Abstract
Background
While rarely used for supplementation trials in the U.S., schools present a practical alternative to a clinical setting.
Purpose
We describe the successful recruitment and retention of urban schoolchildren into a 6-month randomized, double-blind vitamin D3 supplementation trial.
Methods
Boston-area urban schoolchildren, aged 8-15 years, were recruited in 2011-2012 through classroom and auditorium presentations. Informed consent forms in five languages were sent home to parents. Retention methods included regular telephone calls and gift cards for completed study visits.
Results
In total, 691 schoolchildren enrolled. Their mean (SD) age was 11.7 (1.4) years; 59% were from racial/ethnic minorities and 68% qualified for free or reduced-price school meals. Multi-level, culturally-sensitive, creative approaches contributed to success in recruitment and retention. Of 691 participants, 81% completed the 6-month intervention period. Reasons for attrition included missed appointments and fear of a blood draw. More children from households with higher incomes were retained than those from households with lower incomes (85% vs. 79%, respectively, P=0.04).
Limitations
The need for three fasting blood draws over the 6-month supplementation period was a limiting factor in the recruitment and retention of children in this study.
Conclusions
Recruitment of urban children into a school-based randomized controlled trial represents a feasible approach for a supplementation study. Particular attention to children of lower socioeconomic status may enhance participation and retention when conducting intervention studies among diverse populations.
Keywords: recruitment, retention, school-based intervention, vitamin D, randomized controlled trial, children
Introduction
Well-designed recruitment and retention efforts are critical to the success of longitudinal research studies. Schools are a natural setting to conduct studies with children in part due to multiple levels of support,1, 2 but especially when recruiting typically hard-to-reach populations such as those of lower socioeconomic status and racial/ethnic minorities.3 Collecting questionnaire, anthropometric, and biological data1, 2 and distributing a dietary supplement in the school setting4, 5 may circumvent barriers associated with clinical settings, which may include low participation/recruitment,4, 6 poor compliance with a dietary supplement,4 poor attendance at study visits,6 and inadequate inclusion of the population of interest.5, 7 Moreover, conducting research within the familiar school setting can mitigate consequences of a possible distrust of medical research,8-10 which may be magnified when children and clinical procedures such as blood draws are involved.11, 12 Though schools have been used as the site for randomized supplementation trials internationally,13, 14 very few supplementation studies have been conducted within a school setting in the U.S.4, 5
Recruiting participants into school-based research requires a multi-level strategy1-3, 15 directed at the district, school, and individual parent and child levels. Understanding effective strategies for and barriers to recruitment and retention of children to a school-based randomized trial may provide valuable insight into a practical alternative to a clinical setting. Engagement of racial/ethnic minorities and individuals of lower socioeconomic status in research has been found to be impeded by a possible mistrust of scientific investigators,8 resulting in lower participation and higher dropout rates. In recent pediatric weight management interventions, for example, non-white non-Hispanic children were 1.5 times as likely to drop out as white non-Hispanic children,16 and lower household income predicted lower treatment attendance (β=0.265, P=0.03).17 Thus, strategies tailored to these specific populations are expected to promote recruitment and retention.
Previous research in Boston-area children has demonstrated a high prevalence of inadequate blood concentrations of vitamin D,18-20 assessed through the serum biomarker 25-hydroxyvitamin D [25(OH)D]. Vitamin D supplementation trials conducted in U.S. children and adolescents have reported improvements in vitamin D status21-23 and in other health markers such as fasting glucose24 and insulin.23 However, few have utilized a randomized double-blind controlled trial protocol,23, 25 and none has done so within schools. Thus, little is known about successful strategies for recruitment and retention when conducting a supplementation intervention in schools in the U.S. Our objective is to describe the methods of recruitment and retention of urban schoolchildren from racially-diverse and socioeconomically-challenged communities into a randomized, double-blind vitamin D3 supplementation trial, the Daily D Health Study, and to present the recruitment and retention results achieved.
Methods
Participants and study design
Schoolchildren in the 4th through 8th grades (aged 8-15 years) during the 2011-2012 and 2012-2013 school years were recruited from public elementary and middle schools in four urban school districts in the Boston, MA, area to participate in the study. Recruitment was conducted in two waves in fall 2011 and fall 2012; the same time period in both years was selected to control for seasonal effects on vitamin D status. All children enrolled in the 4th through 8th grades were invited to participate. The study protocol and study documents were approved by Tufts University's Institutional Review Board (IRB), and both parental informed consent and the child's assent were obtained before inclusion in the study. Eligibility was determined upon review of returned parental informed consent forms and an accompanying parent questionnaire, which included questions about medical conditions and medication and supplement use by the child.
All study visits were conducted in person at the school of enrollment; parents attended the baseline visit and some but not all of the remaining study visits with participating children. At the baseline visit, children were randomized in a double-blind manner to one of three daily vitamin D3 supplementation doses (600 IU, 1000 IU, or 2000 IU) for 6 months. Thereafter, children completed follow-up visits at 3, 6, and 12 months. The primary outcomes of this study were changes in serum 25(OH)D and blood glucose and lipids over the 6-month supplementation period. Study supplements were distributed directly to parents at the school at the baseline and 3-month visits, but in rare instances the supplements were mailed to the parent at the 3-month visit.
Recruitment strategies
Our recruitment strategies were implemented at two primary levels: (1) the district and school level and (2) the child and parent level (Table 1). Boston-area urban school districts were recruited based on racial/ethnic diversity and having greater than 60% of enrolled children qualifying for free or reduced-price meals. Selection of the districts and schools to recruit was initially derived from previous successes in community intervention research by the study investigators18, 26 and the recognized need for a vitamin D supplementation trial in these communities of children at greater risk for inadequate serum 25(OH)D.18, 20 Once the superintendent of each school district agreed to participate, the principal investigator and study staff met with school principals to enroll interested schools within the district, and to establish primary contacts, which included school health teachers, nurses, and secretaries. Study staff collaborated with these contacts to determine the best recruitment methods and agree upon data collection logistics. Participating schools were given a monetary incentive, divided between the beginning and end of the study.
Table 1. Multi-level Recruitment and Retention Approaches.
| School/District | Child/Parent |
|---|---|
| RECRUITMENT | |
|
|
| RETENTION | |
|
|
Further interest and support was generated for the study at Parent Teacher Association meetings, teacher and staff meetings, and open houses. Study information was included in school newsletters, and informational flyers were distributed to the children. Parents were instructed that they would receive a letter informing them of their child's blood lipid results and vitamin D status [serum 25(OH)D] from the baseline study visit upon completion of study participation.
Schoolchildren were recruited predominantly through classroom and auditorium presentations. Informed consent and assent forms and study information materials were provided to all 4th through 8th grade children primarily by study staff, but in some cases were distributed by school administrative staff, teachers, or nurses. Study materials were available in the major languages spoken in the communities: English, Spanish, Portuguese, Haitian/Creole, and Mandarin. Children who expressed interest in participating in the study were instructed to take the materials home to share with their parents and to return the completed informed consent and assent forms and questionnaires to a specific location or person within the school by a specified date, which ranged from 1 to 3 weeks after distribution. Children who were taking dietary supplements containing vitamin D, receiving oral glucocorticoids, or had chronic diseases that affect bone metabolism, including rickets, cystic fibrosis, kidney disease, sarcoidosis, irritable bowel syndrome, epilepsy, or HIV/AIDS, were excluded from study participation. Some principals and other school staff assisted with recruitment by organizing presentations and collecting consent and assent forms.
Retention strategies
As outlined in Table 1, our retention strategies took place at multiple levels. At the district and school level, they were designed to keep the superintendents, principals, and school staff interested and supportive of the study team's efforts. Study staff maintained communication with the primary contacts with regular emailed messages and telephone calls, which provided updates on the study's progress and timeline. Study staff also received important logistical help from these communications, especially contact information for children who had moved or whose telephone numbers had changed.
We also employed various retention strategies at the parent and child level. Adherence and retention telephone calls were conducted in the household's language one week after the baseline visit, monthly thereafter, and before each study visit. The call script focused on troubleshooting any issues that could reduce adherence or retention and reminded families about gift cards being given to the children at the visit. The main study incentive provided to children was a $25 gift card to a national retailer at each visit. Children also were given clip-magnets to display their study calendars and to help them remember to take the supplement. Other retention strategies included follow-up letters for those not reached by telephone and birthday and Valentine's Day cards. On rare occasions, vitamin D supplements were mailed home for hard-to-reach parents (3-month visit only).
Another strategy at the parent and child level that aimed to strengthen the study's identity and facilitate a bond with the study, as reported elsewhere,9 was the creation of a study name (the Daily D Health Study) and logo. All distributed study materials, including instruction sheets, supplement bottles, calendars, magnets, and wrist-bands, had this logo.
Study procedures
Study visits were conducted at the child's school, prior to the start of the school day to facilitate a fasting blood draw. Supplementation began during late fall/early winter months and was completed in the spring. At the baseline and 3-month visits, each child's parent received one bottle containing 100 vitamin D supplements, an instruction sheet that included the study timeline, and a calendar and stickers to mark off the days the supplement was taken. At the 3-and 6-month visits, children returned used pill bottles and calendars for assessment of adherence to the supplement.
Sociodemographics
During the informed consent procedure, birth date, race/ethnicity, and data on socioeconomic status were reported by parental questionnaire. Parents identified their child's racial/ethnic background by selecting one of the following categories: white/Caucasian, black/African American, Mexican/Mexican-American, other Hispanic/Latino, Asian/Asian-American/Asian-Indian, Native American/American Indian, multiracial/ethnic, or other.27 Parents also reported whether their child was eligible for free or reduced-price school meals and the highest level of education attained by the mother and/or primary caregiver, using categories ranging from no formal schooling to graduate work/higher degree. Annual household income was estimated from responses to a question that presented income categories ranging from <$15,000 to ≥$75,000.
Anthropometric measurements and blood sample
At each visit except at 3 months, height and weight were measured in triplicate with light clothing and without shoes. Height was measured using a portable stadiometer (Model 214, Seca Weighing and Measuring Systems, Hanover, MD) with the head in the Frankfurt plane made with a right angle height procedure28 and recorded to the closest 1/8th inch. Weight was measured on a portable balance beam scale (Seca Clera 803, Hamburg, Germany) and recorded to the closest 0.25 pound. Body mass index was calculated and expressed as a percentile and z-score using the U.S. Centers for Disease Control sex-specific growth charts.29 At each study visit, blood was drawn in private by a trained phlebotomist after an overnight fast, for measurement of serum 25(OH)D and biomarkers of cardiometabolic risk, including blood lipids and glucose.
Data analysis
Characteristics of trial participants in the total sample and by study wave were summarized using descriptive statistics; comparisons between study waves were assessed using t-tests and chi-square tests. Retention rates by characteristics of participants were compared using chi-square tests. Statistical analyses were conducted using SAS 9.3 software (SAS Institute Inc, Cary, NC).
Results
Participant recruitment and baseline sample characteristics
The recruitment presentation to an estimated 4400 4th through 8th grade children yielded an initial enrollment of 693 children and final inclusion of 691 participants accrued over two waves (Wave 1: 2011, n=310; Wave 2: 2012, n=381). Figure 1 shows the recruitment and retention flow through the 6-month study visit. Table 2 presents characteristics of the study sample at baseline. Of 691 children at baseline with a mean (SD) age of 11.7 (1.4) years, 59% were classified as racial/ethnic minorities, and 68% were eligible for free or reduced-price school meals. Based on BMI percentiles for age and sex, almost half (47%) of children were considered overweight or obese. Participants enrolled in Waves 1 and 2 were not significantly different, with the exception of a larger number of Asian children enrolled in Wave 1 vs. Wave 2 [n=37 (11.9%) vs. n=20 (5.3%), respectively, P=0.006, data not shown]. Participants were representative of children in their school districts for gender, race/ethnicity, and eligibility for free or reduced-price meals during the years of recruitment,30 and for prevalence of overweight/obesity;18, 26 across these districts, approximately 47-49% were girls, 32-40% were classified as white, 60-77% qualified for free or reduced-price meals, and 44-45% were overweight or obese.
Figure 1. Daily D Health Study Diagram of Participation and Retention through 6 Months.
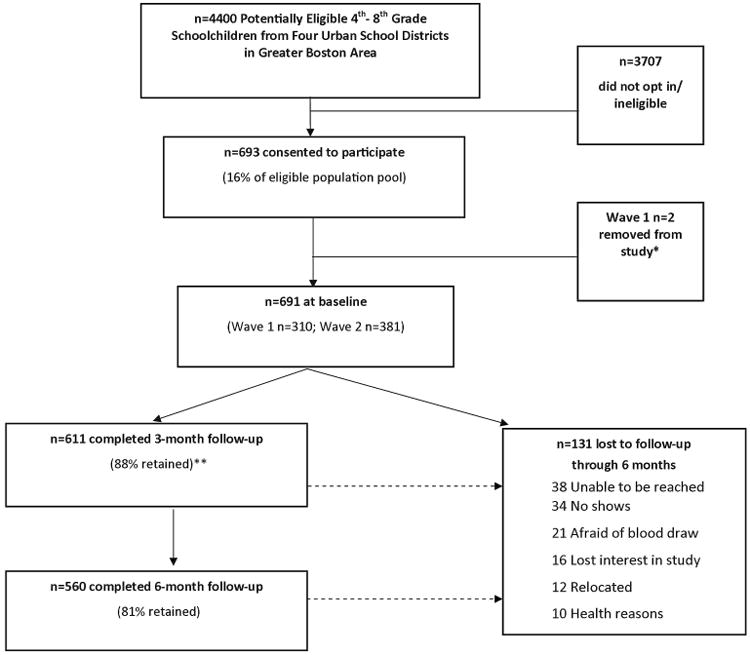
*One child was removed due to diagnosis of rickets, unrelated to the study, by the family's physician, and the other due to insufficient serum from blood draw.
** Two no-shows at 3 months returned for the 6-month study visit.
Table 2. Baseline Characteristics of Daily D Health Study Participants (n = 691).
| Characteristic | Mean (SD) or n (%) |
|---|---|
| Age (years) | 11.7 (1.4) |
| Female | 354 (51.2%) |
| Race/ethnicity | |
| White/Caucasian | 286 (41.4%) |
| Black/African American | 100 (14.5%) |
| Mexican/Mexican American | 3 (0.4%) |
| Other Hispanic/Latino | 150 (21.7%) |
| Asian/Asian American/Asian Indian | 57 (8.3%) |
| Native American/American Indian | 1 (0.1%) |
| Multi-racial/Multi-ethnic | 66 (9.6%) |
| Other | 28 (4.1%) |
| BMI z-score | 0.81 (1.1) |
| Overweight/obese | 321 (46.5%) |
| Eligible for free or reduced-price mealsa | 467 (67.9%) |
| Maternal post-secondary educationb | 366 (54.8%) |
| Household income <$30,000c,d | 335 (51.9%) |
n=688 due to missing responses
n=668 due to missing responses
n=646 due to missing responses
Household income was categorized as <$30,000 or ≥$30,000, as an approximation of the poverty guidelines updated periodically in the Federal Register by the U.S. Department of Health and Human Services under the authority of 42 U.S.C. 9902(2).
Participant retention
A total of 88% (n=611) and 81% (n=560) of children completed the 3- and 6-month visits, respectively (see Figure 1). Approximately the same percentage of children was retained over the 6-month supplementation period in Waves 1 and 2 (P=0.88), but the percentage retained differed significantly by school (P=0.009, data not shown), with no clear school-level factors explaining these differences. Reasons for attrition over 6 months (n=131) included: being unable to be reached (n=38, 29%) or a no-show (n=34, 26%), fear of a blood draw (n=21, 16%), losing interest in the study (n=16, 12%), relocation (n=12, 9%), and health reasons (n=10, 8%). As shown in Table 3, children who completed the 6-month intervention did not differ significantly from those who did not, with the exception of a greater proportion completing the 6-month study visit among children with higher household incomes: 79% of children with a household income <$30,000 compared to 85% with a household income ≥$30,000 (P=0.04). Among the racial/ethnic groups, 74% of black/African-American children were retained compared to 82% of all other racial/ethnic groups combined (P=0.05).
Table 3. Retention Rates by Characteristics of Children During the 6-Month Interventiona.
| Characteristic | Number (n) Enrolled | n (%) Retainedb |
|---|---|---|
| Age | ||
| < 12 years | 383 | 310 (80.9 %) |
| ≥ 12 years | 308 | 250 (81.2%) |
| Sex | ||
| Male | 337 | 277 (82.2%) |
| Female | 354 | 283 (79.9%) |
| Race/ethnicity | ||
| White/Caucasian | 286 | 233 (81.5%) |
| Black/African American | 100 | 74 (74.0%) |
| Mexican/Mexican American | 3 | 3 (100%) |
| Other Hispanic/Latino | 150 | 120 (80.0%) |
| Asian/Asian American/Asian Indian | 57 | 49 (86.0%) |
| Native American/American Indian | 1 | 1 (100%) |
| Multi-racial/Multi-ethnic | 66 | 54 (81.8%) |
| Other | 28 | 26 (92.9%) |
| Overweight/obese | ||
| No | 370 | 302 (81.6%) |
| Yes | 321 | 258 (80.4%) |
| Eligible for free or reduced-price meals | ||
| No | 221 | 184 (83.3%) |
| Yes | 467 | 374 (80.1%) |
| Maternal education | ||
| Secondary | 302 | 240 (79.5%) |
| Post-secondary | 366 | 302 (82.5%) |
| Household income | ||
| <$30,000 | 335 | 265 (79.1%) |
| ≥$30,000 | 311 | 265 (85.2%) |
Total retention over the 6-month intervention period was 560 (81% of participants enrolled)
Chi-square test for homogeneity among categories of each characteristic showed no significant differences with the exception of household income (P=0.04)
Discussion
Conducting a randomized, double-blind supplementation trial in children within a school setting presented a unique opportunity to recruit and retain a diverse sample of participants. In the Daily D Health Study, we achieved an 81% retention rate over 6 months, but had lower retention of children from households with lower incomes and of black/African American participants. The school setting and multi-level recruitment and retention approaches, including translating study materials into appropriate languages, utilizing translators for telephone contact, frequent and direct contact with parents and children, and distributing incentives at each study visit, contributed to successful inclusion of the study population of interest.
The multi-level approach to recruitment and retention relied upon establishing school contacts and trusting relationships among study staff, school personnel, participants, and parents. The study incorporated health relevance, as recruited schools had socioeconomically challenged and racially/ethnically-diverse populations of children, enabling the intervention to reach children likely to need vitamin D supplementation.18, 20 Also noteworthy is that the relationships and trust developed during previous studies conducted by the study co-investigators18, 26 provided an important foundation for the present study.
The primary method of recruiting participants was through classroom and auditorium presentations, a form of active recruitment reported to engender success in recruiting children in schools1, 3, 31 that may be particularly important for hard-to-reach populations, such as racial/ethnic minorities and those of lower socioeconomic status.3, 8, 32, 33 In addition to helping with recruitment logistics, recruitment efforts were facilitated by schools that made special efforts to communicate with potential study participants and parents. Facilitation included use of public address systems for study-related announcements and the school's telephone alert system to remind parents about the study. Thus, recruitment benefited from overall flexibility in approach, as reported for other school-based studies.2, 3, 33, 34 Furthermore, the convenience and timing provided by the familiar school setting in early mornings likely made participation more feasible for participants and schools by not interfering with the school day.2
Participant retention was aided by the multi-level support available in schools, which helped facilitate ongoing communication with parents and children. Many of the school staff members became strong supporters of the study, in some cases joining the study staff at follow-up visits, thereby providing a trusted face to help ease uncertainties. Having multiple channels of persistent communication with each family, through study staff, school teachers, nurses, or secretaries, helped minimize the number of lost participants and enhance adherence. Thus, despite conduct of the study outside of school hours, the support of school staff was crucial to retention.
The study's retention rates are noteworthy for several reasons. First, the sample was derived from an urban population that was socioeconomically challenged and at high risk of attrition. Other intervention studies in racial/ethnic minority or multi-ethnic samples of children or adolescents have reported retention rates of 8235 to 98%11 after 3 months, and 5932 to 81%35 after 6 months, with considerably smaller samples than the present study. Our study required blood draws (three over the 6-month supplementation period), which has been shown to deter participation, particularly among children.12 Finally, other supplementation studies of vitamin D in children or adolescents report retention rates between 74 and 95% over shorter follow-up periods than the present study, from 8 to 16 weeks,21, 22, 25, 36, 37 with only 66% retention for a 6-month trial.23 None of these studies was conducted in a school setting. In contrast, a multivitamin supplementation study conducted in a predominantly non-white sample of children in a U.S. school, in which the study tablets were administered by teachers and no blood draws were involved, reported a 94% retention over 4 months.4
Despite good retention rates overall, children from households with lower incomes less often completed the 6-month intervention. In randomized studies of weight management interventions for children or adolescents, lower household income has been associated with lower treatment attendance17 and dropping out of the intervention.6 For a randomized intervention study of low birth weight premature infants, low maternal education significantly predicted dropping out.38 Moreover, families of lower socioeconomic status may be more difficult to contact for follow-up visits.39 In the present study, another factor that may have influenced retention was varying levels of involvement by school staff or parents; however, data on the level of school and parental involvement were not collected.
Incentives were found to be an important strategy for both recruitment and retention, as commonly observed for intervention studies in children and adolescents.12, 15, 33, 40 Incentives were used for both schools (monetary; $1500 over one school year) and children (gift cards at each study visit). In addition, the “Daily D Health Study” study name and logo likely enhanced both recruitment and retention efforts.
Limitations
Due to popular recognition and discussion of vitamin D deficiencies among the general population, this study may have had broad acceptance and thus have been easier to implement within the school-setting than other types of supplementation studies. However, with school policies and IRB oversight, there were barriers to the extent to which study researchers could track down study participants (for example, calling the family no more than three times after a missed visit without a returned call) in addition to financial constraints and time limitations of the study team. Additional resources might have allowed for the team to retain a small number of the children who had moved out of the school district. Other possible barriers included the necessity of parental involvement in accompanying the child to school at an early morning hour to receive the pills, as school policy required that the supplements not remain on the school property and were to be administered at home. The fasting blood draw requirement proved difficult or uncomfortable for some children. This intervention required heavy resources, including a large number of staff; however, staff also provided credibility, consistency, and support to the study, allowing for maximum efficiency in the relatively short time interval between recruitment and implementation of supplement distribution.
Conclusion
Schools are a logical location for research in children and adolescents, specifically interventions such as supplementation trials. Given its familiarity and accessibility to diverse populations, as compared to a clinical setting, schools are a convenient place to conduct an intervention study, making it easier to capture a representative population of generally healthy children from a given community. A school-based study may avoid many issues raised in a clinic-based setting, such as enrollment of children with chronic diseases that pose associated challenges related to compliance, among others.6, 7, 9
Recruitment and retention of urban children to longitudinal supplementation trials conducted in schools is feasible and practical. The use of culturally-sensitive recruitment materials and creative retention methods are likely to be critical to the success of school-based studies within racially/ethnically and socioeconomically diverse communities. Establishing relationships with school personnel and conducting data collection outside of school hours enhance “buy-in” of the study by key administrators as it does not directly impact the school-day curricula and activities. Results of the Daily D Health Study have revealed characteristics distinctive to a school setting that may inform future recruitment and retention efforts in school-based trials conducted among children.
Acknowledgments
The authors would like to thank the administrators, staff, teachers, and nurses at the Everett, Malden, Medford, and Somerville Public Schools for allowing us to conduct this research within their schools. In addition, we gratefully acknowledge the support of Peter Bakun at the Friedman School of Nutrition Science and Policy at Tufts University for his assistance with data management, and Charlette Steed, along with the rest of the Daily D Health Study staff and team, who helped with data collection.
Funding: This work was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R01HL106160. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
Footnotes
Trial registration number: ClinicalTrials.gov, NCT01537809
Conflict of Interest: The authors declare that they have no competing interests.
References
- 1.Cline A, Schafer-Kalkhoff T, Strickland E, et al. Recruitment strategies for the Princeton (Ohio) City School District epidemiological study. J Sch Health. 2005;75:189–191. [PubMed] [Google Scholar]
- 2.Geller AC, Oliveria SA, Bishop M, et al. Study of health outcomes in school children: key challenges and lessons learned from the Framingham Schools' Natural History of Nevi Study. J Sch Health. 2007;77:312–318. doi: 10.1111/j.1746-1561.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- 3.Berry DC, Neal M, Hall EG, et al. Recruitment and retention strategies for a community-based weight management study for multi-ethnic elementary school children and their parents. Public Health Nurs. 2013;30:80–86. doi: 10.1111/phn.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perlman AI, Worobey J, O'Sullivan Maillet J, et al. Multivitamin/Mineral supplementation does not affect standardized assessment of academic performance in elementary school children. J Am Diet Assoc. 2010;110:1089–1093. doi: 10.1016/j.jada.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Schoenthaler SJ, Bier ID, Young K, et al. The effect of vitamin-mineral supplementation on the intelligence of American schoolchildren: a randomized, double-blind placebo-controlled trial. J Altern Complement Med. 2000;6:19–29. doi: 10.1089/acm.2000.6.19. [DOI] [PubMed] [Google Scholar]
- 6.Williams NA, Coday M, Somes G, et al. Risk factors for poor attendance in a family-based pediatric obesity intervention program for young children. J Dev Behav Pediatr. 2010;31:705–712. doi: 10.1097/DBP.0b013e3181f17b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy BM, Newton RL, Jr, York-Crowe E, et al. Recruiting African American girls and parents for a secondary weight gain prevention study. J Cult Divers. 2008;15:181–186. [PubMed] [Google Scholar]
- 8.Yancey AK, Ortega AN, Kumanyika SK. Effective recruitment and retention of minority research participants. Annu Rev Public Health. 2006;27:1–28. doi: 10.1146/annurev.publhealth.27.021405.102113. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson LM, Schwirian PM, Klein EG, et al. Recruitment and retention strategies in longitudinal clinical studies with low-income populations. Contemp Clin Trials. 2011;32:353–362. doi: 10.1016/j.cct.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swanson GM, Ward AJ. Recruiting minorities into clinical trials: toward a participant-friendly system. J Natl Cancer Inst. 1995;87:1747–1759. doi: 10.1093/jnci/87.23.1747. [DOI] [PubMed] [Google Scholar]
- 11.Story M, Sherwood NE, Obarzanek E, et al. Recruitment of African-American pre-adolescent girls into an obesity prevention trial: the GEMS pilot studies. Ethn Dis. 2003;13:S78–87. [PubMed] [Google Scholar]
- 12.Kafka T, Economos C, Folta S, et al. Children as subjects in nutrition research: a retrospective look at their perceptions. J Nutr Educ Behav. 2011;43:103–109. doi: 10.1016/j.jneb.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Rich-Edwards JW, Ganmaa D, Kleinman K, et al. Randomized trial of fortified milk and supplements to raise 25-hydroxyvitamin D concentrations in schoolchildren in Mongolia. Am J Clin Nutr. 2011;94:578–584. doi: 10.3945/ajcn.110.008771. [DOI] [PubMed] [Google Scholar]
- 14.Ward KA, Das G, Roberts SA, et al. A randomized, controlled trial of vitamin D supplementation upon musculoskeletal health in postmenarchal females. J Clin Endocrinol Metab. 2010;95:4643–4651. doi: 10.1210/jc.2009-2725. [DOI] [PubMed] [Google Scholar]
- 15.Harrington KF, Binkley D, Reynolds KD, et al. Recruitment issues in school-based research: lessons learned from the High 5 Alabama Project. J Sch Health. 1997;67:415–421. doi: 10.1111/j.1746-1561.1997.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 16.Dolinsky DH, Armstrong SC, Ostbye T. Predictors of attrition from a clinical pediatric obesity treatment program. Clin Pediatr (Phila) 2012;51:1168–1174. doi: 10.1177/0009922812458355. [DOI] [PubMed] [Google Scholar]
- 17.Jensen CD, Aylward BS, Steele RG. Predictors of attendance in a practical clinical trial of two pediatric weight management interventions. Obesity (Silver Spring) 2012;20:2250–2256. doi: 10.1038/oby.2012.96. [DOI] [PubMed] [Google Scholar]
- 18.Sacheck J, Goodman E, Chui K, et al. Vitamin D deficiency, adiposity, and cardio metabolic risk in urban schoolchildren. J Pediatr. 2011;159:945–950. doi: 10.1016/j.jpeds.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Au LE, Economos CD, Goodman E, et al. Vitamin D intake and serum vitamin D in ethnically diverse urban schoolchildren. Public Health Nutr. 2012;15:2047–2053. doi: 10.1017/S1368980012003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon CM, DePeter KC, Feldman HA, et al. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158:531–537. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 21.Dong Y, Stallmann-Jorgensen IS, Pollock NK, et al. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J Clin Endocrinol Metab. 2010;95:4584–4591. doi: 10.1210/jc.2010-0606. [DOI] [PubMed] [Google Scholar]
- 22.Aguirre Castaneda R, Nader N, Weaver A, et al. Response to vitamin D3 supplementation in obese and non-obese Caucasian adolescents. Horm Res Paediatr. 2012;78:226–231. doi: 10.1159/000343446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belenchia AM, Tosh AK, Hillman LS, et al. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. Am J Clin Nutr. 2013;97:774–781. doi: 10.3945/ajcn.112.050013. [DOI] [PubMed] [Google Scholar]
- 24.Ashraf AP, Alvarez JA, Gower BA, et al. Associations of serum 25-hydroxyvitamin D and components of the metabolic syndrome in obese adolescent females. Obesity (Silver Spring) 2011;19:2214–2221. doi: 10.1038/oby.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Putman MS, Pitts SA, Milliren CE, et al. A randomized clinical trial of vitamin D supplementation in healthy adolescents. J Adolesc Health. 2013;52:592–598. doi: 10.1016/j.jadohealth.2012.10.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Economos CD, Hyatt RR, Goldberg JP, et al. A community intervention reduces BMI z-score in children: Shape Up Somerville first year results. Obesity (Silver Spring) 2007;15:1325–1336. doi: 10.1038/oby.2007.155. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Race and Ethnicity Code Set. [accessed 29 September 2011];2000 http://wonder.cdc.gov/wonder/help/populations/bridged-race/Directive15.html.
- 28.Lohman T. Current Issues in Exercise Science. Champaign, IL: Human Kinetics; 1992. Advances in body composition assessment. [Google Scholar]
- 29.Centers for Disease Control and Prevention. CDC Table for Calculated Body Mass Index Values for Selected Heights and Weights for Ages 2 to 20. [accessed 3 December 2007];2000 http://www.cdc.gov/nccdphp/dnpa/healthyweight/assessing/bmi/00binaries/bmi-tables.pdf.
- 30.Massachusetts Department of Elementary and Secondary Education. School and District Profiles. [accessed 15 February 2013]; http://profiles.doe.mass.edu.
- 31.Frye FH, Baxter SD, Thompson WO, et al. Influence of school, class, ethnicity, and gender on agreement of fourth graders to participate in a nutrition study. J Sch Health. 2002;72:115–120. doi: 10.1111/j.1746-1561.2002.tb06528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guzman A, Richardson IM, Gesell S, et al. Recruitment and retention of Latino children in a lifestyle intervention. Am J Health Behav. 2009;33:581–586. [PMC free article] [PubMed] [Google Scholar]
- 33.Elder JP, Shuler L, Moe SG, et al. Recruiting a diverse group of middle school girls into the trial of activity for adolescent girls. J Sch Health. 2008;78:523–531. doi: 10.1111/j.1746-1561.2008.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lytle LA, Johnson CC, Bachman K, et al. Successful recruitment strategies for school-based health promotion: experiences from CATCH. J Sch Health. 1994;64:405–409. doi: 10.1111/j.1746-1561.1994.tb03261.x. [DOI] [PubMed] [Google Scholar]
- 35.Villarruel AM, Jemmott LS, Jemmott JB, et al. Recruitment and retention of Latino adolescents to a research study: lessons learned from a randomized clinical trial. J Spec Pediatr Nurs. 2006;11:244–250. doi: 10.1111/j.1744-6155.2006.00076.x. [DOI] [PubMed] [Google Scholar]
- 36.Economos CD, Moore CE, Hyatt RR, et al. Multinutrient-fortified juices improve vitamin D and vitamin E status in children: a randomized controlled trial. J Acad Nutr Diet. 2014;114:709–717. doi: 10.1016/j.jand.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 37.Lewis RD, Laing EM, Hill Gallant KM, et al. A randomized trial of vitamin D(3) supplementation in children: dose-response effects on vitamin D metabolites and calcium absorption. J Clin Endocrinol Metab. 2013;98:4816–4825. doi: 10.1210/jc.2013-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Constantine WL, Haynes CW, Spiker D, et al. Recruitment and retention in a clinical trial for low birth weight, premature infants. J Dev Behav Pediatr. 1993;14:1–7. [PubMed] [Google Scholar]
- 39.Cotter RB, Burke JD, Loeber R, et al. Predictors of contact difficulty and refusal in a longitudinal study. Crim Behav Ment Health. 2005;15:126–137. doi: 10.1002/cbm.46. [DOI] [PubMed] [Google Scholar]
- 40.Coday M, Boutin-Foster C, Goldman Sher T, et al. Strategies for retaining study participants in behavioral intervention trials: retention experiences of the NIH Behavior Change Consortium. Ann Behav Med. 2005;29(Suppl):55–65. doi: 10.1207/s15324796abm2902s_9. [DOI] [PubMed] [Google Scholar]


