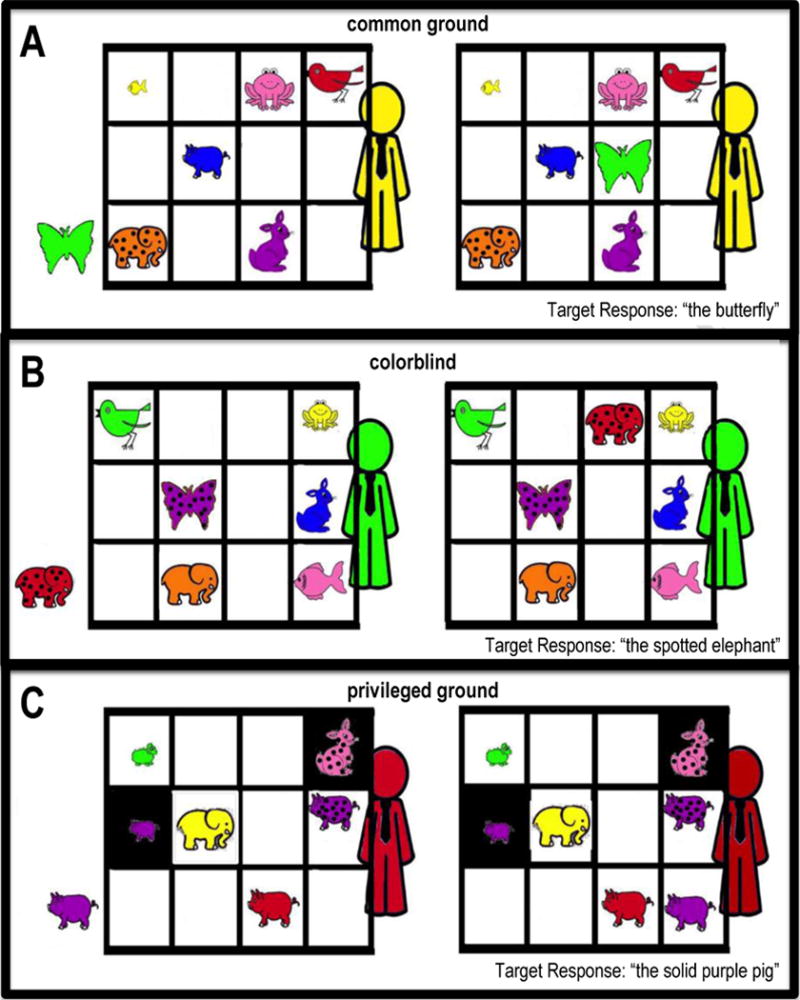
Experimental Design. Participants were presented two-scene stories in which a target animal moves from one location to another. We illustrate this with sample stimuli from the three conditions (A: common ground, B: colorblind, C: privileged ground). The stimuli above also vary in the number of competitors (i.e. animals of the same species as the target animal) visible in the scene (A: 0 competitors, B: 1 competitor, C: 3 competitors). Target responses are indicated in the lower right hand corner of each panel.
To assess coordination and perspective-taking, trials varied in the amount of information available to the avatar. In “common ground” trials, the avatar had equal access to visual information. In “colorblind” trials, the avatar was said to be completely colorblind (i.e. only able to see in grayscale). In “privileged ground” trials, there was a physical obstruction blocking the avatar’s view of selected portions of the shelf so that only the participant could see some objects. The latter two conditions were hypothesized to put increasing demand on the participant’s perspective-taking ability. In the privileged ground condition, there was a physical reminder of the different perspectives available to the participant and the avatar; in the colorblind condition, there was no such physical reminder, and instead the phrase “colorblind” was placed in front of the participant.
In order to manipulate resource demands, the stimuli differed according to the number of competitors (i.e. objects displaying a shared feature). The number of competitors visible in the scene (0, 1, or 3) partially determined the number and type of adjectives necessary for the participant to adequately distinguish the target animal when describing its movement to the avatar.
Following presentation of each story, the subject was asked to describe the scene with sufficient detail so that the avatar could identify which animal was moving. There were eight stories for each level of competitor (0, 1, 3) for each condition (common ground, colorblind, privileged ground), with a total of 72 stimuli equally distributed across conditions. Stimuli were presented in a pseudo-randomized order to ensure that a single condition was not repeated across consecutive trials. Subjects were trained prior to testing by familiarizing them with task materials and providing feedback to their responses. All patients appeared to understand the task. In total, task administration took approximately one hour.
2.2.2 Scoring
Subject responses were digitally recorded and later transcribed using the speech analysis program Praat. Responses were coded by the first author, who was blind to group membership. Responses were categorized as precise, superfluous, or insufficient, depending on the adjectives used to describe the moved object. Precise responses used the exact number of adjectives necessary to distinguish the target animal; superfluous responses used an excess number of adjectives, and insufficient responses were lacking necessary adjectives. For example, when the target animal differed from its competitors only in terms of color, the precise response would be “the red pig move…”, a superfluous response would be “the solid red pig moved…” and an insufficient response would be “the pig moved…”. Precise and superfluous responses were also summed to create an overall accuracy score, since both types of responses would allow the avatar to correctly identify the target animal. We used non-parametric statistics to analyze behavioral performance because the data were not normally distributed according to Levene’s tests.
2.3 Imaging Procedure and Analysis
High-resolution volumetric T1-weighted MRI was available within an average of 6.04 months (SEM=1.95 months) from the date of behavioral testing for 9 bvFTD patients. MRI images were not available for a subset of individuals with bvFTD (n=3) for health and safety reasons, including claustrophobia and metallic implants (e.g. pacemakers, shrapnel) in the body. MRI volumes were acquired using an MPRAGE sequence from a SIEMENS 3.0T Trio scanner with an 8-channel head coil and the following acquisition parameters: repetition time=1620 msec; echo time=3.87 msec; slice thickness=1.0 mm; flip angle=15°; matrix=192×256, and in-plane resolution=0.98×0.98 mm. Whole-brain MRI volumes were preprocessed using PipeDream (https://sourceforge.net/projects/neuropipedream/) and Advanced Normalization Tools (http://www.picsl.upenn.edu/ANTS/) using a state-of-the-art procedure described previously (Avants et al., 2008; Klein et al., 2010; Tustison et al., 2014). Briefly, PipeDream deforms each individual dataset into a standard local template space. A diffeomorphic deformation was used for registration that is symmetric to minimize bias toward the reference space for computing the mappings, and topology-preserving to capture the large deformation necessary to aggregate images into a common space. Template-based priors are used to guide GM segmentation and compute GM probability, which reflects a quantitative measure of GM density. Resulting images were warped into MNI space, smoothed using a 5 mm full-width half-maximum Gaussian kernel and downsampled to 2 mm resolution to account for variation in individual gyral anatomy.
Permutation-based imaging analyses were performed with threshold-free cluster enhancement using the randomise tool in FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki). Briefly, permutation-based t-tests evaluate a true assignment of GM density across groups (signal) relative to many (10,000) random assignments of GM density across groups (noise) and thus is a statistically robust procedure that is much less susceptible to multiple comparisons problems compared to traditional parametric-based t-tests. GM density was compared in patients relative to healthy seniors (an independent group of 35 healthy seniors with imaging who were comparable to the patient group for age (t(42)=0.52, ns) and education (t(42)=0.86, ns). Analyses were run with 10,000 permutations and restricted to voxels containing GM using an explicit mask generated from the average gray matter probability map of all groups. We report clusters that survived a threshold of p<0.005 (uncorrected) and containing a minimum of 200 adjacent voxels.
To relate behavioral performance to regions of significant GM disease, we used regression analyses with the randomise tool of FSL and threshold-free cluster enhancement, as described above. Permutations were run exhaustively up to a maximum of 10,000 for each analysis. To constrain our interpretation to areas of known GM disease, we restricted our regression analyses to an explicit mask containing voxels of GM atrophy in the patients as defined in the group comparison. Regressions outside these regions of known disease would be difficult to interpret since they could be attributed to a variety of factors associated with individual differences in GM density, including healthy aging and genetic factors. We report clusters surviving a height threshold of p<0.005(uncorrected) and containing a minimum of 10 adjacent voxels.
3. Results
3.1 Behavioral Results
Our first analysis focused on overall accuracy (whether or not participants correctly produced necessary adjectives) and revealed that patients with bvFTD (mean=52.03% correct, SD=8.65) are less accurate overall compared to healthy seniors [mean=78.45% correct, SD=11.04, U(24)=6.00, p<0.001)]. Correlation analyses using Spearman’s rank correlation coefficient indicated that accuracy in patients did not correlate with MMSE (r=0.174, p=0.588), any of the language measures [Boston Naming Test: r=0.51, p = 0.09; Language Scale, PBAC: r=0.44, p=0.150; Pyramids and Palm Trees: r = −0.45, p = 0.167], or with the caregiver-based apathy measure (total frequency × severity domain score) of the Neuropsychiatric Inventory (r=0.13, p=0.685). These data suggest that performance was not related to a language-specific impairment or to apathetic behavior.
When conditions were compared using a Friedman test, we found a significant difference performance across common ground, colorblind, and privileged ground conditions (Q=12.58, p<0.01). As illustrated in Figure 2A, subsequent Mann-Whitney tests investigating group differences within each condition showed that patients with bvFTD are significantly less accurate than healthy seniors in describing the movement of a target animal in both the common ground (U(24)=12.00, p<0.001) and colorblind conditions (U(24)=3.00, p<0.001). Patients did not differ significantly from healthy seniors in the privileged ground condition (U(22)=35.50, Z=1.843, p=0.10), suggesting that the physical reminder of the avatar’s obstructed view prompted patients to be more sensitive to a conversational partner.
Figure 2.
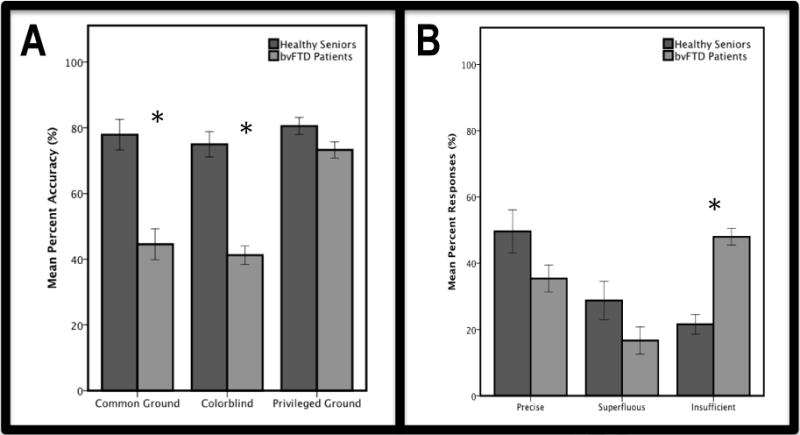
Panel A: Mean (±SEM) percent accuracy of responses in common ground, colorblind, and privileged ground conditions for healthy seniors (blue) and bvFTD patients (green). bvFTD patients performed significantly worse than healthy controls on common ground and colorblind trials, but not on privileged ground trials. Panel B: Mean (±SEM) percent responses classified as precise, superfluous, and insufficient for healthy seniors (blue) and bvFTD patients (green). bvFTD patients provide significantly more insufficient responses than healthy seniors.
Next, we conducted a qualitative error analysis by investigating the types of responses that patients produced when they erred, collapsing across all conditions. The results of this analysis, illustrated in Figure 2B, demonstrated that patients are significantly more likely than controls to give responses that are categorized as insufficient (U(24)=6.00, Z=44.02, p<0.001). Thus, patients omitted adjectives that would have been useful in identifying the target object for a conversational partner. Importantly, patients never gave responses that include factually incorrect information (e.g. misidentifying blue as green), which provides further evidence that the observed social coordination deficits are not related to lexical retrieval or visuospatial difficulty.
Within-group comparisons using Wilcoxon signed ranks test revealed that patients are equally impaired on common ground and colorblind trials (Z=−1.56, p=0.120), despite the hypothesized difference in perspective-taking demands associated with these conditions. Therefore, we conducted a follow-up analysis specifically examining the use of superfluous color terms across conditions. In common ground and privileged ground trials, the use of a color term was superfluous if it was not needed for the conversational partner to identify the target. In colorblind trials, use of color terms was always superfluous. As illustrated in Figure 3, we found a significant difference for the colorblind condition (U(24)=24.00, p<0.01). Patients demonstrated their insensitivity to the colorblindness of the conversational partner by using superfluous color terms significantly more often than healthy seniors. Indeed, according to within-group comparisons using the Wilcoxon signed ranks test, they used color terms in the colorblind condition as often as they did in the common ground condition, where color terms are informative and appropriate (Z= −0.51, p=0.959). Healthy seniors, on the other hand, adopted an effective strategy and decreased their use of color terms in the colorblind condition compared to the common ground condition (Z= −2.20, p<0.05). There were no group differences in color term use for the common ground condition (U(26)=82.00, p=0.940) or the privileged ground condition (U(23)=52.00, p=0.446).
Figure 3.
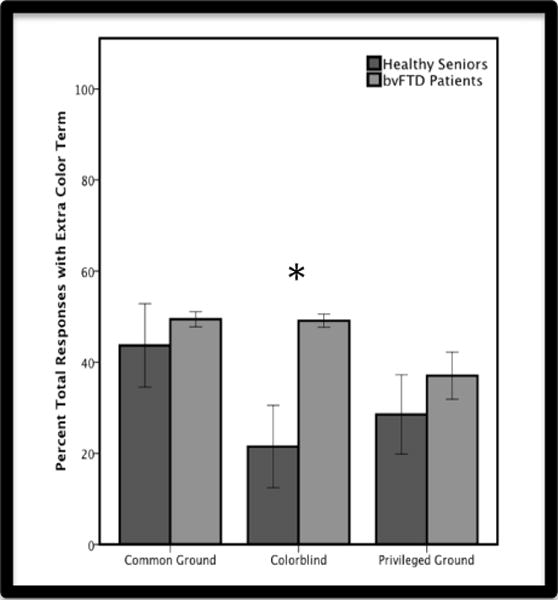
Mean (±SEM) percent responses that include a superfluous color term in common ground, colorblind, and privileged ground conditions for healthy seniors (blue) and bvFTD patients (green). Patients use significantly more color terms in colorblind trials than healthy controls, but not in common ground or privileged ground trials.
Finally, we examined the effect of resource demands across groups. No difference in performance was observed across groups for 0-competitor trials. This confirms that patients understood the task structure and were capable of visualizing and describing the materials appropriately. We computed a normalized difference score by calculating the percent difference in accuracy between 3-competitor and 1-competitor trials, and dividing this by percent accuracy in 1-competitor trials ([3COMP – 1COMP]/1COMP]), in order to account for differences in baseline performance across subjects and groups. We found that bvFTD patients (mean= 2212 79.77%; SD=18.22) are significantly more affected by resource demands than healthy seniors (mean= −46.04%; SD=27.03; U=27.50, p<0.01). However, Wilcoxon tests for within-group comparisons revealed that there are no significant differences across coordination conditions for either group (healthy seniors: p=0.40, bvFTD: p=0.50). Therefore, bvFTD patients showed reduced working memory, but this did not appear to interact with coordination and perspective-taking, suggesting the deficit observed during social coordination is largely independent of a limitation in executive resources per se.
3.2 Imaging Results
We contrasted GM density in bvFTD patients relative to healthy seniors. This revealed significantly reduced GM density throughout the frontal and temporal lobes, as summarized in Table 2 and illustrated in Figure 4A. To relate deficits in coordination to GM density, we performed a regression analysis within patients using the accuracy score from all trials as the independent variable and restricted to regions of known GM disease. This analysis revealed that impaired performance on the coordination task is associated with reduced GM density in medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC), dorsolateral prefrontal cortex (dlPFC), and anterior cingulate cortex (ACC) (see Table 2 and green regions in Figure 4B). Results of a whole-brain regression are also reported in the Supplementary Materials (see Supplementary Figure 1 and Supplementary Table 1).
Table 2.
Clusters of reduced gray matter density in patients with bvFTD relative to healthy seniors, and clusters of reduced gray matter density related to performance (accuracy score across all trials) in bvFTD patients. BA, Brodmann Area; MNI, Montreal Neurological Institute.
| MNI Coordinates
|
||||||
|---|---|---|---|---|---|---|
| Neuroanatomic Region (BA) | L/R | x | y | z | p value | voxels |
| Clusters of reduced gray matter density in patients with bvFTD relative to controls | ||||||
|
| ||||||
| orbitofrontal cortex (11) | R | 26 | 34 | −16 | <0.001 | 29,345 |
| fusiform gyrus (37) | R | 40 | −50 | −16 | <0.001 | 145 |
| fusiform gyrus(37) | L | −46 | −54 | −20 | <0.001 | 124 |
| inferior temporal gyrus (37) | R | 50 | −66 | −14 | <0.001 | 77 |
| posterior cingulate gyrus (23) | R | 20 | −64 | 4 | <0.001 | 56 |
| Clusters of reduced gray matter density related to overall accuracy in patients | ||||||
|
| ||||||
| dorsolateral prefrontal cortex (46) | L | −46 | 22 | 26 | <0.001 | 45 |
| anterior cingulate cortex (24) | L | −6 | 36 | −4 | 0.001 | 32 |
| superior frontal gyrus (6) | R | 24 | 10 | 52 | 0.001 | 22 |
| anterior cingulate cortex (32) | L | −4 | 30 | 30 | <0.001 | 18 |
| orbitofrontal cortex (11) | L | −28 | 44 | −16 | 0.001 | 18 |
| anterior cingulate cortex (32) | R | 10 | 32 | 28 | 0.002 | 17 |
| orbitofrontal cortex (47) | L | −36 | 32 | −14 | 0.001 | 17 |
| middle frontal gyrus (8) | R | 30 | 30 | 46 | <0.001 | 15 |
| medial prefrontal cortex (10) | L | −12 | 50 | 6 | 0.001 | 11 |
| dorsolateral prefrontal cortex (9) | R | 30 | 36 | 34 | <0.001 | 10 |
Figure 4.
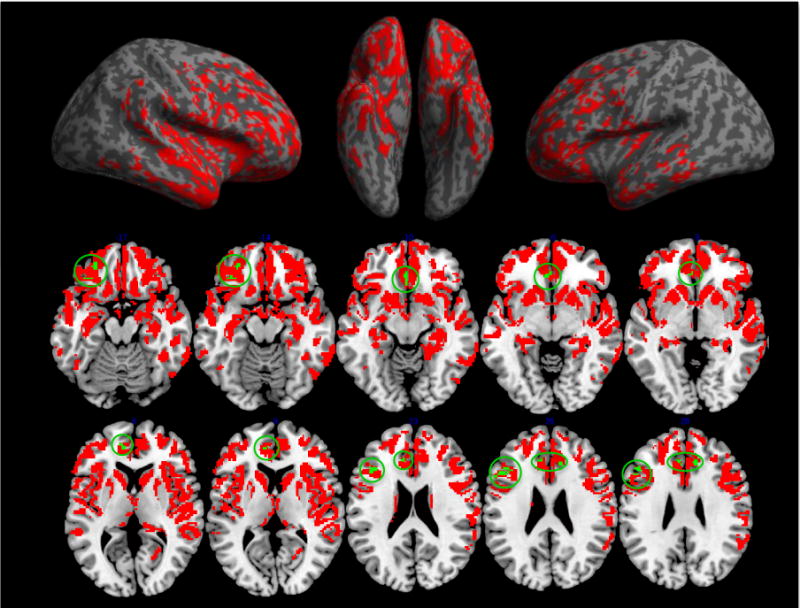
Panel A. Surface renderings depicting regions of significantly reduced GM density in bvFTD patients relative to healthy seniors. Panel B. Regions of significantly reduced GM density in bvFTD patients relative to healthy seniors (all colored areas) and regions of significantly reduced GM density associated with impaired social coordination (accuracy score across all trials) in bvFTD patients (green areas, circled).
To specifically examine the neuroanatomic basis for coordination and perspective-taking, we performed regression analyses using the accuracy scores from the colorblind and privileged ground conditions, both of which were hypothesized to put increasing demand on perspective-taking due to differences in the information available to the patient and conversational partner. Results indicated that performance in the colorblind condition was uniquely related to reduced GM density in OFC and mPFC, as well as portions of ACC and inferior temporal gyrus (see Table 3 and green regions in Figure 5A). The privileged ground condition implicated a unique, non-overlapping set of brain regions, including dlPFC (see Table 3 and green regions in Figure 5B).
Table 3.
Clusters of reduced gray matter density related to performance on colorblind and privileged ground trials. BA, Brodmann Area; MNI, Montreal Neurological Institute.
| MNI Coordinates
|
||||||
|---|---|---|---|---|---|---|
| Neuroanatomic Region (BA) | L/R | x | y | z | p value | voxels |
| Clusters of reduced GM density related to patient performance in the colorblind condition. | ||||||
|
| ||||||
| orbitofrontal cortex (11) | L | −14 | 14 | −24 | <0.001 | 112 |
| anterior cingulate cortex (24) | L | −2 | 24 | 20 | <0.001 | 23 |
| medial orbitofrontal cortex (11) | R | 10 | 52 | −4 | 0.002 | 22 |
| orbitofrontal cortex (11) | R | 8 | 44 | −20 | 0.002 | 20 |
| orbitofrontal cortex (47) | L | −28 | 22 | −16 | 0.001 | 18 |
| middle frontal gyrus (8) | R | 20 | 20 | 46 | 0.002 | 16 |
| orbitofrontal cortex (11) | L | −28 | 44 | −14 | 0.002 | 13 |
| anterior cingulate cortex (32) | R | 10 | 24 | 38 | 0.002 | 11 |
| medial orbitofrontal cortex (11) | R | 4 | 58 | −16 | 0.001 | 10 |
| orbitofrontal cortex (11) | L | −6 | 46 | −22 | 0.001 | 10 |
| inferior temporal gyrus (20) | L | −64 | −6 | −24 | 0.001 | 10 |
| Clusters of reduced GM density related to patient performance in the privileged ground condition | ||||||
|
| ||||||
| dorsolateral prefrontal cortex (46) | L | −46 | 18 | 26 | 0.001 | 21 |
| superior frontal gyrus (10) | L | −32 | 56 | 2 | <0.001 | 20 |
| inferior frontal gyrus (44) | L | −58 | 18 | 12 | 0.001 | 13 |
| orbitofrontal cortex (47) | L | −30 | 32 | −12 | 0.003 | 12 |
Figure 5.
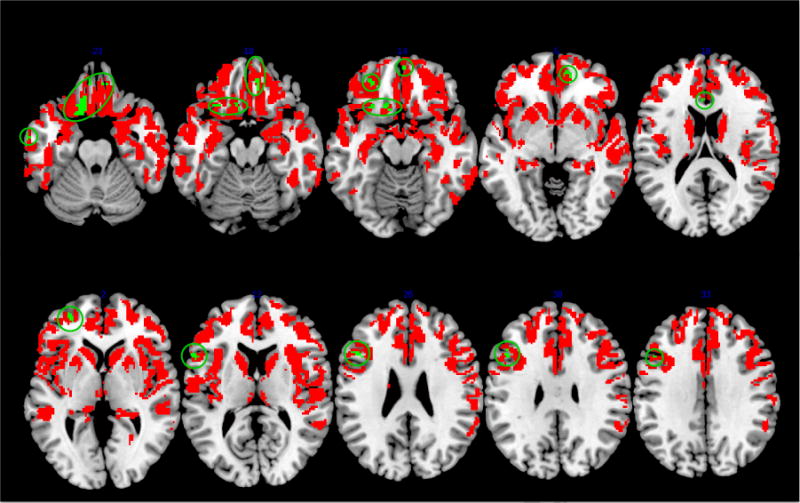
Regions of significantly reduced gray matter density selectively related to performance in colorblind (panel A) and privileged ground (panel B) conditions (green areas). Red areas represent regions of significant cortical thinning in bvFTD patients relative to healthy controls.
4. Discussion
This study examined the neuroanatomic basis of social coordination deficits observed in bvFTD. We used a novel task that actively engages patients in demonstrating their perspective-taking by asking them to describe an object, and we carefully minimized task-related demands. Our results suggest that bvFTD patients have perspective-taking difficulties, offering insufficient descriptions of the given conversation topic and providing colored attributes that were inaccessible to a colorblind partner. This impairment was related to reduced GM density in medial, dorsolateral, and orbital frontal cortices. Performance on colorblind trials was specifically related to mPFC and OFC, suggesting roles for these areas in social perspective-taking. Overall, these results are consistent with social coordination theory and support the view that successful communication involves social processing and is supported in part by prefrontal activity. We discuss the behavioral and anatomic results in turn below.
4.1 Social coordination during discourse in behavioral variant frontotemporal degeneration
Only a limited number of studies to date have examined perspective-taking in a communicative context (Dumontheil et al., 2010; Hillebrandt et al., 2013; Wardlow, 2013; Wardlow et al., 2014). For example, in an fMRI study of healthy volunteers, Dumontheil et al., (2010) used a variant of the director task, originally described by Keysar and colleagues (Keysar et al., 2000, 2003). The authors report activation in mPFC and left temporal pole for the critical condition (director present, 3-objects), which partially overlaps with our findings. In a follow-up study, Hillebrandt et al., (2013) used dynamic causal modeling to show that the social demands of the task modulate backward connections from mPFC. The paradigm in both studies, however, is comprehension-based and requires subjects to identify the target object via a motor response. Some referential communication studies in healthy adults and patients with Alzheimer’s disease (Wardlow, 2013; Wardlow et al., 2014) do involve overt speech responses, but not embedded within a discourse context. These studies also used smaller arrays of objects that may not adequately mimic the complex nature of real-world conversation. Therefore, while the current study constrains conversation to brief exchanges, it benefits from increased ecological validity as natural, self-generated speech is used to describe the stimuli to a conversational partner.
Our results suggest that bvFTD patients, who provide too few adjectives for a conversational partner to correctly identify the target animal, have perspective-taking limitations. This cannot be fully explained by a language deficit since the patients show largely intact language as assessed by a brief battery of language tests (i.e. Boston Naming Test, Philadelphia Brief Assessment of Cognition, Pyramids and Palm Trees). Moreover, their performance matched controls’ performance when no competitors were present. Apathy is frequently documented in bvFTD (Massimo et al., 2009), and we cannot entirely dismiss its contribution to the phenotype observed here. However, the lack of correlation between the Neuropsychiatric Inventory’s apathy score and performance on the coordination task suggests that apathy alone is also unlikely to explain the patients’ reduced production of adjectives.
Additional support implicating limitations in perspective-taking comes from the novel “colorblind” condition. Previous studies examining perspective-taking (Dumontheil et al., 2010; Hillebrandt et al., 2013; Wardlow, 2013; Wardlow et al, 2014) used stimuli that physically obstruct one partner’s view, much like our privileged ground condition. However, the perspective-taking required during most conversations involves not necessarily the alignment of visuospatial references, but rather the alignment of mental representations and situation models. The colorblind condition utilized here, which is identical in visual appearance to the common ground condition and does not include any physical reminder of the avatar’s limited knowledge state beyond the written condition label, requires the subject to consider which attributes will be informative for the avatar. In this sense, the colorblind condition involves appreciating the avatar’s mental state and recognizing shared information. This is a more complex and abstract form of social perspective-taking that may account for the dissociation we observe behaviorally: while patients perform as well as healthy seniors on privileged ground trials when there is a physical prompt (i.e. the opaque portions of shelf), they are significantly impaired on colorblind trials.
Although patients appeared to perform comparably on common ground and colorblind trials, closer examination revealed a crucial distinction. An error analysis evaluating use of color terms demonstrated that patients are essentially insensitive to the avatar’s colorblind status and refer to color terms significantly more than healthy seniors in the colorblind condition. Furthermore, while healthy seniors showed evidence of perspective-taking and decreased color term use in the colorblind versus common ground condition, patients showed no such modulation of behavior.
While our behavioral results align well with the existing literature on ToM, the design used here represents an important methodological improvement. Many previous studies on ToM used “false belief and other story-based paradigms that involve extensive task-related performance demands, including maintaining complex relationships between actors throughout multi-sentence narratives. Caution must be used when interpreting the results of such tasks as they may have conflated theory of mind with executive function. Our measure minimized such confounds, as patients were merely asked to describe a target object using one or two adjectives and natural self-generated speech. Furthermore, we manipulated perspective-taking (i.e. the knowledge state of the avatar) and working memory (i.e. number of competitors) independently, finding that patients show a greater decrease in performance relative to healthy seniors as working memory demands (i.e. competitor number) increased. This is consistent with previous observations of bvFTD patients (Kramer et al., 2003; Libon et al., 2007). Nevertheless, we failed to observe an interaction between working memory and perspective-taking, since patients did not appear to show an effect of increased competitor number for a specific coordination condition. Thus, we were able to demonstrate more conclusively that patients with bvFTD do have a deficit in perspective-taking per se that is largely independent of any deficit in executive function. Additional work is needed to assess the subtle ways in which social perspective-taking and executive resources may interact during social coordination.
4.2. Neuroanatomic basis for social coordination during discourse in behavioral variant frontotemporal degeneration
We identified a large-scale neural network associated with task performance that encompassed dlPFC, mPFC, OFC, insula and ACC. These areas are elements of the “social brain network” described previously by others (Adolphs, 2003; Frith and Frith, 2007). Interestingly, even though we examined spoken discourse, these areas overlap minimally with the peri-Sylvian language network. These data are consistent with our conclusion that, the limitations in perspective-taking we observed during discourse in bvFTD cannot by entirely explained by a language deficit. Accordingly, our data are less consistent with strict interpretations of interactive-alignment, which hypothesizes that alignment in conversation is supported predominantly by co-activation of the language network (particularly BA44 and BA21) in speakers and listeners (Menenti et al., 2011, 2012). Although language clearly contributes to conversational competence, areas beyond the traditional language network alone appear to be involved in real-world communication. Our findings are also somewhat inconsistent with the idea that successful communication is purely the result of simulation that is driven by mirror neuron activity in premotor cortex. Instead, our data appear to be more consistent with social coordination theory and suggest that successful communication is at least partially dependent upon additional prefrontal regions that supplement traditional brain regions thought to support language processing. It is important to emphasize that these accounts (i.e. social coordination theory, interactive alignment, simulation theory) are not necessarily mutually exclusive but may operate in concert. Future fMRI studies in healthy adults using a whole-brain approach can address the possibility that both linguistic and social neuroanatomic networks contribute to the success of communication and mutual understanding between two or more partners.
Subsequent regression analyses examined the potential role that these frontal regions may play in social coordination in more detail. Accuracy on colorblind trials was specifically associated with reduced GM density in mPFC and OFC. mPFC has been implicated consistently in fMRI studies of healthy adults examining ToM and the ability to interpret other’s beliefs or intentions (Gallagher and Frith, 2003; Saxe and Kanwisher, 2003; Van Overwalle, 2011). OFC, on the other hand, has been implicated in decision-making, including the ability to encode stimulus-outcome contingencies, assess potential risk and reward, and perform tasks involving reversal learning or response inhibition (Murray et al., 2007; Viskontas et al, 2007; Wallis, 2007). In the current experiment, OFC damage may thus contribute to the inappropriate and superfluous references to color seen in patients in the colorblind condition. Furthermore, the current data may also implicate OFC in social perspective-taking, a multi-component process that likely involves several of the aforementioned functions. Indeed, some have considered perspective-taking to be a two-stage process: 1) inhibiting one’s own perspective and 2) belief reasoning (i.e. interpreting and adopting another’s perspective) (van der Meer et al., 2011). Although findings have been inconsistent, other studies assessing perspective-taking and mentalizing in clinical populations have also suggested a role for OFC (Stone et al., 1998; Sabbagh, 2004; Channon et al., 2007; Kipps et al., 2009; Goodkind et al., 2012). For example, patients with OFC disease appear to have difficulty tracking the dynamically changing emotions of a character in a film clip (Goodkind et al., 2012) and deciding whether an actor in a video is expressing sarcastic or sincere statements (Kipps et al., 2009). Interestingly, these areas implicated in the colorblind condition are unique when compared to those implicated by the privileged ground condition. This again confirms our conclusion that social but not visual perspective-taking is specifically impaired in bvFTD and associated with a partially distinct cortical network.
As described above, the regression on performance across all trials also implicated the dlPFC and ACC. Briefly, the dlPFC is classically associated with top-down attentional regulation, working memory, and selection amongst competing responses (Petrides, 2005; Suzuki and Gottlieb, 2013). Additional research has suggested that dlPFC is associated with second-order relational complexity and assessing concrete relationships amongst objects, which is relevant in the given context, as subjects needed to identify which features of a given object were shared versus unique (Badre, 2008). The ACC was also related to performance across all trials and is commonly associated with error detection, response conflict, and performance monitoring (Chang et al., 2013; Alexander and Brown, 2010; Carter et al., 1998).
It is important to point out that previous work has demonstrated that right anterior temporal regions may also contribute to social and emotional processing (Olson et al., 2007; Wong and Gallate, 2012; Irish et al., 2014). Interestingly, we find little evidence for ATL involvement in social coordination, suggesting that perhaps its function is more tied to social knowledge rather than coordination and perspective-taking per se.
While these findings allow us to begin disentangling the roles of specific prefrontal regions in social coordination, several caveats should be kept in mind when interpreting our results. Despite observations consistent with our hypotheses, we studied a small group of these rare patients and used somewhat lenient statistical thresholds. Furthermore, given the wide range of observed MMSE scores, there may be subgroups present in our patient population, although we did not see evidence of this. As a result, replication in an independent cohort with a patient control group would be valuable. Converging evidence from fMRI studies in healthy adults using the same stimulus materials would also lend additional support to our findings. These future studies might also adopt a more exploratory, whole-brain approach and examine potential effects of aging and individual differences on social coordination. Next, while we performed a detailed analysis of non-aphasic patients’ speech production in a structured context, it would be valuable to develop a comprehension-based paradigm that further reduces task-related demands. Although we were able to demonstrate that performance on the current measure is not correlated with confrontation naming or semantic knowledge, a comprehension-based measure also would minimize any difficulties that could be attributed to impaired lexical retrieval. Finally, our paradigm was designed to directly engage patients in perspective-taking (versus passive observation), but we used an avatar to represent a conversational partner. Future studies might benefit from using paradigms that examine truly interactive exchanges between two (or more) human partners. With these caveats in mind, our observations offer preliminary support for the claim that social coordination in a discourse context is compromised in bvFTD due to perspective-taking limitations and degradation of a prefrontal network that supports perspective-taking and social coordination.
Supplementary Material
Highlights.
bvFTD patients show coordination and perspective-taking deficits in conversation.
Deficits in social coordination are independent of deficits in executive function.
Coordination during discourse is dependent upon prefrontal regions.
Acknowledgments
This work was supported in part by grants from NIH (AG017586, NS044266, NS088341, AG032953, AG038490, AG043503, 5-T32-GM007517) and the Wyncote Foundation.
We thank Jonathan Peelle and Michael Bonner for helpful comments on this manuscript, the radiographers at the Hospital of the University of Pennsylvania for their assistance with data collection, and all our volunteers for their participation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Ash S, Moore P, Antani S, McCawley G, Work M, Grossman M. Trying to tell a tale: discourse impairments in progressive aphasia and frontotemporal dementia. Neurology. 2006;66:1405–1413. doi: 10.1212/01.wnl.0000210435.72614.38. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Channon S, Rule A, Maudgil D, Martinos M, Pellijeff A, Frankl J, Drury H, Shieff C. Interpretation of mentalistic actions and sarcastic remarks: effects of frontal and posterior lesions on mentalising. Neuropsychologia. 2007;45:1725–1734. doi: 10.1016/j.neuropsychologia.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Clark R. Meaningful Games: Exploring Language With Game Theory. Cambridge, MA: The MIT Press; 2011. [Google Scholar]
- Cooke A, Grossman M, DeVita C, Gonzalez-Atavales J, Moore P, Chen W, Gee J, Detre J. Large-scale neural network for sentence processing. Brain Lang. 2006;96:14–36. doi: 10.1016/j.bandl.2005.07.072. [DOI] [PubMed] [Google Scholar]
- Cosentino S, Chute D, Libon D, Moore P, Grossman M. How does the brain support script comprehension? A study of executive processes and semantic knowledge in dementia. Neuropsychology. 2006;20:307–318. doi: 10.1037/0894-4105.20.3.307. [DOI] [PubMed] [Google Scholar]
- Couto B, Manes F, Montañés P, Matallana D, Reyes P, Velasquez M, Yoris A, Baez S, Ibáñez A. Structural neuroimaging of social cognition in progressive non-fluent aphasia and behavioral variant of frontotemporal dementia. Front Hum Neurosci. 2013;7:467. doi: 10.3389/fnhum.2013.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I, Küster O, Apperly Ia, Blakemore S-J. Taking perspective into account in a communicative task. Neuroimage. 2010;52:1574–1583. doi: 10.1016/j.neuroimage.2010.05.056. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Moore P, Troiani V, Antani S, Cross K, Kwok S, Grossman M. Oops! Resolving social dilemmas in frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2007;78:457–460. doi: 10.1136/jnnp.2006.098228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag C, Troiani V, Bonner M, Powers C, Avants B, Gee J, Grossman M. Hierarchical organization of scripts: converging evidence from FMRI and frontotemporal degeneration. Cereb Cortex. 2010;20:2453–2463. doi: 10.1093/cercor/bhp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Duque D, Baird Ja, Black SE. False-belief understanding in frontotemporal dementia and Alzheimer’s disease. J Clin Exp Neuropsychol. 2009;31:489–497. doi: 10.1080/13803390802282688. [DOI] [PubMed] [Google Scholar]
- Ferstl EC, Neumann J, Bogler C, von Cramon DY. The extended language network: a meta-analysis of neuroimaging studies on text comprehension. Hum Brain Mapp. 2008;29:581–593. doi: 10.1002/hbm.20422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M, Binns Ma, Black SE, Murphy C, Stuss DT. Theory of mind and recognition of facial emotion in dementia: challenge to current concepts. Alzheimer Dis Assoc Disord. 2013;27:56–61. doi: 10.1097/WAD.0b013e31824ea5db. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Social cognition in humans. Curr Biol. 2007;17:R724–R732. doi: 10.1016/j.cub.2007.05.068. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of “theory of mind. Trends Cogn Sci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gallese V. Before and below “theory of mind”: embodied simulation and the neural correlates of social cognition. Philos Trans R Soc Lond B Biol Sci. 2007;362:659–669. doi: 10.1098/rstb.2006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind MS, Sollberger M, Gyurak A, Rosen HJ, Rankin KP, Miller B, Levenson R. Tracking emotional valence: the role of the orbitofrontal cortex. Hum Brain Mapp. 2012;33:753–762. doi: 10.1002/hbm.21251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C, Lough S, Stone V, Erzinclioglu S, Martin L, Baron-cohen S, Hodges JR. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer’s disease : theoretical and practical implications. 2002:752–764. doi: 10.1093/brain/awf079. [DOI] [PubMed] [Google Scholar]
- Grossman M, Eslinger PJ, Troiani V, Anderson C, Avants B, Gee JC, McMillan C, Massimo L, Khan A, Antani S. The role of ventral medial prefrontal cortex in social decisions: converging evidence from fMRI and frontotemporal lobar degeneration. Neuropsychologia. 2010;48:3505–3512. doi: 10.1016/j.neuropsychologia.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P. Nodes and networks in the neural architecture for language: Broca’s region and beyond. Curr Opin Neurobiol. 2014;28C:136–141. doi: 10.1016/j.conb.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Henry JD, Phillips LH, von Hippel C. A meta-analytic review of theory of mind difficulties in behavioural-variant frontotemporal dementia. Neuropsychologia. 2014;56:53–62. doi: 10.1016/j.neuropsychologia.2013.12.024. [DOI] [PubMed] [Google Scholar]
- Hillebrandt H, Dumontheil I, Blakemore S-J, Roiser JP. Dynamic causal modelling of effective connectivity during perspective taking in a communicative task. Neuroimage. 2013;76:116–124. doi: 10.1016/j.neuroimage.2013.02.072. [DOI] [PubMed] [Google Scholar]
- Irish M, Hodges JR, Piguet O. Right anterior temporal lobe dysfunction underlies theory of mind impairments in semantic dementia. 2014 doi: 10.1093/brain/awu003. [DOI] [PubMed] [Google Scholar]
- Keysar B, Barr DJ, Balin Ja, Brauner JS. Taking Perspective in Conversation: The Role of Mutual Knowledge in Comprehension. Psychol Sci. 2000;11:32–38. doi: 10.1111/1467-9280.00211. [DOI] [PubMed] [Google Scholar]
- Keysar B, Lin S, Barr DJ. Limits on theory of mind use in adults. Cognition. 2003;89:25–41. doi: 10.1016/s0010-0277(03)00064-7. [DOI] [PubMed] [Google Scholar]
- Kipps CM, Hodges JR. Theory of mind in frontotemporal dementia. Soc Neurosci. 2006;1:235–244. doi: 10.1080/17470910600989847. [DOI] [PubMed] [Google Scholar]
- Kipps CM, Nestor PJ, Acosta-Cabronero J, Arnold R, Hodges JR. Understanding social dysfunction in the behavioural variant of frontotemporal dementia: the role of emotion and sarcasm processing. Brain. 2009;132:592–603. doi: 10.1093/brain/awn314. [DOI] [PubMed] [Google Scholar]
- Klein A, Ghosh SS, Avants B, Yeo BTT, Fischl B, Ardekani B, Gee JC, Mann JJ, Parsey RV. Evaluation of volume-based and surface-based brain image registration methods. Neuroimage. 2010;51:214–220. doi: 10.1016/j.neuroimage.2010.01.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, Miller BL. Distinctive Neuropsychological Patterns in Frontotemporal Dementia, Semantic Dementia, And Alzheimer Disease. Cogn Behav Neurol. 2003;16:211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Kuo W-J, Sjöström T, Chen Y-P, Wang Y-H, Huang C-Y. Intuition and deliberation: two systems for strategizing in the brain. Science. 2009;324:519–522. doi: 10.1126/science.1165598. [DOI] [PubMed] [Google Scholar]
- Le Bouc R, Lenfant P, Delbeuck X, Ravasi L, Lebert F, Semah F, Pasquier F. My belief or yours? Differential theory of mind deficits in frontotemporal dementia and Alzheimer’s disease. Brain. 2012;135:3026–3038. doi: 10.1093/brain/aws237. [DOI] [PubMed] [Google Scholar]
- Libon DJ, Xie SX, Moore P, Farmer J, Antani S, McCawley G, Cross K, Grossman M. Patterns of neuropsychological impairment in frontotemporal dementia. Neurology. 2007;68:369–375. doi: 10.1212/01.wnl.0000252820.81313.9b. [DOI] [PubMed] [Google Scholar]
- Lough S, Kipps CM, Treise C, Watson P, Blair JR, Hodges JR. Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsychologia. 2006;44:950–958. doi: 10.1016/j.neuropsychologia.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Massimo L, Powers C, Moore P, Vesely L, Avants B, Gee J, Libon DJ, Grossman M. Neuroanatomy of Apathy and Disinhibition in Frontotemporal Lobar Degeneration. Dement Geriatr Cogn Disord. 2009;27:96–104. doi: 10.1159/000194658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan CT, Rascovsky K, Khella MC, Clark R, Grossman M. The neural basis for establishing a focal point in pure coordination games. Soc Cogn Affect Neurosci. 2012;7:881–887. doi: 10.1093/scan/nsr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF, Shapira JS. Altered emotional morality in frontotemporal dementia. Cogn Neuropsychiatry. 2009;14:165–179. doi: 10.1080/13546800902924122. [DOI] [PubMed] [Google Scholar]
- Menenti L, Gierhan SME, Segaert K, Hagoort P. Psychological Science. 2011 doi: 10.1177/0956797611418347. [DOI] [PubMed] [Google Scholar]
- Menenti L, Pickering MJ, Garrod SC. Toward a neural basis of interactive alignment in conversation. 2012;6:1–9. doi: 10.3389/fnhum.2012.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray Ea, O’Doherty JP, Schoenbaum G. What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. J Neurosci. 2007;27:8166–8169. doi: 10.1523/JNEUROSCI.1556-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novais-Santos S, Gee J, Shah M, Troiani V, Work M, Grossman M. Resolving sentence ambiguity with planning and working memory resources: Evidence from fMRI. Neuroimage. 2007;37:361–378. doi: 10.1016/j.neuroimage.2007.03.077. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering MJ, Garrod S. Toward a mechanistic psychology of dialogue. Behav Brain Sci. 2004;27:169–225. doi: 10.1017/s0140525x04000056. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh Ma. Understanding orbitofrontal contributions to theory-of-mind reasoning: implications for autism. Brain Cogn. 2004;55:209–219. doi: 10.1016/j.bandc.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking peopleThe role of the temporoparietal junction in “theory of mind.”. Neuroimage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. J Cogn Neurosci. 1998;10:640–656. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Gottlieb J. Distinct neural mechanisms of distractor suppression in the frontal and parietal lobe. Nat Neurosci. 2013;16:98–104. doi: 10.1038/nn.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torralva T, Kipps CM, Hodges JR, Clark L, Bekinschtein T, Roca M, Calcagno ML, Manes F. The relationship between affective decision-making and theory of mind in the frontal variant of fronto-temporal dementia. Neuropsychologia. 2007;45:342–349. doi: 10.1016/j.neuropsychologia.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Torralva T, Roca M, Gleichgerrcht E, Bekinschtein T, Manes F. A neuropsychological battery to detect specific executive and social cognitive impairments in early frontotemporal dementia. Brain. 2009;132:1299–1309. doi: 10.1093/brain/awp041. [DOI] [PubMed] [Google Scholar]
- Troiani V, Fernández-Seara Ma, Wang Z, Detre Ja, Ash S, Grossman M. Narrative speech production: an fMRI study using continuous arterial spin labeling. Neuroimage. 2008;40:932–939. doi: 10.1016/j.neuroimage.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Meer L, Groenewold Na, Nolen Wa, Pijnenborg M, Aleman A. Inhibit yourself and understand the other: neural basis of distinct processes underlying Theory of Mind. Neuroimage. 2011;56:2364–2374. doi: 10.1016/j.neuroimage.2011.03.053. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. A dissociation between social mentalizing and general reasoning. Neuroimage. 2011;54:1589–1599. doi: 10.1016/j.neuroimage.2010.09.043. [DOI] [PubMed] [Google Scholar]
- Viskontas IV, Possin KL, Miller BL. Symptoms of frontotemporal dementia provide insights into orbitofrontal cortex function and social behavior. Ann N Y Acad Sci. 2007;1121:528–545. doi: 10.1196/annals.1401.025. [DOI] [PubMed] [Google Scholar]
- Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annu Rev Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- Wardlow L. Individual differences in speakers’ perspective taking: the roles of executive control and working memory. Psychon Bull Rev. 2013;20:766–772. doi: 10.3758/s13423-013-0396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlow L, Ivanova I, Gollan TH. The cognitive mechanisms underlying perspective taking between conversational partners: evidence from speakers with Alzheimer’s disease. Neuropsychologia. 2014;56:184–195. doi: 10.1016/j.neuropsychologia.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Gallate J. The function of the anterior temporal lobe: a review of the empirical evidence. Brain Res. 2012;1449:94–116. doi: 10.1016/j.brainres.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Yoshida W, Seymour B, Friston KJ, Dolan RJ. Neural mechanisms of belief inference during cooperative games. J Neurosci. 2010;30:10744–10751. doi: 10.1523/JNEUROSCI.5895-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


