Abstract
Objective
Clinical and research settings often require sequencing multiple respiratory tests in a brief visit. Guidelines recommend measuring the concentration of exhaled nitric oxide (FeNO) before spirometry, but evidence for a spirometry carryover effect on FeNO is mixed. Only one study has investigated spirometry carryover effects on multiple flow FeNO analysis. The objective of this study was to evaluate evidence for carryover effects of recent spirometry on three exhaled NO summary measures: FeNO at 50 ml/s, airway wall NO flux [J’awNO], and alveolar NO concentration [CANO] in a population-based sample of schoolchildren.
Methods
Participants were 1146 children (191 with asthma), ages 12–15, from the Southern California Children’s Health Study who performed spirometry and multiple flow FeNO on the same day. Approximately half the children performed spirometry first. Multiple linear regression was used to estimate differences in exhaled NO summary measures associated with recent spirometry testing, adjusting for potential confounders.
Results
In the population-based sample, we found no evidence of spirometry carryover effects. However, for children with asthma, there was a suggestion that exhaled NO summary measures assessed ≤6 minutes after spirometry were lower (FeNO: 25.8% lower, 95% CI: −6.2%, 48.2%; J’awNO: 15.1% lower 95% CI: −26.5%, 43.0%; and CANO 0.43 parts per billion lower, 95% CI: −0.12, 0.98).
Conclusions
In clinical settings, it is prudent to assess multiple flow FeNO before spirometry. In studies of healthy subjects, it may not be necessary to assess FeNO first.
Keywords: alveolar nitric oxide, asthma, bronchial nitric oxide, pulmonary function test, spirometry
INTRODUCTION
In clinical and research assessments of respiratory health, it is often necessary to conduct multiple tests during a brief visit. Although closely spaced tests may be unavoidable, concern that one test could influence the results of subsequent tests may lead to test ordering recommendations to minimize possible carryover effects. Furthermore, carryover effects may vary by health status, leading to different test ordering recommendations for clinical assessment (e.g., in children with asthma) and research assessment (e.g., in healthy children).
The use of spirometry and the fractional concentration of exhaled nitric oxide (FeNO) in the assessment of respiratory health raises the question of whether test ordering affects results. Early evidence showed that prior spirometric maneuvers reduced FeNO [1–4], so current American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines recommend collecting FeNO before spirometry [5]. Subsequent evidence for this carryover effect has been mixed, despite the increasingly standardized methods of subsequent studies [6–9]. FeNO is conventionally measured at a 50 ml/s exhalation flow rate and ATS/ERS guidelines are for FeNO at 50 ml/s. However, there is growing interest in the measurement of FeNO at multiple flow rates (“extended NO analysis”) to fractionate exhaled NO into airway and alveolar sources that are quantified by the NO parameters: maximum airway wall NO flux [J’awNO] and alveolar NO concentration [CANO]. Guidelines have yet to be developed for the measurement of FeNO at multiple flow rates. To our knowledge, only one study of 26 adults with asthma has investigated spirometry carryover effects on NO parameters and this study found no evidence for spirometry carryover effects on J’awNO or CANO [10].
The aim of our study was to evaluate evidence for carryover effects of recent spirometry on three “exhaled NO summaries” (we use this term to refer generically to measured FeNO or estimated NO parameters): FeNO at 50 ml/s, J’awNO, and CANO in a population-based sample of school children 12–15 years of age.
METHODS
Participants and study design
Children included in this study were participants of the Southern California Children’s Health Study (CHS), a large population-based cohort study of school children designed to study respiratory effects of air pollution. From March—June 2010, spirometric and multiple flow FeNO maneuvers were conducted concurrently in a CHS cohort for the first time. In accordance with ATS/ERS guidelines [5], the CHS protocol called for FeNO testing prior to spirometry. However, the testing schedule became compressed due to resource constraints and academic calendar scheduling. Field technicians found it necessary to conduct spirometry prior to FeNO tests in approximately half of the children, quasi-randomly. Here, we take advantage of this feature of the CHS data (similar to a natural experiment) to investigate spirometry carryover effects in a large, population-based sample of children. Details of the study protocol, testing procedures, and cohort characteristics have been described previously [11–12]. The protocol was approved by the University of Southern California Health Sciences Campus Institutional Review Board. Written informed consent was obtained from a parent or guardian on behalf of each child participant.
In this analysis, we included data from 1146 children (955 without asthma and 191 reporting doctor-diagnosed asthma, but not taking inhaled corticosteroids in the past 12 months) who had valid spirometric maneuvers and valid FeNO maneuvers at each of the 4 target flow rates on the same day. We excluded 176 children who had inadequate data for calculation of NO summary measures (n=139 children without at least one valid FeNO maneuver at each of the 4 target flow rates or with FeNO maneuver(s) at 50 ml/s that were not adequately reproducible) and/or who reported both having asthma and having taken inhaled corticosteroids (ICS) in the past 12 months (n=42). Children with asthma taking ICS were excluded because their relatively small number limited our ability to perform appropriate analyses of this subgroup and we expected this subgroup to have different exhaled NO summaries.
Spirometry and FeNO testing
Spirometry and FeNO were assessed by experienced, well-trained technicians who also measured weight, and height, and collected information about recent acute respiratory illness in a protocol similar to one described in detail previously [13]. Spirometry was assessed using pressure-transducer-based spirometers (Screenstar Spirometers, Morgan Scientific, Haverhill, MA). FeNO was assessed using a chemiluminescence analyzer (Model CLD88-SP with DeNOx accessory, EcoMedics, Duernten, Switzerland/Ann Arbor, Michigan, USA) in a protocol described previously [11, 14]. Briefly, children were requested to perform 9 FeNO maneuvers, in the following order: 3 at the conventional 50 ml/s target flow rate and 2 at each of the following target flow rates: 30, 100, and 300 ml/s. Procedures conformed to ATS/ERS guidelines for assessment of FeNO at 50 ml/s [5], except for slightly relaxed reproducibility requirements (for FeNO above 10 parts per billion (ppb): <15% difference and for FeNO at or below 10 ppb: <15% or <1 ppb difference). These relaxed requirements allowed for inclusion of 35 children who would have otherwise been deemed to not have valid (reproducible) maneuvers at 50 ml/s. For the other children, Pearson’s correlation of FeNO at 50 ml/s using the strict versus the relaxed reproducibility requirements was >0.999. For assessment of FeNO at multiple flow rates, the NO concentration from a maneuver was determined from the 3-second plateau interval with minimum coefficient of variation, rather than the first acceptable interval. Raw data were screened to remove maneuvers with technical problems [11, 15].
NO parameter estimation
We estimated NO parameters using an adaptation of the Högman and Meriläinen Algorithm (HMA) [16] to CHS data [11, 15]. The HMA is based on a third order approximation to the deterministic two compartment model of NO in the lower respiratory tract [17]. The HMA includes internal data consistency checks [16, 18] that have been found to impose bias in NO parameter estimates [15]; so we considered all available HMA estimates of NO parameters. Inputs to the HMA were the average FeNO values at the target flow rates of 30, 100, and 300 ml/s.
Data analysis
We used t-tests and Fisher’s exact tests, as appropriate, to test for differences in demographic and health characteristics when comparing the 1146 children included in this study to the 176 children excluded.
As shown in Figure 1, FeNO was assessed before spirometry in 526 children (437 without asthma, 89 with asthma) whom we will refer to as the “reference group” since test ordering for these children followed ATS/ERS guidelines. We split the remaining 620 children (518 without asthma, 102 with asthma) into four similarly-sized groups based on the observed time interval (t) between the last spirometric maneuver and first FeNO maneuver: 22.3% had FeNO tested ≤6 minutes after spirometry (t ≤ 6), 26.0% had 6 < t ≤ 10, 20.0% had 10 < t ≤ 15, and 31.8% had 15 < t ≤ 138.
Figure 1.

Histogram of the time intervals (t) between the first FeNO maneuver and the last spirometry test. The 526 children with FeNO assessed before spirometry (according to ATS/ERS guidelines) are treated as a reference group and the 620 children with FeNO assessed after spirometry are divided into 4 similarly sized groups (t ≤ 6, 6 < t ≤ 10, 10 < t ≤ 15, 15 < t ≤ 138).
Because previous studies have shown the carryover effects of spirometry to be transitory, our primary goal was to estimate the difference in mean exhaled NO summaries comparing children with FeNO assessed immediately after spirometry (t ≤ 6) to children with FeNO assessed before spirometry (reference group). We used multiple linear regression to adjust for potential confounders [sex, age, race/ethnicity, height, weight, allergic rhinitis history (never, not current, current), month of test, ambient NO at time of FeNO test, FeNO analyzer, field technician, and community of residence]. Our secondary goal was to evaluate temporal trends in spirometry carryover effects so we estimated adjusted mean exhaled NO summaries for the reference group and each of the four time interval groups using multiple linear regression and plotted the results. Finally, F-tests were used to evaluate whether the variance in NO summary measures differed when comparing children with FeNO testing prior to spirometry to children with FeNO tested ≤6 minutes after spirometry.
We conducted analyses for all children and then conducted separate analyses for the subgroups of children with and without asthma. To better satisfy modeling assumptions, FeNO and J’awNO were natural log transformed. CANO was analyzed on the original ppb scale. All hypothesis tests were two-sided, with a significance level of 0.05. Analyses were performed using R version 3.0.0 [19].
RESULTS
Briefly, the 1146 children from 8 communities in southern California were 47.5% male, predominantly Hispanic white and non-Hispanic white (56.8% Hispanic white and 32.8% non-Hispanic white), 16.7% reported having doctor-diagnosed asthma, and 31.2% reported current allergic rhinitis. The 176 children excluded from this study were 1.4 months younger on average (p = 0.02) than the 1146 children included, but were otherwise similar in terms of race, gender, BMI, asthma status (when not considering those excluded for taking ICS), and allergic rhinitis history (all p ≥ 0.10).
The median (25th to 75th percentile) for the exhaled NO summaries were: 12.9 (8.8 to 22.3) ppb for FeNO, 697.8 (431.8 to 1251.0) pl/s for J’awNO and 1.18 (0.71 to 1.81) ppb for CANO. As expected, log(FeNO) and log(J’awNO) were highly correlated (Pearson’s R = 0.96), since FeNO at 50 ml/s primarily reflects NO from proximal airway sources [20]. CANO had low correlation with log(FeNO) and log(J’awNO) (0.19 and 0.03, respectively).
In analyses of all children and in analyses of the subset of children without asthma, FeNO and J’awNO assessed ≤6 minutes after spirometry were similar to reference group levels after adjusting for potential confounders (differences of ≤6.6%, Table 1). A 6.6% decrease in FeNO translates, for example, to a decrease in FeNO from 12.9 ppb (median FeNO in the study population) when assessed before spirometry to 12.0 ppb when assessed ≤6 minutes after spirometry. For children with asthma, adjusted differences in FeNO and J’awNO were suggestive of reduced levels following spirometry as compared to the reference group, but these differences were not statistically significant. FeNO was 25.8% lower (95% CI: −6.2%, 48.2%) and J’awNO was 15.1% lower (95% CI: −26.5%, 43.0%) ≤6 minutes after spirometry, after adjusting for potential confounders (Table 1). A 25.8% decrease in FeNO translates, for example, to a decrease in FeNO from 17.4 ppb (median in children with asthma) to 12.9 ppb. There were no apparent temporal trends in FeNO or J’awNO by test timing groups (Figure 2).
Table 1.
Estimated percent difference in FeNO and J’awNO and estimated absolute difference in mean CANO when comparing FeNO tests performed within 6 minutes of spirometry to FeNO tests performed before spirometry, adjusted for potential confounders.a
| All childrenb | Children without asthmab | Children with asthmab | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Exhaled NO summary | Estimate | 95% Confidence Interval | p Value | Estimate | 95% Confidence Interval | p Value | Estimate | 95% Confidence Interval | p Value |
| FeNO, % difference | −6.6 | (−18.7, 7.3) | 0.34 | −4.5 | (−17.7, 10.8) | 0.54 | −25.8 | (−48.2, 6.2) | 0.10 |
| J’awNO, % difference | −4.8 | (−18.3, 10.9) | 0.53 | −4.9 | (−19.3, 12.0) | 0.55 | −15.1 | (−43.0, 26.5) | 0.42 |
| CANO, ppb difference | −0.12 | (−0.31, 0.08) | 0.24 | −0.09 | (−0.29, 0.12) | 0.41 | −0.43 | (−0.98, 0.12) | 0.13 |
Potential confounders: sex, age, race/ethnicity, height, weight, allergic rhinitis history, month of test, ambient NO, FeNO analyzer, field technician, and community of residence.
The number of children with FeNO tests before spirometry is 526 (437 without asthma and 89 with asthma) and the number of children with FeNO tests ≤6 min after spirometry is 138 (114 without asthma and 24 with asthma).
Figure 2.
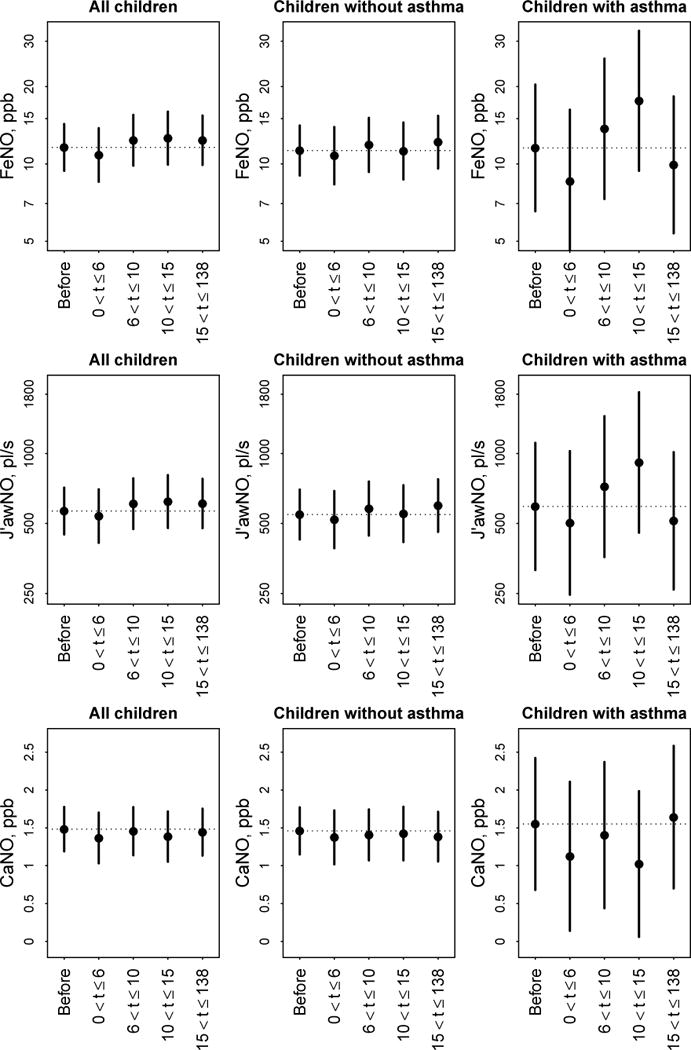
Adjusted mean exhaled NO summary (top row: FeNO, middle row: J’awNO, bottom row: CANO) and 95% confidence intervals by FeNO test timing group, separately for: all children, children without asthma, and children with asthma. For FeNO and J’awNO, means and confidence intervals are plotted on the natural log-scale and the y-axis is labeled on the original scale. Dotted horizontal reference lines are drawn from the mean in the “Before” reference group.
In analyses of all children and in analyses of the subset of children without asthma, adjusted mean CANO in each test timing group was similar to that in the reference group (differences of ≤0.12 ppb), with no apparent temporal trend (Figure 2). For children with asthma, adjusted mean CANO was 0.43 ppb lower (95% CI: −0.12, 0.98) in the t ≤ 6 group (n=24), 0.15 ppb lower (95% CI: −0.44, 0.73) in the 6 < t ≤ 10 group (n=23), 0.53 ppb lower (95% CI: −0.03, 1.08) in the 10 < t ≤ 15 group (n=23), and 0.09 ppb higher (95% CI: −0.41, 0.59) in the 15 < t ≤ 138 group (n=32) as compared to the reference group (n=89). Because there appeared to be some consistency in the results for the first three test timing groups, we pooled the children with asthma with t ≤ 15 (n=70) and found that adjusted mean CANO was 0.38 (95% CI: 0.00, 0.76) ppb lower for children with asthma with t ≤ 15 than in the reference group (p = 0.05). These estimated differences in CANO were relatively large given that the interquartile range of CANO in the subset of children with asthma in this study was 1.14 ppb. For example, a 0.43 ppb reduction of CANO from 1.19 ppb (median in children with asthma) to 0.76 ppb translates to a 36.1% reduction.
There was no evidence that the variance of any of the NO summary measures was different after spirometry (p ≤ 0.15 for all children and for subgroups based on asthma status).
DISCUSSION
This large, cross-sectional study provided the opportunity to investigate the carryover effect of prior spirometry on three NO summary measures in a population-based sample of children. In the population-based sample and in the subset of children without asthma, we found no evidence of spirometry carryover effects. For children with asthma, there was a suggestion that FeNO and J’awNO were lower when assessed ≤6 minutes after spirometry and CANO was lower when assessed ≤15 minutes after spirometry. This study provides the first evidence, to our knowledge, supporting a carryover effect of recent spirometry on NO parameters (CANO and J’awNO) in children with asthma. This should be confirmed in studies with larger samples of children with asthma and/or repeated measures designs.
It has been suggested that spirometry may decrease FeNO in subjects with asthma due to changes in airway caliber or airway closure caused by spirometry [2–3]. Prior repeated measures studies found that FeNO at 50 ml/s decreased 10–13% in the 1–5 minutes following spirometry and returned to baseline after 1 hour in 18 subjects (7 healthy and 11 with mild asthma) (p < 0.05) [2] or by 6–8% in the 5–15 minutes following spirometry in 24 children with asthma (p = 0.04) [7]. Other studies with similar designs, but larger sample sizes failed to find strong evidence for spirometry carryover effects. For example, mean FeNO was 2.2 ppb lower 5 minutes after spirometry (p = 0.09) in 30 patients with asthma (ages 13–80) [6] or relatively unchanged 3 minutes after spirometry (≤1.1 ppb different, all p > 0.16) in 105 atopic asthmatic adults, 44 atopic asthmatic children, 15 healthy adults, and 15 healthy children [9]. Our estimated spirometry carryover effect on FeNO at 50 ml/s (25.8% lower FeNO at 50 ml/s comparing children with asthma who were assessed ≤6 minutes after spirometry to those assessed before spirometry) was qualitatively consistent with these previous findings, though the magnitude of our between-subject, cross-sectional study estimate was larger than previous within-subject estimates from repeated measures studies. Using our cross-sectional data, we did not replicate previously reported trends at longer time intervals. It has also been reported that prior spirometry increased the within-subject coefficient of variation (standard deviation/mean) of FeNO for healthy children (from 4.3% to 10.5%) and for children with asthma (from 3.9% to 16.9%) [8]. In our study, we found no evidence that prior spirometry affected the between-subject variance of any of the NO summary measures.
Prieto et al have conducted the only other study, to our knowledge, that investigates spirometry carryover effects on NO parameters (CANO and J’awNO) and found no evidence for spirometry carryover effects [10]. Their study had a repeated measures design, with multiple flow FeNO assessed before and 10 minutes after spirometry in 26 adults with asthma [10]. Prieto et al found that CANO was, on average, 0.05 ppb lower (p = 0.87) when assessed 10 minutes after spirometry as compared to CANO assessed in the same adults with asthma before spirometry [10]. We found that adjusted mean CANO was 0.38 ppb lower for a group of children with asthma assessed ≤15 minutes after spirometry as compared to another group of children with asthma assessed before spirometry (p = 0.05). Prieto et al also found that J’awNO was, on average, 27 pl/s higher (p = 0.85) 10 minutes after spirometry in adults with asthma [10]. After adjusting for potential confounders, we found that J’awNO was 15.1% lower for a group of children with asthma assessed ≤6 minutes after spirometry as compared to another group of children with asthma assessed before spriometry (p = 0.42).
As described above, the effect estimates from our cross-sectional study have different interpretations than those from the Prieto et al repeated measures study, limiting direct comparison. The two studies also had different study populations (our study: healthy or mildly asthmatic schoolchildren versus Prieto et al: adults with mild to moderately persistent asthma recruited from a hospital-based allergy clinic [10]) and used different methods to estimate NO parameters. We used the HMA method to estimate NO parameters, while Prieto et al used a linear approximation to the two compartment model of NO (model did not fit for 7 subjects, who were excluded), with the Condorelli et al method to correct for back diffusion [21] (8 negative CANO estimates were set to zero) [10]. In summary, acknowledging the difficulties of direction comparision, our cross-sectional study provides evidence supporting reduced CANO levels in children with asthma following spirometry while the repeated measures study by Prieto et al in adults does not. Our cross-sectional estimate of reduced J’awNO could be considered consistent with the previous literature on within-subject reductions in FeNO at 50 ml/s following spirometry (relevant due to the high correlation between J’awNO and FeNO at 50 ml/s).
Strengths of our study included the large population-based sample of children, the ability to investigate temporal trends in exhaled NO summaries after spirometry testing, the assessment of FeNO at 4 flow rates (with a protocol of 9 maneuvers per subject), allowing for estimation of J’awNO and CANO in addition to FeNO at 50 ml/s, and the use of the HMA estimation method. The HMA is based on a more refined (third order) approximation to the two compartment model of NO exchange dynamics in the lower respiratory tract than the linear approximation method applied in the Prieto et al study. In earlier work in our study population, we evaluated a number of NO parameter estimation methods, but found that—given the target flow rates available in our data—linear approximation based methods inadequately partitioned airway NO from alveolar NO (estimated CANO and J’awNO had correlations of ~0.5) and that correction for back diffusion produced a large number of negative estimates of CANO [15].
Limitations of our study included the relatively small number of children with asthma—due to the population-based CHS sampling design—and the reduced power from a cross-sectional study design as compared to a repeated measures design. Our cross-sectional study design did not have the power to detect as statistically significant the relatively small, transient reductions in FeNO reported previously in people with asthma. Repeated measures and cross-sectional studies provide different, complementary evidence, with each study design having its own strengths and weaknesses. Repeated measures studies estimate within-subject effects by using each subject as her or her own control, resulting in fewer sources of excess variability (and increased power). However, repeated measures studies are more reflective of well-controlled experimental settings and may have biases resulting from intensive repeat testing on the same subject. Cross-sectional studies estimate population-level effects that may be more representative of the impacts of prior spirometry in epidemiological or clinical settings where only one FeNO testing session and one spirometry testing session will be conducted. However, differences across subjects leads to increased variability (reducing power) and potentially confounding the comparison of interest. We measured and accounted for a large set of potential confounders in our analysis, but unmeasured confounders could affect our results. Detailed allergic sensitization information from serum or skin-prick testing was not available in this study, although questionnaire-based allergic rhinitis history was available and we adjusted for allergic rhinitis history as a potential confounder. Our study was an analysis of existing data in which unanticipated constraints caused quasi-randomization of a large cohort of children to FeNO testing before or after spirometry and hence we did not have experimental control over the time interval between spirometry and FeNO testing. We divided the children with FeNO assessed after spirometry into similarly sized groups—based on the time interval since their last spirometry test (t ≤ 6, 6 < t ≤ 10, 10 < t ≤ 15, 15 < t ≤ 138)—in order to have similar power to detect differences in exhaled NO summaries when comparing each group to the reference group. However, prospective studies might select different time intervals.
In summary, our results are suggestive of a novel carryover effect of prior spirometry on CANO in children with asthma, independent of the carryover effect of spirometry on FeNO at 50 ml/s. This finding should be confirmed in future studies with larger samples of children with asthma and/or repeated measures designs. Beyond the need for replication, our findings highlight the need for more research that will inform the development of specific recommendations for multiple flow FeNO. Existing guidelines for FeNO at 50 ml/s cannot necessarily be translated immediately to FeNO collected at other flow rates. For example, Puckett et al [22] showed that the determination of the time-based determination of the analysis interval for calculation of NO concentration during a given maneuver was not appropriate for higher flow maneuvers and suggested volume-based analysis intervals instead. Studies of experimental and subject-level factors that affect multiple flow FeNO can inform future guidelines tailored to this method.
CONCLUSIONS/KEY FINDINGS
Our study demonstrates the practical implications of conducting spirometry prior to multiple flow FeNO testing in a population-based, epidemiological setting. For children without asthma, there was no evidence of spirometry carryover effects. For children with asthma, there was a novel finding that CANO was lower when assessed ≤15 minutes after spirometry. Similar to previous studies, there was a suggestion that FeNO (and J’awNO) were lower when assessed ≤6 minutes after spirometry. In conclusion, as with FeNO at 50 ml/s, it remains a sensible precaution to assess multiple flow FeNO before spirometry or at least 15 minutes after spirometry when the study population includes children with asthma. In studies of healthy subjects, it may not be necessary to assess multiple flow FeNO before spirometry. When analyzing data from population-based studies where practical constraints necessitated assessing multiple flow FeNO before and after spirometry, it may be adequate to adjust for FeNO assessment ≤15 minutes after spirometry in children with asthma.
Acknowledgments
Funding sources:
This work was supported by the National Heart, Lung and Blood Institute (grants 5R01HL61768 and 5R01HL76647); the Southern California Environmental Health Sciences Center (grant 5P30ES007048) funded by the National Institute of Environmental Health Sciences; the Children’s Environmental Health Center (grants 5P01ES009581, R826708-01 and RD831861-01) funded by the National Institute of Environmental Health Sciences and the Environmental Protection Agency; the National Institute of Environmental Health Sciences (grants 5P01ES011627, 1R01ES023262-01, 1K22ES022987); the James H. Zumberge Research and Innovation Fund, and the Hastings Foundation.
Footnotes
Declaration of interest:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Deykin A, Halpern O, Massaro AF, Drazen JM, Israel E. Expired nitric oxide after bronchoprovocation and repeated spirometry in patients with asthma. Am J Respir Crit Care Med. 1998 Mar;157(3):769–775. doi: 10.1164/ajrccm.157.3.9707114. [DOI] [PubMed] [Google Scholar]
- 2.Silkoff PE, Wakita S, Chatkin J, Ansarin K, Gutierrez C, Caramori M, McClean P, Slutsky AS, Zamel N, Chapman KR. Exhaled nitric oxide after beta2-agonist inhalation and spirometry in asthma. Am J Respir Crit Care Med. 1999 Mar;159(3):940–944. doi: 10.1164/ajrccm.159.3.9805044. [DOI] [PubMed] [Google Scholar]
- 3.Deykin A, Massaro AF, Coulston E, Drazen JM, Israel E. Exhaled nitric oxide following repeated spirometry or repeated plethysmography in healthy individuals. Am J Respir Crit Care Med. 2000 Apr;161(4 Pt 1):1237–1240. doi: 10.1164/ajrccm.161.4.9904086. [DOI] [PubMed] [Google Scholar]
- 4.Kissoon N, Duckworth LJ, Blake KV, Murphy SP, Lima JJ. Effect of beta2-agonist treatment and spirometry on exhaled nitric oxide in healthy children and children with asthma. Pediatr Pulmonol. 2002 Sep;34(3):203–208. doi: 10.1002/ppul.10154. [DOI] [PubMed] [Google Scholar]
- 5.ATS/ERS. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005 Apr 15;171(8):912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 6.Tee AK, Hui KP. Effect of spirometric maneuver, nasal clip, and submaximal inspiratory effort on measurement of exhaled nitric oxide levels in asthmatic patients. Chest. 2005 Jan;127(1):131–134. doi: 10.1378/chest.127.1.131. [DOI] [PubMed] [Google Scholar]
- 7.Gabriele C, Pijnenburg MW, Monti F, Hop W, Bakker ME, de Jongste JC. The effect of spirometry and exercise on exhaled nitric oxide in asthmatic children. Pediatr Allergy Immunol. 2005 May;16(3):243–247. doi: 10.1111/j.1399-3038.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- 8.Barreto M, Villa MP, Montesano M, Rennerova Z, Monti F, Darder MT, Martella S, Ronchetti R. Reduced exhaled nitric oxide in children after testing of maximal expiratory pressures. Pediatr Pulmonol. 2006 Feb;41(2):141–145. doi: 10.1002/ppul.20358. [DOI] [PubMed] [Google Scholar]
- 9.Garriga T, Labrador-Horrillo M, Guillen M, Luengo O, Eseverri JL, Guilarte M, Marin AM, Cardona V. Spirometric maneuvers and inhaled salbutamol do not affect exhaled nitric oxide measurements among patients with allergic asthma. Respiration. 2012;83(3):239–244. doi: 10.1159/000329440. [DOI] [PubMed] [Google Scholar]
- 10.Prieto L, Ruiz-Jimenez L, Marin J. The Effect of Spirometry on Bronchial and Alveolar Nitric Oxide in Subjects with Asthma. J Asthma. 2013 May 9; doi: 10.3109/02770903.2013.790418. [DOI] [PubMed] [Google Scholar]
- 11.Linn WS, Rappaport EB, Eckel SP, Berhane KT, Zhang Y, Salam MT, Bastain TM, Gilliland FD. Multiple-Flow Exhaled Nitric Oxide, Allergy, and Asthma in a Population of Older Children. Pediatr Pulmonol. 2013 May 17; doi: 10.1002/ppul.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Islam T, Urman R, Gauderman WJ, Milam J, Lurmann F, Shankardass K, Avol E, Gilliland F, McConnell R. Parental stress increases the detrimental effect of traffic exposure on children’s lung function. Am J Respir Crit Care Med. 2011 Oct 1;184(7):822–827. doi: 10.1164/rccm.201104-0720OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters JM, Avol E, Gauderman WJ, Linn WS, Navidi W, London SJ, Margolis H, Rappaport E, Vora H, Gong H, Thomas DC. A study of twelve Southern California communities with differing levels and types of air pollution. II. Effects on pulmonary function. Am J Respir Crit Care Med. 1999 Mar;159(3):768–775. doi: 10.1164/ajrccm.159.3.9804144. [DOI] [PubMed] [Google Scholar]
- 14.Linn WS, Rappaport EB, Berhane KT, Bastain TM, Salam MT, Gilliland FD. Extended exhaled nitric oxide analysis in field surveys of schoolchildren: a pilot test. Pediatr Pulmonol. 2009 Oct;44(10):1033–1042. doi: 10.1002/ppul.21101. [DOI] [PubMed] [Google Scholar]
- 15.Eckel SP, Linn WS, Berhane K, Rappaport EB, Salam MT, Zhang Y, Gilliland FD. Estimation of Parameters in the Two-Compartment Model for Exhaled Nitric Oxide. PLoS One. 2014;9(1):e85471. doi: 10.1371/journal.pone.0085471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogman M, Holmkvist T, Wegener T, Emtner M, Andersson M, Hedenstrom H, Merilainen P. Extended NO analysis applied to patients with COPD, allergic asthma and allergic rhinitis. Respir Med. 2002 Jan;96(1):24–30. doi: 10.1053/rmed.2001.1204. [DOI] [PubMed] [Google Scholar]
- 17.George SC, Hogman M, Permutt S, Silkoff PE. Modeling pulmonary nitric oxide exchange. J Appl Physiol. 2004 Mar;96(3):831–839. doi: 10.1152/japplphysiol.00950.2003. [DOI] [PubMed] [Google Scholar]
- 18.Hogman M, Merilainen P. Extended NO analysis in asthma. J Breath Res. 2007 Dec;1(2):024001. doi: 10.1088/1752-7155/1/2/024001. [DOI] [PubMed] [Google Scholar]
- 19.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 20.Paraskakis E, Brindicci C, Fleming L, Krol R, Kharitonov SA, Wilson NM, Barnes PJ, Bush A. Measurement of bronchial and alveolar nitric oxide production in normal children and children with asthma. Am J Respir Crit Care Med. 2006;174(3):260–267. doi: 10.1164/rccm.200506-962OC. [DOI] [PubMed] [Google Scholar]
- 21.Condorelli P, Shin HW, Aledia AS, Silkoff PE, George SC. A simple technique to characterize proximal and peripheral nitric oxide exchange using constant flow exhalations and an axial diffusion model. J Appl Physiol. 2007 Jan;102(1):417–425. doi: 10.1152/japplphysiol.00533.2006. [DOI] [PubMed] [Google Scholar]
- 22.Puckett JL, Taylor RW, Galant SP, George SC. Impact of analysis interval on the multiple exhalation flow technique to partition exhaled nitric oxide. Pediatr Pulmonol. 2010 Feb;45(2):182–191. doi: 10.1002/ppul.21182. [DOI] [PubMed] [Google Scholar]


