Abstract
Objectives
Examine the validity of an induced pain paradigm in which people stand while performing simulated light work tasks (standing paradigm).
Methods
Initially, people with LBP reported the quality and location of their typical symptoms on a body pain diagram. Then, people with LBP and back-healthy people stood for 2 hours and reported the intensity, quality, and location of symptoms at baseline and every 15 minutes. Quality and location of typical symptoms of people with LBP were compared to their symptoms during standing. Back-healthy people were separated into pain developers (PDs) and non-pain developers. Symptom quality and location were compared between people with LBP and PDs.
Results
There were no differences in the quality and location of typical symptoms and symptoms during standing in people with LBP (P-values > 0.05). Three symptom descriptors were used by more than 30% of people with LBP to describe typical symptoms. Only 2 people with LBP used these descriptors to describe typical symptoms but not during standing. There were no differences in the quality and location of symptoms reported in standing between people with LBP and PDs (P-values > 0.05). Four symptom descriptors were used by more than 30% of participants with LBP during standing. There were no symptoms reported by PDs that were not reported by people with LBP.
Discussion
This study provides evidence that symptoms experienced during the standing paradigm are similar to symptoms experienced by people with LBP and, thus, provides support for the validity of the paradigm.
Keywords: Prolonged standing, low back pain
INTRODUCTION
Epidemiological studies indicate that occupations that require standing for prolonged periods are associated with increased reports of low back pain (LBP).1,2 Previous studies have used an exposure-based, induced pain paradigm to examine characteristics that may predispose people to initial LBP development during prolonged standing (standing paradigm).3–12 The standing paradigm involves back-healthy people standing for 2 hours performing simulated, light work tasks while rating their LBP intensity on a 100 mm visual analogue scale (VAS). Any person that has an increase of ≥10 mm on the VAS is classified as a pain developer (PD); all others are classified as non-pain developers (NPD). Characteristics are then examined to determine factors that might make back-healthy people more susceptible to becoming PDs.
A consistent finding across previous studies using the standing paradigm is that symptoms are induced in 40–71% of back-healthy people which has allowed examination of baseline differences between PDs and NPDs. Compared to NPDs, PDs have displayed (1) difficulty controlling the trunk during the clinical test of active hip abduction,6 (2) more trunk extensor muscle activity in response to a trunk perturbation,4 and (3) altered timing of activation of the trunk extensor muscles during the clinical test of return from forward bending.11 Identification of baseline differences between PDs and NPDs can lend insight into the characteristics that may make a back-healthy person more susceptible to developing LBP symptoms during standing.
The paradigm is appealing from an experimental perspective because transient LBP symptoms are induced in back-healthy people during an occupational activity associated with increased reports of LBP.1,2 However, it is currently not known whether symptoms induced in PDs during the standing paradigm are similar to symptoms typically experienced by people with LBP. In order for the paradigm to be considered a valid method for investigating risk factors for initial LBP symptom development during standing, the symptoms typically experienced by people with LBP should be similar to symptoms that people develop during the paradigm. Therefore, it is critical to know if the standing paradigm results in reports of symptoms in people with LBP that are (1) similar to symptoms they typically experience, and (2) the same as symptoms induced in back-healthy people classified as PDs. No previous studies have tested the validity of the standing paradigm by comparing the symptoms induced during the standing paradigm to symptoms experienced by people with LBP.
The purpose of this study was to examine the validity of the standing paradigm. First we compared the quality and location of symptoms “typically” experienced by people with LBP to symptoms they experienced during standing. This comparison was to confirm that the symptoms produced in a person with LBP during standing were similar to the symptoms the person identified as part of his LBP condition. We then compared the quality and location of symptoms experienced during the standing paradigm between people with LBP and back-healthy people classified as PDs. This comparison was to confirm that symptoms experienced by back healthy people classified as PDs were similar to those experienced by participants with LBP. We hypothesized that the quality and location of the symptoms experienced by people with LBP during standing would be similar to (1) their “typical” symptoms, and (2) symptoms induced in back-healthy people classified as PDs. If our hypotheses are supported, the data would provide support for the validity of the paradigm as a method for investigating risk factors for initial LBP development during prolonged standing.
MATERIALS AND METHODS
Participants
People with LBP and back-healthy people were recruited using flyers posted at local universities in the surrounding area and venues across the St. Louis metropolitan area. Participants also were recruited through 2 community-based, university operated, recruitment organizations. People interested called the laboratory and were screened. Based on the most recent studies using the standing paradigm, LBP was defined as any lifetime episode of LBP that resulted in 3 or more consecutive days of missed work or school or seeking some type of health intervention (e.g., physician, physical therapist, chiropractor). For the current study, we also required that a person could not have had 3 or more consecutive days of altered activity due to LBP. People with LBP were excluded if they had a history of pain, numbness or tingling below the knee during a LBP episode, disc herniation, previous spinal surgery, or were diagnosed with a specific spinal condition that caused their LBP symptoms (e.g., spinal stenosis). Back-healthy people were excluded if they (1) reported an episode of LBP as operationally defined, or (2) had been employed in a job that involved standing in one place for more than 1 hour per day during the last 12 months. Exclusion criteria for all people included being unable to stand for > 4 hours or a body mass index > 30. All participants read and signed an informed consent form that was approved by the Human Research Protection Office at Washington University School of Medicine.
All participants were recruited during the same time period. If a back-healthy participant was classified as a PD, a participant with LBP then was recruited that was matched for sex, age, height, weight, and BMI. If a participant with LBP was recruited, back-healthy participants were recruited that were matched for sex, age, height, weight and BMI until one was classified as a PD.
Procedures
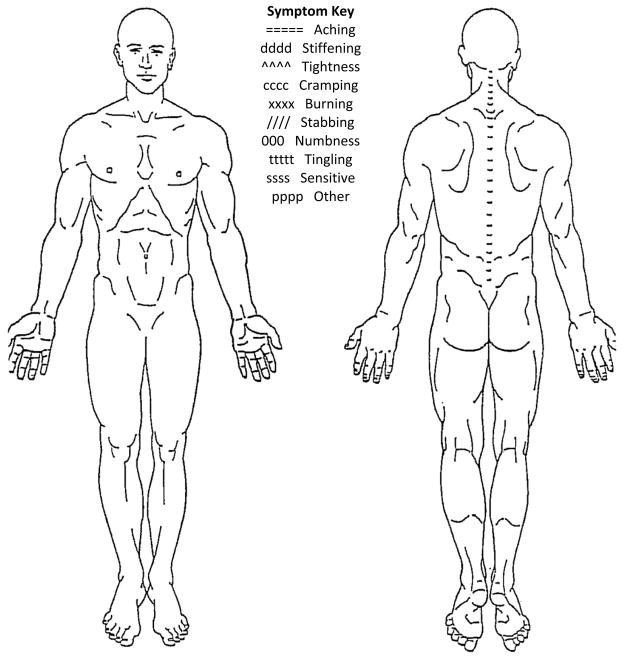
Upon arrival at the laboratory, all participants reported their current LBP symptom intensity level on a VAS while seated. The VAS is a 100 mm horizontal line with the anchors of “no pain” and “worst pain imaginable.” Participants placed an ‘X’ through the line at the point that best represented their current perception of LBP symptom intensity. To quantify intensity, the distance between the left end of the scale and the ‘X’ was measured with a ruler. Greater distances indicated higher symptom intensities. Participants with LBP also completed a LBP history questionnaire and the modified Oswestry LBP disability questionnaire.13 Included in the LBP history questionnaire was a numeric rating scale (NRS)14 in which the participants with LBP rated their average and worst symptoms over the 7 days prior to participation. The NRS is a scale ranging from 0 to 10 with the anchors of “no pain” to “worst pain imaginable.” The number of acute flare-ups experienced over the 12 months prior to participation also was included in the LBP history questionnaire. Flare-ups were operationally defined as LBP symptoms that were “markedly more severe than usual and lasted at least 2 consecutive days.”15,16 Participants with LBP then reported the quality and location of the LBP symptoms they typically experience on a body pain diagram (BPD, Figure 1). Symptom quality was based on descriptors from a symptom key provided on the BPD. Symptom descriptors included aching, stiffening, tightness, cramping, burning, numbness, tingling, and sensitive.17 There also was an “other” option participants used if they perceived their symptoms to be different from any of the descriptors provided. If the participant used the “other” response, he or she was given the option to provide a word that described the symptoms. Symptom location was based on the region of the BPD that the participant indicated he experienced symptoms. The regions of interest were the low back, gluteal, and thigh. The VAS and BPD have been shown to be reliable methods for describing symptoms of musculoskeletal pain, including LBP.18
Figure 1.
Body pain diagram.
All participants then stood for 2 hours in a 2′ × 4′ confined workspace. A table where the simulated work tasks were performed was adjusted to 5 cm below the participant’s wrist while their elbows were flexed to 90°.7 Participants were allowed to shift their weight as often as desired but were told to keep both feet on the ground the majority of the time, and were not allowed to rest their feet or arms on the table. While standing, the participant performed either a light simulated work task (shuffling cards, sorting poker chips, assembly task) or stood quietly. The work tasks and quiet standing were completed in 15 minute blocks of time with the order of tasks randomized. Randomization was performed using random.org/lists. Each task and quiet standing was completed twice throughout the two hours, with the added constraint that the same task could not be performed in consecutive sequence. Following randomization, if any of the 3 work tasks or quiet standing was to be performed in consecutive sequence, randomization was repeated. At baseline and every 15 minutes during the standing, participants reported the quality and location of their symptoms on the BPD and intensity of their symptoms on the VAS.
Statistical analyses
Participants
Back-healthy participants were separated into PDs and NPDs based on the ≥ 10 mm change in VAS score criterion. This criterion was chosen because 9 mm has been found to be the minimum clinically significant difference in VAS.19 Initially, an independent t-test was used to examine if there was a significant difference between the PDs and NPDs in change in VAS score (maximum VAS score during standing minus VAS score at the beginning of standing). The analysis was performed to confirm that the PDs and NPDs were different with regard to VAS ratings. Subsequent analyses included only participants with LBP and PDs. A Chi-square analysis was conducted to test for differences in the distribution of sex in participants with LBP and PDs. Independent groups t-tests were conducted to test for differences in demographics and activity level (age, height, weight, body mass index (BMI), Baecke Habitual Activity Questionnaire20) between participants with LBP and PDs.
Participants with LBP: Typical versus standing symptoms
Symptom Quality. For each symptom descriptor, (1) frequency counts of yes and no responses were calculated for the 2 conditions, (2) an agreement (yes/yes and no/no responses) to disagreement (no/yes and yes no responses) ratio was calculated, and (3) a McNemar’s chi-square test was performed to test for differences in the proportions of participants with LBP who did and did not report each descriptor as “typical” and who did and did not report each descriptor during standing. For the symptom descriptors in which 30% or more participants reported as typical an agreement to disagreement ratio was calculated (1) across all descriptors, and (2) for the participants that reported at least one of the descriptors as typical and during standing. Symptom Location. For each region of interest, (1) frequency counts of yes and no responses were calculated for the 2 conditions, (2) an agreement (yes/yes and no/no responses) to disagreement (no/yes and yes no responses) ratio was calculated, and (3) a McNemar’s chi-square test was used to test for differences in the proportions of participants with LBP who did and did not report symptoms in each region as “typical” and who did and did not report symptoms in each region during standing.
Participants with LBP and PDs: Symptoms in standing
Symptom Quality. For each symptom descriptor, a Fisher’s exact test was used to compare the proportions of participants with LBP and PDs who did and did not report each descriptor during standing. A w statistic was calculated to index effect size for each comparison.21 Symptom Location. For each region of interest, a Fisher’s exact test was used to compare the proportions of participants with LBP and PDs who did and did not report symptoms in the region during standing. A w statistic was calculated to index effect size for each comparison.21
RESULTS
Group characteristics
Descriptive statistics for demographic, activity, and LBP-related variables (LBP only) are provided in Table 1. Fifty-three back-healthy people participated. Of these, 15 people were classified as PDs (28%) and 38 (72%) were classified as NPDs. As expected, there was a significant difference between PDs and NPDs in change in VAS score (mean difference: 18.7 mm, 95% CI: 13.6 mm to 23.9 mm, P-value < 0.01). PDs had a higher mean change in VAS score compared to NPDs. There were 15 people with LBP who participated. All reported having symptoms within the 7 days prior to participation. There were no differences between the participants with LBP and PDs for sex, age, height, weight, BMI, or activity level (P-values > 0.05, Table 1).
Table 1.
Participant characteristics.
| Characteristic | Group
|
Statistical Value* | P-value | |
|---|---|---|---|---|
| LBP (n = 15) | PDs (n = 15) | |||
| Sex (female, male)# | 9, 6 | 9, 6 | Χ2 = 0.0 | 1.0 |
| Age (years) | 23.5 ± 3.1 | 22.7 ± 1.6 | t = −1.0 | 0.3 |
| Height (cm) | 169.5 ± 7.7 | 172.8 ± 6.9 | t = 1.2 | 0.2 |
| Weight (kg) | 68.3 ± 12.7 | 71.5 ± 10.4 | t = 0.7 | 0.5 |
| BMI (kg/m2) | 23.7 ± 3.2 | 23.8 ± 1.8 | t = 0.2 | 0.9 |
| Baecke Questionnaire of Habitual Physical Activity (3–15)20 | 8.6 ± 1.2 | 8.6 ± 1.4 | t = 1.0 | 1.0 |
| Oswestry low back pain disability questionnaire (0–100%)13 | 11.1 ± 9.1 | NA** | NA** | NA** |
| Current low back pain intensity (Visual analog scale; 0–100 mm) | 8.3 ± 9.3 | NA** | NA** | NA** |
| Time since first low back pain episode (years) | 7.7 ± 4.0 | NA** | NA** | NA** |
| Number of LBP flare-ups15, 16 in the 12 months prior to participation | 5.8 ± 3.8 | NA** | NA** | NA** |
| Average symptoms over the 7 days prior to participation (Numeric rating scale; 0–10)14 | 2.7 ± 1.3 | NA** | NA** | NA** |
| Worst symptoms over the 7 days prior to participation (Numeric rating scale; 0–10)14 | 6.3 ± 1.8 | NA** | NA** | NA** |
Chi-square test was used to test for differences in proportions of gender between groups. An independent t-test was used to test for differences for all other variables.
Sex is number of females and males in each group; all other values are the mean ± standard deviation
PDs do not have a history of LBP.
Fritz and Irrgang, 2001.
Downie, 1978.
Von Korff, 1994.
McGorry et al., 2000
Baecke et al., 1982.
LBP, low back pain; PDs, pain developers; NA, not applicable.
Participants with LBP: Typical versus standing symptoms
Symptom quality. Three descriptors, aching, stiffening, and tightness, were reported as “typical” by 5 (33%) or more of the participants with LBP. Results for these descriptors are provided here and in Table 2. Data for all 10 descriptors are provided in the Supplemental Digital Content 1. There were no significant differences in the proportions of participants with LBP who did and did not report aching, stiffening, and tightness as “typical” and during standing (all P-values > 0.05, Table 2). In addition, the agreement to disagreement ratios for aching, stiffening, and tightness were 15:0, 8:7, and 10:5, respectively. For stiffening only 1 disagreement was due to a participant reporting stiffening as typical but not during standing. The remaining 6 disagreements were participants that reported stiffening only during standing. For tightness only 1 disagreement was due to a participant reporting tightness as typical but not during standing. The remaining 4 disagreements were participants that reported tightness only during standing. The overall agreement to disagreement ratio for the 3 symptom descriptors was 33:12. All 15 participants with LBP reported at least one of the 3 descriptors as typical and during standing. Symptom location. There were no significant differences in the proportions of participants with LBP who did and did not report symptoms in any of the three regions as “typical” and during standing (P-values > 0.05, Table 3). The agreement to disagreement ratios for the low back, gluteal, and thigh regions were 15:0, 9:6, and 11:4, respectively. For the gluteal region 3 of the disagreements were due to participants reporting symptoms in the gluteal region as typical but not during standing. The remaining 3 disagreements were due to participants reporting symptoms in the gluteal region only during standing. For the thigh region 2 of the disagreements were due to participants reporting symptoms in the thigh region as typical but not during standing. The remaining 2 disagreements were participants that reported symptoms in the thigh region only during standing. The overall agreement to disagreement ratio for the 3 regions of interest was 35:10.
Table 2.
The number of participants with LBP (n = 15) that reported the descriptors aching, stiffening, and tightness as “typical” and during standing.
| Descriptor | Typical
|
Χ2* | P-value | Agreement: Disagreement¶ | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Total | ||||||
| Aching | Standing | Yes | 14 | 0 | 14 | NA# | NA# | 15:0 |
| No | 0 | 1 | 1 | |||||
| Total | 14 | 1 | 15 | |||||
|
| ||||||||
| Stiffening | Standing | Yes | 5 | 6 | 11 | 2.29 | 0.13 | 8:7 |
| No | 1 | 3 | 4 | |||||
| Total | 6 | 9 | 15 | |||||
|
| ||||||||
| Tightness | Standing | Yes | 4 | 4 | 8 | 0.80 | 0.37 | 10:5 |
| No | 1 | 6 | 7 | |||||
| Total | 5 | 10 | 15 | |||||
|
| ||||||||
| Aching, Stiffening, or Tightness** | Standing | Yes | 15 | 0 | 15 | NA# | NA# | 15:0 |
| No | 0 | 0 | 0 | |||||
| Total | 15 | 0 | 15 | |||||
For each symptom descriptor, a McNemar’s chi-square test was calculated.
Ratio of agreements (yes/yes and no/no responses) to disagreements (no/yes to yes/no responses).
Unable to calculate statistic because of lack of discordant pairs.
Numbers of participants that reported at least one of the 3 descriptors as “typical” and during standing.
LBP, low back pain; NA, not applicable.
Table 3.
The number of participants with LBP (n = 15) that reported symptoms in each region as “typical” and reported symptoms in the region during standing.
| Region | Typical
|
Χ2* | P-value | Agreement: Disagreement¶ | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Total | ||||||
| Low Back | Standing | Yes | 15 | 0 | 15 | NA# | NA# | 15:0 |
| No | 0 | 0 | 0 | |||||
| Total | 15 | 0 | 15 | |||||
|
| ||||||||
| Gluteal | Standing | Yes | 1 | 3 | 4 | 0.17 | 0.68 | 9:6 |
| No | 3 | 8 | 11 | |||||
| Total | 4 | 11 | 15 | |||||
|
| ||||||||
| Thigh | Standing | Yes | 2 | 2 | 4 | 0.25 | 0.62 | 11:4 |
| No | 2 | 9 | 11 | |||||
| Total | 4 | 11 | 15 | |||||
For each region of interest, a McNemar’s chi-square test was calculated.
Ratio of agreements (yes/yes and no/no responses) to disagreements (no/yes to yes/no responses).
Unable to calculate statistic because of lack of discordant pairs.
LBP, low back pain; NA, not applicable.
Participants with LBP and PDs: Symptoms in standing
Symptom quality. Four descriptors, aching, stiffening, tightness, and cramping were reported by 5 (33%) or more of the participants with LBP. Results for these descriptors are provided here and in Table 4. Data for all descriptors are provided in the Supplemental Digital Content 2. There were no significant differences in the proportions of participants with LBP and PDs who did and did not report aching, stiffening, tightness and cramping during standing (P-values > 0.05, Table 4). Effect sizes for the comparisons ranged from 0 to .34. Additionally, all 15 participants in the LBP group and in the PD group reported at least one of the 4 descriptors (aching, stiffening, tightness, cramping) during standing (Table 4). Symptom location. There were no significant differences in the proportions of participants with LBP and PDs who did and did not report symptoms in any of the three regions during standing (P-values > 0.05, Table 5). Effect sizes for the comparisons ranged from 0.0 to 0.27.
Table 4.
The number of participants with LBP and PDs that reported the descriptors aching, stiffening, tightness, and cramping during standing.
| Descriptor | Group
|
P-value* | w¶ | |||
|---|---|---|---|---|---|---|
| LBP (n = 15) | PDs (n = 15) | Total | ||||
| Aching | Yes | 14 | 10 | 24 | 0.17 | 0.34 |
| No | 1 | 5 | 6 | |||
| Total | 15 | 15 | 30 | |||
|
| ||||||
| Stiffening | Yes | 11 | 8 | 19 | 0.45 | 0.21 |
| No | 4 | 7 | 11 | |||
| Total | 15 | 15 | 30 | |||
|
| ||||||
| Tightness | Yes | 8 | 6 | 14 | 0.72 | 0.13 |
| No | 7 | 9 | 16 | |||
| Total | 15 | 15 | 30 | |||
|
| ||||||
| Cramping | Yes | 5 | 1 | 6 | 0.17 | 0.34 |
| No | 10 | 14 | 24 | |||
| Total | 15 | 15 | 30 | |||
|
| ||||||
| Aching, Stiffening, Tightness, or Cramping# | Yes | 15 | 15 | 30 | 1.0 | 0 |
| No | 0 | 0 | 0 | |||
| Total | 15 | 15 | 30 | |||
For each symptom descriptor, a Fisher’s exact test was calculated.
Effect size (range 0–1).21
Numbers of participants that reported at least one of the 4 descriptors as “typical” and during standing.
LBP, low back pain; PDs, pain developers.
Table 5.
The number of participants with LBP and PDs that reported symptoms in each region during standing.
| Region | Group
|
P-value* | w¶ | |||
|---|---|---|---|---|---|---|
| LBP (n = 15) | PDs (n = 15) | Total | ||||
| Low Back | Yes | 15 | 15 | 30 | 1.0 | 0.0 |
| No | 0 | 0 | 0 | |||
| Total | 15 | 15 | 30 | |||
|
| ||||||
| Gluteal | Yes | 4 | 1 | 5 | 0.33 | 0.27 |
| No | 11 | 14 | 25 | |||
| Total | 15 | 15 | 30 | |||
|
| ||||||
| Thigh | Yes | 4 | 2 | 6 | 0.65 | 0.17 |
| No | 11 | 13 | 24 | |||
| Total | 15 | 15 | 30 | |||
For each region of interest, a Fisher’s exact test was calculated.
Effect size (range 0–1).21
LBP, low back pain; PDs, pain developers.
DISCUSSION
The purpose of this study was to examine the validity of the standing paradigm by comparing characteristics of symptoms experienced by people with LBP during the standing paradigm to (1) their “typical” symptoms, and (2) symptoms induced in back-healthy people classified as PDs. In participants with LBP there was no difference in the quality and location of the symptoms they experienced during standing and their “typical” symptoms. In addition, for the most prevalent symptom descriptors and their locations the overall agreement to disagreement ratios indicated there were 3 times more agreements than disagreements (33:12 and 35:10, respectively). There also was no difference in the quality and location of symptoms reported during standing by participants with LBP and PDs. These data provide key evidence that symptoms induced during the standing paradigm are similar to symptoms typically experienced by people with LBP and, therefore, support the validity of the standing paradigm as a method to investigate risk factors for initial LBP development during prolonged standing.
Participants with LBP did not have perfect agreement between reports of “typical” symptoms and symptoms during standing (Table 2). We consider, however, that our data support the initial hypothesis that the quality of “typical” symptoms would be similar to the quality of symptoms during standing. In only 2 (13%) instances did participants with LBP not report a symptom in standing that they had reported to be typical. The remaining disagreements were because participants only reported a symptom during standing, i.e., the symptom was not reported as typical. Since all participants had a long-standing LBP condition (Table 1), a symptom not typically experienced may be produced in a circumstance that is less than typical for a participant, i.e., loading the spine in standing for a prolonged period of time.
There were no significant differences between the quality and location of symptoms reported during the paradigm by people with LBP and PDs (Table 4). There also were no symptom descriptors or locations that were reported by PDs during standing that were not reported by participants with LBP. Therefore, we consider that our data supports the initial hypothesis that symptoms developed and locations reported during the standing by PDs are not different from those developed by people with LBP.
The standing paradigm is appealing from an experimental perspective because prior studies have documented that acute, transient LBP symptoms can be induced in back-healthy people during an occupational activity associated with increased reports of LBP.1,2 Prior studies using the standing paradigm have resulted in reports of symptoms in 40–71% of back-healthy people3–11 which has allowed identification of baseline differences between PDs and NPDs. However, none of the prior studies have considered whether the symptoms induced during the standing paradigm are similar to symptoms experienced by people with LBP. In order for the standing paradigm to be considered a valid method for investigating risk factors for initial LBP development during prolonged standing, it is necessary to know that the symptoms typically experienced by people with LBP are (1) similar to their symptoms in standing, and (2) symptoms experienced during the standing paradigm by back-healthy people who are considered PDs. The current study provides evidence that the acute, transient symptoms induced during the standing paradigm in PDs are the not different from symptoms experienced by people with LBP.
To our knowledge, only one previous study has attempted to compare characteristics of symptoms experienced by people with musculoskeletal pain to those of healthy controls during an induced pain paradigm.22 Madeleine et al (1998) compared symptoms experienced in butchers with and without chronic neck and shoulder pain during a standardized repetitive work task. Before performing the task butchers without chronic neck and shoulder pain were given an injection of hypertonic saline in the trapezius and infraspinatus muscles. Injection of hypertonic saline to muscles has been shown to induce pain in asymptomatic people.23 After participation, both groups reported similar quality and intensity of symptoms. The authors concluded that the induced pain mimicked symptoms experienced by people with chronic neck and shoulder pain and, thus, supported the use of the paradigm to investigate aspects of work-related pain. However, injection of hypertonic saline to muscles has been shown to induce pain in the absence of activity.23 Therefore, it is not known if the butchers without chronic neck and shoulder pain experienced pain because of the injection, the work task, or a combination of the injection and work task. A strength of the paradigm examined in the current study is that the symptoms are induced in back-healthy people during the standing task without the confound of an agent (hypertonic saline) known to induce pain. Therefore, symptoms developed by back-healthy people during the standing paradigm can better be attributed directly to conditions associated with the standing task.
We chose to recruit people with LBP who were not in an acute flare-up of their symptoms. As a result the participants with LBP had relatively low levels of symptom intensity and disability at the beginning of the study (Table 1). We chose to recruit people who were not acutely involved so that the quality and location of the LBP symptoms they would be experiencing would be more similar to the symptoms they typically experienced. Including participants who were not acutely involved also increased the likelihood that the participants with LBP could complete the standing task. In addition, any increase in LBP symptoms during the paradigm more likely could be attributed to the standing task.
One limitation of the study is the small sample size. We were unable to make an a priori estimate of sample size because this was the first study to compare symptom descriptors and locations in people with LBP and PDs. Post-hoc analyses revealed that the effect sizes were small (range 0 to 0.39) suggesting the sample size to detect significant differences would need to be larger than what was recruited. The primary goal of the study, however, was to determine whether the quality and location of symptoms developed by PDs was similar to that reported by people with LBP. Inspection of the data (Tables 2–5) supports that the PDs did not report symptoms that were different in quality or location than symptoms reported by participants with LBP. A second limitation is that our sample consisted of people between the ages of 18 to 30 years (Table 1) so our results may not be generalizable to people older than our sample. Similar to many people with LBP, however, the participants with LBP in our sample had longstanding symptoms, experienced moderate levels of symptom intensity, and had frequent LBP flare-ups (Table 1).15,16 Given the similarity in LBP behavior, we consider that it is likely the symptom characteristics of our participants with LBP are similar to those of people with LBP across a range of ages. A final limitation is that the symptoms PDs experienced were acute and transient. Therefore, findings regarding LBP symptoms developed during the standing paradigm in subsequent studies may not be generalizable to all types of LBP. However, it is important to understand factors that contribute to the initial development of acute, transient symptoms as most people that experience an acute episode of LBP will go on to have recurrences of LBP in the future.16
During the standing paradigm, people with LBP reported symptoms similar to (1) their “typical” symptoms, and (2) symptoms reported by back-healthy people who were classified as PDs. The current study provides essential evidence that symptoms experienced during the standing paradigm are similar to symptoms experienced by people with LBP and, thus, provides partial support for the validity of the paradigm. Therefore, one can be confident in using the paradigm to identify characteristics of people susceptible to initial LBP symptom during prolonged standing. Understanding of characteristics related to initial LBP symptom development can inform strategies for early detection in order to prevent chronic and recurrent LBP conditions.
Supplementary Material
Acknowledgments
Sources of Funding: Christopher J. Sorensen was supported by the Washington University Institute of Clinical and Translational Sciences grants UL1 TR000448 and TL1 TR000449 from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH). Dr. Van Dillen was receiving a grant (R01 HD047709-04) from the NIH.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Reference List
- 1.Frymoyer JW. Back pain and sciatica. N Engl J Med. 1988;318:291–300. doi: 10.1056/NEJM198802043180506. [DOI] [PubMed] [Google Scholar]
- 2.Picavet HS, Schouten JS. Physical load in daily life and low back problems in the general population - the MORGEN Study. Prev Med. 2000;31:506–12. doi: 10.1006/pmed.2000.0737. [DOI] [PubMed] [Google Scholar]
- 3.Gregory DE, Callaghan JP. Prolonged standing as a precursor for the development of low back discomfort: an investigation of possible mechanisms. Gait Posture. 2008;28:86–92. doi: 10.1016/j.gaitpost.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Gregory DE, Brown SH, Callaghan JP. Trunk muscle responses to suddenly applied loads: do individuals who develop discomfort during prolonged standing respond differently? J Electromyogr Kinesiol. 2008;18:495–502. doi: 10.1016/j.jelekin.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Nelson-Wong E, Gregory DE, Winter DA, et al. Gluteus medius muscle activation patterns as a predictor of low back pain during standing. Clin Biomech. 2008;23:545–53. doi: 10.1016/j.clinbiomech.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Nelson-Wong E, Flynn T, Callaghan JP. Development of active hip abduction as a screening test for identifying occupational low back pain. J Orthop Sports Phys Ther. 2009;39:649–57. doi: 10.2519/jospt.2009.3093. [DOI] [PubMed] [Google Scholar]
- 7.Nelson-Wong E, Callaghan JP. Is muscle co-activation a predisposing factor for low back pain development during standing? A multifactorial approach for early identification of at-risk individuals. J Electromyogr Kinesiol. 2010;20:256–63. doi: 10.1016/j.jelekin.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Nelson-Wong E, Callaghan JP. Changes in muscle activation patterns and subjective low back pain ratings during prolonged standing in response to an exercise intervention. J Electromyogr Kinesiol. 2010;20:1125–33. doi: 10.1016/j.jelekin.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Nelson-Wong E, Howarth SJ, Callaghan JP. Acute biomechanical responses to a prolonged standing exposure in a simulated occupational setting. Ergonomics. 2010;53:1117–28. doi: 10.1080/00140139.2010.500400. [DOI] [PubMed] [Google Scholar]
- 10.Nelson-Wong E, Callaghan JP. Repeatability of clinical, biomechanical, and motor control profiles in people with and without standing-induced low back pain. Rehabil Res Pract. 2010;2010:1–9. doi: 10.1155/2010/289278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson-Wong E, Alex B, Csepe D, et al. Altered muscle recruitment during extension from trunk flexion in low back pain developers. Clin Biomech. 2012;27:994–8. doi: 10.1016/j.clinbiomech.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Marshall PW, Patel H, Callaghan JP. Gluteus medius strength, endurance, and co-activation in the development of low back pain during prolonged standing. Hum Mov Sci. 2011;30:63–73. doi: 10.1016/j.humov.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Fritz JM, Irrgang JJ. A comparison of a modified Oswestry Low Back Pain Disability Questionnaire and the Quebec Back Pain Disability Scale. Phys Ther. 2001;81:776–88. doi: 10.1093/ptj/81.2.776. [DOI] [PubMed] [Google Scholar]
- 14.Downie WW, Leatham PA, Rhind VM, et al. Studies with pain rating scales. Ann Rheum Dis. 1978;37:378–81. doi: 10.1136/ard.37.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Von Korff M. Studying the natural history of back pain. Spine. 1994;19:2041S–6S. doi: 10.1097/00007632-199409151-00005. [DOI] [PubMed] [Google Scholar]
- 16.McGorry RW, Webster BS, Snook SH, et al. The relation between pain intensity, disability, and the episodic nature of chronic and recurrent low back pain. Spine. 2000;25:834–41. doi: 10.1097/00007632-200004010-00012. [DOI] [PubMed] [Google Scholar]
- 17.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 18.Margolis RB, Chibnall JT, Tait RC. Test-retest reliability of the pain drawing instrument. Pain. 1988;33:49–51. doi: 10.1016/0304-3959(88)90202-3. [DOI] [PubMed] [Google Scholar]
- 19.Kelly AM. Does the clinically significant difference in visual analog scale pain scores vary with gender, age, or cause of pain? Acad Emerg Med. 1998;5:1086–90. doi: 10.1111/j.1553-2712.1998.tb02667.x. [DOI] [PubMed] [Google Scholar]
- 20.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 21.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates, Pub; 1988. Chi-Square Tests for Goodness of Fit and Contingency Tables; pp. 215–71. [Google Scholar]
- 22.Madeleine P, Lundager B, Voigt M, et al. Sensory manifestations in experimental and work-related chronic neck-shoulder pain. Eur J Pain. 1998;2:251–60. doi: 10.1016/s1090-3801(98)90021-0. [DOI] [PubMed] [Google Scholar]
- 23.Graven-Nielsen T. Fundamentals of muscle pain, referred pain, and deep tissue hyperalgesia. Scand J Rheumatol Suppl. 2006;122:1–43. doi: 10.1080/03009740600865980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.