Abstract
Background
Ruxolitinib is a potent and specific JAK1/JAK2 inhibitor recently approved for the treatment of myelofibrosis.
Patients and Methods
We conducted a single-center phase I/II clinical study testing 3 dose levels (50 mg BID [n=4], 100 mg BID [n=5], and 200 mg BID [n=18]). We enrolled 27 patients older than 14 years with relapsed or refractory acute myeloid leukemia (n=26) or acute lymphoid leukemia (n=1).
Results
Median age was 66 years (range, 25–88). Thirteen patients were evaluable for dose-limiting toxicities. The most common grade 3 or 4 non-hematologic event was infection (n= 26 events; most frequently pneumonia; 15/26, 58%). One patient with multiple relapses after 7 lines of therapy had a CRp at ruxolitinib dose of 200 mg BID.
Conclusion
In this cohort of heavily pre-treated patients with relapsed or refractory acute leukemias, ruxolitinib was overall reasonably well tolerated, with one patient achieving CRp. This study was registered at www.clinicaltrials.gov (NCT01251965).
Keywords: myelofibrosis, ruxolitinib, acute myeloid leukemia, JAK2V617 mutation
Introduction
Acute myeloid leukemia (AML) is an aggressive hematologic malignancy representing a diverse group of clonal hematopoietic cell disorders. Despite progress in leukemia therapy, most adult patients with acute leukemia still die from disease progression. Approximately 13,780 new cases of AML were estimated to be diagnosed in the US in 2012, while an estimated 10,200 died from this disease during that year1. Treatment outcomes in AML remain poor, particularly in the relapsed or refractory setting2. Because of continued poor prognosis, predominance of older patients who are unable to tolerate intensive chemotherapy, and high relapse rates despite achieving complete remission (CR), novel therapies are urgently needed in both the upfront and relapsed settings. Assorted molecular markers have been identified in AML that represent potential therapeutic targets. One example is FMS-like tyrosine kinase 3 (FLT3), which harbors a constitutively active mutation in approximately 25%–30% of AML patients3. Consequently, several FLT3 inhibitors are in clinical trials in AML. Another set of molecular lesions that have received increasing attention and research focus are activating mutations in the genes encoding the Janus kinase (JAK) family of enzymes, which have been initially identified as the main drivers of pathogenesis for BCR-ABL1-negative 'classic' myeloproliferative neoplasms (MPNs)4–7. In addition to its importance in MPNs, the JAK-STAT pathway has been found to be dysregulated in a number of different hematologic malignancies8, including AML9 and acute lymphoid leukemia (ALL) 10–12. Further, JAKs have been identified as potential targets for development of novel therapies for AML on the basis of pre-clinical studies implicating abnormalities in JAK family kinases in progression/transformation to AML or ALL (either from antecedent MPN or de novo)13–16. Ruxolitinib, a potent, selective JAK1 and JAK2 inhibitor was approved by the US Food and Drug Administration for the treatment of intermediate- or high-risk myelofibrosis in 2011 and is being actively investigated as a treatment for patients with acute leukemia and other hematologic malignancies17–19. Notably, MPN patients derive clinically meaningful benefit, including enhanced survival, from ruxolitinib regardless of whether they harbor the JAK2V617F mutation, an important driver of pathogenesis in MPNs. The fact that the salutary effects of ruxolitinib are independent of the presence of a mutant clone in MPNs denotes that this drug inhibits the entire JAK-STAT pathway, which is universally overactive in these neoplasms, a situation distinct from most of the other kinase inhibitors (the activity of which is almost totally dependent on the presence of a mutated enzyme).
Recently, investigators from our group studied ruxolitinib in a phase 2 study20 enrolling patients with relapsed myeloid malignancies, including MDS (n=1), CMML (n=4), CML (n=2), ALL (n=3) and AML (n=28) at a starting dose of 25 mg given orally twice daily (BID), with dose escalation up to 50 mg BID (20). In that study of 28 patients with AML (18 with AML secondary to MPN) who had received a median of 2 (range, 1–4) prior lines of therapy, 3 had a complete response (CR; one with incomplete recovery of peripheral blood counts [CRp]). In all 3 responding patients, the response occurred in the absence of preceding myelosuppression, and disappearance of blasts from the BM occurred slowly over a varying number of cycles. Responding patients also experienced a significant reduction in spleen size and improvement in their quality of life. Overall, ruxolitinib given at 25 and 50 mg BID was well tolerated, with only 4 patients developing grade 3 or 4 toxicities. In the context of that phase 2 pilot trial, because ruxolitinib was overall well tolerated at these lower doses, we hypothesized that even higher doses of ruxolitinib might also be tolerated and potentially yield further clinical benefit in acute leukemia patients. In the present study, we sought to determine the safety, maximum tolerated dose (MTD), and dose-limiting toxicity (DLT) of ruxolitinib at doses of 50, 100, and 200 mg BID in patients with relapsed/refractory acute leukemia.
Patients and Methods
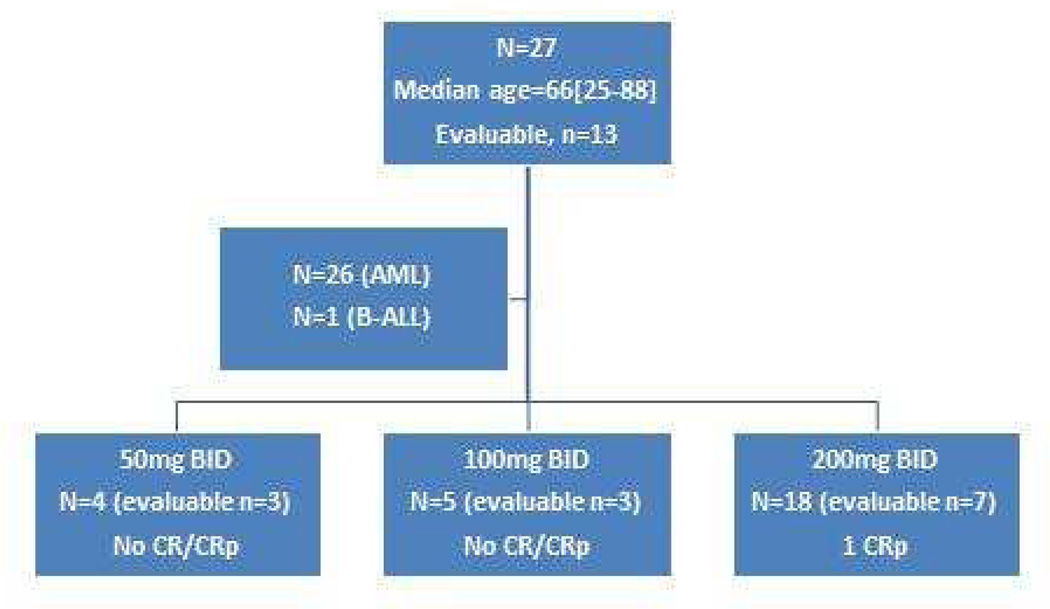
This phase I/II clinical trial enrolled patients with relapsed or refractory acute leukemia who were treated at The University of Texas MD Anderson Cancer Center (MDACC). The primary objectives were to determine safety, including DLT and MTD of ruxolitinib in patients with relapsed/refractory AML or ALL, as well as to describe the clinical activity of ruxolitinib in this patient population. Inclusion criteria included the following: age >14 years; diagnosis of relapsed or refractory AML or ALL; adequate organ function (ALT and/or AST ≤ 1.5× upper limit of normal and serum creatinine ≤ 2.5mg/dL); Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤ 2; a period of at least 2 weeks having elapsed since the last specific antileukemic therapy (with the exception of hydroxyurea); and no other active malignancies (with the exception of basal cell or squamous cell carcinoma of the skin, or carcinoma “in situ” of the cervix or breast). Patients with known HIV positivity, active hepatitis A, B, or C infection, or serious psychiatric illness that would prevent them from yielding informed consent were excluded. Also excluded were pregnant or lactating women and patients with acute promyelocytic leukemia. The study was approved by the MDACC institutional review board and was conducted according to the principles of the Declaration of Helsinki. All patients signed written informed consent. Figure 1 depicts the patient enrollment algorithm in this clinical trial and includes disposition by study drug dosage groups.
Figure 1.
Clinical Study Algorithm for Phase I/II Study of Ruxolitinib in Patients with Relapsed/Refractory Acute Leukemia
The starting dose of ruxolitinib was 50 mg BID. Patients received daily oral ruxolitinib continuously in 28-day cycles. In the Phase I part of the study, cohorts of three patients were enrolled at each ruxolitinib dose level with expansion to six subjects if necessary to assess toxicity. Subjects at each dose level were treated and observed through the end of the first cycle before treatment of subjects at the next higher dose level of ruxolitinib began. Patients remained on study treatment in the absence of disease progression or toxicity warranting discontinuation of therapy as long as there was evidence of clinical benefit, as judged by the treating physician. Adverse events were assessed on a continuous basis using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 (NCI-CTCAE, v4.0). The dose for each level was determined based on the treatment-related toxicities observed during the first cycle of treatment (at the prior dose level) using the following guidelines for a standard 3+3 study design: if no DLT is observed during the first month of therapy, the dose will be increased by up to 100%; if treatment-related non-hematologic Grade 2 toxicity is observed at any dose level, dose escalation will be adjusted by up to 50%; the dose will be escalated up to a maximum dose of 200 mg BID or until the MTD is reach; when the MTD is reached, additional patients with AML and ALL can added at the MTD level to confirm tolerability and explore efficacy, for a total of 14 patients with each disease type.
A non-hematologic DLT was defined as a clinically significant grade 3 or 4 adverse event or abnormal laboratory value occurring during the first 28 days on study that is assessed by the treating physician as related to study drug (and unrelated to disease progression, intercurrent illness, or concomitant medications). Myelosuppression and associated complications are expected events during leukemia therapy and are part of the treatment goals (marrow emptying of leukemia cells). Therefore, myelosuppression and associated complications such as fever, infections, bleeding, and related hospitalization were reported in the study summary, but not considered as DLTs. Only prolonged myelosuppression, as defined by the NCI-CTCAE, v4.0 criteria specific for leukemia (marrow cellularity of <5% on day 42 or later from start of therapy, without evidence of leukemia) were reported as DLT.
Patients who received at least 80% of the originally assigned doses in the first cycle were evaluable for DLT assessment of each cohort. Patients who received less than 80% of originally assigned doses in the first cycle for reasons other than DLT were replaced. The response criteria followed definitions of complete remission (CR) as set by the International Working Group (IWG) criteria21, aiming at the mere documentation of response (rather than a formal assessment of efficacy). Serial assessments of blood counts and chemistry laboratory tests were performed at least weekly during cycle 1, at the start of cycles 2 and 3, and then with every other cycle. Bone marrow aspirates were performed at screening, on Day 28 of the first cycle, and then as needed to confirm response or when clinically indicated.
Results
Patient Characteristics
Twenty-seven patients with acute leukemia were sequentially enrolled in 3 cohorts [50 mg (n=4), 100 mg (n=5), 200 mg (n=18)] from December 2010 to June 2012. The study was stopped by the principal investigator before enrollment of all planned patients due to a lack of satisfactory clinical benefit even in patients treated at the highest dose (200 mg BID). Baseline characteristics of all patients on study are shown in Table 1. Twenty-six patients had AML and 1 patient had pre-B cell-ALL. Median age at diagnosis was 66 years (range, 25–88 years). The majority of patients (23 patients, 85%) were treated in salvage 2 (S2) or beyond: S2-S3 (n=11), ≥S4 (n=12). Seven patients had a prior non-leukemia malignancy (mantle cell lymphoma, breast, colon, lung, prostate, melanoma, T-cell lymphoma) and had been treated with chemotherapy, radiation, or surgery (or combinations thereof). Sixteen patients had a prior diagnosis of MPN and/or MDS. Among all 27 patients enrolled, the median number of treatment cycles received was 1 (range, 1–4). Of these, 13 patients who received therapy for at least one month were evaluable for DLT as well as response assessments (all with AML; Table 2). Fourteen patients who had discontinued treatment before completing cycle 1 were not evaluable for DLT: 6 of the inevaluable patients died while on study, and 8 had progressive disease. Among all patients (n=27), the 4- and 8-week mortality rates were 37% and 52%, respectively. Two patients died from Stenotrophomonas maltophilia pneumonia, 1 from Streptococcus viridans sepsis/acute stroke, 1 from myocardial infarction, and 2 of unknown causes (died at an outside hospital or home). The median number of prior therapies for the 6 patients that died was 3, and 4 of 6 patients had a PS of 2.
Table 1.
Baseline characteristics of acute leukemia patients treated with ruxolitinib (n=27)
| Baseline Characteristics of All Patients (N=27) N (%) [range] |
||||
|---|---|---|---|---|
| Ruxolitinib (mg, po, BID) | ||||
| 50 | 100 | 200 | Overall | |
| Total # enrolled | 4 | 5 | 18 | 27 |
| Also on hydrea | 1 | 4 | 6 | 11 |
| AML | 4 | 5 | 17 | 26 |
| Pre-B ALL | 0 | 0 | 1 | 1 |
| Age, y | 62 [51 – 69] | 70 [41 – 85] | 67 [25 – 88] | 66 [25 – 88] |
| PS = 2 | 3 (75) | 1 (20) | 7 (39) | 11 (41) |
| Splenomegaly, # of patients | 1 | 1 | 3 | 5 (19) |
| Hb, g/dL | 9.1 [8.5 – 10.6] | 9.7 [9.1 – 10.9] | 9.2 [7.9 – 10.6] | 9.2 [7.9 – 10.9] |
| Plt × 109/L | 31 [17 – 161] | 62 [10 – 207] | 17 [3 – 271] | 22 [3 – 271] |
| WBC × 109/L | 10.9 [2.1 – 34.8] | 19.2 [13.3 – 42.8] | 3.9 [0.3 – 34.6] | 7.4 [0.3 – 42.8] |
| % Blast (BM) | 39 [1 – 75] | 78 [10 – 95] | 51 [15 – 99] | 56 [1 – 99] |
| Diploid | 1 (25) | 2 (40) | 6 (33) | 9 (33) |
| +8 | 0 | 0 | 1 (6) | 1 (4) |
| −5/−7/Both | 1 (25) | 1 (20) | 6 (33) | 8 (30) |
| abn11q | 0 | 1 (20) | 0 | 1 (4) |
| MISC | 2 (50) | 1 (20) | 5 (28) | 8 (30) |
| JAK2 = V617F | 3 (75) | 0 | 2 (11) | 5 (19) |
| FLT3 = ITD | 0 | 1 (20) | 0 | 1 (4) |
| S1 | 0 | 0 | 4 (22) | 4 (15) |
| S2–S3 | 3 (75) | 2 (40) | 6 (33) | 11 (41) |
| ≥ S4 | 1 (25) | 3 (60) | 8 (44) | 12 (44) |
Abbreviations used: po, BID = orally, twice per day; PS = performance status; Hb = hemoglobin; Plt = platlets; WBC = white blood cell count; S1, S2-S3, S≥ 4 = Salvage treatment #1, 2, 3, 4 and beyond
Table 2.
Baseline characteristics and outcomes of AML patients evaluable for DLT and efficacy assessments (n=13)
| Pt | Age | Prior dx |
# prior tx |
Ruxo dose |
cyto | PS | # courses |
Response/Current Status |
COD | OS (wks) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51 | PV | 3 | 50 | complex | 2 | 2 | NR/died | PNA/DAH | 8 |
| 2 | 66 | CMML | 3 | 50 | −7 | 2 | 1 | NR/died | CVA/cerebral edema | 6 |
| 3 | 69 | MDS | 4 | 50 | del6 | 1 | 4 | NR/died | E. coli sepsis | 15 |
| 4 | 70 | PV | 3 | 100 | del6 | 2 | 1 | NR/died | unknown | 22 |
| 5 | 41 | none | 8 | 100 | Abnl11q | 1 | 1 | NR/died | PNA/DAH | 4 |
| 6 | 85 | MDS | 5 | 100 | dip | 1 | 1 | NR/died | PNA | 4 |
| 7 | 81 | Colon cancer | 3 | 200 | dip | 1 | 3 | NR/died | PNA | 12 |
| 8 | 55 | MF | 2 | 200 | complex | 1 | 1 | NR/died | unknown | 35 |
| 9 | 83 | MDS | 4 | 200 | dip | 1 | 1 | NR/died | unknown | 5 |
| 10 | 67 | ET/MF | 1 | 200 | dip | 2 | 4 | NR/died | unknown | 17 |
| 11 | 71 | ET | 1 | 200 | complex | 2 | 3 | NR/died | unknown | 13 |
| 12 | 66 | MDS | 7 | 200 | +8 | 1 | 3 | CRp/died | unknown | 12 |
| 13 | 75 | MDS | 7 | 200 | Dip | 1 | 2 | NR/alive | Alive | 16 |
Abbreviations used: NR=Non-responder, CRp=complete remission with incomplete recovery of platelet count, PNA=pneumonia, DAH=diffuse alveolar hemorrhage, CVA =cerebrovascular accident (stroke)
Adverse Events
Of the 13 patients evaluable for DLT, only one required a dose reduction during the study period (from 200 to 150 mg BID during cycle 3 due to grade 3 drug-related thrombocytopenia). Therefore, this study did not achieve a goal of identifying formal DLT for ruxolitinib in acute leukemia. Table 3 lists all ≥ grade 3 adverse events (AEs). Among all 27 patients, the most common non-hematologic ≥ grade 3 event was infection, which occurred in 21 of 27 patients (4 of these 21 patients had multiple infectious events). These infectious events (n=26) were pneumonia (n=15, of which 4 were fungal pneumonia), sepsis (n=5), sepsis with another infectious complication (n=2), and other infection, including viral infection and cellulitis (n=4). The majority of these infectious events (19/26, 73%) occurred in patients in the 200 mg BID dose cohort, which corresponded to a majority of patients receiving this dose. The most common hematologic AEs ≥ grade 3 were thrombocytopenia (n=10, 1 possibly caused by hydroxyurea treatment) and neutropenia (n=9, 1 possibly caused by hydroxyurea treatment). Other grade 3 or 4 events included stroke (n=3; all three were ischemic), cerebral edema (n=1), fatigue (n=1), mucositis (n=1), and elevation of alkaline phosphatase (n=1).
Table 3.
Adverse Events (AE) Grade ≥3
| AE Grade ≥3 | ||||
|---|---|---|---|---|
| Ruxolitinib (mg, PO, BID) | ||||
| N=27 patients | 50 (n=4) |
100 (n=5) |
200 (n=18) |
Overall (n=27) |
| Thrombocytopenia | 2 | 3 | 6 | 11 |
| Neutropenia | 2 | 2 | 6 | 10 |
| Stroke (ischemic) | 1 | 0 | 2 | 3 |
| Hemorrhage (Pulmonary) | 1 | 1 | 0 | 2 |
| Alkaline phosphatase elevation | 1 | 0 | 0 | 1 |
| Other liver test abrnomality (ALT, AST, Total bilirubin) | 0 | 1 | 0 | 1 |
| Cerebral edema | 1 | 0 | 0 | 1 |
| Fatigue | 0 | 0 | 1 | 1 |
| Mucositis | 0 | 0 | 1 | 1 |
| Headache | 0 | 0 | 1 | 1 |
| INFECTIONS: | ||||
| Pneumonia | 3 | 1 | 11 | 15 |
| Sepsis | 0 | 1 | 4 | 5 |
| Sepsis + other infection | 1 | 0 | 1 | 2 |
| Other (Viral, cellulitis) | 1 | 0 | 3 | 4 |
Efficacy
After a median follow-up of 27 weeks (range, 11–48 weeks), no CR was observed. The study was stopped due to a lack of satisfactory clinical benefit, as judged by the principal investigator. One 68 year-old patient with AML in the 200 mg BID cohort had a CRp after 2 cycles of therapy (out of a total of 3 cycles given). This patient had received 7 prior therapies for MDS/AML, had trisomy 8 cytogenetic abnormality, and was JAK2V617F-mutation negative. Seven patients were diagnosed with a classical MPN prior to the AML diagnosis, 4 of whom (57%) were JAK2V617F-mutation positive at time of enrollment. Among all patients, 5 tested positive for the JAK2V617F mutation (4 patients with classical MPNs and 1 with CMML). Of these, 4 were evaluable for response. The median number of cycles of ruxolitinib in these patients was 1 (range, 1–3). None of the MPN patients or those with a JAK2V617F mutation achieved an objective response; however, one patient who was on ruxolitinib for 3 cycles experienced a reduction in spleen size and significant improvement in bone pain.
Discussion
In this cohort of heavily pre-treated patients with acute leukemia, we found that ruxolitinib administration at doses considerably higher than those used for its FDA-approved indication was deliverable, but only one patient, on the highest dose level (200 mg BID), had a response. Although this patient had a transient response (CRp for 1 cycle), it is notable that this patient had received 7 prior therapies. The most common grade 3 and 4 adverse events were infections, with pneumonia being the most common infectious event. This observation is probably explained by several factors. First, this study included patients with multiply-relapsed or refractory acute leukemia (85% of patients had had 2 or more relapses), a group with poor prognosis who are at increased risk for both opportunistic and classic bacterial infections. For example, Atallah et al. analyzed data from a large group of patients with AML treated in the frontline setting who received induction chemotherapy 22. In that study, 723 (47%) patients were older than 60 years. Although this group of patients were newly diagnosed and untreated, baseline rates of toxicity were still quite remarkable, with 86% of patients experiencing grade 3 to 4 cytopenias at baseline prior to therapy. In addition 28% of patients were admitted to the intensive care unit and 20% of patients died. In a study focusing on relapsed patients (similar to the present study), Ravandi et al studied a group of patients with AML who did not respond to a course of a high-dose cytarabine-based regimen during induction23. In that study of 285 patients with primary refractory AML, the median overall survival was only 3.8 months with multiple associated complications. These two studies highlight the expected high rates of co-morbidities and complications often encountered in patients with AML. Second, because JAK-STAT signaling and cytokine activity are important for immune system function8, JAK inhibition and concomitant down-regulation of certain chemokine- and cytokine-dependent pathways in cells responsible for immune competence and surveillance may underlie an increased risk of infection at extremely high ruxolitinib doses24, 25.
Future directions for clinical studies using ruxolitinib in patients with relapsed or refractory acute leukemia (more specifically post-MPN AML) should focus on investigation of regimens combining ruxolitinib at lower doses (i.e., 25 or 50 mg BID) with other agents (e.g., hypomethylating agents, cytoreductive therapies, or other targeted therapies) because higher doses do not appear to be more effective. To that extent, the Myeloproliferative Disease Consortium has a study underway combining low dose ruxolitinib and decitabine as frontline therapy for patients with post-MPN AML. It would also be of interest to study the possibility of pre-selecting patients with acute leukemia based on molecular biomarkers (genomic, transcriptomic or proteomic) that denote activation of the JAK/STAT cascade in a given patient. Such studies would be pivotal to clarify the exact role of inhibition of this pathway as a therapeutic approach in acute leukemia, not only in the salvage setting, but eventually in front-line regimens.
Clinical Practice Points.
Ruxolitinib, a potent JAK 1/2 inhibitor, is approved for treatment of intermediate to high-risk myelofibrosis
We investigated ruxolitinib for treatment in patients with relapsed acute leukemia
Ruxolitinib, at higher doses than previously utilized, was overall reasonably well tolerated, with one patient achieving CRp
Acknowledgements
This research is supported in part by a Cancer Center Support Grant from the National Cancer Institute (CA016672), as well as Incyte Corporation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures/Conflicts: JC, FR, and SV have received research support from Incyte Corporation for conduct of clinical research studies.
Authorship Contributions: SV and HK designed the study. NP and SV wrote the manuscript. NP, SP, SD provided data collection and statistical analysis. TK, JC, GB, GGM, FR, EJ, SV contributed and treated patients on the study. All authors provided critical review and analysis of the final manuscript prior to publication.
References
- 1.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2.Burnett AK. Treatment of acute myeloid leukemia: are we making progress? Hematology Am Soc Hematol Educ Program. 2012;2012:1–6. doi: 10.1182/asheducation-2012.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Pemmaraju N, Kantarjian H, Ravandi F, Cortes J. FLT3 inhibitors in the treatment of acute myeloid leukemia: the start of an era? Cancer. 2011;117:3293–3304. doi: 10.1002/cncr.25908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 6.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 7.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 8.O'Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368:161–170. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiang Z, Zhao Y, Mitaksov V, et al. Identification of somatic JAK1 mutations in patients with acute myeloid leukemia. Blood. 2008;111:4809–4812. doi: 10.1182/blood-2007-05-090308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flex E, Petrangeli V, Stella L, et al. Somatically acquired JAK1 mutations in adult acute lymphoblastic leukemia. J Exp Med. 2008;205:751–758. doi: 10.1084/jem.20072182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asnafi V, Le Noir S, Lhermitte L, et al. JAK1 mutations are not frequent events in adult T-ALL: a GRAALL study. Br J Haematol. 2010;148:178–179. doi: 10.1111/j.1365-2141.2009.07912.x. [DOI] [PubMed] [Google Scholar]
- 12.Mullighan CG, Zhang J, Harvey RC, et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2009;106:9414–9418. doi: 10.1073/pnas.0811761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee HJ, Daver N, Kantarjian HM, Verstovsek S, Ravandi F. The Role of JAK Pathway Dysregulation in the Pathogenesis and Treatment of Acute Myeloid Leukemia. Clin Cancer Res. 2013;19:327–335. doi: 10.1158/1078-0432.CCR-12-2087. [DOI] [PubMed] [Google Scholar]
- 14.Lee JW, Kim YG, Soung YH, et al. The JAK2 V617F mutation in de novo acute myelogenous leukemias. Oncogene. 2006;25:1434–1436. doi: 10.1038/sj.onc.1209163. [DOI] [PubMed] [Google Scholar]
- 15.Walters DK, Mercher T, Gu TL, et al. Activating alleles of JAK3 in acute megakaryoblastic leukemia. Cancer Cell. 2006;10:65–75. doi: 10.1016/j.ccr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Ikezoe T, Kojima S, Furihata M, et al. Expression of p-JAK2 predicts clinical outcome and is a potential molecular target of acute myelogenous leukemia. Int J Cancer. 2011;129:2512–2521. doi: 10.1002/ijc.25910. [DOI] [PubMed] [Google Scholar]
- 17.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 20.Eghtedar A, Verstovsek S, Estrov Z, et al. Phase 2 study of the JAK kinase inhibitor ruxolitinib in patients with refractory leukemias, including postmyeloproliferative neoplasm acute myeloid leukemia. Blood. 2012;119:4614–4618. doi: 10.1182/blood-2011-12-400051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 22.Atallah E, Cortes J, O'Brien S, et al. Establishment of baseline toxicity expectations with standard frontline chemotherapy in acute myelogenous leukemia. Blood. 2007;110:3547–3551. doi: 10.1182/blood-2007-06-095844. [DOI] [PubMed] [Google Scholar]
- 23.Ravandi F, Cortes J, Faderl S, et al. Characteristics and outcome of patients with acute myeloid leukemia refractory to 1 cycle of high-dose cytarabine-based induction chemotherapy. Blood. 2010;116:5818–5823. doi: 10.1182/blood-2010-07-296392. quiz 6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heine A, Brossart P, Wolf D. Ruxolitinib is a potent immunosuppressive compound: is it time for anti-infective prophylaxis? Blood. 2013;122:3843–3844. doi: 10.1182/blood-2013-10-531103. [DOI] [PubMed] [Google Scholar]
- 25.Heine A, Held SA, Daecke SN, et al. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo. Blood. 2013;122:1192–1202. doi: 10.1182/blood-2013-03-484642. [DOI] [PubMed] [Google Scholar]