Abstract
Objectives
To test our hypothesis that obese adolescents have left ventricular (LV) dysfunction and remodeling that are associated with markers of cardiovascular risk and insulin resistance (IR).
Study design
In a cross-sectional study of 44 obese and 14 lean age-, sex-, Tanner stage-, and race-matched adolescents, IR, markers of cardiovascular risks, conventional and 2-dimensional speckle tracking echocardiography (2DSTE) measures of LV function and structure were evaluated and compared.
Results
The obese adolescents had significantly increased body mass index (BMI) Z-score, systolic blood pressure, fasting insulin, IR, and atherogenic lipids compared with the lean adolescents. A subgroup of obese adolescents had LV remodeling characterized by significantly increased LV mass index (LVMI, g/m2.7) and relative wall thickness (RWT). Almost all obese adolescents had LV dysfunction with peak LV global longitudinal strain (GLS,%), systolic global GLS rate (GLSR, %/s) and early diastolic GLSR significantly lower than in lean adolescents and in the normal pediatric population. BMI Z-score predicted LV remodeling [LVMI (R2= 0.34) and RWT (R2 0.10], and peak LV GLS (R2 0.15), and along with systolic blood pressure, predicted systolic GLSR (R2 0.16); (P ≤ 0.01 for all). Fasting insulin predicted early diastolic GLSR (R2 0.17, P ≤ 0.01).
Conclusions
Obese adolescents have subclinical ventricular dysfunction associated with the severity of obesity, increased systolic blood pressure, and IR. Ventricular remodeling is present in a subgroup of obese adolescents in association with the severity of obesity. These findings suggest that obesity may have an early impact on the cardiovascular health of obese adolescents.
Keywords: cardiac function, structure, obesity, adolescence
Emerging evidence suggests that although obese adults have increased conventional cardiovascular risk factors, hemodynamic load, and neuro-hormonal activation, but those contributing factors cannot entirely explain the reported changes in ventricular structure and function that lead to heart failure6,7. There are intrinsic alterations in the myocardium that are independent of load. Insulin resistance (IR) promotes alterations in myocardial substrate metabolism, evident by increases in myocardial fatty acid uptake, utilization and oxidation that may play a role in the pathogenesis of decreased myocardial efficiency and cardiac dysfunction in obese individuals8. Studies in obese children and adolescents have shown ventricular remodeling and subclinical impairment in diastolic function but have not discerned the association between ventricular dysfunction and markers of cardiovascular risks or IR in this population9-11.
We measured clinical and metabolic markers of cardiovascular risk and IR, and assessed the left ventricular (LV) structure and function in lean and obese adolescents Our hypothesis was that obese adolescents have subclinical LV dysfunction and remodeling associated with markers of modifiable cardiovascular risk and IR.
Methods
Using a cross-sectional study approach we studied a group of obese adolescents (body mass index, BMI ≥95th percentile for age and sex)14 referred to the Washington University Outpatient Pediatric Preventive Cardiology Clinic at St. Louis Children's Hospital from January 2007 to December 2012. Subjects were eligible for inclusion if they were 12 to 18 years of age, Tanner stage III or higher, and had complete echocardiographic and laboratory evaluations performed during the study period. Subjects were excluded if they had heart disease, diabetes, or other endocrinopathies, pregnancy, or a history of substance and alcohol abuse, smoking, obstructive sleep apnea, use of pharmaceutical agents that affect IR or had a poor acoustic windows for echocardiographic evaluation. A cohort of healthy, lean (BMI < 85th and > 5th percentile for age and sex), age, sex, race, and Tanner stage matched (for minimizing the potential confounding effects on IR)2 subjects from a simultaneous study in our institution was used as a control group15. In addition, data from normal healthy, lean, age- (12 to 18 years) and sex-matched pediatric cohort (n = 51) from our echocardiography laboratory were included to provide the range of normal pediatric values of echocardiographic functional 16 ,17. The Institutional Review Board for human studies at Washington University approved the study. Written informed consent was obtained from the parents/guardians of the study subjects.
All subjects underwent a comprehensive clinical evaluation as part of the standard of care. Demographic and anthropometric variables were collected. BMI (kg/m2) was transformed into BMI Z-score to adjust for age and sex. Three measurements of systemic blood pressure (mmHg) were taken using a manual sphygmomanometer with the appropriate cuff size in resting position. Hypertension was defined based on the average systolic or diastolic blood pressure percentiles for age, sex, and height: pre-hypertension (≥ 90th but < 95th), stage I (≥ 95th but < 99th, plus 5 mmHg) and stage II (>99th, plus 5 mmHg)19. The metabolic assessment was performed after a 12-hour overnight fasting and included plasma glucose (mg/dl), plasma insulin (μU/mL), and lipids (total cholesterol, triglycerides, high-density lipoprotein - HDL cholesterol, low-density lipoprotein - LDL cholesterol, mg/dl). Dyslipidemia and hyperglycemia were defined according the published criteria for the pediatric population20, 21. Variables used to define metabolic syndrome were considered as markers of cardiovascular risk and included BMI Z-score, systemic blood pressure, fasting glucose and lipid profiles22. For IR indices, we used the reciprocals of homeostasis model assessment of insulin sensitivity [HOMA-IS = 22.4/ Glucose (mg/dL) x Insulin (μU/mL)] and the fasting plasma insulin (mL/μU).
Assessment of cardiac structure and function
Left ventricular structure
A transthoracic complete M-mode, 2-dimensional (2D) and Doppler echocardiographic examination was performed with a commercially available ultrasound imaging system using a phased array transducer of appropriate frequency (Vivid 7 and 9; General Electric Medical Systems, Milwaukee, WI, USA). Using M-mode imaging of the LV in the parasternal short-axis view, relative wall thickness (RWT) and LV mass (Devereux formula) were calculated and LV mass was indexed to height2.7 (LVMI, g/m2.7)24, 25. The 95th percentiles for LVMI for children older than 9 years of age (> 40 g/height2.7 in females and >45 g/height2.7 in males) and the 95th percentile value for RWT for normal children and adolescents (RWT > 0.41) were used as cut-off values to categorize LV structure (geometry)26, 27.
Left ventricular function
LV fractional shortening (FS) and biplane LV ejection fraction (EF) were measured according to the guideline of the American Society of Echocardiography24. Myocardial mechanics were analyzed by the quantification of LV longitudinal strain and strain rate. Strain (%) describes the fractional changes in the dimension of a myocardial fiber/segment. Myocardial strain rate (%/s) is the rate of change in strain and represents fiber contractility13. LV global longitudinal strain (GLS, %) and systolic and diastolic global longitudinal strain rates (GLSR, %/s) were measured by using validated 2DSTE 28. A single observer, who was blinded to the subjects’ clinical and metabolic values analyzed peak LV GLS, systolic GLSR, and early and late diastolic GLSR values using vendor software (EchoPAC,TM General Electric Medical Systems, Waukesha, WI, USA). Our echocardiography laboratory has previously demonstrated high reproducibility of strain measurements28. We further tested the reproducibility of GLS and GLSR in 7 age- (12 – 18 years), sex- (4 male) and BMI z score- (1.82 – 2.32) matched obese adolescents at two time points at 6 weeks interval.
Statistical analyses
Continuous data were tested for normality with the Shapiro-Wilk W test and equality of variances was tested with the O'Brien, Brown-Forsythe, Levene, Bartlett, and F Tests. The t test and the Wilcoxon rank sums test were used for data with normal and non-normal distribution and/or variances respectively. Because structure and function in the heart are closely linked29 the adolescent cohort was further divided into obese with normal LV structure (normal LVMI and RWT, “Normal Obese”) and obese with abnormal LV structure (abnormal LVMI and RWT, “Abnormal Obese”). Comparisons were made among the “abnormal” and “normal” obese adolescents and lean control groups by using oneway ANOVA, with two planned contrasts for the comparisons of interest: obesity (lean vs. all obese adolescents) and abnormal LV structure (“Normal Obese” vs. “Abnormal Obese” adolescents). Bivariate analyses and backward stepwise multiple linear regression analyses were used to determine which variables best predicted changes in LV structure and function for the entire cohort of lean and obese subjects as well as for the cohort of obese subjects only. To predict LV structure and function, BMI Z-score, systolic blood pressure, and fasting insulin were used as independent variables27, 30, 31. The maximum R2 from K-fold cross validation was used for the multiple linear regression analyses. A p value ≤ 0.05 was considered statistically significant. Using data on differences in fasting insulin levels between obese and lean children from a previous study15 and with alpha set at 0.05, a two-tailed test determined a sample size of 13 in each group and a power of 80.1%. We enrolled more than 13 obese adolescents in the study group at the outset. Statistical analyses were performed with JMP Statistical Software 10.0.0 (SAS Institute, Inc., Cary, NC), StatXact 10 (Cytel, Inc., Cambridge, MA), and Power and Precision 4.0 (Biostat, Inc., Englewood, NJ).
Results
A total of 44 out of 57 obese adolescents were included in this study. Thirteen subjects were excluded due to incomplete laboratory data and inadequate acoustic windows. Fourteen lean adolescents from a simultaneous study in our institution were used as a control group15. Tables I and II display the demographic, clinical and metabolic characteristics of the study population. Obese adolescents had significantly increased HOMA-IR, fasting insulin levels, systolic blood pressure and lipid measurements. Pre-hypertension and stage I hypertension were found in 9 (20%) and 4 (9%) obese adolescents, respectively. None had stage II hypertension. Thirty-seven (84%) obese adolescents had dyslipidemia but none had hyperglycemia. All lean subjects had normal blood pressure, fasting glucose and lipids.
Table 1.
Subject demographics by LV structure
| Control subjects | Obese subjects | P value | P value | ||
|---|---|---|---|---|---|
| Lean (n=14) | Normal (n=34) | Abnormal (n=10) | Control subjects vs. Obese subjects | Obese subjects Normal vs. Abnormal | |
| Age, years | 15 (13–17) | 14 (13–16) | 14 (12–15) | 0.11 | 0.94 |
| *Gender (M/F) | 8/6 | 21/13 | 7/3 | 0.76 | 0.91 |
| †Tanner stage | 1/9/04 | 5/13/16 | 1/4/5 | 0.12 | 0.86 |
| ‡Race | 12/2/0/0 | 20/7/7/0 | 5/2/0/3 | 0.19 | 0.32 |
| BMI, kg/m2 | 20 (17–23) | 32 (30–38) | 41 (30–53) | <0.001 | 0.52 |
Values are median (25th– 75th percentile).
Normal, Normal LV structure; Abnormal, Abnormal LV structure
For gender, Tanner stage and race, values are the number of subjects.
Gender: Male/ Female.
Tanner stage: III/IV/V.
Race: White/ Black/ Asian/ Unknown.
Table 2.
Insulin resistance indices and markers of cardiovascular risk in obese adolescents with normal and abnormal LV structure
| Control subjects | Obese subjects | P value | P value | ||
|---|---|---|---|---|---|
| Lean (n=14) | Normal (n=34) | Abnormal (n=10) | Control subjects vs. Obese subjects | Obese subjects Normal vs. Abnormal | |
| Insulin resistance indices | |||||
| Fasting Insulin, mL/uU | 8.3 (2.70–14.80) | 15.65 (5.00–26.00) | 16.50 (3.50–24.00) | 0.004 | 0.79 |
| HOMA-IR, Umol | 1.8 (0.57–2.66) | 3.45 (1.01–5.13) | 3.46 (0.67–5.08) | 0.007 | 0.66 |
| Markers of cardiovascular risk | |||||
| BMI Z-score | −0.115(−0.46-0.145) | 2.24 (1.78–2.38) | 2.35 (1.61–2.65) | <0.001 | 0.28 |
| Systolic blood pressure (mm/Hg) | 109.00 (98.00-120.00) | 115.50 (100.00–122.00) | 109.50 (101.00–116.50) | 0.03 | 0.35 |
| Diastolic blood pressure (mm/Hg) | 68 (57.00 -76.00) | 66.00 (60.00–72.00) | 69.00 (55.50–77.00) | 0.49 | 0.59 |
| Fasting glucose, mg/dL | 88.80 (81.00–98.00) | 86.00 (77.90–90.00) | 82.50 (75.50–80.00) | 0.34 | 0.49 |
| Total cholesterol, mg/dL | 126.70 (92–176.00) | 212.00 (146.20–238.00) | 185.50 (157.00–208.00) | <0.001 | 0.46 |
| Triglycerides, mg/dL | 64 (30.00–160.00) | 170.50 (90.80–236.00) | 131.50 (77.00–281.00) | <0.001 | 0.78 |
| LDL cholesterol, mg/dL | 69 (53.00 - 110.00) | 126.00 (67.00–155.50) | 120.00(59.50 - 133.00) | <0.001 | 0.21 |
| HDL cholesterol, mg/dL | 44.70 (31.00–60.00) | 42.00 (26.90–48.00) | 36.50 (33.00–39.00) | 0.39 | 0.41 |
Values are median (25 th– 75 th percentile).
Normal, Normal LV structure; Abnormal, Abnormal LV structure
Assessment of cardiac structure and function
Left ventricular structure
The results of analyses of the measurements of LV structure are shown in Table III. Ten (“Abnormal Obese”) of the 44 obese adolescents (23%) had abnormal LV geometry based on their LVMI and RWT measurements; 4 had concentric remodeling, 1 had concentric hypertrophy and 5 had eccentric hypertrophy. There was no difference in age between the obese subjects with normal and abnormal cardiac geometry (14.4 ± 1.8 vs. 14.5 ± 2.4, p=0.86). All the lean adolescents had normal LV geometry. There was no significant correlation with fasting glucose and lipid variables. Backward stepwise multiple linear regression analyses retained only BMI Z score as a predictor for LVMI (P < 0.01, R2 = 0.34) as well as for RWT (P = 0.01, R2 = 0.10). The association was positive, and residuals were normally distributed (Shapiro-Wilk W test, P = 0.43 and 0.41respectively).
Table 3.
Echocardiographic assessment of left ventricular structure and function in obese adolescents with normal and abnormal LV structure.
| Control subjects | Obese subjects | P value | P value | Normal Pediatric | ||
|---|---|---|---|---|---|---|
| Lean (n=14) | Normal (n=34) | Abnormal (n=10) | Control subjects vs. Obese subjects | Obese subjects Normal vs. Abnormal | Values (n=51) | |
| Left ventricular structure | ||||||
| LVMI, g/height2.7 | 23.6 (18.85-29.34) | 33.50 (23.00–36.89) | 44.24 (19.94–45.33) | <0.001 | <0.001 | 26.25 (22.87–27.45) |
| RWT | 0.3 (0.17-0.49) | 0.30 (0.22–0.34) | 0.41 (0.29–0.44) | 0.03 | 0.05 | |
| Standard echocardiographic parameters of left ventricular systolic function | ||||||
| Fractional shortening % | 37.00 (32.00-4.002) | 36.00 (28.00–39.00) | 34.00 (29.50–38.00) | 0.43 | 0.18 | 37.5 (34.92–39.75) |
| Ejection fraction % | 62.5 (58-70) | 60.00 (55.00–64.25) | 62.00 (54.00–66.00) | 0.64 | 0.87 | 64.0 (59.0–69.0) |
| 2D-speckle tracking echocardiographic parameters of left ventricular function | ||||||
| Peak GLS (%) | −18.8 (−16.5 - −22.5) | −16.85 (−21.14- −15.60) | −13.35 (−17.85– −12.50) | 0.01 | 0.01 | −22.3 (−24.93– −19.50) |
| Peak systolic GLSR (1/s) | −1.1 (−0.9 - −1.2) | −0.95 (−1.20– −0.90) | −0.85 (−1.15– −0.80) | 0.06 | 0.11 | −1.20 (−1.40– −1.04) |
| Peak early diastolic GLSR (1/s) | 1.6 (1.2 -2.1) | 1.50 (1.10–1.70) | 1.20 (0.70–1.60) | 0.02 | 0.02 | 1.61 (1.55–1.75) |
| Peak late diastolic GLSR (1/s) | 0.6 (0.2 -0.8) | 0.50 (0.39–0.70) | 0.50 (0.30–0.70) | 0.64 | 0.24 | 0.77 (0.56–0.92) |
Values are median (25 th– 75 th percentile).
Normal Pediatric Values, these normal pediatric values represent the 25-75 percentile strain and strain rate values in a normal healthy age (14 to 18 years) and gender matched pediatric cohort (n = 51) from our echocardiography laboratory. The values are similar to the reference values reported in a separate large pediatric population by Lorch et al 2008. (−22.7±6.5%) Echo, echocardiographic; LVMI, left ventricle mass index; RWT; relative wall thickness; GLS, global longitudinal strain; GLSR, global longitudinal strain rate.
Left ventricular function
The LV GLS was significantly lower in obese compared with lean subjects and significantly lower in “abnormal obese” than in “normal obese” (Figure and Table III), indicating greater systolic dysfunction in the “abnormal obese” group. The LV early diastolic GLSR was significantly lower in obese than in lean subjects and significantly lower in “Abnormal Obese” than in “Normal Obese” (Figure and Table III), indicating greater diastolic dysfunction in the “Abnormal Obese” group. In obese cohort, only 5 (11%) subjects had GLS values (mean -18.7 %; 25th – 75th percentile -16.9 % to -19.2 %) and early diastolic GLSR (mean 1.4 1/s; 25th – 75th percentile 1.3 to 1.5 1/s) values > 25th percentile of the control subjects (-16.5 % and 1.2 1/s respectively) and normal pediatric values (-19.5 % and 1.55 1/s respectively). The mean values of GLS and early diastolic GLSR for the entire cohort were less than the 5th percentile of the normal pediatric values. Thus, although LV FS and EF were normal, almost all obese adolescents had subclinical systolic and early diastolic dysfunction.
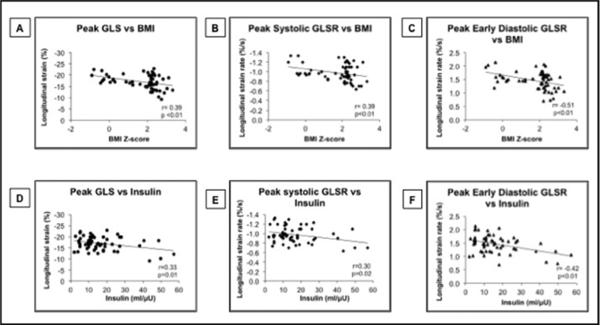
Figure.
Relationship between BM Z-score and fasting insulin with LV longitudinal deformation variables. (A) Correlation between LV peak GLS and BMI Z-score. (B) Correlation between LV peak systolic GLSR and BMI Zscore. (C) Correlation between LV peak early diastolic GLSR and BMI Z-score. (D) Correlation between LV peak GLS and fasting insulin. (E) Correlation between LV peak systolic GLSR and fasting insulin. (F) Correlation between LV peak early diastolic GLSR and fasting insulin.
Backward stepwise multiple linear regression analyses for the entire cohort of lean and obese subjects retained BMI Z-score as the only predictor for peak GLS (P ≤ 0.01, R2 = 0.15). The residuals were normally distributed (Shapiro-Wilk W test, P = 0.39). BMI Z-score and systolic blood pressure were found to be predictors for systolic GLSR. The model was significant (P ≤ 0.01, R2 = 0.16) and the residuals were normally distributed. Fasting insulin was the only predictor for early diastolic GLSR (P = 0.03, R2 = 0.22). The residuals were again normally distributed (Shapiro-Wilk W test, P = 0.62). When the data were analyzed by backward stepwise multiple linear regression for the obese subjects only, BMI Z-score was retained as the only predictor for peak GLS (P = 0.05, R2 = 0.12), systolic blood pressure for systolic GLSR (P = 0.03, R2 = 0.15), and fasting insulin for early diastolic GLSR (P = 0.01, R2 = 0.17). The residuals were again normally distributed for all.
Reproducibility
The intra-observer and inter-observer reproducibility for LV GLS had a bias of 7% with LOA -3.7 to 3.3 and 8% with LOA -4.5 to 3.5 respectively by Bland Altman analysis, coefficient of variation 6 and 7 respectively, and linear correlation of 0.84 and 0.78 (p-value <0.001 for both) respectively. The intra-observer and inter-observer reproducibility for LV early diastolic GLSR had a bias of 16% with LOA -1.1 to 1.1 and 17% with LOA -1.2 to 1.2 respectively, coefficient of variation 13 and 17 respectively, and linear correlation of 0.81 and 0.70 (p-value <0.001 for both) respectively. Both intra- and inter-observer reproducibility for late diastolic GLSR were poor with wider LOA (-2.1 to 2.5), higher coefficient of variation (>25) and modest linear correlation (<0.35). Thus, the reproducibility in obese adolescents was the most robust for GLS, good for early diastolic GLSR but poor for late diastolic GLSR.
Discussion
A subgroup of obese adolescents (“Abnormal Obese”) had LV remodeling characterized by significantly increased LVMI and RWT that were exclusively and positively correlated with BMI Z-score. This was in agreement with similar findings in previous studies in obese adolescents that suggested that the severity of obesity may directly affect LVM and ventricular remodeling33. None of the metabolic and cardiovascular risk factors were predictive of ventricular remodeling as they were not significantly different between “Abnormal Obese” and “Normal Obese”. It is possible that the duration of obesity may play an important role in the development of ventricular remodeling34. Those who have longer duration of obesity appears to develop more severe alterations in LV geometry34. This could explain the presence of LV remodeling in only ten (23%) of the obese adolescents in our study. Potential mechanisms involved in LV remodeling could be increased neuro-hormonal activity due to excessive visceral adipose tissue and overall increased lean mass, predisposing obese individuals to a high preload and afterload state6, 34. Studies in children and adolescents also have shown that IR has an incremental effect on LV mass in those with a greater degree of adiposity30.
Our study demonstrates that the majority of obese adolescents had subclinical systolic and early diastolic dysfunction, even when conventional echocardiographic measurements showed normal values. The magnitude of the decrement in LV GLS and early diastolic GLSR was greater in obese adolescents with abnormal LV structure than in those with normal LV structure. The LV GLS and early diastolic GLSR in 90% of obese adolescent were significantly lower than those in the lean control group, were well below the 5th percentile of the normal pediatric values17; and were present even in those with less severe obesity and normal LV structure (“Normal Obese”). These results confirm previous reports that obese adolescents and adults, with normal function by conventional measures have decreased GLS and early diastolic GLSR in association with the severity of obesity.7, 31 These results raise the question of whether ventricular dysfunction may precede the development of ventricular structural abnormalities in some of obese adolescents. The development of ventricular dysfunction may serve as an early marker of cardiovascular involvement before the manifestation of cardiac clinical and structural signs of the impact of obesity.
Decreased diastolic GLSR in obese adolescents in our study was predicted by IR, which is consistent with a previous study that showed that the peak LV systolic strain rate correlated with HOMA-IR and insulin levels, but not with BMI, duration of obesity, and LVMI14. Another study assessed cardiac function in obese children using 2DSTE and showed that LV GLS negatively correlated with HOMA-IR and BMI Z-score35. However, the multivariate analysis performed in this study did not include measures of IR. Our results show that IR may be a predictor of decreased diastolic GLSR, independently of BMI, lipids, and systolic blood pressure.
This study had several limitations, including small sample size. The absence of direct measurement of lipoprotein subclasses by nuclear magnetic resonance spectroscopy39 may have led to underestimation of lipoproteins and the possible association with LV mechanics. There is evidence that obese adolescents have compensatory increase in LV radial deformation and torsion measured with three-dimensional wall motion tracking imaging40. However, reliable radial and circumferential deformation measurement is often difficult due to an inadequate view and suboptimal myocardium tracking in the parasternal short-axis view, we only evaluated LV longitudinal myocardial deformation, which is the most reliably measured component of LV mechanics13, 28. The correlations of LV functional measures with BMI, systolic blood pressure and IR were modest. The findings in this study suggest that obesity may have an early impact on cardiac function in obese adolescents. This finding deserves further study in larger populations.
Acknowledgments
Supported by the National Institutes of Health (R21 HL106417, 5 T32 HD043010-09, UL1 TR000448).
Abbreviations
- IR
Insulin resistance
- LV
Left ventricular
- 2DSTE
2-dimensional speckle tracking echocardiography
- BMI
Body mass index
- HOMA-IR
Homeostasis model assessment of insulin resistance
- LVMI
Left ventricular mass index
- RWT
Relative wall thickness
- GLS
Global longitudinal strain
- GLSR
Global longitudinal strain rate
- MetS
Metabolic syndrome
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- FS
Fractional shortening
- EF
Ejection fraction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The NIH was not involved in the study design, data collection, statistical analysis, writing of the manuscript or decision to submit this paper for publication.
The authors declare no conflicts of interest.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. Prevalence and determinants of insulin resistance among U.S. adolescents: a population-based study. Diabetes Care. 2006;29:2427–32. doi: 10.2337/dc06-0709. [DOI] [PubMed] [Google Scholar]
- 3.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 4.Bremer AA, Mietus-Snyder M, Lustig RH. Toward a unifying hypothesis of metabolic syndrome. Pediatrics. 2012;129:557–70. doi: 10.1542/peds.2011-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294:334–41. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 6.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321:225–36. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Wong CY, O'Moore-Sullivan T, Leano R, Byme B, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110:3081–3087. doi: 10.1161/01.CIR.0000147184.13872.0F. [DOI] [PubMed] [Google Scholar]
- 8.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–6. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 9.Khositseth ASU, Chongviriyaphan N. Left Ventricular Mass and Geometry in Obese Children. Asian Journal of Clinical Nutrition. 2009:58–64. [Google Scholar]
- 10.Di Salvo G, Pacileo G, Del Giudice EM, Natale F, Limongelli G, Verrengia M, et al. Abnormal myocardial deformation properties in obese, non-hypertensive children: an ambulatory blood pressure monitoring, standard echocardiographic, and strain rate imaging study. Eur Heart J. 2006;27:2689–95. doi: 10.1093/eurheartj/ehl163. [DOI] [PubMed] [Google Scholar]
- 11.Barbosa JA, Mota CC, Simoes ESAC, Nunes MD, Barbosa MM. Assessing pre-clinical ventricular dysfunction in obese children and adolescents: the value of speckle tracking imaging. Eur Heart J Cardiovasc Imaging. 2013;14(9):882–9. doi: 10.1093/ehjci/jes294. [DOI] [PubMed] [Google Scholar]
- 12.The NS, Suchindran C, North KE, Popkin BM, Gordon-Larsen P. Association of adolescent obesity with risk of severe obesity in adulthood. JAMA. 2010;304:2042–7. doi: 10.1001/jama.2010.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg NL, Firstenberg MS, Castro PL, Main M, Travaglini A, Odabashian JA, et al. Doppler-derived myocardial systolic strain rate is a strong index of left ventricular contractility. Circulation. 2002;105:99–105. doi: 10.1161/hc0102.101396. [DOI] [PubMed] [Google Scholar]
- 14.Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. Natl Health Stat Report. 2010:1–5. [PubMed] [Google Scholar]
- 15.Singh GK, Vitola BE, Holland MR, Sekarski T, Patterson BW, Magkos F, et al. Alterations in ventricular structure and function in obese adolescents with nonalcoholic fatty liver disease. J Pediatr. 2013;162:1160–8. doi: 10.1016/j.jpeds.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorch SM, Ludomirsky A, Singh GK. Maturational and growth-related changes in left ventricular longitudinal strain and strain rate measured by 2-dimensional speckle tracking echocardiography in healthy pediatric population. J Am Soc Echocardiogr. 2008;21:1207–15. doi: 10.1016/j.echo.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Marcus KA, Mavinkurve-Groothuis AM, Barends M, van Dijk A, Feuth T, de Korte C, et al. Reference values for myocardial two-dimensional strain echocardiography in a healthy pediatric and young adult cohort. J Am Soc Echocardiogr. 2011;24:625–36. doi: 10.1016/j.echo.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Koren D, Marcus CL, Kim C, Gallagher PR, Schwab R, Bradford RM, et al. Anthropometric predictors of visceral adiposity in normal-weight and obese adolescents. Pediatr Diabetes. 2013;14:575–84. doi: 10.1111/pedi.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National High Blood Pressure Education Program Working Group on High Blood Pressure in C, Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76. [PubMed] [Google Scholar]
- 20.Daniels SR, Greer FR, Committee on N Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):S64–71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinberger J, Daniels SR, Eckel RH, Hayman L, Lustig RH, McCrindle B, et al. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119:628–47. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]
- 23.George L, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Surrogate estimates of insulin sensitivity in obese youth along the spectrum of glucose tolerance from normal to prediabetes to diabetes. J Clin Endocrinol Metab. 2011;96:2136–45. doi: 10.1210/jc.2010-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 26.Khoury PR, Mitsnefes M, Daniels SR, Kimball TR. Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr. 2009;22:709–14. doi: 10.1016/j.echo.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Daniels SR, Loggie JM, Khoury P, Kimball TR. Left ventricular geometry and severe left ventricular hypertrophy in children and adolescents with essential hypertension. Circulation. 1998;97:1907–11. doi: 10.1161/01.cir.97.19.1907. [DOI] [PubMed] [Google Scholar]
- 28.Singh GK, Cupps B, Pasque M, Woodard PK, Holland MR, Ludomirsky A. Accuracy and reproducibility of strain by speckle tracking in pediatric subjects with normal heart and single ventricular physiology: a two-dimensional speckle-tracking echocardiography and magnetic resonance imaging correlative study. J Am Soc Echocardiogr. 2010;23:1143–52. doi: 10.1016/j.echo.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buckberg G, Hoffman JI, Mahajan A, Saleh S, Coghlan C. Cardiac mechanics revisited: the relationship of cardiac architecture to ventricular function. Circulation. 2008;118(24):2571–87. doi: 10.1161/CIRCULATIONAHA.107.754424. [DOI] [PubMed] [Google Scholar]
- 30.Sivanandam S, Sinaiko AR, Jacobs DR, Jr., Steffen L, Moran A, Steinberger J. Relation of increase in adiposity to increase in left ventricular mass from childhood to young adulthood. Am J Cardiol. 2006;98:411–5. doi: 10.1016/j.amjcard.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 31.Labombarda F, Zangl E, Dugue AE, Bougle D, Pellissier A, Ribault V, et al. Alterations of left ventricular myocardial strain in obese children. Eur Heart J Cardiovasc Imaging. 2013;14:668–76. doi: 10.1093/ehjci/jes238. [DOI] [PubMed] [Google Scholar]
- 32.Ortega FB, Lee DC, Katzmarzyk PT, et al. The intriguing metabolically healthy but obese phenotype: cardiovascular prognosis and role of fitness. Eur Heart J. 2013;34:389–97. doi: 10.1093/eurheartj/ehs174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinik ST, Varan B, Yildirim SV, Tokel K. The effect of obesity on echocardiographic and metabolic parameters in childhood. J Pediatr Endocrinol Metab. 2006;19:1007–14. doi: 10.1515/jpem.2006.19.8.1007. [DOI] [PubMed] [Google Scholar]
- 34.Alpert MA, Lambert CR, Panayiotou H, Terry BE, Cohen MV, Massey CV, et al. Relation of duration of morbid obesity to left ventricular mass, systolic function, and diastolic filling, and effect of weight loss. Am J Cardiol. 1995;76:1194–7. doi: 10.1016/s0002-9149(99)80338-5. [DOI] [PubMed] [Google Scholar]
- 35.Labombarda F, Zangl E, Dugue AE, Bougle D, Pellissier A, Ribault V, et al. Alterations of left ventricular myocardial strain in obese children. Eur Heart J Cardiovasc Imaging. 2013;14:668–76. doi: 10.1093/ehjci/jes238. [DOI] [PubMed] [Google Scholar]
- 36.Turer AT, Hill JA, Elmquist JK, Scherer PE. Adipose tissue biology and cardiomyopathy: translational implications. Circ Res. 2012;111:1565–77. doi: 10.1161/CIRCRESAHA.111.262493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Færch K, Witte DR, Tabák AG, Perreault L, Herder C, Brunner EJ, et al. Trajectories of cardiometabolic risk factors before diagnosis of three subtypes of type 2 diabetes: a post-hoc analysis of the longitudinal Whitehall II cohort study. The Lancet Diabetes & Endocrinology. 2013;1:43–51. doi: 10.1016/S2213-8587(13)70008-1. [DOI] [PubMed] [Google Scholar]
- 38.Prevalence of abnormal lipid levels among youths --- United States, 1999-2006. MMWR Morb Mortal Wkly Rep. 2010;59:29–33. [PubMed] [Google Scholar]
- 39.Otvos J. Measurement of triglyceride-rich lipoproteins by nuclear magnetic resonance spectroscopy. Clin Cardiol. 1999;22:II21–27. doi: 10.1002/clc.4960221405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saltijeral A, Isla LP, Perez-Rodriguez O, Rueda S, Fernandez-Golfin C, Almeria C, et al. Early myocardial deformation changes associated to isolated obesity: a study based on 3D-wall motion tracking analysis. Obesity. 2011;19:2268–73. doi: 10.1038/oby.2011.157. [DOI] [PubMed] [Google Scholar]