Abstract
Our understanding of the role of somatosensory feedback in regulating motility during chicken embryogenesis and fetal development in general has been hampered by the lack of an approach to selectively alter specific sensory modalities. In adult mammals, pyridoxine overdose has been shown to cause a peripheral sensory neuropathy characterized by a loss of both muscle and cutaneous afferents, but predominated by a loss of proprioception. We have begun to explore the sensitivity of the nervous system in chicken embryos to the application of pyridoxine on embryonic days 7 and 8, after sensory neurons in the lumbosacral region become post-mitotic. Upon examination of the spinal cord, DRG and peripheral nerves, we find that pyridoxine causes a loss of TrkC-positive neurons, a decrease in the diameter of the muscle innervating nerve tibialis, and a reduction in the number of large diameter axons in this nerve. However, we found no change in the number of Substance P or CGRP-positive neurons, the number of motor neurons or the diameter or axonal composition of the femoral cutaneous nerve. Therefore, pyridoxine causes a peripheral sensory neuropathy in embryonic chickens largely consistent with its effects in adult mammals. However, the lesion may be more restricted to proprioception in the chicken embryo. Therefore, pyridoxine lesion induced during embryogenesis in the chicken embryo can be used to asses how the loss of sensation, largely proprioception, alters spontaneous embryonic motility and subsequent motor development.
Keywords: pyridoxine, proprioception, DRG, motor neurons, chicken embryo, TrkC
Studies on chicken embryos have been instrumental for gaining an understanding of the development of motor activity during embryogenesis (Bekoff et al., 1975; O’Donovan and Landmesser, 1987; Sharp et al., 1999; Bradley, 2001; Bradley et al., 2005). However, we do not have a clear understanding of how sensory information modulates spontaneous embryonic movement. It has been shown that sensory synapses are present in the lumbosacral spinal cord as early as embryonic day (E) 7 in the chicken embryo (Lee et al., 1988; Davis et al., 1989) and that peripheral stimulation can initiate reflexic activity starting at this time (Oppenheim, 1972). Additionally, behavioral observations and manipulations have suggested that somatosensory feedback may be used to modify embryonic leg and wing motility as early as E9 (Bradley, 1997; Sharp et al., 1999). To verify that sensation can modulate embryonic motility, it is important to develop a model that alters or eliminates specifically a defined population of somatosensory neurons.
It has been know since the 1940s that large dosages of pyridoxine, the essential vitamin B6, cause a significant peripheral neuropathy in adult mammals resulting in severe ataxia (Antropol and Tarlov, 1942). The effects of pyridoxine toxicity have been demonstrated in humans (Schaumburg et al., 1983), dogs (Antropol and Tarlov, 1942), cats (Stapley et al., 2002; Pearson et al., 2003), guinea pigs (Xu et al., 1989) and rats (Antropol and Tarlov, 1942; Xu et al., 1989; Helgren et al., 1997). Pyridoxine lesion in mammals is characterized by the loss of sensory neurons with large diameter axons. This sensitivity to pyridoxine is present in both skin and muscle innervating populations. Despite the loss of cutaneous sensory neurons, pyridoxine lesion results predominantly in a loss of proprioception from a behavioral perspective. Interestingly, pyridoxine does not appear to be toxic to motor neurons. Despite the lack of a mechanistic understanding of pyridoxine toxicity, pyridoxine overdose has been used in cats to study the role of sensation in response to postural perturbations (Stapley et al., 2002) and recovery from peripheral injury (Pearson et al., 2003).
It should be noted that pyridoxine is often used to ameliorate nausea during pregnancy in humans (up to 100 mg/day). The use of pyridoxine during pregnancy is somewhat controversial, but Shrim and colleagues (2006) have shown that pyridoxine usage during pregnancy does not give rise to major malformations in new born children. However, more subtle neurological effects, such as a reduction in proprioception, were not examined.
The goal of this study was to begin to characterize the effects of pyridoxine administration on the peripheral nervous system of embryonic chickens to determine if pyridoxine lesion can be used to study the role of sensory feedback during embryogenesis. Since these studies are likely to take place on E9 or later after sensory synapses are formed, it was necessary to begin pyridoxine application prior to that time. However, it was important to begin administration of pyridoxine after sensory neurons become post-mitotic around E6 (Hamburger et al., 1981). This was to avoid the complication of additional cell divisions replacing neurons that had been killed by pyridoxine. Therefore, pyridoxine was administered on E7 and E8 and the effects on motor neurons and various sensory neuron types were assayed on E13 allowing time for the removal of pyridoxine-sensitive neurons.
EXPERIMENTIAL PROCEDURES
Incubation and pyridoxine treatment
All procedures on chicken embryo were in compliance with the guidelines of the Southern Illinois University Institutional Animal Care and Use Committee.
White leghorn chicken eggs (Charles River Laboratories) were incubated on their sides in a forced-air incubator (99.5°F, 55% relative humidity) until E7. Eggs were then removed from the incubator and candled. A location free of underlying blood vessels, towards the pointed end of the egg, was marked. A fine file was used to create a groove in the shell at the marked location up to the shell membrane. For control embryos, 100 μl of 0.1 M phosphate buffered saline (PBS: 25 mM NaH2PO4·H2O, 75 mM Na2HPO4, 150 mM NaCl, pH 7.2) was then injected under the shell membrane through a 32 gauge syringe needle. Animals treated with pyridoxine were injected with 3.75 mg of pyridoxine hydrochloride (Sigma) dissolved in 100 μm of PBS. This dosage was selected after a preliminary dose-response study (see Results). Holes were sealed with a small piece of label tape and the eggs were returned to the incubator. Injections were repeated on E8 after candling to ensure embryo viability.
Tissue collection
Eggs were removed from the incubator on E13 and cracked into a small glass dish. The membranes were quickly incised and the embryos decapitated. Then, the bodies were rinsed in PBS, pinned in small dissecting dishes and eviscerated. An incision through the ventral vertebrae of the lumbosacral region was made with a scalpel blade to allow for better penetration of fixative. The spinal column was then severed at the last thoracic segment and the lumbosacral spinal column, including DRGs, was removed and placed into fixative. For comparisons of skin and muscle innervating nerves, the lateral branch of the femoral sensory nerve and the lateral branch of the tibialis nerve were dissected and placed into fixative. Care was taken such that no axons from a given nerve would be lost due to variations in branch points.
Tissue fixation
Spinal columns isolated for the various assays were fixed differently. Tissue for TrkC immunostaining and spinal morphology was emersion fixed in Bouin’s fixative for two days at 4°C and then washed in PBS (5X). Tissue for immunofluorescence was emersion fixed in 4% buffered paraformaldehyde (PBS, pH 7.2) overnight at 4°C and then rinsed in PBS (3X). Nerves collected for cross-sectional analysis were emersion fixed in 2.5% glutaraldehyde for 3-4 hours at 4°C and then rinsed in Sorensen’s phosphate buffer at 4°C for 24 hr. Tissue was then post-fixed in 1% osmium tetroxide (OsO4) for two hours at room temperature and rinsed twice in distilled water.
TrkC immunocytochemistry
Spinal columns used for TrkC immunocytochemistry were dehydrated and embedded in paraffin, sectioned at 10 μm on a rotary microtome and mounted on slides. The slides were then examined to identify those that contained the third lubosacral DRG. These slides were then deparaffinized and rehydrated.
The primary antiserum used (courtesy of Frances Lefcort, Montana State University) was generated previously against the extracellular domain of the chicken TrkC receptor and its specificity has been described (Lefcort et al., 1996). The standard ABC peroxidase method with diaminobenzidine visualization was used. Sections from two embryos, one control and one pyridoxine treated, were reacted simultaneously to control for variability in reaction intensity that can sometimes occur. Briefly, sections were treated with 0.5% hydrogen peroxide. Then, sections were placed in blocking solution (1% bovine serum albumin, 1% normal horse serum, 0.3% Triton X-100 in PBS) for one hour prior to reaction with the primary antiserum. Sections were incubated overnight at room temperature in the primary antiserum (diluted 1:1000 in blocking solution). Standard ABC protocols (Vector Labs) followed by diaminobenzidine reaction (12.5 mg/50 ml PBS with 30 μL H2O2) were used for visualization and slides were coverslipped with Permount.
Substance P and CGRP immunocytochemistry
Spinal columns for immunofluorescence were cryoprotected overnight in 20% sucrose (w/v) dissolved in PBS. Sections were cut at 14 μm on a cryostat and collected directly onto charged slides. Sections were collected alternately onto a series of three slides. Each series was examined under low power magnification and a series that contained completely intact sections of the third lumbosacral DRG was selected for immunofluorescence.
A mouse monoclonal antibody directed against amino acids 1-11 of human Substance P (SP-DE4-21, ab14184) and a rabbit polyclonal antibody to the full length rat CGRP (ab43873) were obtained commercially (abcam, Cambridge, MA). Standard dual-immunofluorescence methods were used. Briefly, sections were treated for one hour at room temperature with blocking solution (see above) then incubated over night at room temperature in a mixture of both primary antibodies diluted in blocking solution (anti-Substance P, 1:10,000; anti-CGRP, 1:5000). Lastly, sections were reacted for one hour in a mixture of Alexa Fluor 488 labeled donkey anti-mouse IgG and Alexa Fluor 546 labeled donkey anti-rabbit IgG each diluted 1:1000 in blocking solution. Slides were then coverslipped in Fluoromount G (Southern Biotechnology Associates, Birmingham, AL).
Toluidine Blue staining of peripheral nerves
Nerves were embedded in epoxy resin (Polybed 812 A (soft) and B (hard), 3:7, EMS) according to the procedure in Bozzola and Russell (1999). The blocks were thin sectioned using an ultramicrotome and stained with 1% Toluidine Blue O (EMS).
Cresyl Violet staining of spinal cords
For assaying the effects of pyridoxine on spinal cord morphology, slides containing sections from the second lumbosacral segment were used from the same animals as for TrkC immunolabeling. Sections were deparaffined, rehydrated, stained with cresyl violet and mounted with Permount.
Quantification
Cell counts were performed on every fourth section when the tissue was sectioned at 10 μm and every third section when sectioned at 14 μm. This allowed for similar sampling rates even thought no direct comparisons were made between sections of different thicknesses. To quantify the number of immuno-positive or cresyl violet stained neurons, neurons were only counted if they contained both cytoplasmic and nuclear compartments in the same section. This requirement along with the sectional sampling interval prevented the same cell from being counted twice.
Sampling errors can arise from differences in the numbers of sections obtained from each animal for analysis. This can be due to changes in the orientation of the DRG during sectioning or due to changes in the number of neurons (e.g. Fig. 1G). Therefore, total cell counts were normalized in proportion to the number of sections analyzed for each animal with respect to the greatest number of sections counted for any animal (total cell count X number of sections/greatest number of sections). Both uncorrected (uc) and corrected (c) counts are provided.
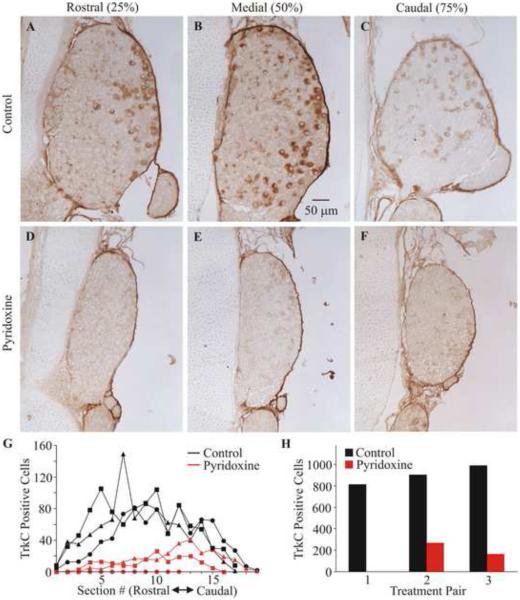
Fig. 1.
Effect of pyridoxine treatment on TrkC immuno-reactivity of neurons in the third lumbosacral DRG. Representative sections of the third lumbosacral DRG immuno-stained for TrkC are shown from a control (A-C) and a pyridoxine-treated (D-F) embryo. Sections are from positions 25%, 50% and 75% from the rostral to caudel extent of the DRG. Panel (G) shows a plot of the number (uc) of TrkC positive neurons in each assayed section for each of the animals. The symbol shape denotes the reaction pairs. The total number (uc) of TrkC positive neurons for each animal are shown grouped by pairs as they were reacted (H).
Sections stained with cresyl violet or immuno-labeled for TrkC were imaged at 200X with white light. Quantifications of neurons were performed by observing the sections directly on the microscope to allow for focal plane adjustments to be used during the quantifications. The images were then annotated to log cells that were counted.
Optical sections of tissues treated for immunofluorescence were imaged using structured illumination (200X, Zeiss AxioImager equipped with an Apotome) at 1 μm intervals. Fluorophores were imaged independently at each image plane. Image stacks were then assayed in ImageJ. Positive neurons were determined by manually moving between image planes and annotating the images where positive neurons were determined.
Nerve cross-sections were imaged at 630X with white light. Composite images of the nerves were composed using the photo merge function in Photoshop and then assayed in ImageJ. If sections were prepared ideally, such that each axon was cut in perfect cross-section, then the axons should appear as circles. However, most axons appear ovoid due to offsets in sectioning angle. Therefore, lines were drawn across the short axis of the ovoid as this best represents the diameter of the axon. These line lengths (axon diameters) were calculated by the ImageJ software and then tabulated.
Approximate cross-sectional areas of nerves were assayed in ImageJ. The outside of each nerve was traced by hand and the area was then calculated by the software in arbitrary units. It should be noted that these are approximations as we made no attempt to correct for the angle of sectioning.
Statistical comparisons between treatment groups were performed using the Student t-test. A p-value of less than 0.05 was considered to be significant. To protect against Type 1 errors when more than one dependent variable was analyzed per animal, the p-value was divided evenly between groups to produce a total group p-value of 0.05 (Bonferonni correction).
RESULTS
Selection of pyridoxine dosage
Since there was no direct way to equate pyridoxine dosage between chicken embryos and adult mammals, a preliminary dose-response series was performed to establish a dosage for this study. Embryos were treated with 1, 3.75, 7.5 or 15 mg of pyridoxine, 3 embryos at each concentration, on E7 and E8 and checked for viability on E13. All embryos treated with 15 mg of pyridoxine died. Two embryos treated with 7.5 mg of pyridoxine died, but one of these appeared to have died due to vascular damage from injection. All of the embryos injected with 1 or 3.75 mg of pyridoxine survived. All embryos that survived to E13 exhibited spontaneous motor activity, but these movements were not analyzed quantitatively. While there was no definitive evidence that pyridoxine at the higher concentrations was lethal, a dosage of 3.75 mg of pyridoxine was initially selected. As initial anatomical observation of the DRG showed apparent changes, this dosage was used for the entire study. A complete dose response relationship for pyridoxine would require a significantly greater study and was not our purpose here.
Effect on TrkC expression
The sensory neuropathy caused by pyridoxine overdose in mammals has its greatest effect on proprioception (Antropol and Tarlov, 1942; Helgren et al., 1997; Stapley et al., 2002; Pearson et al., 2003). Therefore, we began by assaying the effects of pyridoxine on TrkC receptor expressing neurons in the lumbosacral DRG using immunocytochemistry as proprioceptive neurons express the NT-3 specific TrkC receptor (Oakley et al., 1995). Figure 1 shows representative sections through the third lumbosacral DRG of a control and a pyridoxine treated animal. Notice that in the control animal there are TrkC positive neurons throughout the DRG. However, there is a greater apparent concentration of these neurons on the lateral side (the right side in Fig. 1) of the DRG. Pyridoxine caused a very obvious reduction in the cross sectional area of the DRG and a significant loss of TrkC positive neurons in the DRG (n=3, p=0.003 (uc), p=0.006 (c)). This loss was not specific to any one part of the DRG as can be seen both in the images and the plot of positive neurons as a function of rostral to caudal location in the DRG (Fig. 1G). The loss of TrkC positive neurons varied between 70% and 100% for these embryos (85 +/− 14% (uc), 84 +/− 16% (c); average +/− s.d.). The TrkC positive neuron counts for each of the three control and pyridoxine treated animals are shown as a function of location in the ganglion and as total counts (uc) to provide a more complete demonstration of the magnitude and generality of this effect.
Effect on Substance P and CGRP expression
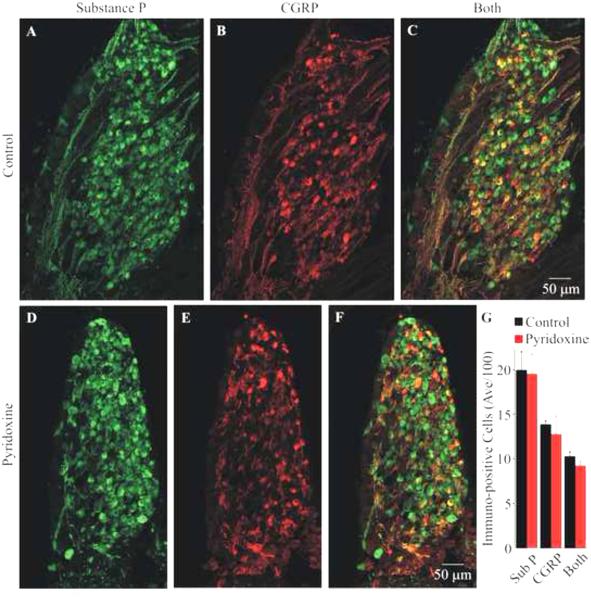
The largest populations of neurons in the DRG are small diameter pain and thermal sensitive neurons. Many of these neurons are known to express the peptides Substance P and/or CGRP (reviewed in Willis and Coggeshall, 2004). To determine the effect of pyridoxine on these populations of neurons, sections of control and pyridoxine treated DRGs were immunolabeled for both peptides. Figure 2 shows maximum projections of the medial section from the third lumbosacral DRG of a control and pyridoxine treated embryo. Notice that the cross-sectional area of the DRG was decreased by pyridoxine as was seen for the TrkC labeled DRGs. This was observed in all cases. The average and standard deviation of immuno-positive neurons (uc) are shown in Figure 2G. The corrected counts for Substance P, CGRP and both Substance P and CGRP for control vs. pyridoxine DRGs were 1660 +/− 478 vs. 1874 +/− 351, 1139 +/− 209 vs. 1229 +/− 279 and 860 +/− 139 vs. 876 +/− 124 (average +/− s.d.); respectively. Pyridoxine did not cause a significant change in the number of Substance P or CGRP positive neurons (p=0.848 and 0.440 (uc), p=0.566 and 0.677 (c); respectively).
Fig. 2.
Effect of pyridoxine treatment on Substance P and CGRP immuno-reactivity in the third lumbosacral DRG. Panels (A-C) show images of the immuno-reactivity for Substance P (green), CGRP (red) and a superposition of the images, respectively, from the medial section of a control DRG. Panels (D-F) show similar images from the medial section of a pyridoxine-treated embryo. All images are maximum projections from optical sections collected using structured illumination. Notice that the treated DRG is smaller than the control DRG. The total number of cells (uc) from all embryos (mean and standard deviation) expressing Substance P, CGRP or both are shown in (G). There was no significant loss of Substance P or CGRP expressing cells.
Effect on peripheral nerves
As pyridoxine caused a loss of sensory neurons, it would be expected to cause a decrease in the diameter of peripheral nerves containing the axons from the types of neurons killed by pyridoxine. As can be seen from the images in Figure 3A and B, pyridoxine caused slightly more than a 50% decrease in the diameter of the tibialis nerve, a muscle innervating nerve (n=3, p=0.006). However, there was no change in the diameter of the femoral cutaneous nerve, a skin innervating nerve (n=3, p=0.343). These changes in nerve diameter are consistent with the results from the immunocytochemistry results from the DRGs. While there was a very large change in the diameter of the tibialis nerve, it is important to remember that determining whether a section from a nerve is truly orthogonal to the nerve is difficult at best.
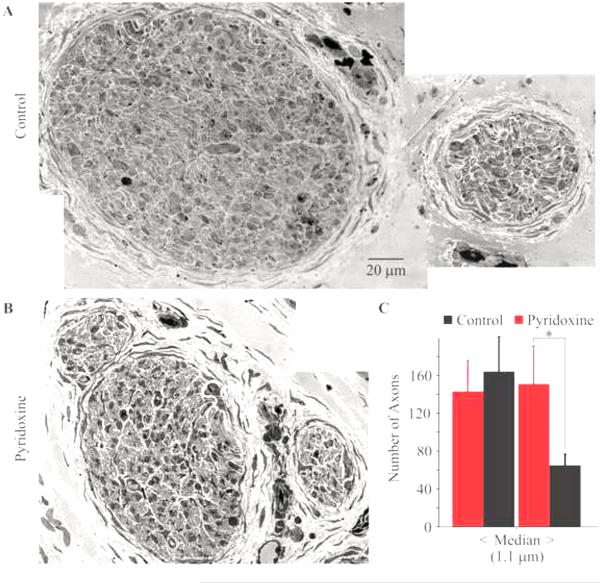
Fig. 3.
Effect of pyridoxine treatment on axon diameter distribution in the tibialis nerve. Representative cross-sections of the tibialis nerve from a control (A) and pyridoxine treated embryo (B). Images of the two nerves are at the same magnification. The average number of axons greater than and less than the median diameter are plotted in (C). Pyridoxine treatment caused a significant decrease in large diameter axons. The error bars represent the standard deviations. The asterisk denotes a statistically different change.
A more informative assay of changes in peripheral nerves is to assay for changes in the size distribution of axons in a nerve. The diameter of each axon from the same nerve cross-sections as described above was determined. Since pyridoxine kills neurons with large diameter axons in mammals, axonal diameters were tabulated as either greater than or less than the average median diameter determined from measurements of control axons. Pyridoxine caused a significant decrease in the number of axons larger than the median diameter of 1.1 μm (Fig. 3C) in the tibialis nerve (p=0.011), but did not cause a significant change in axon number for the smaller diameter groups (p=0.497). Together, this indicates that pyridoxine killed some of the larger axons rather than causing an axonal atrophy. Also, pyridoxine did not cause a significant change in either size range for the femoral cutaneous nerve. However, it should be noted that it was not possible to determine the diameter of axons less than 0.3 μm and most of the axons in the femoral cutaneous nerve were smaller than this. In contrast, very few axons in the tibialis nerve were too small to measure.
Effects on the spinal cord
The loss of large diameter axons in a muscle innervating nerve could be consistent with pyridoxine killing motor neurons in addition to large diameter proprioceptive neurons. Therefore, the number of motor neurons in one side of the second lumbosacral segment was assayed in the same embryos used for TrkC immunoreactivity. As can be seen in Figure 4C and D, pyridoxine caused the motor column to appear more condensed and the motor neurons appeared to be somewhat smaller. However, pyridoxine did not cause a significant change in the number of motor neurons (n=3; average +/− s.d.; control, 452 +/−17; pyridoxine, 448 +/−40; p=0.881).
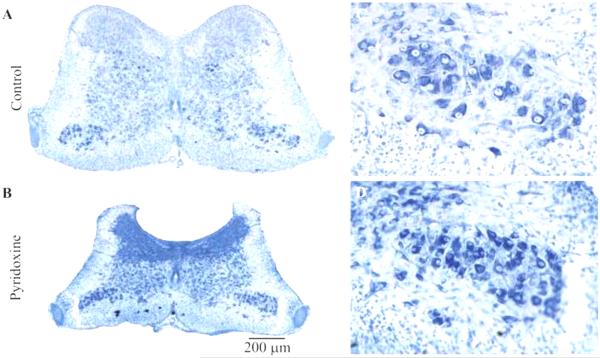
Fig. 4.
Effect of pyridoxine treatment on spinal cord morphology in the second lumbosacral segment. Images of representative sections of the lumbosacral spinal cord (LS2) stained with cresyl violet from a control (A) and a pyridoxine treated embryo (B). Images are at the same magnification. Notice the significant reduction in volume caused by pyridoxine. (C) and (D) show enlargements of the motor column from (A) and (B) respectively.
While the number of motor neurons was not altered by pyridoxine treatment, the general morphology of the spinal cord (Fig. 4A and B) was grossly altered. In particular, pyridoxine caused a decrease in the cross-sectional area of the spinal cord. The apparent density of nissl staining was increased and appears to be due to a greater density of somata. Additionally, the dorsal and lateral white matter appears to have been decreased in size. These decreases in volume could be the result of the loss of axons from proprioceptive neurons, but these observations will have to be explored further in future studies.
DISCUSSION
The goal of this study was to begin to describe the effects of high dosages of pyridoxine on embryonic chickens in the hope that pyridoxine lesion can be used as a selective peripheral sensory lesion in this model organism. Our findings are similar to those reported for pyridoxine lesion in adult mammals. Specifically, pyridoxine kills large diameter, TrkC-positive, sensory neurons, but spares motor neurons and small diameter, CGRP- and Substance P-positive sensory neurons. We found that the number of large diameter axons in the muscle innervating tibialis nerve was decreased by pyridoxine application. This decrease in large diameter axons did not represent axonal atrophy as the number of small diameter axons in the tibialis nerve did not increase. Interestingly, we did not find evidence for loss of large diameter sensory neurons that innervate the skin in chick embryos though this has been reported for adult mammals (Stapley et al., 2002).
Prior studies on the effects of pyridoxine have relied on assays of axon diameter and axon conduction velocities (e.g. Stapley et al., 2002; Pearson et al., 2003), but have not sought to describe pyridoxine-sensitive neurons based on specific molecular markers for subtypes of sensory neurons. While our findings are consistent with those from adult mammals, our findings demonstrate that TrkC positive neurons are likely the largest component of the pyridoxine-sensitive, large diameter sensory neuron population in chicken embryos. This is consistent with behavioral observations in adult mammals which indicate that proprioception is the sensory modality most affected by pyridoxine lesion. Interesting, Helgren and colleagues (1997) showed in rats that co-administration of NT-3, which binds specifically to the TrkC receptor, along with pyridoxine blocks the deleterious effects seen when pyridoxine is administered alone. While their intention appeared to have been to apply NT-3 as a general “neuroprotectant”, it raises the possibility that pyridoxine may exert its damaging effects via some step of the NT-3/TrkC signaling pathway that can be overcome by exogenously applied NT-3. Our findings do not demonstrate this as the mechanism of action for pyridoxine, but they certainly present an intriguing correlation.
We were not able to demonstrate that pyridoxine causes the loss of large diameter skin innervating neurons as has been found in adult mammals. This may be a trivial matter stemming from the possibility that at this stage of development pyridoxine-sensitive axons in cutaneous nerves are still smaller than our ability to resolve at the resolution allowed by light microscopy. Likewise, direct uptake of pyridoxine into the extra-embryonic blood supply could result in a somewhat different effects in chicken embryos as opposed to mammals that ingest pyridoxine or receive it through intra-muscular injection. However, there is a more interesting possibility related to the difference between chickens and mammals in the distribution of TrkC positive neurons. It has been shown that between 90 and 95% of TrkC positive neurons in chick embryos is muscle innervating (Oakley et al., 1995; 2000) and that these neurons comprise between 60 and 65% of muscle afferents (Oakley et al., 1995; 2000). TrkC-positive neurons that innervate the skin represent approximately 10% of total cutaneous afferents in rats (McMahon et al., 1994) whereas they represent only 2% of large cutaneous afferents in chicken (Oakley et al., 1995). Therefore, there are significantly fewer TrkC-positive cutaneous neurons in chicken embryos than in rats. It would be interesting to determine if the pyridoxine-sensitive neurons that innervate the skin of mammals are TrkC-positive as well.
For this study, we chose to treat embryos with pyridoxine beginning on E7 as sensory synapses begin to form in the spinal cord (Lee et al., 1988; Davis et al., 1989). There were several reasons for the timing used in this study. First, behavioral studies on chick embryos have indicated that sensory information may be modulating spontaneous embryonic behavior as early as E9. Therefore, it is important to initiate a sensory lesion prior to this interesting period of development in order to allow for the assessment of pyridoxine lesion on embryonic behavior. Next, to avoid replacement of neurons sensitive to pyridoxine from cells still undergoing mitosis, it was necessary to wait until most sensory neurons become post-mitotic on E6 (Hamburger et al., 1981) Lastly, it is possible that pyridoxine may affect sensory neurons through some NT-3/TrkC specific mechanism. It is known that many cutaneous neurons that will ultimately express TrkA or TrkB require NT-3 prior to entering the post-mitotic programmed cell death period (Lefcort et al., 1996) that occurs roughly at E6 in the lumbosacral region (Hamburger et al., 1981). Therefore, delaying treatment until E7 may help preserve a more specific, proprioceptive lesion. Conversely, it may be possible to generate a more generalized sensory lesion if administration of pyridoxine was performed even earlier.
CONCLUSION
In conclusion, pyridoxine administration during early chicken embryogenesis produces a large fiber sensory neuropathy similar to that produced in adult mammals. Since pyridoxine sensitivity is largely restricted to TrkC positive neurons in the chick, pyridoxine toxicity should result largely in a loss of proprioception. However, future studies will need to determine if some small population of neurons excluded from this study are sensitive to pyridoxine. Likewise, it will be important to determine to what degree muscle spindle afferents and golgi tendon organ afferents are affected as the distribution of these groups within the TrkC-positive muscle afferents has not been defined in chickens. None the less, the administration of pyridoxine during chicken embryogenesis should be a useful approach to study how somatosensory feedback contributes to embryonic motility and to determine the need for somatosensation for proper development of sensorimotor circuitry.
Highlights.
Embryonic pyridoxine administration kills TrkC-positive sensory neurons in chicken embryos.
Pyridoxine does not kill Substance P- or CGRP-positive sensory neurons or motor neurons.
Embryonic pyridoxine lesion is suitable for studies on the role of somatosensation in embryonic motility and motor development.
ACKNOWLEDGEMENTS
The authors would like to thank Robin Michaels and Maureen Doran for technical assistance. This work was supported by the National Institutes for Health (1 R03 HD039241 to A.A.S.) and the Southern Illinois University School of Medicine. Y.F. was supported by a Saluki Scholars Research Opportunity fellowship through the Southern Illinois University Honors Program.
Abbreviations
- DRG
dorsal root ganglion
- CGRP
calcitonin gene-related peptide
- E
embryonic day
- PBS
phosphate buffered saline
- TrkC
neurotrophic tyrosine kinase receptor type 3
- NT-3
neurotrophin-3
- s.d.
standard deviation of the mean
- uc
uncorrected
- c
corrected
- vs.
versus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Antropol W, Tarlov IM. Experimental study of the effects produced by large doses of vitamin B6. J Neuropathol Exp Neurol. 1942;1:330–336. [Google Scholar]
- Bekoff A, Stein PS, Hamburger V. Coordinated motor output in the hindlimb of the 7-day chick embryo. Proc Natl Acad Sci USA. 1975;72(4):1245–1248. doi: 10.1073/pnas.72.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzola JJ, Russell LD. Electron microscopy: principles and techniques for biologists. 2nd Jones and Bartlett Publishers, Inc.; Sudbury, MA: 1999. [Google Scholar]
- Bradley NS. Reduction in buoyancy alters parameters of motility in E9 chick embryos. Physiol Behav. 1997;62:591–595. doi: 10.1016/s0031-9384(97)00168-6. [DOI] [PubMed] [Google Scholar]
- Bradley NS. Age-related changes and condition-dependent modifications in distribution of limb movements during embryonic motility. J Neurophysiol. 2001;86(4):1511–1522. doi: 10.1152/jn.2001.86.4.1511. [DOI] [PubMed] [Google Scholar]
- Bradley NS, Solanki D, Zhao D. Limb movements during embryonic development in the chick: evidence for a continuum in limb motor control antecedent to locomotion. J Neurophysiol. 2005;94(6):4401–4411. doi: 10.1152/jn.00804.2005. [DOI] [PubMed] [Google Scholar]
- Davis BM, Frank E, Johnson FA, Scott SA. Development of central projections of lumbosacral sensory neurons in the chick. J Comp Neurol. 1989;279:556–566. doi: 10.1002/cne.902790405. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Brunso-Bechtold JK, Yip JW. Neuronal death in the spinal ganglia of the chick embryo and its reduction by nerve growth factor. J Neurosci. 1981;1:60–72. doi: 10.1523/JNEUROSCI.01-01-00060.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgren M, Cliffer K, Torrento K, Cavnor C, Curtis R, DiStefano P, Wiegand S, Lindsay R. Neurotrophin-3 administration attenuates deficits of pyridoxine-induced large-fiber sensory neuropathy. J Neurosci. 1997;17:372–382. doi: 10.1523/JNEUROSCI.17-01-00372.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MT, Koebbe MJ, O’Donovan MJ. The development of sensorimotor synaptic connections in the lumbosacral spinal cord of the chick embryo. J Neurosci. 1988;8:2530–2543. doi: 10.1523/JNEUROSCI.08-07-02530.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefcort F, Clary DO, Rusoff AC, Reichardt LF. Inhibition of the NT-3 receptor TrkC, early in chick embryogenesis, results in severe reductions in multiple neuronal subpopulations in the dorsal root ganglia. J Neurosci. 1996;16:3704–3713. doi: 10.1523/JNEUROSCI.16-11-03704.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Oakley RA, Garner AS, Large TH, Frank E. Muscle sensory neurons require neurotrophin-3 from peripheral tissues during the period of normal cell death. Development. 1995;121:1341–1350. doi: 10.1242/dev.121.5.1341. [DOI] [PubMed] [Google Scholar]
- Oakley RA, Lefcort FB, Plouffe P, Ritter A, Frank E. Neurotrophin-3 promotes the survival of a limited subpopulation of cutaneous sensory neurons. Dev Biol. 2000;224:415–427. doi: 10.1006/dbio.2000.9804. [DOI] [PubMed] [Google Scholar]
- O'Donovan MJ, Landmesser L. The development of hindlimb motor activity studied in the isolated spinal cord of the chick embryo. J Neurosci. 1987;7(10):3256–3264. doi: 10.1523/JNEUROSCI.07-10-03256.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RW. An experimental investigation of the possible role of tactile and proprioceptive stimulation in certain aspects of embryonic behavior in the chick. Dev Psychobiol. 1972;5:71–91. doi: 10.1002/dev.420050109. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Misiaszek JE, Hulliger M. Chemical ablation of sensory afferents in the walking system of the cat abolishes the capacity for functional recovery after peripheral nerve lesions. Exp Brain Res. 2003;150:50–60. doi: 10.1007/s00221-003-1445-1. [DOI] [PubMed] [Google Scholar]
- Schaumburg H, Kaplan J, Windebank A, Vick N, Rasmus S, Pleasure D, Brown MJ. Sensory neuropathy from pyridoxine abuse. N Engl J Med. 1983;309:445–448. doi: 10.1056/NEJM198308253090801. [DOI] [PubMed] [Google Scholar]
- Sharp AA, Ma E, Bekoff A. Developmental changes in leg coordination of the chick at embryonic days 9, 11, 13: uncoupling of ankle movements. J Neurophysiol. 1999;82:2406–2414. doi: 10.1152/jn.1999.82.5.2406. [DOI] [PubMed] [Google Scholar]
- Shrim A, Boskovic R, Maltepe C, Navios Y, Garcia-Bournissen F, Koren G. Pregnancy outcome following use of large doses of vitamin B6 in the first trimester. J Obstet Gynaecol. 2006;26:749–51. doi: 10.1080/01443610600955826. [DOI] [PubMed] [Google Scholar]
- Stapley PJ, Ting LH, Hulliger M, Macpherson JM. Automatic postural responses are delayed by pyridoxine-induced somatosensory loss. J Neurosci. 2002;22:5803–5807. doi: 10.1523/JNEUROSCI.22-14-05803.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Sensory mechanisms of the spinal cord: vol. 1: primary afferent neurons and the spinal dorsal horn. Kluwer Academic/Plenum Publishers; New York: 2004. [Google Scholar]
- Xu Y, Sladky JT, Brown MJ. Dose-dependent expression of neuronopathy after experimental pyridoxine intoxication. Neurology. 1989;39:1077–1083. doi: 10.1212/wnl.39.8.1077. [DOI] [PubMed] [Google Scholar]