Abstract
Adenoviral infection is a major risk factor for otitis media. We hypothesized that adenovirus promotes bacterial ascension into the middle ear through the disruption of normal function in the Eustachian tubes due to inflammation-induced changes. An intranasal infection model of the chinchilla was used to test the ability of type 5 adenovirus to promote middle ear infection by Streptococcus pneumoniae. The hyperinflammatory adenovirus mutant dl327 and the nonreplicating adenovirus mutant H5wt300ΔpTP were used to test the role of inflammation and viral replication, respectively, in promotion of pneumococcal middle ear infection. Precedent infection with adenovirus resulted in a significantly greater incidence of middle ear disease by S. pneumoniae as compared to nonadenovirus infected animals. Infection with the adenovirus mutant dl327 induced a comparable degree of bacterial ascension into the middle ear as did infection with the wild-type virus. By contrast, infection with the nonreplicating adenovirus mutant H5wt300ΔpTP resulted in less extensive middle ear infection compared to the wild-type adenovirus. We conclude that viral replication is necessary for adenoviral-induced pneumococcal middle ear disease.
Keywords: coinfection, pneumococcal disease, animal model
Little is known about the interplay in the host between pathogens associated with otitis media. This important study demonstrates that precedent infection with adenovirus increases the incidence of middle ear disease by Streptococcus pneumoniae.
Little is known about the interplay in the host between pathogens associated with otitis media. This important study demonstrates that precedent infection with adenovirus increases the incidence of middle ear disease by Streptococcus pneumoniae.
INTRODUCTION
Otitis media is an inflammatory disease of the middle ear. The disease extols a high socioeconomic burden as both the greatest cause of lost work time for parents of children with an infectious disease and the most common reason for pediatric antibiotic prescription (Alsarraf et al., 1999; Gonzales et al., 2001). Most children will encounter at least one incident of otitis media by the age of 7, and many will experience recurrent otitis media (Klein 2000). Otitis media can profoundly impact normal development by causing hearing impairment, thus contributing to learning disabilities (Teele et al., 1990). The prevalence of otitis media among children is attributed to many factors, including immature Eustachian tube structure and function, an immature immune system, and prevalence of viral upper respiratory tract infections (for review, see Bakaletz 2010). The three bacterial species most commonly isolated from individuals with otitis media, nontypeable Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis, frequently reside commensally in the host nasopharynx (García-Rodríguez and Fresnadillo Martínez 2002). The shift to a disease state in the middle ear requires ascension of the bacteria through the Eustachian tubes.
Epidemiological studies indicate an association between viral upper respiratory infection and development of otitis media. Respiratory syncytial virus, influenza A, and adenovirus are major risk factors for developing otitis media and are frequently detected along with known bacterial agents of otitis media (Laufer et al., 2011). Children with adenovirus infection are 3–4 times more likely to develop otitis media (Pettigrew et al., 2011). Additionally, adenovirus has been detected in the middle ear fluid of children with otitis media (Sarkkinen et al., 1985). Experimental evidence supports a causative role for viral upper respiratory infection in promoting otitis media. Influenza A and respiratory syncytial virus have been shown to promote development of bacterial otitis media in chinchilla and mouse models (Short et al., 2011; Giebink et al., 1980; Brockson et al., 2012). In a chinchilla model, type 1 adenovirus induces otitis media when inoculated directly into the middle ear and promotes ascension of the otopathogen nontypeable H. influenzae during intranasal infection (Bakaletz et al., 1993; Suzuki and Bakaletz 1994; Miyamoto and Bakaletz 1997). Another study found that type 1 adenoviral infection did not promote ascension by S. pneumoniae in the chinchilla model (Tong et al., 2000).
Type 5 adenovirus, along with type 1 adenovirus, belongs to species C adenoviruses, which cause upper respiratory tract infections in humans. In this study, we examined the capacity of type 5 adenovirus to promote ascension of S. pneumoniae into the middle ear in the chinchilla model of otitis media. Streptococcus pneumoniae resides commensally within the nasopharynx of healthy individuals but is capable of causing invasive disease, including sinusitis, meningitis, bacteremia, pneumonia, and otitis media. Streptococcus pneumoniae is one of the most commonly isolated bacterial species from patients with otitis media and has been positively associated with adenovirus in children with recurrent otitis media (Wiertsema et al., 2011).
Viral coinfection can alter the course of S. pneumoniae infection. Infection with influenza A virus promotes pneumococcal pneumonia in mice (McCullers and Rehg 2002). In the chinchilla model of otitis media, influenza A virus promotes ascension of S. pneumoniae into the middle ear (Giebink et al., 1980). In vitro, influenza A virus infection increases adherence of S. pneumoniae to respiratory epithelial cells (Hakansson et al., 1994).
We hypothesized that infection with type 5 adenovirus would promote ascension of S. pneumoniae into the middle ear. To test the possible contribution of adenovirus-induced host inflammation in the development of otitis media, we used a mutant adenovirus that has been deleted of early region three (E3) of the viral genome. The E3 region is nonessential for viral replication in cell culture, but in vivo serves to modulate the host inflammatory response via the actions of several encoded gene products (Burgert et al., 2002). These gene products serve such functions as downregulation of MHC class I antigen presentation, inhibition of TNF-mediated apoptosis, and downregulation of the TRAIL, TNF, and Fas receptors. Deletion of the E3 region induces a more extensive inflammatory response in a cotton rat model of pneumonia (Ginsberg et al., 1989; Ginsberg and Prince 1994) and a BALB/c mouse model of intradermal edema (Schaack et al., 2004).
We also tested whether viral replication was necessary for promotion of middle ear infection by S. pneumoniae using the nonreplicating adenovirus mutant H5wt300ΔpTP. The adenoviral precursor terminal protein (pTP) directs viral genome replication; deletion from adenovirus results in an adenovirus capable of infecting host cells and expressing gene products but unable to replicate (Schaack et al., 2004).
Our results show that infection with wild-type adenovirus significantly increased the incidence of pneumococcal middle ear infection. The hyperinflammatory adenovirus mutant induced bacterial ascension to the same extent as the wild-type virus, while the nonreplicating adenovirus mutant did not promote ascension. These findings suggest that factors other than the initial host inflammatory response to adenovirus play a role in adenovirus-induced promotion of pneumococcal otitis media.
MATERIALS AND METHODS
Cell lines
Cell culture media from Lonza (Hopkinton, MA) and sera from Invitrogen (Gaithersburg, MD) were obtained through the Tissue Culture and Virus Vector Core Laboratory of the Comprehensive Cancer Center of Wake Forest University. HEK 293 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% newborn calf serum and were cultured at 3°C with 5% CO2 by passaging twice weekly at a 1 : 10 dilution.
Injection-quality virus production
Of the three adenoviruses used, type 5 adenovirus wt300 served as the wild-type control. The virus dl327 bears a deletion in the immunomodulatory E3 region (Ginsberg et al., 1989; Ginsberg and Prince 1994) and is otherwise isogenic with wt300. The H5wt300ΔpTP virus bears a deletion in the terminal protein gene, rendering it completely incapable of replicating (Schaack 2005). The H5wt300ΔpTP virus was grown in HEK 293 cells expressing the E2B gene. All other viruses were grown in HEK 293 cells and prepared in a form appropriate for use in animals by the following method. HEK 293 cells were infected at an MOI of 3 and harvested at 4 dpi. After harvesting the cells by centrifugation, the cell pellet was suspended in 10 mM Tris-HCl, pH 8.0, and subjected to three freeze–thaw cycles. Virus was harvested after two rounds of centrifugation through step and equilibrium CsCl gradients as described previously (Shepard and Ornelles 2004). The purified virus was dialyzed at 4°C against three changes of buffered saline with glycerol (10 mM Tris-HCl, pH 8.0; 135 mM NaCl; 1 mM MgCl2; 50% v/v glycerol). The resulting injection-quality virus was then titered on HEK 293 cells.
Bacterial strains and growth conditions
Streptococcus pneumoniae strain EF3030 is a well-characterized serotype 19F clinical isolate from the nasopharynx of an adult with otitis media (Andersson et al., 1981; Briles et al., 1992). For animal inoculations, S. pneumoniae bacterial suspensions were prepared by diluting frozen stocks of known concentration in phosphate-buffered saline with 0.04% gelatin.
Adenovirus quantitation
Adenoviral loads in chinchilla tissue samples were quantitated by limiting dilution assay in HEK 293 cells (Pacini et al., 1984). Harvested chinchilla homogenates were freeze-thawed three times, centrifuged, and passed through a 0.2-μm Whatman syringe-top filter (GE Healthcare). The filtered homogenate was serially diluted in serum-free media, added to 96-well plates preseeded overnight with 2 × 103 cells per well, and allowed to incubate with gentle agitation. An equal volume of media with 20% serum was added after 1 h, and the plates were maintained for 2 weeks. The culture media were regularly supplemented with complete growth media during this time. The cultures were monitored for appearance of the cytopathic effect, which is revealed by a sharp decrease in pH due to replication and amplification of an infectious virus added to the well.
Chinchilla infection studies
The chinchilla is a well-established model for otitis media (Bakaletz 2009). Two- to three-month-old chinchillas were purchased from Rauscher's Chinchilla Ranch and allowed to acclimate for 1 week prior to infection. Animals were evaluated by veterinary staff prior to experimental infection to ensure that they were in healthy condition. Intranasal inoculations were performed by first anesthetizing the animals via isoflurane inhalation and then administering bacterial or viral suspensions by dispensing 50–100μL into each nostril with a pipette. Chinchillas were monitored daily for signs of illness; otoscopy was performed every 48 h to assess changes to the tympanic membrane. Extent of middle ear disease was assessed by monitoring the tympanic membrane via otoscopy; each ear was scored on a scale of 0–3 based on coloration of the tympanic membrane, presence of fluid behind the tympanic membrane, protrusion of the tympanic membrane, and rupture of the tympanic membrane; scores were assigned by two independent observers. Chinchillas were pair-housed in individually ventilated cages, with no contact between experimental groups, to avoid viral or bacterial transmission. At indicated times following infection, chinchillas were euthanized. Nasal tissue, Eustachian tubes, and middle ear bullae and associated mucosal tissue were aseptically removed and homogenized in phosphate-buffered saline using a PowerGen 1000 homogenizer. In some cases, one Eustachian tube and one middle ear bulla were preserved from each animal for histopathology processing.
To quantitate S. pneumoniae, homogenized tissue samples were serially diluted and plated on trypticase soy agar (BD) supplemented with 5% sheep's blood (HemoStat Laboratories) and 4μg mL−1 gentamicin (Sigma). The bacterial CFU limit of quantitation was calculated for each sample volume based on a colony count equal to 20 CFUs on the undiluted spread plate; colony counts below 20 CFUs on a standard size agar plate have been considered to be inaccurate (Sutton 2011). Values less than this limit of quantitation were not included in analyses involving the geometric mean bacterial CFUs but were included as zero counts in nonparametric analyses. In addition to bacterial counts, chinchilla middle ears were categorized as culture positive or culture negative using the bacterial CFU limit of quantitation as a cutoff. Animal experiments described here were approved by the IACUC of Wake Forest and performed in compliance with the USDA Animal Welfare Act and relevant policies on the humane care and use of laboratory animals.
Histopathology
Animal tissue samples were fixed in 2–4% paraformaldehyde overnight at 4°C and then decalcified in Decalcifier II solution. Tissue samples were embedded in Tissue-Tek OCT compound (Sakura Finetek) and frozen at −80°C. A Microm HM 525 cryotome was used to cut sections at 5μm thickness. Cryosections were allowed to adhere to Superfrost Plus microscope slides (Fisher Scientific) and stored at −20°C. Hematoxylin and eosin staining was performed on sections for histopathological analysis.
RESULTS
Adenovirus infection in the chinchilla model
Chinchillas were intranasally inoculated with 3 × 106, 3 × 107, or 6 × 108 IU of Ad5 wild-type strain wt300, as described in the Materials and methods. At 7 and 10 days postinoculation, animals were euthanized and the extent of adenoviral infection in the nasopharynx and Eustachian tubes was quantitated by limiting dilution assay in HEK 293 cells. Adenovirus was detected in the Eustachian tubes of animals from each of the three inocula groups at 7 days postinoculation (Fig. 1b), but was only detected in a single Eustachian tube from the 3 × 107 IU inocula group at 10 days postinoculation. Adenovirus was detected in the nasopharynx of animals from the highest inocula group at 7 days postinoculation but not by 10 days postinoculation; virus was not detected in the nasopharynx of animals from the two lower dose groups at either time point (Fig. 1a). Middle ear bullae of animals from the high-dose group mostly contained low levels of virus or no virus (Fig. 1c). During the course of the study, chinchillas did not display any acute signs of otitis media including any changes evident in the tympanic membrane as determined by otoscopy. A single animal from the 3 × 107 IU inocula group died 9 days postinoculation, but did not show previous signs of illness. The cause of death was determined to be severe bronchopneumonia with intralesional bacterial cocci, which corresponded to culture-positive beta-hemolytic Streptococcus species.
Figure 1.
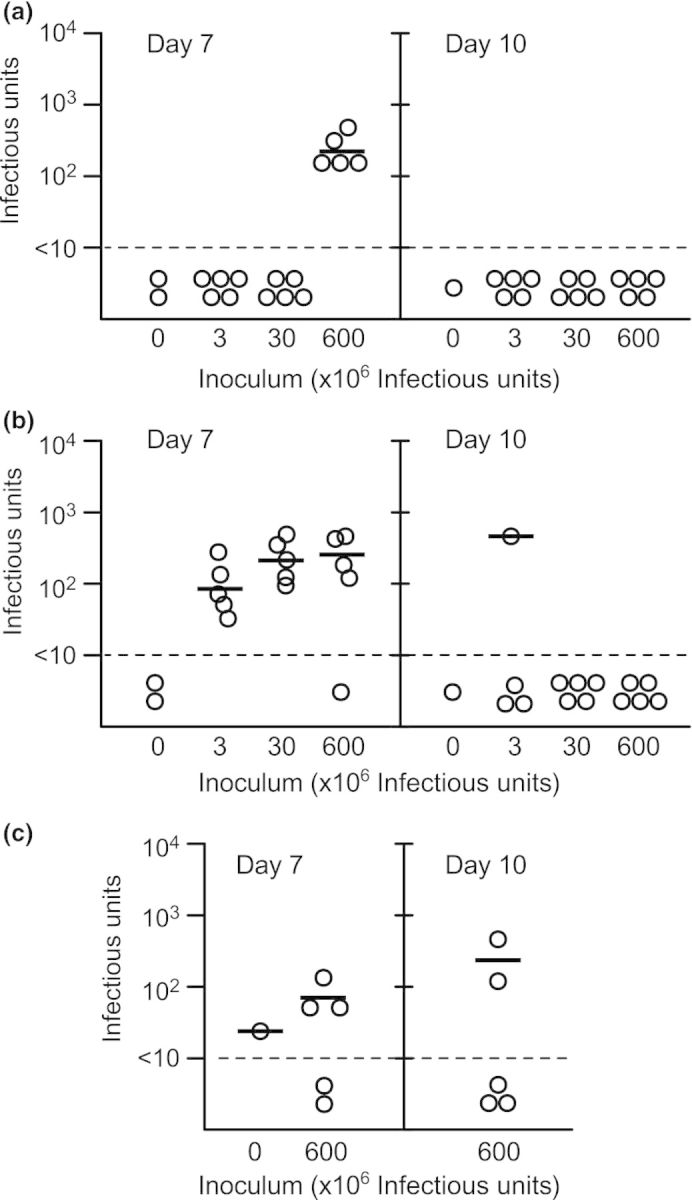
Type 5 adenovirus can be detected in the Eustachian tubes and middle ear bullae of chinchillas following intranasal inoculation. Chinchillas were inoculated with type 5 adenovirus at an inoculum of 6 × 108, 3 × 107, or 3 × 106 infectious units. At 7 and 10 days postinoculation, animals were euthanized and adenovirus was quantitated in the nasopharynx (a), Eustachian tubes (b), and middle ear (c). The solid lines indicate geometric mean viral infectious units. The dashed line indicates the limit of detection. Each data point in (b) and (c) represents a single Eustachian tube or single middle ear bulla, respectively.
Adenoviral infection promotes colonization of S. pneumoniae in the chinchilla middle ear
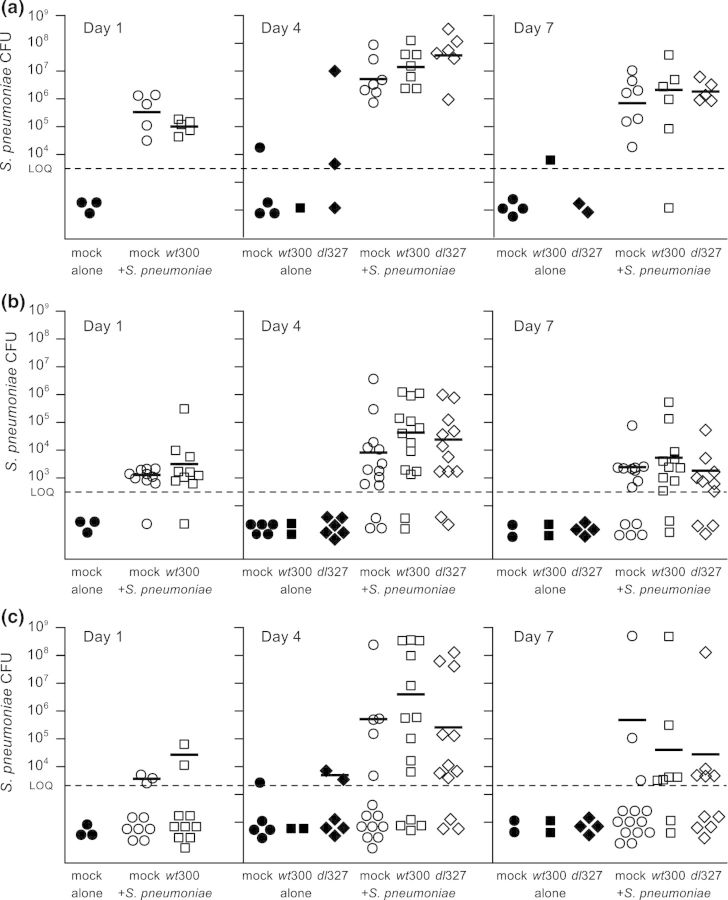
We have previously observed that intranasal inoculation of chinchillas with S. pneumoniae strain EF3030 results in ascension of bacteria into c. 30% of ears (data not shown). We hypothesized that adenoviral infection would increase the propensity of S. pneumoniae to ascend into the middle ear, as suggested by the epidemiological association between adenovirus and otitis media. We used a staggered coinfection model, in which chinchillas were intranasally inoculated with 6 × 108 IU of wild-type Ad5 wt300 or PBS, and 7 days later intranasally inoculated with 107 CFUs of S. pneumoniae strain EF3030. This time course was selected based on our detection of adenovirus in the Eustachian tubes at 7 days postinoculation. Additionally, Suzuki and associates previously reported that coinfection with nontypeable H. influenzae and type 1 adenovirus induced the greatest middle ear disease when adenovirus inoculation preceded H. influenzae inoculation by 7 days (Suzuki and Bakaletz 1994). Bacterial counts in the nasopharynx, Eustachian tubes, and middle ear were determined at 1, 4, and 7 days post-bacterial-inoculation. Streptococcus pneumoniae counts in the nasopharynx were comparable for virus-infected and mock-infected animals (P > 0.08, Kruskal–Wallis test), indicating no change in carriage levels of the bacteria (Fig. 2a). Similarly, bacterial loads in the Eustachian tubes were indistinguishable among virus-infected and mock-infected animals (P > 0.18, Fig. 2b). Although the mean bacterial CFUs recovered from the middle ears of animals inoculated with adenovirus also did not differ significantly from that recovered from nonvirally infected animals (Fig. 2c), the virus infection increased the likelihood of bacterial ascension into the middle ear. There was a significant increase (P = 0.016, Fisher's exact test) in the proportion of ears identified as culture positive for S. pneumoniae in animals coinfected with wt300 Ad5 as compared to animals infected with S. pneumoniae alone at days 4 and 7 post-bacterial-inoculation (Table 1). Counts obtained on both days were pooled for the analysis because of the similar proportion of culture-positive ears at 4 and 7 days post-bacterial-inoculation. Acute signs of middle ear disease, as determined by otoscopic examination of the tympanic membrane, were most severe at 2 days postinoculation with S. pneumoniae in the coinfected cohort (data not shown). These three animals were subsequently euthanized due to development of severe ataxia and lethargic behavior; blood cultures from these animals revealed bacteremia. By contrast, only one animal from the cohort inoculated with S. pneumoniae alone developed symptoms severe enough to warrant euthanasia; this animal was also determined to be bacteremic based on blood culture. Preserved Eustachian tubes from randomly selected animals were sectioned and stained for histopathological assessment (see Materials and methods). However, the results were inconclusive due to the small sample size and limited amount of tissue examined (data not shown).
Figure 2.
Type 5 adenoviral infection promotes colonization of Streptococcus pneumoniae in the chinchilla middle ear with minimal impact on the bacterial load at bacteria-positive sites. Chinchillas were inoculated with the wild-type adenovirus wt300, adenovirus mutant dl327, or PBS. Seven days later, chinchillas were inoculated with S. pneumoniae (day 0). Streptococcus pneumoniae colony-forming units (CFUs) were quantitated in the nasopharynx (a), Eustachian tubes (b), and middle ear bullae and associated tissue (c) at 1, 4, and 7 days post-bacterial-inoculation. The results of two experiments are represented here. Each data point in (b) and (c) represents a single Eustachian tube or single middle ear bulla, respectively. The solid lines indicate geometric mean bacterial CFUs for bacterial counts above the limit of quantitation for S. pneumoniae-infected animals. The dashed line (LOQ) indicates the limit of quantitation as described in the Materials and methods.
Table 1.
Culture-positive ears in adenovirus-infected animals.*
| Virus | ||||
|---|---|---|---|---|
| Analyze† | None | wt300 | dl327 | ΔpTP |
| d1 | 3/10 (30) | 2/10 (20) | nd | nd |
| d3 (terminated) | 2/2 (100) | 2/2 (100) | 4/4 (100) | 2/2 (100) |
| d4 | 5/14 (36) | 15/22 (68) | 9/12 (75) | 6/18 (33) |
| d7 | 3/14 (21) | 6/8 (75) | 5/10 (50) | nd |
Data are reported as culture-positive ears/total ears (percentage culture positive).
Animals were harvested on the indicated day after bacterial inoculation. Individual ears were categorized as either culture positive or culture negative using a cutoff value of 2 × 103 CFU Streptococcus pneumoniae, corresponding with our limit of quantitation of 20 CFU on the undiluted sample plate. Data include results from three experiments. Note that all d3 animals were euthanized due to distress and not as part of a scheduled endpoint. Combinations that were not performed are indicated by nd.
Deletion of adenovirus early region 3 does not affect pneumococcal ascension
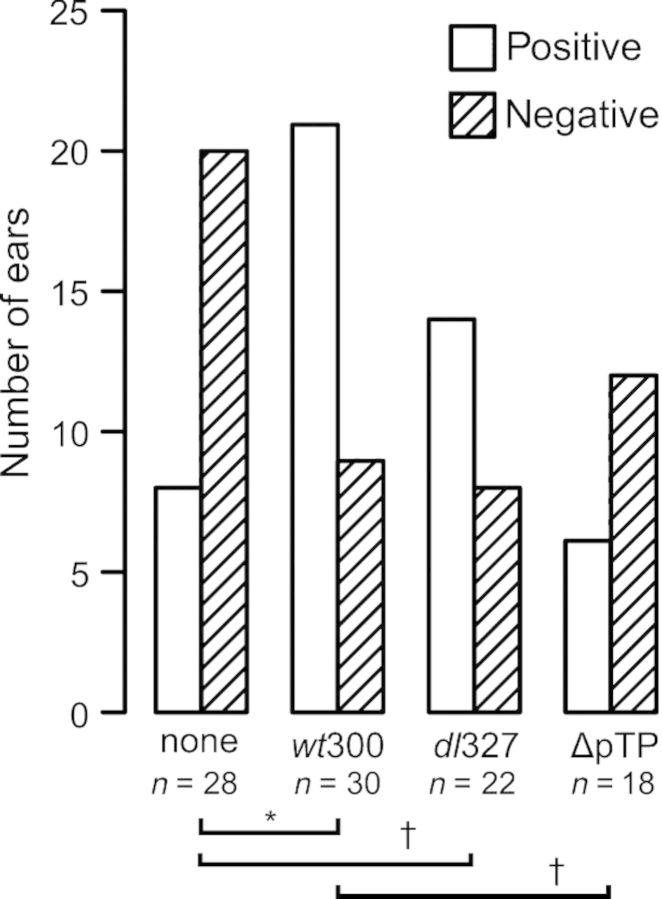
Early region three (E3) of the Ad5 adenoviral genome modulates the host immune response (Burgert et al., 2002). Viruses bearing deletion in the E3 region elicit a more vigorous host inflammatory response in a cotton rat (Ginsberg et al., 1989) and a BALB/c mouse model (Schaack et al., 2004) of infection. We therefore used dl327, an adenoviral mutant which has been deleted of the E3 region, to test the contribution of the inflammatory response to ascension of S. pneumoniae. We expected that infection with dl327 would increase the rate of bacterial ascension by inducing a more pronounced host inflammatory response. In contrast to our expectation, infection with dl327 resulted in a similar outcome as infection with the wild-type virus. Streptococcus pneumoniae colonized the nasopharynx at similar levels (P > 0.07, Kruskal–Wallis test) in all infected animals (Fig. 2a), and there was a comparable level of bacterial CFUs (P > 0.18, Kruskal–Wallis test) in the Eustachian tubes and middle ear bullae for mock-, wt300-, and dl327-infected animals (Fig. 2b and c). The incidence of middle ear infection was also similar for animals infected with either virus, which was significantly higher (P = 0.005, Fisher's exact test) than the occurrence of middle ear infection compared to non-virus-infected animals (Fig. 4). Otoscopic examination did not reveal any differences between wt300-infected animals and dl327-infected animals with respect to the tympanic membrane (data not shown).
Figure 4.
The wild-type and E3-deleted type 5 adenoviruses promote comparable levels of Streptococcus pneumoniae ascension into the chinchilla middle ear, while the viral DNA replication-deficient virus fails to promote ascension. Middle ear samples from chinchillas euthanized at 4 (none, wt300, dl327, ΔpTP) and 7 (none, wt300, dl327) days post-bacterial-inoculation (see Table 1) were categorized as culture positive or culture negative for Streptococcus pneumoniae using the limit of quantitation as a cutoff value (2 × 103 CFU). The number of ears (n) included in each group is indicated. The proportion of S. pneumoniae positive ears was significantly affected by the preceding virus infection (P = 0.0036, Fisher's exact test). The basis for the variation was identified by the Marascuilo post hoc test for multiple proportions and is indicated by the horizontal bars (*P < 0.05, - P < 0.075). Days 4 and 7 postinoculation results were combined for this analysis as there was no difference between middle ear incidence at these time points.
Infection with a nonreplicating adenovirus does not promote bacterial colonization in the middle ear
We sought to determine whether the early events of infection by adenovirus were sufficient to promote ascension of S. pneumoniae into the middle ear. We infected animals with the adenovirus mutant H5wt300ΔpTP (ΔpTP), which expresses all of the early genes except for E2B and therefore does not direct viral genome replication. After 7 days, the virus-infected animals were inoculated with S. pneumoniae. Bacterial loads were assessed at 4 days post-bacterial-inoculation; a day 7 time point was considered unnecessary for this experiment due to our observation that incidence of bacterial middle ear infection was similar between days 4 and 7 post-bacterial-inoculation in our previous experiments. Chinchillas inoculated with ΔpTP had similar loads of S. pneumoniae in the nasopharynx and Eustachian tubes as animals inoculated with the replicating wild-type virus (Fig. 3a and b). While we did not observe a significant difference in mean bacterial CFUs in the middle ears of animals (Fig. 3c), there was a lower incidence of middle ear infection by S. pneumoniae in the ΔpTP-inoculated animals than in wt300-inoculated animals (Fig. 4).
Figure 3.
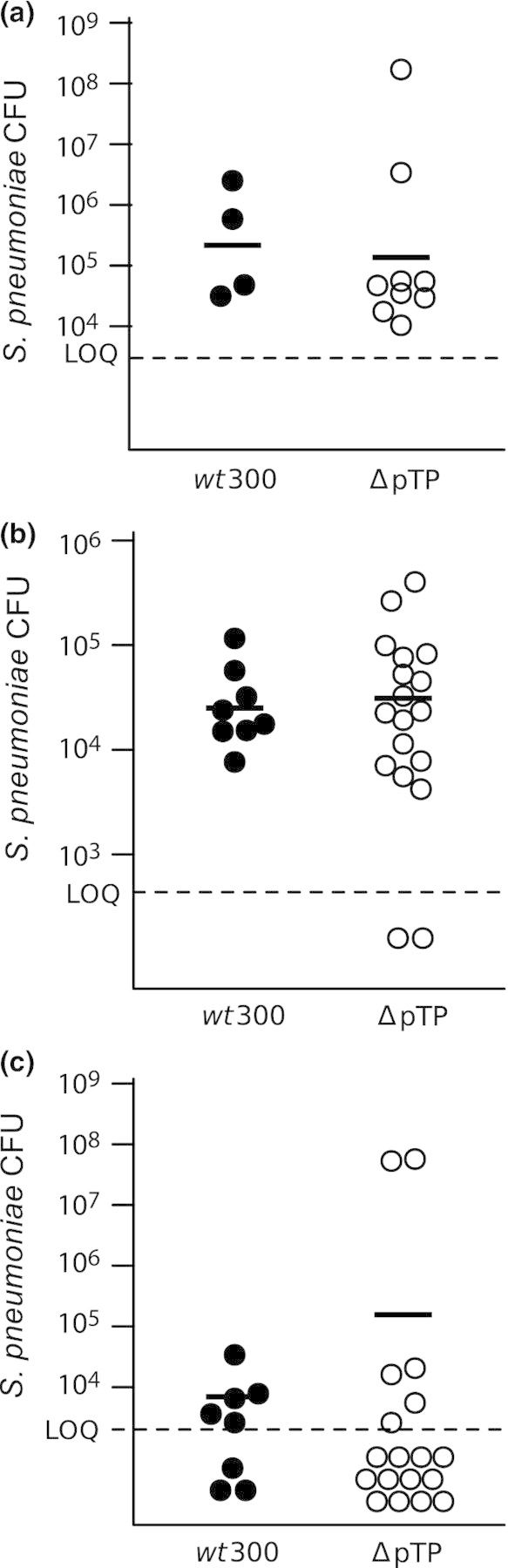
Intranasal inoculation of a nonreplicating type 5 adenovirus fails to promote bacterial ascension to the chinchilla middle ear with no effect on bacterial loads detected in the nasopharynx or Eustachian tube. Chinchillas were inoculated with adenovirus strain wt300 or H5wtΔpTP (ΔpTP) 7 days prior to inoculation with Streptococcus pneumoniae. At 4 days post-bacterial-inoculation, S. pneumoniae loads were quantitated within the nasopharynx (a), Eustachian tubes (b), and middle ear bullae (c). Solid lines indicate the geometric mean bacterial CFUs for values above the dashed line, which indicates the limit of quantitation.
DISCUSSION
Epidemiological evidence indicates an association between adenovirus infection and otitis media (Pettigrew et al., 2011). We therefore investigated the capacity of type 5 adenovirus to infect chinchillas and promote middle ear infection by S. pneumoniae in a coinfection model. It has previously been reported that chinchillas are permissible to infection with type 1 human adenovirus (Bakaletz et al., 1993). The limited data on adenovirus types associated with otitis media suggest that the relative frequency of the respiratory adenoviruses linked to otitis media differs from that associated with severe respiratory infections. For example, type 1 adenovirus was repeatedly identified in children with acute otitis media and an upper respiratory infection (Kalu et al., 2010). By contrast, this virus was 2–3 times less frequent than types 2 or 3 adenovirus in patients requiring hospitalization for respiratory disease (Gray et al., 2007; Qurei et al., 2012). These observations show that the respiratory adenoviruses differ in their association with otitis media and may therefore differ in their ability to promote otitis media. In this report, we show evidence indicating that 2- to 3-month-old chinchillas are permissive to infection with type 5 human adenovirus. Type 5 human adenovirus was detected at 7 days postinoculation in the Eustachian tubes of chinchillas inoculated with any of the three viral dosages. The comparable viral titers in the Eustachian tubes of animals from the 6 × 108 and 3 × 107 IU infection groups may indicate a saturation of infected cells at the 3 × 107 IU inoculum; it is possible that only a subset of the cells in the Eustachian tubes are permissive for adenoviral infection. Interestingly, we initially attempted to establish an adenovirus infection model in adult chinchillas, but were unable to recover virus at any time point after inoculation (data not shown). This observed difference in infection outcome based on age suggests that adult chinchillas may be resistant to adenoviral infection; whether this is due to a more mature immune system, prior exposure to adenovirus, or some other factor remains to be determined.
When chinchillas were inoculated with S. pneumoniae 7 days after inoculation with adenovirus, there was a significantly increased incidence of pneumococcal middle ear infection in adenovirus-infected animals. Streptococcus pneumoniae loads in the nasopharynx were comparable for virus-infected and non-virus-infected animals. In children, nasal carriage loads of Streptococcus pneumonia were associated with an increased risk of otitis media (Smith-Vaughan et al., 2006), which has led to the hypothesis that viral upper respiratory infections may promote bacterial ascension by increasing bacterial loads in the nasopharynx. However, our experimental results do not support this as a mechanism by which adenovirus promotes pneumococcal middle ear disease.
Deletion of the adenovirus E3 gene region, which encodes gene products that modulate the host inflammatory response to adenovirus, did not significantly alter the outcome of middle ear infection. Although the E3-deleted virus elicits a robust inflammatory response in the cotton rat (Ginsberg et al., 1989; Ginsberg and Prince 1994) and mouse (Schaack et al., 2004), it is not known whether this virus elicits a similar host response in the chinchilla. Nonetheless, infection with the nonreplicating adenovirus mutant ΔpTP did not promote an increase in bacterial middle ear infection.
In humans, most adenovirus serotypes enter respiratory epithelial cells by first binding to the coxsackievirus and adenovirus receptor (CAR); the interaction of the HAdV type 5 fiber with CAR induces inflammatory gene expression in human epithelial cells (Tamanini et al., 2006). In our experiment, infection with the ΔpTP mutant did not promote pneumococcal middle ear infection, suggesting that the initial host response to the components of the viral capsid was not sufficient to induce bacterial ascension. Of note, while the geometric mean bacterial CFUs in the middle ears were comparable for wt300-coinfected and ΔpTP-coinfected animals in this experiment (Fig. 3c), the proportion of culture-positive ears in the ΔpTP-coinfected group was more similar to that of S. pneumoniae-alone-infected animals (Fig. 4). The geometric mean bacterial CFUs of the wt300-coinfected group in this experimental cohort was low compared to previous cohorts (Fig. 2), but the proportion of culture-positive ears was consistent. These differences in observed geometric mean bacterial CFUs between experiments illustrate the biological variability that is frequently observed among bacterial counts in culture-positive ears. In these experiments, the range of S. pneumoniae in culture-positive middle ears varied over nearly eight orders of magnitude. We observed this spread of pneumococcal loads in all S. pneumoniae-challenged experimental groups, including animals coinfected with the adenovirus ΔpTP mutant, a group which experienced a low incidence of pneumococcal middle ear infection. Indeed, a small number of control animals not challenged with S. pneumoniae were scored as culture positive for S. pneumoniae (Fig. 2). The wide range of bacterial loads in the middle ear may be attributable to the outbred chinchilla model or intrinsic limitations of this animal model.
Type 1 adenovirus has been shown to promote ascension of nontypeable H. influenzae in the chinchilla model of otitis media (Suzuki and Bakaletz 1994), but few published studies to date have assessed the ability of adenovirus to promote ascension of S. pneumoniae. Tong and associates reported that type 1 adenovirus did not promote ascension of S. pneumoniae into the chinchilla middle ear (Tong et al., 2000). The difference in outcome from our experiment may be due to differences between type 1 and type 5 adenovirus; although both belong to species C, significant differences map to the fiber and hexon capsid proteins as well as the E3 immunomodulatory region (data not shown). The degree of association of different adenoviral serotypes with otitis media and bacterial otopathogens has not been thoroughly addressed in epidemiological studies, and interactions with bacterial otopathogens may differ between adenoviral serotypes. Bacterial species within similar niches can also differ in their interactions with the same adenovirus serotype. For example, type 1 adenovirus did not promote culture-positive otitis media during coinfection with M. catarrhalis in the chinchilla model (Bakaletz et al., 1995). It is possible that the mechanism by which adenovirus promotes pneumococcal ascension does not affect M. catarrhalis.
Bacterial ascension into the middle ear is a critical step in the development of otitis media. In this paper, we have shown that adenovirus promotes ascension of S. pneumoniae in a chinchilla model. Understanding the means by which adenovirus infection contributes to the burden of otitis media will help to determine appropriate treatment for the disease.
Acknowledgments
The authors gratefully acknowledge colleagues in the Swords and Ornelles laboratory groups for expert technical assistance. We thank Jerry Schaack (University of Denver) for kindly providing the H5wt300ΔpTP virus. This work was supported by the National Institute on Deafness and Other Communication Disorders at the National Institute of Health (RO1 DC10051 to W. E. S) and the National Institute of Allergy and Infectious Disease at the National Institute of Health (Training Grant T32 AI07401 in support of K.AM. and R.L.T).
Conflict of interest statement. None declared.
REFERENCES
- Alsarraf R, Jung CJ, Perkins J, Crowley C, Alsarraf NW, Gates GA. Measuring the indirect and direct costs of acute otitis media. Arch Otolaryngol Head Neck Surg. 1999;125:12–8. doi: 10.1001/archotol.125.1.12. [DOI] [PubMed] [Google Scholar]
- Andersson B, Eriksson B, Falsen E, Fogh A, Hanson LA, Nylen O, Peterson H, Eden C Svanborg. Adhesion of Streptococcus pneumoniae to human pharyngeal epithelial cells in vitro: differences in adhesive capacity among strains isolated from subjects with otitis media, septicemia, or meningitis or from healthy carriers. Infect Immun. 1981;32:311–7. doi: 10.1128/iai.32.1.311-317.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaletz LO. Chinchilla as a robust, reproducible and polymicrobial model of otitis media and its prevention. Expert Rev Vaccines. 2009;8:1063–82. doi: 10.1586/erv.09.63. [DOI] [PubMed] [Google Scholar]
- Bakaletz LO. Immunopathogenesis of polymicrobial otitis media. J Leukoc Biol. 2010;87:213–22. doi: 10.1189/jlb.0709518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaletz LO, Daniels RL, Lim DJ. Modeling adenovirus type 1-induced otitis media in the chinchilla: effect on ciliary activity and fluid transport function of eustachian tube mucosal epithelium. J Infect Dis. 1993;168:865–72. doi: 10.1093/infdis/168.4.865. [DOI] [PubMed] [Google Scholar]
- Bakaletz LO, Murwin DM, Billy JM. Adenovirus serotype 1 does not act synergistically with Moraxella (Branhamella) catarrhalis to induce otitis media in the chinchilla. Infect Immun. 1995;63:4188–90. doi: 10.1128/iai.63.10.4188-4190.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles DE, Crain MJ, Gray BM, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:111–6. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockson ME, Novotny LA, Jurcisek JA, McGillivary G, Bowers MR, Bakaletz LO. Respiratory syncytial virus promotes Moraxella catarrhalis-induced ascending experimental otitis media. PLoS ONE. 2012;7:e40088. doi: 10.1371/journal.pone.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgert HG, Ruzsics Z, Obermeier S, Hilgendorf A, Windheim M, Elsing A. Subversion of host defense mechanisms by adenoviruses. Curr Top Microbiol Immunol. 2002;269:273–318. doi: 10.1007/978-3-642-59421-2_16. [DOI] [PubMed] [Google Scholar]
- García-Rodríguez JÃ, Fresnadillo Martínez MJ. Dynamics of nasopharyngeal colonization by potential respiratory pathogens. J Antimicrob Chemother. 2002;50:59–74. doi: 10.1093/jac/dkf506. [DOI] [PubMed] [Google Scholar]
- Giebink GS, Berzins IK, Marker SC, Schiffman G. Experimental otitis media after nasal inoculation of Streptococcus pneumoniae and influenza A virus in chinchillas. Infect Immun. 1980;30:445–50. doi: 10.1128/iai.30.2.445-450.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg HS, Prince GA. The molecular basis of adenovirus pathogenesis. Infect Agents Dis. 1994;3:1–8. [PubMed] [Google Scholar]
- Ginsberg HS, Lundholm-Beauchamp U, Horswood RL, Pernis B, Wold WS, Chanock RM, Prince GA. Role of early region 3 (E3) in pathogenesis of adenovirus disease. P Natl Acad Sci USA. 1989;86:3823–7. doi: 10.1073/pnas.86.10.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales R, Malone DC, Maselli JH, Sande MA. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33:757–62. doi: 10.1086/322627. [DOI] [PubMed] [Google Scholar]
- Gray GC, McCarthy T, Lebeck MG, et al. Genotype prevalence and risk factors for severe clinical adenovirus infection, United States 2004–2006. Clin Infect Dis. 2007;45:1120–31. doi: 10.1086/522188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson A, Kidd A, Wadell G, Sabharwal H, Svanborg C. Adenovirus infection enhances in vitro adherence of Streptococcus pneumoniae. Infect Immun. 1994;62:2707–14. doi: 10.1128/iai.62.7.2707-2714.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalu SU, Loeffelholz M, Beck E, Patel JA, Revai K, Fan J, Henrickson KJ, Chonmaitree T. Persistence of adenovirus nucleic acids in nasopharyngeal secretions: a diagnostic conundrum. Pediatr Infect Dis J. 2010;29:746–50. doi: 10.1097/INF.0b013e3181d743c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JO. The burden of otitis media. Vaccine. 2000;19:S2–8. doi: 10.1016/s0264-410x(00)00271-1. [DOI] [PubMed] [Google Scholar]
- Laufer AS, Metlay JP, Gent JF, Fennie KP, Kong Y, Pettigrew MM. Microbial communities of the upper respiratory tract and otitis media in children. mBio. 2011;2:e00245–10. doi: 10.1128/mBio.00245-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullers JA, Rehg JE. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis. 2002;186:341–50. doi: 10.1086/341462. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Bakaletz LO. Kinetics of the ascension of NTHi from the nasopharynx to the middle ear coincident with adenovirus-induced compromise in the chinchilla. Microb Pathog. 1997;23:119–26. doi: 10.1006/mpat.1997.0140. [DOI] [PubMed] [Google Scholar]
- Pacini DL, Dubovi EJ, Clyde WA., Jr A new animal model for human respiratory tract disease due to adenovirus. J Infect Dis. 1984;150:92–7. doi: 10.1093/infdis/150.1.92. [DOI] [PubMed] [Google Scholar]
- Pettigrew MM, Gent JF, Pyles RB, Miller AL, Nokso-Koivisto J, Chonmaitree T. Viral-bacterial interactions and risk of acute otitis media complicating upper respiratory tract infection. J Clin Microbiol. 2011;49:3750–5. doi: 10.1128/JCM.01186-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qurei L, Seto D, Salah Z, Azzeh M. A molecular epidemiology survey of respiratory adenoviruses circulating in children residing in Southern Palestine. PLoS ONE. 2012;7:e42732. doi: 10.1371/journal.pone.0042732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkkinen H, Ruuskanen O, Meurman O, Puhakka H, Virolainen E, Eskola J. Identification of respiratory virus antigens in middle ear fluids of children with acute otitis media. J Infect Dis. 1985;151:444–8. doi: 10.1093/infdis/151.3.444. [DOI] [PubMed] [Google Scholar]
- Schaack J. Adenovirus vectors deleted for genes essential for viral DNA replication. Front Biosci. 2005;10:1146–55. doi: 10.2741/1607. [DOI] [PubMed] [Google Scholar]
- Schaack J, Bennett ML, Colbert JD, Torres AV, Clayton GH, Ornelles D, Moorhead J. E1A and E1B proteins inhibit inflammation induced by adenovirus. P Natl Acad Sci USA. 2004;101:3124–9. doi: 10.1073/pnas.0303709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard RN, Ornelles DA. Diverse roles for E4orf3 at late times of infection revealed in an E1B 55-kilodalton protein mutant background. J Virol. 2004;78:9924–35. doi: 10.1128/JVI.78.18.9924-9935.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Diavatopoulos DA, Thornton R, Pedersen J, Strugnell RA, Wise AK, Reading PC, Wijburg OL. Influenza virus induces bacterial and nonbacterial otitis media. J Infect Dis. 2011;204:1857–65. doi: 10.1093/infdis/jir618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Vaughan H, Byun R, Nadkarni M, Jacques N, Hunter N, Halpin S, Morris P, Leach A. Measuring nasal bacterial load and its association with otitis media. BMC Ear Nose Throat Disord. 2006;6:10. doi: 10.1186/1472-6815-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton S. Accuracy of plate counts. J Valid Technol. 2011;17:42–6. [Google Scholar]
- Suzuki K, Bakaletz LO. Synergistic effect of adenovirus type 1 and nontypeable Haemophilus influenzae in a chinchilla model of experimental otitis media. Infect Immun. 1994;62:1710–8. doi: 10.1128/iai.62.5.1710-1718.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamanini A, Nicolis E, Bonizzato A, Bezzerri V, Melotti P, Assael BM, Cabrini G. Interaction of adenovirus type 5 fiber with the coxsackievirus and adenovirus receptor activates inflammatory response in human respiratory cells. J Virol. 2006;80:11241–54. doi: 10.1128/JVI.00721-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teele DW, Klein JO, Chase C, Menyuk P, Rosner BA Greater Boston Otitis Media Study G. Otitis media in infancy and intellectual ability, school achievement, speech, and language at age 7 years. J Infect Dis. 1990;162:685–94. doi: 10.1093/infdis/162.3.685. [DOI] [PubMed] [Google Scholar]
- Tong HH, Fisher LM, Kosunick GM, DeMaria TF. Effect of adenovirus type 1 and influenza A virus on Streptococcus pneumoniae nasopharyngeal colonization and otitis media in the chinchilla. Ann Otol Rhinol Laryngol. 2000;109:1021–7. doi: 10.1177/000348940010901106. [DOI] [PubMed] [Google Scholar]
- Wiertsema SP, Chidlow GR, Kirkham LA, Corscadden KJ, Mowe EN, Vijayasekaran S, Coates HL, Harnett GB, Richmond PC. High detection rates of nucleic acids of a wide range of respiratory viruses in the nasopharynx and the middle ear of children with a history of recurrent acute otitis media. J Med Virol. 2011;83:2008–17. doi: 10.1002/jmv.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]