Abstract
OBJECTIVE
Efficient treatments to phase advance human circadian rhythms are needed to attenuate circadian misalignment and the associated negative health outcomes that accompany early morning shift work, early school start times, jet lag, and delayed sleep phase disorder. This study compared three morning bright light exposure patterns from a single light box (to mimic home treatment) in combination with afternoon melatonin.
METHODS
Fifty adults (27 males) aged 25.9±5.1 years participated. Sleep/dark was advanced 1 hour/day for 3 treatment days. Participants took 0.5 mg melatonin 5 hours before baseline bedtime on treatment day 1, and an hour earlier each treatment day. They were exposed to one of three bright light (~5000 lux) patterns upon waking each morning: four 30-minute exposures separated by 30 minutes of room light (2 h group); four 15-minute exposures separated by 45 minutes of room light (1 h group), and one 30-minute exposure (0.5 h group). Dim light melatonin onsets (DLMOs) before and after treatment determined the phase advance.
RESULTS
Compared to the 2 h group (phase shift=2.4±0.8 h), smaller phase advance shifts were seen in the 1 h (1.7±0.7 h) and 0.5 h (1.8±0.8 h) groups. The 2-hour pattern produced the largest phase advance; however, the single 30-minute bright light exposure was as effective as 1 hour of bright light spread over 3.25 h, and produced 75% of the phase shift observed with 2 hours of bright light.
CONCLUSIONS
A 30-minute morning bright light exposure with afternoon melatonin is an efficient treatment to phase advance human circadian rhythms.
Keywords: bright light, melatonin, circadian rhythms, DLMO, circadian misalignment, jet lag
INTRODUCTION
Misalignment between the circadian clock and 24-hour rhythmic behaviors, like sleep/wake and fasting/feeding (“circadian misalignment”) is associated with sleep disruption, excessive sleepiness and cognitive decrements during wake, and gastrointestinal problems [1–12]. The most recognized cause of circadian misalignment is jet lag after crossing multiple time zones, though night shift work and early school or work times are other situations in which individuals can experience circadian misalignment. In laboratory studies that experimentally imposed severe acute circadian misalignment, healthy participants showed adverse metabolic responses that are risk factors for cardiovascular disease and type 2 diabetes [1, 8, 9]. When experienced chronically like in night-shift work, circadian misalignment increases the risk for a number of diseases, including cancer [13–18].
Appropriately timed sleep (dark), light, and exogenous melatonin can phase shift circadian rhythms, and therefore can be used to reduce the degree of circadian misalignment and attenuate risks for negative health and daily functioning outcomes [7, 11, 12]. The direction and magnitude of the shift is predicted by phase response curves (PRCs) [19–29]. Advancing the system (shifting it earlier) is more difficult and typically takes longer than delaying (shifting it later). This may be in part because most humans have an endogenous period that is slightly longer than 24 hours [30–34] which favors the ability to delay. Of note, however, mice also have more difficulty advancing despite their average free-running period being less than 24 hours [35]. Our laboratory has focused on testing methods to advance rhythms to attenuate circadian misalignment, which could be utilized by travelers flying east, shift workers who need to wake early for early-morning shifts or who want to take all of their daily sleep before a night shift, and extreme night owls or patients with Delayed Sleep Phase Disorder (DSPD) who struggle to wake up for work or school. Indeed, the most recent American Academy of Sleep Medicine Practice Parameters for treatment of Circadian Rhythm Sleep Disorders [36] indicated (guideline) timed light exposure and timed melatonin administration for shift work disorder and DSPD. In a gradual sleep/dark shift paradigm, we have examined phase advances in response to afternoon melatonin alone [37], morning bright light alone [38–40], as well as the combination of afternoon melatonin and morning bright light [41] in healthy young adults. The latter combination of afternoon melatonin and morning bright light exposure produced the largest phase advance shifts (~2.5 hours) over three days of treatment.
One of the criticisms of bright light treatment, however, is that it is time-consuming, which could impact compliance to treatment. Little data exist on compliance to the use of bright light (either sunlight or light boxes) for reducing circadian misalignment, though a few reports describing light therapy for Seasonal Affective Disorder (SAD) suggest that compliance rates to using bright light boxes are mediocre in this particular group of patients. Michalak and colleagues [42] reported that over a four-week intervention, mean adherence to the prescribed bright light treatment was 59%. Others have reported rates of long-term use of prescribed bright light therapy for SAD ranging between 11% and 42% [43, 44]. Therefore, identifying an effective and efficient duration of bright light treatment that patients can realistically follow is warranted.
Many studies of phase shifting designed for practical purposes used long durations (3 or more hours) of continuous bright light exposures [38, 45–54]; however, intermittent bright light exposure is likely more feasible and mimics real-life treatment patterns compared to continuous light exposure [55, 56]. For this reason, we often utilize intermittent bright light in our studies [57–59]. Furthermore, previous studies from our laboratory [38] and others [60, 61] showed that intermittent bright light patterns can be almost as effective as continuous exposure producing about 60% to 90% of the phase shift obtained with continuous exposure. These data may suggest that the total time that the light is on may not be as important as the amount of time light exposure spans the appropriate portion of the PRC to light. Alternatively, or in addition, the beginning of a bright light exposure may be the most effective in eliciting a phase resetting response due to light adaptation [62].
While using a sleep schedule that was advanced by 1 hour/day for 3 days, we previously tested the combination of intermittent morning bright light and afternoon melatonin [41] to produce a phase advance while maintaining circadian alignment. The purpose of the current study was to identify whether there was a more efficient bright light treatment that could be implemented in combination with 0.5 mg exogenous melatonin to phase advance rhythms. The light pattern in the previous study [41] was intermittent such that the bright light box was turned on four times for 30 minutes with 30 minutes of normal room lighting in between. Therefore, the total bright light exposure was 2 hours, but the total treatment time was spread over 3.5 hours. Using this “2 h” group as a comparison group, the aims of this study were to examine whether phase advance shifts were smaller when total bright light treatment duration was shortened using the following strategies: (1) reduce the duration of the intermittent bright light exposures from 30 minutes to 15 minutes (“1 h” group); and (2) reduce the number of bright light exposures from four to one 30-minute exposure (“0.5 h” group). Two additional groups of participants completed the study and were compared to the historical comparison group.
METHODS
Participants
Data from 50 participants (27 males) aged 18 to 40 years (mean = 25.9 ± 5.1 years) were included in this analysis. The 2 h group included 16 participants (10 male), the 1 h group included 17 participants (9 male), and the 0.5 h group included 17 participants (8 males). Age, sex distribution, morningness/eveningness, and body mass index (BMI) did not differ among the 3 bright light groups. Morningness-eveningness was measured using the Horne-Östberg questionnaire [63]; 32 participants were intermediate types, 10 were moderate morning types, 6 were moderate evening types, 1 was a definite morning type, and 1 was a definite evening type (mean = 52.2, SD=8.6). BMI was < 30 (mean ± SD = 23.4 ± 2.8), and they weighed between 47 and 96 kg (mean ± SD = 68.5 ± 10.5 kg). The majority of participants (66%) reported their race as White/Caucasian (12% Black/African American; 10% Asian; 6% more than one race; 6% other); race distribution did not differ among groups. Inclusion/exclusion criteria and procedures for all groups were similar, and are described together below.
All participants were free of medical and psychiatric disorders, as assessed by in-person interviews, the Minnesota Multiphasic Personality Inventory-2 (MMPI-2), and part of a health questionnaire [64]. Participants reported not taking any prescription medications, except for 5 women who were taking oral contraceptives. Participants reported no more than moderate alcohol (2 or fewer drinks per day) and caffeine (< 500 mg per day) intake, and were non-smokers. Inclusion criteria included habitual sleep duration between 6.5 and 9 hours per night, habitual bedtimes between 23:00 and 02:00, and habitual wake times between 07:00 and 10:00. Participants reported no sleep problems over the proximal month of enrollment as assessed by a Pittsburgh Sleep Quality Index (PSQI) [65] score of 5 or less, and no problems with excessive daytime sleepiness as assessed by an Epworth Sleepiness Scale [66] score of less than 10. Participants reported not working night shifts during the 2 months before the start of the study or crossing more than 3 times zones in the month before beginning the study.
The Rush University Medical Center Institutional Review Board approved the study protocols, and therefore, the study was performed in accordance with the ethical standards outlined in the 1964 Declaration of Helsinki. Each participant gave written informed consent.
Procedures
Experimental Protocol
Participants completed the 14-day protocol illustrated in Figure 1. Each participant was given a fixed sleep schedule to follow at home during baseline days (1–6 and 8–11) providing 8 hours of time in bed. The sleep schedule assigned to each participant was based on their reported average bedtime and wake time before starting the study. Assigned baseline bedtimes ranged from 23:00 to 02:00 (mean = 00:10, SD=56 minutes), and thus wake time ranged from 07:00 to 10:00 (mean = 08:10, SD = 56 minutes). Each baseline morning, participants were required to go outside for at least 10 minutes during the second hour after waking for daylight exposure. The fixed sleep schedule and morning light was designed to stabilize circadian phase and ensure that participants were not sleep deprived before the phase advancing treatment in the laboratory. Participants slept at home on days 1–6 and 8–11; on the other days they slept in private temperature-controlled bedrooms in the laboratory. Participants lived in the laboratory from days 12 through 15. Wake-up times in the laboratory were gradually advanced by 1 hour per day over the three treatment days (days 12, 13, and 14). Bedtimes were also advanced by 1 hour per day, except in the 2 h group. Participants in the 2 h group were put to bed at their baseline bedtime on the first treatment day instead of one hour earlier, and then bedtime shifted by 2 h relative to baseline on the second treatment day (day 13), and by 3 h relative to baseline on the third treatment day (day 14). On the second and third treatment days, the sleep schedule among the 3 treatment groups were the same relative to their baseline sleep schedules. The gradual advance of sleep/dark over the 3 treatment days was designed to maintain alignment between sleep and circadian rhythms during the treatment and to facilitate a phase advance shift [38, 67].
Figure 1.
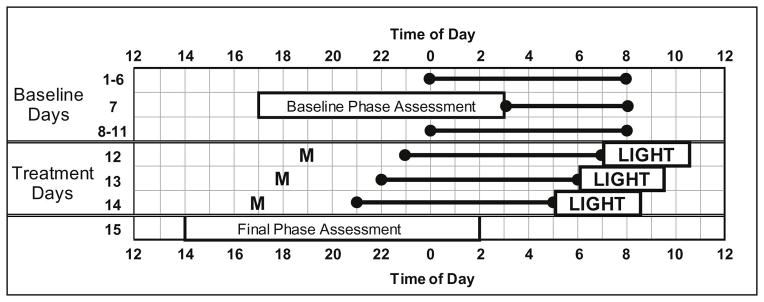
Protocol Diagram. The 2-week protocol included Baseline Days (1–11) and Treatment Days (12–14). Days 12, 13, and 14 are also referred to as Treatment Days 1, 2, and 3 respectively in the text. The horizontal lines anchored with circles illustrate scheduled sleep times. A participant with a baseline bedtime of midnight (0) and baseline wake-up time of 08:00 is shown as an example. Baseline sleep schedules always allowed for 8 hours of time in bed, but bed and wake times varied among participants as they were based on self-reported sleep times before beginning the study. The sleep schedule was gradually advanced on days 12, 13 and 14. Participants ingested 0.5 mg melatonin (M) 5 hours before their baseline bedtime on the first treatment day and then 1 hour earlier on each subsequent treatment day. Bright light (~ 5000 lux) from a single light box was administered within 5 minutes of waking on each of the 3 treatment days. On Days 7 and 15, participants completed a circadian phase assessment to determine the dim light melatonin onset (DLMO).
Participants were permitted to consume caffeine (up to 100 mg) during the first 3 hours of wake on baseline days 1–3. A maximum of 2 standard alcoholic drinks were permitted on Fridays and Saturdays (days 1, 2, 8, and 9) of the study. Participants were tested for alcohol using a breathalyzer before each phase assessment. Non-steroidal anti-inflammatory drugs were not permitted during the entire study, as these drugs suppress melatonin [68]. Recreational drugs and nicotine were prohibited throughout the study.
The study ran throughout the year (January through December), and therefore occurred during all seasons. The number of participants that ran in each season (defined as spring (Mar 1 – May 31), summer (Jun 1 – Aug 31), fall (Sept 1 – Nov 30), and winter (Dec 1 – Feb 28) did not differ among groups [Χ2 (6) = 4.89, p=0.56].
Bright Light
Soon after waking on the three treatment days (12, 13, and 14 in Figure 1), participants sat at a desk in their private bedroom in front of a single bright light box (54 × 54 cm diffuser screen; Enviro-Med, Vancouver, WA) approximately 40 cm from their eyes. The light box contained four 54-cm, 40-watt cool white fluorescent horizontal lamps (PL-L 40W/41/RS/IS, 4100K; Philips).
Figure 2 illustrates the three light patterns we compared in this between subjects design. In each group, the light box was turned on within 5 minutes of scheduled wake time. In the “2 h” group, participants received four 30-minute exposures of bright light with 30 minutes of room light in between. To test whether reducing the duration of the intermittent bright light exposures affects phase advances, a second group of participants (“1 h” group) received four 15-minute exposures of bright light with 45 minutes of room light in between. To test whether reducing the duration of bright light by reducing the number of exposures affect phase advances, a third group (“0.5 h” group) received one 30-minute bright light exposure only. Light intensity measured with a Minolta TL1 illuminance meter (KONICA MINOLTA, INC. Tokyo, Japan) ranged from 3500 to 6880 lux at the angle of gaze (mean = 4829, SD = 831 lux). A similar illuminance range was measured in each group: 0.5 h group: 3500–6440 lux; 1 h group: 3520 – 6490 lux; and 2 h group: 3510–6880 lux. The illuminance meter came from the manufacturer calibrated; we did not calibrate the meter. While awake and while the bright light box was off, one ceiling fixture with fluorescent tubes (4100 K) was on and dimmed to the lowest setting in the bedroom. Light intensity in the angle of gaze averaged 18.6 ± 16.6 lux and ranged from 4.1 to 93.9 lux. The ceiling lights were controlled by staff members in a separate control room. Angle of gaze was not controlled during the study because this is not how bright light treatment is delivered in the home.
Figure 2.
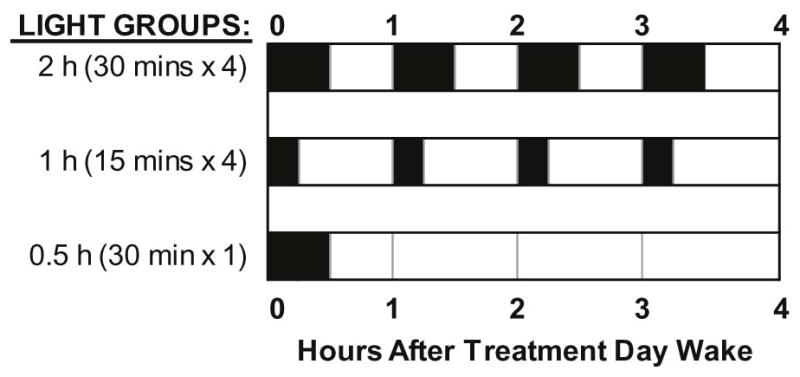
Morning bright light patterns (~ 5000 lux) for each group. Dark bars indicate when lights were on relative to wake-up time. Between bright light pulses, participants remained in room light (~20 lux at the angle of gaze).
Exogenous Melatonin
All participants were given 0.5 mg melatonin (Ecological Formulas, Cardiovascular Research Ltd., Concord CA) on each treatment day. Pill administration was double-blind; research staff administered the pill and were told that it was melatonin or placebo. Participants did not eat or drink anything 2 hours before until 30 minutes after ingesting the pills. Participants provided saliva samples 1 and 2 hours after pill ingestion to confirm that melatonin pills were correctly administered. These samples were later assayed for melatonin concentration. No melatonin doses were missed. We chose a small dose of melatonin (0.5 mg) to reduce the risk of evening sleepiness [37]. The half life of exogenous melatonin ranges from about 45 to 60 minutes [23, 69–71]. Based on previous findings [23, 69–71], the peak melatonin values in the current study likely occurred approximately 1 to 2 h after ingestion.
On the first treatment day we administered the 0.5 mg dose of melatonin 5 hours before baseline bedtime (9 hours before the baseline mid-sleep time), which targets the phase advance portion of the 0.5 mg PRC [25]. The timing of the pills was advanced by 1 hour each day because theoretically the circadian system is advancing each day; timing the dose earlier each day increased the likelihood that exogenous melatonin fell near the optimal time for phase advances according to the 0.5 mg melatonin PRC. We did not administer melatonin according to body weight because this is not the way it is sold to consumers. Based on the weight (in kg) of the participants, the dose averaged 0.007 ± 0.001 mg/kg, and ranged from 0.005 to 0.011 mg/kg.
Dim Light Melatonin Onset (DLMO) Phase Assessments
Endogenous salivary melatonin concentration was measured from approximately 2 mL of saliva collected every 30 minutes using Salivettes (Starstedt, Nümbrecht, Germany). Saliva collection for the baseline phase assessment began 7 hours before and ended 3 hours after assigned baseline bedtime. Saliva collection for the final phase assessment began 10 hours before and ended 2 hours after baseline bedtime. Participants remained awake in dim light (< 5 lux), sitting in comfortable recliners. Each sample was immediately centrifuged to extract saliva and then frozen. Saliva samples were later radioimmunoassayed (RIA) for melatonin concentration (Pharmasan Labs, Inc. Osceola, WI, USA). An individual’s samples were analyzed in the same batch. The sensitivity of the assay was between 0.7 and 1 pg/ml. The intra-assay coefficient of variation for low daytime levels of salivary melatonin was 12%. The inter-assay coefficients of variation for low daytime levels of salivary melatonin ranged from 13.2 to 15.9%.
Sleep and Ambient Light Monitoring
Participants completed daily sleep logs to record bedtime, estimated sleep onset time, night time awakenings > 5 minutes, final wake time, and the time they got out of bed. Participants wore actigraphs (Actiwatch-L, Philips Respironics, Inc., Bend OR, USA) on their wrist. They wore another Actiwatch-L (without the wrist band) on a cord around their necks as a medallion to measure compliance to the 10-minute outdoor morning light requirement during baseline. When sleeping at home, participants called the laboratory’s time-stamped voice mail system at bedtime and wake-up time. Activity and light data with the daily logs were reviewed with participants every 2 to 3 days at the laboratory to verify compliance.
Subjective Symptoms
Within 30 minutes of scheduled bedtime on baseline and treatment days, participants completed the Columbia Jet Lag Scale [72] to rate how they felt all day. This 9-item scale asked participants to rate how much they were bothered by sleepiness, fatigue, decreased daytime alertness, trouble concentrating or thinking clearly, lethargy/sluggish feeling, light-headed/dizziness, feelings of weakness, physical clumsiness, and trouble with memory on a scale of 0 (not at all) to 4 (extremely). A total sum score was computed with a possible range of 0 to 36. Participants also completed the Stanford Sleepiness Scale (SSS) [73] 4 or 5 times throughout the day during baseline and treatment days. Participants completed the SSS 15 to 30 minutes after waking, 15 to 60 minutes before bedtime, and 2 to 3 other times evenly spaced throughout the day. The SSS is a 7-point Likert Scale with the following anchored descriptions: 1=feeling active and vital; alert, wide awake; 2= functioning at high level, but not at peak; able to concentrate; 3= relaxed; awake; not at full alertness; responsive; 4= a little foggy; not at peak; let down; 5= fogginess; beginning to lose interest in remaining awake; slowed down; 6= sleepiness; prefer to be lying down; fighting sleep; woozy; and 7=almost in reverie; sleep onset soon; must struggle to remain awake.
Data and Statistical Analyses
DLMO
Raw melatonin curves were smoothed using a locally weighted least squares (LOWESS) curve generated using Prism software (GraphPad, Inc., San Diego CA, USA). The threshold for each phase assessment was the average of the five lowest continuous daytime values from the beginning of the phase assessment plus 15% of the average of the five highest continuous values. Baseline and final phase assessment thresholds for an individual were averaged. The DLMOs were the clock times at which the smoothed curves crossed this averaged threshold. We have implemented this method for calculating the DLMO previously [40, 59, 74–77] because it takes into account differences in the amplitude of the absolute levels of melatonin, which vary substantially between participants. In contrast to the DLMO20% (e.g., [78]) or DLMO25% (e.g., [79]) method, this threshold is typically lower on a melatonin profile (i.e., closer to the actual time of melatonin secretion onset), but above the low daytime background level of melatonin. The phase shift is the baseline DLMO minus the final DLMO; by convention, phase advances are positive numbers and phase delays are negative numbers.
To determine whether the DLMO phase advance differed among the three bright light groups, a 2 (time: baseline versus final)-by-3 (group: 2h versus 1h versus 0.5 h) repeated measures omnibus ANOVA was computed. To determine whether DLMO phase advances differed after reducing the duration of the light exposures from 30 minutes to 15 minutes, a 2 (time: baseline versus final)-by-2 (bright light group: 2 h versus 1 h) repeated measures ANOVA was computed. To test whether DLMO phase advances differed after reducing the number of light exposures from four to one, a 2 (time: baseline versus final)-by- 2 (bright light group: 2 h versus 0.5 h) repeated measures ANOVA was computed. When the assumption of sphericity was violated, Greenhouse-Geisser corrections were used, though the original degrees of freedom are reported here.
Sleep
Wrist activity data were analyzed using the Actiware 5 software (version 5.59, Respironics, Inc., Bend Oregon, USA) to estimate sleep/wake (medium threshold; immobile minutes algorithm). Each sleep episode was inspected within a rest interval beginning with the participants’ reported bedtime and ending with their reported wake-up time on their daily sleep log. The following variables were derived: Sleep Onset Time, Wake Time, and Total Sleep Time. To examine whether sleep timing or duration differed among the three groups, 3 (bright light group)-by-4 (time: baseline, treatment days 1, 2, and 3) repeated measures ANOVAs were computed. When the assumption of sphericity was violated, Greenhouse-Geisser corrections were used, though the original degrees of freedom are reported here.
Subjective Symptoms
Subjective symptoms were derived from the Columbia Jet Lag Scale and SSS. Ratings on days 2–6 were averaged to define baseline and were compared to Treatment Day 3 (from waking for the second light treatment on day 13 until bedtime on day 14), because this day followed one night of similar time in bed among the groups. A 2 (time: baseline versus Treatment Day 3)-by-3 (bright light group) repeated measures ANOVA was used to determine whether symptoms near the end of treatment became worse, and whether the change in symptoms differed among the bright light groups.
RESULTS
DLMO Phase Advance
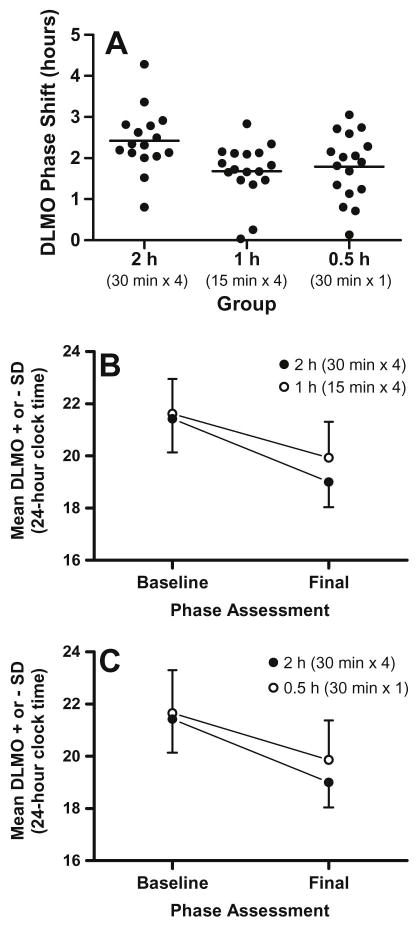
Table 1 shows the sex distribution and sample size for each group and the magnitude of the phase shifts in the DLMO. All three groups phase advanced from the baseline to final phase assessment [time: F(1,47)=336.9, p<.001]. In comparison to the 2 h group, however, smaller phase advance shifts were seen in the 1 h and 0.5 h groups [time x group: F(2,47)=4.6, p=.02]. The 1 h and 0.5 h groups showed similar phase advance shifts (see Table 1 and Figure 3A).
Table 1.
Phase shifts and circadian phase (mean ± SD) marked by the dim light melatonin onset (DLMO) for all bright light groups.
| Bright Light Group
|
|||
|---|---|---|---|
| 2 h (30 min × 4) | 1 h (15 min × 4) | 0.5 h (30 min × 1) | |
| N | 16 (10 M, 6 F) | 17 (9 M, 8 F) | 17 (8 M, 9 F) |
| DLMO phase advance (h) | 2.4 ± 0.8 | 1.7 ± 0.7** | 1.8 ± 0.8* |
| Baseline DLMO | 21:25 ± 01:17 | 21:36 ± 01:20 | 21:39 ± 01:30 |
| Final DLMO | 19:00 ± 00:58 | 19:56 ± 01:22 | 19:52 ± 01:30 |
| Baseline DLMO to baseline bedtime (h) | 2.6 ± 1.2 | 2.7 ± 0.7 | 2.3 ± 1.2 |
| Final DLMO to treatment day 3 bedtime (h) | 2.1 ± 0.7 | 1.4 ± 0.6 | 1.2 ± 1.0 |
| Administration Times (Treatment Day 1) | |||
| Melatonin pill to baseline DLMO (h) | 2.4 ± 1.2 | 2.3 ± 0.7 | 2.6 ± 1.1 |
| Baseline DLMO to start of bright light (h) | 9.7 ± 1.2 | 9.7 ± 0.7 | 9.4 ± 1.1 |
p<.05,
p<.01 compared to 2 h group.
Figure 3.
Phase shifts to intermittent morning bright light plus afternoon melatonin. (A) Black circles illustrate the phase advance shifts for each individual, and horizontal lines illustrate mean phase shifts for each bright light group. (B) Mean DLMO at baseline and final assessments for the 2 h group in comparison to the 1 h group, which differed in bright light exposure duration (30 minutes versus 15 minutes). (C) Mean DLMO at baseline and final assessments for the 2 h group in comparison to the 0.5 h group, which differed in light exposure number (four versus one 30-minute light exposure). The phase advance was significantly larger in the 2 h group.
Figure 3 B illustrates average baseline and final DLMOs for the 2 h and 1 h groups. In combination with a gradually advancing sleep/dark schedule and afternoon melatonin, reducing the duration of the four morning light exposures from 30 minutes to 15 minutes decreased the average phase advance shift by 42 minutes. There was a significant time [F(1,31)=260.9, p < .001] and time-by-group interaction [F(1,31)=8.43, p=.007].
Figure 3 C illustrates baseline and final DLMOs for the 2 h and 0.5 h groups. When the number of 30-minute bright light exposures was reduced from four to one, the phase advance shift decreased by an average of 36 minutes. There was a significant time [F(1,31)=234.6, p < .001] and time-by-group interaction [F(1,31)=5.35, p=.028].
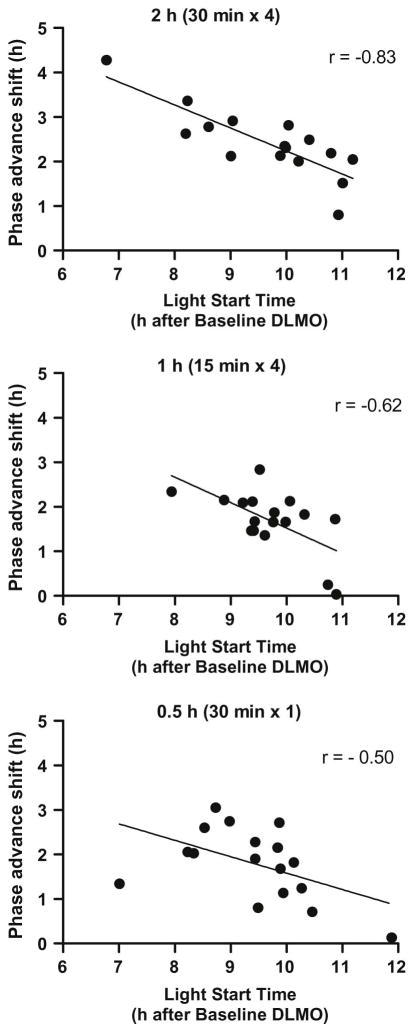
Baseline DLMOs were similar among the three bright light groups, and the timing of morning bright light and 0.5 mg of melatonin on the first treatment day in the laboratory (Day 12) relative to baseline DLMO did not differ among groups (see Table 1). Bright light started 6.8 to 11.9 h after baseline DLMO. As expected based on properties of the light PRC, bright light that started in the earlier part of this range showed larger phase advance shifts compared to those that started later in this range (Figure 4). A significant negative linear association was seen between phase shift and light start time in the 2 h group [r = −0.83, p < .001], the 1 h group [r = −0.62, p= .008], and the 0.5 h group [r = −0.50, p = .041]. The melatonin pill was always given 12 h before scheduled wake-up time and the start of light treatment; therefore, the correlation coefficients showing the association between the phase advance shift and the timing of the melatonin pill relative to DLMO are the same.
Figure 4.
Scatter plots showing the association between the time of the morning bright light (relative to the DLMO) and the magnitude of the phase advance. Light start time is the interval between the baseline DLMO and the start of morning light exposure on the first treatment morning. A linear regression line (solid line) was fit separately for each group.
Sleep
Actigraphic estimates of sleep during baseline and during treatment days for each bright light group are included in Table 2. Sleep timing and duration did not differ among groups during baseline or on treatment days 2 and 3. The 2 h group had 7 hours in bed on treatment day 1, because their bedtime was not advanced on that day, so their total sleep time was about 1 hour less than for the other 2 groups who had 8 hours in bed.
Table 2.
Mean ± SD actigraphic estimates of sleep onset time, wake time, and total sleep time during baseline (days 1–6 and 8–11) and the 3 treatment days (days 12, 13, and 14) for each bright light group.
| Bright Light Group
|
|||
|---|---|---|---|
| 2 h (30 min × 4) | 1 h (15 min × 4) | 0.5 h (30 min × 1) | |
| Baseline: | |||
| Sleep Onset Time | 00:16 ± 0:48 | 00:31 ± 1:03 | 00:17 ± 1:04 |
| Wake Time | 7:55 + 0:43 | 8:08 ± 1:01 | 7:54 + 0:59 |
| Total Sleep Time (h) | 6.9 ± 0.4 | 7.0 ± 0.37 | 7.0 ± 0.2 |
| Treatment Day 1: | |||
| Sleep Onset Time | 00:10 ± 0:44 | 23:27 ± 1:05 | 23:10 ± 1:03* |
| Wake Time | 6:54 ± 0:45 | 7:13 ± 1:02 | 6:54 ± 0:58 |
| Total Sleep Time (h) | 6.1 ± 0.5 | 7.1 ± 0.4* | 7.2 ± 0.4* |
| Treatment Day 2: | |||
| Sleep Onset Time | 22:12 ± 0:45 | 22:24 ± 1:05 | 22:13 ± 1:04 |
| Wake Time | 5:58 ± 0:46 | 6:11 ± 1:03 | 5:48 ± 0:58 |
| Total Sleep Time (h) | 7.0 ± 0.5 | 7.1 ± 0.4 | 7.0 ± 0.5 |
| Treatment Day 3: | |||
| Sleep Onset Time | 21:13 ± 0:45 | 21:29 ± 1:06 | 21:15 ± 1:04 |
| Wake Time | 4:51 ± 0:47 | 5:11 ± 1:01 | 4:57 ± 0:58 |
| Total Sleep Time (h) | 6.8 ± 0.7 | 7.1 ± 0.5 | 7.0 ± 0.3 |
p<.05 compared to 2 h group.
- Sleep Onset Time [time: F(3,141) = 6281.5, p <.001]; [time × group: F(6,141) = 91.0, p <.001]
- Wake Time [time: (F(3,141) = 2682.6, p < .001]
- Total Sleep Time [time: F(3,141) = 5.0, p = .002]; [time × group: F(6,141) = 17.7, p <.001]
Subjective Symptoms
Figure 5 shows that jet lag symptoms measured from the Columbia Jet Lag Scale showed a small increase compared to baseline on Treatment Day 3 (from wake on day 13 in Figure 1 to right before bed on day 14) for all bright light groups compared to baseline [time: F(1,45) = 9.7, p = .003]; scores for all three groups increased by about 2 points on this scale, which has a maximum value of 36. Despite smaller phase advance shifts in the 1 h and 0.5 h groups in comparison to the 2 h group, jet lag symptoms did not differ among the groups. Participants did not rate their sleepiness differently on Treatment Day 3 compared to baseline, and subjective sleepiness ratings did not differ among the three bright light groups.
Figure 5.
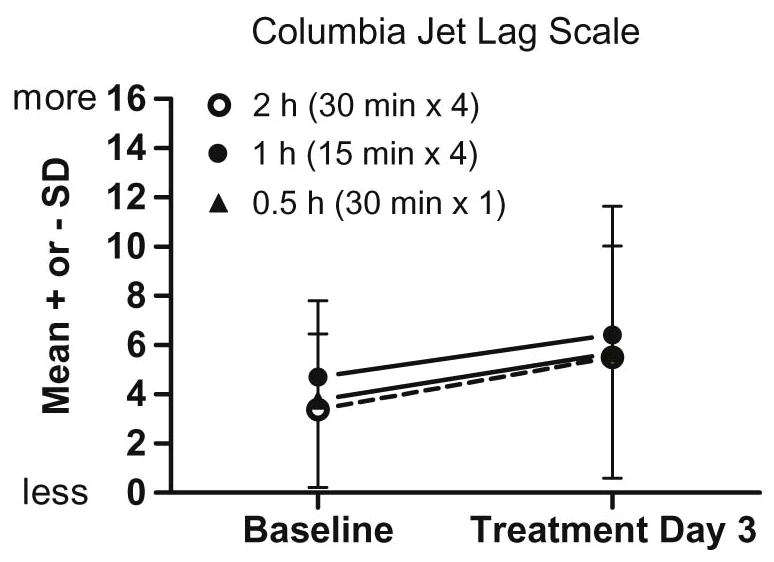
Mean subjective ratings of jet lag at baseline (days 2–6) and Treatment Day 3 (from wake up on day 13 to bedtime on day 14) for the three bright light groups. The 2 h group is differentiated by an open symbol and dotted line to more easily compare it to the 1 h (closed circle) and 0.5 h (closed triangle) groups. There was a slight increase in subjective jet lag (the maximum score is 36), but there were no differences among the groups.
DISCUSSION
This study is part of a series of studies in our laboratory aimed at testing methods to phase advance human circadian rhythms using afternoon melatonin and/or morning bright light from a single light box [37–41]. We combined these zeitgebers with a gradually advancing sleep/dark schedule rather than an abrupt advance of the sleep schedule to limit the degree of circadian misalignment between the internal circadian system and sleep/wake during treatment. This method is designed to advance circadian rhythms before flying east to reduce or eliminate jet lag or to advance the rhythms of people who want to have an earlier schedule for other reasons, such as shift workers on early morning shifts or extreme night owls and people with DSPD who want to be able to fall asleep and wake up earlier.
In this report we compared 3 different morning light patterns combined with a low dose of afternoon melatonin (0.5 mg) and a sleep/dark schedule that was advanced by 1 hour/day for 3 days. The group that received the longest duration of morning bright light each day (2 h group; four 30-minute exposures interspersed with 30 minute of room light), showed the largest phase advance. This occurred despite not shifting bedtime earlier on treatment day 1 in the 2 h group as was done for the two other groups, which could have reduced the advance in the 2 h group. Reducing the duration of the 4 bright light exposures from 30 minutes to 15 minutes (1 h group) significantly reduced the phase advance, as did reducing the number of 30-minute light exposures from four to one (0.5 h group). The 1-h and 0.5-h bright light exposures, however, still produced about 70 to 75% of the phase advance as that produced by the 2-hour exposure, even though the total duration of bright light was only 50% and 25% of the 2-hour condition. Average phase advances in the 1 h and 0.5 h groups did not differ from one another, which showed that given 3 days of afternoon melatonin and a gradually advancing sleep schedule, the single 30-minute bright light exposure immediately after waking was just as effective as one hour of intermittent light exposure spread across the first 3.25 h after waking.
These data suggest that the first morning light exposure likely contributes the most to the observed phase advance shift. The single 30-minute light exposure of the 0.5 h group produced about 75% of the phase shift of the 2 h group with four 30-minute exposures, indicating that the subsequent exposures contributed less to the overall phase advance shift. This may also explain the similar phase advances seen in the 0.5 h group and the 1 h group; the first 15-minute exposure contributed the most to the phase advance – about the same as the 30-minute exposure – and the subsequent 15-minute exposures of the 1 h group may have contributed less to the overall shift. These data mimic the findings of Chang and colleagues [80], who showed similar phase delay shifts (1.1 h and 1.6 h) in response to 12 minutes and 60 minutes of bright light (> 6000 lux), respectively. The relative potency of the first exposure may be explained by studies showing that the beginning of the light exposure (the transition from dark to light) is the most effective to produce a phase shift compared to the subsequent hours of light exposure [62, 81]. Alternatively or in addition, the first bright light exposure of all groups was timed closer to the peak of the phase advance region of the light phase response curve compared to the subsequent intermittent exposures. Data from the current study indicate that light treatment beginning at an earlier circadian time produced greater phase advance shifts than light treatment beginning later (see Figure 4). This pattern is consistent with previously published PRCs to bright light [21, 82]. Of note, the overall magnitude of the phase shift was also driven by the exogenous melatonin timed close to the peak of the phase advance region of the 0.5 mg melatonin PRC [25] over the three treatment days.
Three previous studies [38, 60, 61] compared the phase shifting effects of long continuous bright light exposures to intermittent bright light patterns that spanned the same time interval and showed that intermittent light patterns can be very effective. Rimmer and colleagues [60] tested 3 days of bright light exposures (up to ~10,000 lux) with 4 abruptly shifted sleep/dark episodes to produce phase advances. They showed that intermittent patterns spanning 5.8 hours – one with 38- and 46-minute exposures totaling 3.8 hours, and another with 5.3-minute exposures totaling 1.9 hours – produced about 60–90% of the phase shift produced by 5.8 hours of continuous bright light despite bright light being on for 33% and 66% of the time. Gronfier and colleagues [61] tested one day of bright light and two abruptly shifted sleep/dark episodes designed to produce phase delays. They showed that an intermittent pattern spanning 6.5 hours with 15-minute exposures totaling 1.5 hours produced about 80% of the shift produced by 6.5 hours of continuous bright light even though it was on for only 23% of the time. Using a similar protocol to the current study with 3 days of a 1 hour /day advance of sleep/dark, we compared 3.5 hours of continuous morning bright light (~ 5000 lux) to 2 hours of intermittent light spanning the same 3.5-h time interval (four 30-minute bright light exposures interspersed with 30 minute of room light) [38]. The intermittent pattern produced about 70% of the phase shift produced by 3.5 hours of continuous bright light. In the current study, we compared two intermittent light patterns that spanned a similar time interval. The 15-minute light exposure pattern produced 71% of the phase shift as the pattern with 30-minute exposures even though the bright light was on for only 50% of the time. The low-cost-to-high-benefit ratio shown in all of these studies may be explained by animal data suggesting that the system is able to integrate these intermittent light stimuli over time [83]. More recently in humans, Zeitzer and colleagues [84, 85] reported that brief 2-msec flashes of light in an otherwise dark room spaced over an hour can delay circadian rhythms.
Previous studies compared different durations of continuous bright light exposures, and showed that longer durations produced larger phase shifts [49, 80, 86]. Previously in our lab, we compared daily bright light (~5000 lux) with durations of 3 or 6 hours along with an abrupt 12-hour shift of sleep/dark for 8 days [49]. The phase shift averaged over the last 4 days was 8.1 hours for the 3-hour duration and 9.4 hours for the 6-hour duration. A second study [86] tested three bright light durations (1, 2, and 3 hours) with different light intensities (2000, 4000, and 8000 lux). Subjects were awakened for a single bright light exposure in the middle of their sleep period before the temperature minimum to produce phase delays. When averaged over the 3 light intensities the phase shifts were 0.4, 1.1 and 1.7 hours for the 1-, 2- and 3-hour durations, respectively. Another study [80] measured phase shifts to bright light exposures (> 6000 lux) in a protocol that included one day with bright light and two abruptly shifted sleep/dark episodes designed to produce phase delays. Bright light durations of 0.2, 1.0, 2.5, 4.0, or 6.5 hours produced phase shifts of 1.1, 1.6, 2.3, 2.7 and 3.1 hours. From these data, they constructed a duration response curve, which was approximately linear between 0.2 and 2.5 hours and then appeared to level off (saturate). Taken together, these studies suggest that increasing the duration of continuous bright light by more than about 3 hours results in minimal gains to phase shift the circadian system. In the current study, the intermittent light pattern with the longest duration (2 hours) produced the largest phase shifts. It is possible that adding more light exposures would increase the phase shift; however, increasing the time may reduce feasibility of the bright light treatment.
Thus far we have discussed studies that compared different durations and patterns of bright light; however, the current study also included exogenous melatonin to phase shift circadian rhythms. Outside of our laboratory, we know of two studies that combined afternoon melatonin and morning light to produce a phase advance in circadian rhythms [87, 88]. Paul and colleagues [87] administered 3 mg sustained release melatonin at 4:00 pm, after which participants went to bed (approximately 8 hours before their usual bedtime). Participants were then awakened about 2.5 hours before their usual wake time and exposed to green light for 1 hour. This single day of afternoon melatonin, morning light and an abrupt advance of the sleep schedule produced a phase advance of 1.0 hour. Burke and colleagues [88] administered 5 mg of melatonin 5.75 hours before habitual bedtime, and 3 hours of bright light (~3000 lux) starting 1 hour before habitual wake time. This single day of afternoon melatonin and morning light produced a phase advance of 1.3 hours. In the current study, 0.5 mg afternoon melatonin and 3 different morning light patterns applied over 3 days produced descriptively larger phase advances than the two studies described above, especially for the 2 h group (2.4 hours). Similarly, 3 mg of afternoon melatonin combined with 2 hours of bright intermittent light over 3 days also produced descriptively larger phase advance shifts (2.6 hours) [41] than these previous studies. These larger phase shifts from our laboratory are expected because the treatment was repeated over 3 days.
Despite different protocols, different melatonin doses, and different types of morning light among previous studies, the phase advancing effect of morning light alone and afternoon melatonin alone are roughly similar and when combined they are roughly additive. In our gradually advancing sleep/dark protocol, we have shown that 3 mg of afternoon melatonin alone produced a phase advance of 1.3 hours [37], morning light alone (30 minute x 4 exposures) produced phase advances of 1.5 hours [38] and 1.7 hours [41], and the combination produced a phase advance of 2.6 hours [41]. Paul and colleagues [87] also show this additive pattern; afternoon melatonin produced a phase advance of 0.7 hours, morning light advanced rhythms by 0.3 hours and the combination advanced rhythms by 1.0 hour. Finally, in the hands of Burke and colleagues [88], afternoon melatonin alone, morning light alone and the combination produced phase advances of 0.6, 0.7 and 1.1 hours, respectively. Therefore, all of these studies show an additive effect of melatonin and bright light, and all of these studies illustrate that the largest phase advances are produced with the combination of melatonin and light.
The largest phase advance in the current study was seen in the 2 h group and averaged 2.4 hours, but the sleep/wake schedule advanced by 3 hours by the last treatment day. Thus, by the end of the protocol there was a small degree of circadian misalignment because the advance in the circadian system was not enough to keep up with the advance in the sleep/wake schedule. In the other two groups with less morning bright light exposure, the phase advances were smaller (1.7 and 1.8 h); thus, there was a slightly greater amount of circadian misalignment by the end of treatment in these groups compared to the 2 h group. The degree of circadian misalignment in each group by the end of the protocol can be estimated by comparing the interval between the DLMO and bedtime before and after the phase advancing treatment (i.e., baseline DLMO to baseline bedtime vs. final DLMO to treatment day 3 bedtime in Table 1). The average difference was 0.5 h in the 2 h group, 1.3 h in the 1 h group, and 1.1 h in the 0.5 h group. Although there was a small degree of misalignment between circadian rhythms and sleep timing, total sleep time did not differ and sleepiness ratings were not worse at the end of the protocol compared to baseline for any of the groups. Jet lag scores from the Columbia scale were slightly higher than baseline near the end of the protocol, with no differences among the 3 groups. These elevated scores likely reflect the cumulative amount of circadian misalignment by the last treatment day in all groups. A previous study from our laboratory [38] tested morning bright light without melatonin (continuous versus intermittent bright light exposure over 3.5 hours) versus dim light in the same phase advancing protocol as the current study. On average, continuous bright light advanced rhythms by 2.1 h, intermittent bright light advanced rhythms by 1.5 h, and dim light phase advanced rhythms by 0.6 h. Again, none of these groups were completely aligned with the 3-hour shifted sleep schedule by the end of treatment; however, smaller phase advances (i.e., greater circadian misalignment) were associated with more jet lag symptoms.
In addition to circadian misalignment, the slightly elevated Columbia Scale jet lag scores in the current study might also be due to the acute side effect of melatonin (sleepiness), especially since the questionnaire was filled out a few hours after ingesting the pill. Our previous studies using evening melatonin showed an increase in subjective sleepiness in the hours after taking 3.0 mg melatonin [37, 41], but not after taking 0.5 mg melatonin (see Figure 6 in [41]). The lower dose of melatonin (0.5 mg) was used in the current study to reduce the risk of sleepiness. Therefore, we think it unlikely that residual or acute effects of melatonin are contributing significantly to these slightly elevated jet lag symptoms. Generally, this method for advancing circadian rhythms produced only a small degree of circadian misalignment and jet-lag-type symptoms. If our treatment protocol was continued for more than 3 days, however, it is possible that the circadian system would lag behind the sleep schedule even more, producing a greater degree of circadian misalignment and eventually jet lag symptoms, including sleep difficulties.
There are several ways to reduce the potential for circadian misalignment when using our method for advancing circadian rhythms. First, use the longest practical duration of continuous bright light as early in the morning after waking as possible as this study and the others reviewed above show larger phase shifts with more hours of bright light and larger advances with earlier morning light. We also recommend using a light box as large as possible to cover as much of the visual field as possible [89] and seeking outdoor sunlight exposure when it is daytime rather than using a light box [11]. Another way to reduce circadian misalignment while advancing the circadian system is to reduce the amount of time that sleep/dark is shifted each day; for example, advancing sleep/dark times by 30 or 45 minutes per day instead of 1 hour may keep the sleep schedule more aligned with circadian rhythms. Reducing the daily sleep/dark shift to less than 1 hour, however, still needs to be tested. Another way to reduce circadian misalignment with our method is to start using the melatonin and bright light a day or two before the sleep/wake schedule is advanced (see Figures 30–5 and 30–7 in Revell & Eastman [11]) rather than waiting until the first day that sleep/dark is advanced. Finally, for cases in which bedtime advances to a time when there is daylight, exposure to outdoor light in the hours before bed should be avoided as this could delay rhythms. Dimming indoor lights or even wearing sunglasses in the few hours before bedtime could also reduce this risk as we know that even normal indoor lighting in the evening can delay rhythms [90]. Creating a dark environment to sleep (e.g., with black-out shades or dark material over windows) is also recommended because the advance of the sleep/dark period removes the light from the phase delay portion of the PRC and thus facilitates the desired phase advance.
In real-life settings, the recommendation of which melatonin dose to use depends on the individual’s circumstances. For coping with an abrupt advance of sleep after it occurs, for example, after flying east across several time zones with no pre-flight adjustment, taking sustained release melatonin before the advanced bedtime in the new time zone, as simulated by Paul and colleagues [87] is appropriate. Our method of a gradual advance of sleep/dark with afternoon melatonin and morning bright light is designed to be used a few days before the earlier sleep schedule is required (e.g., before eastward jet travel, before early morning shift work, before the transition back to early school start times after a vacation) and will not produce the extreme circadian misalignment that comes from a large abrupt shift of sleep. Exogenous melatonin is taken several hours before bedtime when using this method. We [37, 41] and others [91–94] have found that high doses of afternoon melatonin (e.g., 2–10 mg) will likely produce unwanted evening sleepiness. Thus, for those who are sensitive to the sleepiness produced by melatonin, we recommend the 0.5 mg dose, which we showed did not produce more sleepiness than placebo [41]. Our melatonin PRC studies [25] show that the average magnitude of the phase shifts is similar between a 0.5 and 3 mg dose; however, the 3 mg dose is more likely to produce reliable phase shifts compared to the 0.5 mg dose. Therefore, we recommend the 3 mg dose if possible, especially if there are no evening activities that require vigilance, such as driving.
The current study is limited by only studying healthy young adult participants. Future studies may consider testing these phase advancing strategies on older adults or patients with circadian-based sleep disorders, such as DSPD. The scope of the study was limited to comparing three morning bright light patterns with one melatonin dose. A number of light patterns and melatonin doses are possible, however, and some could be more effective than, yet as feasible as, a single 30-minute exposure (e.g., one 30-minute exposure with 3.0 mg melatonin). Questioning participants about feasibility of such treatments at home is also missing, and we suggest collecting such feasibility data in future work.
In summary, the current study tested 3 different morning bright light patterns combined with a low dose of afternoon melatonin and a gradual advance of sleep/dark over three days to advance circadian rhythms. Such a strategy could be used to help night owls or people with DSPD, to help people adapt to an early work or school schedule, to help a shift worker who has to work early morning shifts, or help travelers to reduce or prevent jet lag. The 2-hour (30 minutes x 4) morning bright light exposure produced the largest phase advance; however, one 30-minute bright light exposure immediately upon waking each day is effective and takes a quarter of the time.
HIGHLIGHTS.
Morning bright light, afternoon melatonin, & early sleep advances circadian rhythms
With advancing sleep and melatonin, 3 morning bright light patterns are compared
A 2-h light exposure spread over 3.5 h produces the largest advance shift
A single 0.5-h light exposure is as effective as a 1-h exposure spread over 3.25 h
A single 0.5-h light exposure produces 75% of the advance produced by a 2-h pattern
Acknowledgments
Melatonin and matching placebo were provided by Ecological Formulas (a division of Cardiovascular Research Ltd., Concord, CA). We are grateful to Thomas Molina, Christina Suh, Carlo Legasto, Heather Gunn, Sarah Garcia, Rose Diskin, Liz Sorokin, and Marissa Dziepak for their assistance with data collection and data management. This work was supported by a grant from the National Institutes of Health (R01 NR007677) awarded to C.I. Eastman.
ABBREVIATIONS
- DLMO
Dim Light Melatonin Onset
- PRC
phase response curve
- DSPD
delayed sleep phase disorder
- SAD
seasonal affective disorder
- h
hour
- min
minute
- BMI
Body Mass Index
- PSQI
Pittsburgh Sleep Quality Index
- SSS
Stanford Sleepiness Scale
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Institute of Nursing Research. The National Institute of Nursing Research and the National Institutes of Health had no involvement in designing the study, data collection, data analysis, and interpretation, writing of the manuscript, nor in the decision to submit the manuscript for publication.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hampton SM, Morgan LM, Lawrence N, Anastasiadou T, Norris F, Deacon S, et al. Postprandial hormone and metabolic responses in simulated shift work. J Endocrinol. 1996;151:259–67. doi: 10.1677/joe.0.1510259. [DOI] [PubMed] [Google Scholar]
- 2.Knutsson A. Health disorders of shift workers. Occupational Medicine. 2003;53:103–8. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- 3.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–62. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 4.Sack R, Auckley D, Auger R, Carskadon M, Wright K, Vitiello M, et al. Circadian rhythm sleep disorders: Part 1, basic principles, shift work and jet lag disorders. Sleep. 2007;30:1460–83. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akerstedt T, Wright K. Sleep loss and fatigue in shift work and shift work disorder. Sleep Med Clin. 2009;4:257–71. doi: 10.1016/j.jsmc.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arendt J. Managing jet lag: Some of the problems and possible new solutions. Sleep Medicine Reviews. 2009;13:249–56. doi: 10.1016/j.smrv.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Eastman CI, Burgess HJ. How to travel the world without jet lag. Sleep Med Clin. 2009;4:241–55. doi: 10.1016/j.jsmc.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–8. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129ra43. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright KP, Jr, Bogan RK, Wyatt JK. Shift work and the assessment and management of shift work disorder (SWD) Sleep Med Rev. 2012;17:41–54. doi: 10.1016/j.smrv.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Revell VL, Eastman CI. Jet lag and its prevention. In: Barkoukis TJ, Matheson JK, Ferber R, Doghramji K, editors. Therapy in Sleep Medicine. Elsevier; 2012. pp. 390–401. [Google Scholar]
- 12.Smith MR, Eastman CI. Shift work: health, performance and safety problems, traditional countermeasures, and innovative management strategies to reduce circadian misalignment. Nat Sci Sleep. 2012;4:111–32. doi: 10.2147/NSS.S10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rafnsson V, Tulinius H, Jonasson JG, Hrafnkelsson J. Risk of breast cancer in female flight attendants: a population-based study (Iceland) Cancer Causes Control. 2001;12:95–101. doi: 10.1023/a:1008983416836. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds P, Cone J, Layefsky M, Goldberg DE, Hurley S. Cancer incidence in California flight attendants (United States) Cancer Causes Control. 2002;13:317–24. doi: 10.1023/a:1015284014563. [DOI] [PubMed] [Google Scholar]
- 15.Hansen J. Risk of breast cancer after night- and shift work: current evidence and ongoing studies in Denmark. Cancer Causes Control. 2006;17:531–7. doi: 10.1007/s10552-005-9006-5. [DOI] [PubMed] [Google Scholar]
- 16.Viswanathan AN, Hankinson SE, Schernhammer ES. Night shift work and the risk of endometrial cancer. Cancer Res. 2007;67:10618–22. doi: 10.1158/0008-5472.CAN-07-2485. [DOI] [PubMed] [Google Scholar]
- 17.Blask DE. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev. 2009;13:257–64. doi: 10.1016/j.smrv.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Erren TC, Falaturi P, Morfeld P, Knauth P, Reiter RJ, Piekarski C. Shift work and cancer: the evidence and the challenge. Dtsch Arztebl Int. 2010;107:657–62. doi: 10.3238/arztebl.2010.0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czeisler CA, Kronauer RE, Allan JS, Duffy JF, Jewett ME, Brown EN, et al. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–33. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- 20.Minors DS, Waterhouse JM, Wirz-Justice A. A human phase-response curve to light. Neurosci Lett. 1991;133:36–40. doi: 10.1016/0304-3940(91)90051-t. [DOI] [PubMed] [Google Scholar]
- 21.Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549(3):945–52. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kripke DF, Elliott JA, Youngstedt SD, Rex KM. Circadian phase response curves to light in older and young women and men. J Circadian Rhythms. 2007;5:4. doi: 10.1186/1740-3391-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewy AJ, Bauer VK, Ahmed S, Thomas KH, Cutler NL, Singer CM, et al. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int. 1998;15:71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- 24.Burgess HJ, Revell VL, Eastman CI. A three pulse phase response curve to three milligrams of melatonin in humans. J Physiol. 2008;586(2):639–47. doi: 10.1113/jphysiol.2007.143180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgess HJ, Revell VL, Molina TA, Eastman CI. Human phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mg. J Clin Endocr Metab. 2010;95:3325–31. doi: 10.1210/jc.2009-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buxton OM, L’Hermite-Baleriaux M, Turek FW, Van Cauter E. Daytime naps in darkness phase shift the human circadian rhythms of melatonin and thyrotropin secretion. Am J Physiol Regulatory Integrative Comp Physiol. 2000;278:R373–R82. doi: 10.1152/ajpregu.2000.278.2.R373. [DOI] [PubMed] [Google Scholar]
- 27.Revell VL, Molina TA, Eastman CI. Human phase response curve to intermittent blue light using a commercially available device. J Physiol. 2012;590:4859–68. doi: 10.1113/jphysiol.2012.235416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruger M, St Hilaire MA, Brainard G, Khalsa SB, Kronauer RE, Czeisler CA, et al. Human phase response curve to a single 6.5-h pulse of short-wavelength light. J Physiol. 2013;591:353–63. doi: 10.1113/jphysiol.2012.239046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.St Hilaire MA, Gooley JJ, Khalsa SB, Kronauer RE, Czeisler CA, Lockley SW. Human phase response curve to a 1h pulse of bright white light. J Physiol. 2012;590:3035–45. doi: 10.1113/jphysiol.2012.227892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wever RA. The Circadian System of Man: Results of Experiments Under Temporal Isolation. New York-Heidelberg-Berlin: Springer-Verlag; 1979. [Google Scholar]
- 31.Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–81. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 32.Burgess HJ, Eastman CI. Human tau in an ultradian light-dark cycle. J Biol Rhythms. 2008;23:374–6. doi: 10.1177/0748730408318592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith MR, Burgess HJ, Fogg LF, Eastman CI. Racial differences in the human endogenous circadian period. PLoS One. 2009;4:e6014. doi: 10.1371/journal.pone.0006014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eastman CI, Molina TA, Dziepak ME, Smith MR. Blacks (African Americans) have shorter free-running circadian periods than whites (Caucasian Americans) Chronobiol Int. 2012;29:1072–7. doi: 10.3109/07420528.2012.700670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Curr Biol. 2006;16:R914–6. doi: 10.1016/j.cub.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgenthaler T, Lee-Chiong T, Alessi C, Friedman L, Aurora N, Boehlecke B, et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. Sleep. 2007;30:1445–59. doi: 10.1093/sleep/30.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crowley SJ, Eastman CI. Melatonin in the afternoons of a gradually advancing sleep schedule enhances the circadian rhythm phase advance. Psychopharmacol (Berl) 2013;225:825–37. doi: 10.1007/s00213-012-2869-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burgess HJ, Crowley SJ, Gazda CJ, Fogg LF, Eastman CI. Preflight adjustment to eastward travel: 3 days of advancing sleep with and without morning bright light. J Biol Rhythms. 2003;18:318–28. doi: 10.1177/0748730403253585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eastman CI, Gazda CJ, Burgess HJ, Crowley SJ, Fogg LF. Advancing circadian rhythms before eastward flight: A strategy to prevent or reduce jet lag. Sleep. 2005;28:33–44. doi: 10.1093/sleep/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith MR, Revell VL, Eastman CI. Phase advancing the human circadian clock with blue-enriched polychromatic light. Sleep Med. 2009;10:287–94. doi: 10.1016/j.sleep.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Revell VL, Burgess HJ, Gazda CJ, Smith MR, Fogg LF, Eastman CI. Advancing human circadian rhythms with afternoon melatonin and morning intermittent bright light. J Clin Endocr Metab. 2006;91:54–9. doi: 10.1210/jc.2005-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michalak EE, Murray G, Wilkinson C, Dowrick C, Lam RW. A pilot study of adherence with light treatment for seasonal affective disorder. Psychiatry Res. 2007;149:315–20. doi: 10.1016/j.psychres.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz PJ, Brown C, Wehr TA, Rosenthal NE. Winter seasonal affective disorder: a follow-up study of the first 59 patients of the National Institute of Mental Health Seasonal Studies Program. Am J Psychiatry. 1996;153:1028–36. doi: 10.1176/ajp.153.8.1028. [DOI] [PubMed] [Google Scholar]
- 44.Rohan KJ, Roecklein KA, Lacy TJ, Vacek PM. Winter depression recurrence one year after cognitive-behavioral therapy, light therapy, or combination treatment. Behav Ther. 2009;40:225–38. doi: 10.1016/j.beth.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Czeisler CA, Johnson MP, Duffy JF, Brown EN, Ronda JM, Kronauer RE. Exposure to bright light and darkness to treat physiologic maladaptation to night work. N Engl J Med. 1990;322:1253–9. doi: 10.1056/NEJM199005033221801. [DOI] [PubMed] [Google Scholar]
- 46.Eastman CI. High intensity light for circadian adaptation to a 12-h shift of the sleep schedule. Am J Physiol. 1992;263:R428–R36. doi: 10.1152/ajpregu.1992.263.2.R428. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell PJ, Hoese EK, Liu L, Fogg LF, Eastman CI. Conflicting bright light exposure during night shifts impedes circadian adaptation. J Biol Rhythms. 1997;12:5–15. doi: 10.1177/074873049701200103. [DOI] [PubMed] [Google Scholar]
- 48.Martin SK, Eastman CI. Medium-intensity light produces circadian rhythm adaptation to simulated night-shift work. Sleep. 1998;21:154–65. [PubMed] [Google Scholar]
- 49.Eastman CI, Liu L, Fogg LF. Circadian rhythm adaptation to simulated night shift work: Effect of nocturnal bright-light duration. Sleep. 1995;18:399–407. doi: 10.1093/sleep/18.6.399. [DOI] [PubMed] [Google Scholar]
- 50.Dawson D, Encel N, Lushington K. Improving adaptation to simulated night shift: Timed exposure to bright light versus daytime melatonin administration. Sleep. 1995;18:11–21. doi: 10.1093/sleep/18.1.11. [DOI] [PubMed] [Google Scholar]
- 51.Horowitz TS, Cade BE, Wolfe JM, Czeisler CA. Efficacy of bright light and sleep/darkness scheduling in alleviating circadian maladaptation to night work. Am J Physiol Endocrinol Metab. 2001;281:E384–E91. doi: 10.1152/ajpendo.2001.281.2.E384. [DOI] [PubMed] [Google Scholar]
- 52.Yoon IY, Jeong DU, Kwon KB, Kang SB, Song BG. Bright light exposure at night and light attenuation in the morning improve adaptation of night shift workers. Sleep. 2002;25:351–6. [PubMed] [Google Scholar]
- 53.Lack L, Wright H, Kemp K, Gibbon S. The treatment of early-morning awakening insomnia with 2 evenings of bright light. Sleep. 2005;28:616–23. doi: 10.1093/sleep/28.5.616. [DOI] [PubMed] [Google Scholar]
- 54.Santhi N, Aeschbach D, Horowitz TS, Czeisler CA. The impact of sleep timing and bright light exposure on attentional impairment during night work. J Biol Rhythms. 2008;23:341–52. doi: 10.1177/0748730408319863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boivin DB, James FO. Circadian adaptation to night-shift work by judicious light and darkness exposure. J Biol Rhythms. 2002;17:556–67. doi: 10.1177/0748730402238238. [DOI] [PubMed] [Google Scholar]
- 56.Boivin DB, Boudreau P, James FO, Kin NM. Photic resetting in night-shift work: impact on nurses’ sleep. Chronobiol Int. 2012;29:619–28. doi: 10.3109/07420528.2012.675257. [DOI] [PubMed] [Google Scholar]
- 57.Baehr EK, Fogg LF, Eastman CI. Intermittent bright light and exercise to entrain human circadian rhythms to night work. Am J Physiol. 1999;277:R1598–R604. doi: 10.1152/ajpregu.1999.277.6.R1598. [DOI] [PubMed] [Google Scholar]
- 58.Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. J Biol Rhythms. 2003;18:513–23. doi: 10.1177/0748730403258422. [DOI] [PubMed] [Google Scholar]
- 59.Smith MR, Fogg LF, Eastman CI. Practical interventions to promote circadian adaptation to permanent night shift work: Study 4. J Biol Rhythms. 2009;24:161–72. doi: 10.1177/0748730409332068. [DOI] [PubMed] [Google Scholar]
- 60.Rimmer DW, Boivin DB, Shanahan TL, Kronauer RE, Duffy JF, Czeisler CA. Dynamic resetting of the human circadian pacemaker by intermittent bright light. Am J Physiol. 2000;279:R1574–R9. doi: 10.1152/ajpregu.2000.279.5.R1574. [DOI] [PubMed] [Google Scholar]
- 61.Gronfier C, Wright KP, Kronauer RE, Jewett ME, Czeisler CA. Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am J Physiol. 2004;287:E174–E81. doi: 10.1152/ajpendo.00385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Comas M, Beersma DG, Spoelstra K, Daan S. Phase and period responses of the circadian system of mice (Mus musculus) to light stimuli of different duration. J Biol Rhythms. 2006;21:362–72. doi: 10.1177/0748730406292446. [DOI] [PubMed] [Google Scholar]
- 63.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 64.Tasto DL, Colligan MJ, Skjei EW, Polly SJ. Health Consequences of Shift Work. Cincinnati: NIOSH Publication; 1978. pp. #78–154. [Google Scholar]
- 65.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 66.Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 67.Sharkey KM, Carskadon MA, Figueiro MG, Zhu Y, Rea MS. Effects of an advanced sleep schedule and morning short wavelength light exposure on circadian phase in young adults with late sleep schedules. Sleep Med. 2011;12:685–92. doi: 10.1016/j.sleep.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murphy PJ, Myers BL, Badia P. Nonsteroidal anti-inflammatory drugs alter body temperature and suppress melatonin in humans. Physiol Behav. 1996;59:133–9. doi: 10.1016/0031-9384(95)02036-5. [DOI] [PubMed] [Google Scholar]
- 69.Waldhauser F, Waldhauser M, Lieberman HR, Deng MH, Lynch HL, Wurtman RJ. Bioavailability of oral melatonin in humans. Neuroendocrinology. 1984;39:307–13. doi: 10.1159/000123997. [DOI] [PubMed] [Google Scholar]
- 70.Wyatt JK, Dijk DJ, Ritz-de Cecco A, Ronda JM, Czeisler CA. Sleep-facilitating effect of exogenous melatonin in healthy young men and women is circadian-phase dependent. Sleep. 2006;29:609–18. doi: 10.1093/sleep/29.5.609. [DOI] [PubMed] [Google Scholar]
- 71.Breslow ER, Phillips AJ, Huang JM, St Hilaire MA, Klerman EB. A mathematical model of the circadian phase-shifting effects of exogenous melatonin. J Biol Rhythms. 2013;28:79–89. doi: 10.1177/0748730412468081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spitzer RL, Terman M, Williams JB, Terman JS, Malt UF, Singer F, et al. Jet lag: clinical features, validation of a new syndrome-specific scale, and lack of response to melatonin in a randomized, double-blind trial. Am J Psychiatry. 1999;156:1392–6. doi: 10.1176/ajp.156.9.1392. [DOI] [PubMed] [Google Scholar]
- 73.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: A new approach. Psychophysiology. 1973;10:431–6. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 74.Lee C, Smith MR, Eastman CI. A compromise phase position for permanent night shift workers: circadian phase after two night shifts with scheduled sleep and light/dark exposure. Chronobiol Int. 2006;23:859–75. doi: 10.1080/07420520600827160. [DOI] [PubMed] [Google Scholar]
- 75.Smith MR, Cullnan EE, Eastman CI. Shaping the light/dark pattern for circadian adaptation to night shift work. Physiol Behav. 2008;95:449–56. doi: 10.1016/j.physbeh.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 76.Smith MR, Eastman CI. Night shift performance is improved by a compromise circadian phase position: Study 3. Circadian phase after 7 night shifts with an intervening weekend off. Sleep. 2008;31:1639–45. doi: 10.1093/sleep/31.12.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith MR, Fogg LF, Eastman CI. A compromise circadian phase position for permanent night work improves mood, fatigue, and performance. Sleep. 2009;32:1481–9. doi: 10.1093/sleep/32.11.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L’Hermite-Baleriaux M, Zee PC. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms. 2005;20:178–88. doi: 10.1177/0748730404273983. [DOI] [PubMed] [Google Scholar]
- 79.Santhi N, Duffy JF, Horowitz TS, Czeisler CA. Scheduling of sleep/darkness affects the circadian phase of night shift workers. Neurosci Lett. 2005;384:316–20. doi: 10.1016/j.neulet.2005.04.094. [DOI] [PubMed] [Google Scholar]
- 80.Chang AM, Santhi N, St Hilaire MA, Gronfier C, Bradstreet DS, Duffy JF, et al. Human responses to bright light of different durations. J Physiol. 2012;590:3103–12. doi: 10.1113/jphysiol.2011.226555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beersma DG, Comas M, Hut RA, Gordijn MC, Rueger M, Daan S. The progression of circadian phase during light exposure in animals and humans. J Biol Rhythms. 2009;24:153–60. doi: 10.1177/0748730408330196. [DOI] [PubMed] [Google Scholar]
- 82.Revell VL, Eastman CI. How to trick Mother Nature into letting you fly around or stay up all night. J Biol Rhythms. 2005;20:353–65. doi: 10.1177/0748730405277233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nelson DE, Takahashi JS. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster. J Physiol. 1991;439:115–45. doi: 10.1113/jphysiol.1991.sp018660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zeitzer JM, Ruby NF, Fisicaro RA, Heller HC. Response of the human circadian system to millisecond flashes of light. PLoS One. 2011;6:e22078. doi: 10.1371/journal.pone.0022078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zeitzer JM, Fisicaro RA, Ruby NF, Heller HC. Millisecond Flashes of Light Phase Delay the Human Circadian Clock during Sleep. J Biol Rhythms. 2014;29:370–6. doi: 10.1177/0748730414546532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dewan K, Benloucif S, Reid K, Wolfe LF, Zee PC. Light-Induced Changes of the Circadian Clock of Humans: Increasing Duration is More Effective than Increasing Light Intensity. Sleep. 2011;34:593–9. doi: 10.1093/sleep/34.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paul MA, Gray GW, Lieberman HR, Love RJ, Miller JC, Trouborst M, et al. Phase advance with separate and combined melatonin and light treatment. Psychopharmacology (Berl) 2011;214:515–23. doi: 10.1007/s00213-010-2059-5. [DOI] [PubMed] [Google Scholar]
- 88.Burke TM, Markwald RR, Chinoy ED, Snider JA, Bessman SC, Jung CM, et al. Combination of light and melatonin time cues for phase advancing the human circadian clock. Sleep. 2013;36:1617–24. doi: 10.5665/sleep.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eastman CI. How to get a bigger dose of bright light. Sleep. 2011;34:559–60. doi: 10.1093/sleep/34.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burgess HJ, Molina TA. Home lighting before usual bedtime impacts circadian timing: A field study. Photochem Photobiol. 2014;90:723–6. doi: 10.1111/php.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arendt J, Borbely AA, Franey C, Wright J. The effects of chronic, small doses of melatonin given in the late afternoon on fatigue in man: a preliminary study. Neurosci Lett. 1984;45:317–21. doi: 10.1016/0304-3940(84)90245-3. [DOI] [PubMed] [Google Scholar]
- 92.Dollins AB, Zhdanova IV, Wurtman RJ, Lynch HJ, Deng MH. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc Natl Acad Sci U S A. 1994;91:1824–8. doi: 10.1073/pnas.91.5.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cajochen C, Krauchi K, von Arx MA, Mori D, Graw P, Wirz-Justice A. Daytime melatonin administration enhances sleepiness and theta/alpha activity in the waking EEG. Neurosci Lett. 1996;207:209–13. doi: 10.1016/0304-3940(96)12517-9. [DOI] [PubMed] [Google Scholar]
- 94.Cajochen C, Krauchi K, Wirz-Justice A. The acute soporific action of daytime melatonin administration: effects on the EEG during wakefulness and subjective alertness. J Biol Rhythms. 1997;12:636–43. doi: 10.1177/074873049701200619. [DOI] [PubMed] [Google Scholar]