Abstract
Objective:
Children with attention-deficit hyperactivity disorder (ADHD) may have oppositional behaviour, conduct problems, and aggression. These symptoms vary in severity, and may be related to a comorbid diagnosis of oppositional defiant disorder (ODD) or conduct disorder (CD). Critical evaluation of the efficacy of ADHD medications may guide the clinician regarding the usefulness of medications for these symptoms.
Method:
We performed a systematic review and meta-analysis of psychostimulants, alpha-2 agonists, and atomoxetine for oppositional behaviour, conduct problems, and aggression in youth with ADHD, ODD, and CD. The quality of evidence for medications was rated using the Grading of Recommendations Assessment, Development and Evaluation approach.
Results:
Two systematic reviews and 20 randomized controlled trials were included. There is high-quality evidence that psychostimulants have a moderate-to-large effect on oppositional behaviour, conduct problems, and aggression in youth with ADHD, with and without ODD or CD. There is very-low-quality evidence that clonidine has a small effect on oppositional behaviour and conduct problems in youth with ADHD, with and without ODD or CD. There is moderate-quality evidence that guanfacine has a small-to-moderate effect on oppositional behaviour in youth with ADHD, with and without ODD. There is high-quality evidence that atomoxetine has a small effect on oppositional behaviour in youth with ADHD, with and without ODD or CD.
Conclusions:
Evidence indicates that psychostimulants, alpha-2 agonists, and atomoxetine can be beneficial for disruptive and aggressive behaviours in addition to core ADHD symptoms; however, psychostimulants generally provide the most benefit.
Keywords: alpha-2 agonists, atomoxetine, clonidine, guanfacine, systematic review, meta-analysis, psychostimulants, attention-deficit hyperactivity disorder, oppositional defiant disorder, conduct disorder
Abstract
Objectif :
Les enfants souffrant du trouble de déficit de l’attention avec hyperactivité (TDAH) peuvent avoir un comportement oppositionnel, des problèmes de conduite, et de l’agressivité. La gravité de ces symptômes peut varier, et ils peuvent être liés à un diagnostic comorbide de trouble oppositionnel avec provocation (TOP) ou de trouble des conduites (TC). L’évaluation critique de l’efficacité des médicaments du TDAH peut guider le clinicien à l’égard de l’utilité des médicaments pour ces symptômes.
Méthode :
Nous avons effectué une revue systématique et une méta-analyse des psychostimulants, des agonistes alpha-2 et de l’atomoxétine pour le comportement oppositionnel, les problèmes de conduite, et l’agressivité chez les adolescents souffrant de TDAH, de TOP et de TC. La qualité des données probantes pour les médicaments a été cotée à l’aide de l’approche de Classement de l’analyse, de l’élaboration et de l’évaluation des recommandations.
Résultats :
Deux revues systématiques et 20 essais randomisés contrôlés ont été inclus. Des données probantes de qualité élevée indiquent que les psychostimulants ont un effet de modéré à grand sur le comportement oppositionnel, les problèmes de conduite, et l’agressivité chez les adolescents souffrant du TDAH, avec et sans TOP ou TC. Des données probantes de très faible qualité indiquent que la clonidine a un effet modeste sur le comportement oppositionnel et les problèmes de conduite chez les adolescents souffrant du TDAH, avec et sans TOP ou TC. Des données probantes de qualité modérée montrent que la guanfacine a un effet de modeste à modéré sur le comportement oppositionnel chez les adolescents souffrant de TDAH, avec et sans TOP. Des données probantes de qualité élevée indiquent que l’atomoxétine a un effet modeste sur le comportement oppositionnel chez les adolescents souffrant du TDAH, avec et sans TOP ou TC.
Conclusions :
Les données probantes indiquent que les psychostimulants, les agonistes alpha-2, et l’atomoxétine peuvent être bénéfiques pour les comportements perturbateurs et agressifs qui s’ajoutent aux symptômes de base du TDAH. Cependant, les psychostimulants sont généralement plus avantageux.
Attention-deficit hyperactivity disorder is the most common neuropsychiatric disorder of childhood, with a prevalence of 4.1% in Canadian school-aged children.1 Children with ADHD show a persistent pattern of inattention and (or) hyperactivity-impulsivity that interferes with functioning and is inappropriate for developmental level. In addition to these core features, many children with ADHD and their families struggle with oppositional behaviour, conduct problems, and aggression. These symptoms vary in severity among children, and in up to 60% of those with ADHD, may be related to a comorbid diagnosis of ODD or CD.2 Children with ODD have severe and persistent negative, defiant, hostile, and oppositional behaviour, while children with CD violate the rights of others or societal norms through repeated acts of aggression to people and animals, destruction of property, deceitfulness or theft, or serious violations of rules.
The Canadian ADHD Practice Guidelines3 outline 5 tiers of holistic-based care, including: adequate education of patients and their families; behavioural interventions; psychological treatment; educational accommodations; and medical management. In children with comorbid ODD, these guidelines advise optimization of pharmacotherapy of ADHD, as well as psychosocial treatments, including parent and other behavioural treatments. For children with comorbid CD, the guidelines state that while medications are usually effective in reducing the symptoms of ADHD and impulsive aggression, these children usually benefit from multimodal treatment, and may require additional pharmacotherapy with antipsychotics or mood stabilizers. Until now, the relative benefits of using ADHD medications rather than adding an antipsychotic or mood stabilizer have been unclear.
Clinical Implications
Among the medications used for the treatment of ADHD, psychostimulants have the most evidence for efficacy in the treatment of oppositional behaviour, conduct problems, and aggression.
There is evidence to support the use of guanfacine and atomoxetine for oppositional behaviour, though effect sizes are small to moderate.
The effect of clonidine on oppositional behaviour and conduct problems may not be clinically significant.
Limitations
There are a very limited number of studies of guanfacine and clonidine for the treatment of oppositional behaviour, conduct problems, and aggression.
In light of the disability related to oppositional behaviour, conduct problems, and aggression in youth with ADHD, and the high rate of comorbidity between ADHD, ODD, and CD, a critical evaluation of the efficacy of ADHD medications in treating these types of behaviours can help guide the clinician regarding pharmacotherapy for these target symptoms. The objective of this systematic review is to assess the efficacy and safety of ADHD medications—psychostimulants, alpha-2 agonists, and atomoxetine—for oppositional behaviour, conduct problems, and aggression in youth with ADHD that is comorbid with ODD or CD. A companion article in this 2-part series provides a systematic review of antipsychotics and mood stabilizers for the same purpose.4
Methods
Criteria for Considering Studies for This Review
Types of Studies
We included systematic reviews and RCTs, both parallel group and crossover designs. Studies had to include outcomes on oppositional behaviour, conduct problems, or aggression, and had to include a placebo phase or group. We searched for studies regardless of publication type.
Types of Participants
We included children and adolescents with a diagnosis of ADHD, ODD, or CD, made by an established classification system. Given the high rate of comorbidity between these disorders and both subaverage IQ and chronic tic disorders, we did not exclude studies that included children with these comorbidities.
Types of Interventions
Psychostimulants (methylphenidate and amphetamine) at any dosage or formulation (for example, short- or long-acting medications).
Alpha agonists (clonidine and guanfacine) at any dosage or formulation.
Atomoxetine at any dosage.
Types of Outcome Measures
Oppositional behaviour, conduct problems, or aggression as measured by validated clinician-, parent-, or teacher-reported scales.
Adverse effects, including physical, laboratory, or electrocardiogram abnormalities, and adverse event–related drop outs.
Search Methods for Identification of Studies
Electronic Searches
We searched the CENTRAL (Cochrane Central Register of Controlled Trials), MEDLINE, and PsycInfo databases in October 2013 (start date from database inception) using the strategies in online eAppendix 1. Searches were performed by medication class. Language restrictions were not imposed. Studies were not excluded based on publication status.
Searching Other Resources
We searched ClinicalTrials.gov database for unpublished studies, and checked the references of all review articles found in our searches.
Data Collection and Analysis
Selection of Studies
Two authors independently reviewed titles and abstracts from the searches and selected potentially relevant studies. Full-text articles were read in detail to determine if they fulfilled inclusion criteria. Disagreements were resolved through discussion between the reviewers. We did not need to refer any disagreement to an independent arbiter.
Data Extraction and Management
Two authors independently extracted data from studies and entered them into a predesigned data extraction form. The following data were extracted and entered:
Study design.
Randomization method.
Allocation concealment.
Blinding of participants, personnel, and outcome assessors.
Inclusion and exclusion criteria.
Number of participants.
Age distribution.
Sex.
Loss to follow-up.
Premature discontinuation and reasons for such.
Outcomes.
Incomplete outcome data and selective outcome reporting.
Method of analysis.
Comparability of groups at baseline.
We compared extracted data to ensure accuracy, and we resolved discrepancies through discussion.
Assessment of Methodological Quality of Individually Included Studies and Systematic Reviews
Two authors independently assessed the risk of bias of each study according to predetermined criteria. For each criterion, included studies were given ratings of low risk of bias, high risk of bias, or uncertain risk of bias. Based on the combination of these individual factors, studies were given an overall rating of class I, class II, or class III for methodological quality. See online eAppendix 2 for the methodological quality rating system. Included systematic reviews were evaluated for methodological quality using the R-AMSTAR tool and scored up to 44 points.5
Measures of Treatment Effect
For continuous outcomes, we used SMDs between treatment and placebo. The SMD is used to pool outcomes measured on different scales across studies. Depending on the presentation of individual study results, SMDs were calculated based on end point or change scores. For binary outcomes, we used odds ratios. Intention-to-treat analyses were performed: all randomized participants were included in the analyses and retained in the group to which they were allocated.
Unit of Analysis Issues
For crossover trials, we analyzed first period data only, when reported. When this was not reported, we took all measurements from treatment periods and placebo periods and analyzed these as if the trial were a parallel group trial. In crossover trials reporting results from multiple dosages of methylphenidate, only the data on the highest-included dosage were used in the meta-analysis.
Dealing With Missing Data
When standard deviations were missing, they were calculated from given standard errors and group sample size. When both the standard deviation and standard error were not provided, standard deviations were imputed from a study using the same measurement instrument, treatment, and population type. This was required for one study.
Assessment of Heterogeneity
We assessed clinical heterogeneity by comparing trial design and the distribution of participant factors. We assessed statistical heterogeneity by examining the I2 statistic, an approximate quantity that describes the proportion of variation in point estimates that is due to heterogeneity of studies rather than to sampling error. We performed a chi-square test of homogeneity to determine the strength of evidence that heterogeneity is genuine.
Assessment of Reporting Biases
Given the limited number of RCTs, we did not formally assess for reporting bias. Both included systematic reviews (for psychostimulants and atomoxetine) had an adequate number of RCTs to perform tests of reporting bias, which are described in the Results.
Data Synthesis
We performed meta-analysis on the data using both random-effects and fixed-effects models, and compared the results of these 2 approaches to assess statistical heterogeneity. If the I2 statistic was less than 40%, a fixed-effect model was used. Meta-analysis was performed on efficacy data only, as there was great variability in the reporting of adverse effects. Data on the type and frequency of adverse effects are described for each included study.
Overall Evidence Quality Rating for Each Medication
Each medication was given an overall quality of evidence rating based on the GRADE approach.6 The overall quality of evidence is rated as high, moderate, low, or very low, based on the confidence in the estimate of effect. With high-quality evidence, further research is very unlikely to change our confidence in the estimate of effect. With moderate-quality evidence, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. With low-quality evidence, any estimate of effect is very uncertain, and further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
We determined an initial rating of the quality of evidence for each medication based on the number and quality of the individual studies. We required multiple RCTs without serious limitations to make an initial rating of high-quality evidence. Multiple RCTs with some serious limitations resulted in an initial rating of moderate-quality evidence. An initial rating of low-quality evidence was given when RCTs had very serious limitations and inconsistent results. GRADE then specifies 5 reasons for rating down the quality of evidence (risk of bias, imprecision, inconsistency, indirectness, and publication bias) and 3 reasons for rating up the quality of evidence (a large magnitude of effect, a dose–response gradient, and a situation in which all plausible biases would serve to increase our confidence in the estimated effect). Each of these factors was evaluated for each medication, leading to a final determination of the quality of evidence, which are presented in the evidence profile table (Table 1).
Table 1.
Grading of Recommendations Assessment, Development and Evaluation evidence profiles
| Outcome assessed; number of studies; number of patients; duration of studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Dose–response effect present? | Summary of findings | Overall quality |
|---|---|---|---|---|---|---|---|---|
| Psychostimulants for aggression, oppositional behaviour, and conduct problems in youth with ADHD, with and without ODD and CD | ||||||||
|
| ||||||||
| Aggression, oppositional behaviour, conduct problems; | Some limitations in study quality | No serious inconsistency | No indirectness | No serious imprecision | Publication bias unlikely | Evidence of dose–response effect for this outcome | Studies 1970 to 2001 | High |
| Effect size (Cohen d) | ||||||||
| Clinician 0.77, 95% CI 0.63 to 0.88 | ||||||||
| 40 RCTs; | Parent 0.71, 95% CI 0.42 to 1.15 | |||||||
| Teacher 1.04, 95% CI 0.79 to 1.32 | ||||||||
| 2364 patients; | Studies 2002 to 2013 | |||||||
| 2 to 16 weeks | SMD | |||||||
| Parent 0.55, 95% CI 0.36 to 0.73 | ||||||||
| Teacher 0.84, 95% CI 0.59 to 1.10 | ||||||||
|
| ||||||||
| Clonidine (monotherapy or in combination with a psychostimulant) for oppositional behaviour, and conduct problems in youth with ADHD, with and without ODD and CD | ||||||||
|
| ||||||||
| Oppositional behaviour and conduct problems; | Major limitations in study quality | Inconsistency in results between studies | No indirectness | Some imprecision of results | Possible publication bias | No evidence of dose–response effect for this outcome | SMD, clonidine, compared with placebo: 0.27, 95% CI 0.04 to 0.51 | Very lowa |
| 6 RCTs; | ||||||||
| 545 patients; | ||||||||
| 6 to 16 weeks | ||||||||
|
| ||||||||
| Guanfacine (monotherapy or in combination with a psychostimulant) for oppositional behaviour in youth with ADHD, with and without ODD | ||||||||
|
| ||||||||
| Oppositional behaviour; | Minor limitations in study quality | No inconsistency | No indirectness | No serious imprecision | Possible publication bias | No evidence of dose–response effect for this outcome | SMD, guanfacine, compared with placebo: 0.43, 95% CI 0.18 to 0.68 | Moderateb |
| 2 RCTs; | ||||||||
| 678 patients; | ||||||||
| 8 to 9 weeks | ||||||||
|
| ||||||||
| Atomoxetine for oppositional behaviour in youth with ADHD, with and without ODD and CD | ||||||||
|
| ||||||||
| Oppositional behaviour; | No serious limitations in study quality | No major inconsistency | No indirectness | No serious imprecision | Publication bias unlikely | Dose–response effect not demonstrated for this outcome | Effect size, atomoxetine, compared with placebo: 0.33, 95% CI 0.24 to 0.43 | High |
| 15 RCTs; | ||||||||
| 1907 patients; | ||||||||
| 4 to 18 weeks | ||||||||
Overall quality was downgraded based on study quality and inconsistency in results between studies.
Overall quality was downgraded based on study quality.
ADHD = attention-deficit hyperactivity disorder; CD = conduct disorder; ODD = oppositional defiant disorder; RCT = randomized controlled trial; SMD = standardized mean difference
Results
Results of the Search
We included 2 systematic reviews, 1 on psychostimulants and 1 on atomoxetine. For RCTs, we included 12 of psychostimulants and 8 of alpha agonists. See online eAppendix 3 for flow diagrams by drug class.
Study Participants
Most studies included youth with ADHD with and without ODD or CD. All studies included more boys than girls.
Study Outcomes
Several different scales were used to measure oppositional behaviour, conduct problems, or aggression. Most studies used rating scales developed by Keith Conners, which have been extensively revised and updated over 4 decades. The version of the instrument used was largely dependent on the time the RCT was performed. Items on the Conners instruments are based on the Diagnostic and Statistical Manual of Mental Disorders criteria for ADHD, ODD, and CD. These scales are widely used, both in clinical practice and in research, with a large sample of normative data available for comparison. The scales have overall excellent reliability and validity, and are available in full-length, short, and index forms, both for parents and for teachers.7 Other rating scales used in the included studies were the Attention Deficit Disorder with/without Hyperactivity Comprehensive Teacher Rating Scale, the Disruptive Behaviour Scale, the Child Conflict Index, the Swanson Nolan and Pelham 4 ODD Subscale, the ADHD Symptom Checklist-4 Oppositional Defiant Subscale, the CGI—Improvement scale, and the CGI—Severity scale. Descriptions of each scale can be found in online eAppendix 4.
Treatment Effects
Psychostimulants
Our search found 1 systematic review of psychostimulants for the treatment of aggression in youth with ADHD; it included 28 RCTs published between 1970 to 2001.8 Therefore, we included this systematic review in our analysis and restricted our own systematic review to studies published from 2001 to 2013.
Connor et al8 performed a meta-analysis to evaluate the effect size for stimulants on overt and covert aggression in children with ADHD. This meta-analysis received an R-AMSTAR score of 29/44 points. The authors included 28 RCTs of methylphenidate (21 studies), amphetamine (5), and pemoline (2). These studies had a total of 683 subjects, with sample sizes ranging from 6 to 46 (mean 24, SD not provided in the article). The mean age of subjects was 9.7 years (range 7.7 to 14.4), and 75% had comorbid ODD or CD. The mean duration of treatment was 13 days (SD not provided in the article), and mean doses of methylphenidate, amphetamine, and pemoline were 22.18, 23.74, and 145.15 mg/day, respectively. Each study was given a quality rating out of 7, and the mean rating was 5.8 (range 5 to 7).
Overt aggression-related behaviours were defined as “aggression resulting in a direct confrontation with the environment”8,p 254 (for example, physical assault, verbal threats, oppositional and defiant behaviour, conduct problems, rage attacks, and irritability), whereas covert aggression-related behaviours were defined as “aggression that is furtive and hidden from the environment”p 254 (for example, cheating, lying, stealing, and fire-setting). Where possible, separate effect sizes (Cohen d) were calculated for clinician-, parent-, and teacher-rated overt and covert aggression. Overall effect sizes for overt and covert aggression were calculated as well. Adverse effects were not considered.
For overt aggression, weighted effect sizes comparing psychostimulant and placebo were significant (P < 0.001) based on clinician ratings in 18 studies (d = 0.77; 95% CI 0.63 to 0.88), parent ratings in 13 studies (d = 0.71; 95% CI 0.42 to 1.15), teacher ratings in 16 studies (d = 1.04; 95% CI 0.79 to 1.32), and overall ratings in all 28 studies (d = 0.84; 95% CI 0.70 to 1.02). Significant heterogeneity was detected among the effect sizes in each of these analyses, but calculation of a so-called file-drawer statistic suggested that publication bias was highly unlikely. In general, the presence of comorbid ODD or CD correlated negatively with effect size. Effect sizes based on overall ratings were comparable for studies of methylphenidate (d = 0.80) and amphetamine (d = 0.83), and dose was not significantly associated with effect size.
Seven of 28 studies reported effects of stimulant treatment on covert aggression. Clinician ratings were available in 6 studies (d = 0.81; 95% CI 0.20 to 1.43, P < 0.001), and overall ratings in all 7 studies were lower at d = 0.54 (95% CI 0.21 to 1.29). In each of these analyses, heterogeneity across results was observed. The file-drawer statistics suggested that publication bias was unlikely. Drug type and dose were not associated with effect size.
We found 12 RCTs comparing psychostimulants with placebo, published from the end date of the Connor systematic review8 to 2013, that met our inclusion criteria. Eleven evaluated methylphenidate9–19 and 1 studied lisdexamphetamine20 in children with ADHD. These 12 RCTs included a total of 1681 participants. Trial size ranged from 24 to 327 participants (mean 140, SD 113.1). All trials included children with ADHD comorbid with ODD or CD (44% to 93%). Both immediate-release and long-acting forms of methylphenidate were studied. Trial length ranged from 2 to 16 weeks. Two trials received a quality rating of class I, 4 were class II, and 6 were class III. Measures of aggression, oppositional behaviour, and conduct problems were included as a secondary outcome in all studies, and all studies reported a significant benefit with psychostimulants, compared with placebo. See online eAppendix 5 for study characteristics, quality ratings, results of individual RCTs, and details regarding adverse effects. See eAppendix 6 for a list of excluded studies.
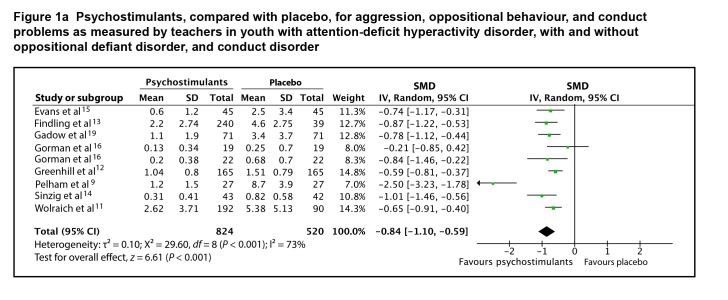
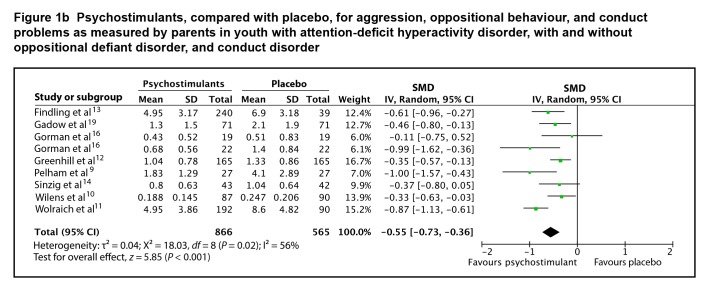
Eight studies provided adequate data on end point scores to be included in a meta-analysis. The SMD between psychostimulants and placebo for teacher-rated oppositional behaviour, conduct problems, and aggression was 0.84 (95% CI 0.59 to 1.10; I2 = 73%, P < 0.001), using a random effects model (Figure 1a). The SMD between psychostimulants and placebo for parent-rated oppositional behaviour, conduct problems, and aggression was 0.55 (95% CI 0.36 to 0.73; I2 = 56%, P < 0.001), using a random-effects model (Figure 1b). The evidence profile for psychostimulants is presented in Table 1. There is high-quality evidence that psychostimulants have a moderate-to-large effect in the management of oppositional behaviour, conduct problems, and aggression in youth with ADHD, with or without ODD or CD.
Figure 1a.
Psychostimulants, compared with placebo, for aggression, oppositional behaviour, and conduct problems as measured by teachers in youth with attention-deficit hyperactivity disorder, with and without oppositional defiant disorder, and conduct disorder
Figure 1b.
Psychostimulants, compared with placebo, for aggression, oppositional behaviour, and conduct problems as measured by parents in youth with attention-deficit hyperactivity disorder, with and without oppositional defiant disorder, and conduct disorder
Alpha Agonists
Eight studies met our inclusion criteria; 2 of guanfacine extended release, 1 of clonidine extended release, and 5 of clonidine immediate release. Six studies evaluated children with ADHD, 1 study evaluated children with ADHD and a chronic tic disorder, and 1 study evaluated children with mental retardation and hyperkinetic disorder. See online eAppendix 5 for study characteristics, quality ratings, results of individual RCTs, and details regarding adverse effects. See online eAppendix 6 for a list of excluded studies.
Clonidine
These 6 studies included 545 children with ADHD.17,21–25 All but 1 study23 did not exclude children with ODD or CD, with rates ranging from 46% to 100%. Trial size ranged from 10 to 198 participants (mean 91, SD 74.6). Trials ranged in length from 6 to 16 weeks. Two studies evaluated clonidine added to psychostimulant therapy, 2 studies evaluated clonidine monotherapy, and 2 studies evaluated both clonidine monotherapy and clonidine added to a psychostimulant. Methodological quality was rated as class II for 4 studies and class III for 2 studies. One study specified conduct problems as the primary outcome. Among the 4 class II quality studies, 3 were negative regarding oppositional behaviour and conduct problem outcomes; at least 2 of the 3 negative studies were adequately powered to detect a difference between treatments. Both class III quality studies were positive; 1 provided no data on outcome measures, thus we could not include the results in the meta-analysis.
Only 3 of the 6 studies provided adequate data on change or end-point scores to be included in the meta-analysis. The SMD between clonidine and placebo for oppositional behaviour and conduct problems was 0.27 (95% CI 0.04 to 0.51; I2 = 0%, P = 0.02), by fixed-effects model (Figure 2). The evidence profile for clonidine is presented in Table 1. There is very-low-quality evidence that clonidine has a small effect on oppositional behaviour and conduct problems in youth with ADHD, with and without ODD or CD. The lower limit of the 95% confidence interval for the effect of clonidine approaches zero; thus, it is possible that the true effect of clonidine for the treatment of oppositional behaviour and conduct problems is not clinically important.
Figure 2.
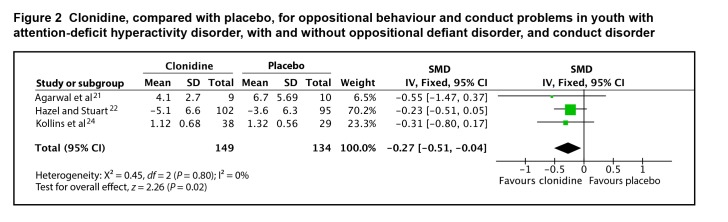
Clonidine, compared with placebo, for oppositional behaviour and conduct problems in youth with attention-deficit hyperactivity disorder, with and without oppositional defiant disorder, and conduct disorder
Guanfacine
The 2 studies of guanfacine extended release included 678 children with ADHD (children with a diagnosis of ODD were permitted in the studies, but the rate of comorbidity was not specified).26,27 One study evaluated guanfacine extended release for 9 weeks as an adjunct to psychostimulants; the other study evaluated guanfacine monotherapy for 8 weeks. Both trials were class II in quality. One trial had oppositional behaviour as the primary outcome. Both studies demonstrated a significant effect of guanfacine extended release, compared with placebo, for oppositional behaviour.
Both studies provided adequate data on change scores to be included in the meta-analysis. The SMD between guanfacine extended release and placebo for oppositional behaviour was 0.43 (95% CI 0.18 to 0.68; I2 = 5 4%, P < 0.001), by random-effects model (Figure 3). The evidence profile for guanfacine extended release is presented in Table 1. Overall, there is moderate-quality evidence that guanfacine has a small-to-moderate effect on oppositional behaviour in youth with ADHD, with and without ODD.
Figure 3.
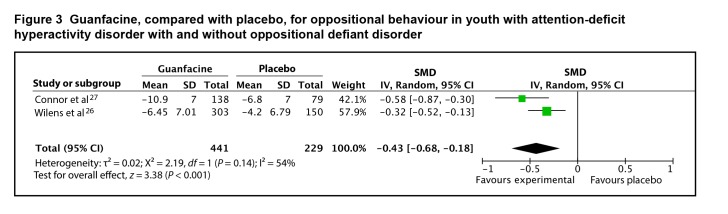
Guanfacine, compared with placebo, for oppositional behaviour in youth with attention-deficit hyperactivity disorder with and without oppositional defiant disorder
Atomoxetine
We found one systematic review and meta-analysis of atomoxetine, published in 2014, with ODD symptoms as an outcome (see online eAppendix 5 for details).28 The authors included all published and unpublished data on atomoxetine from placebo-controlled, double-blind, randomized trials in youth with ADHD and average intelligence. Twenty-five trials were included, comprising 3928 youth. The majority of studies (23/25) were industry-funded, with a mean trial duration of 8.6 weeks (range 4 to 18 weeks), and a mean atomoxetine dose of 1.17 mg/kg/day. Comorbid ODD and (or) CD was present in 44.6% of study participants. The results for ODD outcomes are based on 15 comparisons involving 1907 patients. The overall effect size for ODD symptoms was 0.33 (95% CI 0.24 to 0.43; I2 = 0%, P < 0.001). The authors found that the proportion of subjects with a diagnosis of ODD did not significantly influence the effect size for ODD symptoms (P = 0.41). Funnel plots were inspected to assess for publication bias for all outcomes; no indication of publication bias favouring atomoxetine was identified.
Ratings of trial methodological quality were not performed by the authors of the systematic review.28 Our own review of methodological quality of the included studies found 4 studies rated class I, 5 studies rated class II, and 2 studies rated class III. The evidence profile for atomoxetine is presented in Table 1. Overall, there is high-quality evidence that atomoxetine has a small effect on oppositional defiant behaviour in youth with ADHD, with and without ODD or CD.
Discussion
Summary of Main Results
First, there is high-quality evidence that psychostimulants have a moderate-to-large effect on oppositional behaviour, conduct problems, and aggression in youth with ADHD, with and without ODD or CD. Second, there is very-low-quality evidence that clonidine has a small effect on oppositional behaviour and conduct problems in youth with ADHD, with and without ODD or CD; the 95% confidence interval approaches zero, suggesting that the true effect may not be clinically significant. Third, there is moderate-quality evidence that guanfacine has a small-to-moderate effect on oppositional behaviour in youth with ADHD, with and without ODD. Fourth, there is high-quality evidence that atomoxetine has a small effect on oppositional behaviour in youth with ADHD, with and without ODD or CD.
Overall Completeness and Applicability of Evidence
The outcomes that were measured differed between drugs. Only the studies of psychostimulants evaluated all 3 outcomes of oppositional behaviour, conduct problems, and aggression, while studies of guanfacine and atomoxetine evaluated outcomes related to oppositional behaviour only, and studies of clonidine evaluated outcomes related to oppositional behaviour and conduct problems. Thus, when applying this evidence base clinically, the clinician must consider if the target symptoms for a particular patient involve oppositional behaviour, conduct problems, and (or) aggression, although of course considerable overlap exists between these 3 symptom domains.
While most studies included children with ADHD alone, ADHD with ODD, or ADHD with CD, none of them subanalyzed results based on diagnostic groups; rather, they presented aggregate data for all included subjects. Nonetheless, the systematic review of psychostimulants found that the presence of comorbid ODD or CD correlated negatively with effect size, suggesting that the benefits for disruptive and aggressive behaviour may be smaller in patients with more severe symptoms. Therefore, the clinician should bear in mind that there may be differences in treatment response based on the presence or absence of comorbid ODD and CD. Conversely, the systematic review of atomoxetine found that a diagnosis of ODD did not significantly moderate the effect size for oppositional symptoms. As the benefit of atomoxetine is small, compared with psychostimulants, the ability to detect potential moderators may be limited.
Conclusions
Implications for Practice
The largest effect size for symptoms of oppositional behaviour, conduct problems, and aggression was seen with the psychostimulants, which also show the largest effect size for the core symptoms of inattention, hyperactivity, and impulsivity,29 and are generally considered first-line medication treatment for ADHD.3,30 Therefore, the evidence confirms that in the child with ADHD, with or without ODD or CD, who suffers from disabling symptoms of oppositional behaviour, conduct problems, or aggression, psychostimulants are a medication of first choice. Treatment with other medications for ADHD, including atomoxetine, guanfacine, and clonidine, may also provide some symptom relief, though effect sizes are smaller for these outcomes.
In general, adverse effects related to psychostimulants in RCTs were mild, and infrequently led to discontinuation of treatment. The most commonly reported adverse effects were appetite suppression and difficulty sleeping. The most common adverse events related to alpha-2 agonists were sedation, headache, and decreased blood pressure or heart rate. Discontinuation due to adverse effects was also uncommon with atomoxetine, with gastrointestinal symptoms, loss of appetite, and fatigue most commonly reported.
Implications for Research
Longer-duration studies to evaluate symptom control over time would be valuable to determine if improvement in oppositional behaviour, conduct problems, and aggression are sustained, as well as to evaluate long-term safety of treatment. Authors reporting the results of clinical trials should be encouraged to adhere to the CONSORT (Consolidated Standards of Reporting Trials) Statement guidelines, as the failure to report important methodological factors results in lower-quality ratings for individual studies and the overall body of evidence.
Acknowledgments
Sources of support for individual studies included in the systematic review can be found in online eAppendix 4, Table of Included Studies. Funding support for the systematic review came from the Royal Bank of Canada Knowledge Translation Fund and the SickKids Foundation.
Abbreviations
- ADHD
attention-deficit hyperactivity disorder
- CD
conduct disorder
- CGI
Clinical Global Impression
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- ODD
oppositional defiant disorder
- RCT
randomized controlled trial
- R-AMSTAR
Revised Assessment of Multiple Systematic Reviews
- SMD
standardized mean difference
Footnotes
Editor’s Note
The opinions expressed in this paper are those of the authors and do not necessarily reflect the opinions of either The Canadian Journal of Psychiatry (The CJP) or the Canadian Psychiatric Association (CPA). This paper is not related to work done by the CPA’s Committee on Professional Standards and Practice or its Subcommittee on Clinical Practice Guidelines, and its publication in The CJP should not be construed as an endorsement of the content.
References
- 1.Brault MC, Lacourse E. Prevalence of prescribed attention-deficit hyperactivity disorder medications and diagnosis among Canadian preschoolers and school-age children: 1994–2007. Can J Psychiatry. 2012;57(2):93–101. doi: 10.1177/070674371205700206. [DOI] [PubMed] [Google Scholar]
- 2.Maughan B, Rowe R, Messer J, et al. Conduct disorder and oppositional defiant disorder in a national sample: developmental epidemiology. J Child Psychol Psychiatry. 2004;45(3):609–621. doi: 10.1111/j.1469-7610.2004.00250.x. [DOI] [PubMed] [Google Scholar]
- 3.Canadian Attention Deficit Hyperactivity Disorder Research Alliance (CADDRA) Canadian ADHD practice guidelines. 3rd ed. Toronto (ON): CADDRA; 2011. [Google Scholar]
- 4.Pringsheim T, Hirsch L, Gardner DM, et al. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 2: antipsychotics and traditional mood stabilizers. Can J Psychiatry. 2015;60(2):52–61. doi: 10.1177/070674371506000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kung J, Chiappelli F, Cajulis OO, et al. From systematic reviews to clinical recommendations for evidence-based health care: validation of Revised Assessment of Multiple Systematic Reviews (R-AMSTAR) for grading of clinical relevance. Open Dent J. 2010;4:84–91. doi: 10.2174/1874210601004020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Conners CK. Conners. 3rd ed. Toronto (ON): Multi-Health Systems Inc; 2008. [Google Scholar]
- 8.Connor DF, Glatt SJ, Lopez ID, et al. Psychopharmacology and aggression. I: A meta-analysis of stimulant effects on overt/covert aggression-related behaviours in ADHD. J Am Acad Child Adolesc Psychiatry. 2002;41(3):253–261. doi: 10.1097/00004583-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Pelham WE, Burrows-Maclean L, Gnagy EM, et al. Transdermal methylphenidate, behavioral, and combined treatment for children with ADHD. Exp Clin Psychopharmacol. 2005;13(2):111–126. doi: 10.1037/1064-1297.13.2.111. [DOI] [PubMed] [Google Scholar]
- 10.Wilens TE, McBurnett K, Bukstein O, et al. Multisite controlled study of OROS methylphenidate in the treatment of adolescents with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2006;160(1):82–90. doi: 10.1001/archpedi.160.1.82. [DOI] [PubMed] [Google Scholar]
- 11.Wolraich ML, Greenhill LL, Pelham W, et al. Randomized, controlled trial of oros methylphenidate once a day in children with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108(4):883–892. doi: 10.1542/peds.108.4.883. [DOI] [PubMed] [Google Scholar]
- 12.Greenhill L, Kollins S, Abikoff H, et al. Efficacy and safety of immediate-release methylphenidate treatment for preschoolers with ADHD. J Am Acad Child Adolesc Psychiatry. 2006;45(11):1284–1293. doi: 10.1097/01.chi.0000235077.32661.61. Erratum in: J Am Acad Child Adolesc Psychiatry. 2007;46(1):141. [DOI] [PubMed] [Google Scholar]
- 13.Findling RL, Quinn D, Hatch SJ, et al. Comparison of the clinical efficacy of twice-daily Ritalin and once-daily Equasym XL with placebo in children with attention deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry. 2006;15(8):450–459. doi: 10.1007/s00787-006-0565-0. [DOI] [PubMed] [Google Scholar]
- 14.Sinzig J, Dopfner M, Lehmkuhl G. Long-acting methylphenidate has an effect on aggressive behavior in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2007;17(4):421–432. doi: 10.1089/cap.2007.0011. [DOI] [PubMed] [Google Scholar]
- 15.Evans SW, Pelham WE, Smith BH, et al. Dose–response effects of methylphenidate on ecologically valid measures of academic performance and classroom behavior in adolescents with ADHD. Exp Clin Psychopharmacol. 2001;9(2):163–175. doi: 10.1037//1064-1297.9.2.163. [DOI] [PubMed] [Google Scholar]
- 16.Gorman EB, Klorman R, Thatcher JE, et al. Effects of methylphenidate on subtypes of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45(7):808–816. doi: 10.1097/01.chi.0000214191.57993.dd. [DOI] [PubMed] [Google Scholar]
- 17.Palumbo D, Sallee F, Pelham W, et al. Clonidine for attention-deficit/hyperactivity disorder: I. Efficacy and tolerability. J Am Acad Child Adolesc Psychiatry. 2008;47(2):180–188. doi: 10.1097/chi.0b013e31815d9af7. [DOI] [PubMed] [Google Scholar]
- 18.Pearson DA, Santos CW, Roache JD, et al. Treatment effects of methylphenidate on behavioral adjustment in children with mental retardation and ADHD. J Am Acad Child Adolesc Psychiatry. 2003;42(2):209–216. doi: 10.1097/00004583-200302000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Gadow KD, Sverd J, Nolan EE, et al. Immediate-release methylphenidate for ADHD in children with comorbid chronic multiple tic disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):840–848. doi: 10.1097/chi.0b013e31805c0860. [DOI] [PubMed] [Google Scholar]
- 20.Lopez FA, Ginsbeg LD, Arnold VA. Effect of lisdexamfetamine dimesylate on parent-rated measures in children aged 6 to 12 years with attention-deficit/hyperactivity disorder: a secondary analysis. Postgrad Med. 2008;120(3):89–102. doi: 10.3810/pgm.2008.09.1910. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal V, Sitholey P, Kumar S, et al. Double-blind, placebo-controlled trial of clonidine in hyperactive children with mental retardation. Ment Retard. 2001;39(4):259–267. doi: 10.1352/0047-6765(2001)039<0259:DBPCTO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Hazell PL, Stuart JE. A randomized controlled trial of clonidine added to psychostimulant medication for hyperactive and aggressive children. J Am Acad Child Adolesc Psychiatry. 2003;42(8):886–894. doi: 10.1097/01.CHI.0000046908.27264.00. [DOI] [PubMed] [Google Scholar]
- 23.Hunt RD, Minderaa RB, Cohen DJ. Clonidine benefits children with attention deficit disorder and hyperactivity: report of a double-blind placebo-crossover therapeutic trial. J Am Acad Child Psychiatry. 1985;24(5):617–629. doi: 10.1016/s0002-7138(09)60065-0. [DOI] [PubMed] [Google Scholar]
- 24.Kollins S, Jain R, Brams M, et al. Clonidine extended-release tablets as add-on therapy to psychostimulants in children and adolescents with ADHD. Pediatrics. 2011;127(6):e1406–e1413. doi: 10.1542/peds.2010-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurlan R. Treatment of ADHD in children with tics: a randomized controlled trial. Neurology. 2002;58(4):527–536. doi: 10.1212/wnl.58.4.527. [DOI] [PubMed] [Google Scholar]
- 26.Wilens T, Bukstein O, Brams M, et al. A controlled trial of extended-release guanfacine and psychostimulants for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(1):74–85. doi: 10.1016/j.jaac.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Connor DF, Findling RL, Kollins S, et al. Effects of guanfacine extended release on oppositional symptoms in children aged 6–12 years with attention-deficit hyperactivity disorder and oppositional symptoms: a randomized, double-blind, placebo-controlled trial. CNS Drugs. 2010;24(9):755–768. doi: 10.2165/11537790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz S, Correll CU. Efficacy and safety of atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder: results from a comprehensive meta-analysis and metaregression. J Am Acad Child Adolesc Psychiatry. 2014;53(2):174–187. doi: 10.1016/j.jaac.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Faraone SV. Understanding the effect size of ADHD medications: implications for clinical care [Internet] Medscape Psychiatry & Mental Health; 2003. [cited 2014 Jun 25]. Available from: http://www.medscape.com/psychiatry. [Google Scholar]
- 30.Seixas M, Weiss M, Muller U. Systematic review of national and international guidelines on attention-deficit hyperactivity disorder. J Psychopharmacol. 2012;26(6):753–765. doi: 10.1177/0269881111412095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.