Abstract
Aims
To evaluate the efficacy and safety of extended dosing with mipomersen in patients with familial hypercholesterolaemia (HC) taking maximally tolerated lipid-lowering therapy.
Methods and results
A planned interim analysis of an ongoing, open-label extension trial in patients (n = 141) with familial HC receiving a subcutaneous injection of 200 mg mipomersen weekly plus maximally tolerated lipid-lowering therapy for up to 104 weeks. The mean changes in low-density lipoprotein cholesterol (LDL-C) from baseline to weeks 26 (n = 130), 52 (n = 111), 76 (n = 66), and 104 (n = 53) were −28, −27, −27, and −28%; and in apolipoprotein B −29, −28, −30, and −31%, respectively. Reductions in total cholesterol, non-high-density lipoprotein-cholesterol, and lipoprotein(a) were comparable with decreases in LDL-C and apolipoprotein B levels. Mean high-density lipoprotein cholesterol increased from baseline by 7 and 6% at weeks 26 and 52, respectively. The long-term safety profile of mipomersen was similar to that reported in the associated randomized placebo-controlled Phase 3 trials. Adverse events included injection site reactions and flu-like symptoms. There was an incremental increase in the median liver fat during the initial 6–12 months that appeared to diminish with continued mipomersen exposure beyond 1 year and returned towards baseline 24 weeks after last drug dose suggestive of adaptation. The median alanine aminotransferase level showed a similar trend over time.
Conclusion
Long-term treatment with mipomersen for up to 104 weeks provided sustained reductions in all atherosclerotic lipoproteins measured and a safety profile consistent with prior controlled trials in these high-risk patient populations.
Clinicaltrials.gov
Keywords: Familial hypercholesterolaemia, Hypercholesterolaemia, Lipid-lowering, Adverse events, Long-term safety
Introduction
Mipomersen is a second-generation antisense oligonucleotide that inhibits apolipoprotein B 100 (apoB) protein synthesis.1 The liver produces apoB, which is the primary structural protein of atherogenic lipoproteins including low-density lipoprotein (LDL).2 Having a mechanism of action different from that of statins, mipomersen binds apoB messenger RNA, reducing apoB production, which in turn reduces very-low-density lipoprotein (VLDL) and consequently LDL production.1,3 Mipomersen 200 mg once weekly added to maximally tolerated lipid-lowering therapy has demonstrated lipid-lowering efficacy in a variety of populations including subjects with statin intolerance,4 homozygous familial hypercholesterolaemia (HoFH),5 heterozygous familial hypercholesterolaemia (HeFH),6 severe HeFH,7 and severe hypercholesterolaemia (HC) at high cardiovascular risk.8 The mean percent change in LDL cholesterol (LDL-C) from baseline to 2 weeks after treatment completion in these 6-month studies ranged from −25 to −37% (P < 0.001) compared with placebo at −5 to +13%. Reductions in LDL-C in HoFH and severe FH averaged over 100 mg/dL (2.6 mmol/L). In the HeFH population, 45% of patients achieved a LDL-C < 100 mg/dL and in the HC at high risk for CHD population, 50% of patients achieved an LDL-C <70 mg/dL (1.8 mmol/L). Hence, mipomersen resulted in clinically meaningful LDL-C lowering.
In 6-month clinical trials, the safety profile of mipomersen was preliminarily characterized.1,3 This analysis reports interim safety and efficacy results of the open-label extension period for three randomized, placebo-controlled trials with treatment up to 104 weeks.
Methods
Patients
We enrolled 142 patients with HeFH (n = 103) and HoFH (n = 38) taking maximally tolerated lipid-lowering therapy who had successfully completed a Phase 3 placebo-controlled mipomersen trial [NCT00607373-HoFH, n = 38 (86%); NCT00706849-HeFH, n = 94 (82%); NCT00794664-HeFH, n = 9 (100%)] had an acceptable safety profile [no significant liver blood test abnormalities that met stopping criteria or intolerable injections site reactions/flu-like symptoms (FLS)], no new or worsening conditions that interfered with study completion, and were willing to limit alcohol consumption to moderate amounts [males, 2 drinks (20 g) per day, 8 drinks (80 g) per week; females, 1 drink (10 g) per day, 4 drinks (40 g) per week] for the study duration.
Dosing
Mipomersen 200 mg was administered subcutaneously weekly (or 160 mg weekly for patients weighing <50 kg). A protocol amendment (May 2011) allowed patients to extend treatment to 4 years, which is ongoing. There was a 2-week screening period and a 24-week post-treatment follow-up, during which patients did not receive mipomersen. Dose reductions to 100 or 150 mg weekly were allowed if elevations in hepatic transaminases ≥5x ULN, moderate or severe injection site reactions (ISRs), FLS, or excessive reductions in LDL-C occurred; however, sufficient efficacy, defined as ≥15% LDL-C reduction from the patient's index (Phase 3) study baseline value, was required. Concomitant medications were allowed and patients were counselled to maintain a consistent diet and activity level.
Assessments
Efficacy endpoints included percent change in LDL-C, apoB, total cholesterol (TC), non-high-density lipoprotein cholesterol (non-HDL-C), LDL/HDL ratio, triglycerides (TG), VLDL cholesterol, high-density lipoprotein cholesterol (HDL-C), and apolipoprotein A-I (apo A-I), lipoprotein(a) (Lp[a]). Safety was assessed by adverse events, and clinical and laboratory parameters. All patients who received at least one injection of mipomersen (n = 141; HoFH, n = 38; HeFH, n = 103) were evaluated. Adverse events (AEs) were classified using MedDRA 13.0.
Laboratory evaluations
Fasting blood, urine samples, and vital signs were obtained at each study visit approximately every 4–7 weeks. Medpace Reference Laboratories, Cincinnati OH, USA and Leuven, Belgium performed analyses that included routine haematology with differential, blood chemistry, coagulation parameters, full serum lipid panel, high-sensitivity C-reactive protein, complement C3, and urinalysis. Physical examinations and ECGs, for the determination of corrected QT (QTc) intervals, were performed periodically. Adverse events were monitored and recorded at each visit. Magnetic resonance imaging (MRI) scans were performed at 6-month intervals during treatment to determine hepatic fat content.9 Magnetic resonance imagings were acquired at the clinical site but read at MR3T Bydder Lab, University of California, San Diego, CA, USA.
Statistics
The data cut-off date for this planned interim analysis was 30 November 2011. Baseline was defined to be the assessments before the first dose of mipomersen. Patients randomized to mipomersen received their first dose during the index study, whereas patients randomized to placebo during the index study received their first mipomersen dose at the start of the extension study. Data during the 24-week washout was not included. Descriptive statistics were used to summarize the data. Individual plasma trough concentration-vs.-time-curves were generated with Phoenix WinNonlin software.
This study is being performed in accordance with the Declaration of Helsinki. Locally appointed ethics committees approved the protocol (33 centres, 7 countries). Informed consent was obtained.
Results
Patients
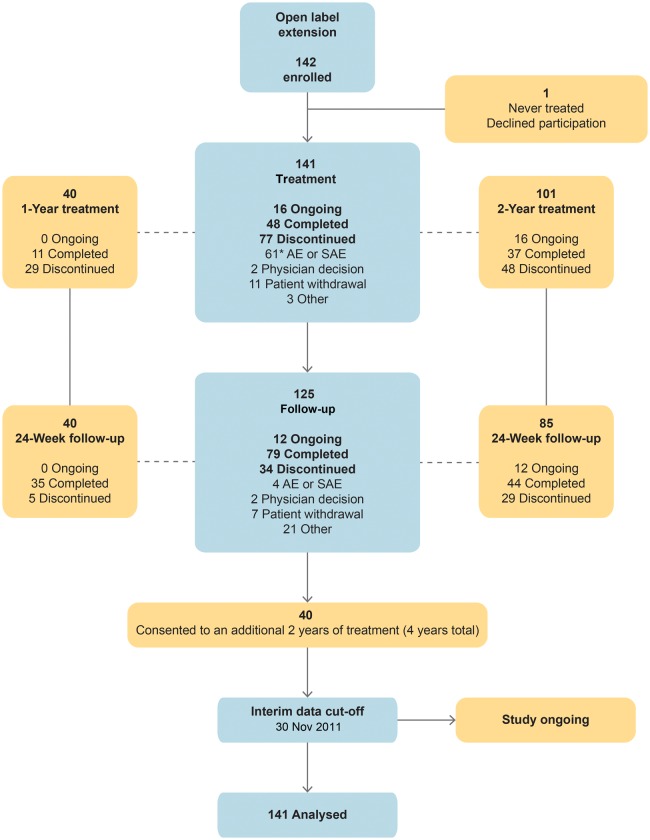
Upon completion of a Phase 3 index study, 141 patients (58 from the placebo and 83 from the mipomersen arms) were treated in this open-label extension study with median drug exposure 18.2 months (Figure 1). One patient declined participation and was not dosed. See the Supplementary material online, Table for demographic and baseline characteristics.
Figure 1.
Patient disposition. *One patient discontinued after completing the initial treatment period.
Efficacy
Efficacy results were available for 111 patients at 1 year and 53 patients at 2 years (Table 1).
Table 1.
Change from baseline for select lipoproteins
| Lipoprotein, mean (95% CI) | Baseline, n = 141 | Week 26, n = 130 | Week 52, n = 111 | Week 76, n = 66 | Week 104, n = 53 |
|---|---|---|---|---|---|
| LDL-C | 6.0 (3.8) | −28 (−32, −25) | −27 (−31, −23) | −27 (−33, −22) | −28 (−35, −22) |
| Completers | 4.8 (2.9) | −31 (−37, −24) | −29 (−36, −23) | −31 (−39, −23) | −29 (−35, −22) |
| ApoB | 1.8 (0.8) | −29 (−32, −26) | −28 (−32, −24) | −30 (−35, −26) | −31 (−37, −26) |
| Lp(a) | 0.6 (0.6) | −21 (−25, −17) | −16 (−23, −10) | −17 (−24, −10) | −14 (−22, −5) |
| Triglycerides | 1.3 (0.7) | −14 (−19, −9) | −7 (−13, −1) | −3 (−12, 6) | −12 (−21, −2) |
| Total cholesterol | 7.8 (3.8) | −22 (−24, −19) | −20 (−24, −17) | −20 (−25, −15) | −20 (−25, −15) |
| Non-HDL-C | 6.6 (3.9) | −27 (−30, −24) | −25 (−29, −21) | −25 (−30, −20) | −27 (−33, −21) |
| HDL-C | 1.2 (0.4) | +7 (3, 10) | +6 (2, 10) | +3 (−1, 8) | +10 (3, 17) |
Baseline values are the mean (SD) in mmol/L, except apoB and Lp(a) which are in g/L. Patients who remained in the study up to the November 2011 cut-off date are considered completers, although this trial is ongoing.
Low-density lipoprotein cholesterol
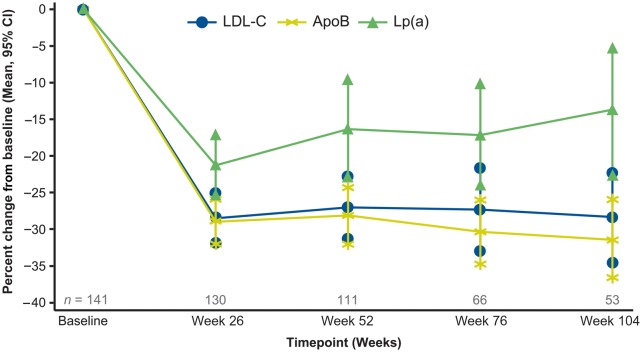
The mean changes in LDL-C from baseline to weeks 26 (n = 130), 52 (n = 111), 76 (n = 66), and 104 (n = 53) were −28, −27, −27, and −28%, respectively (Figure 2). If treatment was stopped, LDL-C gradually returned to near baseline over 24 weeks.
Figure 2.
Sustained reductions in LDL-C, ApoB, and Lp(a) with long-term mipomersen treatment.
ApoB
The mean changes in apoB from baseline to weeks 26, 52, 76, and 104 were −29, −28, −30, and −31%, respectively (P < 0.001).
Other lipids
Mean reductions in TC and non-HDL-C and median percent reductions in Lp(a) were observed throughout treatment, consistent with the mean reductions in LDL-C and apoB. Median changes in Lp(a) from baseline to weeks 26, 52, 76, and 104 were −21, −19, −18, and −17, respectively (P < 0.001). Mean increases in HDL-C from baseline at weeks 26, 52, 76, and 104 were 6% (P < 0.01), 6% (P < 0.05), 3%, and 10% (P < 0.01), respectively.
Pharmacokinetics
Most patients achieved steady-state plasma trough levels with no gender differences noted. The terminal-elimination plasma half-life ranged from 14.3 to 105 days, mean 43.8 (±24.3) and median 35.1 days.
Safety
Safety variables included AEs (including additional analyses of FLS and ISRs), clinical laboratory parameters (blood pressure and QTc measurements on the ECG), and liver MRI.
Drug exposure
Sixty-four patients either completed or remain on mipomersen; 40 patients consented to an additional 2-year extension for a total of 4 years. Mean treatment exposure was 18.8 months (median, 18.2 months). At the time of this interim analysis, including exposure to mipomersen in the Phase 3 studies, 17 (12%) patients had received treatment for 0 to 6 months; 27 (19%) for >6–12 months; 23 (16%) for >12–18 months; 25 (18%) for >18–24 months; and 49 (35%) for >24 months. The number of patients completing at least 1 year of treatment was 97 (69%); the number of patients completing at least 2 years of treatment was 49 (35%).
Adverse events
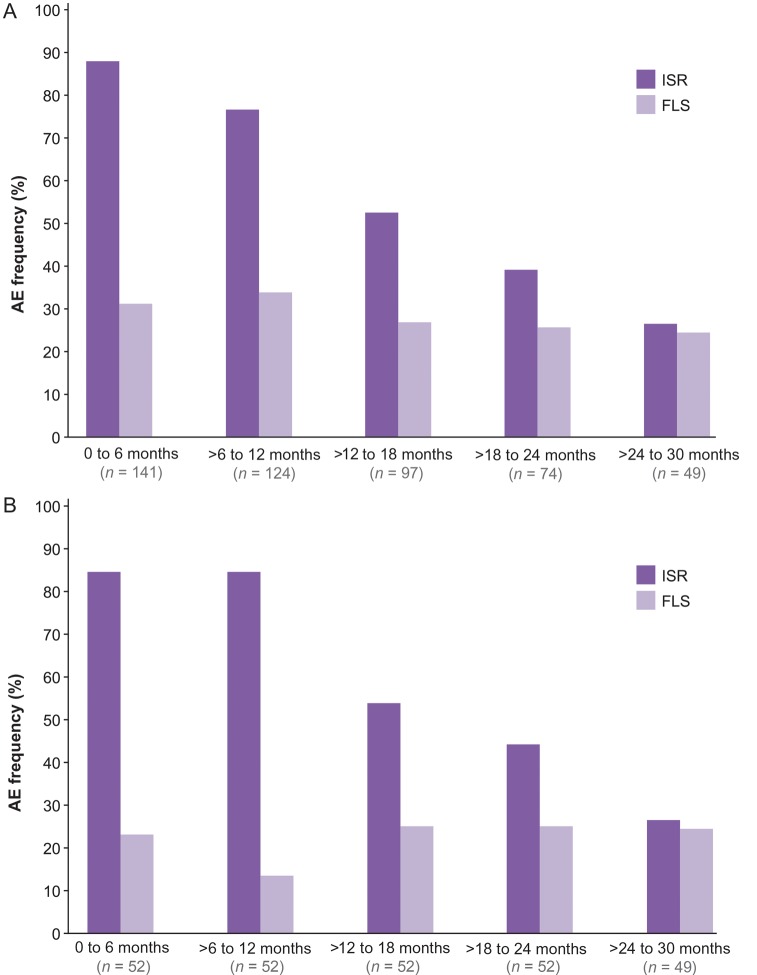
All patients had at least one treatment-emergent adverse event (TEAE); 138 (98%) experienced at least one ISR, and 92 (65%) had at least one episode of FLS (Table 2). Flu-like symptoms were the most frequent cause of drug discontinuation. Figure 3 displays the incidence of ISRs and FLS over 130 weeks; the frequency of ISRs progressively decreased by two-thirds after 24 months of follow-up. Serious AEs occurred in 33 (23%) patients; 4 were considered possibly drug related, which included membranous glomerulonephritis, atrial fibrillation, appendicitis, and biliary colic. The biliary colic resulted in treatment discontinuation.
Table 2.
On-treatment adverse event summary and ALT results
| Adverse event | n (%), N = 141 |
|---|---|
| Injection-site reactionsa | 138 (98) |
| Flu-like symptomsb | 92 (65) |
| Serious adverse eventsc | 33 (23) |
| SAEs not or unlikely drug-related | 29 (21) |
| Adverse events leading to dose adjustmentd | 41 (29) |
| Treatment emergent adverse events leading to discontinuatione | 62f (44) |
| General disorders and injection site conditionsg | 40 (28.4) |
| Abnormal laboratory resultsh | 10 (7.1) |
| GI disorders | 8 (5.7) |
| Nervous system disorders | 5 (3.5) |
| Hepatobiliary disordersi | 2 (1.4) |
| Psychiatric disorders | 2 (1.4) |
| Metabolism and nutrition disorders | 2 (1.4) |
| Cardiac disorders | 2 (1.4) |
| Eye disorders | 1 (0.7) |
| Immune system disorders | 1 (0.7) |
| Respiratory, thoracic, and mediastinal disorders | 1 (0.7) |
| Skin and subcutaneous tissue disorders | 1 (0.7) |
| Alanine aminotransferase levelsi | |
| ≥3xULN, two consecutive results ≥7 days apart | 18 (13) |
| ALT ≥3xULN with concomitant increases in total bilirubin ≥2xULN | 0 (0) |
aISRs included erythema, pain or discomfort, haematoma, discoloration, pruritus, swelling, induration, recall reaction, nodule, haemorrhage, oedema, warmth, rash, inflammation, uticaria, papule, macule, paresthaesia, vesicles, pallor, hypersensitivity, anaesthesia, hypertrophy, eczema, exfoliation, or lymphadenopathy; and were generally self-limiting and mild to moderate in severity.
bFlu-like symptoms included influenza-like illness, pyrexia, chills, myalgia, arthralgia, malaise, or fatigue that started within 2 days after an injection.
cSAEs included 1 death considered not drug-related.
dDose interruption or dose decrease.
eTreatment-emergent adverse events were adverse events with start dates/times on or after the date/time of the first study drug dose.
fOne patient discontinued after completing the initial treatment period.
gMost common were influenza-like illness and injection site reactions.
hMost common were ALT and AST abnormalities.
iNo Hy's law.
Figure 3.
Incidence of injection site reactions (ISRs) and flu-like symptom (FLS) events occurring at least once over time. (A) All patients. (B) Patients completing 2 years of treatment.
Discontinuations
Of the 141 patients treated, 77 (55%) discontinued treatment within the first 2 years of treatment; 61 (43%) due to TEAEs and 16 (11%) for reasons that were not AEs such as investigator drop-out, patient study fatigue, or starting LDL apheresis. Discontinuations varied among the sites (0–100%). Tolerability increases over time and can be enhanced with appropriate physician and patient education.
Laboratory values
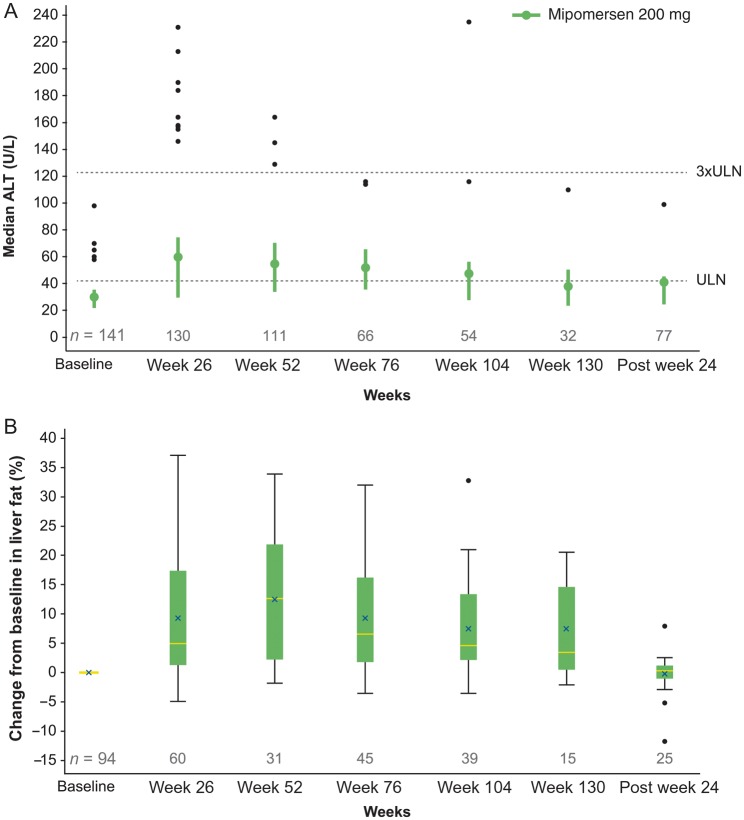
Eighteen of 141 patients (13%) had an ALT ≥3xULN on two consecutive occasions at least 7 days apart (Table 2). The time to the first occurrence of persistent ALT elevations was predominantly 6–12 months. Increases in transaminase levels were not accompanied by hyperbilirubinaemia, and specifically, there were no subjects who met Hy's law criteria.10 ALT and liver fat content increased initially, but trended towards baseline in many cases with extended dosing and in follow-up (Figure 4).
Figure 4.
Change from baseline in ALT levels and liver fat fraction over time. Values shown represent: (A) box plot of ALT levels (U/L); (B) box plot of change from baseline in liver fat fraction (%).
There were no notable changes in other biochemistry or haematology parameters. There was no change in renal function as determined by serum creatinine levels and calculated glomerular filtration rates. Protein in the urine (dipstick ≥2+) was found in nine (6.4%) patients and deemed sporadic and incidental in nature. There were no cases of progressive proteinuria. All other laboratory test results were unremarkable.
Liver magnetic resonance imaging
Twenty-seven of 65 (42%) patients with MRI assessments at baseline and 12 months or longer of treatment had minimal change (<5%) in liver fat content over time. On the other hand, 16 of 65 (25%) patients had an increase in liver fat content that exceeded 20% on at least one occasion. Of this subset of 16 patients, 6 had adverse events leading to dose discontinuation unrelated to their liver effects, consistent with the discontinuation rate in the overall study population. Of importance, repeat liver MRI 24 weeks after mipomersen discontinuation (n = 25) showed regression of liver fat towards baseline.
Measures of cardiac risk
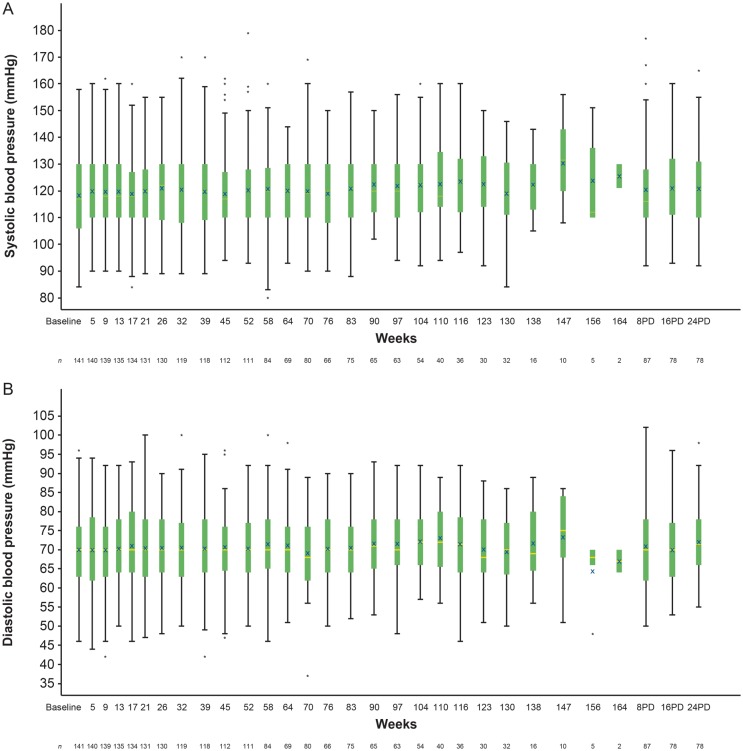
This study was not powered to evaluate any possible association of mipomersen with cardiovascular disease; however, there were no significant changes in systolic/diastolic blood pressures (Figure 5A and B) or QTc measurements (Figure 6).
Figure 5.
Evolution of systolic and diastolic blood pressure over time. The horizontal line in the middle of each box indicates the median, an X indicates the mean and the top and the bottom borders of the box mark the 75th and 25th percentiles, respectively. Whiskers are presented to the most extreme value within ±1.5 times the interquartile range. The data points above and below the whiskers are defined as outliers. BL, baseline, PD, post last dose.
Figure 6.
Evolution of corrected QT (QTc) intervals over time. The horizontal line in the middle of each box indicates the median, an X indicates the mean and the top and the bottom borders of the box mark the 75th and 25th percentiles, respectively. Whiskers are presented to the most extreme value within ±1.5 times the interquartile range. The data points above and below the whiskers are defined as outliers. BL, baseline; PD, post last dose.
Discussion
We report 2-year interim results of an ongoing, open-label study to evaluate the efficacy and safety of the apoB antisense oligonucleotide, mipomersen, administered to HoFH and HeFH patients in combination with maximally tolerated lipid-lowering therapy. The previously demonstrated5–8 favourable effects of mipomersen on atherogenic lipoproteins were maintained long term for both the intent-to-treat and 2-year completer data sets. Safety findings, including transaminase elevations and liver fat accumulation—both reversible with drug discontinuation—were similar to findings from the 26-week double-blind, placebo-controlled, randomized Phase 3 trials and Phase 2 trials that assessed changes in liver fat content by magnetic resonance spectroscopy or MRI.4,11 Injection site reactions were the most common AEs encountered; ISR and FLS were the most frequent reasons for drug discontinuation.
The sustained beneficial effects of mipomersen on LDL-C and other pro-atherogenic lipoproteins paralleled the 28–31% average fall in plasma apoB concentrations. In addition to these beneficial changes, there was a small reduction in plasma TG and increase in HDL-C concentrations.
FH is characterized by severely elevated LDL-C and high risk of early CHD and mortality. This risk is particularly high in HoFH where severely high LDL-C levels persist despite maximum pharmacologic therapy.12 Unfortunately, alternative treatments for HoFH, such as LDL-apheresis and liver transplantation, are not widely available, are expensive, and are associated with numerous co-morbidities.13 Similarly, a high risk for CHD and mortality exists for HeFH patients who have elevated LDL-C levels despite pharmacological treatment.14 This risk is highest for those who present with a previous cardiovascular event and for those who cannot tolerate statin treatment.4 Therefore, newer therapies to reduce plasma concentration of atherogenic lipoproteins are being developed, such as mipomersen and lomitapide (both now approved in the USA for the treatment of HoFH; mipomersen was not approved by the European Union).15 In addition, anti-PCSK9 antibodies are also being developed for this difficult to treat patient population.16 We await more evidence regarding the safety and efficacy of these newer therapies and future treatment recommendations from leaders in this field.
A recent meta-analysis of various statin regimens (n = 170 000) showed a risk reduction of 24% in the incidence of major vascular events for every 39 mg/dL (1 mmol/L) decrease in LDL-C,17 which suggests that the >60 mg/dL (1.5 mmol/L) mean decrease in LDL-C in this study may be associated with cardiovascular benefit. Additional studies are required to assess the potential cardiovascular benefits of treatment with mipomersen. The already high risk of cardiovascular events in patients with FH due to increased LDL-C levels may be significantly worsened in some of these patients by concomitant elevation of plasma Lp(a) concentrations. Lp(a), which is frequently elevated in HoFH and HeFH,18 is an independent risk factor for CHD, is both an atherothrombotic and inflammatory marker,19 and is minimally affected by statin treatment in HeFH and other populations.20 Our study shows that the previously reported reduction in plasma Lp(a) by mipomersen is sustained long term. Further mechanistic data are necessary to explain the effects of apoB antisense therapy on Lp(a) concentrations in humans; however, experiments in Lp(a) transgenic mice treated with mipomersen suggest that apoB-100 production is a key factor in the generation of Lp(a) particles.21 A favourable reduction in Lp(a) by mipomersen is a therapeutic distinction, not shared by approved lipid-lowering therapies other than niacin; effects of lomitapide on Lp(a) have been inconsistent.15 Finally, the small but significant changes in plasma TG levels may be a consequence of a reduction in VLDL production due to decreased apoB synthesis. The smaller content of available TG may also be the basis for the observed increase in HDL-C levels.
This interim report of a long-term extension trial with antisense oligonucleotide therapy in a large FH patient population evaluated the tolerability and safety of mipomersen. This open-label, non-blinded, extension design excludes placebo comparison and limits the complete evaluation of possible biases of patients towards adverse events. Only patients who completed Phase 3 studies were included making this a select population. Mipomersen provided an acceptable benefit to risk safety and tolerability profile that was similar to that reported in four placebo-controlled Phase 3 trials with 6-month safety data.5–8 However, safety data need to be interpreted with caution; bias may have been introduced into the study analysis results due to the high discontinuation rate.
The majority of patients experienced at least one ISR during the study. Injection site reactions were generally self-limiting and mild to moderate in severity. The incidence of ISR tended to decrease over time, potentially reflecting greater patient tolerance, increased patient experience, and understanding of subcutaneous administration, as well as greater physician experience in patient counselling of subcutaneous mipomersen administration.
Flu-like symptoms, the most common reason for drug discontinuation, were reported by 65% (92/141) of patients at least once. Unlike ISRs, the incidence of FLS remained relatively constant over time. Flu-like symptoms may be secondary to pro-inflammatory activation following mipomersen, but no significant changes in hs-C-reactive protein or complement levels were found during long-term exposure to mipomersen.
Inhibition of hepatic apoB synthesis by mipomersen reduces TG export from hepatocytes and may contribute to hepatic steatosis. Phase 3 studies have shown elevations of ALT and AST as well as increased liver fat (measured by MRI)9 in some patients. This extension study provides evidence that the transaminase elevations and hepatic steatosis do not progress during long-term treatment beyond 1 year, and there were no cases meeting Hy's law criteria to suggest potentially serious hepatocellular injury.10 It is also notable that many patients experienced no increase in liver fat with long-term mipomersen therapy (42% of patients experienced <5% increase in liver fat).
There was an incremental increase in the median liver fat during the initial 6–12 months that appeared to diminish with continued mipomersen exposure beyond 1 year and returned towards baseline 24 weeks after last drug dose. These results suggest that there may be liver adaptation to the reduction in TG export that attenuates hepatic fat accumulation. The clinical significance of hepatic steatosis in the context of reduced apoB production is unclear.22 Liver biopsies from mipomersen-treated patients in Phase 2/3 trials showed minimal signs of inflammation4 and resembled the histology seen in non-alcoholic fatty liver disease, rather than non-alcoholic steatohepatitis, which may lead to cirrhosis and liver-related death.23
Discontinuation rates were not different from those found in similar studies with subcutaneously injected drugs.24–26 Since MRI was not performed on all patients, additional data are needed to evaluate mipomersen-associated liver TG export and fat accumulation.
Long-term mipomersen use lowered LDL-C, apoB, Lp(a), non-HDL-C, TC, and TG (modest reduction) with a concurrent increase in HDL-C in FH in this non-placebo-controlled setting. Mipomersen had a safety and tolerability profile consistent with that seen in the randomized placebo-controlled Phase 3 studies. Longer term assessments of transaminase elevations and hepatic steatosis are needed, but the current data are reassuring. Forthcoming data from the 4-year time point will help substantiate these findings.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by Genzyme Corporation, a Sanofi company, Cambridge, MA. Funding to pay the Open Access publication charges for this article was provided by unrestricted grant from Genzyme a Sanofi Company.
Conflict of interest: R.D.S. is a consultant for Aegerion, AMGEM, Astra Zeneca, Biolab, Bristol Myers Squibb, Genzyme Corporation a Sanofi Company, Lilly, Merck, Novo-Nordisk Laboratories, Pfizer, and Renegeron. He has received speaker honoraria from Astra Zeneca, Bristol Myers Squibb, Novartis, Pfizer, Merck and Abbott. P.B.D. has been a speaker, consultant, or received grant funds from Merck, Genzyme, Pfizer, Bristol Meyers Squibb, Aegerion, Cerenis, and Vivus. C.E. is on the speaker's bureau for Boehringer-Ingelheim (Pradaxa) and is currently involved in research studies with Aastrom, Anthera Pharmaceuticals, Baxter, Baylor Health Care System Foundation, Boehringer-Ingelheim, Bristol-Myers Squibb, Genzyme, GlaxoSmithKline, National Institutes of Health, Novartis, Pfizer, Sanofi Aventis, and Yale University School of Medicine. J.R.G. consults for Merck, Sanofi-Aventis, and Kowa; is a speaker for Merck; owns stock in Eli Lilly; and has participated in a research capacity for Abbott, Amarin Pharma, Genzyme/Sanofi, GlaxoSmithKline, Merck, and Regeneron/Sanofi. P.M.M. consults for Genzyme and Regeneron, is on the speaker's bureau for Takeda and GlaxoSmithKline, and has participated in a research capacity for Abbott Laboratories, Amgen, Inc, Beijing Peking University, WBL Biotech Company, LTD, B. Braun, Genzyme, Kaneka Corporation, Kowa Pharmaceuticals America Inc, Lilly USA, LLC, Novartis Pharmaceuticals Corporation, Regeneron, and Sanofi-Aventis. W.C. and R.S.M. are full-time employees of Genzyme Corporation, the sponsoring company.
Supplementary Material
Acknowledgements
The authors wish to acknowledge Joanne M. Donovan former Vice President of Clinical Research at Genzyme for her contribution to the study design and protocol development. The authors also acknowledge the contributions of the investigators and site coordinators for their diligence in data acquisition. Barbara Rinehart, MS of ReSearch Pharmaceutical Sciences assisted with article preparation. Brenda Baker, Isis Pharmaceuticals, Inc. provided critical manuscript review.
References
- 1.Haddley K. Mipomersen sodium: a new option for the treatment of familial hypercholesterolemia. Drugs Today (Barc) 2011;47:891–901. doi: 10.1358/dot.2011.47.12.1722069. [DOI] [PubMed] [Google Scholar]
- 2.Bel DA, Hooper AJ, Burnett JR. Mipomersen, an antisense apolipoprotein B synthesis inhibitor. Expert Opin Investig Drugs. 2011;20:1–8. doi: 10.1517/13543784.2011.547471. [DOI] [PubMed] [Google Scholar]
- 3.Ricotta DN, Frishman W. Mipomersen: a safe and effective antisense therapy adjunct to statins in patients with hypercholesterolemia. Cardiol Rev. 2012;20:90–95. doi: 10.1097/CRD.0b013e31823424be. [DOI] [PubMed] [Google Scholar]
- 4.Visser ME, Wagener G, Baker BF, Geary RS, Donovan JM, Beuers UH, Nederveen AJ, Verheij J, Trip MD, Basart DC, Kastelein JJ, Stroes ES. Mipomersen, an apolipoprotein B synthesis inhibitor, lowers low-density lipoprotein cholesterol in high-risk statin-intolerant patients: a randomized, double-blind, placebo-controlled trial. Eur Heart J. 2012;33:1142–1149. doi: 10.1093/eurheartj/ehs023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raal FJ, Santos RD, Blom DJ, Marais AD, Charng MJ, Cromwell WC, Lachmann RH, Gaudet D, Tan JL, Chasan-Taber S, Tribble DL, Flaim JD, Crooke ST. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:998–1006. doi: 10.1016/S0140-6736(10)60284-X. [DOI] [PubMed] [Google Scholar]
- 6.Stein EA, Dufor R, Gagne C, Gaudet D, East C, Donovan JM, Chin W, Tribble DL, McGowan M. Apolipoprotein B synthesis inhibition with mipomersen in heterozygous familial hypercholesterolemia: results of a randomized, double-blind, placebo controlled trial to assess efficacy and safety as add-on therapy in patients with coronary artery disease. Circulation. 2012;126:2283–2292. doi: 10.1161/CIRCULATIONAHA.112.104125. [DOI] [PubMed] [Google Scholar]
- 7.McGowan M, Tardif JC, Ceska R, Burgess LJ, Soran H, Gouni-Berthold I, Wagener G, Chasan-Taber S. Randomized, placebo-controlled trial of mipomersen in patients with severe hypercholesterolemia receiving maximally tolerated lipid-lowering therapy. PLoS ONE. 2012;7:e49006. doi: 10.1371/journal.pone.0049006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas GS, Cromwell WC, Ali S, Chin W, Flaim JD, Davidson M. Mipomersen, an apolipoprotein B synthesis inhibitor, reduces atherogenic lipoproteins in patients with severe hypercholesterolemia at high cardiovascular risk: a randomized, double-blind, placebo-controlled trial. JACC. 2013 doi: 10.1016/j.jacc.2013.07.081. [DOI] [PubMed] [Google Scholar]
- 9.Yokoo T, Bydder M, Hamilton G, Middleton MS, Gamst AC, Wolfson T, Hassanein T, Patton HM, Lavine JE, Schwimmer JB, Sirlin CB. Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5T. Radiology. 2009;251:67–76. doi: 10.1148/radiol.2511080666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Department of Health and Human Services Food and Drug Administration. Guidance for Industry. Drug-induced liver injury: premarketing clinical evaluation. 2009 http://www.fdanews.com/ext/files/UCM174090.pdf (Accessed 5 September 2012) [Google Scholar]
- 11.Visser ME, Akdim F, Tribble DL, Nederveen AJ, Kwoh TJ, Kastelein JJ, Trip MD, Stroes ES. Effect of apolipoprotein-B synthesis inhibition on liver triglyceride content in patients with familial hypercholesterolemia. J Lipid Res. 2010;51:1057–1062. doi: 10.1194/jlr.M002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raal FJ, Pilcher GJ, Panz VR, van Deventer HE, Brice BC, Blom DJ, Marais AD. Reduction in mortality in subjects with homozygous familial hypercholesterolemia associated with advances in lipid-lowering therapy. Circulation. 2011;124:2202–2207. doi: 10.1161/CIRCULATIONAHA.111.042523. [DOI] [PubMed] [Google Scholar]
- 13.Nemati MH, Astaneh B. Optimal management of familial hypercholesterolemia: treatment and management strategies. Vasc Health Risk Manag. 2010;6:1079–1088. doi: 10.2147/VHRM.S8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mortality in treated heterozygous familial hypercholesterolaemia: implications for clinical management. Scientific Steering Committee on behalf of the Simon Broome Register Group. Atherosclerosis. 1999;142:105–112. [PubMed] [Google Scholar]
- 15.Cuchel M, Meagher EA, du Toit Theron H, Blom DJ, Marais AD, Hegele RA, Averna MR, Sirtori CR, Shah PK, Gaudet D, Stefanutti C, Vigna GB, Du Plessis AM, Propert KJ, Sasiela WJ, Bloedon LT, Rader DJ Phase 3 HoFH Lomitapide Study investigators. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet. 2013;381:40–46. doi: 10.1016/S0140-6736(12)61731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, Wu R, Pordy R. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380:29–36. doi: 10.1016/S0140-6736(12)60771-5. [DOI] [PubMed] [Google Scholar]
- 17.Cholesterol Treatment Trialist's Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seed M, Hoppichler F, Reaveley D, McCarthy S, Thompson GR, Boerwinkle E, Utermann G. Relation of serum lipoprotein(a) concentration and apolipoprotein(a) phenotype to coronary heart disease in patients with familial hypercholesterolemia. N Engl J Med. 1990;322:1494–1499. doi: 10.1056/NEJM199005243222104. [DOI] [PubMed] [Google Scholar]
- 19.Tsimikas S, Hall JL. Lipoprotein(a) as a potential causal genetic risk factor of cardiovascular disease: a rationale for increased efforts to understand its pathophysiology and develop targeted therapies. J Am Coll Cardiol. 2012;60:716–721. doi: 10.1016/j.jacc.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 20.Nordestgaard BG, Chapman MJ, Ray K, Borén J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps O, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Reiner Z, Taskinen MR, Tokgozoglu L, Hansen AT European Atherosclerosis Society Consensus Panel. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merki E, Graham MJ, Mullick AE, Miller ER, Crooke RM, Pitas RE, Witztum JL, Tsimikas S. Antisense oligonucleotide directed to human apolipoprotein B-100 reduces lipoprotein(a) levels and oxidized phospholipids on human apolipoprotein B-100 particles in lipoprotein(a) transgenic mice. Circulation. 2008;118:743–753. doi: 10.1161/CIRCULATIONAHA.108.786822. [DOI] [PubMed] [Google Scholar]
- 22.Tarugi P, Averna M. Hypobetalipoproteinemia: genetics, biochemistry, and clinical spectrum. Adv Clin Chem. 2011;54:81–107. [PubMed] [Google Scholar]
- 23.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterol. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 24.Fleischmann RM, Tesser J, Schiff MH, Schechtman J, Burmester GR, Bennett R, Modafferi D, Zhou L, Bell D, Appleton B. Safety of extended treatment with anakinra in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:1006–1012. doi: 10.1136/ard.2005.048371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheffield CA, Kane MP, Busch RS, Bakst G, Abelseth JM, Hamilton RA. Safety and efficacy of exenatide in combination with insulin in patients with type 2 diabetes mellitus. Endocr Pract. 2008;14:285–292. doi: 10.4158/EP.14.3.285. [DOI] [PubMed] [Google Scholar]
- 26.Hetland ML, Christensen IJ, Tarp U, Dreyer L, Hansen A, Hansen IT, Kollerup G, Linde L, Lindegaard HM, Poulsen UE, Schlemmer A, Jensen DV, Jensen S, Hostenkamp G, Østergaard M All Departments of Rheumatology in Denmark. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62:22–32. doi: 10.1002/art.27227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.