Abstract
Rationale
Endocardial fibroelastosis (EFE) is a unique form of fibrosis which forms a de novo subendocardial tissue layer encapsulating the myocardium and stunting its growth, and which is typically associated with congenital heart diseases of heterogeneous origin, such as hypoplastic left heart syndrome. Relevance of EFE was only recently highlighted through establishment of staged biventricular repair surgery in HLHS infant patients, where surgical removal of EFE tissue has resulted in improvement in the restrictive physiology leading to growth of the left ventricle in parallel with somatic growth. However, pathomechanisms underlying EFE formation are still scarce and specific therapeutic targets are not yet known.
Objective
Here we aimed to investigate the cellular origins of EFE tissue and to gain insights into underlying molecular mechanisms to ultimately develop novel therapeutic strategies.
Methods and Results
By utilizing a novel EFE model of heterotopic transplantation of hearts from newborn reporter mice and by analyzing human EFE tissue, we demonstrate for the first time that fibrogenic cells within EFE tissue originate from endocardial endothelial cells via aberrant endothelial mesenchymal transition (EndMT). We further demonstrate that such aberrant EndMT involving endocardial endothelial cells is caused by dysregulated TGFβ/BMP signaling and that this imbalance is at least in part caused by aberrant promoter methylation and subsequent transcriptional suppression of BMP5 and BMP7. Finally, we provide evidence that supplementation of exogenous recombinant BMP7 effectively ameliorates EndMT and experimental EFE in rats.
Conclusions
In summary our data point to aberrant EndMT as a common denominator of infant EFE development in heterogeneous, congenital heart diseases, and to BMP7 as an effective treatment for EFE and its restriction of heart growth.
Keywords: Endocardial fibroelastosis, hypoplastic left heart syndrome, endothelial mesenchymal transition, endocardium, fibrosis, endothelial cell, congenital heart disease
INTRODUCTION
Endocardial fibroelastosis refers to a pronounced, fibro-elastic thickening of the ventricular endocardium1. The term endocardial fibroelastosis (EFE) was introduced by Weinberg and Himelfarb in 1943, when EFE presented as unexplained heart failure and common cause of death in infants and children1, 2. While, for unknown reasons, the primary form, which is not associated with any significant structural anomaly of the heart has become rare, EFE is still a major feature of several congenital heart diseases, most notably lesions with left heart obstructions including hypoplastic left heart syndrome (HLHS).
HLHS is a term to describe a spectrum of congenital heart disease, which is lethal if it is not treated3. Children diagnosed with HLHS suffer from left ventricular outflow tract obstruction with various degrees of left ventricular hypoplasia3. To prevent the evolution of the full syndrome, balloon aortic valvotomy was introduced to reestablish flow through the LV outflow tract which led to renewed LV growth in some fetuses4–6. In untreated children, as well as in non-responders, a thick endocardial layer of cellular fibroelastic tissue, termed endocardial fibroelastosis (EFE) forms, which restricts growth of the LV. Clinical observation indicates a direct observation between LV growth in the fetus and the extent of EFE present at the time of the intervention on the valve4, 7. Thus, fetal intervention was combined with post-natal surgical removal of EFE tissue8. Technically successful EFE removal in both previously untreated children as well as in the fetal non-responders to valvotomy resulted in catch-up growth of left-sided structures of the heart, restoring biventricular physiology in a subset of cases, whereas in the children, where EFE could not be removed surgically, there was limited LV growth 8, 9. These clinical findings underscore the likely fundamental role of EFE in HLHS. Therefore, preventive measures to inhibit formation of EFE tissue would be highly desirable. However, pathomechanisms underlying EFE formation are still scarce and specific therapeutic targets are not yet known. Here we aimed to investigate the cellular origins of EFE tissue and to gain insights into underlying molecular mechanisms.
EFE is a special type of fibrosis, which forms a de novo subendocardial tissue layer encapsulating the myocardium. It can be surgically removed without myocardial contamination, suggesting that it is derived from the endocardium and not from the myocardial stroma9. Furthermore, during embryonic heart development, the endocardial endothelium plays a significant role in the development of the valves and septa. Through a process termed endothelial to mesenchymal transition (EndMT), the endocardial endothelial cells form the (mesenchymal) endocardial cushion, which later gives rise to the valves and septum10 Thus, we hypothesized that fibroblasts within the EFE layer originate from the endothelial cells of the endocardium via EndMT. Here we demonstrate that the fibroblasts within the EFE layer indeed derive from endocardial endothelial cells via EndMT. While several of the mutations which have been linked to HLHS directly impact EndMT, previous studies detected no genetic mutation in the vast majority of patients. We therefore explored if transcription level of affected genes could be regulated by the epigenetic mechanism of promoter hypermethylation. We demonstrate here that transcriptional suppression of BMP7 through promoter methylation contributes to EndMT and thus EFE formation and that supplementation with exogenous recombinant BMP7 impedes EFE formation.
METHODS
Ethics statement
This research was approved by and conducted in accordance with the standards of the local institutional review committees of the Boston Children’s Hospital and the Beth Israel Deaconess Medical Center. All studies with human tissue were performed with approval of the institutional review board of Boston Children’s Hospital.
All animals received humane care from the Animal Resources of Boston Children’s Hospital or the Beth Israel Deaconess Medical Center respectively, and all protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Boston Children’s Hospital or Beth Israel Deaconess Medical Center.
Subjects
EFE tissue form patients with HLHS were obtained as discarded material during open heart surgery between May 2009 and June 2010 at the Boston Children’s Hospital, and snap frozen for further analysis. Left ventricular control tissue was obtained from autopsy of infants who have passed away for reasons other than congenital heart disease.
Statistical analysis
All qPCR data for RNA expression analysis (two or more biological replicates) were calculated using the ΔΔCT method. Student t-test (GraphPad Prism 5.1) was used to obtain calculations of statistical significance. For multiple parameter comparison, One-way Anova was used with Bonferroni adjustment for the VE-Cadherin luciferase experiment.
All further methods are described in detail in the supplementary information.
RESULTS
Evidence for EndMT in EFE tissue from HLHS patients
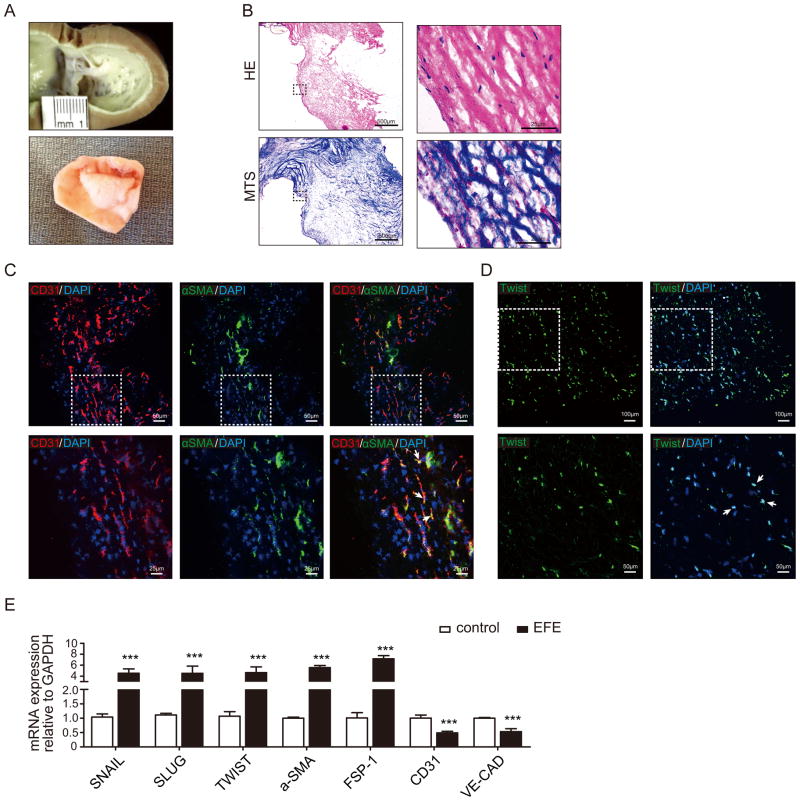
In young infants with HLHS, the endocardial fibroelastic tissue forms a distinct subendocardial tissue layer with high collagen and elastin content encapsulating the underlying myocardium (Figure 1A, B). This EFE layer is removed from the underlying myocardium to allow the left ventricle to grow, as part of the operative therapy during biventricular repair11, enabling us to directly analyze fresh EFE tissue (for RNA, DNA and microscopic analysis) without “contamination” from myocardial tissue. The main cell population in patients’ EFE tissue is fibroblasts. In order to gain insights into possible endocardial origin of EFE fibroblasts, we performed confocal analysis of the EndMT marker TWIST as well as of double immunofluorescence for fibroblast markers FSP1 and αSMA and the endothelial marker CD31, and expression analysis of the EndMT marker genes SNAIL, TWIST and SLUG12. In 27 out of 30 patients we detected both FSP1+/CD31+ and αSMA+/CD31+ double positive cells as well as twist-positive cells. 54.4% of FSP1-positive fibroblasts and 30.3% of αSMA-positive fibroblasts also expressed CD31, suggesting that they are transitioning from endocardial cells into fibroblasts via EndMT (Figure 1C, D and Online Figure I). SNAIL, TWIST and SLUG as well as mesenchymal markers αSMA and FSP1 were all significantly upregulated in EFE tissue as compared to healthy control samples, whereas endothelial markers CD31 and VE-Cadherin were downregulated (Figure 1E), suggesting that fibroblasts within EFE tissue possibly derive from the endocardium via undergoing EndMT.
Figure 1. Evidence for EndMT in EFE tissue from HLHS patients.
(A) Macroscopic pictures of EFE tissue lining the left ventricle of a human heart from an HLHS patient at autopsy (upper panel) and resected from an HLHS patient during surgery (lower panel). (B) Representative photomicrographs of HE and Masson’s Trichome stained sections of human EFE tissue obtained during surgery. (C, D) Representative confocal photomicrographs of surgically removed EFE tissue from HLHS patients (C) double stained for endothelial (CD31) and fibroblast specific markers (αSMA) or (D) single stained with EndMT transcription factor (TWIST). White arrows denote representative double positive cells; dotted areas denote the regions were magnified in the lower panel. (E) Quantitative real- time PCR analysis of the mRNA expression levels of EndMT transcriptional factors (SNAIL, SLUG, and TWIST), mesenchymal markers (FSP-1, a-SMA) and endothelial markers (CD31, VE-Cadherin) in healthy controls and EFE tissues. Results were normalized to reference gene GAPDH. The size of the scale bars represents as noted above. Data are shown as the mean ± SD of three experiments. ***, P<0.001.
BMP7 promoter is hypermethylated in EFE tissue
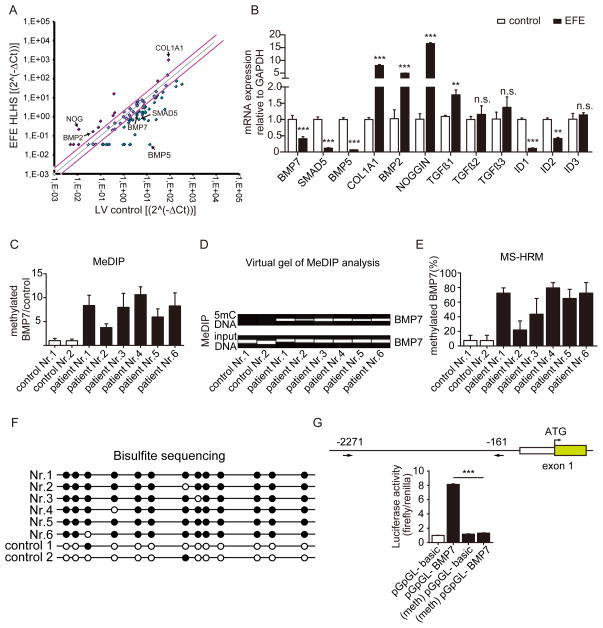
During normal embryonic heart development, the endocardial endothelial cells which line the AV canal undergo an endothelial to mesenchymal transition to form the endocardial mesenchymal cushion which later gives rise to the septum and mitral and tricuspid valves10, prompting us to hypothesize that the EndMT observed in EFE formation is a reflection of the unique susceptibility of the embryonic endocardial endothelium to undergo EndMT. Because EndMT during embryonic heart development is regulated to a great extent by the TGFβ/BMP signaling pathways13–18, we hypothesized that aberrant TGFβ/BMP signaling contributes to aberrant EndMT associated with EFE. We performed a qPCR array, which included 84 genes of the TGFβ/BMP signaling family, and we compared EFE tissue to normal neonatal left ventricular myocardium without heart disease (Figure 2A). Select regulated genes were then validated by real time PCR (Figure 2B). Among the most regulated genes, we found BMP7, BMP5 and their signaling molecule smad5 downregulated (Figure 2B). In contrast, BMP2 and Noggin as well as collagen1A1 (the main constituent of cardiac matrix) were markedly upregulated in EFE tissue (Figure 2B). TGFβ1 was similarly significantly upregulated albeit less pronounced (Figure 2B). In addition, we tested relevant genes in the TGFβ/signaling pathway ID1-3 (as target genes for BMP signals) as well as TGFβ3 (which were not included in the PCR array) by real time analysis. Both ID1 and ID2 were downregulated in EFE, whereas ID3 and TGFβ3 were not significantly regulated in EFE as compared to healthy control tissue (Figure 2B).
Figure 2. BMP7 promoter is hypermethylated in EFE.
(A) TGF-β/BMP qPCR Array compared EFE with healthy control heart tissues. The black arrow highlights the candidate genes which were altered in the EFE sample. (B) Quantitative real-time PCR analysis to validate the candidate genes selected from the TGF-β/BMP qPCR Array. (C, D) Methylated DNA immunoprecipitation (MeDIP) to assess BMP7 methylation in healthy and EFE hearts. (C) Real-time PCR analysis quantitatively showed the methylation levels in control and EFE samples and (D) virtual gel images of BMP7 PCR products (upper panel) of immunoprecipitated 5mC DNA and the lower panel shows PCR products of input DNA as loading controls. (E) BMP7 promoter methylation was determined by methylation-sensitive high resolution melting (MS-HRM) analysis. (F) BMP7 promoter methylation status of the individual CpG sites in the EFE samples and in healthy hearts by bisulfite sequencing. Open and filled circles indicate unmethylated and methylated status, respectively. (G) Luciferase assay assessed promoter activity of methylated human BMP7 promoter. Around 2.1kb (from 2271 to 161 upstream of transcriptional starting site) human BMP7 promoter fragment was cloned into a CpG- free luciferase vector (upper panel). The luciferase activity from the methylated BMP7 promoter construct was significantly reduced as compared to unmethylated control construct. Data are shown as the mean ± SD of three experiments. n.s. no significance, **, P<0.01, ***, P<0.001.
We next aimed to gain insights into the mechanisms underlying the persistent transcriptional suppression of select members of the BMP/TGF signaling family. Because BMP7, BMP5 and SMAD5 all contain CpG rich motifs within their promoter regions, we hypothesized that observed persistent transcriptional suppression could be due to promoter hypermethylation. To explore if decreased expression of BMP7, BMP5 and SMAD5 in EFE tissue is associated with promoter hypermethylation of these genes, a common epigenetic mechanism to reduce gene expression, we performed immunoprecipitation of methylated DNA, followed by PCRs for the BMP7, BMP5 and SMAD5 promoters (MeDIP assay, Figures 2C–D and Online Figure II). Results revealed higher BMP7 and BMP5 promoter methylation in EFE tissue compared to controls, whereas SMAD5 promoter methylation levels were unchanged in EFE tissue (Figures 2C–D and Online Figure II). In order to validate MeDIP results, we also performed methylation-sensitive high resolution melting analysis (MS-HRM, Figure 2E and Online Figure V) as well as bisulfite genomic sequencing (BGS, Figure 2F) for the BMP7 promoter, which both confirmed significantly increased both relative as well as absolute BMP7 promoter CpG methylation in EFE as compared to healthy control tissue (Figure 2E–F).
In order to test the biological impact of BMP7 promoter methylation we cloned the BMP7 promoter into a CpG free backbone and performed an in vitro methylation luciferase assay, demonstrating that hypermethylation of BMP7 promoter leads to reduced BMP7 expression (Figure 2G). This result suggests that the observed BMP7 promoter hypermethylation in EFE tissue likely causally contributes to the similarly documented decrease of BMP7 expression.
BMP7 blocks TGFβ-induced EndMT through regulation of VE-Cadherin promoter activity
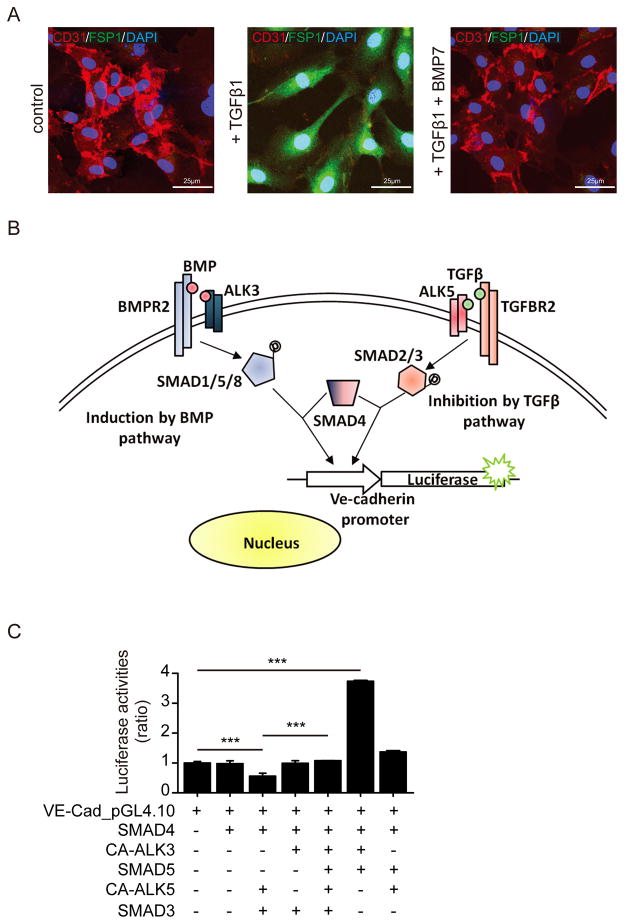
Because analysis of EFE tissues revealed that observed EndMT is associated with persistent suppression of BMP signaling and increased TGFβ signaling we next aimed to gain further insights into the impact of BMP/TGFβ dysregulation on EndMT. While BMP7 antagonizes TGFβ1-induced EndMT (Figure 3A), the molecular level of this mechanism has not yet been addressed. BMP7 and TGFβ1 each bind to distinct type II receptors, which then form complexes with specific type I serine-threonine kinase receptors (ALK receptors)19–22. BMP7 binds specifically to ALK3 and ALK6 receptors, whereas TGFβ1 binds to ALK2 and ALK5 receptors19, 20, 22. Complex formation between BMP7 or TGFβ1 with type II and ALK type I receptors then leads to the intracellular signaling pathway mediated by SMAD proteins. SMAD1, SMAD5 and SMAD8 transduce BMP7 action, whereas SMAD2 and SMAD3 mediate TGFβ action in epithelial cells. SMAD4 is common to both pathways (Figure 3B).
Figure 3. BMP7 blocks TGFβ-induced EndMT through regulation of VE-Cadherin promoter activity.
(A) Representative confocal photomicrographs of HCAEC double stained for CD31 and FSP1. In non-treated HCAEC (left), the endothelial specific marker CD31 is visible (red), while there is FSP1 expression (green) detectable. Upon TGFβ1-treatment (middle), CD31 expression is reduced whereas expression of FSP1 is induced. Addition of BMP7 to TGFβ1 (right) leads to loss of FSP1 and re-induction of CD31. (B) Human telomerase reverse transcriptase (hTERT) immortalized HCAEC cells were transfected with VE-Cadherin luciferase reporter construct and control Renilla expression vector. To mimic TGFβ1 and BMP7 signaling, the cells were also transfected with expression vectors encoding constitutively active type 1 receptor (ALK5 or ALK3) or SMAD3, SMAD4 or SMAD5. (C) Different plasmid combinations (+) or with empty control vector (−) were used to co-transfect the cells. The bar graphs display relative VE-Cadherin promoter activities presented as ratio to Renilla control activities. ***, P<0.001. The size of the scale bars represents as noted above.
We therefore transfected endothelial cells with a VE-cadherin luciferase construct and mimicked TGFβ1 and BMP7 signaling by additionally transfecting endothelial cells with constitutively active ALK3 (to mimic BMP7) and ALK5 (to mimic TGFβ1) receptors or with SMAD3, SMAD5 or SMAD4. Mimicry of the BMP7 signaling pathway increased the activity of full-length VE-cadherin promoter in HCAEC. In contrast, transfection with CA-ALK5 and SMAD3 (TGFβ pathway) resulted in a >50% decrease in VE-cadherin promoter activity (Figure 3B–C). Additional transfection in the same cells with CA-ALK3 and SMAD5 (BMP7 pathway) stimulated re-induction of VE-cadherin promoter activity by about 2fold, restoring it back to the basal level (Figure 3C).
Because Smad signaling pathways are not entirely specific for BMP7 and TGFβ, similar cross talk by other members of the TGFβ-superfamily is possible. Nevertheless, these results provide evidence for a direct SMAD-dependent counteraction of the TGFβ pathway by BMP7 signaling, and vice versa, in cardiac endothelial cells.
In order to investigate potential therapeutic efficacy of exogenous recombinant human BMP7 (rhBMP7) in HLHS we analyzed presence of BMP7 type 1 (ALK3) and type 2 (BMPR2) receptors in human EFE tissue by immunofluorescence labeling. Both receptors were present abundantly in all human EFE tissues. An average of 91% of fibroblasts co-expressed ALK3 and 71% BMPRII. An average of 44% of CD31-positive endothelial cells also co-expressed ALK3 and 65% BMPRII (Online Figures III and IV).
EFE in the Tie2Cre;Rosa-Stop-YFP reporter mouse is derived from EndMT
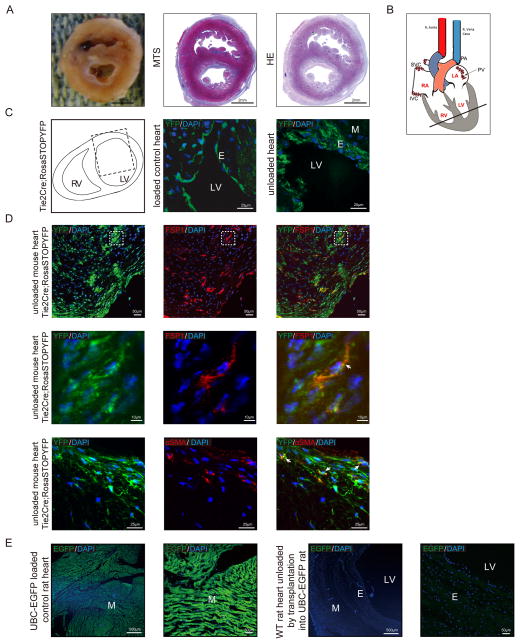
In order to clarify the origin of EFE tissue in the left ventricle, a rodent model was developed in which neonatal hearts were heterotopically transplanted in the abdomen of adult animals 23. The rationale for this heterotopic transplantation was that human EFE develops in the fetus and in association with reduced blood flow, and this surgical technique allows to mimic this situation, by using immature donor hearts where intracavitary blood flow is reduced. Implantation of the heart was performed as a heterotopic infrarenal graft unloaded with aorta to aorta and pulmonary artery (PA) to inferior vena cava (IVC) anastomoses (Figure 4A, B). These “unloaded” neonatal rodent hearts develop EFE tissue resembling human EFE tissue observed in HLHS patients (Figure 4A). We have applied this technique, which was originally developed in rats to transgenic Tie2Cre;Rosa-Stop-YFP reporter mice (Figure 4C, D). In these mice, cells of (Tie2-positive) endothelial origin express YFP, and they continue to express YFP, even if they change their phenotype. These neonatal donor reporter mouse hearts developed massive EFE two weeks after surgery, similar to the previously used neonatal rat hearts. Confocal analysis of the thickened endocardium revealed presence of YFP throughout the endocardium, revealing that cells within the EFE tissue are of endocardial origin (Figure 4C, D), whereas neonatal control reporter mouse hearts display only one thin (YFP-expressing) layer of endocardium (Figure 4C). Co-labeling of these hearts with the fibroblast markers FSP1 and αSMA showed that the FSP1- and αSMA-positive cells co-expressed YFP, indicating the endothelial origin of the fibroblasts (Figure 4D).
Figure 4. EFE in the Tie2Cre;Rosa-Stop-YFP reporter mouse is derived from EndMT.
(A) Representative macroscopic picture and photomicrographs of Masson’s Trichome and HE stained sections of unloaded rodent hearts after heterotopic transplantation. (B) Sketch of the rodent unloaded heterotopic heart transplant model, the bold line indicates the position of the cross-sections (C) Sketch of heart cross-section, dotted line rectangle indicates the area used for confocal microscopy (left panel). Representative confocal photomicrographs of heart sections from neonatal unloaded Tie2Cre; Rosa-Stop-YFP mice two weeks after heterotopic transplantation (right panel) and Tie2Cre; Rosa-Stop-YFP control hearts (middle panel). (D) Representative confocal photomicrographs of sections from neonatal unloaded heterotopic transplanted Tie2Cre; Rosa-Stop-YFP mouse heart stained for mesenchymal marker FSP1 (red, upper panel) or αSMA (red, bottom panel). Endogenous YFP expression is shown in green. Dotted areas denote the region which is shown magnified in the middle panel; white arrows denote representative double positive cells. (E) Representative confocal photomicrographs of sections from loaded control UBC-EGFP rat heart and unloaded rat heart. M, myocardium; E, endocardium; LV, left ventricular lumen.
Because Tie2 is also expressed on hematopoietic cells, we aimed to address if immune cells infiltrate into the fibroelastic endocardium (and thereby account for YFP-labeling not derived from Tie2-positive endocardial cells but from Tie2-positive hematopoietic progenitor cells). We therefore obtained ubiquitous EGFP rats, where all cells are labeled green (Figure 4E, upper panel) and performed additional transplantation experiments, engrafting wildtype donor hearts into ubiquitously EGFP-positive recipient rats. No EGFP-positive cells are detected in the newly formed EFE tissue of the donor heart, indicating that immune cells from the recipient do not contribute to EFE formation (Figure 4E, lower panel).
Exogenous BMP7 ameliorates experimental EFE
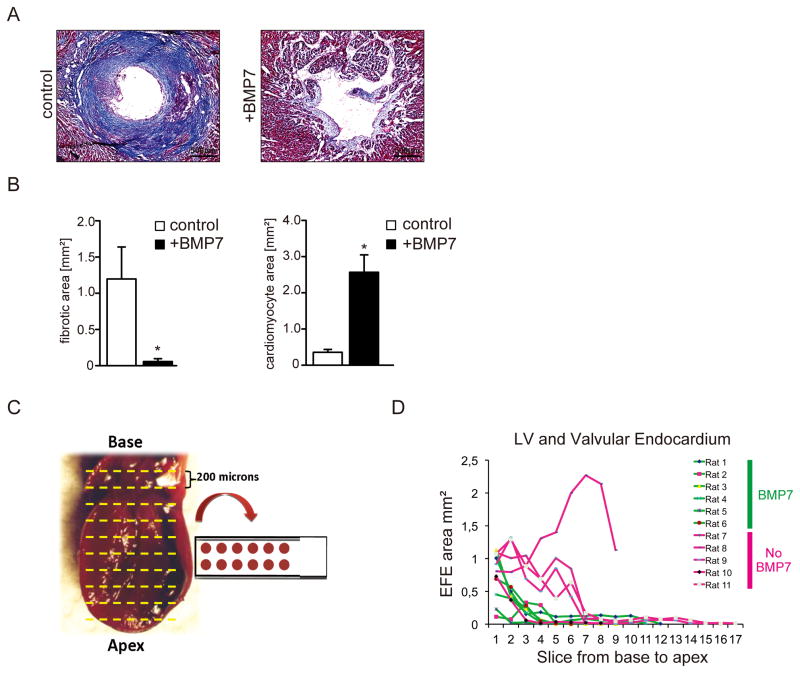
We next sought to test the in vivo effect of rhBMP7 on inhibition of EFE. Because of the substantially bigger size of neonatal rat hearts as compared to neonatal mice we chose to use the rat heart transplantation model for the BMP7 treatment study in order to decrease impact due to handling artifacts. Wild type neonatal rat hearts were unloaded by heterotopic heart transplantation into wild type adult rats as previously described. Recipient rats were administered 30μg/kg rhBMP7 or vehicle buffer i.p. every other day, starting on the day of surgery, with n=6 in each group. Transplanted hearts were harvested 2 weeks after surgery; sectioned from base to apex and analyzed for fibrosis with Masson’s Trichrome stains (Figure 5A–D, Online Figure VII). The fibrotic area was significantly reduced, and cardiomyocyte area was significantly increased in BMP7 administered rats as compared to vehicle-treated animals (Figure 5AD).
Figure 5. Exogeneous BMP7 ameliorates experimental EFE.
(A) Representative photomicrographs of Masson’s Trichrome–stained sections from rat hearts two weeks after heterotopic transplantation without (left) and after rhBMP7 treatment (right) and (B) Quantification of fibrotic and cardiomyocyte area in control and rhBMP7 treated hearts. (C, D) Measurement of the EFE area of slices located from the base to the apex of rat hearts from the left ventricle and the valvular endocardium after heterotopic transplantation. The left picture shows the anatomic position of the slices. The Graph shows the measurement of the mean EFE area in mm2. Each data point reflects at least six measured slices. Data series of slices from hearts without rhBMP7 treatment are highlighted in red; those from slices of hearts after rhBMP7 treatment are highlighted in green. (*p<0.05).
DISCUSSION
“Fibroelastosis of the endocardium is a congenital condition of unknown etiology in which there occurs a diffuse thickening of the mural endocardium associated in most cases with myocardial hypertrophy, and leading to early death.” This definition by Gowing from 1953 is still valid today 11. Since then various attempts have been made to unravel its etiology, but until now a definite mechanism of EFE development could not be identified. Genetic predispositions have been proposed as well as infectious causes or hypoxia during fetal cardiac development24–30. Today, most forms of EFE are associated with congenital heart disease, most notably with hypoplastic left heart syndrome (HLHS), where clinical data suggest that EFE plays a role in the pathogenesis of the disease and is functionally most relevant4, 9, 31. Here we provide evidence that EFE tissue derives from the endocardial endothelium involving aberrant EndMT associated with epigenetically induced BMP/TGFβ signaling dysregulation. We also provide evidence that such insight can be utilized to inhibit EndMT and EFE formation through supplementation of exogenous recombinant BMP7.
Our finding that EFE tissue derives via aberrant EndMT from the endocardial endothelium is in agreement with several independent lines of research: EFE comprises a distinct tissue layer which encapsulates the myocardium and which can be surgically removed without affecting the myocardial tissue, suggesting that it is of endocardial and not of myocardial stroma origin. Furthermore, clinical observations suggest that EFE development is directly associated with reduction of blood flow through the left ventricular cavity (in case of HLHS due to obstruction of the aortic valve), which further suggests that EFE formation is sensed and initiated in the endocardium and not within the myocardial compartment4, 9, 31. During embryonic heart development the endocardial endothelial cells of the AV canal undergo an endothelial-mesenchymal transition to form the mesenchymal endocardial cushion, which later develops into the septum and the valves of the heart suggesting that even in later stages the endocardial endothelial cells retain a unique propensity to undergo EndMT10, 32. In this regard previous studies demonstrated that endocardial cells generate the endothelial cells of coronary arteries32, which may explain, why human coronary endothelial cells retain the capacity to undergo EndMT33. A recent study which questioned contribution of EndMT to cardiac fibrosis also reported presence of fibroblasts of endocardial ancestry within myocardial fibrosis34. In this regard, our findings of EndMT involving endocardial endothelial cells provide additional evidence that heterogeneous embryonic ancestry of endothelial cells impacts their susceptibility to undergo EndMT and that the clinical context of EFE highlights the unique propensity of endocardial endothelial cells to undergo EndMT. Causal contribution of EndMT involving endocardial endothelial cells in formation of EFE is also compatible with several lines of research which went alternate routes to identify underlying mechanisms: Several genetic mutations or copy number variations found in HLHS patients this far, while overall rare, are in genes which regulate EndMT via different pathways (e.g. Notch1, Smad3, GJA1, NR2F2, GATA4)26–30, 35–38. Hypoxia, another possible factor in development of EFE is also a strong inducer of EMT and EndMT in cell culture experiments39, 40. Various viruses have been suspected to cause primary EFE without other associated cardiac anomaly, and frequency of primary EFE has declined in association with eradication of the mumps virus through vaccination25. Several viruses have been shown to induce EMT which makes viruses potential inducers of aberrant EndMT 41–43. Of note, it has been described that in patients with primary EFE, adventitial perivascular hyperelastosis and fibrosis are often found in medium-sized and small arteries of parenchymatous organs other than the heart, suggesting a generalized activation of EndMT in these patients2.
We further provide evidence that observed EndMT in EFE tissue is at least in part caused by imbalance of TGFβ /BMP signaling (TGFβ is increased and BMP signaling is impaired) and that EndMT and ultimately formation of EFE can be ameliorated through supplementation of exogenous recombinant BMP7. Through utilization of a ligand free cell culture system, we demonstrate that TGF and BMP signaling directly counteract each other in the regulation of EndMT. Our studies further suggest that impaired BMP signaling in the context of HLHS-associated EFE is directly linked to transcriptional suppression of BMP5 and BMP7. During mouse development, BMP5 and BMP7 are co-expressed in the heart, and they can substitute for each other. The simultaneous lack of both BMP5 and BMP7 leads to embryonic lethality with endocardial cushion defects whereas the individual BMP5 or BMP7 mutants have negligible developmental defects44. BMP2, which is not downregulated in EFE tissue, on the other hand is necessary and sufficient to drive EndMT of endocardial cells to form the endocardial cushion 45.
In the vast majority of patients with hypoplastic left heart syndrome whole-exome sequencing so far failed to detect causal mutations. Our studies demonstrate that the observed imbalance of TGFβ /BMP in EFE tissue is at least in part due to aberrant promoter hypermethylation of BMP7. Thus, our approach to link HLHS to epigenetic silencing of select genes may provide an important new aspect of the molecular roots of this heterogeneous congenital heart disease. Mechanisms why specific genes are subject to aberrant hypermethylation in the context of EFE remain unclear, but to the best of our knowledge this issue has not been solved in any disease context yet.
Finally, we provide evidence that EndMT involving endocardial endothelial cells and formation of experimental EFE can be ameliorated through supplementation of exogenous recombinant BMP7. This is in line with previous studies from our group which demonstrated that aberrant EndMT of vascular endothelial cells contributes to adult cardiac fibrosis and that by counteraction of TGFβ, BMP7 inhibits EndMT of adult cardiac endothelial cells and thereby ameliorates cardiac fibrosis in several mouse models of cardiac fibrosis33, suggesting that EndMT of coronary artery and of endocardial endothelial cells is controlled by common mechanisms. Because the two BMP7 receptors ALK3 and BMPRII are present on a significant portion of both endothelial cells and fibroblasts in EFE tissue, it is conceivable that rhBMP7 could be effective in ameliorating EFE in HLHS patients. Because the different pathways of EndMT ultimately all lead to VE-cadherin suppression, and BMP7- induced SMAD5 activity is capable of reinducing VE-Cadherin expression (even outside the TGFβ pathway), we believe that the ubiquitous rescue mechanism by BMP7 is most relevant, irrespective of the underlying EndMT mechanism, which may be not be same in all children with EFE. A schematic to illustrate the effect of BMP7 in different settings of known EndMT pathways is shown in Figure 6. Of note, our data does not exclude the possibility that other BMPs are equally effective in inhibiting EndMT and EFE formation, but those to our knowledge have not yet been developed for systemic use, (while recombinant pro-BMP7 is in clinical use for compound fractures).
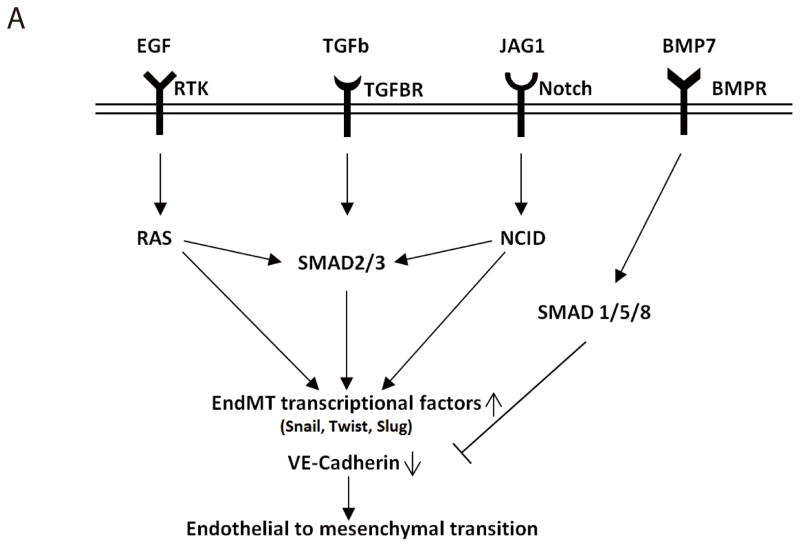
Figure 6. BMP7 inhibits EndMT induced by different pathways.
(A) Schematic representation of EndMT regulated by different signaling pathways. Extrinsic signals stimulate endothelial cells to undergo EndMT through ligands binding to various receptors such as receptor tyrosine kinases (RTK), transforming growth factor receptor (TGFBR), and NOTCH receptor. Once getting activated, these pathways either independently trigger unknown mechanisms or work in concert through common pathway intermediates like SMAD2/3, thereby activating EndMT transcription factor, which leads to loss of endothelial cell characteristics, i.e. expression of VE-Cadherin. The presence of BMP7 can activate the BMP pathway through binding to bone morphogenetic protein receptors (BMPR), thereby activating SMAD1/5/8 which can block the EndMT-inducing signals to ultimately maintain endothelial cell characteristics.
We are aware of a few limitations of our studies: Because human endocardial endothelial cells to our knowledge have not yet been successfully cultured, we have used primary human coronary artery endothelial cells (HCAEC), which were isolated from the coronary arteries from a single donor, in order to induce EndMT in vitro. Coronary artery endothelial cells (unlike aortic) originate to a significant extent from endocardial cells32, and, at least to some degree retain similar biological potential as original endocardial cells, such as the capability to undergo EndMT upon TGFβ treatment. Thus we believe that HCAEC are well suited to substitute for the lack of primary endocardial cells with respect to understanding molecular mechanisms of both vascular and endocardial EndMT.
Furthermore, we induced EFE in neonatal Tie2Cre;Rosa-Stop-YFP mouse hearts and demonstrate here that in this animal model cells of the EFE tissue are of endothelial origin. Because Tie2, in addition to endothelial cells, is also expressed in hematopoietic progenitor cells in the bone marrow, we also induced EFE in wildtype rats transplanted in recipient rats with ubiquitous EGFP expression. No EGFP-positive cells were found in the donor heart EFE tissue, ruling out contribution of bone marrow cells to EFE formation.
No EFE develops if two week old mouse hearts are used in this model of unloading, suggesting that immaturity is a necessary factor during EndMT in EFE formation23. We therefore believe that most EndMT of endocardial cells happens during fetal EFE development. On the other hand, we still detect a fraction of cells undergoing active EndMT (by co-labeling of CD31 and mesenchymal markers or of CD31 and EndMT transcription factors such as TWIST) in EFE tissue from children with HLHS, aged between a few days until preschool, suggesting that part of EFE tissue is “maintained” by active EndMT even at later developmental stages, while naturally, an absolute number of EndMT-derived fibroblasts cannot be provided by this technique.
While we provide evidence that aberrant EndMT causes EFE, further studies are needed to address how disturbed EndMT relates to abnormal valve formation and reduced ventricular growth in HLHS and in other congenital heart diseases associated with EFE. Because aberrant EndMT also contributes to cardiac fibrosis in adult forms of heart disease, studying EndMT in the context of fetal EFE may well contribute valuable knowledge to unravel fibrogenesis in chronic heart disease.
Supplementary Material
Novelty and Significance.
What Is Known?
Endocardial fibroelastosis (EFE) is a unique form of subendocardial fibrosis that complicates congenital heart disease.
During embryonic heart development the endocardial endothelium undergoes an endothelial to mesenchymal transition (EndMT) to form the mesenchymal endocardial cushion.
What New Information Does This Article Contribute?
Fibroblasts contributing to EFE originate from the endocardium via an aberrant EndMT.
Hypermethylation of CpG island promoters of Bone Morphogenic Proteins (BMP)-5 and -7 cause an imbalanced growth factor microenvironment and subsequently aberrant EndMT.
In rodent models of EFE, administration of recombinant BMP-7 ameliorates EndMT and the formation of EFE tissue.
Endocardial fibroelastosis (EFE) is a unique form of subendocardial fibrosis that complicates congenital heart disease, including hypoplastic left heart syndrome (HLHS). While surgical removal of EFE tissue improves outcome of HLHS, preventive measures to inhibit EFE would be highly desirable. However, the mechanisms underlying EFE formation remain unclear and specific therapeutic targets have not been identified. During embryonic heart development the endocardial endothelium undergoes an endothelial to mesenchymal transition (EndMT) to form the (mesenchymal) endocardial cushion (which later gives rise to the valves and septum reference). Hence, we hypothesized that fibroblasts within the EFE layer originate from the endothelial cells of the endocardium via EndMT. Here we demonstrate that the fibroblasts within the EFE layer derive from endocardial endothelial cells via EndMT. We also report that transcriptional suppression of BMP7 through promoter methylation contributes to EndMT and EFE formation and that supplementation with exogenous recombinant BMP7 impedes EFE formation. Based on these findings, it might be useful to assess the utility of intrauterine administration of BMP7 in fetuses diagnosed with HLHS.
Acknowledgments
SOURCES OUF FUNDING
This study was funded in part by funds of the University Medical Center of Göttingen, the Boston Children’s Hospital, the Beth Israel Deaconess Medical Center Boston, the Harvard Catalyst Pilot Grant NIH-1UL1RR025758-01 (to E.Z. and I.M.), NHLB-KO8 HL-075430 (to I.F.) and NIH RO1 – HL63095 (to PJdN). EZ is further supported by grants SFB1002/C01 from the DFG and F/55/13 from the German Heart Foundation. MZ is supported by DFG grant ZE523/2-1, ZE523/3-1 and the Else-Kröner Memorial Stipend 2005/59. XX and BT were supported by seed funding “Research program” of the Faculty of Medicine, Georg-August-University Göttingen. Confocal microscopy was performed at the Molecular & Optical Live Cell Imaging (MOLCI) core facility at the University Medicine Göttingen.
The authors are strongly indebted to Prof. Kohei Miyazono (The University of Tokyo, Japan) for providing us with the smad3-5 as well as the constitutively active ALK3 and ALK5 plasmids and to Prof. Philippe Huber (University Joseph Fourier, Grenoble, France) for providing the VE-cadherin_pGL3 plasmid. We also would like to especially thank Prof. Michael Rehli (University of Regensburg, Germany) for sharing pCpGL-basic plasmids.
Nonstandard Abbreviations and Acronyms
- ALK
activin receptor-like kinase
- AV
atrioventricular
- BGS
bisulfite genomic sequencing
- BMP
bone morphogenetic proteins
- EFE
endocardial fibroelastosis
- EndMT
endothelial to mesenchymal transition
- HCAEC
human coronary artery endothelial cells
- HLHS
hypoplastic left heart syndrome
- HRM
high resolution melting
- i.p
intraperitoneal
- IVC
inferior vena cava
- LV
left ventricle
- MeDIP
methylated DNA immunoprecipitation
- PA
pulmonary artery
- PCR
polymerase chain reaction
- TGFβ
transforming growth factor beta
- YFP
yellow fluorescent protein
Footnotes
DISCLOSURES
None.
References
- 1.Weinberg T, Himelfarb AJ. Endocardial fibroelastosis (socalledfoetal endocarditis) Bull Johns Hopkins Hosp. 1943;72:299. [Google Scholar]
- 2.Rosahn PD. Endocardial Fibroelastosis: Old And New Concepts. New York: Pathological Society; 1954. pp. 453–474. [PMC free article] [PubMed] [Google Scholar]
- 3.Hickey EJ, Caldarone CA, McCrindle BW. Left ventricular hypoplasia: a spectrum of disease involving the left ventricular outflow tract, aortic valve, and aorta. J Am Coll Cardiol. 2012;59(1 Suppl):S43–54. doi: 10.1016/j.jacc.2011.04.046. [DOI] [PubMed] [Google Scholar]
- 4.McElhinney DB, Vogel M, Benson CB, Marshall AC, Wilkins-Haug LE, Silva V, Tworetzky W. Assessment of left ventricular endocardial fibroelastosis in fetuses with aortic stenosis and evolving hypoplastic left heart syndrome. Am J Cardiol. 2010;106(12):1792–1797. doi: 10.1016/j.amjcard.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Vogel M, Wilkins-Haug LE, McElhinney DB, Marshall AC, Benson CB, Silva V, Tworetzky W. Reversible ductus arteriosus constriction due to maternal indomethacin after fetal intervention for hypoplastic left heart syndrome with intact/restrictive atrial septum. Fetal Diagn Ther. 2010;27(1):40–45. doi: 10.1159/000268290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selamet Tierney ES, Wald RM, McElhinney DB, Marshall AC, Benson CB, Colan SD, Marcus EN, Marx GR, Levine JC, Wilkins-Haug L, Lock JE, Tworetzky W. Changes in left heart hemodynamics after technically successful in-utero aortic valvuloplasty. Ultrasound Obstet Gynecol. 2007;30(5):715–720. doi: 10.1002/uog.5132. [DOI] [PubMed] [Google Scholar]
- 7.McElhinney DB, Marshall AC, Wilkins-Haug LE, Brown DW, Benson CB, Silva V, Marx GR, Mizrahi-Arnaud A, Lock JE, Tworetzky W. Predictors of technical success and postnatal biventricular outcome after in utero aortic valvuloplasty for aortic stenosis with evolving hypoplastic left heart syndrome. Circulation. 2009;120(15):1482–1490. doi: 10.1161/CIRCULATIONAHA.109.848994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emani SM, Bacha EA, McElhinney DB, Marx GR, Tworetzky W, Pigula FA, del Nido PJ. Primary left ventricular rehabilitation is effective in maintaining two-ventricle physiology in the borderline left heart. J Thorac Cardiovasc Surg. 2009;138(6):1276–1282. doi: 10.1016/j.jtcvs.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emani SM, McElhinney DB, Tworetzky W, Myers PO, Schroeder B, Zurakowski D, Pigula FA, Marx GR, Lock JE, del Nido PJ. Staged left ventricular recruitment after single-ventricle palliation in patients with borderline left heart hypoplasia. J Am Coll Cardiol. 2012;60(19):1966–1974. doi: 10.1016/j.jacc.2012.07.041. [DOI] [PubMed] [Google Scholar]
- 10.Eisenberg LM, Markwald RR. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ Res. 1995;77(1):1–6. doi: 10.1161/01.res.77.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Gowing NFC. Congenital fibroelastosis of the endocardium. J Path Bact. 1953;65:13–28. doi: 10.1002/path.1700650103. [DOI] [PubMed] [Google Scholar]
- 12.Kokudo T, Suzuki Y, Yoshimatsu Y, Yamazaki T, Watabe T, Miyazono K. Snail is required for TGFbeta-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells. J Cell Sci. 2008;121(Pt 20):3317–3324. doi: 10.1242/jcs.028282. [DOI] [PubMed] [Google Scholar]
- 13.Liebner S, Cattelino A, Gallini R, Rudini N, Iurlaro M, Piccolo S, Dejana E. Beta-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J Cell Biol. 2004;166(3):359–367. doi: 10.1083/jcb.200403050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakajima Y, Yamagishi T, Hokari S, Nakamura H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP) Anat Rec. 2000;258(2):119–127. doi: 10.1002/(SICI)1097-0185(20000201)258:2<119::AID-AR1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 15.Boyer AS, Ayerinskas II, Vincent EB, McKinney LA, Weeks DL, Runyan RB. TGFbeta2 and TGFbeta3 have separate and sequential activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev Biol. 1999;208(2):530–545. doi: 10.1006/dbio.1999.9211. [DOI] [PubMed] [Google Scholar]
- 16.Camenisch TD, Molin DG, Person A, Runyan RB, Gittenberger-de Groot AC, McDonald JA, Klewer SE. Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev Biol. 2002;248(1):170–181. doi: 10.1006/dbio.2002.0731. [DOI] [PubMed] [Google Scholar]
- 17.Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-beta(1) Mol Genet Metab. 2000;71(1–2):418–435. doi: 10.1006/mgme.2000.3032. [DOI] [PubMed] [Google Scholar]
- 18.Ramsdell AF, Moreno-Rodriguez RA, Wienecke MM, Sugi Y, Turner DK, Mjaatvedt CH, Markwald RR. Identification of an autocrine signaling pathway that amplifies induction of endocardial cushion tissue in the avian heart. Acta Anat (Basel) 1998;162(1):1–15. doi: 10.1159/000046463. [DOI] [PubMed] [Google Scholar]
- 19.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390(6659):465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 20.Wrana JL. Regulation of Smad activity. Cell. 2000;100(2):189–192. doi: 10.1016/s0092-8674(00)81556-1. [DOI] [PubMed] [Google Scholar]
- 21.Derynck R, Zhang Y, Feng XH. Smads: transcriptional activators of TGF-beta responses. Cell. 1998;95(6):737–740. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- 22.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1(3):169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 23.Friehs I, Illigens B, Melnychenko I, Zhong-Hu T, Zeisberg E, Del Nido PJ. An animal model of endocardial fibroelastosis. J Surg Res. 2012 doi: 10.1016/j.jss.2012.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purevjav E, Varela J, Morgado M, Kearney DL, Li H, Taylor MD, Arimura T, Moncman CL, McKenna W, Murphy RT, Labeit S, Vatta M, Bowles NE, Kimura A, Boriek AM, Towbin JA. Nebulette mutations are associated with dilated cardiomyopathy and endocardial fibroelastosis. J Am Coll Cardiol. 2010;56(18):1493–1502. doi: 10.1016/j.jacc.2010.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ni J, Bowles NE, Kim YH, Demmler G, Kearney D, Bricker JT, Towbin JA. Viral infection of the myocardium in endocardial fibroelastosis. Molecular evidence for the role of mumps virus as an etiologic agent. Circulation. 1997;95(1):133–139. doi: 10.1161/01.cir.95.1.133. [DOI] [PubMed] [Google Scholar]
- 26.Iascone M, Ciccone R, Galletti L, Marchetti D, Seddio F, Lincesso AR, Pezzoli L, Vetro A, Barachetti D, Boni L, Federici D, Soto AM, Comas JV, Ferrazzi P, Zuffardi O. Identification of de novo mutations and rare variants in hypoplastic left heart syndrome. Clin Genet. 2012;81(6):542–554. doi: 10.1111/j.1399-0004.2011.01674.x. [DOI] [PubMed] [Google Scholar]
- 27.Fitzgerald KK, Bhat AM, Conard K, Hyland J, Pizarro C. Novel SMAD3 Mutation in a Patient with Hypoplastic Left Heart Syndrome with Significant Aortic Aneurysm. Case Rep Genet. 2014;2014:591516. doi: 10.1155/2014/591516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dasgupta C, Martinez AM, Zuppan CW, Shah MM, Bailey LL, Fletcher WH. Identification of connexin43 (alpha1) gap junction gene mutations in patients with hypoplastic left heart syndrome by denaturing gradient gel electrophoresis (DGGE) Mutat Res. 2001;479(1–2):173–186. doi: 10.1016/s0027-5107(01)00160-9. [DOI] [PubMed] [Google Scholar]
- 29.Al Turki S, Manickaraj AK, Mercer CL, Gerety SS, Hitz MP, Lindsay S, D’Alessandro LC, Swaminathan GJ, Bentham J, Arndt AK, Low J, Breckpot J, Gewillig M, Thienpont B, Abdul-Khaliq H, Harnack C, Hoff K, Kramer HH, Schubert S, Siebert R, Toka O, Cosgrove C, Watkins H, Lucassen AM, O’Kelly IM, Salmon AP, Bu’lock FA, Granados-Riveron J, Setchfield K, Thornborough C, Brook JD, Mulder B, Klaassen S, Bhattacharya S, Devriendt K, Fitzpatrick DF, Consortium UK, Wilson DI, Mital S, Hurles ME. Rare variants in NR2F2 cause congenital heart defects in humans. Am J Hum Genet. 2014;94(4):574–585. doi: 10.1016/j.ajhg.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warburton D, Ronemus M, Kline J, Jobanputra V, Williams I, Anyane-Yeboa K, Chung W, Yu L, Wong N, Awad D, Yu CY, Leotta A, Kendall J, Yamrom B, Lee YH, Wigler M, Levy D. The contribution of de novo and rare inherited copy number changes to congenital heart disease in an unselected sample of children with conotruncal defects or hypoplastic left heart disease. Hum Genet. 2014;133(1):11–27. doi: 10.1007/s00439-013-1353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharland GK, Chita SK, Fagg NL, Anderson RH, Tynan M, Cook AC, Allan LD. Left ventricular dysfunction in the fetus: relation to aortic valve anomalies and endocardial fibroelastosis. Br Heart J. 1991;66(6):419–424. doi: 10.1136/hrt.66.6.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu B, Zhang Z, Lui W, Chen X, Wang Y, Chamberlain AA, Moreno-Rodriguez RA, Markwald RR, O’Rourke BP, Sharp DJ, Zheng D, Lenz J, Baldwin HS, Chang CP, Zhou B. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell. 2012;151(5):1083–1096. doi: 10.1016/j.cell.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13(8):952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 34.Bursac N. Cardiac fibroblasts in pressure overload hypertrophy: the enemy within? J Clin Invest. 2014;124(7):2850–2853. doi: 10.1172/JCI76628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mihira H, Suzuki HI, Akatsu Y, Yoshimatsu Y, Igarashi T, Miyazono K, Watabe T. TGF-beta-induced mesenchymal transition of MS-1 endothelial cells requires Smad-dependent cooperative activation of Rho signals and MRTF-A. J Biochem. 2012;151(2):145–156. doi: 10.1093/jb/mvr121. [DOI] [PubMed] [Google Scholar]
- 36.Nakano Y, Oyamada M, Dai P, Nakagami T, Kinoshita S, Takamatsu T. Connexin43 knockdown accelerates wound healing but inhibits mesenchymal transition after corneal endothelial injury in vivo. Invest Ophthalmol Vis Sci. 2008;49(1):93–104. doi: 10.1167/iovs.07-0255. [DOI] [PubMed] [Google Scholar]
- 37.Rivera-Feliciano J, Lee KH, Kong SW, Rajagopal S, Ma Q, Springer Z, Izumo S, Tabin CJ, Pu WT. Development of heart valves requires Gata4 expression in endothelial-derived cells. Development. 2006;133(18):3607–3618. doi: 10.1242/dev.02519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noseda M, McLean G, Niessen K, Chang L, Pollet I, Montpetit R, Shahidi R, Dorovini-Zis K, Li L, Beckstead B, Durand RE, Hoodless PA, Karsan A. Notch activation results in phenotypic and functional changes consistent with endothelial-to-mesenchymal transformation. Circ Res. 2004;94(7):910–917. doi: 10.1161/01.RES.0000124300.76171.C9. [DOI] [PubMed] [Google Scholar]
- 39.Luo D, Wang J, Li J, Post M. Mouse snail is a target gene for HIF. Mol Cancer Res. 2011;9(2):234–245. doi: 10.1158/1541-7786.MCR-10-0214. [DOI] [PubMed] [Google Scholar]
- 40.Misra A, Pandey C, Sze SK, Thanabalu T. Hypoxia activated EGFR signaling induces epithelial to mesenchymal transition (EMT) PLoS One. 2012;7(11):e49766. doi: 10.1371/journal.pone.0049766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horikawa T, Yoshizaki T, Kondo S, Furukawa M, Kaizaki Y, Pagano JS. Epstein-Barr Virus latent membrane protein 1 induces Snail and epithelial-mesenchymal transition in metastatic nasopharyngeal carcinoma. Br J Cancer. 2011;104(7):1160–1167. doi: 10.1038/bjc.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimamura M, Murphy-Ullrich JE, Britt WJ. Human cytomegalovirus induces TGF-beta1 activation in renal tubular epithelial cells after epithelial-to-mesenchymal transition. PLoS Pathog. 2010;6(11):e1001170. doi: 10.1371/journal.ppat.1001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sides MD, Klingsberg RC, Shan B, Gordon KA, Nguyen HT, Lin Z, Takahashi T, Flemington EK, Lasky JA. The Epstein-Barr virus latent membrane protein 1 and transforming growth factor--beta1 synergistically induce epithelial--mesenchymal transition in lung epithelial cells. Am J Respir Cell Mol Biol. 2011;44(6):852–862. doi: 10.1165/rcmb.2009-0232OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solloway MJ, Robertson EJ. Early embryonic lethality in Bmp5; Bmp7 double mutant mice suggests functional redundancy within the 60A subgroup. Development. 1999;126(8):1753–1768. doi: 10.1242/dev.126.8.1753. [DOI] [PubMed] [Google Scholar]
- 45.Rivera-Feliciano J, Tabin CJ. Bmp2 instructs cardiac progenitors to form the heart-valve-inducing field. Dev Biol. 2006;295(2):580–588. doi: 10.1016/j.ydbio.2006.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.