Abstract
The cardiovascular science community has pursued the quest to identify vulnerable atherosclerotic plaque in patients for decades, hoping to prevent acute coronary events. However, despite major advancements in imaging technology that allow visualization of rupture-prone plaques, clinical studies have not demonstrated improved risk prediction compared to traditional approaches. Considering the complex relationship between plaque rupture and acute coronary event risk suggested by pathology studies and confirmed by clinical investigations, these results are not surprising. This review summarizes the evidence supporting a multifaceted hypothesis of the natural history of atherosclerotic plaque rupture. Managing patients at risk of suffering acute coronary events mandates a greater focus on the atherosclerotic disease burden, rather than on features of individual plaques.
Keywords: atherosclerosis, coronary heart disease, coronary imaging, prevention
Introduction
Cardiovascular atherosclerotic disease is the leading cause of death in Western industrialized nations and in developing countries (1). Strategies to prevent acute coronary events and their sequelae are among our most important public health priorities (2). Identifying patients at increased risk of suffering an acute coronary event who may benefit from intensified preventative measures is a major, ongoing challenge (2). Numerous factors (e.g., dyslipidemia, diabetes, and others), are associated with increased rates of adverse events; however, their hazard rates are too small for accurate individual risk assessments (3). The DIAD trial revealed that after 4.8 years of follow-up, 97% of asymptomatic patients with diabetes mellitus remained free of myocardial infarction and cardiac death (4). Even when combined as comprehensive risk scores (e.g., by the Framingham study (5)), predictive accuracy is insufficient for adequate individual risk assessments, leading to substantial overtreatment and undertreatment, with associated morbidity and societal costs (6).
The mechanisms leading to adverse events from atherosclerotic disease are clearly more complex than initially assumed, explaining our difficulties in accurately predicting events in individuals (7, 8). In addition to the presence, extent, and metabolic activity of atherosclerotic disease, individual adaptations and responses to thrombogenic stimulation from altered vascular function are critical for determining the risk of acute coronary events (7, 9). Despite a consensus on the complexity of acute coronary event risk evaluation and the necessity for comprehensive patient assessment (10,11), recent efforts to identify high-risk patients focus on using advanced imaging methods to detect single “vulnerable” atherosclerotic plaques (12). This narrow focus neglects the complexity of the processes leading to risk and lacks supporting evidence. This review summarizes the shift from conceptualizing acute coronary event risk as a simple cause-and-effect principle centered on high-risk plaques to a complex model involving numerous factors.
“The Vulnerable Plaque” Concept
Pathology studies demonstrated the common association of acute myocardial infarction with the rupture or erosion of a coronary atherosclerotic plaque (13,14), most frequently a thin-cap fibroatheroma (TCFA), characterized by a large lipid or necrotic core separated from the coronary arterial lumen by a thin membrane cap (15). Thus, identification of TCFAs in humans was assumed to signify a high risk of ensuing acute coronary events, which then might necessitate directed treatment or specific preventative measures (16). Accordingly, enormous efforts have been undertaken to enable identification of TCFAs and other high-risk plaque features in humans. The search term vulnerable plaque finds more than 400 current National Institutes of Health (NIH) research awards totaling more than $150,000,000 per year (17) and almost 2,000 research papers in the U.S. National Library of Medicine database. While not all of these efforts aim to identify “vulnerable plaques”, this topic is clearly central to many investigations involving large amounts of research dollars. Industry has also been keenly interested in developing technologies for visualization of “vulnerable plaques”, with progression of several catheter-based inventions, notably virtual histology intravascular ultrasound, thermography, infrared spectroscopy, palpography, and optical coherence tomography to preclinical or clinical stages (12,18).
Limitations of Studies Supporting the High-Risk Atherosclerotic Plaque Concept
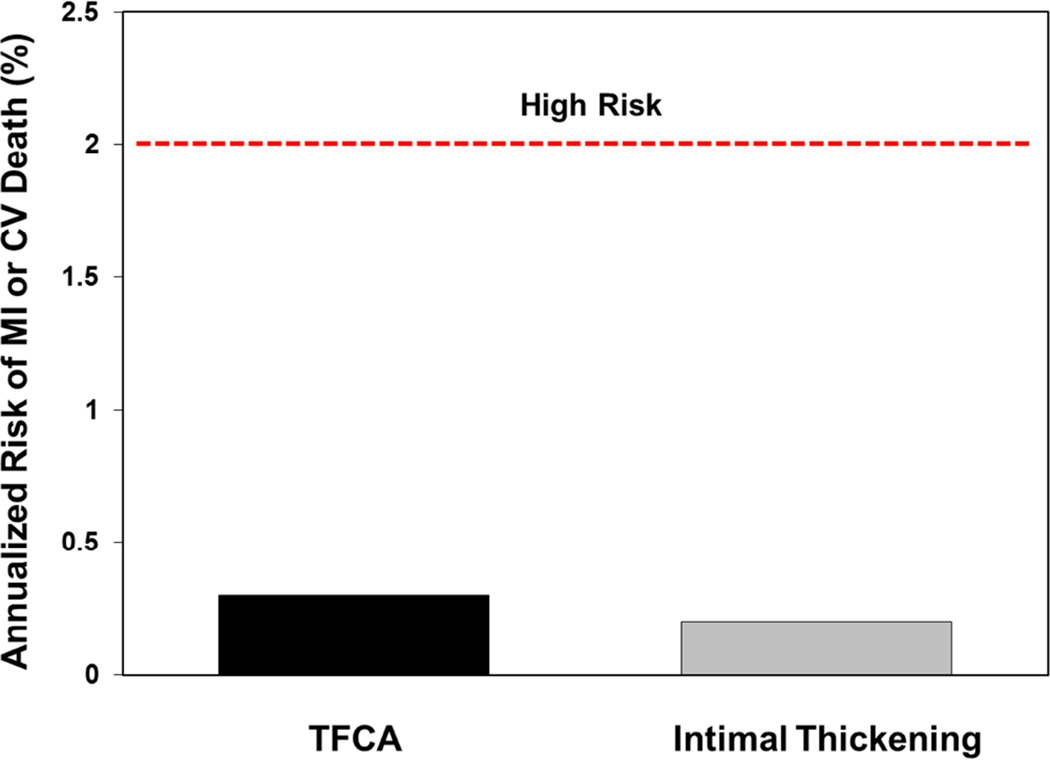
A number of clinical investigations reported using various imaging tools to identify high-risk atherosclerotic plaque features in order to predict an increased risk of adverse events. A large, prospective clinical study, PROSPECT, investigated the rates of adverse cardiac events according to types of coronary atherosclerotic plaque in more than 600 high-risk patients studied with virtual histology intravascular ultrasound (19). While 596 TCFAs were identified, only repeat hospitalization for chest pain was associated with events. This was expected, given the typically larger lumen encroachment of TCFAs compared to pathological intimal thickening (the prevalent type of lesion in the study). However, the risk of myocardial infarction or sudden cardiac death related to these lesions was very low (Figure 1). A similar study using virtual histology intravascular ultrasound (VIVA) reported nearly identical findings (20). Studies using optical coherence tomography (OCT) revealed greatly detailed plaque characteristics in patients with acute coronary syndrome and other at-risk populations (21). Similar to the information provided by intravascular ultrasound (IVUS), OCT studies suggest that a larger lesion plaque burden might indicate an increased risk of acute coronary events (22). Noninvasive imaging studies of the coronary arteries using CT angiography reported increased rates of acute coronary syndromes in patients with low-attenuation plaques (presumably high in lipid content) with external remodeling compared to those without such plaques (23–25). Puchner et al. recently reported independent prediction of acute coronary syndrome using CT to identify similar high-risk characteristics in patients presenting with acute chest pain (26). Finally, plaque hemorrhage, assessed by magnetic resonance imaging, has been implicated in poor outcomes of patients with cerebrovascular disease (27,28). However, all of these studies claiming independent risk prediction of certain plaque features share the fundamental limitation that the atherosclerotic disease burden was not considered as a potential confounder. These “high-risk” features are conceivably mere markers of more extensive and/or active atherosclerotic disease compared to the control group. Given the overwhelming evidence for disease burden as a powerful predictor of outcome, any additional risk features should be assessed against it before we assume independent risk prediction. Therefore, despite promising results from a number of clinical studies, there is no conclusive evidence for truly independent risk prediction associated with high-risk plaque features. High-risk features may still be valuable as markers for disease burden or activity; however, such value has not been established.
Figure 1. Risk of MI/Death Associated With Individual Plaques in the PROSPECT Study.
Maximum annualized risks (%) of MI or CV death associated with individual coronary atherosclerotic plaques identified at baseline by virtual histology intravascular ultrasound in the PROSPECT study are shown (18). The rates are on the basis of the occurrence of 6 events (6 myocardial infarctions and 0 deaths) after 3.4 years of follow-up among 1,005 coronary arterial sites with pathological intimal thickening and 595 TCFAs, assuming all events were caused by the respective plaque type, thus representing the worst-case scenario. Considering equal risk among the 3,160 plaques detected at baseline (best-case scenario), the event rate associated with each plaque would be only 0.06%/year. The risk of MI or death associated with individual TCFA or vulnerable plaques is much smaller than what is conventionally considered high-risk, even when maximal risk is assumed. CV = cardiovascular; MI = myocardial infarction; TCFA = thin-cap fibroatheroma.
Evidence Diminishing the Significance of Identifying “High-Risk” Plaques
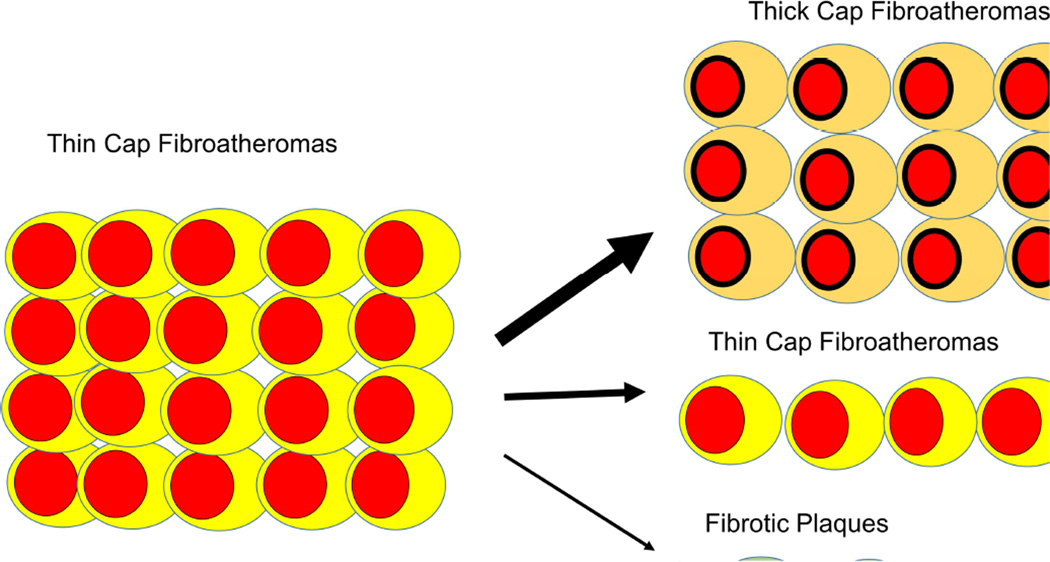
Despite our ability to identify atherosclerotic lesions that exhibit vulnerable characteristics using various imaging tools, clinical studies have failed to demonstrate meaningful clinical utility for plaque imaging. These negative results are explained by the numerous pathological and clinical investigations demonstrating that many (if not most) plaques rupture without clinical syndromes (22,29–38). The percentage of patients with subclinical plaque ruptures varies with their risk profile and the sensitivity of assessment methods, ranging from 4% to 79% (Table 1). Plaque rupture and its healing are frequently clinically silent, but lead to progressive lumen obstruction (39,40). In lesions with advanced lumen narrowing (>50% stenosis), histopathology almost invariably reveals 1 or more healed subclinical plaque ruptures (39–41). Because healed plaque ruptures can only be detected by pathology or imaging within a certain time frame after the initial plaque disruption, the true rate of asymptomatic plaque ruptures is probably underestimated (40). In most individuals with advanced atheroma, plaque rupture and subsequent healing have already occurred (40). Pathology studies show that approximately 10% of the subclinical U.S. adult population exhibits advanced coronary atheroma (42), thus, it is reasonable to assume that many millions of persons unknowingly experience plaque ruptures each year. Several longitudinal imaging studies in humans demonstrate that plaque morphology changes over a few months, gaining or losing “vulnerable” characteristics (43–45). Using IVUS, Kubo et al. found that 75% of TCFAs transition to thick-cap fibroatheromas or fibrotic plaques within a 12-month interval, presumably secondary to rupture and healing (Figure 2). None of these patients experienced an event during this period, providing further evidence of frequent subclinical plaque alterations. A recent study used OCT to confirm the dynamic nature of coronary atherosclerotic disease, demonstrating that TCFAs in various stages of development are highly prevalent in patients with coronary atherosclerotic disease (46). In patients with acute coronary syndrome, plaque ruptures are frequently found apart from the culprit lesions, indicating that vulnerability is disseminated throughout the coronary tree (41). This suggests that detection of a state of vulnerability in a patient (e.g., widespread inflammation) is more important than detection of individual sites of vulnerability. The emerging picture of acute coronary event pathophysiology suggests a widespread, systemic condition with great unpredictability as to which particular lesion will be associated with clinically-significant vascular thrombosis (47). While plaque ruptures and erosions are, indeed, responsible for most culprit lesions in patients with acute events, because the frequency of subclinical plaque ruptures is vastly underestimated, the assumption that identifying lesions prone to rupture will prevent acute coronary events was unrealistic. Of the many plaque ruptures occurring in patients with atherosclerotic disease, very few will trigger symptomatic events, rendering it exceedingly difficult to predict adverse outcomes associated with particular lesions. Identifying a single TCFA or other “high-risk plaque”, without considering other clinical or imaging characteristics, is unlikely to be of incremental benefit for risk prediction over established factors (e.g., extent and distribution of atherosclerotic plaque burden), because of the low risk associated with a given individual plaque and the temporal relationship of its vulnerable characteristics.
Table 1.
Prevalence of Subclinical Coronary Atherosclerotic Plaque Ruptures (%) in Patients With Stable Coronary Heart Disease or Healthy Controls and in Individuals With Acute Coronary Syndromes
| Study | Mode of Assessment |
Number of Stable CHD Patients or Controls* |
Nonculprit Plaque Ruptures (%) |
Number of ACS Patients |
Nonculprit Plaque Ruptures (%) |
|
|---|---|---|---|---|---|---|
| Arbustini et al. (29) | Pathology | 77* | 17 | 106 | 58 | |
| Cheruvu et al. (37) | Pathology | 13* | 31 | 33 | 45 | |
| Riofol et al. (40) | IVUS | - | - | 24 | 79 | |
| Kotani et al. (36) | IVUS | 48 | 6 | 38 | 11 | |
| Schoenhagen et al. (35) | IVUS | 92 | 4 | 105 | 19 | |
| Hong et al. (34) | IVUS | 113 | 5 | 122 | 17 | |
| Tanaka et al. (28) | IVUS | - | - | 45 | 24 | |
| Takano et al. (33) | Angioscopy | - | - | 327 | 9 | |
| Tanaka et al. (32) | OCT | - | - | 45 | 24 | |
| Tian et al. (21) | OCT | - | - | 82 | 10 | |
| Shimamura et al. (31) | OCT | 191 | 17 | - | - | |
| Total N | 444 | 927 | ||||
| Median | 11.5 | 21.5 | ||||
Multiple asymptomatic plaque ruptures are more frequently found in ACS patients than in individuals with stable CHD or healthy controls, indicating the systemic inflammatory state in the coronary arteries with acute events. ACS = acute coronary syndrome; CHD = coronary heart disease; IVUS = intravascular ultrasound; OCT = optical coherence tomography.
Indicates healthy controls.
Figure 2. Changes in TCFAs During 12 Months Follow-Up.
Changes in TFCAs observed with virtual histology intravascular ultrasound 12 months after baseline imaging. Of 20 TFCAs, only 5 remained unchanged while 15 (75%) lost vulnerable characteristics and revealed thickening of the fibrous cap or transformed into fibrous plaques. The data (Kubo et al. (43)) demonstrate high metabolic activity in atherosclerotic lesions and the short-lived nature of vulnerable plaque characteristics. None of the 99 patients experienced any events during follow up; thus many of the observed changes were likely the result of subclinical plaque ruptures. Abbreviations as in Figure 1.
Current Paradigm of Acute Coronary Event Pathophysiology
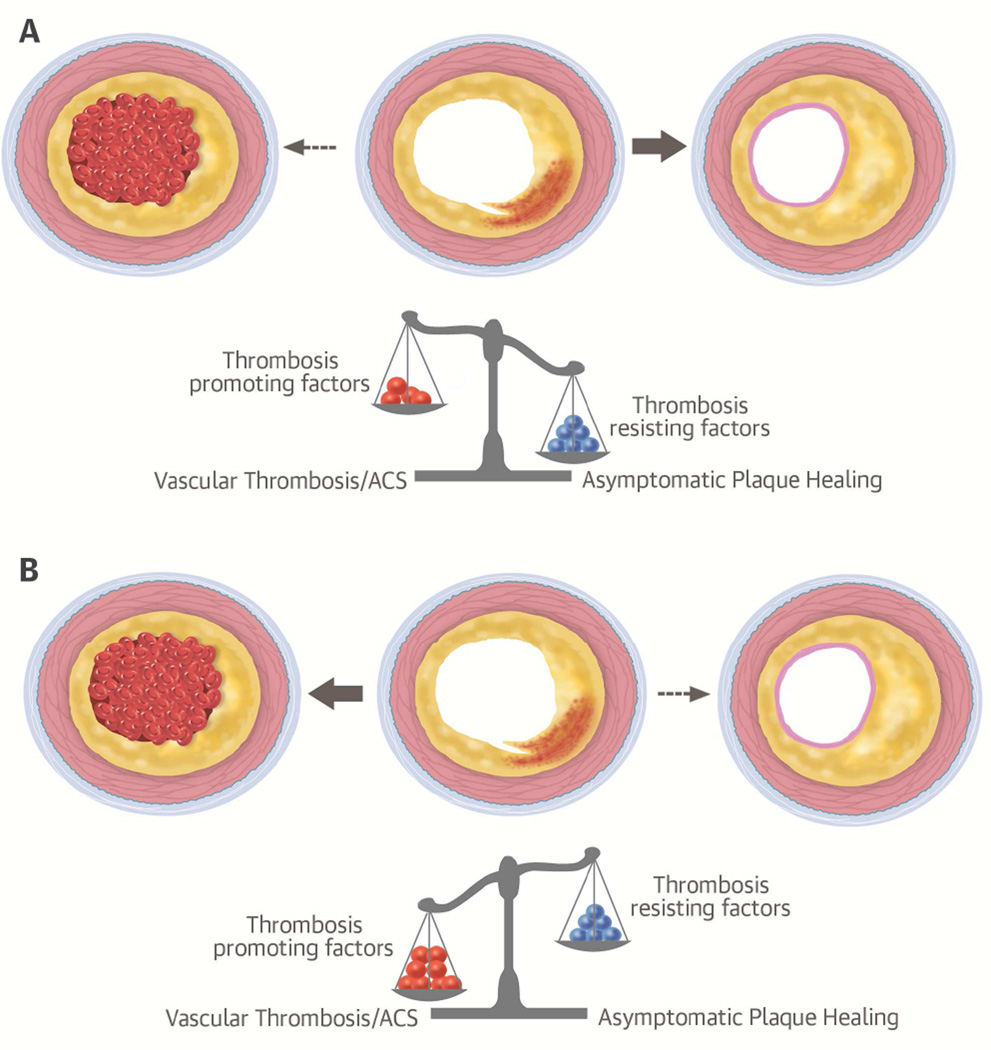
Over the past few decades, clinical and laboratory investigations have led to a more complex concept of the pathophysiology of acute coronary events, involving numerous processes, many with poorly-understood interactions (7,8). While the occurrence of acute coronary events typically requires alterations of coronary atherosclerotic plaques (rupture or erosion), a thrombosis-promoting milieu is necessary to allow a clinically significant decrease in coronary blood flow and associated myocardial ischemia (7,9). Such a setting appears to result from an unfortunate constellation of prothrombotic features, for example, in patients with increased inflammatory activity and systemic or local suppression of fibrinolytic performance, an extraordinarily large stimulus for thrombosis, vasoconstriction, and/or others (7). The respective contributions of these factors (some hereditary, some environmental) and their temporal relationships necessary to trigger clinically meaningful vascular thrombosis are unknown. Factors favoring thrombosis need to be collectively sufficient to tilt the scale away from localized thrombus and towards extensive vascular thrombosis (Central Illustration). Because numerous factors influence the performance of the coagulation system at any given point in time, acute coronary events may arise as result of a “perfect storm” scenario, in which plaque disruption occurs in a specific, thrombosis-promoting setting (7). The risk of an acute coronary event equals the probability of plaque rupture/erosion coinciding with vascular thrombosis-promoting conditions that cannot contain the thrombus in the vascular wall. Frequent plaque ruptures, as with a large, metabolically active atherosclerotic disease burden, increase the chance that a plaque rupture coincides with a thrombosis-conducive setting. Accordingly, the strongest predictors of adverse events are the magnitude and activity of the coronary atherosclerotic plaque burden and the number of risk factors for a prothrombotic milieu, a concept supported by many clinical studies and epidemiologic data (3,5,48–50).
Central Illustration. Fate of Ruptured Coronary Atherosclerotic Plaques According to Thrombotic Milieu.
The hypothesized interplay of prothrombotic and thrombosis resisting/containing factors that presumably determine the outcome of a ruptured coronary atherosclerotic plaque is shown. (A) In the most common scenario, small thrombus formation associated with plaque rupture is contained and vascular occlusive thrombus is inhibited. (B) In the less common scenario of several prothrombotic factors coinciding (e.g., inflammatory state, large lesion plaque burden, vasoconstriction, circadian rheological changes), local thrombosis associated with plaque rupture cannot be contained and clinically significant vascular thrombosis occurs, triggering an acute coronary syndrome (ACS). The constellation of factors leading to these different outcomes is unknown.
Lesion Focused Versus Disease Burden for Risk Assessment and Management
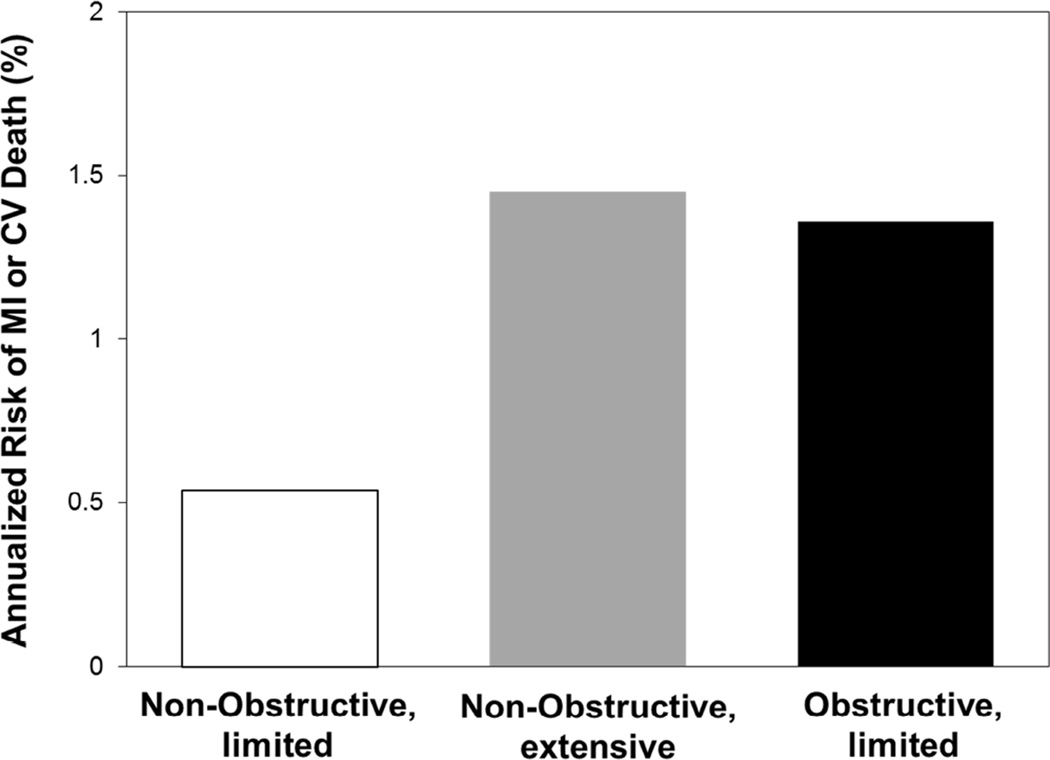
Numerous clinical studies using conventional invasive coronary angiography, IVUS, and cardiac CT (8,48,51) confirm the strong relationship between atherosclerotic disease burden and risk of adverse events. Despite capturing only calcified atherosclerotic disease, when compared directly, coronary calcium scoring was equivalent to traditional stenosis assessment for predicting mortality and myocardial infarction in asymptomatic patients (52). Using a comprehensive imaging approach in several vascular beds, the BIOIMAGE study recently revealed a high prevalence of atherosclerotic disease in individuals categorized as high-risk for cardiovascular events based on clinical predictors (53). Halting coronary atherosclerotic disease progression and/or altering the vascular thrombosis-promoting milieu via platelet inhibition and risk factor interdiction are approaches proven to lower myocardial infarction and death rates (54–56). Conversely, meta-analyses have not demonstrated reduced rates of myocardial infarction or death with lesion-based treatment (i.e., percutaneous coronary intervention) compared to medical therapy in patients with stable coronary artery disease (57,58). Contradicting some earlier reports (59–62), no benefit was shown, even when selecting patients with hemodynamically significant stenoses. These results confirm that risk is most strongly conveyed by the extent of coronary artery disease, but not necessarily by individual lesions, even when highly obstructive. This supports the controversial idea that severe coronary artery stenoses are no greater risk for triggering acute coronary events than mild lesions (63). Earlier angiographic studies suggested that most myocardial infarctions arise from mild coronary artery stenoses (64,65), but recent data question this paradigm (66,67). Thrombus material accumulates over several days following a plaque disruption, which may not allow lesion size and a partly organized thrombus to be accurately distinguished (66). Pathology studies in patients who died suddenly found culprit lesions to have an average diameter stenosis of approximately 50%, with no clear relationship between stenosis severity and risk of death (68,69). Conversely, acute coronary death rarely results from lesions with <30% lumen stenosis (66,69). Thus, a certain local plaque volume appears necessary to trigger vascular thrombosis. However, given the lack of benefit with coronary stenting, as well as the large number of obstructive lesions found by imaging and autopsy in patients without symptoms of acute coronary events, stenosis severity is unlikely to substantially alter such risk beyond a particular threshold. Patients with high-grade coronary artery stenoses may conceivably carry an increased risk of suffering myocardial infarction and death because these lesions are markers for advanced atherosclerotic disease in the coronary tree (7). Consistent with this notion, nonobstructive and obstructive coronary artery disease are associated with similar risks of myocardial infarction and death if the former affects a larger number of coronary arterial segments (Figure 3)(70). Overall, strong evidence supports addressing the extent and activity of the atherosclerotic burden and thrombosis-promoting risk factors for improved patient outcomes, but there is no conclusive evidence of incremental risk reduction with lesion-specific treatment.
Figure 3. Risk from Non-Obstructive Versus Obstructive Coronary Artery Disease.
Annualized risk (%) of MI or CV death in 3,242 patients followed for a median of 3.6 years after a baseline CT coronary angiogram, according to the extent and severity of coronary artery disease (70). Risk is low in patients with nonobstructive disease (<50% stenosis) involving 4 or fewer coronary artery segments (limited disease). Conversely, the risk is similarly high in patients with nonobstructive disease if more than 4 segments are affected (extensive disease) compared to patients with obstructive disease (≥50% stenosis). Modified from Bittencourt et al. (70). CT = computed tomography. Other abbreviations as in Figure 1.
Areas of Uncertainty: Influence of Study Population
The atherosclerotic disease burden is a powerful predictor of outcomes for patients without prior history of coronary artery disease, facilitated by the ease of coronary calcium scanning, which approximates the total coronary atherosclerotic disease volume (71). However, it is infrequently performed in patients with established coronary artery disease; thus, in this population, plaque burden data are more limited. Risk characteristics and the need for assessment may conceivably differ in patients with established coronary artery disease compared with those with a history of acute coronary syndrome. Aside from calcium scanning, plaque burden assessment is technically difficult and most available data were from IVUS imaging. Atherosclerotic plaque burden assessment using CT angiography has recently become feasible, but long-term outcome data are not yet available (72). Aggregate data from IVUS-derived plaque burden assessment reveal its strong predictive power for outcomes in patients with established coronary artery disease (73). Conventional angiography data for atherosclerotic disease burden are similarly predictive of patient outcome and superior to myocardial ischemia testing in an analysis of the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) study (74). Thus, the risk associated with plaque burden applies to both asymptomatic individuals without prior cardiac events and those with established, stable coronary artery disease. Whether lesion-based treatment (aside from the culprit lesion) is beneficial in patients with acute coronary syndrome is unclear. Two recent small clinical studies suggested reduced rates of myocardial infarction and death following percutaneous coronary intervention of nonculprit lesions in patients with ST-elevation myocardial infarction (75,76), in stark contrast to large, aggregate data suggesting the opposite (77). In addition to concerns about the effect of chance, given the small numbers of events in these 2 studies, the contributions of event allocation (e.g., differentiating spontaneous and periprocedural events) and varying levels of expertise at the study centers to these results remain unclear. PROSPECT, conducted in patients with acute coronary syndrome, found low risk of myocardial infarction and death associated with nonculprit lesions within 3 years of follow-up (19). A large clinical study, COMPLETE, is underway to conclusively address the question of benefit of stenting nonculprit lesions in patients with ST-elevation myocardial infarction (78).
Is there a Role for Individual Plaque Imaging?
Coronary artery imaging has provided insights into numerous lesion characteristics, but we have yet to identify which are useful for guiding management. Changing patterns of lesion characteristics, resulting from widespread use of lipid-modifying medications, pose an additional challenge (79). There are promising data for characterizing coronary arterial lesions prior to percutaneous interventions (80,81). Heavily calcified lesions adversely affect the outcome of coronary artery revascularization and imaging data may help weigh treatment options (82–83). Similarly, knowledge of complex bifurcation lesions prior to cardiac catheterization may avoid high-risk interventions (80,81). Plaque imaging also may elucidate the effects of drugs on atherosclerotic disease (84). Individual plaque features may have particular significance in specific settings: for example, a TCFA may have different implications in patients with or without known susceptibility to vascular thrombosis. Thus, integration of lesion characteristics with risk factors may be valuable. Currently unknown features of atherosclerotic plaque may conceivably independently herald poor outcome. Advanced imaging techniques (e.g., molecular imaging), may elucidate such features and allow further insights into mechanisms of acute coronary event pathophysiology (85). To determine truly independent risk prediction, any plaque assessment should be measured against the predictive power of atherosclerotic burden and its metabolic activity.
Implications for Future Investigations
While general morphologic patterns of atherosclerotic disease influence the probability of acute coronary syndromes, they are clearly modified by individual characteristics. Pathology and IVUS studies show that most U.S. adults above 50 years of age have evidence of coronary atherosclerotic disease, but only a minority will suffer acute events (42,86). Furthermore, the patterns and morphologic features of atherosclerotic disease appear similar among populations, suggesting that the individual’s response is critical for determining the probability of events. Traditional risk factors for coronary artery disease (e.g., diabetes, smoking, dyslipidemia), and genetic predisposition modify such responses. Several mutations are associated with increased event hazard and individualized risk characterization may soon be available (87–89).
We need a better understanding of which combination of imaging information and risk factors yields the most accurate individual risk prediction. Research is needed to investigate mechanisms influencing the coagulation system’s response to various internal and external modifiers, both locally and systemically. Specifically, we need to understand and potentially to predict the response of the coagulation system to stimuli occurring with atherosclerotic plaque alterations. Variability in the coagulation system’s performance depends on numerous hormonal, dietary, and environmental influences, hampering our ability to predict its function at a given time, for example, when plaque ruptures (90–92). Thus, we must strive for comprehensive risk assessment that integrates specific information on the atherosclerotic plaque burden and systemic factors that increase the risk of disease activity and vascular thrombosis, and is tailored to specific patient populations and individuals. This would enable effective, efficient triaging of patients into treatment categories ranging from continued risk factor control to coronary arterial revascularization.
Conclusions
Despite major advancements in coronary artery imaging and identification of atherosclerotic lesion morphology associated with rupture, there is no conclusive evidence that individual plaque assessment better predicts acute coronary event risk than established risk factors, such as the extent and severity of coronary artery disease. Pathology and clinical studies consistently demonstrate that atherosclerotic plaques rupture without clinical symptoms much more frequently than is widely acknowledged, challenging the notion of a close association between plaque rupture and clinical event. Conversely, the atherosclerotic disease burden is a consistent, strong predictor of adverse cardiovascular events and deserves greater attention. Current data suggest that, rather than focusing on individual coronary arterial lesions, we need a comprehensive, integrative approach for identifying and managing patients at risk of suffering adverse cardiovascular events.
Acknowledgments
Dr. Zadeh is supported by NIH grant K23-HL098368
Abbreviations
- CT
computed tomography
- IVUS
intravascular ultrasound
- OCT
optical coherence tomography
- TCFA
thin-cap fibroatheroma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2014 update: A report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franco M, Cooper RS, Bilal U, et al. Challenges and opportunities for cardiovascular disease prevention. Am J Med. 2011;124:95–102. doi: 10.1016/j.amjmed.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Greenland P, Knoll MD, Stamler J, et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA. 2003;290:891–897. doi: 10.1001/jama.290.7.891. [DOI] [PubMed] [Google Scholar]
- 4.Young LH, Wackers FJ, Chyun DA, et al. DIAD Investigators. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009;301:1547–1555. doi: 10.1001/jama.2009.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 6.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 7.Arbab-Zadeh A, Nakano M, Virmani R, et al. Acute coronary events. Circulation. 2012;125:1147–1156. doi: 10.1161/CIRCULATIONAHA.111.047431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bentzon JF, Otsuka F, Virmani R, et al. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 9.Ueda Y, Ogasawara N, Matsuo K, et al. Acute coronary syndrome: insight from angioscopy. Circ J. 2010;74:411–417. doi: 10.1253/circj.cj-09-0795. [DOI] [PubMed] [Google Scholar]
- 10.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 11.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation. 2003;108:1772–1778. doi: 10.1161/01.CIR.0000087481.55887.C9. [DOI] [PubMed] [Google Scholar]
- 12.Fleg JL, Stone GW, Fayad ZA, et al. Detection of high-risk atherosclerotic plaque: Report of the NHLBI working group on current status and future directions. J Am Coll Cardiol Img. 2012;5:941–955. doi: 10.1016/j.jcmg.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finn AV, Nakano M, Narula J, et al. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282–1292. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- 14.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 15.Virmani R, Burke AP, Farb A, et al. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–C18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 16.Narula J, Garg P, Achenbach S, et al. Arithmetic of vulnerable plaques for noninvasive imaging. Nat Clin Pract Cardiovasc Med. 2008;5(Suppl 2):S2–S10. doi: 10.1038/ncpcardio1247. [DOI] [PubMed] [Google Scholar]
- 17.NIH Research Portfolio Online Reporting Tools (RePORT) [Accessed December 4, 2014]; Available at: http://report.nih.gov.
- 18.Hlatky MA, Douglas PS, Cook NL, et al. Future directions for cardiovascular disease comparative effectiveness research: report of a workshop sponsored by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol. 2012;60:569–580. doi: 10.1016/j.jacc.2011.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stone GW, Maehara A, Lansky AJ, et al. PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 20.Calvert PA, Obaid DR, O'Sullivan M, et al. Association between IVUS findings and adverse outcomes in patients with coronary artery disease: the VIVA (VH-IVUS in Vulnerable Atherosclerosis) study. J Am Coll Cardiol Img. 2011;4:894–901. doi: 10.1016/j.jcmg.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Yonetsu T, Bouma BE, Kato K, et al. Optical coherence tomography- 15 years in cardiology. Circ J. 2013;77:1933–1940. doi: 10.1253/circj.cj-13-0643.1. [DOI] [PubMed] [Google Scholar]
- 22.Tian J, Ren X, Vergallo R, et al. Distinct morphological features of ruptured culprit plaque for acute coronary events compared to those with silent rupture and thin-cap fibroatheroma: A combined optical coherence tomography and intravascular ultrasound study. J Am Coll Cardiol. 2014;63:2209–2216. doi: 10.1016/j.jacc.2014.01.061. [DOI] [PubMed] [Google Scholar]
- 23.Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54:49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 24.Maurovich-Horvat P, Hoffmann U, Vorpahl M, et al. The napkin-ring sign: CT signature of high-risk coronary plaques? J Am Coll Cardiol Img. 2010;3:440–444. doi: 10.1016/j.jcmg.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Otsuka K, Fukuda S, Tanaka A, et al. Napkin-ring sign on coronary CT angiography for the prediction of acute coronary syndrome. J Am Coll Cardiol Img. 2013;6:448–457. doi: 10.1016/j.jcmg.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 26.Puchner SB, Liu T, Mayrhofer T, et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: Results from the ROMICAT-II trial. J Am Coll Cardiol. 2014;64:684–692. doi: 10.1016/j.jacc.2014.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hellings WE, Peeters W, Moll FL, et al. Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: a prognostic study. Circulation. 2010;121:1941–1950. doi: 10.1161/CIRCULATIONAHA.109.887497. [DOI] [PubMed] [Google Scholar]
- 28.Saam T, Hetterich H, Hoffmann V, et al. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol. 2013;62:1081–1091. doi: 10.1016/j.jacc.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka A, Shimada K, Sano T, et al. Multiple plaque rupture and C-reactive protein in acute myocardial infarction. J Am Coll Cardiol. 2005;45:1594–1599. doi: 10.1016/j.jacc.2005.01.053. [DOI] [PubMed] [Google Scholar]
- 30.Arbustini E, Grasso M, Diegoli M, et al. Coronary atherosclerotic plaques with and without thrombus in ischemic heart syndromes: a morphologic, immunohistochemical, and biochemical study. Am J Cardiol. 1991;68:36B–50B. doi: 10.1016/0002-9149(91)90383-v. [DOI] [PubMed] [Google Scholar]
- 31.Hudson J, McCaughey WT. Mural thrombosis and atherogenesis in coronary arteries and aorta. An investigation using antifibrin and antiplatelet sera. Atherosclerosis. 1974;19:543–553. doi: 10.1016/s0021-9150(74)80018-3. [DOI] [PubMed] [Google Scholar]
- 32.Shimamura K, Ino Y, Kubo T, et al. Difference of ruptured plaque morphology between asymptomatic coronary artery disease and non-ST elevation acute coronary syndrome patients: an optical coherence tomography study. Atherosclerosis. 2014;235:532–537. doi: 10.1016/j.atherosclerosis.2014.05.920. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka A, Imanishi T, Kitabata H, et al. Distribution and frequency of thin-capped fibroatheromas and ruptured plaques in the entire culprit coronary artery in patients with acute coronary syndrome as determined by optical coherence tomography. Am J Cardiol. 2008;102:975–979. doi: 10.1016/j.amjcard.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 34.Takano M, Inami S, Ishibashi F, et al. Angioscopic follow-up study of coronary ruptured plaques in nonculprit lesions. J Am Coll Cardiol. 2005;45:652–658. doi: 10.1016/j.jacc.2004.09.077. [DOI] [PubMed] [Google Scholar]
- 35.Hong MK, Mintz GS, Lee CW, et al. Comparison of coronary plaque rupture between stable angina and acute myocardial infarction: a three-vessel intravascular ultrasound study in 235 patients. Circulation. 2004;110:928–933. doi: 10.1161/01.CIR.0000139858.69915.2E. [DOI] [PubMed] [Google Scholar]
- 36.Schoenhagen P, Stone GW, Nissen SE, et al. Coronary plaque morphology and frequency of ulceration distant from culprit lesions in patients with unstable and stable presentation. Arterioscler Thromb Vasc Biol. 2003;23:1895–1900. doi: 10.1161/01.ATV.0000084811.73196.1C. [DOI] [PubMed] [Google Scholar]
- 37.Kotani J, Mintz GS, Castagna MT, et al. Intravascular ultrasound analysis of infarct-related and non-infarct-related arteries in patients who presented with an acute myocardial infarction. Circulation. 2003;107:2889–2893. doi: 10.1161/01.CIR.0000072768.80031.74. [DOI] [PubMed] [Google Scholar]
- 38.Cheruvu PK, Finn AV, Gardner C, et al. Frequency and distribution of thin-cap fibroatheroma and ruptured plaques in human coronary arteries: a pathologic study. J Am Coll Cardiol. 2007;50:940–949. doi: 10.1016/j.jacc.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 39.Burke AP, Kolodgie FD, Farb A, et al. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103:934–940. doi: 10.1161/01.cir.103.7.934. [DOI] [PubMed] [Google Scholar]
- 40.Mann J, Davies MJ. Mechanisms of progression in native coronary artery disease: role of healed plaque disruption. Heart. 1999;82:265–268. doi: 10.1136/hrt.82.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rioufol G, Finet G, Ginon I, et al. Multiple atherosclerotic plaque rupture in acute coronary syndrome: a three-vessel intravascular ultrasound study. Circulation. 2002;106:804–808. doi: 10.1161/01.cir.0000025609.13806.31. [DOI] [PubMed] [Google Scholar]
- 42.Nemetz PN, Roger VL, Ransom JE, et al. Recent trends in the prevalence of coronary disease: a population-based autopsy study of nonnatural deaths. Arch Intern Med. 2008;168:264–270. doi: 10.1001/archinternmed.2007.79. [DOI] [PubMed] [Google Scholar]
- 43.Kubo T, Maehara A, Mintz GS, et al. The dynamic nature of coronary artery lesion morphology assessed by serial virtual histology intravascular ultrasound tissue characterization. J Am Coll Cardiol. 2010;55:1590–1597. doi: 10.1016/j.jacc.2009.07.078. [DOI] [PubMed] [Google Scholar]
- 44.Motreff P, Rioufol G, Finet G. Seventy-four-month follow-up of coronary vulnerable plaques by serial gray-scale intravascular ultrasound. Circulation. 2012;126:2878–2879. doi: 10.1161/CIRCULATIONAHA.112.132449. [DOI] [PubMed] [Google Scholar]
- 45.Uemura S, Ishigami K, Soeda T, et al. Thin-cap fibroatheroma and microchannel findings in optical coherence tomography correlate with subsequent progression of coronary atheromatous plaques. Eur Heart J. 2012;33:78–85. doi: 10.1093/eurheartj/ehr284. [DOI] [PubMed] [Google Scholar]
- 46.Tian J, Dauerman H, Toma C, et al. Prevalence and characteristics of TCFA and degree of coronary artery stenosis: an OCT, IVUS, and angiographic study. J Am Coll Cardiol. 2014;64:672–680. doi: 10.1016/j.jacc.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 47.Narula J, Kovacic JC. Putting TCFA in clinical perspective. J Am Coll Cardiol. 2014;64:681–683. doi: 10.1016/j.jacc.2014.06.1163. [DOI] [PubMed] [Google Scholar]
- 48.Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 49.Budoff MJ, Hokanson JE, Nasir K, et al. Progression of coronary artery calcium predicts all-cause mortality. J Am Coll Cardio Img. 2010;3:1229–1236. doi: 10.1016/j.jcmg.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 50.Yokoya K, Takatsu H, Suzuki T, et al. Process of progression of coronary artery lesions from mild or moderate stenosis to moderate or severe stenosis: A study based on four serial coronary arteriograms per year. Circulation. 1999;100:903–909. doi: 10.1161/01.cir.100.9.903. [DOI] [PubMed] [Google Scholar]
- 51.Yusuf S, Zucker D, Peduzzi P, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994;344:563–570. doi: 10.1016/s0140-6736(94)91963-1. [DOI] [PubMed] [Google Scholar]
- 52.Cho I, Chang HJ, Sung JM, et al. CONFIRM Investigators. Coronary computed tomographic angiography and risk of all-cause mortality and nonfatal myocardial infarction in subjects without chest pain syndrome from the CONFIRM registry (coronary CT angiography evaluation for clinical outcomes: an international multicenter registry) Circulation. 2012;126:304–313. doi: 10.1161/CIRCULATIONAHA.111.081380. [DOI] [PubMed] [Google Scholar]
- 53.Baber U, Mehran R, Sartori S, et al. Detection and impact of subclinical coronary and carotid atherosclerosis on cardiovascular risk prediction and reclassification in asymptomatic US adults: Insights from the high RIsk plaque BioImage study. J Am Coll Cardiol. 2014;63 http://dx.doi.org/10.1016/S0735-1097(14)60998-0 (Abstr). [Google Scholar]
- 54.Collins R, Armitage J, Parish S, et al. Heart Protection Study Collaborative Group. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004;363:757–767. doi: 10.1016/S0140-6736(04)15690-0. [DOI] [PubMed] [Google Scholar]
- 55.Cannon CP, Braunwald E, McCabe CH, et al. Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 56.Berger JS, Brown DL, Becker RC. Low-dose aspirin in patients with stable cardiovascular disease: a meta-analysis. Am J Med. 2008;121:43–49. doi: 10.1016/j.amjmed.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Stergiopoulos K, Brown DL. Initial coronary stent implantation with medical therapy vs medical therapy alone for stable coronary artery disease: meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172:312–319. doi: 10.1001/archinternmed.2011.1484. [DOI] [PubMed] [Google Scholar]
- 58.Boden WE. Mounting evidence for lack of PCI benefit in stable ischemic heart disease : What more will it take to turn the tide of treatment?: comment on "initial coronary stent implantation with medical therapy vs medical therapy alone for stable coronary artery disease". Arch Intern Med. 2012;172:319–321. doi: 10.1001/archinternmed.2011.2321. [DOI] [PubMed] [Google Scholar]
- 59.Stergiopoulos K, Boden WE, Hartigan P, et al. Percutaneous coronary intervention outcomes in patients with stable obstructive coronary artery disease and myocardial ischemia: A collaborative meta-analysis of contemporary randomized clinical trials. JAMA Intern Med. 2014;174:232–240. doi: 10.1001/jamainternmed.2013.12855. [DOI] [PubMed] [Google Scholar]
- 60.Tonino PA, De Bruyne B, Pijls NH, et al. FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 61.Arbab-Zadeh A. Fractional flow reserve guided percutaneous coronary intervention is not a valid concept. Circulation. 2014;129:1871–1878. doi: 10.1161/CIRCULATIONAHA.113.003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Bruyne B, Fearon WF, Pijls NH, et al. FAME 2 Trial Investigators. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014;371:1208–1217. doi: 10.1056/NEJMoa1408758. [DOI] [PubMed] [Google Scholar]
- 63.Niccoli G, Stefanini GG, Capodanno D, et al. Are the culprit lesions severely stenotic? J Am Coll Cardiol Img. 2013;6:1108–1114. doi: 10.1016/j.jcmg.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 64.Little WC, Constantinescu M, Applegate RJ, et al. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation. 1988;78:1157–1166. doi: 10.1161/01.cir.78.5.1157. [DOI] [PubMed] [Google Scholar]
- 65.Fishbein MC, Siegel RJ. How big are coronary atherosclerotic plaques that rupture? Circulation. 1996;94:2662–2666. doi: 10.1161/01.cir.94.10.2662. [DOI] [PubMed] [Google Scholar]
- 66.Ojio S, Takatsu H, Tanaka T, et al. Considerable time from the onset of plaque rupture and/or thrombi until the onset of acute myocardial infarction in humans: coronary angiographic findings within 1 week before the onset of infarction. Circulation. 2000;102:2063–2069. doi: 10.1161/01.cir.102.17.2063. [DOI] [PubMed] [Google Scholar]
- 67.Frobert O, van't Veer M, Aarnoudse W, et al. Acute myocardial infarction and underlying stenosis severity. Catheter Cardiovasc Interv. 2007;70:958–965. doi: 10.1002/ccd.21280. [DOI] [PubMed] [Google Scholar]
- 68.Davies MJ, Thomas A. Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. N Engl J Med. 1984;310:1137–1140. doi: 10.1056/NEJM198405033101801. [DOI] [PubMed] [Google Scholar]
- 69.Farb A, Burke AP, Tang AL, et al. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93:1354–1363. doi: 10.1161/01.cir.93.7.1354. [DOI] [PubMed] [Google Scholar]
- 70.Bittencourt MS, Hulten E, Ghoshhajra B, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014;7:282–291. doi: 10.1161/CIRCIMAGING.113.001047. [DOI] [PubMed] [Google Scholar]
- 71.Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 72.Boogers MJ, Broersen A, van Velzen JE, et al. Automated quantification of coronary plaque with computed tomography: comparison with intravascular ultrasound using a dedicated registration algorithm for fusion-based quantification. Eur Heart J. 2012;33:1007–1016. doi: 10.1093/eurheartj/ehr465. [DOI] [PubMed] [Google Scholar]
- 73.Nicholls SJ, Hsu A, Wolski K, et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol. 2010;55:2399–2407. doi: 10.1016/j.jacc.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 74.Mancini GB, Hartigan PM, Shaw LJ, et al. Predicting outcome in the COURAGE trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation): coronary anatomy versus ischemia. J Am Coll Cardio; Intv. 2014;7:195–201. doi: 10.1016/j.jcin.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 75.Wald DS, Morris JK, Wald NJ, et al. PRAMI Investigators. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115–1123. doi: 10.1056/NEJMoa1305520. [DOI] [PubMed] [Google Scholar]
- 76.Kelly DJ, McCann GP, Blackman D, et al. Complete versus culprit-lesion only PRimary PCI trial (CVLPRIT): a multicentre trial testing management strategies when multivessel disease is detected at the time of primary PCI: rationale and design. EuroIntervention. 2013;8:1190–1198. doi: 10.4244/EIJV8I10A183. [DOI] [PubMed] [Google Scholar]
- 77.Bainey KR, Mehta SR, Lai T, et al. Complete vs culprit-only revascularization for patients with multivessel disease undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: a systematic review and meta-analysis. Am Heart J. 2014;167:1.e2–14.e2. doi: 10.1016/j.ahj.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 78.Complete vs Culprit-only Revascularization to Treat Multi-vessel Disease After Primary PCI for STEMI (COMPLETE) [Accessed December 4, 2014];2012 Nov 27; Updated April 24, 2013. Available at: http://clinicaltrials.gov/ct2/show/NCT01740479.
- 79.van Lammeren GW, den Ruijter HM, Vrijenhoek JE, et al. Time-dependent changes in atherosclerotic plaque composition in patients undergoing carotid surgery. Circulation. 2014;129:2269–2276. doi: 10.1161/CIRCULATIONAHA.113.007603. [DOI] [PubMed] [Google Scholar]
- 80.McDaniel MC, Eshtehardi P, Sawaya FJ, et al. Contemporary clinical applications of coronary intravascular ultrasound. J Am Coll Cardiol Intv. 2011;4:1155–1167. doi: 10.1016/j.jcin.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 81.Rodriguez-Granillo GA, Rosales MA, Llaurado C, et al. Guidance of percutaneous coronary interventions by multidetector row computed tomography coronary angiography. EuroIntervention. 2011;6:773–778. doi: 10.4244/EIJV6I6A131. [DOI] [PubMed] [Google Scholar]
- 82.Madhavan MV, Tarigopula M, Mintz GS, et al. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol. 2014;63:1703–1714. doi: 10.1016/j.jacc.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 83.Ertelt K, Genereux P, Mintz GS, et al. Impact of the severity of coronary artery calcification on clinical events in patients undergoing coronary artery bypass grafting (from the Acute Catheterization and Urgent Intervention Triage Strategy Trial) Am J Cardiol. 2013;112:1730–1737. doi: 10.1016/j.amjcard.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 84.Hattori K, Ozaki Y, Ismail TF, et al. Impact of statin therapy on plaque characteristics as assessed by serial OCT, grayscale and integrated backscatter-IVUS. J Am Coll Cardiol Img. 2012;5:169–177. doi: 10.1016/j.jcmg.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 85.Sadat U, Jaffer FA, van Zandvoort MA, et al. Inflammation and neovascularization intertwined in atherosclerosis: imaging of structural and molecular imaging targets. Circulation. 2014;130:786–794. doi: 10.1161/CIRCULATIONAHA.114.010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tuzcu EM, Kapadia SR, Tutar E, et al. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation. 2001;103:2705–2710. doi: 10.1161/01.cir.103.22.2705. [DOI] [PubMed] [Google Scholar]
- 87.Damani SB, Topol EJ. Emerging genomic applications in coronary artery disease. J Am Coll Cardiol Intv. 2011;4:473–482. doi: 10.1016/j.jcin.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Traylor M, Farrall M, Holliday EG, et al. Australian Stroke Genetics Collaborative, Wellcome Trust Case Control Consortium 2 (WTCCC2), et al.; International Stroke Genetics Consortium. Genetic risk factors for ischaemic stroke and its subtypes (the METASTROKE collaboration): a meta-analysis of genome-wide association studies. Lancet Neurol. 2012;11:951–962. doi: 10.1016/S1474-4422(12)70234-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Makinen VP, Civelek M, Meng Q, et al. Coronary ARtery DIsease Genome-Wide Replication And Meta-Analysis (CARDIoGRAM) Consortium, et al. Integrative genomics reveals novel molecular pathways and gene networks for coronary artery disease. PLoS Genet. 2014;10:e1004502. doi: 10.1371/journal.pgen.1004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Montagnana M, Salvagno GL, Lippi G. Circadian variation within hemostasis: an underrecognized link between biology and disease? Semin Thromb Hemost. 2009;35:23–33. doi: 10.1055/s-0029-1214145. [DOI] [PubMed] [Google Scholar]
- 91.Phang M, Lazarus S, Wood LG, et al. Diet and thrombosis risk: nutrients for prevention of thrombotic disease. Semin Thromb Hemost. 2011;37:199–208. doi: 10.1055/s-0031-1273084. [DOI] [PubMed] [Google Scholar]
- 92.Servoss SJ, Januzzi JL, Muller JE. Triggers of acute coronary syndromes. Prog Cardiovasc Dis. 2002;44:369–380. doi: 10.1053/pcad.2002.123470. [DOI] [PubMed] [Google Scholar]