Abstract
MHC class II (MHC-II) molecules function by binding peptides derived from either self-or foreign proteins and expressing these peptides on the surface of antigen presenting cells (APCs) for recognition by CD4 T cells. MHC-II is known to exist on clusters on the surface of APCs, and a variety of biochemical and functional studies have suggested that these clusters represent lipid raft microdomain-associated MHC-II. This review will summarize data exploring the biosynthesis of raft-associated MHC-II and the role that lipid raft association plays in regulating T cell activation by APCs.
Introduction
Glycosphingolipid-, sphingomyelin-, and cholesterol-enriched lipid raft membrane microdomains reside on the plasma membrane and throughout the secretory and endocytic membranes of eukaryotic cells [1]. Lipid rafts are dynamic structures, are approximately 4 to 200 nm in diameter [2], and have been implicated in a variety of cellular events including signal transduction, endocytosis, vesicular transport, and cell adhesion [3, 4]. Biochemically, rafts are “defined” as detergent-resistant membrane microdomains; this is based on their relative insolubility in mild nonionic detergents at low temperature (e.g. Triton X-100) and rafts (and raft-associated proteins) can be isolated using discontinuous sucrose density gradient centrifugation [5]. This “definition” has a number of caveats that revolve around the potential that detergent extraction per se “creates” lipid rafts [6, 7]; these will be dealt with elsewhere in this issue. A diverse array of plasma membrane proteins are detected in lipid rafts either constitutively [8], or more commonly, after ligand-induced receptor clustering. Treatment with cholesterol-binding compounds that perturb lipid raft integrity can impact raft-associated membrane protein’s functions [9]. The ability of proteins to become (and maintain) raft associated thus has important implications in understanding the coordination of a wide variety of cellular events.
Professional antigen presenting cells (APCs, including dendritic cells (DCs), macrophages, B cells, and certain epithelial cell types) generate peptides fragments of internalized protein antigens, and these peptide fragments in turn bind MHC-II molecules (MHC-II) in lysosome-like antigen processing compartments [10]. Peptide binding to MHC-II in these compartments results in either constitutive or inducible transport of the molecules to the cell surface, depending upon the APC subtype. Once at the plasma membrane, specific peptide-MHC-II (pMHC-II) complexes interact with peptide-specific CD4 helper T cells and induce antigen-specific T cell activation, proliferation, generation of immunological memory, and stimulate production and secretion of a variety of cytokines. The recognition of pMHC-II complexes by T cells forms the basis of the adaptive immune response and there is considerable evidence that lipid rafts (both on APCs and on CD4 T cells) play an important role in antigen presentation. In this review we will discuss the evidence for lipid raft microdomain-mediated clustering of MHC-II, the biosynthesis of lipid raft-associated pMHC-II complexes, and the role that lipid rafts plays in facilitating antigen presentation to T cells for the initiation of adaptive immune responses.
A History of MHC-II Lipid Rafts
Electron microscopy, fluorescence resonance energy transfer, and confocal microscopy imaging first suggested that MHC-II are not evenly dispersed on lymphocytes, but instead a portion of the molecules are clustered [11–13]. The first indication that MHC-II was associated with membrane rafts came from MHC-II antibody cross-linking studies in a macrophage cell line that revealed cross-linking induced intracellular tyrosine kinase activity together with increased ability to detect MHC-II in Triton X-100 insoluble lipid rafts [14]. Treatment with the cholesterol chelating agent methyl-β-cyclodextrin (MCD) prevented MHC-II raft association and tyrosine kinase activity, leading the authors to conclude that antibody-induced clustering of MHC-II initiated signal transduction via plasma membrane rafts [14]. Confocal fluorescence microscopy analysis confirmed MHC-II raft association after cross-linking [14, 15], since the clusters co-localized with cholera toxin B subunit, a commonly used lipid raft marker that binds GM1 glycosphingolipids. We have recently shown that treatment with MCD leads to a redistribution of MHC-II on the surface of DCs such that the molecules no longer have a clustered distribution, thereby supporting the idea that these clusters represent lipid raft-associated MHC-II [16].
Subsequent to the study of Huby et al. [14], a number of laboratories have shown that a substantial fraction of MHC-II is constitutively lipid raft-associated in APCs. MHC-II is present in lipid rafts isolated from a wide variety of human and mouse APC subtypes including transformed B cells lines [17–19], primary tonsil B cells [20], monocytes [20–22], and DCs [16, 23–25]. In each of these studies cross-linking was not required to reveal MHC-II lipid raft association, and as is found for many raft “resident” proteins, it is likely that cross-linking simply stabilizes MHC-II/lipid raft associations that constitutively exist. The inconsistency in the literature regarding the extent to which MHC-II (or other molecules) associate with lipid rafts is likely due to the raft isolation procedure itself. The amount of MHC-II detected in lipid rafts varies and is critically dependent on a variety of experimental factors including temperature, the detergent used, the ratio of protein-to-detergent in the cell lysate, and even the serum type [18, 26]. Few have found that in the mild detergent Brij-58 more than 70% of MHC-II on mouse DCs is lipid raft associated, however when the same number of DCs are lysed instead in an equivalent volume of Triton X-100 this value drops to 10%. Similarly, small numbers of DCs solubilized in a given volume of lysis buffer shows less apparent lipid raft association as compared to a large number of DCs lysed in this same volume of buffer (PAR, unpublished data). By biotinylating surface proteins on ice we have found that more than 50% of cell surface MHC-II molecules are lipid raft associated in human and mouse B cell lines [17]. In all of our studies we are careful to ensure that sufficient detergent was added to completely solubilize “non-raft” proteins (such as ICAM-1 and calnexin), giving us confidence that a value greater than 50% accurately represents the “average” extent of lipid raft association of MHC-II on the surface of APCs.
The Biosynthesis of the MHC-II Lipid Raft
MHC-II acquires its’ peptide fragments in late endosomal/pre-lysosomal antigen processing compartment following degradation of internalized proteins [10]. The ability of newly synthesized MHC-II to traffic to these intracellular compartments is dependent on MHC-II association with a chaperone protein termed the Invariant chain (Ii). Ii binds MHC-II α- and β-chains shortly after synthesis in the endoplasmic reticulum, and dileucine sorting motifs present in the Ii cytoplasmic tail direct the MHC-II-Ii complex to endosome/lysosomes [27]. Once in these compartments Ii is degraded and dissociates from MHC-II, antigenic peptides bind to Ii-free MHC-II, and functional pMHC-II complexes are transported to the plasma membrane. Pulse-chase biosynthetic labeling studies using [35S]methionine [28] and intracellular transport inhibition studies using Brefeldin A [29] indicate that MHC-II-Ii complexes first becomes lipid raft-associated during transit through the Golgi apparatus. Up to 60% of newly synthesized MHC-II remain lipid raft-associated during the processes of Ii removal, peptide binding, and pMHC-II transport to the cell surface [28, 29].
The lysosome-like antigen processing compartments in APCs usually have a multi-vesicular morphology and represent what is known in non-APCs as multivesicular bodies (MVBs). The intraluminal vesicles (ILVs) in the MVB are approximately 50 nm in diameter and contain many of the lipids associated with rafts including cholesterol, sphingomyelin, and gangliosides [30, 31]. Using the cholesterol-binding toxin perfringolysin-O Mobius et al. have shown that the MHC-II-positive ILVs of MVB possess large amounts of raft lipid membranes [32]. Since the ILVs of MVB are likely to be the site of peptide-loading onto MHC-II, it has been proposed that these vesicles “back-fuse” with the MVB limiting membrane prior to vesicle-mediated transport of pMHC-II to the plasma membrane of APCs [33]. A hypothetical model depicting the steps involved in back-fusion of ILV “raft” with the MVB limiting membrane and budding, docking, and fusion of pMHC-II raft vesicles with the plasma membrane is shown in Figure 1. Occasionally, the intact MVB fuses directly with the plasma membrane of cells, thereby liberating the intraluminal vesicles into the extracellular space. These secreted vesicles, termed exosomes, are released by a variety of cell types and have been reported to serve a variety of distinct functions [34]. The MHC-II present on isolated exosomes is relatively detergent-insoluble [31], a finding that is in agreement with the idea that exosome-associated MHC-II is present in lipid rafts. While MHC-bearing exosomes secreted from APCs can stimulate both CD4 and CD8 T cells [35–37], the significance of exosomes as “mini-APCs” in vivo still needs to be determined.
Figure 1. Topological Model of MHC-II movement from intralumenal vesicles of MVB to the plasma membrane.
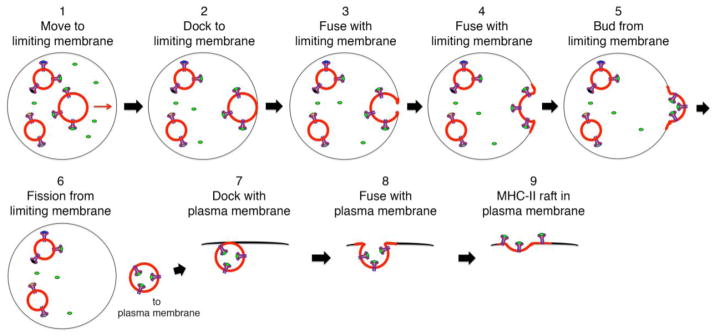
MHC-II are present on lipid raft intraluminal vesicles (ILVs) with their peptide-binding grooves exposed to the antigenic peptide-containing lumen of the MVB. A pMHC-II containing ILV (indicated by red membrane) moves toward the MVB limiting membrane (step 1), docks with (step 2) and fuses with the limiting membrane (steps 3 & 4). Continued outward budding from the limiting membrane (step 5) leads to ILV fission from the limiting membrane and release of a membrane-derived transport vesicle (step 6). This transport vesicles docks with the plasma membrane (step 7) and the docked membranes fuse (steps 8 & 9), thereby depositing a “MHC-II raft” generated from ILV membrane into the plasma membrane.
Linking Lipid Rafts to Efficient pMHC-II Expression on the APC Surface
The demonstration that MHC-II becomes lipid raft-associated in the Golgi apparatus, before Ii dissociation and peptide binding in endo/lysosomes, has allowed us to generate a model describing how MHC-II lipid raft association may enhance the ability of APCs to present pMHC-II complexes to CD4 T cells. It is known that newly synthesized MHC-II molecules consist of three pairs of MHC-II αβ dimers assembled around a core of trimeric Ii molecules [38], and it is also known that this nine-chain MHC-II-Ii complex is already lipid-raft associated before its arrival in lysosome-like antigen processing compartments [28]. Following Ii degradation and dissociation from the MHC-II-Ii complex, the three liberated peptide-free MHC-II αβ dimers would be predicted to disseminate by diffusion. We speculate that upon removal of Ii, the three MHC-II αβ dimers remain “associated” due to their localization to the same lipid raft (Figure 2). This model further predicts that newly-liberated MHC-II αβ dimers would be likely to acquire the same antigenic peptides in these compartments, be transported to the plasma membrane, and at the cell surface remain associated with the same lipid raft. The dissociation and re-association of MHC-II at the cell surface would be predicted to result in a “mixing” of raft protein components and led to a more heterogeneous lipid raft, thus diluting any single peptide determinant on MHC-II.
Figure 2. Model of MHC-II-Invariant chain complex-dependent pMHC-II concentration in lipid rafts.
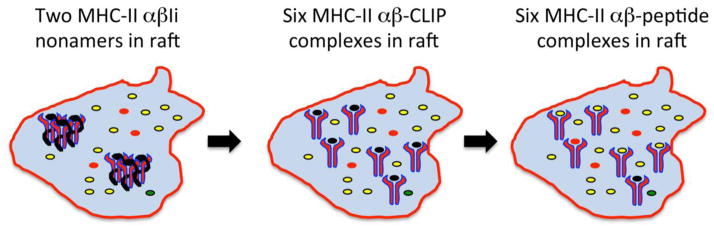
Two MHC-II αβ-Ii nonameric complexes are present in an ILV-derived lipid raft microdomain (surrounded by the red border) containing large amounts of “relevant” antigenic peptides (indicated in yellow, left panel). Following Ii degradation, six MHC-II αβ-CLIP complexes are present in this same microdomain (center panel). Following CLIP removal by HLA-DM, six MHC-II αβ-peptide complexes are formed that are enriched in “relevant” antigenic peptides (in yellow) that are all present within a single membrane microdomain (right panel).
One prediction of any model proposing that lipid raft association is important for MHC-II function in APCs is that there is insertion of a “quantum” of identical pMHC-II into the plasma membrane of APC after pMHC-II complex formation in antigen processing compartments. We have recently obtained evidence that clusters of specific pMHC-II complexes generated in late endosomes and remain clustered in lipid rafts with the same peptide at the cell surface [16]. Using confocal immunofluorescence microscopy, DCs pulsed with HEL protein first generate MHC-II-HEL(46–61) complexes with MHC-II microdomains in lysosome-like antigen processing compartments. When these complexes arrive at the DC surface, they are visualized by both immunoelectron microscopy and confocal microscopy to exist in 80 nm clusters (Figure 3). The integrity of these MHC-II-HEL(46–61) clusters is disrupted by MCD, suggesting that these clusters represent newly arrived lipid raft-associated MHC-II-HEL(46–61) complexes [16]. While there is much we do not know about the organization or molecular mechanisms stabilizing these structures on the APC surface, the visualization of raft-associated clusters of identical pMHC-II complexes is at least a beginning in our quest to understand this process.
Figure 3. Newly-arrived pMHC-II complexes are clustered on the DC surface.
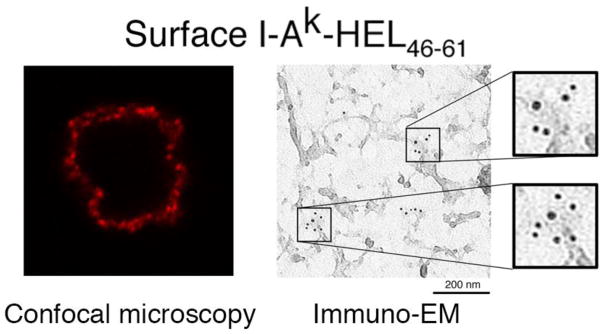
Immature DCs were incubated in with HEL protein, washed, and activated with LPS for 12 hr. The DCs were stained live with a mAb that only recognizes the I-Ak-HEL(46–61) complexes. Aw3.18.14. The cells were analyzed by confocal immunofluorescence microscopy and a single 0.8 μm thick optical section is shown (left panel). The distribution of these newly-arrived pMHC-II complexes on the DC surface was also determined using “plasma membrane rips” and analysis by immunoelectron microscopy (right panel) (Reprinted with permission from reference [16].
MHC-II Lipid Rafts and MHC-II Tetraspanin Domains
Tetraspanin protein family members contain four transmembrane domains, intracellular amino and carboxy termini, and bind to a variety of proteins to form the basis of the “tetraspan web”. Like association with lipid raft microdomains, the tetraspan web is a relatively detergent-insoluble membrane microdomain and association with the tetraspan web has been implicated in regulating a variety of biological events [39]. There are many reports documenting the ability of MHC-II to association with different tetraspanin family members. The endosomal/lysosomal tetraspanins CD81 and CD82 co-immunoprecipitate with MHC-II in human B cell lines [40, 41]. MHC-II associates with the CD63 in endosomes and with CD9, CD53, and CD81 at the cell surface of DCs [42]. MHC-II also associates with CD9 and CD38 in human monocytes [22], with CD9, CD53, CD81, and CD82 in human DCs [41], and the association of CD9 with MHC-II has been reported to lead to clustering of MHC-II on the surface of mouse DCs [43]. It has been proposed that tetraspan web-association facilitates MHC-II clustering to form membrane domains that are distinct from lipid rafts [44]. This study was based on the use of a mAb that was proposed to recognize a subset of tetraspanin-associated pMHC-II complexes [45]. A follow-up study did not find any evidence that this mAb selectively recognized a subset of MHC-II [46], calling into question the idea that MHC-II is differentially associated with either lipid raft or tetraspanin membrane microdomains.
In fact, a number of studies have indicated that tetraspanin molecules can be isolated in detergent-insoluble fractions that, like lipid rafts themselves, are cholesterol-dependent [47–49]. The tetraspanin-web resident protein CD9 has also been reported to be a constituent in “lipid rafts” isolated from activated eosinophil cells [50] and DCs [42]. Such studies suggest to us that the distinction between a detergent-insoluble lipid raft microdomain and a tetraspanin microdomain is a somewhat artificial one, and the most likely scenario is that tetraspanin proteins, present in “lipid rafts”, help stabilize raft associations of their binding partners (such as MHC-II) with these membrane microdomains.
Importance of MHC-II Lipid Raft Association for APC Function
Each CD4 T cell contains a single T cell antigen receptor (TCR) specificity that recognizes a combinatorial epitope containing both MHC-II and its associated peptide fragment [51]. There are approximately 106 MHC-II molecules on professional APCs, however this number can fluctuate depending upon cell type, stage of differentiation, or activation status. The pMHC-II repertoire is diverse and under normal conditions the vast majority of MHC-II-bound peptides are derived from cellular “self” proteins. During viral or microbial infections the foreign invader MHC-II-bound peptides are recognized and responsible for antigen-specific T cell activation. Less than 500 specific pMHC-II complexes are required to activate antigen-specific naïve T cells [52, 53] and this number may be lower for particular high-affinity peptides. Even less pMHC-II required to activate memory T cells, due to changes in the TCR oligomers after responding to the original antigen [54]. These vanishingly small amounts of specific pMHC-II on the APC surface (representing less than 0.01% of the total pool of MHC-II) must somehow efficiently interact with a single T cell, and we have proposed that MHC-II clustering in lipid rafts is immunologically important since it increases the local concentration of relevant pMHC-II complexes required to activate antigen-specific CD4 T cells [17, 28, 55].
The first evidence to suggest that APC lipid rafts are important for T cell antigen recognition was the demonstration that treatment of antigen-pulsed B cells with the cholesterol disrupting agents MCD or nystatin significantly reduced their ability to stimulate an antigen-specific T cell hybridoma [17]. The suppressive effect of nystatin on antigen-pulsed APC function was fully reversible, demonstrating that cholesterol perturbation did not affect specific pMHC-II expression levels or other factors necessary for efficient T cell activation by APCs. Curiously, raft disruption preferentially affected the APCs pulsed with small amounts of antigen; raft disruption had no effect on T cell activation when the B cells were pulsed with large amounts of antigen [17]. The ability of lipid raft associated MHC-II to preferentially facilitate T cell activation at low antigen concentration has now been observed in a variety of systems including human DCs and mouse B cells presenting influenza virus haemagglutinin epitopes to T cell clones [24, 56] and mouse DCs presenting HEL epitopes to naïve T cells [16]. The antigen-dose dependence of MHC-II lipid raft association has been seen not only when APCs were pulsed with intact protein antigens (which require intracellular processing) but also when APCs were pulsed with pre-processed antigenic peptides. Although it is often assumed the peptide-pulsing results in a “homogenous” distribution of pMHC-II on the APC surface, the ability of APCs to internalize extracellular material (including peptides) by macropinocytosis leaves open the possibility that even pre-processed peptides bind primarily to intracellular (nascent) MHC-II. While this possibility remains to be tested, the well-documented ability of cholesterol-depletion to suppress T cell activation by APCs loaded with limiting amounts of antigenic-peptides is entirely consistent with the hypothesis that lipid raft association allows small numbers of specific pMHC-II to stimulate antigen-specific CD4 T cells.
MHC-II Lipid Rafts and T Cell Co-Stimulation at the Immunological Synapse
TCR recognition of pMHC-II complexes triggers a cascade of events leading to the formation of a macromolecular complex between the APC and T cell referred to as the immunological synapse [57]. The formation of this structure triggers dramatic changes in the APC and T cell membrane, ultimately resulting in T cell differentiation and acquisition of effector function. Imaging studies have shown that T cell lipid rafts coalesce at the immunological synapse [58–60], with many T cell-associated signaling molecules becoming raft-associated and synapse-localized upon TCR engagement [58, 61]. We have shown that formation of an immunological synapse between antigen-loaded B cells and antigen-specific T cells results in APC raft accumulation at the immunological synapse [15]. T cell:APC conjugate formation also leads to synaptic clustering of MHC-II that is both co-stimulation [62] and ICAM-dependent [62, 63]. Curiously, immunologically “relevant” pMHC-II preferentially accumulates at the synapse under conditions of low, but not high, antigen dose [15], suggesting that concentration of pMHC-II at the synapse is important primarily when antigenic pMHC-II amounts are limiting. These data are in excellent agreement with data showing a concentration-dependent role for APC rafts in T cell activation [16, 17, 24, 56].
When the antigen-specific TCR recognizes relevant pMHC-II on DCs, the maturing DCs respond by generating elongated MHC-II endo/lysosomal tubules that emanate toward the immunologic synapse [64, 65] where they ultimately fuse with the plasma membrane [65]. The association of MHC-II with lipid rafts is likely to be critical for this event since most of endosomal MHC-II in DCs is already raft-associated [28] and any nascent MHC-II molecules are likely to load with antigenic peptides derived from the pathogen (or antigen) that initially stimulated the DC. Thus, insertion of relevant pMHC-II directly into the immunological synapse would enhance T cell signaling and activation, a hallmark of efficient antigen presentation.
Lipid rafts may also be involved in concentrating the APC co-stimulatory molecules CD80, CD86, and CD40 at the immunological synapse. CD40 weakly associates with MHC-II in resting B cells, and either B cell activation or cross-linking with MHC-II or CD40 mAb greatly increases their association [66]. Unlike MHC-II, only a small portion of the total pool of CD40 is lipid raft associated in resting APCs, but engagement of CD40 with CD40 Ligand (CD154) dramatically enhances CD40 clustering [67] and raft association [68, 69]. Similarly, shortly after CD40-mediated B cell activation total MHC-II and CD80 cell surface expression remains unchanged but there is a rapid increase in clustering of MHC-II with CD80 at the plasma membrane that is blocked by cholesterol depletion [70]. In addition, CD86 and MHC-II begin to co-localize in endosome-like vesicles in maturing DCs, and upon full DC maturation these proteins co-localize extensively on the APC surface in small clusters [71]. Although it is not yet known whether these interactions are by direct protein-protein interactions or are facilitated by their co-association with lipid rafts, these studies raise the interesting possibility that one of the functions of MHC-II lipid raft localization is to provide a lipid environment conducive to APC co-stimulatory protein concentration at the developing immunological synapse.
Conclusion
Since the first description of clustering of MHC-II on the surface of APCs nearly 20 years ago, we have made great progress in understanding the role MHC-II microdomain association plays in APC function. There are, however, considerable gaps in our knowledge, such as what are the structural elements that regulate association of MHC-II with lipid raft (or other) membrane microdomains, how can the reported short life-time of lipid rafts be reconciled with extended stability of MHC-II clusters on the APC surface, and whether/how the mechanisms of peptide-binding onto MHC-II and MHC-II microdomain association are linked. Undoubtedly these questions will be answered in the near future and this information will give us a more clear understanding of the wide variety of mechanisms APCs use to interact with and stimulate antigen-specific T cells.
Highlights.
MHC class II-peptide complexes are present in lipid rafts on the surface of APCs
MHC class II is present in cholesterol-rich microclusters on the APC surface
MHC class II lipid raft-association promotes efficient CD4 T cell activation by APCs
Acknowledgments
The work from the Roche laboratory cited in this review was supported by the Intramural Research Program of the National Cancer Institute. H.A.A. is supported by the U.S. Food and Drug Administration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simons K, Sampaio JL. Membrane organization and lipid rafts. Cold Spring Harbor perspectives in biology. 2011;3:a004697. doi: 10.1101/cshperspect.a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson RG, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- 3.Hanzal-Bayer MF, Hancock JF. Lipid rafts and membrane traffic. FEBS Lett. 2007;581:2098–2104. doi: 10.1016/j.febslet.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Horejsi V, Hrdinka M. Membrane microdomains in immunoreceptor signaling. FEBS Lett. 2014 doi: 10.1016/j.febslet.2014.05.047. [DOI] [PubMed] [Google Scholar]
- 5.Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 6.Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 7.Nichols B. Cell biology: without a raft. Nature. 2005;436:638–639. doi: 10.1038/436638a. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nature cell biology. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 9.Keller P, Simons K. Cholesterol is required for surface transport of influenza virus hemagglutinin. J Cell Biol. 1998;140:1357–1367. doi: 10.1083/jcb.140.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annual review of immunology. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu I, Davis DM, Strominger JL. Trafficking of spontaneously endocytosed MHC proteins. Proc Natl Acad Sci U S A. 1999;96:13944–13949. doi: 10.1073/pnas.96.24.13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenei A, Varga S, Bene L, Matyus L, Bodnar A, Bacso Z, Pieri C, Gaspar R, Jr, Farkas T, Damjanovich S. HLA class I and II antigens are partially co-clustered in the plasma membrane of human lymphoblastoid cells. Proc Natl Acad Sci U S A. 1997;94:7269–7274. doi: 10.1073/pnas.94.14.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vereb G, Matko J, Vamosi G, Ibrahim SM, Magyar E, Varga S, Szollosi J, Jenei A, Gaspar R, Jr, Waldmann TA, Damjanovich S. Cholesterol-dependent clustering of IL-2Ralpha and its colocalization with HLA and CD48 on T lymphoma cells suggest their functional association with lipid rafts. Proc Natl Acad Sci U S A. 2000;97:6013–6018. doi: 10.1073/pnas.97.11.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huby RD, Dearman RJ, Kimber I. Intracellular phosphotyrosine induction by major histocompatibility complex class II requires co-aggregation with membrane rafts. J Biol Chem. 1999;274:22591–22596. doi: 10.1074/jbc.274.32.22591. [DOI] [PubMed] [Google Scholar]
- 15.Hiltbold EM, Poloso NJ, Roche PA. MHC class II-peptide complexes and APC lipid rafts accumulate at the immunological synapse. J Immunol. 2003;170:1329–1338. doi: 10.4049/jimmunol.170.3.1329. [DOI] [PubMed] [Google Scholar]
- 16.Bosch B, Heipertz EL, Drake JR, Roche PA. Major histocompatibility complex (MHC) class II-peptide complexes arrive at the plasma membrane in cholesterol-rich microclusters. J Biol Chem. 2013;288:13236–13242. doi: 10.1074/jbc.M112.442640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson HA, Hiltbold EM, Roche PA. Concentration of MHC class II molecules in lipid rafts facilitates antigen presentation. Nat Immunol. 2000;1:156–162. doi: 10.1038/77842. [DOI] [PubMed] [Google Scholar]
- 18.Knorr R, Karacsonyi C, Lindner R. Endocytosis of MHC molecules by distinct membrane rafts. J Cell Sci. 2009;122:1584–1594. doi: 10.1242/jcs.039727. [DOI] [PubMed] [Google Scholar]
- 19.Busman-Sahay K, Sargent E, Harton JA, Drake JR. The Ia.2 epitope defines a subset of lipid raft-resident MHC class II molecules crucial to effective antigen presentation. Journal of immunology. 2011;186:6710–6717. doi: 10.4049/jimmunol.1100336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouillon M, El FY, Girouard J, Khalil H, Thibodeau J, Mourad W. Lipid raft-dependent and -independent signaling through HLA-DR molecules. J Biol Chem. 2003;278:7099–7107. doi: 10.1074/jbc.M211566200. [DOI] [PubMed] [Google Scholar]
- 21.Buatois V, Baillet M, Becart S, Mooney N, Leserman L, Machy P. MHC class II-peptide complexes in dendritic cell lipid microdomains initiate the CD4 Th1 phenotype. J Immunol. 2003;171:5812–5819. doi: 10.4049/jimmunol.171.11.5812. [DOI] [PubMed] [Google Scholar]
- 22.Zilber MT, Setterblad N, Vasselon T, Doliger C, Charron D, Mooney N, Gelin C. MHC class II/CD38/CD9: a lipid-raft-dependent signaling complex in human monocytes. Blood. 2005;106:3074–3081. doi: 10.1182/blood-2004-10-4094. [DOI] [PubMed] [Google Scholar]
- 23.Setterblad N, Roucard C, Bocaccio C, Abastado JP, Charron D, Mooney N. Composition of MHC class II-enriched lipid microdomains is modified during maturation of primary dendritic cells. J Leukoc Biol. 2003;74:40–48. doi: 10.1189/jlb.0103045. [DOI] [PubMed] [Google Scholar]
- 24.Eren E, Yates J, Cwynarski K, Preston S, Dong R, Germain C, Lechler R, Huby R, Ritter M, Lombardi G. Location of major histocompatibility complex class II molecules in rafts on dendritic cells enhances the efficiency of T-cell activation and proliferation. Scand J Immunol. 2006;63:7–16. doi: 10.1111/j.1365-3083.2006.01700.x. [DOI] [PubMed] [Google Scholar]
- 25.Khandelwal S, Roche PA. Distinct MHC class II molecules are associated on the dendritic cell surface in cholesterol-dependent membrane microdomains. J Biol Chem. 2010;285:35303–35310. doi: 10.1074/jbc.M110.147793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babiychuk EB, Draeger A. Biochemical characterization of detergent-resistant membranes: a systematic approach. Biochem J. 2006;397:407–416. doi: 10.1042/BJ20060056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bakke O, Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990;63:707–716. doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- 28.Poloso NJ, Muntasell A, Roche PA. MHC class II molecules traffic into lipid rafts during intracellular transport. Journal of immunology. 2004;173:4539–4546. doi: 10.4049/jimmunol.173.7.4539. [DOI] [PubMed] [Google Scholar]
- 29.Karacsonyi C, Knorr R, Fulbier A, Lindner R. Association of major histocompatibility complex II with cholesterol- and sphingolipid-rich membranes precedes peptide loading. J Biol Chem. 2004;279:34818–34826. doi: 10.1074/jbc.M404608200. [DOI] [PubMed] [Google Scholar]
- 30.Mobius W, van Donselaar E, Ohno-Iwashita Y, Shimada Y, Heijnen HF, Slot JW, Geuze HJ. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic. 2003;4:222–231. doi: 10.1034/j.1600-0854.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 31.Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Mobius W, Hoernschemeyer J, Slot JW, Geuze HJ, Stoorvogel W. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem. 2003;278:10963–10972. doi: 10.1074/jbc.M207550200. [DOI] [PubMed] [Google Scholar]
- 32.Mobius W, Ohno-Iwashita Y, van Donselaar EG, Oorschot VM, Shimada Y, Fujimoto T, Heijnen HF, Geuze HJ, Slot JW. Immunoelectron microscopic localization of cholesterol using biotinylated and non-cytolytic perfringolysin O. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2002;50:43–55. doi: 10.1177/002215540205000105. [DOI] [PubMed] [Google Scholar]
- 33.Kleijmeer M, Ramm G, Schuurhuis D, Griffith J, Rescigno M, Ricciardi-Castagnoli P, Rudensky AY, Ossendorp F, Melief CJ, Stoorvogel W, Geuze HJ. Reorganization of multivesicular bodies regulates MHC class II antigen presentation by dendritic cells. J Cell Biol. 2001;155:53–63. doi: 10.1083/jcb.200103071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nature medicine. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 36.Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 37.Muntasell A, Berger AC, Roche PA. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. Embo J. 2007;26:4263–4272. doi: 10.1038/sj.emboj.7601842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roche PA, Marks MS, Cresswell P. Formation of a nine-subunit complex by HLA class II glycoproteins and the invariant chain. Nature. 1991;354:392–394. doi: 10.1038/354392a0. [DOI] [PubMed] [Google Scholar]
- 39.Hemler ME. Targeting of tetraspanin proteins--potential benefits and strategies. Nat Rev Drug Discov. 2008;7:747–758. doi: 10.1038/nrd2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammond C, Denzin LK, Pan M, Griffith JM, Geuze HJ, Cresswell P. The tetraspan protein CD82 is a resident of MHC class II compartments where it associates with HLA-DR, -DM, and -DO molecules. Journal of immunology. 1998;161:3282–3291. [PubMed] [Google Scholar]
- 41.Poloso NJ, Denzin LK, Roche PA. CDw78 defines MHC class II-peptide complexes that require Ii chain-dependent lysosomal trafficking, not localization to a specific tetraspanin membrane microdomain. J Immunol. 2006;177:5451–5458. doi: 10.4049/jimmunol.177.8.5451. [DOI] [PubMed] [Google Scholar]
- 42.Engering A, Pieters J. Association of distinct tetraspanins with MHC class II molecules at different subcellular locations in human immature dendritic cells. Int Immunol. 2001;13:127–134. doi: 10.1093/intimm/13.2.127. [DOI] [PubMed] [Google Scholar]
- 43.Unternaehrer JJ, Chow A, Pypaert M, Inaba K, Mellman I. The tetraspanin CD9 mediates lateral association of MHC class II molecules on the dendritic cell surface. Proc Natl Acad Sci U S A. 2007;104:234–239. doi: 10.1073/pnas.0609665104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kropshofer H, Spindeldreher S, Rohn TA, Platania N, Grygar C, Daniel N, Wolpl A, Langen H, Horejsi V, Vogt AB. Tetraspan microdomains distinct from lipid rafts enrich select peptide-MHC class II complexes. Nat Immunol. 2002;3:61–68. doi: 10.1038/ni750. [DOI] [PubMed] [Google Scholar]
- 45.Drbal K, Angelisova P, Rasmussen AM, Hilgert I, Funderud S, Horejsi V. The nature of the subset of MHC class II molecules carrying the CDw78 epitopes. Int Immunol. 1999;11:491–498. doi: 10.1093/intimm/11.4.491. [DOI] [PubMed] [Google Scholar]
- 46.Poloso NJ, Denzin LK, Roche PA. CDw78 defines MHC class II-peptide complexes that require Ii chain-dependent lysosomal trafficking, not localization to a specific tetraspanin membrane microdomain. Journal of immunology. 2006;177:5451–5458. doi: 10.4049/jimmunol.177.8.5451. [DOI] [PubMed] [Google Scholar]
- 47.Claas C, Stipp CS, Hemler ME. Evaluation of prototype transmembrane 4 superfamily protein complexes and their relation to lipid rafts. J Biol Chem. 2001;276:7974–7984. doi: 10.1074/jbc.M008650200. [DOI] [PubMed] [Google Scholar]
- 48.Charrin S, Manie S, Thiele C, Billard M, Gerlier D, Boucheix C, Rubinstein E. A physical and functional link between cholesterol and tetraspanins. Eur J Immunol. 2003;33:2479–2489. doi: 10.1002/eji.200323884. [DOI] [PubMed] [Google Scholar]
- 49.Silvie O, Charrin S, Billard M, Franetich JF, Clark KL, van Gemert GJ, Sauerwein RW, Dautry F, Boucheix C, Mazier D, Rubinstein E. Cholesterol contributes to the organization of tetraspanin-enriched microdomains and to CD81-dependent infection by malaria sporozoites. J Cell Sci. 2006;119:1992–2002. doi: 10.1242/jcs.02911. [DOI] [PubMed] [Google Scholar]
- 50.Akuthota P, Melo RC, Spencer LA, Weller PF. MHC Class II and CD9 in human eosinophils localize to detergent-resistant membrane microdomains. Am J Respir Cell Mol Biol. 2012;46:188–195. doi: 10.1165/rcmb.2010-0335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 52.Harding CV, Unanue ER. Quantitation of antigen-presenting cell MHC class II/peptide complexes necessary for T-cell stimulation. Nature. 1990;346:574–576. doi: 10.1038/346574a0. [DOI] [PubMed] [Google Scholar]
- 53.Demotz S, Grey HM, Sette A. The minimal number of class II MHC-antigen complexes needed for T cell activation. Science. 1990;249:1028–1030. doi: 10.1126/science.2118680. [DOI] [PubMed] [Google Scholar]
- 54.Kumar R, Ferez M, Swamy M, Arechaga I, Rejas MT, Valpuesta JM, Schamel WW, Alarcon B, van Santen HM. Increased sensitivity of antigen-experienced T cells through the enrichment of oligomeric T cell receptor complexes. Immunity. 2011;35:375–387. doi: 10.1016/j.immuni.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 55.Poloso NJ, Roche PA. Association of MHC class II-peptide complexes with plasma membrane lipid microdomains. Curr Opin Immunol. 2004;16:103–107. doi: 10.1016/j.coi.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 56.Gombos I, Detre C, Vamosi G, Matko J. Rafting MHC-II domains in the APC (presynaptic) plasma membrane and the thresholds for T-cell activation and immunological synapse formation. Immunol Lett. 2004;92:117–124. doi: 10.1016/j.imlet.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 57.Dustin ML, Depoil D. New insights into the T cell synapse from single molecule techniques. Nat Rev Immunol. 2011;11:672–684. doi: 10.1038/nri3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains [see comments] Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 60.Burack WR, Lee KH, Holdorf AD, Dustin ML, Shaw AS. Cutting edge: quantitative imaging of raft accumulation in the immunological synapse. J Immunol. 2002;169:2837–2841. doi: 10.4049/jimmunol.169.6.2837. [DOI] [PubMed] [Google Scholar]
- 61.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 62.Wulfing C, Sumen C, Sjaastad MD, Wu LC, Dustin ML, Davis MM. Costimulation and endogenous MHC ligands contribute to T cell recognition. Nat Immunol. 2002;3:42–47. doi: 10.1038/ni741. [DOI] [PubMed] [Google Scholar]
- 63.de la Fuente H, Mittelbrunn M, Sanchez-Martin L, Vicente-Manzanares M, Lamana A, Pardi R, Cabanas C, Sanchez-Madrid F. Synaptic clusters of MHC class II molecules induced on DCs by adhesion molecule-mediated initial T-cell scanning. Mol Biol Cell. 2005;16:3314–3322. doi: 10.1091/mbc.E05-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boes M, Cerny J, Massol R, Op den Brouw M, Kirchhausen T, Chen J, Ploegh HL. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature. 2002;418:983–988. doi: 10.1038/nature01004. [DOI] [PubMed] [Google Scholar]
- 65.Chow A, Toomre D, Garrett W, Mellman I. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature. 2002;418:988–994. doi: 10.1038/nature01006. [DOI] [PubMed] [Google Scholar]
- 66.Leveille C, Chandad F, Al-Daccak R, Mourad W. CD40 associates with the MHC class II molecules on human B cells. Eur J Immunol. 1999;29:3516–3526. doi: 10.1002/(SICI)1521-4141(199911)29:11<3516::AID-IMMU3516>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 67.Grassme H, Jendrossek V, Bock J, Riehle A, Gulbins E. Ceramide-rich membrane rafts mediate CD40 clustering. J Immunol. 2002;168:298–307. doi: 10.4049/jimmunol.168.1.298. [DOI] [PubMed] [Google Scholar]
- 68.Hostager BS, Catlett IM, Bishop GA. Recruitment of CD40 and tumor necrosis factor receptor-associated factors 2 and 3 to membrane microdomains during CD40 signaling. J Biol Chem. 2000;275:15392–15398. doi: 10.1074/jbc.M909520199. [DOI] [PubMed] [Google Scholar]
- 69.Nadiri A, Polyak MJ, Jundi M, Alturaihi H, Reyes-Moreno C, Hassan GS, Mourad W. CD40 translocation to lipid rafts: signaling requirements and downstream biological events. Eur J Immunol. 2011;41:2358–2367. doi: 10.1002/eji.201041143. [DOI] [PubMed] [Google Scholar]
- 70.Clatza A, Bonifaz LC, Vignali DA, Moreno J. CD40-induced aggregation of MHC class II and CD80 on the cell surface leads to an early enhancement in antigen presentation. J Immunol. 2003;171:6478–6487. doi: 10.4049/jimmunol.171.12.6478. [DOI] [PubMed] [Google Scholar]
- 71.Turley SJ, Inaba K, Garrett WS, Ebersold M, Unternaehrer J, Steinman RM, Mellman I. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 2000;288:522–527. doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]


