Abstract
Stone formation and nephrocalcinosis are both very common features of primary hyperoxaluria, yet the extent of each disease varies markedly between patients. Here we studied whether kidney damage from nephrocalcinosis and/or stone related events contributed to end stage kidney disease (ESKD). Clinical information was analyzed from 348 patients enrolled in the Rare Kidney Stone Consortium Primary Hyperoxaluria registry and included demographic, laboratory and imaging features. Among all patients there were 277 with type 1, 37 with type 2, and 34 with type 3 primary hyperoxaluria. Overall, 58% passed a stone (mean 0.3/year) and one or more urologic procedures were required by 70% of patients (mean 0.15/year). Nephrocalcinosis was found in 34% of patients, including 41% with type 1 primary hyperoxaluria. High urine oxalate was associated with increased risk for both nephrocalcinosis and stone number, while low urine citrate was a risk factor for stone events and stone number. After adjustment for the type of primary hyperoxaluria, diagnosis by family screening and age at first image, the overall adjusted hazard ratio for ESKD among those with a history of nephrocalcinosis was 1.7 [95% CI 1.0–3.0], while the risk was 4.0 [1.9–8.5] for new onset nephrocalcinosis during follow-up. In contrast, the number of stones and stone events were not significantly associated with ESKD risk. Thus, nephrolithiasis and nephrocalcinosis appear to be pathophysiologically distinct entities. The presence of nephrocalcinosis implies increased risk for ESKD.
Keywords: End stage kidney disease, Nephrocalcinosis, Nephrolithiasis, Oxalate, Primary Hyperoxaluria
INTRODUCTION
The primary hyperoxalurias (PHs) are autosomal recessive inherited diseases caused by defects in glyoxylate metabolism, resulting in overproduction of oxalate1, 2. Three types of PH have been described to date, each involving a different enzyme within the oxalate metabolic pathways. PH type 1 (PH1) is caused by low or absent activity of liver-specific peroxisomal alanine: glyoxylate aminotransferase (AGT), which results in increased urinary excretion of both oxalate and glycoalate3. In PH type 2 (PH2), deficiency or absence of glyoxylate reductase/hydroxy-pyruvate reductase (GRHPR) leads to elevated urinary excretion of both oxalate and L-glyceric acid4. A third type of PH (PH3) was recently found to be due to abnormality of enzyme 4-hydroxy-2-oxaloglutarate aldolase (HOGA1)2. The exact mechanism of oxalate overproduction in PH3 remains to be determined.
Humans cannot degrade oxalate which is primarily eliminated through the kidneys as an end product of metabolism. Thus PH is characterized by very high urinary oxalate excretion, typically >1 mmol/1.73m2/day. Consequently, tubular fluid becomes supersaturated for calcium oxalate, which can result in the formation of crystals within the lumen. Once formed, crystals can attach to the surface of renal tubular cells. In the medullary collecting duct this can result in tubular plugging and a nidus for stone formation, while in more proximal nephron segments adherent crystals can be endocytosed and/or transcytosed into the renal interstitium leading to nephrocalcinosis (NC). If the glomerular filtration rate (GFR) decreases due to progressive renal damage, oxalate accumulates in the blood stream and crystal deposition in the parenchyma of most solid organs, bones, joints and retina can occur once supersaturation is exceeded (~30µM/L)5.
PH often leads to end-stage kidney disease (ESKD) and the death of many patients if untreated. Stone formation and NC are both very common features of PH, yet the numbers and extent vary markedly between individuals. Thus, we investigated whether NC and/or stone related events contributed to ESKD.
RESULTS
Population characteristics and image finding
A total of 379 PH patients were enrolled in the Rare Kidney Stone Consortium (RKSC) registry through April 2013. After excluding 31 unclassified PH patients (25 in whom PH1, PH2, or PH3 were all negative by genetic analysis and 6 historical patients with PH of unknown type), 348 patients were left for further analysis, with 151 (43%) patients known to have ESKD at the time of this analysis. There were 277 (79.6%) PH1, 37 (10.6%) PH2, and 34 (9.8%) PH3 in the registry. Among them 322 (256 PH1, 32 PH2 and 34 PH3) were diagnosed or confirmed by genetic testing or liver biopsy. Marked hyperoxaluria in the absence of identifiable secondary cause and in combination with hyperglycolic aciduria provided PH1 diagnosis (n=21) and hyperoxaluria in combination with hyperglyceric aciduria provided PH2 diagnosis (n=5). Family screening identified 29 patients, including 21 with PH1, 7 with PH2 and 1 with PH3. As expected, those identified by family screening were diagnosed at a younger age (4.0 vs. 7.2 yrs, P=0.01), but had a similar risk of NC before ESKD (31.0 vs. 34.0%, P=0.75). Median age at first symptoms and at diagnosis of all patients was 4.9 (1.6–13.3) and 10.4 (4.0–27.9) years old, respectively. Median age at last follow up was 22.5 (10.0–40.7) years old. Stone burden and imaging results are shown in Table 1. Altogether, 58.4 % of patients passed a stone, with a mean of 0.3 passed/yr, translating to about 1 stone every 3.3 years. One or more urologic procedures were required by 70.2 % of patients ever (mean 0.15 procedures/yr). Clinical features by PH type are in Supplemental Table S1.
Table 1.
Clinical stone events and imaging findings for all PH patients.
| Mean/Median (25th, 75th) | No. of patients (%) | |
|---|---|---|
| clinical stone events (n=348) | ||
| Stones passed /yr | 0.30 / 0.04 (0.00–0.26) | 121/291 (58.4%) |
| Stone procedures /yr | 0.15 / 0.06 (0.00–0.17) | 172/245 (70.2%) |
| Stone eventsa, /yr | 0.42 / 0.13 (0.00–0.43) | 212/300 (70.7%) |
| Image findings (n=303) | ||
| stones per image before ESKD | 3.5 / 2.0 (1.0–4.3) | 185/303 (61.1%b) |
| NC ever | NA | 112/303 (37.0%) |
| NC before ESKD | NA | 79/235 (33.6%) |
Abbreviations: NC, nephrocalcinosis; ESKD, end stage kidney disease; NA: not applicable
Stone event is defined as any clinical event associated with kidney stones, including stone passage or stone procedure.
Percent of imaged subjects (n=303) who ever had a stone on imaging.
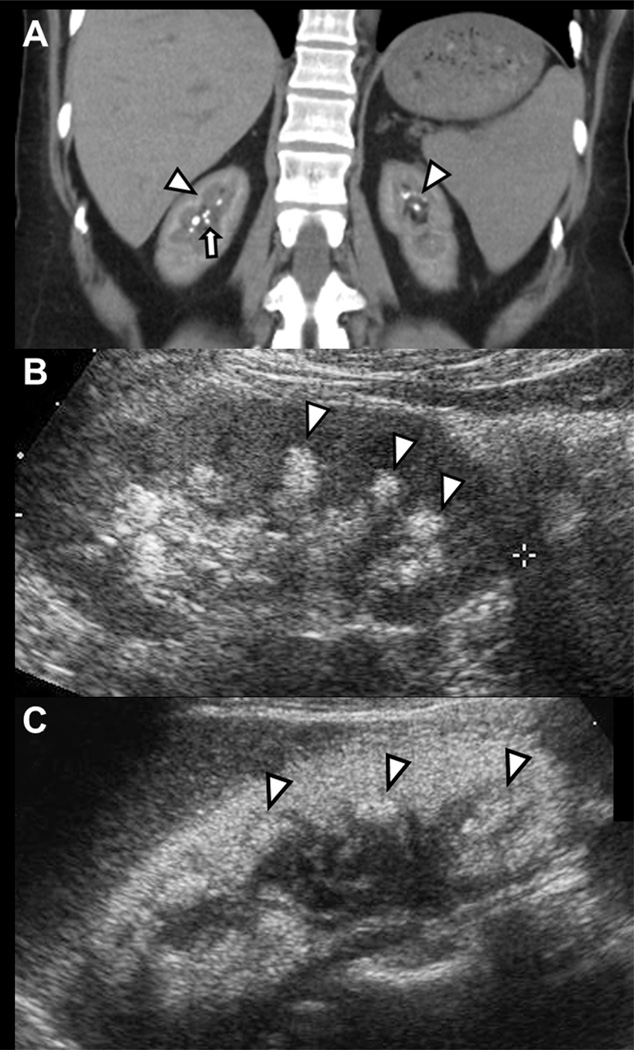
Kidney imaging was documented in 303 (87%) patients, with a median of 2 tests per patient (1,324 images total); 25.2% were computed tomography (CT) scans, 52.6% ultrasounds (US) and 22.2% kidney/ureter/bladder (KUB) X-rays. NC was defined as a pattern of calcification occurring in the renal parenchyma, most prominent at the corticomedullary juncture, while stones were discrete structures ≥ 1 mm in size in the renal papillae or collecting system 6. Figure 1A depicts a patient with diffuse NC at the corticomedullary juncture and medulla as well as a few discrete papillary tip stones, while Figures 1B and 1C show the typical appearance of NC by ultrasound, with increased pyramidal and cortical density. Kidney stone and NC were documented in 61% (185/303) and 37% (112/303) of those with imaging. NC was noted more commonly on US (20%) as opposed to KUB (12%) or CT (11%) (P<0.001). The distribution of imaging method (US/CT/KUB) varied slightly by PH type being 55/26/20% for PH1, 44/18/38% for PH2, and 48/28/24% for PH3 (P<0.001).
Figure 1. Nephrocalcinosis and kidney stones by renal imaging.
Panel A: High attenuation within the kidneys due to nephrocalcinosis is present at the corticomedullary juncture and medulla (arrowhead), while discrete stones are present at the collecting system (arrow). Panels B and C: Nephrocalcinosis by renal ultrasound evidenced by increased pyramidal density (arrowheads) associated with acoustic shadowing. Changes in Panel C are more marked than in Panel B.
Determinants of nephrocalcinosis and stone burdens
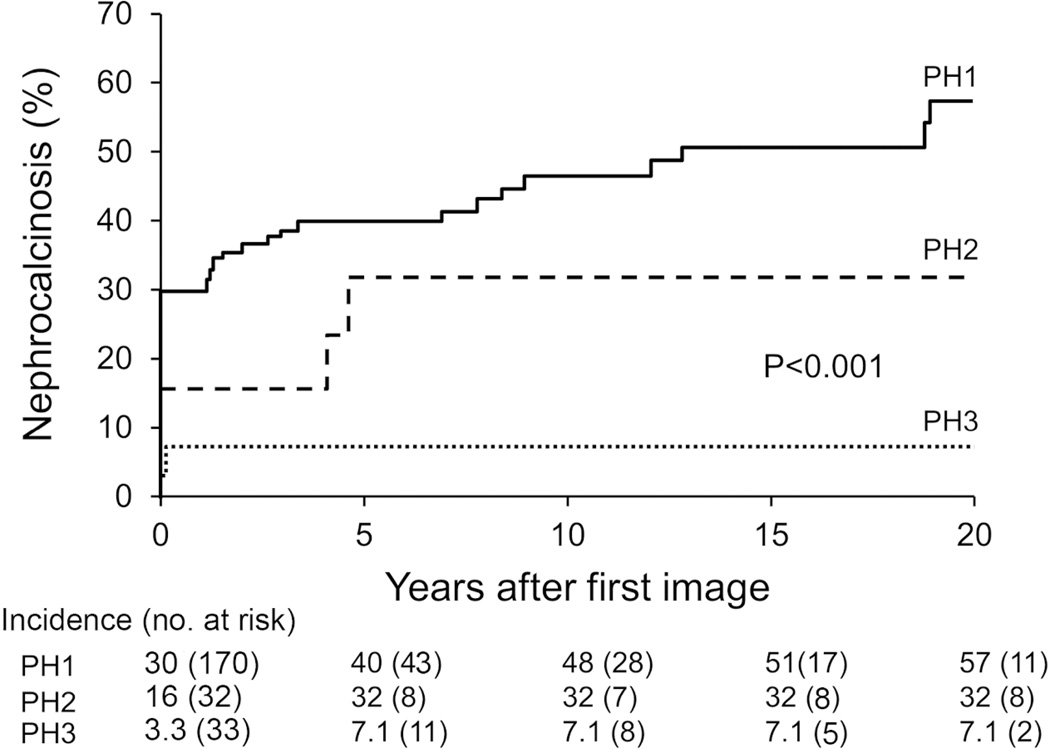
Among the 303 patients with imaging, 68 had ESKD before first image, leaving 235 with a first image prior to ESKD. In these subjects, 24% (56/235) patients had both NC and kidney stone, 10% (23/235) had NC only and 47% (111/235) had kidney stone only. NC (prior to ESKD) was found in 69 (40.6%) PH1, 8 (25%) PH2 and 2 (6.1%) PH3 patients, respectively. Table 2 lists a comparison of clinical and laboratory features of patients with and without NC before ESKD for all PH patients and also the PH1 subgroup. Patients with NC did not differ significantly by gender but were diagnosed at a younger age (median 4.4) compared to those without NC (median 9.3). Urinary oxalate was significantly higher in patients with NC, while the 24h urine calcium was significantly lower. NC and kidney stones were not significantly associated. Kidney stones were found in 70.9% of those with NC compared to 71.2% of those without NC (P=0.93). However, those patients with NC had more stones per image than those without (3.0 versus 2.0, P=0.02). NC was more common both at presentation (prevalent) and during follow up (incident) in PH1 compared to PH2 and PH3 (P<0.001). Trends for NC and stones were similar for PH 2 (Supplemental Table S2). The number of PH3 NC cases was too small to allow for meaningful subanalysis. (As shown in Figure 2, NC was prevalent in 30% of PH1 patients on first image, while the cumulative proportion with NC increased to 48% at 10 years and 57% at 20 years of follow up. In contrast, 32% of PH2 and 7.1% of PH3 patients had NC at 5 years follow-up, and the percentages did not increase further.
Table 2.
Characteristics of all PH patients and the PH1 subgroup with or without nephrocalcinosis.
| PH | PH1 | |||
|---|---|---|---|---|
| Without NC (N=156) |
With NC (N=79) |
Without NC (N=101) |
With NC (N=69) |
|
| Gender | ||||
| Male/Female | 86/70 | 45/34 | 54/47 | 39/30 |
| Age, yrs | ||||
| at diagnosis | 9.3 (4.5, 24.7) | 4.4 (1.8, 10.0)* | 11.5 (5.6, 30.7) | 4.2 (1.9, 9.4)* |
| at last follow up | 22.7 (10.1, 44.7) | 15.4 (5.8, 23.9)* | 25.7 (13.2, 44.8) | 15.1 (5.8, 22.6)* |
| Stone events, /yr | 0.18 (0.06, 0.47) | 0.27 (0.00, 0.52) | 0.16 (0.05, 0.45) | 0.28 (0.00, 0.59) |
| Stones per image | 2.0 (0.8, 4.0) | 3.0 (1.5, 6.1)# | 2.6 (1.0, 4.0) | 3.8 (1.7, 6.8)# |
| P[ox], µmol/L | 4.6 (2.7, 12.0) | 8.7 (3.7, 17.5)# | 6.6 (3.0, 15.6) | 8.8 (4.1, 17.6) |
| U[ox], mmol/24ha | 1.3 (0.9, 1.9) | 2.0 (1.3, 2.6) * | 1.3 (0.9, 2.1) | 2.0 (1.3, 2.6) * |
| U[Ca], mg/24ha | 90.2 (59.2, 149.2) | 63.0 (43.4, 90.9) * | 79.2 (51.5, 112.7) | 55.6 (39.9, 85.7) * |
| U[citrate], mg/24ha | 445 (264, 750) | 346 (190, 608) | 336 (210, 643) | 294 (183, 490) |
| U[volume], L/24ha | 2.4 (1.9, 3.1) | 2.8 (2.2, 3.6) | 2.4 (1.8, 3.2) | 2.8 (2.3, 3.6) |
Patients who had ESKD before first image were excluded in the analysis. All plasma and urinary results were the average of all values prior to ESKD. Gender is expressed as number and statistical analysis was made by Chi-Square. The rest are expressed as median (interquartile range) and statistical analysis was made by rank sum test.
adjusted by body surface area;
P<0.01;
P<0.05
Figure 2. Time to Nephrocalcinosis by PH type.
Follow-up starts at the time of first image and ends at the last image or ESKD. NC risk was highest in PH1 and least in PH3 (P< 0.001).
There was less evidence that stone-related events associated with laboratory characteristics (Table 3). Urine citrate was inversely correlated with stone passage (Spearman correlation coefficient (r) = −0.22, P=0.004), while the average stone number per image positively associated with plasma and urinary oxalate, and negatively with urine citrate and urine calcium at last follow-up. No plasma or urine measurements correlated with the number of surgical interventions. In the PH1 subgroup, correlations between laboratory characteristics and kidney stone events were similar, although the correlation coefficients of urinary citrate with stone number (r =−0.19, P=0.07) and stone passage (r =−0.18, P=0.06) were of borderline significance in this smaller subset of the total group. Trends for correlation between urine chemistries and stone events were similar when analyzed by PH subtype, although most findings no longer achieved statistical significance in these smaller sample sizes (Supplemental Table S3).
Table 3.
Correlation of stone burden with lab results in all PH patients.
| Stones passed /yr |
Stone procedures/yr |
Stone events/yr |
Mean stone number/image |
||
|---|---|---|---|---|---|
|
Plasma oxalate (µmol/L) |
r P |
−0.03 0.69 |
−0.02 0.84 |
−0.06 0.52 |
0.31 0.001 |
|
U[ox] mmol/1.73m2 /24h |
r P |
−0.09 0.19 |
0.03 0.65 |
−0.05 0.46 |
0.16 0.05 |
|
U[Ca] mg/1.73m2/24h |
r P |
0.05 0.54 |
0.10 0.22 |
0.08 0.29 |
−0.26 0.002 |
|
U[citrate] mg/1.73m2/24h |
r P |
−0.22 0.004 |
−0.02 0.82 |
−0.15 0.05 |
−0.35 <0.001 |
|
U[volume] ml/1.73m2/24h |
r P |
−0.02 0.73 |
0.02 0.84 |
−0.04 0.55 |
0.06 0.46 |
|
Current eGFR ml/min/1.73m2 |
r P |
−0.05 0.43 |
0.10 0.18 |
0.03 0.70 |
−0.35 <0.001 |
|
eGFR decline rate ml/min/1.73m2/yr |
r P |
−0.04 0.62 |
0.04 0.61 |
−0.01 0.92 |
−0.13 0.13 |
P: P value; r: correlation coefficients of Spearman rank correlation
Relationships of imaging findings to renal outcome
The impact of NC and other factors on renal outcome was studied in the 235 subjects without ESKD prior to their first image. Altogether, 57 (56 PH1, 1 PH2, 0 PH3) of 235 patients developed ESKD after the first image. Table 4 summarizes the effect of NC on ESKD for all PH patients and the PH1 subgroup separately. The ESKD hazard ratio (HR) among all PH patients with NC-ever was 2.1 [95% confidence interval (CI) 1.2–3.6]. After adjustment for PH type, PH diagnosis by family screening, and age at first image, the overall adjusted HR of ESKD among those with NC ever was 1.7 [1.0–3.0]. The effect on ESKD risk was larger for new onset NC during follow-up (HR 4.0 [1.9–8.5]) as opposed to NC at first image (HR 1.2 [0.6–2.3]). The effect of NC on ESKD risk in the PH1 subgroup was similar with a HR of 1.5 [0.9–2.5] and 3.6 [1.7–7.7] for NC-ever and new onset NC, respectively. After adjustment for baseline urinary oxalate, new onset NC remained a significant risk factor for ESKD in all PH patients (HR 4.3 [1.6–12]) and in PH1 (HR 3.4 [1.2, 10]).
Table 4.
Risk of ESKD among PH patients with and without NC.
| Group of PH patients | Hazard ratio (95% CI) |
||
|---|---|---|---|
| Total NC | Prevalent NC | Incident NCb | |
| All PH types (N=235, 57 ESKD) | |||
| No adjustment | 2.1 (1.2–3.6) | 1.5(0.8–2.8) | 5.1(2.4–11.0) |
| Adjusting PH1, patients by family screening and age on 1st image | 1.7 (1.0–3.0) | 1.2 (0.6–2.3) | 4.0 (1.9–8.5) |
| Adjusting PH1 and stone numbers on 1st imagea | 3.9 (1.7–8.9) | 1.6 (0.4–5.7) | 8.2 (3.2–21.3) |
| PH1 subgroup (N=170, 56 ESKD) | |||
| No adjustment | 1.5 (0.9–2.5) | 1.1 (0.6–2.0) | 3.6(1.7–7.7) |
| Adjusting patients by family screening and age on 1st image | 1.6 (0.9–2.9) | 1.2 (0.6–2.3) | 3.6 (1.6–7.8) |
| Adjusting stone numbers on 1st imagea | 3.5 (1.5–8.3) | 1.6 (0.4–5.7) | 6.9 (2.6–19.0) |
Patients who had ESKD before first image were excluded in the analysis.
Stone number is available for 140 PH patients with 25 ESKD events overall, and for 94 PH1 patients with 24 ESKD events.
patients with prevalent NC are those who found NC on first image. Patients with incident NC are those who had new NC during follow-up.
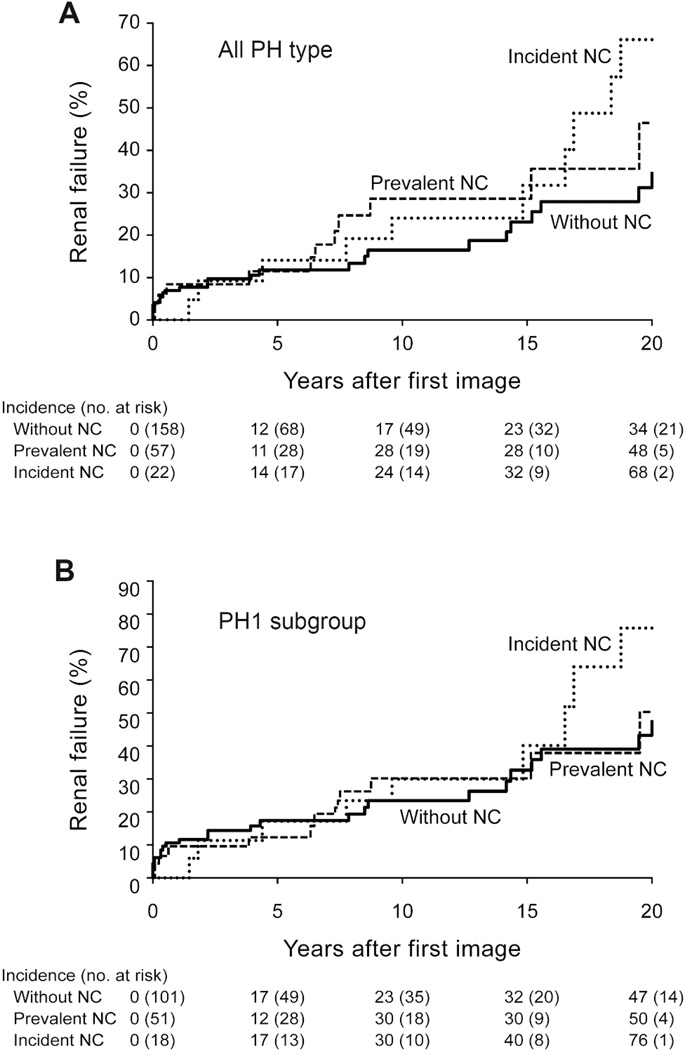
When PH patients were subdivided into three groups according to the presence (prevalent) or absence of NC on the baseline and follow-up images, the decline in eGFR was more significant in those with incident NC on the follow-up images (Table 5). The trend and magnitude of kidney function loss in relation to the presence of NC were similar in the PH1 subgroup, with a median eGFR decline rate among those without NC, with prevalent NC, and with incident NC of −0.25, −0.15 and −1.46 ml/min/1.73m2/yr, respectively (P=0.16). Figure 3 graphically illustrates the cumulative ESKD incidence for all PH patients and the PH1 subgroup divided into the three groups (without NC, prevalent NC, incident NC).
Table 5.
Kidney function among PH patients without NC, with NC on first image and with NC during follow-up.
| Without NC | Prevalent NC | Incident NC | P value | |
|---|---|---|---|---|
| All PH type | 156 | 57 | 22 | |
| eGFR at diagnosis | 74.0 (57.6, 97.5) | 68.2 (50.0, 87.4) | 69.8 (56.7, 91.7) | 0.24 |
| eGFR at last follow-upa | 79.8 (55.6, 104.4) | 69.1 (48.7, 91.9) | 62.6 (30.4, 89.5) | 0.03 |
| eGFR decline rate,/yr | −0.4 (−1.9, 3.0) | −0.4 (−2.3, 1.9) | −1.2 (−3.8, 0.02) | 0.08 |
| ESKD | 21.2% | 24.6% | 45.5% | 0.04 |
| PH1 subgroup | 101 | 51 | 18 | |
| eGFR at diagnosis | 68.9 (51.8, 89.0) | 64.2 (46.8, 85.3) | 69.8 (56.7, 90.2) | 0.56 |
| eGFR at last follow-upa | 67.3 (44.2, 86.9) | 64.2 (34.5, 91.7) | 54.9 (28.5, 85.3) | 0.33 |
| eGFR decline rata,/yr | −0.3 (−2.5, 1.8) | −0.2 (−2.1, 2.8) | −1.5 (−3.8, −0.1) | 0.16 |
| ESKD | 32.7% | 27.5% | 50.0% | 0.21 |
Patients who had ESKD before first image were excluded in the analysis. All eGFR values were expressed as median (interquartile range).
Data were cut at eGFR of 20 ml/min/1.73m2
Figure 3. Renal outcome by nephrocalcinosis on imaging.
Panel A: Among all PH patients the cumulative incidence of ESKD is significantly higher for patients with incident (after first image) NC (dotted line) when compared to those without NC (solid line) (HR [95% CI]) from a time-dependent Cox model (HR=5.1[2.4–11]). Those with prevalent (on first image) NC (dashed line) also trended towards increased risk of ESKD (HR 1.5 [0.8–2.8]). Panel B: In the PH1 subgroup the effect of NC on ESKD risk was similar to the entire PH population. Those with incident NC had a HR of 3.6 [1.7–7.7] and those with prevalent NC a HR of 1.1 [0.6–2.0].
Among 170 PH1 subjects with imaging prior to ESKD, 133 had genetic testing for G170R which appears to confer complete or partial response to pyridoxine responsiveness 7 Of these, 69 had at least one copy of the G170R AGXT genetic change and 64 did not. The rate of NC was not significantly (P=0.29) different between patients with (32% NC) or without (41% NC) a G170R change. Among the 133 PH1 subjects with imaging prior to ESKD, the effect of a G170R change on ESKD was somewhat protective (HR 0.6[0.3–1.0]), when adjusted for NC. Furthermore, incident NC remained significant in this model (HR 3.7[1.6–8.9]) when adjusted for G170R status.
Conversely, the number of stones per image was not significantly associated with ESKD risk (HR 1.07 [0.98–1.17]). Neither the number of stone events nor stones on imaging was associated with the eGFR decline rate (Table 3). Furthermore, the risk of ESKD among PH patients with NC remained increased (HR 3.9 [1.7–8.9]) after adjustment for stone numbers.
DISCUSSION
NC refers to the deposition of radiologically dense calcium salts in the kidney parenchyma (Figure 1A), and is different from crystal deposition in the collecting ducts or collecting system, which typically leads to kidney stones (nephrolithiasis). It is generally accepted that kidney stones and NC are early and common clinical manifestations of PH8, and our study confirmed that impression in a large cohort with good longitudinal follow up. Results demonstrate that 61% of patients had kidney stones by imaging and 58% passed a stone during follow-up, averaging about 1 stone every 3.3 years. NC was slightly less common, being present in 37% of patients ever. Although stones were more common than NC, the latter portended an increased risk of ESKD, while the number of stones did not correlate with kidney function over time. Notably, the percentage of PH patients affected by NC and/or stones could have been underestimated, since we assumed that they were not present if not specifically included in the radiologic reports entered into the registry. It is also possible that the incidence of NC could have been underestimated because of a low sensitivity of radiological imaging techniques9. Recent research has demonstrated that ultrasound is a more accurate technique for evaluation of mild to moderate NC in patients with hypoparathyroidism10. However, CT remains a superior technique for evaluation of renal stone disease11. Therefore, while the most accurate imaging to evaluate PH patients remains unknown, the lower cost and lack of radiation suggests ultrasound can be useful.
PH patients often present with kidney stones, hence the diagnosis can be confused with the far more common idiopathic calcium oxalate stones. Previous studies report the diagnosis of PH was made 1–4 years after the first manifestation, and a high percentage of patients were only diagnosed in ESKD8, 12, 13. Our data also reveal a 5 year delay in the diagnosis, while 43% of patients reached ESKD by the last follow-up. Therefore, more effort is needed to shorten the interval between first symptom and diagnosis in order to improve renal survival.
Our study suggests that increased urinary oxalate excretion is crucial for the development of both NC and the number of kidney stones on imaging, although no evident interaction was found between urine parameters and clinical stone events (stone passage and stone procedures). This finding was consistent with observations in hyperoxaluric animal models in which NC is associated with increased urinary supersaturation 14. Furthermore, in rodent models large crystals can plug terminal collecting ducts and anchor larger stones. It is less clear if collecting duct plugs can anchor stones in humans. Nevertheless, our results stress the importance of an aggressive treatment program to decrease urine calcium oxalate supersaturation and crystallization, with a goal to prevent NC, kidney stones, and possibly ESKD.
Citrate supplements are thought to reduce calcium oxalate stone formation in patients with PH15. Several studies also demonstrate that hypocitraturia is a risk factor for NC in preterm neonates, the post transplanted kidney of children, and Dent disease16–18. However, in the current study we did not detect an effect of urinary citrate levels on NC risk in PH. Hypocitraturia did associate with an increased number of stones on imaging and clinical stone events. One potential explanation could lie in the presumed site of action of citrate. The formation of calcium-citrate complexes may be more important to prevent calcium oxalate crystallization and tubular plugging in the distal tubule, a presumed precursor of stone formation. Conversely, nephrocalcinosis may involve other processes within proximal tubular cells or the renal interstitium. Indeed, the fact that clinical features associated with NC and stones appear to differ supports the hypothesis that these are two distinct pathophysiological entities.
NC correlated with increased risk of subsequent ESKD, while stone events and stone numbers did not, indicating that the ultimate renal consequences of NC and stones are different in PH patients. The role of NC in the destruction of renal parenchyma is still uncertain. Cell culture and animal models have provided evidence that oxalate and calcium oxalate (CaOx) crystals may be injurious to renal tubular cells by both direct and indirect mechanisms, inducing proliferation and/or cell death19–21. Cell culture studies suggest that the response of renal tubular cells is nephron segment-specific, since CaOx crystals are relatively non-toxic to cells obtained from segments of the nephron where their presence is common (collecting ducts), whereas they cause proliferation in low amounts and cell death in high amounts when proximal tubular cells are exposed 20. In markedly hyperoxaluric animals CaOx crystals deposit mainly inside tubules and lead to a lesion mimicking NC in the proximal tubule, associating with cellular injury, inflammation and regeneration 14. Besides a direct effect on tubular cells, CaOx crystals can also promote inflammation. A recent animal study demonstrated that CaOx crystals crystal activated the NLPR3 inflammsome, thus triggering intrarenal secretion of a variety of inflammatory cytokines and chemokines including IL-1β and IL-18 and perpetuating further kidney damage and eventual kidney failure22. Observations among PH1 patients in the Netherlands and Japan also suggested that progression to CKD was associated with the presence of NC13, 23. Therefore, based on the current study demonstrating that in a cohort of PH patients NC rather than kidney stones correlates with renal function decline, we hypothesize that diffuse deposition of CaOx in the proximal tubule and surrounding cortex, visible on imaging as NC, is an important process during the progression to ESKD.
Patients with PH1 have a more severe long-term clinical course than those with PH2 and PH324. Previous data from the RKSC PH registry demonstrated that renal function was preserved in 100% of PH2 and PH3 patients at 20 years of age, while the renal survival of PH1 patients was only 89% at 10 years of age and 75% at 20 years of age 15. Another study of 155 PH1 patients in France found that renal survival was only 81% and 59% at 10 and 20 years old, respectively25. In our study, NC increased the risk of ESKD regardless of PH type. After adjustment for PH type, the ESKD risk remained significant. Since PH1 patients had far more NC than PH2 and PH3, it is logical to hypothesize that increased NC risk could influence their poorer renal prognosis and increase ESKD risk.
Our study has several limitations. Firstly, the voluntary nature of the registry may generate a potentially biased sampling of patients, and has missing data and variable follow-up. Secondly, there might be a selection bias in the ESKD Cox model. While we found new onset NC to be a stronger risk factor for ESKD than existing NC at first image, this could represent a prevalent bias. Those with existing NC and prior ESKD were deleted as they had already had the endpoint of interest. Those with NC on first image without ESKD at that date were included and perhaps had a lower overall risk of ESKD going forward, due to some as yet to be understood factor(s). Thus, new onset NC that occurred during follow-up was a stronger risk factor for ESKD than prevalent NC. Finally, different imaging techniques were used in different patients and across time and may underestimate NC9, and the original images were not available to confirm the presence or lack of stones and NC.
In conclusion, kidney stones and NC are major manifestations of PH. Although NC does not cause pain and suffering, or the need for surgical procedures, it appears to be a pathophysiologically more severe manifestation. Urinary oxalate level correlated with both NC and the stone number, but the presence of NC imparts a much higher ESKD risk than kidney stones. Our observations suggest that greater attention to the mechanisms that underlie NC might lead to strategies to improve outcomes in PH.
MATERIALS AND METHODS
Patients and data collections
Clinical and laboratory information were collected from PH patients enrolled in the RKSC PH registry which was founded in 200426. General information and clinical manifestations such as age, gender, time of first symptom, time of diagnosis, diagnosis (PH1, PH2, PH3) and stone-related events (passed stones, urological procedures) were abstracted from Registry data. PH1 was confirmed by mutations of the AGXT gene, liver biopsy confirming deficiency of AGT, or by marked hyperoxaluria in combination with hyperglycolic aciduria in a patient with no identifiable secondary causes. PH2 was established by mutations of GRHPR, liver biopsy confirming deficiency of GRHPR enzyme, or hyperoxaluria in combination with hyperglyceric aciduria without identifiable secondary cause. PH3 was by demonstrated mutations of HOGA1.
NC and number of stones were assessed and collected according to KUB, US, or CT scan reports. If stones and/or NC were not mentioned on the report, they were assumed to be absent. NC present on the first kidney image was considered prevalent. Incident NC was new NC that developed during follow-up. Laboratory results including serum creatinine, plasma oxalate, 24-hour urine calcium, citrate and oxalate, and calcium oxalate supersaturation (calculated by EQUIL227) were extracted from registry data. All urine samples included in the analysis were timed samples judged complete based on urine creatinine excretion. All results of plasma oxalate and 24-hour urine collections during the follow-up were averaged with a median of 2–3 samples per patient. Renal function was assessed via serum creatinine values in the registry to estimate GFR using the Schwartz formula in children <18 years old and the MDRD formula in adults. ESKD or renal failure was defined as an eGFR <20 ml/min/1.73m2 or start of dialysis or renal transplantation.
Statistical analysis
Results were expressed as median and interquartile range (IQR) for continuous variables, and as percentage for categorical variables. Statistical analysis was performed by rank sum test for continuous variables and by chi-square for nominal variables. Lifetime stone burden was estimated for each subject as number of stone events divided by age at last follow-up. Patients with prevalent ESKD before the first image were excluded from the analysis of ESKD risk factors. Laboratory values and stone number on imaging were based on lifetime averages and were censored at the onset of ESKD. The Kaplan-Meier method was used to compare the number of patients progressing to NC or ESKD. Data were censored at the time of ESKD or last image recorded. The association of NC or kidney stone with ESKD was assessed using hazard ratios from a Cox’s proportional hazard model with and without adjustments. NC was analyzed as both a fixed (present on first image) and as time dependent covariate (seen on follow-up image). Correlations with stone burdens and laboratory results were assessed by Spearman correlation. To confirm the veracity of our conclusions, analyses were performed in all PH patients and those with different type of PH separately. P values < 0.05 were considered to be statistically significant. Analyses were completed using SAS 9.3.
Supplementary Material
ACKNOWLEDGEMENT
This study was supported by the Rare Kidney Stone Consortium (U54KD083908), a member of the NIH Rare Diseases Clinical Research Network (RDCRN), funded by the NIDDK and the National Center For Advancing Translational Sciences (NCATS), the Mayo Hyperoxaluria Center, the Mayo Foundation, and by Shanghai Top Priority Key Clinical Disciplines Construction Project (XT).
We thank the patients and their families for their gracious participation. We are grateful to the investigators of RKSC coordinating sites for generously providing clinical data: Craig Langman (Children's Memorial Hospital), Lawrence Copelvitch (Children's Hospital of Philadelphia), Yaacov Frishberg (Shaare Zedek Medical Center), Vidar Edvardsson (Landspitali University Hospital), David Goldfarb (New York University), Dean Assimos (University of Alabama) and Todd Lowther (Wake Forest University School of Medicine). We also thank the investigators of RKSC participating sites for their contributions: Lavjay Butani (University of California Davis-Cancer Center) and Christine Sethna (North Shore - Long Island Jewish Health System). Finally, we appreciate the contributions of the following physicians to the RKSC: D Adey (San Francisco, CA), S Ahmed (Seattle, WA), M Aigbe (Las Vegas, NV), M Akma (Los Angeles, CA), S Alexander (Palo Alto, CA), M AlFadhel (Saudi Arabia), F Amanallah (Pakistan), N Amin (Los Angeles, CA), M Anders (Argentina), Dr. Ansell (Canada), E Arch (Wilmington, DE), Dr. Ashette (New York, NY), Dr. Ashtari (MI), JR Asplin (Chicago, IL), A Auron (Des Moines, IA), F Ayestarian (Atlanta, GA), N Azam (Houston, TX), N Balakrishnan (India), R Baliga (Jackson, MS), HJ Baluarte (Philadelphia, PA), R Banga (Charlston, SC), M Banks (Denver, CO), S Bartosh (WI), M Baum (Boston, MA), B Becker (Fitchburg, WI), D Beckman (Faribault, MN), Dr. Beiken (New York, NY), S Belani (Petaluma, CA), A Bellucci (Lake Success, NY), R Berkseth (Minneapolis, MN), P Berry (Austin, TX), Dr. Bhadauria (India), N Bhakta (Los Angeles, CA), M Bhaskaran (Great Neck, NY), A Bhat (Roseville, CA), S Bhupalam (East Lansing, MI), M Bia (New Haven, CT), N Blatt (MI), MB Bleicher (Philadelphia, PA), A Bleyer (Winston Salem, NC), R Bloom (Philadelphia, PA), T Blydt-Hansen (Canada), JK Bodner (AZ), F Boineau (Greensville, SC), M Boone (Atlanta, GA), M Borofsky (New York, NY), B Botelho (Oakland, CA), R Bousquet (Canada), I Boydstun (Hollywood, FL), M Brandi (Argentina), J Brandt (NM), M Braun (Houston, TX), E Brewer (Houston, TX), E Brown (Dallas, TX), C Brueggmeyer (Jacksonville, FL), T Bunchman (Grand Rapids, MI), J Burke, J Burns (New York, NY), S Bynon (Birmingham, AL), M Cadnapaphornchai (Aurora, CO), M Cardi (Cincinnati, OH), W Carey (Cleveland, OH), E Carlisle (Canada), S Carmichael (Los Angeles, CA), E Castillo-Velarde (Peru), V Chadha (Richmond, VA), H Chakkera (Phoenix, AZ), J Chandar (Miami, FL), M Chandra (Long Island, NY), S Chandran (San Francisco, CA), Dr. Chatha, B Chavers (MN), MJ Choi (Baltimore, MD), KB Chopra (Phoenix, AZ), F Chybowski (Appleton, WI), F Ciuitarese (Carneigie, PA), F Coe (Chicago, IL), M Collins (Greenville, NC), F Corbin (Canada), H Corey (Morristown, NJ), SD Cramer (Winston-Salem, NC), D Creemers (Netherlands), R Cunningham (Cleveland, OH), K Dalinghaus (Canada), Dr. D'Allessandre-Silva (Hartford, CT), A Dart (Canada), Dr. Davidsson, J Davis, I Davis (Cleveland, OH), C de Souza (Uruguay), M DeBeukelaer (Toledo, OH), V Delaney (Hawthorne, NY), V Dennis (Cleveland, OH), P Devarajan (Cincinnatti, OH), V Dennis (Cleveland, OH), P Devarajan (Cincinnati, OH), M Dhakal (Rochester, NY), Dr. Diaz (TX), D Bradley (Cincinnati, OH), A Djamali (Madison, WI), Z Dolezel (Czech Republic), HA Doll (Tallahasse, FL), N Donin (NY), P Douville (Canada), M Dukeminier (Eugene, OR), M Dummer, K Duncan (Olathe, KS), J Durham (Orangeburg, SC), S Ecklund (Sioux Falls, SD), J Edwards, C Eggert, K Eidman (Minneapolis, MN), L Elangovan (Waukesha, WI), M Emiru (Tyler, TX), M Emmett (Alvardo, TX), S Fathalla-Shaykh (Birmingham, AL), D Fenyves (Canada), M Ferris (Chapel Hill, NC), M Fisher (Greenville, NC), C Flombaum (York Ave, NY), D Ford (Aurora, CO), J Foreman (Durham, NC), A Friedman (Syracuse, NY), C Fritsche (Milwaukee, WI), S Garcia (Spain), E Garin (Gainesville, FL), D Geary (Canada), U Gibbons (Oman), D Gipson (Chapel Hill, NC), M Glather, E Gnessin (Indianapolis, IN), S Goldberger (Farmville, VA), C Gordon (Portland, ME), T Gottheiner (Palo Alto, CA), S Goyal (West Islip NY), O Grandas (Knoxville, TN), D Grapey (Portland, OR), B Greco (Springfield, MA), L Greenbaum (Atlanta, GA), L Gregor (Canada), N Gupta (Worcester, MA), M Haddad (Sacramento,CA), Dr. Hafsteinsdottir, W Haley (Jacksonville, FL), P Hall (Cleveland, OH), M Halty (Uruguay), M Hames (Greenville, NC), CD Hanevold (Seattle, WA), F Harley (Canada), F Harris (Metairle, LA), G Hart (Charlotte, NC), E Harvey (Canada), E Heher (Boston, MA), RL Heilman (Scottsdale, AZ), R Helman (Duluth, MN), J A Herndon (Birmingham, Alabama, USA), J Hernandez (Seattle, WA), A Herndon (Birmingham, AL), J Herrin (Boston, MA), M Hertl (Boston, MA), G Hidalgo (Greenville, NC), P Hmiel (St. Louis, MO), R Holleman (Columbia, SC), P Holloway (England), RP Holmes (Birmingham, AL), B Hoppe (Germany), H Hotchkiss (New Brunswick, NJ), S Hsieh (Phoenix, AZ), C Hughes (Pittsburgh, PA), D Hull (Hartford, CT), T Hunley (Nashville, TN), Dr. Husmann (TN), H Ibrahim (Minneapolis, MN), V Idrovo (Columbia), M Jacobson (Lincoln, NE), T Jarrell (Columbus, GA), T Jaul, S Jernigan (Atlanta, GA), J Jetton (Iowa City, IA), L Jinadu (Baltimore, MD), J Johnston (Pittsburgh, PA), B Kaiser ?Wilington, DE?, A Kalia (Galveston, TX), E Kamil (Los Angeles, CA), U Kannapadi (Pittsburgh, PA), T Kara (New Zealand), C Kashani (Minneapolis, MN), F Kaskel (Bronx, NY), A Katz (Minneapolis, MN), V Keenan, D Kees-Folts (Hershey, PA), E Kendrick (Seattle, WA), O Khan (Chicago, IL), A Khurana (Phoenix, AZ), S Kim (E. Patchogue NY), S Knohl (Syracuse, NY), S Kobrin (Philadelphia, PA), A Kogon (New York, NY), O Kohn (Chicago, IL), B Koneru (Newark, NJ), R Kossman (Santa Fe, NM), T Kovalchik (Torrington, CT), A Krambeck (Indianapolis, IN), J Kropp (Winfield, IL), A Kumar (Long Beach, CA), J Kumar (New York, NY), M Lambert (Shawnee, KS), M Lande (Rochester, NY), V Langlois (Canada), L Larch (Clarksville, IN), J Lebowitz (New Brunswick, NJ), M Lee (San Francisco, CA), WP Lee (Denver, CO), J Leiser (Indianapolis, IN), S Lerman (Los Angeles, CA), DL Levy (Bangor, ME), F Lin (New York, NY), J Lingeman (Indianapolis, IN), DS Lirenman (Canada), A Lowe (Los Angeles, CA), R Loza (Peru), J Lucia (Indianapolis, IN), N Maalouf (Chattanooga, TN), M Maddy (Duluth, MN), D Madhav (India), B Mahadeva (Oakland, CA), S Mahesh (Akron, OH), R Mallavarapu (Frederickburg, VA), C Manning (Australia), MA Mansell (United Kingdom), J Marple (Lincoln, NE), Dr. Marsha, D Martin (Euless, TX), M Martin (Worcester, MA), R Mathias (San Francisco, CA), M Mauer (Minneapolis, MN), H Maxwell, M May (Jacksonville, FL), G Mchedlishvili (York PA), M McHugh (Columbus, OH), E McPhail (Winston-Salem, NC), N Mehta, RS Meisner (WI), S Menon (Detroit, MI), P Metcalfe (Canada), C Meyer (South Africa), A Mian (Rochester, NY), J Misurac (Indianapolis, IN), Y Miyashita (Pittsburgh, PA), P Mohan, D Mohtat (New York, NY), B Morgenstern (Phoenix, AZ), A Mubarak (Dallas, TX), M Muff-Luett (Iowa City, IA), G Murphy (Greenville, NC), C Nailescu (Indianapolis, IN), S Nampoothiri (India), M Narkewicz (Denver, CO), G Nassar (Houston, TX), A Naushabayev (Kazakhstan), M Navarro (Houston, TX), E Neimark (Providence, RI), C Nicely (Columbus, OH), C Norris (Arlington, TX), L Orihuela (Uruguay), NP Pamidi Reddy (India), M Parker, S Patel (India), R Pearl (Canada), M Pearle (Dallas, TX), S Perlman (St. Petersburg, FL), F Perwad (San Francisco, CA), S Peters (NJ), HG Pohl (Washington, DC), C Poortengia (Kingsport, TN), M Porayko (Nashville, TN), H Powell (Australia), G Prasad (India), W Primack, J Pucket (St. Kirksville, MO), D Puliyanda (Los Angeles CA), A Quiroga (Grand Rapids, MI), R Raafat (Norfolk, VA), E Rabin (Canada), E Rademacher (Rochester, NY), S Radhakrishnan (Canada), V Ramanathan (Houston, TX), J I Ramirez (Orlando, FL), Randeree (South Africa), D Rangaswamy (India), Dr. Rao (MA), M Rashid (Rochester, NY), D Raskin (Phoenix, AZ), M Rasoulpour (Hartford, CT), R Ravichandran (India), Dr. Reddy, A Redpath, L Restall (Dallas, TX), D Reyes (Spring Hill, FL), M Rheault (Minneapolis, MN), S Riar (Kansas City, KS), S Rice (GA), C Richardson (Tacoma, WA), L Robinson (Canada), M Rocklin (Denver, CO), R Rosen, G Rumsby (England), M Russo (Charolette, NC), P Sacks (Phoenix, AZ), N Saffarin (Minot, ND), A Saha (India), Dr. Saheny, S Sahney (San Bernardino, CA), J Saland (New York, NY), A Salerno (Worcester, MA), M Sanderson (Saint Paul, MN), D Sas (Charleston, SC), D Saxauer (Sante Fe, NM), JD Scandling (Palo Alto, CA), IM Schmidt (Demark), K Schroeder (Columbus, OH), J Schwimmer (New York NY), S Sech (Asheville, NC), M Seifert (St. Louis, MO), F Sepandj (Canada), SK Sethi (India), E Shahmir (Vacaville, CA), J Sharma (Boston, MA), M Sheehan (Denver, CO), N Shenoy (India), R Sheth (San Bernardino, CA), G Shetty (India), K Sievers (Rolla, MO), E Simon (Albany, NY), D Simon (Austin, TX ), J Simon (Cleveland, OH), R Sirota (Willow Grove, PA), C Smith (Minneapolis, MN), M Sobel (Westerville, OH), Dr. Solemin (Cincinnati, OH), N Soliman (Egypt), Dr. Somasundaram (Indianapolis, IN), D Spencer (Columbus, OH), M Stampfl (Lakeland, FL), T Starzl (Pittsburgh, PA), D Stein (Boston, MA), J Steinke (Grand Rapids, MI), D Steward, M Suarez (Houston, TX), B Sweety, R Swinford (Houston, TX), JM Symons (Seattle, WA), IYS Tang (Chicago, IL), A Tannenbann (Hudson, FL), P Tanpaiboon (Washington, DC), M Tanzer (Ann Arbor, MI), J Tariq (Pakistan), M Teruel (Fort Collins, CO), C Thomas (Iowa City, IA), R Thomas (New York, NY), M Tieg (Huntsville, AL), P Tiwari (Kankakee, IL), A Tolaymat (Jacksonville, FL), MM Tomsho (Summersville, WV), A Torres (Spain), A Traum (Boston, MA), K Treit (Seattle, WA), S Tuchman (Washington, DC), MA Turman (Oklahoma City, OK), R Unwin (United Kingdom), A Valdivieso (Chile), RP Valentini (Detroit, MI), F Varghese (Houston TX), M Velasco (Uruguay), R Venick (Los Angeles, CA), S Venkatesh (Nashville, TN), A Vera (Bogota, Columbia), D Veretnik (Israel), P Verghese (Minneapolis, MN), H Viko (Norway), R Villa (Lubbock, TX), J Wang (Minneapolis, MN), B Warady (Kansas City, KS), B Warshaw (Atlanta, GA), A Wasserstien (Phil, PA), C Weimer (Thibodaux, LA), J Weinstein (Dallas, TX), L Weintraub (Fairfax, VA), RA Weiss (Valhalla, NY), P Wertsch (Madison, WI), J Wesson (Milwaukee, WI), W Wilcox (Los Angeles, CA), K Winden (CA), M Winkelbauer (O'Neill, NE), C Wong (Albuquerque, NM), W Wong (Boston, MA), E Worcester (Chicago, IL), O Yadin (Los Angeles, CA), I Yamaguchi (Seattle, WA), B Ysee (Phoenix, AZ), V Yiu (Canada), A Zolotnitskaya (Valhalla, NY)
Footnotes
DISCLOSURE
All the authors declared no competing interests.
References
- 1.Hoppe B, Beck BB, Milliner DS. The primary hyperoxalurias. Kidney Int. 2009;75:1264–1271. doi: 10.1038/ki.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monico CG, Rossetti S, Belostotsky R, et al. Primary hyperoxaluria type III gene HOGA1 (formerly DHDPSL) as a possible risk factor for idiopathic calcium oxalate urolithiasis. Clin J Am Soc Nephrol. 2011;6:2289–2295. doi: 10.2215/CJN.02760311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danpure CJ. Primary hyperoxaluria: from gene defects to designer drugs? Nephrol Dial Transplant. 2005;20:1525–1529. doi: 10.1093/ndt/gfh923. [DOI] [PubMed] [Google Scholar]
- 4.Cregeen DP, Williams EL, Hulton S, et al. Molecular analysis of the glyoxylate reductase (GRHPR) gene and description of mutations underlying primary hyperoxaluria type 2. Hum Mutat. 2003;22:497. doi: 10.1002/humu.9200. [DOI] [PubMed] [Google Scholar]
- 5.Hoppe B, Kemper MJ, Bokenkamp A, et al. Plasma calcium oxalate supersaturation in children with primary hyperoxaluria and end-stage renal failure. Kidney Int. 1999;56:268–274. doi: 10.1046/j.1523-1755.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 6.Habbig S, Beck BB, Hoppe B. Nephrocalcinosis and urolithiasis in children. Kidney Int. 2011;80:1278–1291. doi: 10.1038/ki.2011.336. [DOI] [PubMed] [Google Scholar]
- 7.Monico CG, Rossetti S, Olson JB, et al. Pyridoxine effect in type I primary hyperoxaluria is associated with the most common mutant allele. Kidney Int. 2005;67:1704–1709. doi: 10.1111/j.1523-1755.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 8.Hoppe B, Latta K, von Schnakenburg C, et al. Primary hyperoxaluria--the German experience. Am J Nephrol. 2005;25:276–281. doi: 10.1159/000086358. [DOI] [PubMed] [Google Scholar]
- 9.Cheidde L, Ajzen SA, Tamer Langen CH, et al. A critical appraisal of the radiological evaluation of nephrocalcinosis. Nephron Clin Pract. 2007;106:c119–124. doi: 10.1159/000102999. [DOI] [PubMed] [Google Scholar]
- 10.Boyce AM, Shawker TH, Hill SC, et al. Ultrasound is superior to computed tomography for assessment of medullary nephrocalcinosis in hypoparathyroidism. J Clin Endocrinol Metab. 2013;98:989–994. doi: 10.1210/jc.2012-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viprakasit DP, Sawyer MD, Herrell SD, et al. Limitations of ultrasonography in the evaluation of urolithiasis: a correlation with computed tomography. J Endourol. 2012;26:209–213. doi: 10.1089/end.2011.0177. [DOI] [PubMed] [Google Scholar]
- 12.Hoppe B, Langman CB. A United States survey on diagnosis, treatment, and outcome of primary hyperoxaluria. Pediatr Nephrol. 2003;18:986–991. doi: 10.1007/s00467-003-1234-x. [DOI] [PubMed] [Google Scholar]
- 13.Takayama T, Nagata M, Ichiyama A, et al. Primary hyperoxaluria type 1 in Japan. Am J Nephrol. 2005;25:297–302. doi: 10.1159/000086361. [DOI] [PubMed] [Google Scholar]
- 14.Khan SR. Nephrocalcinosis in animal models with and without stones. Urol Res. 2010;38:429–438. doi: 10.1007/s00240-010-0303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edvardsson VO, Goldfarb DS, Lieske JC, et al. Hereditary causes of kidney stones and chronic kidney disease. Pediatric nephrology. 2013;28:1923–1942. doi: 10.1007/s00467-012-2329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cebotaru V, Kaul S, Devuyst O, et al. High citrate diet delays progression of renal insufficiency in the ClC-5 knockout mouse model of Dent’s disease. Kidney Int. 2005;68:642–652. doi: 10.1111/j.1523-1755.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 17.Stapenhorst L, Sassen R, Beck B, et al. Hypocitraturia as a risk factor for nephrocalcinosis after kidney transplantation. Pediatr Nephrol. 2005;20:652–656. doi: 10.1007/s00467-005-1831-y. [DOI] [PubMed] [Google Scholar]
- 18.Sikora P, Roth B, Kribs A, et al. Hypocitraturia is one of the major risk factors for nephrocalcinosis in very low birth weight (VLBW) infants. Kidney Int. 2003;63:2194–2199. doi: 10.1046/j.1523-1755.2003.t01-4-00001.x. [DOI] [PubMed] [Google Scholar]
- 19.Scheid C, Koul H, Hill WA, et al. Oxalate toxicity in LLC-PK1 cells: role of free radicals. Kidney Int. 1996;49:413–419. doi: 10.1038/ki.1996.60. [DOI] [PubMed] [Google Scholar]
- 20.Verkoelen CF, Verhulst A. Proposed mechanisms in renal tubular crystal retention. Kidney Int. 2007;72:13–18. doi: 10.1038/sj.ki.5002272. [DOI] [PubMed] [Google Scholar]
- 21.Asselman M. Calcium Oxalate Crystal Adherence to Hyaluronan-, Osteopontin-, and CD44-Expressing Injured/Regenerating Tubular Epithelial Cells in Rat Kidneys. J Am Soc Nephrol. 2003;14:3155–3166. doi: 10.1097/01.asn.0000099380.18995.f7. [DOI] [PubMed] [Google Scholar]
- 22.Mulay SR, Kulkarni OP, Rupanagudi KV, et al. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1beta secretion. J Clin Invest. 2013;123:236–246. doi: 10.1172/JCI63679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Woerden CS, Groothoff JW, Wanders RJ, et al. Primary hyperoxaluria type 1 in The Netherlands: prevalence and outcome. Nephrol Dial Transplant. 2003;18:273–279. doi: 10.1093/ndt/18.2.273. [DOI] [PubMed] [Google Scholar]
- 24.Milliner DS, Wilson DM, Smith LH. Phenotypic expression of primary hyperoxaluria: comparative features of types I and II. Kidney Int. 2001;59:31–36. doi: 10.1046/j.1523-1755.2001.00462.x. [DOI] [PubMed] [Google Scholar]
- 25.Harambat J, Fargue S, Acquaviva C, et al. Genotype-phenotype correlation in primary hyperoxaluria type 1: the p.Gly170Arg AGXT mutation is associated with a better outcome. Kidney Int. 2010;77:443–449. doi: 10.1038/ki.2009.435. [DOI] [PubMed] [Google Scholar]
- 26.Lieske JC, Monico CG, Holmes WS, et al. International registry for primary hyperoxaluria. Am J Nephrol. 2005;25:290–296. doi: 10.1159/000086360. [DOI] [PubMed] [Google Scholar]
- 27.Werness PG, Brown CM, Smith LH, et al. EQUIL2: a BASIC computer program for the calculation of urinary saturation. J Urol. 1985;134:1242–1244. doi: 10.1016/s0022-5347(17)47703-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.