Abstract
Tumour-initiating cells are thought to share features with normal somatic stem cells. In mice, stem cells at the ovarian hilum have been shown to express the stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1A1), and are prone to malignant transformation. The potential relevance of this finding to humans has not been established. In this study, we used immunohistochemistry to assess the distribution of ALDH1A1 staining in the epithelium of human fallopian tubes, with particular reference to the transition of tubal epithelium to mesothelium (i.e. the tubal-mesothelial junction), the ovarian surface epithelium (OSE), putative precursors of ovarian high grade serous carcinoma (HGSC), namely, serous tubal intraepithelial carcinoma (STIC) and p53 signatures, and overt HGSC. Expression of ALDH1A1 was detected in both secretory and ciliated tubal epithelial cells, tubal-mesothelial junctions and OSE, but was absent in STIC and p53 signatures. Positive staining in HGSC, when present, was typically limited to rare tumour cells. In-silico analyses of the mRNA expression dataset from The Cancer Genome Atlas revealed down-regulation of ALDH1A1 transcripts in HGSC relative to normal tubal epithelium and no association between ALDH1A1 expression levels and overall survival. Our results do not support ALDH1A1 as a marker of stem cells in human fallopian tube and demonstrate that its loss of expression is an early event in the development of HGSC.
Keywords: STIC, p53 signature, ALDH1, stem cells, ovarian cancer, tubal-mesothelial junction
Introduction
Mounting evidence has shown that a significant proportion of pelvic high grade serous carcinomas (HGSC) arise from a non-invasive occult carcinoma in the distal fallopian tube, designated “serous tubal intraepithelial carcinoma (STIC)”1. STICs have been identified in 10–15% of prophylactic salpingo-oophorectomy specimens from women with germline BRCA mutations2, 3, and in the tubal fimbriae from up to 60% of women with sporadic ovarian HGSC4. Complete microscopic sectioning of fallopian tubes has also revealed focal proliferations of morphologically normal secretory cells that show diffuse p53 immunoreactivity, termed “p53 signatures,”5 which may represent early clonal expansions of tumour-initiating cells.
Experimental work conducted in a variety of model systems across different tumor types have consistently demonstrated that tumour-initiating cells are derived from either a somatic stem cell or an early progenitor cell that has reacquired stem cell features6. This is in keeping with the concept that the capacity for indefinite proliferation, or self-renewal, is essential for malignant transformation7. Studies have also shown that only a fraction of cancer cells, termed “cancer stem cells” are capable of forming tumours upon serial transplantation into immunocompromised mice, a technique used to assess self-renewal8.
One of the most commonly used markers for both normal and malignant stem cells is aldehyde dehydrogenase isoform 1A1 (ALDH1A1, previously known as ALDH1). ALDH1A1 is an enzyme involved in the metabolism of retinoic acid, which has been implicated in the regulation of cellular differentiation9, 10. Multipotent stem cells in normal tissues have been shown to express ALDH1A110, 11 which has also been studied extensively as a candidate marker for cancer stem cells11–15. In mice, the “ovarian hilum,” where nerves and blood vessels enter the ovary, is covered by a layer of cells, which have been previously described as representing the transition between mesothelium lining the surface of the ovary and ovarian bursa, and ciliated columnar epithelium lining the mouse oviduct16. This region has been reported to be enriched for ALDH1A1-positive stem cells that show increased susceptibility to malignant transformation16.
In humans, the mesothelial lining of the ovarian surface epithelium (OSE) extends over the ovarian hilum and is continuous with the mesovarium and broad ligament. The point of contact between mesothelium and Mullerian epithelium is positioned at multiple foci within the human fallopian tube fimbria and these “tubal-mesothelial junctions” may be analogous to the junctional epithelium overlying the mouse ovarian hilum17. Whether stem cells reside in either the hilar area or tubal-mesothelial junctions in humans, as observed in the mouse model, however, remains unknown.
To gain further insight into the phenotype of the cell of origin and the early events in the pathogenesis of HGSC, we used immunohistochemistry to examine the pattern of ALDH1A1 expression in normal and lesional fallopian tube epithelium, including tubal-mesothelial junctions, endosalpingiosis, p53 signatures, STICs, and HGSCs, as well as in OSE, rete ovarii and primary and recurrent ovarian HGSCs.
Materials and Methods
Case Selection
Formalin-fixed paraffin-embedded surgical specimens were retrieved from the archives of the Department of Pathology, The Johns Hopkins Medical Institutions. Salpingectomy specimens were obtained from 46 patients: 29 normal fallopian tubes (with adjacent ovary and intact OSE in 8 cases), and 17 with STIC. Within these specimens, 28 tubal-mesothelial junctions, 14 foci of endosalpingiosis, 11 p53 signatures and 11 overt HGSCs were identified. Further analyses were performed on a tissue microarray comprised of 28 pairs of matched primary and recurrent ovarian HGSCs.
Diagnoses of STIC and p53 signatures were made according to previously reported criteria18, 19. Tubal-mesothelial junctions were identified based on morphologic features, with questionable foci confirmed by immunohistochemistry for calretinin, as previously described17. The acquisition of tissue samples was approved by the institutional review board.
Western blot analysis
Western blot analysis was performed on the HHL-6 human hepatocyte cell line and protein lysates were separated by electrophoresis on 4% to 12% Tris-glycine gels and transferred onto a poly inylidine difluoride membrane. After being blocked with 5% non-fat milk, the membrane was incubated at 4°C overnight with two separate ALDH1A1 primary antibodies (EP1932Y, Epitomics and clone 44, BD Biosciences), both at 1:500 dilution, washed, incubated with horseradish peroxidase-conjugated secondary antibody and detected with ECL detection reagent (GE Healthcare).
Immunohistochemistry
The following primary antibodies were used: p53 mouse monoclonal (clone Bp53-11, Ventana), calretinin rabbit polyclonal (Cell Marque), ALDH1A1 rabbit monoclonal (clone EP1932Y, Epitomics), and ALDH1A1 mouse monoclonal (clone 44, BD Biosciences). Optimized conditions for p53 and calretinin immunohistochemistry have been reported previously 19, 20.
To determine the expression of ALDH1A1 protein expression on tissue sections, we used two independent antibodies from Epitomics and BD Biosciences. Quality control measures were taken to assess the sensitivity and specificity of the ALDH1A1 antibodies. For both antibodies, western blot was performed on protein extracts from immortalized human hepatocytes to confirm the specificity of the ALDH1A1 antibodies.
For ALDH1A1 immunohistochemistry, antigen retrieval was performed by steaming the sections in citrate buffer (pH 6.0) for 25 minutes. Blocking of endogenous peroxidases was performed by immersing slides in 3% hydrogen peroxide for 15 minutes, followed by blocking using an avidin/biotin blocking kit (Invitrogen), according to the manufacturer’s instructions. Slides were incubated with 5% non-fat milk for 30 minutes at room temperature and overnight at 4°C with ALDH1A1 antibody at 1:300 dilution (EP1932Y) or 1:600 dilution (clone 44). Following wash steps, slides were incubated with peroxidase-conjugated polyclonal goat anti-rabbit antibody (Dako) (for EP1932Y) or polyclonal rabbit anti-mouse antibody (Jackson ImmunoResearch) (for clone 44), and visualized using 3,3’-diaminobenzidene.
Confirmation of appropriate ALDH1A1 staining was performed on control slides of normal colonic and endometrial tissue. The pattern of ALDH1A1 staining was analyzed semi-quantitatively in normal tubal epithelium, OSE, p53 signatures, STIC, endosalpingiosis, rete ovarii and HGSC, subsequent to identification of areas of interest on corresponding H&E- and p53-stained slides. In this study, a case was scored as positive if any of the cells showed strong definitive cytoplasmic staining. In p53 signatures, endosalpingiosis, rete ovarii and STICs, the percentage of ALDH1A1-positive epithelial cells was estimated by counting all observable cells, whereas for tubal-mesothelial junctions, at least 10 cells flanking each side of the junction (i.e. approximately 20 consecutive cells total) were counted. For each sample of normal fallopian tube and HGSC, at least 500 epithelial cells were assessed. Evaluation of OSE was limited to 8 ovaries with intact OSE spatially distributed across different regions of the ovary (i.e. hilum, within cortical clefts, elsewhere). ALDH1A1 staining was also performed on tissue microarrays comprised of 26 pairs of primary and matched recurrent HGSCs. The percentage of ALDH1A1-positive tumour cells was estimated for each core on the tissue microarray and averaged across triplicates. Mean percentages of ALDH1A1-positive cells were compared between groups using the paired t-test (two-tailed). Of note, all samples in this study were stained with ALDH1A1 antibody clone EP1932Y. Confirmation of staining results was subsequently carried out using the clone 44 antibody on control tissues, tissue microarray sections and a subset of STICs.
In-silico analyses
Level 3 Agilent 244K custom gene expression G4502A-07 platform mRNA microarray data were downloaded from the TCGA data portal (https://tcga-data.nci.nih.gov/tcga/, 9/31/2013), which included 558 primary ovarian HGSCs with available clinical data, 8 normal fallopian tube controls, and 15 recurrent ovarian HGSCs. The unpaired Welch 2-sample t-test was performed to compare ALDH1A1 expression between 558 primary tumours and 8 normal (fallopian tubes) samples. Overall survival was compared in patients with ALDH1A1-high and ALDH1A1-low tumours (above and below median levels, respectively) by Kaplan-Meir analysis and log-rank test. Primary and recurrent tumors were compared using the paired t-test.
Results
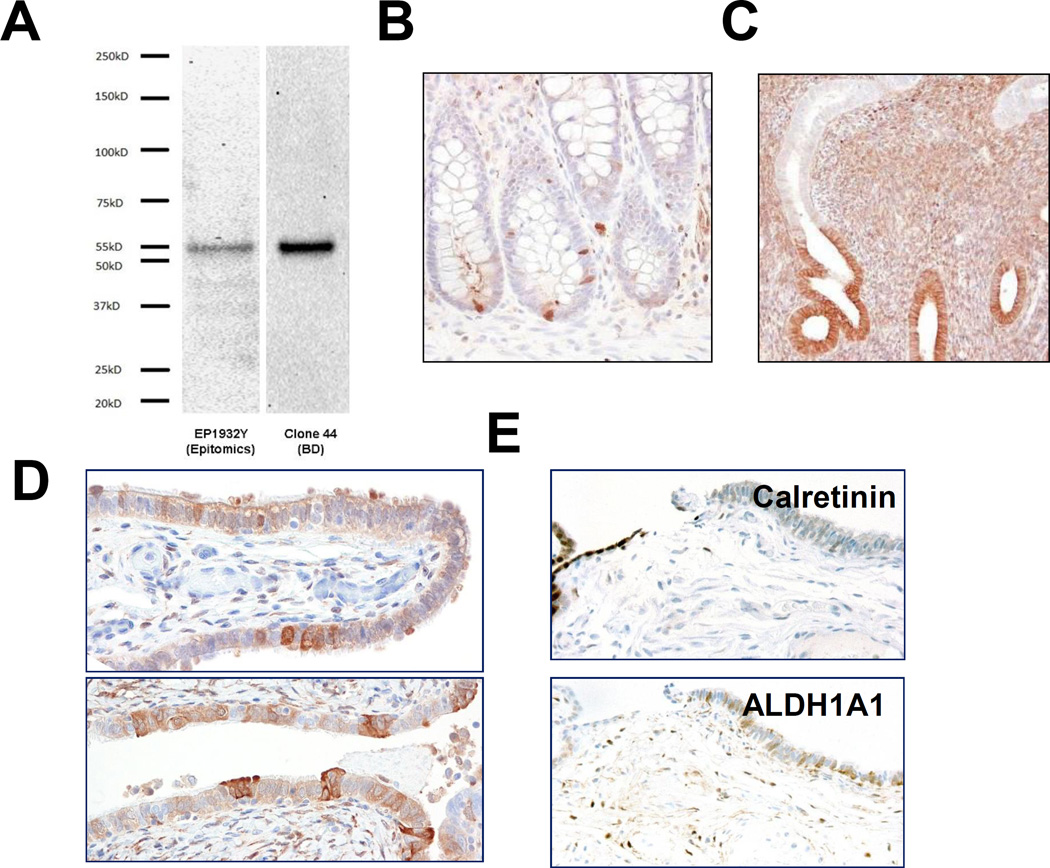
For both ALDH1A1 antibodies, obtained from Epitomics and BD Biosciences, Western blotting revealed a robust band at 55kD, corresponding to the molecular weight of ALDH1A1 (Fig. 1A). This result confirmed the specificity of both antibodies in recognising the ALDH1A1 protein. Immunostaining using both antibodies demonstrated that the positively stained cells were largely restricted to discrete single cells at the colonic crypt bases, where intestinal stem cells are known to reside (Fig. 1B). In endometrial glands, more diffuse immunoreactivity was observed, though localized to the deep portions of glands in the stratum basalis (Fig. 1C). Endometrial stromal cells were also positive for ALDH1A1. Patterns of staining were comparable between the two ALDH1A1 antibodies, with slightly higher background staining observed with the BD antibody. Thus, the results in the present study have been obtained mainly using the Epitomics antibody.
Figure 1.
Specificity of ALDH1A1 antibodies. A. Western blot performed using Epitomics and BD Biosciences antibodies on HHL-6 human hepatocyte cell lysate shows a robust 55 kD band corresponding to the ALDH1A1 protein. B. Section of fallopian tube with HGSC involvement, stained with Epitomics antibody (bottom) and BD Biosciences antibody (top). C. Discrete staining of single cells at colon crypt bases. D. Strong staining of deep portions of endometrium stratum basalis.
In normal tubal epithelial cells, ALDH1A1 staining was detected in all 29 cases. The staining pattern was always patchy and discontinuous, and in some areas, strong staining marked discrete single cells, including both secretory and ciliated cell types (Fig. 1D); however more often, long stretches of epithelium showed diffuse immunoreactivity. There were no clear differences in the percentage of ALDH1A1-positive cells observed in sections of fimbriae compared with more proximal regions of the tube (distal fimbria: 46 ± 29% vs. ampulla cross sections: 42 ± 29%, p = 0.642). As in endometrium, prominent staining was observed in stromal cells.
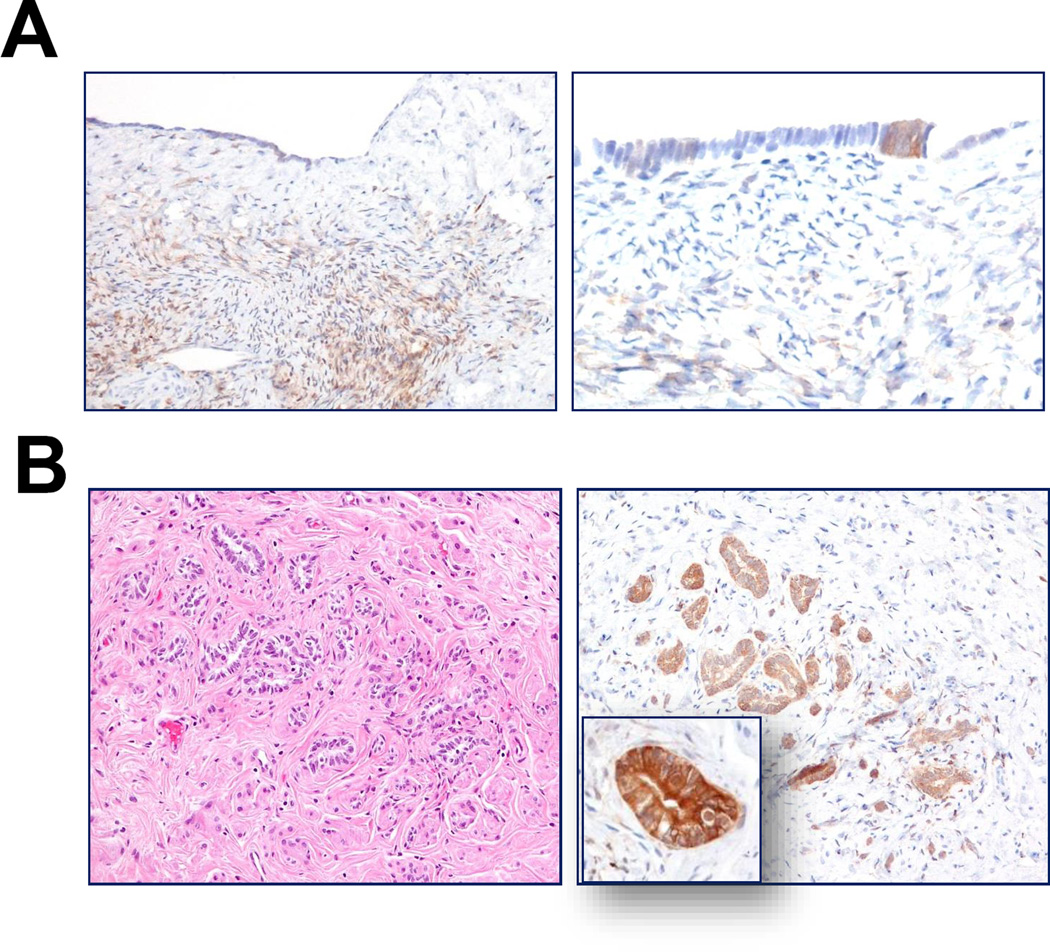
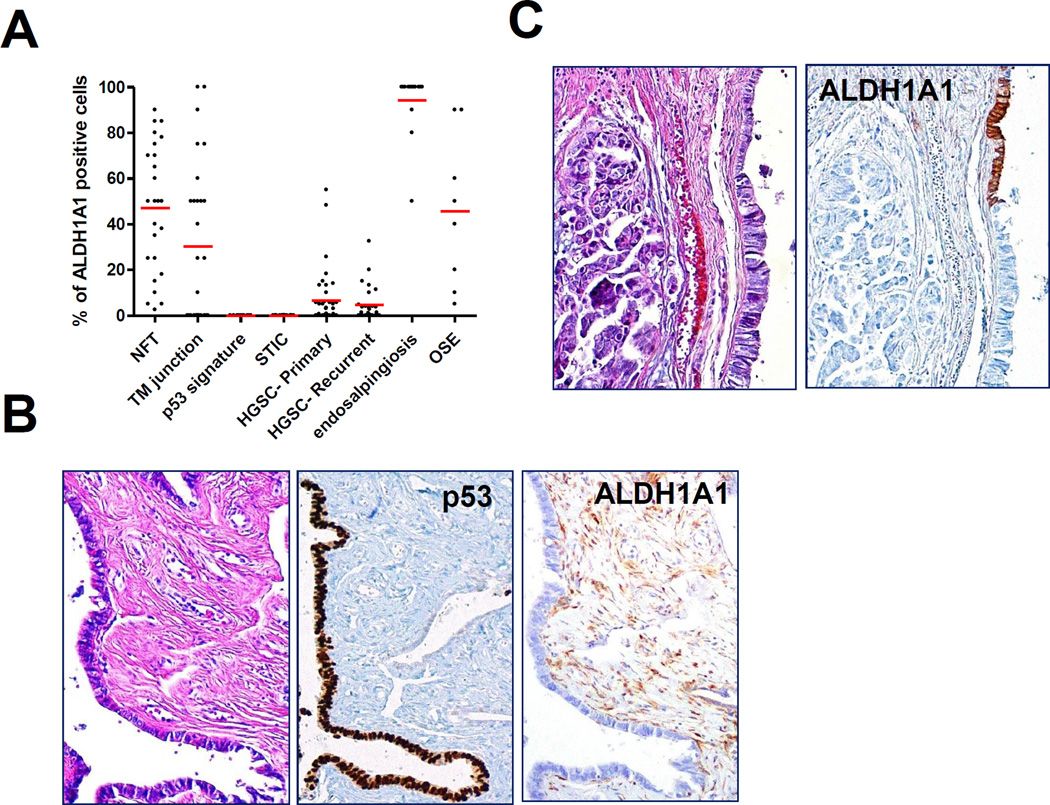
Recent work based on a mouse model has identified ALDH1A1-positive stem cells enriched at the ovarian hilum16. In the mouse, this region represents the transition from mesothelium to tubal-type epithelium; thus, presumably analogous to the tubal-mesothelial junctions present in the human fallopian tube fimbria. In our cohort, ALDH1A1-positive cells were detected in 15 (54%) of 28 junctions. Representative ALDH1A1 staining of a tubal-mesothelial junction is shown in Fig. 1E. ALDH1A1 expression was detected in OSE in all 8 ovaries. However, no consistent differences were observed at the hilar region or tubal-mesothelial junctions compared to normal OSE or fallopian tube epithelium located elsewhere. Of note, evaluation of the OSE revealed heterogeneous ALDH1A1 staining, which occurred more often in cells with cuboidal or columnar morphology, rather than in flat mesothelial cells (Fig. 2A). Interestingly, diffuse and intense ALDH1A1 immunoreactivity was observed in all rete ovarii and foci of endosalpingiosis examined (Fig. 2B). The percentages of ALDH1A1-positive cells in different tissues and lesions are summarized in Fig. 3A.
Figure 2.
A. In normal fallopian tube, ALDH1A1 is variably expressed in secretory and ciliated epithelial cells. B. Tubal-mesothelial junction, representing the transition from mesothelium (calretinin-positive) to tubal epithelium (calretinin-negative); ALDH1A1 staining is positive in epithelial cells in this case. C. Heterogeneous ALDH1A1 staining of OSE, preferentially in cells with columnar morphology. D. Diffuse and strong ALDH1A1 expression in rete ovarii.
Figure 3.
ALDH1A1 staining in tubal precursor lesions of HGSC. A. p53 signature, morphologically normal tubal epithelium with intense nuclear staining for p53 protein, is completely negative for ALDH1A1 immunoreactivity. B. STIC and invasive HGSC show complete absence of ALDH1A1, while adjacent normal epithelium is intensely positive.
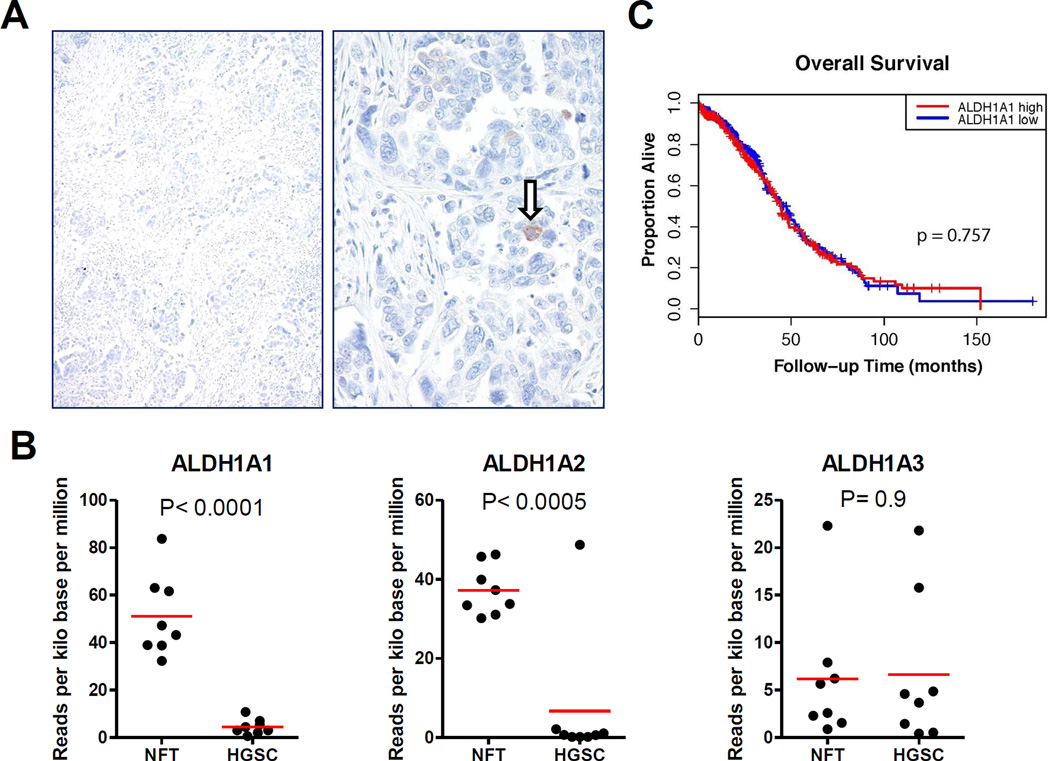
Next, we assessed ALDH1A1 expression in p53 signatures, STICs and HGSCs. A total of 11 p53 signatures, morphologically normal tubal epithelial cells with intense p53 staining, were identified; all were ALDH1A1 negative (Fig. 3B). ALDH1A1 staining was detected in 0 of 17 STICs (Fig. 3C) and in 41 (61%) of 67 HGSCs. Among 17 STIC cases, there were 11 containing HGSC on the same tissue sections, which enabled direct comparison of the staining pattern between STIC and corresponding HGSC from the same patient. Staining patterns were generally consistent between matched STIC and HGSC components, with the exception of two cases, in which focal immunoreactivity was detected in the HGSC, but not within the STIC. Although ALDH1A1 expression was detected to some degree in many HGSCs, in most cases, only rare ALDH1A1-positive cells were observed (Fig. 4A). In our cohort of 26 paired primary and recurrent HGSCs, comparable frequencies of ALDH1A1-positive tumour cells were observed between matched tumour pairs (mean ± SD: 8.0 ± 14.6% versus 4.5 ± 7.7%, p = 0.28). There was a significant decrease in the percentage of ALDH1A1-stained cells in STIC and p53 signatures as compared to normal fallopian tubes (p < 0.0001).
Figure 4.
Dot-plot showing the percentage of ALDH1A1-positive cells in different types of tissues/lesions. Each dot represents a sample, with red lines representing means for each group
To validate our immunohistochemistry results, representative cases were stained using the clone 44/BD Biosciences ALDH1A1 antibody. The performance of both antibodies was comparable, with no appreciable differences in the distribution of staining and the percentage of ALDH1A1 positive cells. In-silico analysis of TCGA mRNA expression data for ovarian HGSC21 revealed decreased expression in HGSC relative to normal fallopian tube (tumour/normal expression ratio = 0.13, p < 0.0001). Moreover, ALDH1A1 expression level in primary ovarian HGSC was not associated with overall survival (HR = 1.04, p = 0.757) (Fig. 4C).
Discussion
ALDH1A1 has been proposed as a marker for cancer stem cells or cancer initiating cells in various types of human cancer, including ovarian carcinoma. Previous experimental work demonstrated ALDH1A1-positive ovarian cancer cells to be more tumorigenic than ALDH1A1-negative cells22, 23. It is therefore plausible that these ALDH1A1-expressing tumor cells may be derived from somatic stem cells, likely from the fallopian tube. Indeed, the mouse ovarian hilum has recently been shown to be enriched for ALDH1A1-positive stem cells with increased susceptibility to malignant transformation16. The purpose of this study was to comprehensively characterize the expression of ALDH1A1 in the normal human fallopian tube, tubal-mesothelial junctions, OSE and in lesions thought to represent different stages of tumor progression, namely, p53 signatures, STICs and primary and recurrent HGSCs.
Somatic stem cells are self-renewing cells that give rise to the differentiated cells necessary for tissue function. They are usually present in small numbers and tend to be located in a discrete anatomic niche such as the colonic crypts and hair follicle bulbs. It is unlikely that the ALDH1A1-positive tubal cells, described in this study, are tubal stem cells as they correspond to ciliated and secretory cells, which are terminally differentiated. Similar staining patterns have been observed in another recent study evaluating ALDH1A1 expression in normal fallopian tube using a polyclonal goat antibody (L15; Santa Cruz) 25. These findings cast doubt on ALDH1A1 as a specific marker for somatic stem cells in human fallopian tube. ALDH1A1 encodes an enzyme belonging to the aldehyde dehydrogenase family that catalyzes the oxidation (dehydrogenation) of aldehydes, which in the fallopian tube may play a role in facilitating the transport of blastocysts26. The finding of diffuse ALDH1A1 positivity within foci of endosalpingiosis is consistent with their presumed origin from fallopian tube epithelium1, whereas the significance of ALDH1A1 staining of rete ovarii, derived from mesonephric remnants, is unknown.
The most interesting observation in this study is that ALDH1A1 expression is completely absent in STIC, the putative precursor of many HGSCs. This finding, along with the observation that all evaluated p53 signatures were also ALDH1A1-negative, suggests that loss of ALDH1A1 expression is an early event in the pathogenesis of HGSC. The cell of origin of HGSC is therefore either a cell that is intrinsically ALDH1A1-negative or an ALDH1A1-positive cell in which expression of this enzyme has been suppressed during neoplastic transformation. Previous work suggests that silencing of ALDH1A1 may be mediated by the histone methyltransferase, EZH2, which is upregulated in HGSC. Knockdown of EZH2 resulted in upregulation of ALDH1A1 in the SKOV3 cell line27, in which ALDH1A1 expression is otherwise suppressed. Of course, there are alternative explanations for the absence of ALDH1A1 expression in HGSC precursors. For example, it remains a possibility that the complete absence of ALDH1A1-positive STICs or p53 signatures may be biased by the relatively limited number of cells assessed, as the number of cells that make up these lesions is small compared to the number of cells present in normal fallopian tubes and HGSCs.
We also observed considerably a lower percentage of ALDH1A1 positive tumor cells in HGSCs relative to normal fallopian tube epithelial cells, by immunohistochemistry, confirming in-silico analysis of TCGA expression data. Accordingly, ALDH1A1 expression in HGSC is probably of minimal biologic significance, as staining was often limited to a few cells in the positive cases and likely reflects intra-tumoral heterogeneity in gene expression28. Nevertheless, it is still possible that the ALDH1A1-positive subpopulation may be enriched for cancer stem cells, as suggested in previous work22, 23. If this were the case, one must conclude that either HGSC does not develop from fallopian tube epithelium, where ALDH1A1 does not appear to label stem cells, or that ALDH1A1 expression has different functions in malignant versus normal Mullerian epithelium. That is to say, in the normal fallopian tube, ALDH1A1 participates in aldehyde metabolism, and not maintaining “stemness,” whereas in HGSC, ALDH1A1 expression is involved in the development of cancer stem cells. This scenario, however, is difficult to reconcile with the absence of ALDH1A1 expression in STICs. Our results mirror recent work showing no differences between ALDH1A1-positive versus negative melanoma cells with respect to colony formation in-vitro, tumour initiation potential or resistance to anti-melanoma drugs31.
While several clinicopathologic studies have suggested ALDH1A1 expression to correlate with poor prognosis in HGSC22, 23, 29, 30, one study found no significant association27. Similarly, our in-silico analysis of the TCGA mRNA expression dataset failed to reveal any prognostic significance, and furthermore, we did not observe a significant difference in ALDH1A1-expressing cells between primary and recurrent HGSCs.
In summary, we demonstrate that ALDH1A1-expressing epithelial cells are abundant in the normal fallopian tube, but not in p53 signatures or STICs. ALDH1A1-positive cells can occasionally be observed in some HGSCs, but usually restricted to only a few tumor cells. These findings suggest that ALDH1A1 is probably not a specific marker for fallopian tube stem cells but demonstrate that its loss of expression is an early event in the development of HGSC. Identification of more specific markers of Müllerian epithelial stem cells is necessary to identify the presumable somatic stem cell population involved in the pathogenesis of pelvic HGSC.
Figure 5.
ALDH1A1 expression in high-grade serous carcinoma. A. HGSC with ALDH1A1 staining restricted to rare tumor cells (arrow). B. No difference in overall survival time when stratified by ALDH1A1 expression levels based on in-silico analysis of TCGA mRNA expression dataset.
Acknowledgements
This study was supported by a grant from US Department of Defense W81XWH-11-2-0230/OC100517.
References
- 1.Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011;42:918–931. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colgan TJ, Murphy J, Cole DE, et al. Occult carcinoma in prophylactic oophorectomy specimens: prevalence and association with BRCA germline mutation status. Am J Surg Pathol. 2001;25:1283–1289. doi: 10.1097/00000478-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Medeiros F, Muto MG, Lee Y, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30:230–236. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 4.Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 5.Lee Y, Miron A, Drapkin R, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 6.Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–322. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- 7.Chui MH. Insights into cancer metastasis from a clinicopathologic perspective: Epithelial-Mesenchymal Transition is not a necessary step. Int J Cancer. 2013;132:1487–1495. doi: 10.1002/ijc.27745. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien CA, Pollett A, Gallinger S, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 9.Douville J, Beaulieu R, Balicki D. ALDH1 as a functional marker of cancer stem and progenitor cells. Stem Cells Dev. 2009;18:17–25. doi: 10.1089/scd.2008.0055. [DOI] [PubMed] [Google Scholar]
- 10.Chute JP, Muramoto GG, Whitesides J, et al. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc Natl Acad Sci U S A. 2006;103:11707–11712. doi: 10.1073/pnas.0603806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jimeno A, Feldmann G, Suarez-Gauthier A, et al. A direct pancreatic cancer xenograft model as a platform for cancer stem cell therapeutic development. Mol Cancer Ther. 2009;8:310–314. doi: 10.1158/1535-7163.MCT-08-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellsten R, Johansson M, Dahlman A, et al. Galiellalactone inhibits stem cell-like ALDH-positive prostate cancer cells. PLoS One. 2011;6:11. doi: 10.1371/journal.pone.0022118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma S, Chan KW, Lee TK, et al. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 2008;6:1146–1153. doi: 10.1158/1541-7786.MCR-08-0035. [DOI] [PubMed] [Google Scholar]
- 15.Clay MR, Tabor M, Owen JH, et al. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck. 2010;32:1195–1201. doi: 10.1002/hed.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flesken-Nikitin A, Hwang CI, Cheng CY, et al. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature. 2013;495:241–245. doi: 10.1038/nature11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seidman JD, Yemelyanova A, Zaino RJ, et al. The fallopian tube-peritoneal junction: a potential site of carcinogenesis. Int J Gynecol Pathol. 2011;30:4–11. doi: 10.1097/PGP.0b013e3181f29d2a. [DOI] [PubMed] [Google Scholar]
- 18.Visvanathan K, Vang R, Shaw P, et al. Diagnosis of serous tubal intraepithelial carcinoma based on morphologic and immunohistochemical features: a reproducibility study. Am J Surg Pathol. 2011;35:1766–1775. doi: 10.1097/PAS.0b013e31822f58bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vang R, Visvanathan K, Gross A, et al. Validation of an algorithm for the diagnosis of serous tubal intraepithelial carcinoma. Int J Gynecol Pathol. 2012;31:243–253. doi: 10.1097/PGP.0b013e31823b8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Movahedi-Lankarani S, Kurman RJ. Calretinin, a more sensitive but less specific marker than alpha-inhibin for ovarian sex cord-stromal neoplasms: an immunohistochemical study of 215 cases. Am J Surg Pathol. 2002;26:1477–1483. doi: 10.1097/00000478-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang YC, Yo YT, Lee HY, et al. ALDH1-bright epithelial ovarian cancer cells are associated with CD44 expression, drug resistance, and poor clinical outcome. Am J Pathol. 2012;180:1159–1169. doi: 10.1016/j.ajpath.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Landen CN, Jr, Goodman B, Katre AA, et al. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther. 2010;9:3186–3199. doi: 10.1158/1535-7163.MCT-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubeau L. The cell of origin of ovarian epithelial tumours. Lancet Oncol. 2008;9:1191–1197. doi: 10.1016/S1470-2045(08)70308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Auersperg N. The Stem-Cell Profile of Ovarian Surface Epithelium is Reproduced in the Oviductal Fimbriae, with Increased Stem-Cell Marker Density in Distal Parts of the Fimbriae. Int J Gynecol Pathol. 2013;32:444–453. doi: 10.1097/PGP.0b013e3182800ad5. [DOI] [PubMed] [Google Scholar]
- 26.Vermot J, Fraulob V, Dolle P, et al. Expression of enzymes synthesizing (aldehyde dehydrogenase 1 and reinaldehyde dehydrogenase 2) and metabolizaing (Cyp26) retinoic acid in the mouse female reproductive system. Endocrinology. 2000;141:3638–3645. doi: 10.1210/endo.141.10.7696. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Bitler BG, Vathipadiekal V, et al. ALDH1A1 is a novel EZH2 target gene in epithelial ovarian cancer identified by genome-wide approaches. Cancer Prev Res (Phila) 2012;5:484–491. doi: 10.1158/1940-6207.CAPR-11-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarin D. Inappropriate gene expression in human cancer and its far-reaching biological and clinical significance. Cancer Metastasis Rev. 2012;31:21–39. doi: 10.1007/s10555-011-9326-8. [DOI] [PubMed] [Google Scholar]
- 29.Liebscher CA, Prinzler J, Sinn BV, et al. Aldehyde dehydrogenase 1/epidermal growth factor receptor coexpression is characteristic of a highly aggressive, poor-prognosis subgroup of high-grade serous ovarian carcinoma. Hum Pathol. 2013 doi: 10.1016/j.humpath.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Deng S, Yang X, Lassus H, et al. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS One. 2010;5:e10277. doi: 10.1371/journal.pone.0010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prasmickaite L, Engesaeter BO, Skrbo N, et al. Aldehyde dehydrogenase (ALDH) activity does not select for cells with enhanced aggressive properties in malignant melanoma. PLoS One. 2010;5:0010731. doi: 10.1371/journal.pone.0010731. [DOI] [PMC free article] [PubMed] [Google Scholar]