Abstract
Objectives
To assess links between comorbid health status, severe excess weight, and weight-related quality of life (WRQOL) in adolescents with severe obesity and undergoing weight loss surgery (WLS) to inform clinical care.
Study design
Baseline (pre-operative) data from Teen-LABS, a prospective multicenter observational study of 242 adolescents with severe obesity (MdnBMI = 50.5 kg/m2; Mage=17.1; 75.6% female; 71.9% White) undergoing WLS, were utilized to examine the impact of demographics, body mass index (BMI), presence/absence of 16 comorbid conditions, and a cumulative comorbidity load (CLoad) index on WRQOL scores (Impact of Weight on Quality of Life-Kids; IWQOL-Kids).
Results
WRQOL was significantly lower than reference samples of healthy weight, overweight, and obese samples. Of 16 comorbid conditions, the most prevalent were dyslipidemia (74.4%), chronic pain (58.3%), and obstructive sleep apnea (56.6%). Males had a higher CLoad (p=.01) and BMI (p=.01), yet less impairment in total WRQOL (p<.01) than females. CLoad was a significant predictor of male WRQOL. For females, psychosocial (versus physical) comorbidities, BMI, and White race were significant predictors of WRQOL impairment. Less prevalent conditions (e.g., stress urinary incontinence) also emerged as contributors to lower WRQOL.
Conclusions
WRQOL impairment is substantial for adolescents with severe obesity undergoing WLS, with predictors varying by sex. These patient-data highlight targets for education, support, and adjunctive care referrals prior to WLS. Further, they provide a comprehensive empirical base for understanding heterogeneity in adolescent WRQOL outcomes following WLS, as weight and comorbidity profiles change over time.
Keywords: Bariatric
A hallmark of the pediatric obesity epidemic is the increased prevalence and associated health burden of severe obesity (ie, body mass index [BMI] ≥ 120% of the BMI-for-age 95th percentile) (1). The Teen Longitudinal Assessment of Bariatric Surgery (Teen-LABS) Consortium recently reported that, prior to weight loss surgery (WLS), 51% of severely obese adolescent participants concurrently managed four or more major medical comorbid conditions (e.g., dyslipidemia, obstructive sleep apnea, hypertension, chronic back pain).(2) However, the psychosocial health of the adolescent with severe obesity undergoing WLS is of equal concern, yet less understood.
Health-related quality of life (HRQOL) is the patient’s perspective on how their current health status impacts their day-to-day life across multiple domains (e.g., physical, social, emotional).(3) HRQOL assessment provides clinicians with information regarding the patient’s well-being within the context of their medical status, identifying targets for support, recommendations, and adjunctive care referrals.(4) Serial HRQOL assessment provides critical information on how change in symptoms and/or treatments impact daily functioning. Condition-specific HRQOL measures capture unique aspects of a disease and demonstrate greater sensitivity to change in disease severity (5) (e.g., weight related quality of life: WRQOL (6, 7)). Finally, HRQOL measurement is regarded as a key patient-reported outcome (PRO) for clinical trials and observational studies (e.g., FDA (8, 9), CONSORT (10)) and has been identified as a new topic area for Healthy People 2020.(11)
It is well established that youth who are obese (BMI ≥ 95th percentile) report greater impairment in HRQOL, whether general (i.e., PedsQL (12)) or weight-related, as compared with “healthy youth”.(7, 13–17) Further, obese youth are characterized by lower HRQOL than youth with other chronic medical conditions with more immediate morbidity and mortality risk (e.g., epilepsy, cystic fibrosis, type 1 diabetes, cancer) (4, 18). Initial studies also suggest adolescents with severe obesity report the most severe impairments in HRQOL/WRQOL within the broader pediatric literature to date.(19–21)
It is unclear as to how are comorbid conditions, severe excess weight, and WRQOL are linked, and might this vary for males versus females? Previous studies suggest greater HRQOL/WRQOL impairment in obese youth (BMI ≥ 95th percentile) with specific comorbidities (ie, chronic pain (22), obstructive sleep apnea [OSA] (13), depressive symptoms (15, 16), binge eating disorder [BED] (23)) and for females relative to males.(15) In obese adults, WRQOL impairment is driven by the severity of excess weight (eg, BMI value), or a specific comorbidity (i.e., gastroesophageal reflux disease [GERD]) relative to others, but also, by the number of comorbidities present.(24–27) Preliminary pediatric evidence from adolescents surveyed at intake in a Metabolic Syndrome clinic lend additional support.(28) Specifically, when evaluating four specific comorbid conditions (triglycerides, systolic or diastolic blood pressure > 95th percentile, high-density lipoprotein cholesterol: < 5th percentile, fasting blood glucose: >100/mg/dl), adolescents experiencing all 4 versus only 2 or 3 conditions, reported greater WRQOL impairment. This concept of cumulative versus individual comorbidity impact on WRQOL may have heightened implications for adolescent WLS patients given the range of obesity-related comorbid conditions present at the time of surgery.(2)
The present study capitalizes on the breadth and scope of the multi-site Teen-LABS Consortium to characterize the domains and extent of WRQOL impairment, and evaluate how BMI and 16 different obesity-related comorbid conditions, both as a cumulative index and individually, are associated with WRQOL for adolescents with severe obesity prior to WLS, and whether this varies by sex. Based on the aforementioned literature, BMI, race, and five individual comorbid conditions (ie, OSA, GERD, BED, DEP, and chronic joint or back pain (PAIN)) as well as a cumulative comorbidity load index (CLoad, range 1–16 comorbidities) were hypothesized to be significantly associated with greater WRQOL impairment. Expanded analyses explored the independent impact of these specific comorbid conditions as well as additional obesity-related comorbidities (11 for females) known to be present in this clinical population.
Methods
Teen-LABS Consortium is a 5 site prospective observational study evaluating safety, efficacy, health, and WRQOL outcomes of WLS for severely obese adolescents (29) and is an ancillary study to the Longitudinal Assessment of Bariatric Surgery (LABS; registered with ClinicalTrials.gov: NCT00465829). Teen-LABS’ rationale, inclusion criterion, and methodology were previously reported.(2, 29) Teen-LABS was approved by each institution’s Institutional Review Board with study conduct overseen by an independent Data and Safety Monitoring Board. The Teen-LABS Data Coordinating Center managed all de-identified data and conducted statistical analyses.
All adolescents (ages 13–19 years) approved for WLS at Teen-LABS centers and their primary caregivers were approached for study enrollment (February 2007–December 2011). Of the 277 eligible patients, 13 declined and 22 consented but did not undergo surgery by the study-imposed deadline, leaving a final cohort of 242. Participants did not differ from non-participants (n=35) in BMI, age, or race/ethnicity but were more likely to be female (p = 0.04). Medical and surgical care were based on patient-care pathways at each institution. No attempts were made to standardize or alter standard care by this observational research protocol.
Teen-LABS modified the standardized methodology of the LABS consortium’s LABS2 study (30, 31) for this adolescent cohort. Adolescents and caregivers provided written assent/consent. Baseline assessments (e.g., anthropometrics, blood and urine sampling, self-report forms) were completed at an in-person visit within 30 days prior to surgery and administered by Teen-LABS trained personnel. Participants were informed that responses on self-report measures were confidential, although the informed consent/assent form specified that investigators would take steps to address significant distress or risk of serious harm (e.g., suicidal ideation). Presence/absence of each comorbid condition was determined by trained Teen-LABS personnel using medical records, physical examination, patient interview, self-report forms, and laboratory values.
WRQOL was assessed via the Impact of Weight on Quality of Life – Kids (IWQOL-Kids©), (7) a self-report instrument for youth (11–19 years) with 4 subscales (Physical Comfort, Body Esteem, Social Life, Family Relations) and a summary Total score. Items begin with the stem “Because of my weight…” to orient a respondent to the condition-specific aspect of each question. Raw scaled scores were transformed to a 0–100 scale with higher scores reflecting better WRQOL (e.g., less impairment). Psychometric evaluation of the IWQOL-Kids has demonstrated excellent psychometric properties, including discrimination among weight status groups, and being responsive to weight change.(7, 32, 33) Internal consistencies of IWQOL-Kids Total and scale scores were strong for the current sample (Total score α=0.92; Physical Comfort α = 0 .82; Body Esteem α = 0.92; Social Life α = 0.87; Family Relations α = 0.88).
Teen-LABS standard definitions were used to determine the presence/absence of hypertension, dyslipidemia, OSA, asthma, GERD, fatty liver disease, cholelithiasis, diabetes, chronic kidney disease (CKD), stress urinary incontinence (SUI), pseudotumor cerebri (PTC), Blount disease, chronic joint or back pain (PAIN), and for females, polycystic ovary syndrome and/or menstrual irregularities (PCOS). Detailed descriptions of study methodology, comorbidity and other data definitions, and laboratory testing are included in the online methodology supplement (Appendix 3; available at www.jpeds.com). A comorbidity load (CLoad) score was computed as the total number of comorbid conditions for each participant.
Depressive symptoms were assessed using the Beck Depression Inventory – II (BDI-II), with the suggested Total score of ≥17 (DEP) utilized as a conservative cut-point for “clinically elevated” depressive symptoms.(34) Screening criteria for binge eating disorder (BED) were assessed using 4 critical items from the Questionnaire of Eating and Weight Patterns – Revised.(35)
Adolescent and caregiver sociodemographic characteristics were self-reported. Height and weight measured via a standardized protocol (2) were used to calculate BMI (kg/m2).
Statistical Analyses
Overall, 1.5% of data values were missing among variables included in statistical models. Most participants (83%) had complete data. Missing data values ranged from 0.4% (n=1) for GERD, FLD, and SUI to 7.4% (n=18) for CKD. Nine (3.7%) participants did not complete the self-reported IWQOL-Kids due to visual impairment or learning difficulties. Multivariate imputation by fully conditional specification was performed to address missing data on all assessment variables. The SAS (version 9.3) MI procedure was used to generate 25 imputed data sets for use in multivariable modeling analyses.
Means, medians, percentages, and counts describe participant demographics, IWQOL-Kids scores, and comorbid conditions. Chi-square, Fisher’s exact, Wilcoxon’s rank-sum and t-tests assessed sex differences. z tests were used to compare IWQOL-Kids scores between the present sample and instrument reference samples of healthy weight (BMI=11–84.9 percentile), overweight (BMI =85–94.9th percentile), and obese (BMI ≥ 95th percentile adolescents, (7) as well as additional published samples of obese (BMI ≥ 95th percentile) (28) and severely obese (BMI ≥ 40) adolescents.(20) Sex-stratified, multivariable mixed-effect linear regression (SAS Proc MIXED) models, incorporating clinical site as a random effect, evaluated predictors of IWQOL-Kids scores (Total, Physical Comfort, Body Esteem, Social Life, log-transformed Family Relations) with the CLoad term forced into each final model. SAS Proc MiAnalyze generated estimates from the multiply imputed datasets. A priori hypothesized variables evaluated for inclusion into the final models included: BMI, race, OSA, PAIN, GERD, BED, and DEP. Additional analyses evaluated the aforementioned covariates (excluding the CLoad), as well as the following: diabetes, PTC, FLD, hypertension, dyslipidemia, CKD, asthma, cholelithiasis, SUI, PCOS, and Blount’s disease. Predictor terms where p < 0.10 were retain in the final models. All reported p-values were two-sided and considered statistically significant at ≤ 0.05. Pseudo-R2 values, defined as the proportion of the variance in the outcome explained by the predictor variables (36) in each linear mixed model, were also computed.
Results
Participants (Mage=17.1, SD=1.6) were primarily female (75.6%) and White (71.9%) with a primary caregiver with at least a high school education (90.0%) (Table I). Median pre-operative BMI was 50.5 kg/m2 for the entire group; BMI was significantly higher for males than females (p = 0.01).
Table 1.
Teen-LABS participant characteristics, comorbid condition prevalence, and IWQOL scores by sex.
| Total (N=242) | Females (n=183) | Males (n=59) | p | |
|---|---|---|---|---|
| Demographics | ||||
| Age at surgery [Mean (SD)] | 17.1 (1.56) | 17.1 (1.54) | 17.1 (1.63) | 0.97 |
| Body Mass Index [Median (Q1, Q3)] | 50.5 (45.2,58.3) | 49.4 (38.7,57.0) | 54.5 (46.1,62.1) | 0.02 |
| White race [%(n)] | 71.9 (174) | 71.6 (131) | 72.9 (43) | 0.85 |
| Primary Caregiver Educationa [%(n)] | 0.86 | |||
| • ≤ High School Graduation | 41.1 (95) | 40.8 (71) | 42.1 (24) | |
| • 1+ Years Post-Secondary | 58.9 (136) | 59.2 (103) | 57.9 (33) | |
|
| ||||
| Comorbid Conditions [%(n)] | ||||
| Dyslipidemia | 74.4 (180) | 70.5 (129) | 86.4 (51) | 0.01 |
| Joint or Back Pain | 58.3 (141) | 57.9 (106) | 59.3 (35) | 0.85 |
| Obstructive Sleep Apnea | 56.6 (137) | 49.7 (91) | 78.0 (46) | <0.01 |
| Hypertension | 45.0 (109) | 38.8 (71) | 64.4 (38) | <0.01 |
| Menstrual Irregularities/PCOS | 43.2 (79) | 43.2 (79) | NA | NA |
| Fatty Liver Diseaseb | 36.9 (89) | 35.5 (65) | 41.4 (24) | 0.42 |
| Chronic Kidney Disease (any stage)c | 19.2 (43) | 20.8 (35) | 14.3 (8) | 0.28 |
| Binge Eating Disorderd | 15.4 (36) | 15.8 (28) | 14.0 (8) | 0.75 |
| GERDb | 14.5 (35) | 14.2 (26) | 15.5 (9) | 0.81 |
| Stress Urinary Incontinenceb | 14.5 (35) | 16.9 (31) | 6.9 (4) | 0.06 |
| Depressione | 14.0 (32) | 16.3 (28) | 7.1 (4) | 0.09 |
| Diabetes | 13.6 (33) | 12.6 (23) | 17.0 (10) | 0.39 |
| Asthma | 9.5 (23) | 7.1 (13) | 17.0 (10) | 0.02 |
| Blount’s Disease | 3.7 (9) | 2.2 (4) | 8.5 (5) | 0.04 |
| Pseudotumor Cerebri | 2.5 (6) | 2.2 (4) | 3.4 (2) | 0.64 |
| Cholelithiasis | 1.2 (3) | 1.1 (2) | 1.7 (1) | 0.57 |
| IWQOL-Kidsf | Total (N=233) | Females (n=176) | Males (n=57) | p |
|---|---|---|---|---|
| Total Score | ||||
| Mean (SD) | 62.9 (18.00) | 61.3 (17.35) | 68.0 (19.14) | <0.01 |
| Median (Q1, Q3) | 62.0 (50.0, 75.0) | 61.1 (50.0, 71.8) | 72.2 (55.6, 83.3) | |
| Physical Comfort | ||||
| Mean (SD) | 52.8 (25.05) | 52.1 (25.42) | 55.2 (23.91) | 0.26 |
| Median (Q1, Q3) | 54.2 (37.5, 70.8) | 50.0 (33.3, 70.8) | 58.3 (41.7, 70.8) | |
| Body Esteem | ||||
| Mean (SD) | 46.6 (27.78) | 42.6 (26.82) | 59.0 (27.28) | <0.01 |
| Median (Q1, Q3) | 44.4 (25.0, 69.4) | 41.7 (20.8, 58.3) | 63.9 (44.4, 80.6) | |
| Social Life | ||||
| Mean (SD) | 68.8 (24.04) | 69.2 (22.78) | 67.6 (27.76) | 0.68 |
| Median (Q1, Q3) | 70.8 (54.2, 87.5) | 70.8 (54.2, 87.5) | 70.8 (58.3, 91.7) | |
| Family Relations | ||||
| Mean (SD) | 91.5 (15.38) | 90.5 (16.73) | 94.8 (9.52) | 0.08 |
| Median (Q1, Q3) | 100.0 (91.7, 100.0) | 100.0 (87.5, 100.0) | 100.0 (95.8, 100.0) |
Q= Quartile, SD= Standard Deviation, PCOS= Polycystic Ovary Syndrome, GERD = Gastroesophageal Reflux Disorder.
P values are based on analyses comparing mean or median scores for females and males.
N=11 missing.
N=1 missing.
N=18 missing.
N=8 missing.
N=14 missing. Clinical range based on Beck Depression Inventory-II total raw score ≥ 17.
N=9 missing.
Comorbidity Prevalence
The most common comorbid conditions were dyslipidemia (74.4%), PAIN (58.3%), OSA (56.6%), and hypertension (45.0%; Table I). Compared with females, males more frequently had dyslipidemia (p = 0.01), OSA (p < 0.01), hypertension (p < 0.01), asthma (p < 0.01), and Blount’s disease (p < 0.05). PCOS/menstrual irregularities were common among females (43.2%). The prevalence of clinically elevated depressive and BED symptoms were 14% and 15.4%, respectively, with no observed sex differences. The CLoad was significantly greater for males (MCLoad= 4.3; range 1–8) than females (MCLoad = 3.9; range 1–9) (p = 0.01).
Weight-Related Quality of Life
Compared with males, females reported lower (i.e., greater impairment) IWQOL-Kids Total and Body-Esteem scores. (Table I; p < 0.01). IWQOL-Kids Total mean scores were significantly lower for Teen-LABS participants than published reference samples of healthy weight, overweight, and obese adolescents, and consistent (ie, not significantly different) than published reference samples of severely obese adolescents (Figure; p < 0.001). IWQOL-Kids Physical Comfort, Body Esteem, and Social Life mean scores were also significantly lower for Teen-LABS participants than healthy weight, overweight, and obese adolescent reference samples, and with one exception (Body-Esteem) consistent with levels reported in published reference samples of severely obese adolescents (Table II; available at www.jpeds.com). IWQOL-Kids Family Relations mean scores were significantly lower for Teen-LABS participants than healthy weight and overweight reference samples, but similar to that of obese and other severely obese adolescent reference samples.
Figure. IWQOL-Kids Total scores from published studies of healthy weight, overweight, obese, severely obese, and Teen-LABS adolescents.
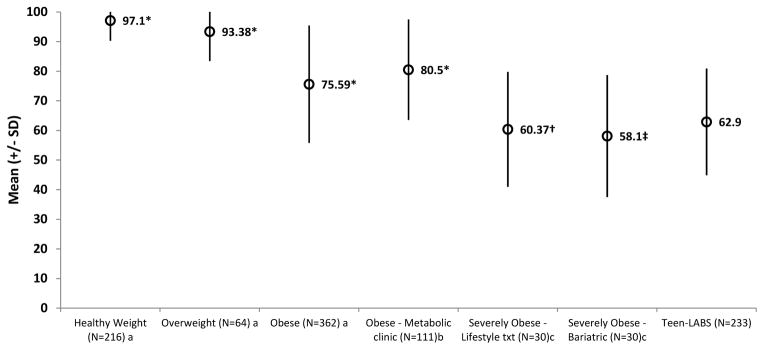
Note: Mean comparisons of each group to Teen-LABS adolescents were made using z-tests. * p ≤ 0.0001; † p = 0.50; ‡ p = 0.22
aPublished means and SDs of 216 healthy weight adolescents (BMI=11–84.9 percentile; Mage 14.1 ± 2.29 years; 53.2% female; 53.7% non-Hispanic White, 29.9% African American, 1.9% Hispanic, 14.5% Other), 64 overweight adolescents (BMI =85–94.9 percentile; Mage 13.52 ± 2.20 years; 57.8% female; 51.6% non-Hispanic White, 32.3% African American, 9.7% Hispanic, 6.5% Other), and 362 obese adolescents (BMI ≥ 95th percentile; Mage 14.04 ± 1.85 years; 63.8% female; 58.0% non-Hispanic White, 35.2% African American, 3.1% Hispanic, 3.7% Other) Kolotkin RL, Zeller MH, Modi AC, Samsa GP, Polanichka Quinlan N, Yanovski JA, et al. Assessing weight-related quality of life in adolescents. Obesity. 2006;14(3):448–57
bPublished means and SDs of 111 obese youth (BMI ≥ 95th percentile) presenting at a Pediatric Metabolic Syndrome clinic (Mage 14.1 ± 2.3 years; 59% female, MeanBMI 35.6 ± 7.1; 65% Non-Hispanic White, 23% Hispanic, 7% African American, 5% Other). Nadeau K, Kolotkin RL, Boex R, Witten T, McFann KK, Zeitler P, et al. Health-related quality of life in adolescents with comorbidities related to obesity. J Adolesc Health. 2011;49:90–2.
cPublished means and SDs of 30 severely obese adolescents presenting for pediatric lifestyle modification program (MeanBMI 51.31 ± 6.0; Mage 15.76 ± 1.2 years; 66.7% female; 83.3% non-Hispanic White, 16.7% non-Hispanic Black) and 30 severely obese adolescents prior to bariatric surgery (MeanBMI 61.81 ± 8.81; Mage 16.23 ± 1.34 years; 66.7% female; 83.3% non-Hispanic White, 16.7% non-hispanic Black). Modi AC, Loux TJ, Bell SK, Harmon CM, Inge TH, Zeller MH. Weight-specific health-related quality of life in adolescents with extreme obesity. Obesity. 2008;16(10):2266 – 71.
Table 2.
IWQOL-Kids mean scores from published studies of healthy weight, overweight, obese, and severely obese adolescents as compared to those of Teen-LABS participants.
| Healthy Weight adolescents a BMI 11th–84.9th percentile (N=216) | Overweight adolescents a BMI 84th–94.9th percentile (N=64) | Obese adolescents a BMI ≥ 95th percentile (N=362) | Obese adolescents Metabolic clinicb (N=111) | Severely Obese Adolescents Lifestyle Txt c BMI ≥ 40 (N=30) | Severely Obese Adolescents Bariatricc BMI ≥ 40 (N=30) | Teen-LABS Bariatric Adolescents BMI ≥ 40 (N=233) | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Total | |||||||
| Mean (SD) | 97.1 (6.82) | 93.38 (9.96) | 75.59 (19.82) | 80.5 (17.0) | 60.37 (19.4) | 58.1 (20.6) | 62.9 (18.00) |
| Z (p-value) d | −26.99 (p<0.001) | −17.77 (p<0.001) | −8.07 (p<0.001) | −8.81 (p<0.001) | 0.68 (p=0.50) | 1.22 (p=0.22) | |
|
| |||||||
| Physical Comfort | |||||||
| Mean (SD) | 98.8 (4.67) | 97.69 (6.73) | 78.34 (23.35) | 81.6 (21.1) | 51.67 (23.0) | 37.1 (24.1) | 52.8 (25.05) |
| Z (p-value) d | −27.52 (p<0.001) | −24.34 (p<0.001) | −12.46 (p<0.001) | −11.12 (p<0.001) | 0.25 (p=.80) | 3.34 (p=0.001) | |
|
| |||||||
| Body Esteem | |||||||
| Mean (SD) | 94.5(11.46) | 87.44 (18.84) | 63.84 (29.08) | 67.4 (27.0) | 45.37 (29.3) | 44.26 (30.9) | 46.6 (27.78) |
| Z (p-value) d | −24.19 (p<0.001) | −13.72 (p<0.001) | −7.25 (p<0.001) | −6.62 (p<0.001) | 0.22 (p=0.83) | 0.39 (p=0.69) | |
|
| |||||||
| Social Life | |||||||
| Mean (SD) | 98.1 (7.33) | 94.66 (11.02) | 75.86 (24.73) | 82.0 (22.5) | 65.83 (26.3) | 64.92 (29.6) | 68.8 (24.04) |
| Z (p-value) d | −17.74 (p<0.001) | −12.36 (p<0.001) | −3.46 (p=0.001) | −4.97 (p<0.001) | 0.59 (p=0.56) | 0.69 (p=0.49) | |
|
| |||||||
| Family Relations | |||||||
| Mean (SD) | 98.6 (6.77) | 96.98 (7.68) | 90.45 (16.83) | 91.4 (13.8) | 86.1 (17.0) | 92.2 (13.0) | 91.5 (15.38) |
| Z (p-value) d | −6.41 (p<0.001) | −3.94 (p<0.001) | 0.78 (p=0.43) | 0.06 (p=0.95) | 1.65 (p=0.10) | −0.27 (p=0.79) | |
SD= standard deviation
Published means and SDs of 216 healthy weight adolescents (BMI=11–84.9 percentile; Mage 14.1 ± 2.29 years; 53.2% female; 53.7% non-Hispanic White, 29.9% African American, 1.9% Hispanic, 14.5% Other), 64 overweight adolescents (BMI =85–94.9 percentile; Mage 13.52 ± 2.20 years; 57.8% female; 51.6% non-Hispanic White, 32.3% African American, 9.7% Hispanic, 6.5% Other), and 362 obese adolescents (BMI ≥ 95th percentile; Mage 14.04 ± 1.85 years; 63.8% female; 58.0% non-Hispanic White, 35.2% African American, 3.1% Hispanic, 3.7% Other) Kolotkin RL, Zeller MH, Modi AC, Samsa GP, Polanichka Quinlan N, Yanovski JA, et al. Assessing weight-related quality of life in adolescents. Obesity. 2006;14(3):448–57
Published means and SDs of 111 obese youth (BMI ≥ 95th percentile) presenting at a Pediatric Metabolic Syndrome clinic (Mage 14.1 ± 2.3 years; 59% female, MeanBMI 35.6 ± 7.1; 65% Non-Hispanic White, 23% Hispanic, 7% African American, 5% Other). Nadeau K, Kolotkin RL, Boex R, Witten T, McFann KK, Zeitler P, et al. Health-related quality of life in adolescents with comorbidities related to obesity. J Adolesc Health. 2011;49:90–2.
Published means and SDs of 30 severely obese adolescents presenting for pediatric lifestyle modification program (MeanBMI 51.31 ± 6.0; Mage 15.76 ± 1.2 years; 66.7% female; 83.3% non-Hispanic White, 16.7% non-Hispanic Black) and 30 severely obese adolescents prior to bariatric surgery (MeanBMI 61.81 ± 8.81; Mage 16.23 ± 1.34 years; 66.7% female; 83.3% non-Hispanic White, 16.7% non-hispanic Black). Modi AC, Loux TJ, Bell SK, Harmon CM, Inge TH, Zeller MH. Weight-specific health-related quality of life in adolescents with extreme obesity. Obesity. 2008;16(10):2266 – 71.
Mean comparisons of each group relative to Teen-LABS adolescents were made using Z-tests.
Predictive Models of WRQOL
IWQOL-Kids Total Score
A significant negative relationship was found between CLoad and Total score among males (β=−4.30; p<0.01; Table III), and approached significance for females (β=−1.32; p=0.06). In females, increasing BMI, white race, BED, and depressive symptoms were independently associated with lower Total scores. Presence of depressive symptoms in males was also associated with lower Total scores.
Table 3.
Evaluation of the impact of comorbidity load and hypothesized predictors on IWQOL-Kids scores for Teen-LABS participants by sex.
| FEMALES | Total | Physical Comfort | Body Esteem | Social Life | Family Relationsa | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P Value | β (95% CI) | P Value | β (95% CI) | P Value | β (95% CI) | P Value | β (95% CI) | P Value | |
| CLoad | −1.32 (−2.70, 0.06) | 0.06 | −1.46 (−3.46, 0.55) | 0.15 | −1.08 (−3.11, 0.95) | 0.30 | −1.81 (−3.72, 0.10) | 0.06 | −0.04 (−0.07, −0.01) | 0.01 |
| BMI | −0.30 (−0.60, −0.01) | 0.04 | −1.03 (−1.43, −0.63) | < 0.01 | b | b | b | |||
| White race | −5.98 (−11.21, −0.75) | 0.03 | b | −10.37 (−18.65, −2.09) | 0.01 | b | b | |||
| DEPc | −15.11 (−21.53, −8.68) | < 0.01 | −10.34 (−19.64, −1.03) | 0.03 | −23.76 (−34.19, −13.13) | <0.01 | −12.44 (−21.81, −3.07) | <0.01 | −0.20 (−0.33, −0.08) | <0.01 |
| BED | −10.00 (−16.43, −3.56) | <0.01 | −14.83 (−24.24, −5.41) | <0.01 | −10.84 (−21.18, −0.51) | 0.04 | −10.25 (−19.59, −0.91) | 0.03 | b | |
| PAIN | b | b | b | b | b | |||||
| GERD | b | b | b | b | −0.15 (−0.29, −0.01) | 0.03 | ||||
| OSA | b | b | b | b | b | |||||
| Pseudo-R2 | 0.27 | 0.24 | 0.21 | 0.11 | 0.08 | |||||
| MALES | ||||||||||
| CLoad | −4.30 (−7.04, −1.56) | <0.01 | −5.47 (−8.82, −2.12) | <0.01 | −3.28 (−7.51, 0.95) | 0.13 | −4.82 (−9.00, −0.65) | 0.02 | −0.02 (−0.04, −0.004) | 0.02 |
| BMI | b | −0.93 (−1.42, −0.45) | <0.01 | b | b | b | ||||
| White race | b | b | b | b | b | |||||
| DEPc | −21.69 (−38.98, −4.40) | 0.01 | b | −31.44 (−37.90, −23.09) | 0.02 | −30.54 (−56.85, −4.22) | 0.02 | b | ||
| BED | b | b | b | b | b | |||||
| PAIN | b | −9.84 (−20.24, 0.56) | 0.06 | b | b | b | ||||
| GERD | b | b | b | b | b | |||||
| OSA | b | b | b | b | b | |||||
| Pseudo-R2 | 0.28 | 0.50 | 0.16 | 0.21 | 0.09 | |||||
BED= Binge Eating Disorder; BMI= Body Mass Index; CLoad= Comorbidity Index; DEP= Depression; GERD = Gastroesophageal Reflux Disorder; OSA= Obstructive Sleep Apnea; PAIN = Joint or Back Pain.
All listed variables were considered for inclusion in the models. Multivariable mixed-effect linear regression models, with clinical site as a random effect, were completed with imputed datasets (M=25) for females and males. P values are for those variables retained in the final model. Pseudo-R2 values refer to the proportion of the variance in the outcome explained by the set of predictor variables.
Log transformed.
Not included in final model.
Clinical range based on the Beck Depression Inventory-II total raw score ≥ 17.
Physical Comfort
CLoad was negatively associated with Physical Comfort scores in males (β=−5.47; p<0.01) but not females (p=0.15). Other significant predictors of decreased Physical Comfort scores were BMI, BED, and depressive symptoms in females, and BMI in males.
Body Esteem
CLoad was not significantly associated with Body Esteem scores in males (p=0.13) or females (p=0.30). White race and presence of BED and depressive symptoms were independently associated with decreased Body Esteem scores in females. Only presence of depressive symptoms was associated with lower Body Esteem scores in males.
Social Life
In males, CLoad was significantly associated with decreased Social Life scores (β=−4.82; p=0.02), and approached significance in females (β=−1.81; p=0.06). Presence of BED and depressive symptoms in females were also significantly associated with lower Social Life scores. In males, only presence of depressive symptoms was significantly associated with lower Social Life scores.
Family Relations
CLoad was a significant predictor of family relations score in both females (p=0.01) and males (p=0.02). Presence of GERD and depressive symptoms in females were each associated with a lower score in Family Relations.
The Role of Individual Comorbidities on WRQOL
Regression analyses examined the relative impact of each individual comorbidity (15 for males, 16 for females), after accounting for BMI and Race, on WRQOL outcomes for each sex. The CLoad was excluded from models (Table IV).
Table 4.
Exploration of the impact of BMI, race, and individual comorbid conditions on IWQOL-Kids scores for Teen-LABS participants by sex.
| FEMALES | Total | Physical Comfort | Body Esteem | Social Life | Family Relationsa | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value | |
| BMI | −0.34 (−0.62, −0.07) | 0.02 | −1.06 (−1.45, −0.67) | <0.01 | b | b | b | |||
| White race | −6.68 (−11.88, −1.49) | 0.01 | b | −10.47 (−18.68, −2.27) | 0.01 | b | b | |||
| DEPc | −15.69 (−22.09, −9.29) | <0.01 | −9.79 (−19.08, −0.50) | 0.04 | −23.94 (−34.24, −13.65) | <0.01 | −12.05 (−21.31, −2.78) | 0.01 | −0.19 (−0.32, −0.06) | <0.01 |
| BED | −10.07 (−16.53, −3.62) | <0.01 | −14.42 (−23.78, −5.07) | <0.01 | −10.85 (−21.15, −0.56) | 0.04 | −9.88 (−19.21, −0.54) | 0.04 | b | |
| PAIN | b | b | b | b | b | |||||
| OSA | b | b | b | b | −0.11 (−0.21, −0.01) | 0.03 | ||||
| PTC | b | b | b | −22.88 (−44.49, −1.28) | 0.04 | b | ||||
| CKD | b | b | b | −10.16 (−18.47, −1.86) | 0.02 | b | ||||
| Asthma | b | b | b | b | b | |||||
| CHOLITH | b | b | b | b | b | |||||
| SUI | b | −10.20 (−18.68, −1.71) | 0.02 | b | b | b | ||||
| MENST | −4.26 (−8.79, 0.27) | 0.07 | b | −6.45 (−13.62, 0.72) | 0.08 | b | b | |||
| Pseudo-R2 | 0.27 | 0.26 | 0.21 | 0.14 | 0.08 | |||||
| MALES | Total | Physical Comfort | Body Esteem | Social Life | Family Relationsa | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| BMI | −0.49 (−0.87, −0.10) | 0.01 | −1.17 (−1.63, −0.71) | <0.01 | b | b | b | |||
| DEPc | −25.02 (−41.34, −8.72) | <0.01 | b | −35.76 (−61.04, −10.47) | <0.01 | −36.40 (−60.63, −12.16) | <0.01 | −0.12 (−0.22, −0.02) | 0.02 | |
| BED | b | b | b | b | b | |||||
| PAIN | −9.19 (−17.75, −0.63) | 0.04 | −15.53 (−25.17, −5.90) | <0.01 | b | b | b | |||
| GERD | b | −18.20 (−31.90, −4.50) | <0.01 | b | b | b | ||||
| DYSLIPID | −10.79 (−23.11, 1.53) | 0.09 | b | −17.73 (−37.69, 2.24) | 0.08 | −19.28 (−37.69, −0.87) | 0.04 | b | ||
| SUI | −31.11 (−40.84, −18.94) | <0.01 | b | −33.17 (−63.38, −2.95) | 0.03 | −37.93 (−69.97, −5.88) | 0.02 | b | ||
| Diabetes | b | b | b | −15.81 (−34.04, 2.04) | 0.09 | b | ||||
| CKD | b | b | b | b | b | |||||
| CHOLITH | b | b | b | b | −0.43 (−0.62, −0.23) | <0.01 | ||||
| Pseudo-R2 | 0.44 | 0.47 | 0.24 | 0.37 | 0.31 | |||||
BMI= Body Mass Index; BED= Binge Eating Disorder; CHOLITH= Cholelithiasis; CKD= Chronic Kidney Disease; DEP= Depression; DYSLIPID= Dyslipidemia; PAIN = Joint or Back Pain; GERD = Gastroesophageal Reflux Disorder; MENST= Menstrual Irregularities and polycystic ovary disease; OSA= Obstructive Sleep Apnea; PTC= Pseudotumor Cerebri; SUI=Stress Urinary Incontinence
Note: Variables not retained in the final models for any of the quality of life domains were not included in the table. For females: Diabetes, Fatty Liver Disease (FLD), Hypertension (Hyperten), DYSLIPID, GERD, and Blount’s Disease (Blounts). For males: White race, OSA, PTC, FLD, Hyperten, Asthma, and Blounts. Multivariable mixed-effect linear regression models, with clinical site as a random effect, were completed with imputed datasets (M=25) for females and males. Pseudo-R2 values refer to the proportion of the variance in the outcome explained by the set of predictor variables.
Log transformed.
Not included in final model.
Clinical range based on the Beck Depression Inventory-II total raw score ≥ 17
For females, significant predictors of the Total score were the same as the main model findings (BMI, White race, BED, DEP). For Physical Comfort, SUI emerged as a new significant negative predictor. Presence of PTC and CKD emerged as new significant negative predictors of Social Life. OSA emerged as the only new significant predictor of Family Relations.
For males, many new comorbid conditions emerged as significant predictors of WRQOL in the absence of CLoad. Specifically, BMI, PAIN, and SUI were new significant negative predictors for the Total score, and PAIN and GERD emerged as significant negative predictors for Physical Comfort. SUI was a significant predictor of Body Esteem, and dyslipidemia and SUI were new significant negative predictors of Social Life. Finally, depressive symptoms and cholelithiasis emerged as significant negative predictors of Family Relations.
Discussion
Prior to WLS, Teen-LABS adolescents present with significant impairment in WRQOL. Severity of impairments were most notable for weight-related physical comfort (eg, fitting into public seating, bending over), body esteem (eg, shame and avoidance of activities due to weight), and social life (eg, weight-based teasing, social exclusion). In contrast, weight-related family relations (eg, weight-based exclusion, stigmatization, shame) are an area of relative strength also reported in previous studies characterizing obese youth engaged in treatment.(7, 32, 33) These large multi-site study findings demonstrate the high costs to day-to-day life associated with severe obesity for the adolescent WLS patient.
Teen-LABS males and females differed in their clinical presentation prior to surgery. Relative to females, males had a greater degree of severe excess weight, a greater overall comorbidity load, a higher prevalence of specific comorbid conditions (ie, dyslipidemia, hypertension, OSA), and yet better overall WRQOL and body-esteem. Similar to recent reports of adults, (37) adolescent males proceeded to WLS with greater disease burden, yet lower perceived burden on their day-to-day life relative to females.
The number of medical comorbidities was a significant predictor of WRQOL for males across the majority of domains, whereas for females the relative impact of comorbidities was less substantial or inconsistent. However, for both sexes, the cumulative burden of managing multiple comorbid conditions had a clear impact on the adolescent’s perception of less positive family relations. Higher BMI and depressive symptoms also were predictors of worse WRQOL. For example, a dose response effect emerged, whereby increasing BMI values were associated with lower Total WRQOL and greater physical discomfort. Chronic pain and binge eating behaviors emerged as unique predictors for male and female WRQOL, respectively. Study findings also highlight several specific comorbid conditions, that have not been previously examined, and their significant impact on adolescent WRQOL, including stress urinary incontinence on both sexes.
With rising rates of severe obesity, (1, 38) understanding the clinical needs of this patient population prior to surgery and across the post-operative course is critically important. The present data highlight clinical concerns of the patient prior to surgery and factors that make those concerns more prominent. For males, physical health, and particularly when comorbid conditions “add up,” appear to play a central role in their daily lives. In contrast, the degree of excess of weight and psychological comorbidities are most salient to female WRQOL. These patient-reported data supplement medical and psychological comorbidity status by highlighting targets for education, support, and potential adjunctive care referrals prior to WLS.
These findings may also have implications for understanding heterogeneity in adolescent post-operative WRQOL outcomes following WLS. Improvement in WRQOL may be more substantial for those who experience better weight loss outcomes.(39, 40) However, for males WRQOL improvement may also vary based on comorbidity load and the degree to which comorbidities are reduced over time. Although not a psychosocial intervention, improvement in depressive symptoms or binge eating behaviors following WLS may prove to be important secondary outcomes that positively impact female WRQOL. However, the adult WLS experience also suggests potential links between these pre-operative psychological comorbid conditions and/or their persistence or post-operative recurrence with poorer weight loss as well as HRQOL outcomes.(41–43) Thus, adolescent symptom profiles should be closely monitored and referrals provided accordingly to optimize patient outcomes.
Strengths of the present study include standardized data collection and clinical definitions and the use of a validated adolescent WRQOL tool. There are also several limitations that suggest directions for future research. Even though Teen-LABS’ high recruitment rate (95%) indicates these data represent the health status and WRQOL typical of this clinical population, these findings may not represent those adolescents with severe obesity who pursue yet do not progress to surgery, those who seek non-surgical intervention, or those who seek no intervention at all. Although consistent with adult surgery trends (37), the Teen-LABS patient population is primarily White and female, resulting in limited information on other race/ethnic groups for whom severe obesity is also a significant concern.(44) Further, published reference samples of adolescents available and used to illustrate the severity of WRQOL impairment in Teen-LABS participants were not demographically matched. Data definitions of 16 comorbid conditions used consistent criteria to create dichotomous presence/absence values, but did not consider more qualitative aspects of each comorbid condition such as the severity of current symptoms, or the condition’s typical course (e.g., progressive, constant, episodic), or potential outcome (e.g., life-shortening, nonfatal). Further, there are likely other psychosocial factors that contribute to WRQOL impairment in severely obese teens (eg, teasing (45), social support.(15)).
Abbreviations
- BED
Binge Eating Disorder
- BMI
Body Mass Index
- CKD
Chronic Kidney Disease
- C-Load
Cumulative Comorbidity Load
- DEP
Depressive Symptom
- HRQOL
Health Related Quality of Life
- OSA
Obstructive Sleep Apnea
- PAIN
Chronic Back or Joint Pain
- SUI
Stress Urinary Incontinence
- WLS
Weight Loss Surgery
- WRQOL
Weight-Related Quality of Life
Appendix
Members of the of the Teen-LABS Consortium include: Cincinnati Children’s Hospital Medical Center (Cincinnati, OH): Victor Garcia, MD, Jennie Noll, PhD, Rosie Miller, RN, CCRC, Rachel Akers, MPH, April Carr, BS, Lindsey Shaw, MS, Cynthia Spikes, CRC, Shelley Kirk, PhD, RD, Linda Kollar, RN, Christine Wright, RN, Stephen Daniels, MD, PhD, Jennifer Andringa, BS, Laurie Bishop, MA, MS, Carolyn Powers, RD, Michelle Starkey Christian, Yanhong Liu, MS, Tawny W. Boyce, MS, MPH, Tara Schafer-Kalkhoff, MA, CCRP, Suzanne Summer, MS, RD; University of Cincinnati (Cincinnati, OH): Mark Simmons, MEng; Texas Children’s Hospital, Baylor Medical Center: Vadim Sherman, MD, Margaret Callie Lee, MPH, David Allen, BS, Trisha Walters-Salas, BSN, Susanne Trout, RD, Gia Washington, PhD; Children’s Hospital of Alabama University of Alabama: Ronald Clements, MD, Richard Stahl, MD, Molly Bray, PhD, Beverly Haynes, BSN, Constance Cushing, DPT; University of Pittsburgh Medical Center: Ramesh Ramanathan, MD, Carol A. McCloskey, MD, George M. Eid, MD, Jessie Eagleton, MPH, William Gourash, MSN, CRNP, Lindsay Lee, MS, RD, Sheila Pierson, BS, Catherine Gibbs, MS, Dana Farrell, BS, Christopher Coburn, PhD, Rebecca Search, MPH, Mark Shaw, MS; Nationwide Children’s Hospital Medical Center: Steven Teich, MD, Allen Browne, MD, Karen Carter, CCRC, Melinda Helton, RN, Bonny Bowen, RN, Cynthia Yensel, RN, MS, Patsy Guittar, MSN, Deanna Lear, RN, MS, Robert David Murray, MD, Ihouma Eneli, MD, Andrea Hedge, Kevin Smith, PhD, Grace Wentzel, CCRP, Paula Davies, CCRC.
Appendix 2
The Teen-LABS Consortium was funded by cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases (U01DK072493, UM1DK072493, UL1 TR000077-04 [to Cincinnati Children’s Hospital Medical Center]; UM1DK095710 [to University of Cincinnati]; UL1RR025755 [to Nationwide Children’s Hospital]; M01-RR00188 [to Texas Children’s Hospital/Baylor College of Medicine]; UL1 RR024153 and UL1TR000005 [to University of Pittsburgh]; and UL1 TR000165 [to University of Alabama, Birmingham]). T.I. has received research grant funding from Ethicon Endosurgery. C.H. has served on an Advisory Panel for Stryker Corporation. A.C. has received research grants from Allergan, Pfizer, Covidien, EndoGastric Solutions, and Nutrisystem, and is on the Scientific Advisory Board of Ethicon J and J Healthcare System. M.M. has received research grant funding from Allergan Medical Corporation and serves as a proctor and speaker for Covidien. The other authors declare no conflicts of interest.
Appendix 3
The Teen-LABS Consortium used a prospective, observational cohort design in which the majority of the data are collected at the same time that routine clinical care of bariatric patients is occurring.
A synopsis of the study protocol, including study design features, inclusion/exclusion criteria, consent/assent, study visits and procedures, physical measurements, laboratory data collection, and other clinical and self-report data collection was previously published1. In addition, methodologic details pertinent specifically to the analyses presented in this article are provided below.
Baseline data elements were collected within 30 days prior to operation: (1) participant identifier (alpha-numeric code); (2) date of consent to participate in Teen-LABS; (3) month and year of birth, sex, height, weight, race, ethnicity; (4) measured height and weight and vital signs (blood pressure); (5) comorbidities; (6) polysomnography (sleep study) data files if available and performed within 12 months of baseline evaluation; and (7) participant self-report on screening measures for depressive symptoms, binge eating, stress urinary incontinence, and weight-related quality of life (Appendix 4; available at www.jpeds.com)
Blood (70mL) and urine (3.5mL) were obtained for research purposes from adolescent participants who were undergoing bariatric surgery. Biochemical testing was performed as described previously1 using fresh specimens at the central laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratories, Seattle, WA).
Definitions of comorbidities used in the analyses
The presence or absence of comorbid conditions was assessed in various ways. A Teen-LABS-certified clinical coordinator or investigator used information (medical records, physical exam, patient interview, and laboratory values) to determine presence or absence of each condition based on standard definitions.
Hypertension was defined as having systolic or diastolic blood pressure ≥ 95th percentile indexed to age, sex, and height during the baseline visit as measured using the standard Teen-LABS protocol, or current use of anti-hypertensive medication.
Specifically, the data for this variable were obtained/analyzed as follows: (1) ≥ 95th percentile of systolic or diastolic indexed to age, sex, and height (Anthropometric [ANTH] form); or (2) Comorbidity Assessment-Baseline (CAB) form, Question 1 – selection equals: “hypertension, treatment with single medication”, or “hypertension, treatment with two or more medications”; or (3) Medications (MED) form, subject-reported use of antihypertensive medications
The presence of dyslipidemia was defined as triglyceride value ≥ 130 mg/dL or LDL cholesterol ≥ 130 mg/dL or HDL cholesterol < 40 mg/dL or use of medication for dyslipidemia. Specifically, the data for this variable were obtained/analyzed as follows: central laboratory measured triglyceride, LDL cholesterol, HDL cholesterol; or (2) Comorbidity Assessment-Baseline (CAB) form, Question 5 – selection equals: “treatment with single medication for dyslipidemia” or “treatment with two or more medications for dyslipidemia”; or (3) Medications (MED) form, subject-reported use of any antilipemic Rx.
The presence of fatty liver disease (FLD) was presumed in the presence of abnormally elevated serum aminotransferases (ALT, AST, or GGT) or if imaging suggested steatosis, or if biopsy confirmed hepatic steatosis, steatohepatitis, or more advanced fibrosis. Specifically, the data for this variable were obtained/analyzed as Comorbidity Assessment-Baseline (CAB) form, Question 13 – selection equals: “abnormal serum aminotransferases (ALT,AST, or GGT)” or “Imaging suggesting steatosis” or “Biopsy confirmed hepatic steatosis” or “Biopsy confirmed steatohepatitis” or “Biopsy confirmed cirrhosis, compensated” or “Decompensated cirrhosis (end-stage liver disease with synthetic dysfunction)”
Obstructive sleep apnea syndrome (OSAS) was defined as provider-diagnosed OSAS. For this assessment, surgical investigators took into account diagnostic polysomnogram findings, or use of continuous positive airway pressure (CPAP). Specifically, the data for this variable were obtained/analyzed as Comorbidity Assessment-Baseline (CAB) form, Question 9 – selection equal: “Yes.”
Diabetes was defined by study investigators taking into consideration patient self-report of prior diagnosis, use of medications for diabetes, baseline HbA1c of ≥6.5%, or fasting glucose of at least 126 mg/dL, or oral glucose tolerance results in prior 6 months.
Specifically, the data for this variable were obtained/analyzed as follows: (1) Preoperative (PO) form, question 9b; (2) Comorbidity Assessment-Baseline (CAB) form, question 6c, 6d, 7, 18; (3) Medical Assessment – Baseline (MAB) form, question 14; (4) all declared medications from the MED form; and (5) central lab measured baseline fasting glucose and HbA1c values.
The data for polycystic ovary syndrome/Menstrual Irregularities represented the when a female met definition(s) for polycystic ovary syndrome (PCOS) and/or experienced menstrual irregularities (PCOS). Presence of PCOS was defined as presence of symptoms of PCOS (features of hirsutism) with treatment with contraceptive or anti-androgens, or, if confirmed by measured androgen levels, the condition was present even whether or not using contraceptives, anti-androgens, or metformin. Specifically, the data for this variable were obtained/analyzed as follows Co-morbidity Assessment-Baseline (CAB) form, question 18 – selection equals: “Symptoms of PCOS present, treatment with contraceptive or anti-androgens” or “Confirmed PCOS, no treatment” or “Confirmed PCOS, treatment with contraceptive or anti-androgens” or “Confirmed PCOS, treatment with metformin” or “Combination treatment (contraceptives, anti-androgens, metformin)”
The presence of menstrual irregularities was by the presence or history of irregular menses. Specifically, the data for this variable were obtained/analyzed as follows: Comorbidity Assessment-Baseline (CAB) form, Question 17 – selection equals: “Irregular menses or oligomenorrhea (> 45 days)” or “History of irregular menses or oligomenorrhea but now on contraceptives.”
The presence of chronic kidney disease (CKD) was defined using glomerular filtration rate (GFR), determined by cystatin C levels, where GFR=77.24 x (Cys C)−1.2623; microalbuminuria was defined as urine albumin to creatinine ratio > 0.03; CKD stages were defined as follows: (1) normal = GFR>60 and no microalbuminuria; (2) CKD stage 1 = Microalbuminuria with GFR ≥ 90; (3) CKD stage 2 = Microalbuminuria with GFR of 60–89; (4) CKD Stage 3 = GFR of 30–59; (5) CKD stage 4 = GFR of 15–29; and (6) CKD stage 5 = GFR < 15.
Presence of pseudotumor cerebri (PTC) was defined as having a prior physician diagnosis of PTC, with or without use of medications or cerebrospinal fluid drainage. Specifically, the data for this variable were obtained/analyzed as Co-morbidity Assessment-Baseline (CAB) form, question 19 – selection equals: “Confirmed PTC, no medications” or “Confirmed PTC, medications used (eg, diuretics)” or “CSF drainage required” or “Persistent symptoms despite medications or drainage.”
Presence of Blount disease was defined as having a prior physician diagnosis as determined from chart review or participant self-report. Specifically, the data for this variable were obtained/analyzed as follows: (1) Preoperative (PO) form, question 9o – selection equals: “Yes”; or (2) Surgeon’s Medical Assessment – Baseline (SMAB) form, question 37 – selection equals: “Yes.”
Presence of chronic pain was defined as meeting definition(s) for joint pain and/or back pain. Presence of joint pain was defined as pain in hips or lower extremity joints with ambulation once a week or less, or pain requiring non-narcotic analgesia used regularly (at least monthly) or narcotic analgesia used regularly (at least monthly). Specifically, the data for this variable were obtained/analyzed as Co-morbidity Assessment-Baseline (CAB) form, question 14 – selection equals: “Pain with ambulation once a week or less” or “Pain with ambulation more than once a week” or “Non-narcotic analgesia used regularly (weekly or monthly)” or “Non-narcotic analgesia used frequently (more than once a week)” or “Narcotic analgesia used regularly (weekly or monthly)” or “Narcotic analgesia used frequently (more than once a week)”.
Presence of back pain was defined as intermittent back pain, not requiring medication or treatment or requiring non-narcotic analgesia regularly (at least monthly), or narcotic analgesia used regularly (at least monthly). Specifically, the data for this variable were obtained/analyzed as Co-morbidity Assessment-Baseline (CAB) form, question 15 – selection equals: “Intermittent back pain, not requiring medication or treatment” or “Nonnarcotic analgesia used regularly (weekly or monthly)” or “Non-narcotic analgesia used frequently (more than once per week)” or “Narcotic analgesia used regularly (weekly or monthly)” or “Narcotic analgesia used frequently (more than once per week).”
Presence of asthma was defined using symptoms present as well as frequency of medication use. Specifically, the data for this variable were obtained/analyzed as Comorbidity Assessment-Baseline (CAB) form, Question 10a – selection equals: “Symptoms present, non-steroid medication used monthly but less than weekly” or “Symptoms present, non-steroid medication used weekly but less than daily” or “Symptoms present, non-steroid medication used daily” or “Symptoms persist with non-steroid medication.”
Presence of gastroesophageal reflux disease (GERD) was determined via formal diagnostic testing and/or presence of symptoms and medication use. Specifically, the data for this variable were obtained/analyzed as follows: (1) Comorbidity Assessment-Baseline (CAB) form, Question 11a – selection equals: “Yes” and CAB form, Question 11a1 – selection equals: “Positive”; or (2) Comorbidity Assessment-Baseline (CAB) form, Question 11b – selection equals: “H2 blockers used daily” or “Proton pump inhibitor used daily” or “Continued symptoms despite regular use of medications.”
The presence of cholelithiasis was determined by documentation of gallstones or documented complications from gallstones. Specifically, the data for this variable were obtained/analyzed as Comorbidity Assessment-Baseline (CAB) form, Question 12 – selection equals: “Documented gallstones with less than monthly symptoms” or “Documented gallstones with weekly or monthly symptoms” or “Documented gallstones with daily symptoms” or “Documented complications of gallstones (eg, pancreatitis).”
The presence of stress urinary incontinence was determined by frequency of symptoms. Specifically, the data for this variable were obtained/analyzed as follows: (1) Comorbidity Assessment-Baseline (CAB) form, question 16 – selection equals: “Weekly symptoms (once or more each week)” or “Daily symptoms (once or more each day)”; or (2) Urinary Incontinence-Baseline (UIB) form, question 3a – response ≥ 1 time per week.
The presence of binge eating disorder was determined using participant self-report of screening criteria from the Questionnaire of Eating and Weight Patterns, Revised.2 Specifically, the data for this variable were obtained/analyzed as follows: (1) Baseline Behavior (BB) form, Question 3 – selection equals: “Yes”; and (2) Baseline Behavior (BB) form, Question 3.1 – selection equals: “Two or three days a week” or “Four or five days a week” or “Nearly every day”; and (3) Baseline Behavior (BB) form, Question 3.2 – “Yes” to 3 or more of the following: “Eating much more rapidly than usual”; “Eating until you felt uncomfortably full”; ‘Eating large amounts of food when you didn’t feel physically hungry”; “Eating alone because you were embarrassed by how much you were eating”; “Feeling disgusted with yourself, depressed, or feeling very guilty after overeating”; and (4) Baseline Behavior (BB) form, Question 3.3 – selection equals: “Moderately” or “Greatly” or “Extremely.”
The presence of depressive symptoms in the clinical range was determined using the cutpoint of a total raw score of ≥ 17 on participant self-report on the Beck Depression Inventory-II.3
- 1.Inge TH, Zeller MH, Jenkins TM, et al. Perioperative Outcomes of Adolescents Undergoing Bariatric Surgery: The Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) Study. JAMA Pediatr. 2014;168(1):47–53. doi: 10.1001/jamapediatrics.2013.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spitzer RL, Yanovski SZ, Marcus MD. The Questionnaire of Eating and Weight Patterns-Revised (QEWP-R, 1993) New York State Psychiaric Institute; 722 West 168th Street, New York, NY 10032: 1993. [Google Scholar]
- 3.Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. San Antonio, TX: Psychological Corp.; 1996. [Google Scholar]
Appendix 4. Data collection forms
| Enrollment | Baseline | ||
|---|---|---|---|
| EF | Enrollment form | x | |
| PO | Pre-op Form | x | |
| ANTH | Anthropometrics | x | |
| RCAB | Research Coordinator Assessment-Baseline | x | |
| CAB | Comorbidity Assessment – Baseline | x | |
| SMAB | Surgeon Medical Assessment - Baseline | x | |
| MAB | Medical Assessment – Baseline | x | |
| CDI | Caregiver Demographic Inventory | x | |
| MED | Medication Form | x | |
| UIB | Urinary Incontinence Form | x | |
| BB | Behavior Baseline Form | x | |
| BDI | Beck Depression Inventory | x | |
| IWQOL | Impact of Weight on Quality of Life-Kids | x | |
Footnotes
Portions of the study were presented as a poster at Obesity Week, November 2013, Atlanta, GA.
Funding and conflict of interest information is available at www.jpeds.com (Appendix 2).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Skinner AC, Skelton JA. Prevalence and Trends in Obesity and Severe Obesity Among Children in the United States, 1999–2012. JAMA Pediatr 2014. 2014 Apr 7; doi: 10.1001/jamapediatrics.2014.21. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Inge TH, King WC, Jenkins TM, Courcoulas AP, Mitsnefes M, Flum DR, et al. The Effect of Obesity in Adolescence on Adult Health Status. Pediatrics. 2013;132(6):1098–104. doi: 10.1542/peds.2013-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schipper H, Clinch JJ, Olweny CL. Quality of life studies: Definitions and conceptual issues. In: Spilker B, editor. Quality of life and pharmacoeconomics in clinical trials. 2. Philadelphia: Lippincott-Raven; 1996. pp. 11–23. [Google Scholar]
- 4.Ingerski LM, Modi AC, Hood KK, Pai AL, Zeller MH, Piazza-Waggoner C, et al. Health-related quality of life across pediatric chronic conditions. J Pediatr. 2010;156(4):639–44. doi: 10.1016/j.jpeds.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guyatt GH, Jaeschke R, Feeny DH, Patrick DL. Measurments in clinical trials: Choosing the right approach. In: Spilker B, editor. Quality of Life and Pharmacoeconomics in Clinical Trials. 2. Minnetonka: Lippincott Williams & Wilkins; 1996. pp. 41–7. [Google Scholar]
- 6.Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res. 2001;9:102–11. doi: 10.1038/oby.2001.13. [DOI] [PubMed] [Google Scholar]
- 7.Kolotkin RL, Zeller MH, Modi AC, Samsa GP, Polanichka Quinlan N, Yanovski JA, et al. Assessing weight-related quality of life in adolescents. Obesity. 2006;14(3):448–57. doi: 10.1038/oby.2006.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Special Issue: The FDA Guidance for Patient-Reported Outcomes. Value in Health. 2007;(Supplement s2):s59–s147. [Google Scholar]
- 9.U.S. Department of Health and Human Services Food and Drug Administration. [Accessed December 10, 2013];Guidance for Industry: Patrient-Reported OUtcome Measures: Use in Medical Product Development to Support Labeling Claims. http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf.
- 10.Calvert M, Brundage M, Jacobsen PB, Schunemann HJ, Efficace F. The CONSORT Patient-Reported Outcome (PRO) extension: implications for clinical trials and practice. Health Qual Life Outcomes. 2013;11(1):184. doi: 10.1186/1477-7525-11-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Healthy People 2020. Healthy People 2020 Framework. The Vision, Mission, and Goals of Healthy People 2020. [Accessed December 4, 2013];Overarching Goals. http://healthypeople.gov/2020/Consortium/HP2020Framework.pdf [PDF - 254KB]
- 12.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3:329–41. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Schwimmer JB, Burwinkle TM, Varni JW. Health-related quality of life of severely obese children and adolescents. JAMA. 2003;289(14):1813–9. doi: 10.1001/jama.289.14.1813. [DOI] [PubMed] [Google Scholar]
- 14.Williams J, Wake M, Hesketh K, Maher E, Waters E. Health-related quality of life of overweight and obese children. JAMA. 2005;293(1):70–6. doi: 10.1001/jama.293.1.70. [DOI] [PubMed] [Google Scholar]
- 15.Zeller MH, Modi AC. Predictors of health-related quality of life in obese youth. Obes Res. 2006;14(1):122–30. doi: 10.1038/oby.2006.15. [DOI] [PubMed] [Google Scholar]
- 16.Janicke DM, Marciel KK, Ingerski LM, Novoa W, Lowry KW, Sallinen BJ, et al. Impact of psychosocial factors on quality of life in overweight youth. Obesity. 2007;15(7):1799–807. doi: 10.1038/oby.2007.214. [DOI] [PubMed] [Google Scholar]
- 17.Wake M, Canterford L, Patton GC, Hesketh K, Hardy P, Williams J, et al. Comorbidities of overweight/obesity experienced in adolescence: longitudinal study. Arch Dis Child. 2010;95(3):162–8. doi: 10.1136/adc.2008.147439. [DOI] [PubMed] [Google Scholar]
- 18.Varni JW, Limbers CW, Burwinkle TM. Impaired health-related quality of life in children and adolescents with chronic conditions: A comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL™ 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5:43. doi: 10.1186/1477-7525-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeller MH, Roehrig HR, Modi AC, Daniels SR, Inge TH. Health-related quality of life and depressive symptoms in adolescents with extreme obesity presenting for bariatric surgery. Pediatrics. 2006;117(4):1155–61. doi: 10.1542/peds.2005-1141. [DOI] [PubMed] [Google Scholar]
- 20.Modi AC, Loux TJ, Bell SK, Harmon CM, Inge TH, Zeller MH. Weight-specific health-related quality of life in adolescents with extreme obesity. Obesity. 2008;16(10):2266– 71. doi: 10.1038/oby.2008.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeller MH, Modi AC, Noll JG, Long JD, Inge TH. Psychosocial functioning improves following adolescent bariatric surgery. Obesity. 2009;17(5):885–90. doi: 10.1038/oby.2008.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hainsworth KR, Davies WH, Khan KA, Weisman SJ. Co-occurring chronic pain and obesity in children and adolescents: the impact on health-related quality of life. Clin J Pain. 2009;25(8):715–21. doi: 10.1097/AJP.0b013e3181a3b689. [DOI] [PubMed] [Google Scholar]
- 23.Ranzenhofer LM, Columbo KM, Tanofsky-Kraff M, Shomaker LB, Cassidy O, Matheson BE, et al. Binge eating and weight-related quality of life in obese adolescents. Nutrients. 2012;4(3):167–80. doi: 10.3390/nu4030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolotkin RL, Crosby RD, Gress RE, Hunt SC, Engel SG, Adam TD. Health and health-related quality of life: differences between men and women who seek gastric bypass surgery. Surg Obes Relat Dis. 2008;4:651–59. doi: 10.1016/j.soard.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doll HA, Petersen SE, Stewart-Brown SL. Obesity and physical and emotional well-being: Associations between body mass index, chronic illness, and the physical and mental components of the SF-36 questionnaire. Obes Res. 2000;8(2):160–70. doi: 10.1038/oby.2000.17. [DOI] [PubMed] [Google Scholar]
- 26.Mannucci E, Petroni ML, Villanova N, Rotella C, Apolone G, Marchesini G, et al. Clinical and psychological correlates of health-related quality of life in obese patients. Health Qual Life Outcomes. 2010;8:90. doi: 10.1186/1477-7525-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warkentin LM, Majumdar SR, Johnson JA, Agborsangaya CB, Rueda-Clausen CF, Sharma AM, et al. Predictors of health-related quality of life in 500 severely obese patients. Obesity. 2014;22(5):1367–72. doi: 10.1002/oby.20694. [DOI] [PubMed] [Google Scholar]
- 28.Nadeau K, Kolotkin RL, Boex R, Witten T, McFann KK, Zeitler P, et al. Health-related quality of life in adolescents with comorbidities related to obesity. J Adoles Health. 2011;49:90–2. doi: 10.1016/j.jadohealth.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Inge TH, Zeller MH, Harmon CM, Helmrath MA, Bean J, Modi AC, et al. Teen-longitudinal assessment of bariatric surgery: Methodological features of the first prospective multicenter study of adolescent bariatric surgery. J Pediatr Surg. 2007;42(11):1969–71. doi: 10.1016/j.jpedsurg.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belle SH, Berk PD, Courcoulas AP, Flum DR, Miles CW, Mitchell JE, et al. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surg Obes Relat Dis. 2007;3(2):116–26. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackey RH, Belle SH, Courcoulas AP, Dakin GF, Deveney CW, Flum DR, et al. Distribution of 10-year and lifetime predicted risk for cardiovascular disease prior to surgery in the longitudinal assessment of bariatric surgery-2 study. Am J Cardiol. 2012;110(8):1130–7. doi: 10.1016/j.amjcard.2012.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Modi AC, Zeller MH. The IWQOL-Kids©: Establishing minimal clinically important difference scores and test-retest reliability. Int J Pediatr Obes. 2011;6(2 Part 2):e94–e6. doi: 10.3109/17477166.2010.500391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeller MH, Reiter-Purtill J, Ratcliff MB, Inge TH, Noll JG. Two-year trends in psychosocial functioning after adolescent Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2011;7(6):727–32. doi: 10.1016/j.soard.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. San Antonio, TX: Psychological Corp; 1996. [Google Scholar]
- 35.Spitzer RL, Yanovski SZ, Marcus MD. The Questionnaire of Eating and Weight Patterns-Revised (QEWP-R, 1993) 1993. (Available from the New York State Psychiaric Institute, 722 West 168th Street, New York, NY 10032) [Google Scholar]
- 36.Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 37.Belle SH, Berk PD, Chapman WH, Christian NJ, Courcoulas AP, Dakin GF, et al. Baseline characteristics of participants in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study. Surg Obes Relat Dis. 2013;9(6):926–35. doi: 10.1016/j.soard.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42(6):563–70. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 39.Karlsson J, Taft C, Ryden A, Sjostrom L, Sullivan M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes. 2007;31(8):1248–61. doi: 10.1038/sj.ijo.0803573. [DOI] [PubMed] [Google Scholar]
- 40.Sarwer DB, Wadden TA, Moore RH, Eisenberg MH, Raper SE, Williams NN. Changes in quality of life and body image after gastric bypass surgery. Surg Obes and Relat Dis. 2010;6(6):608–14. doi: 10.1016/j.soard.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Legenbauer TdZM, Benecke A, Muhlhans B, Petrak F, Herpertz S. Depression and anxiety: their predictive function for weight loss in obese individuals. Obesity Facts. 2009;2:227–34. doi: 10.1159/000226278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wood KV, Ogden J. Explaining the role of binge eating behaviour in weight loss post bariatric surgery. Appetite. 2012;59(1):177–80. doi: 10.1016/j.appet.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 43.Lapidoth JD, Ghaderi A, Norring C. Binge eating in surgical weight-loss treatments. Long-term associations with weight loss, health related quality of life (HRQL), and psychopathology. Eat Weight Dis. 2011;16(4):263–9. doi: 10.1007/BF03327470. [DOI] [PubMed] [Google Scholar]
- 44.Wang CY, Gortkmaker SL, Taveras EM. Trends and racial/ethnic disparities in severe obesity among US children and adolescents, 1976–2006. Int J Pediatr Obes. 2011;6(1):9. doi: 10.3109/17477161003587774. [DOI] [PubMed] [Google Scholar]
- 45.Jensen CD, Steele RG. Longitudinal associations between teasing and health-related quality of life among treatment-seeking overweight and obese youth. J Pediatr Psychol. 2012;37(4):438–47. doi: 10.1093/jpepsy/jsr108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ware JE, Kosinski M. SF-36 Physical and Mental Health Summary Scales: A Manual for Users of Version 1. 2. Lincoln, RI: QualityMetric Incorporated; 2001. [Google Scholar]
- 47.Kolotkin RL, Crosby RD. Psychometric evaluation of the impact of weight on quality of life-lite questionnaire (IWQOL-lite) in a community sample. Qual Life Res. 2002;11:157–71. doi: 10.1023/a:1015081805439. [DOI] [PubMed] [Google Scholar]


