Abstract
Aim
The metabolically healthy obese (MHO) phenotype refers to obese individuals with a favourable metabolic profile. Its prognostic value is unclear and may depend on the health outcome being examined. We examined the association of MHO phenotype with incident cardiovascular disease (CVD) and type 2 diabetes.
Methods and results
Body mass index and metabolic health, assessed using the Adult Treatment Panel-III (ATP-III) criteria, were assessed on 7122 participants (69.7% men) from the Whitehall II study, aged 39–63 years in 1991–93. Incident CVD (coronary heart disease or stroke) and type 2 diabetes were ascertained from medical screenings (every 5 years), hospital data, and registry linkage until 2009. A total of 657 individuals (9.2% of the cohort) were obese and 42.5% of these were classified as MHO in 1991–93. Over the median follow-up of 17.4 years, there were 828 incident cases of CVD and 798 incident cases of type 2 diabetes. Compared with metabolically healthy normal weight individuals, MHO subjects were at increased risk for CVD (HR = 1.97, 95% CI: 1.38–2.80) and type 2 diabetes (3.25, 95% CI: 2.32–4.54). There was excess risk in metabolically unhealthy obese compared with MHO for type 2 diabetes (1.98, 95% CI: 1.39–2.83) but not CVD (1.23, 95% CI: 0.81–1.87). Treating all measures as time varying covariates produced similar findings.
Conclusion
For type 2 diabetes, the MHO phenotype is associated with lower risk than the metabolically unhealthy obese, but for CVD the risk is as elevated in both obesity phenotypes.
Keywords: Cardiovascular diseases, Metabolically healthy obesity, Type 2 diabetes
Introduction
Obesity1 and metabolic syndrome (MetS)2,3 are major public health problems worldwide which frequently co-exist and define obese individuals ‘at-risk’ of adverse health outcomes. Recent studies have identified a subset of obese individuals who have a low burden of adiposity-related metabolic abnormalities compared with ‘at risk’ obese individuals, the so-called ‘metabolically healthy obese’ (MHO) phenotype.4–8 Depending on the definition used for metabolic status, a total of 10–40% of obese individuals9,10 have the MHO phenotype with a good metabolic profile characterized by high levels of insulin sensitivity, low prevalence of hypertension, and a favourable fasting glucose, lipid, and inflammation profile.10
The extent to which the MHO phenotype is associated with a lower risk of adverse health outcomes remains the subject of debate. Some studies show this to be the case11,12 particularly, compared with at-risk obese, whereas other studies show a higher risk for mortality9,13,14 and cardiovascular disease (CVD)11,15,16 in MHO compared with metabolically healthy normal weight (MH-NW) subjects. Overall, the evidence is inconsistent, and the extant research has several limitations. One, the manner in which metabolic health is defined might be critically important. We previously used five possible definitions to show a better prognosis in MHO compared with ‘at risk’ obese only when metabolic health was defined using insulin resistance, for all other definitions, including the widely used Adult Treatment Panel-III (ATP-III) definition, the risk of mortality was similar in all obese individuals.9 However, another report, albeit with a shorter follow-up, showed MHO phenotype to be associated with a lower risk of CVD even using the ATP-III definition.11 Two, it is important to consider potential effect modifications as a function of the length of follow-up as every 2 obese-years has been shown to increase mortality risk by 6%.17 Thus, longer follow-ups may better assess the risk associated with obesity, irrespective of metabolic health status. Three, it is possible that the specific health outcome examined plays a role. Outcomes such as type 2 diabetes are closely tied to parameters used to define metabolic health making it likely that the MHO phenotype carries less risk for type 2 diabetes than CVD.
To better understand the role of MHO as a risk factor for diseases with major public health relevance, we examined the association of the MHO phenotype, defined using the ATP-III criteria, with incident CVD and type 2 diabetes, over a 17-year follow-up period.
Methods
Study population
Data were drawn from the Whitehall II study, an on-going prospective cohort study established in 1985 on 10 308 London-based office staff (6895 males and 3413 females). Details of the cohort and its follow-up have been described previously.18 Briefly, all office staff aged 35–55 years in 20 civil service departments in London, UK, were invited to participate. In total, 73% of those invited agreed to participate at baseline (1985–88) which consisted of a clinical examination and a self-administered questionnaire. Subsequent waves of clinical data collection took place in 1991–93, 1997–99, 2002–04, and 2007–09. Metabolic health was first assessed in 1991–93, the baseline for the present analysis, and repeated in 1997–99 and 2002–04. All the participants provided written consent and the University College London ethics committee approved the study.
Baseline examination (1991–93)
Body mass index
Weight was measured in underwear to the nearest 0.1 kg on digital Soehnle electronic scales (Leifheit AS, Nassau, Germany). With the participant standing erect in bare feet with head in the Frankfurt plane, height was measured to the nearest 1 mm using a stadiometer. Reproducibility of the weight and height measurements over 1 month [correlation coefficient = between-subject variability/total (between + within subject) variability], undertaken on 331 participants, was 0.99 for both weight and height. Body mass index (BMI) was calculated by dividing weight (in kg) by height (in m2) and categorized using the WHO classification:19 <18.5 kg/m2 (underweight), 18.5–24.9 kg/m2(standard weight), 25–29.9 kg/m2 (overweight) ≥30 kg/m2 (obese), with the under 18.5 category (n = 80) removed from the analysis.
Metabolic factors
We used standard operating protocols to measure ATP-III components to define metabolic status.2 Blood pressure was measured twice in the sitting position after 5 min of rest with a Hawksley random-zero sphygmomanometer (Lynjay services Ltd, Worthing, UK). The average of the two readings was taken to be the measured blood pressure. The venous blood was taken in the fasting state or at least 5 h after a light, fat-free breakfast before undergoing a 2-h 75-g oral glucose tolerance test (OGTT). Serum for lipid analyses was refrigerated at −4°C and assayed within 72 h. High-density lipoprotein-cholesterol (HDL-c) was measured by precipitating non-HDL-cholesterol with dextran sulfate-magnesium chloride using a centrifuge and measuring cholesterol in the supernatant. Serum triglyceride was determined by the enzymatic colorimetric method (glycerol-3-phosphate oxidase/phenol and aminophenazone). Blood glucose was measured using the glucose oxidase method20 (YSI MODEL 2300 STAT PLUS Analyzer, YSI Corporation, Yellow Springs, OH, USA) (mean coefficient of variation: 2.9–3.3%). Data on names of medications provided by the participants were coded using the British National Formulary to identify anti-hypertensive and lipid-lowering drugs, and medication for diabetes.
Participants who met <2 of the following four criteria were considered metabolically healthy: high triglycerides (≥1.7 mmol/L) or lipid-lowering drugs, elevated systolic blood pressure (≥130 mmHg) or diastolic blood pressure (≥85 mmHg) or anti-hypertensive drugs, high fasting glucose (≥5.6 mmol/L) or medications for diabetes (insulin and oral anti-diabetic) and low HDL-cholesterol (<1.04 mmol/L for men and <1.29 mmol/L for women). The waist circumference criterion was not used because of collinearity with BMI.
We used this definition alongside data on BMI to create six phenotypes: MH-NW, metabolically healthy overweight, MHO, MU-NW, metabolically unhealthy overweight (MU-OW) and metabolically unhealthy obese (MUO).
Covariates
Our analyses were adjusted for socio-demographic variables, behavioural factors and medications, and procedures related to CVD morbidities. Socio-demographic variables included age, sex, ethnicity (white vs. non-white), marital status (single, married/cohabiting, divorced, widowed) and occupational position, a three level variable representing high (administrative), intermediate (professional or executive), and low (clerical or support) grades. This measure is a comprehensive marker of socioeconomic status (SES) and is related to salary, social status, and level of responsibility at work. Behavioural factors included smoking status (never, current, or ex-smoker), alcohol intake [assessed via questions on the number of alcoholic drinks consumed in the last 7 days and categorized as: no alcohol (none or <1 unit/week), moderate drinkers (1–14 units/week in women and 1–21 units/week in men), heavy drinkers (>14 units in women and >21 units in men)], physical activity (categorized as ‘active’ ≥2.5 h/weeks of moderate or ≥1 h/week of vigorous physical activity, ‘inactive’ <1 h/week of moderate and <1 h/week of vigorous physical activity or ‘moderately active’ if not active or inactive), and consumption of fruits and vegetables (assessed using the question ‘how often do you eat fresh fruit or vegetable?’ and response were on an two-point scale: ≥1 fruit or vegetable/day or < 1 fruit or vegetable/day. Cardiovascular disease medication included nitrates and anti-platelet; procedures consisted of graft replacement of coronary artery, anastomosis of arteries, angioplasty of coronary artery, drugs eluting-stents. Missing data on covariates in 1991–93 (<1%) and at one of the follow-ups (<5%) were replaced with data from the phase immediately before or after that phase.
Covariates over the follow-up, (1997–99, 2002–04, and 2007–09)
All covariates, including BMI and metabolic health, were assessed repeatedly over the follow-up. These measures were used as time varying covariates in the sensitivity analysis in order to assess whether changes in these factors over the course of the follow-up influenced the results.
Outcome ascertainment
Incidence of CVD and type 2 diabetes was assessed over the follow-up from 1991–93 to 2009, a median follow-up of 17.5 years.
Cardiovascular disease
Cardiovascular disease events included fatal CHD [defined by the International Classification of Diseases (ICD) 9 codes 410 to 414 or ICD 10 codes I20 to 25], first non-fatal myocardial infarction (MI), first definite angina, and stroke. Fatal CHD was assessed by flagging participants at the National Health Service (NHS) Central Registry, which provided information on the date and cause of death. Non-fatal MI was determined using data from questionnaires, study electrocardiograms (ECGs), hospital acute ECGs, cardiac enzymes, and physician records following multinational monitoring of trends and determinants in CVD criteria.21 Definite angina was assessed based on the participant's reports of symptoms22 with corroboration in medical records for nitrate medication use or abnormalities on arresting ECG, an exercise ECG or coronary angiogram. We excluded angina cases that were based solely on self-reported data. Stroke included first subarachnoid haemorrhage, intracerebral haemorrhage, cerebral infarction, and not specified stroke (ICD-10 codes I60–I64), and transient cerebral ischaemic attacks (ICD-10 codes G45). The cases were ascertained from participants' general practitioners, information extracted from hospital medical records by study nurses, or data from the NHS Hospital Episode Statistics (ES) database obtained after linking the participants' unique NHS identification numbers to this national database. Self-reported stroke cases without clinical verification were excluded.
Type 2 diabetes
In 1991–93, after the initial venous blood sample, an OGTT (75 g anhydrous glucose) was administered after an overnight fast or in the afternoon after no more than a light fat-free breakfast eaten before 08:00 h. A second blood sample was taken 2 h later. Subsequent clinical assessments for type 2 diabetes took place in 1997–99, 2002–04, and 2007–09. The definition of diabetes used was a 2-h glucose tolerance test finding of at least 200 mg/dL (≥11.1 mmol/L) or, if the 2-h post-load value was missing, a fasting glucose level of 126 mg/dL (≥7.0 mmol/L) or physician-diagnosed diabetes and/or use of diabetes medication. The date of diagnosis of diabetes was assigned according to the interval method as the midpoint between the first visit with a diagnosis of diabetes and the last visit without diabetes.23
Statistical analysis
The characteristics of the sample are presented as mean ± SD or percentage when appropriate, by metabolic status and as a function of BMI categories. We used ANOVA to test for differences between groups in baseline characteristics for continuous variables and the χ2 test for dichotomous measures.
Participants were followed until whichever event occurred first: death, incident disease (CVD/type 2 diabetes) or the end of follow-up (2009). We used logistic regression to examine the extent to which BMI and metabolic status influenced attrition in the study sample over the follow-up. The Cox proportional-hazard regression model with age as the timescale was used to examine the relationship between BMI categories, ATP-III components, metabolic status, the six phenotypes described above and first CVD and then type 2 diabetes. The assumption of proportional hazards was checked by examining the interaction term between the BMI-metabolic status phenotypes and logarithm of the follow-up time. For the CVD outcome, it was P = 0.10 and for type 2 diabetes it was P = 0.39, supporting the proportional hazards assumption. The interaction term between ethnicity (P for interaction >0.60) and sex (P > 0.36) and BMI-metabolic status phenotypes revealed no differences, allowing us to combine them in the analysis.
In the first set of analyses, we used the MH-NW persons as the referent category. We first present survival curves with age as the timescale using Kaplan–Meier survival functions in order to enable visual inspection of trends. Hazard ratio (HR) and 95% confidence intervals (95% CI) were first adjusted for sex, occupational position, marital status and ethnicity and then further for smoking status, alcohol intake, physical activity, fruit and vegetable consumption, CVD medication and procedures. We then ran a second set of analyses, stratified by BMI category to allow us to compare the risk of CVD and type 2 diabetes as a function of metabolic health status in each category. The metabolically healthy group, within each BMI category, was the reference in these analyses.
Sensitivity analyses
We undertook a series of analyses to assess the robustness of our findings. One, we excluded angina from the definition of CVD to examine whether the association between BMI-metabolic status and CVD outcome was similar when CVD was defined using MI, stroke, and CVD death. Two, we re-ran the analysis for the CVD outcome with CHD and stroke in separate analyses to ensure that the results were consistent across both outcomes. Three, we repeated the Cox proportional-hazard regression used in the main analysis, but this time with BMI-metabolic phenotypes and all other measures (including CVD medication and procedures) over the follow-up treated as time varying covariates to assess the impact of changes over time in these measures on the associations with CVD and type 2 diabetes. Of the 7122 participants with data in 1991–93, 5447 had complete data at all subsequent waves of data collection prior to being censored on the date of the CVD/type 2 diabetes or at end of follow-up in 2009. The remaining 1675 participants were censored at the last date at which they had complete data. Four, in order to examine whether changes in metabolic health affected risk of CVD and type 2 diabetes, we created two time windows: one between the 1991–93 and 1997–99 clinical assessments for change in the exposure, the other between 1997–99 and 2007–09 for outcome ascertainment. This analysis was restricted to the metabolically unhealthy and obese or overweight in 1991–93 in order to compare risk of CVD and type 2 diabetes between those who remained metabolically unhealthy and those who became metabolically healthy between 1991–93 and 1997–99. We also examined whether weight loss in this group affected risk of CVD and type 2 diabetes by comparing risk of CVD and type 2 diabetes between those who remained overweight or obese and those who became normal weight. Analyses were undertaken using STATA 11 (StataCorp. College Station, TX, USA). Reported P-values are two-tailed and P-values <0.05 were considered to be statistically significant.
Results
Of the 8815 participants who took part in the 1991–93 examination (the baseline of the analyses reported here), 1693 were excluded for one or more of the following reasons at baseline: BMI < 18.5 kg/m2 (n = 80), missing data on BMI-metabolic status phenotype (n = 995), prevalent type 2 diabetes (n = 262), or prevalent CVD (n = 296); a further 588 persons were excluded because of missing data on type 2 diabetes status over the follow-up. The final sample consisted of 7122 participants. Compared with those excluded, participants included in the analyses were younger (49.3 vs. 51.6 years), more likely to be men (69.7 vs. 64.4%), married or cohabiting (76.8 vs. 73.0%), physically active (44.7 vs. 41.1%), and to have a higher occupational position (39.5 vs. 30.6%), all P < 0.01.
Compared with normal weight persons, overweight (1.19, 95% CI: 1.03, 1.37) and obese (1.29, 95% CI: 1.03, 1.62) persons had a higher odds-ratio of non-participation at the end of the follow-up. This was not the case for poor metabolic status (OR = 1.11, 95% CI: 0.96, 1.28). Crucially, the interaction term between BMI and metabolic status (P = 0.65) suggested that non-participation was not significantly different in the six phenotypes defined by BMI and metabolic health.
Table 1 shows baseline characteristics of the participants included in the analysis by metabolic status and BMI categories; 70.4% (n = 5015) of the participants were metabolically healthy and 9.2% (n = 657) were obese. The MHO phenotype represented 3.9% (n = 279) of the total analytic sample and 42.5% of the obese population. They were more often women and had better health behaviours in terms of smoking, alcohol, and fruit and vegetables consumption compared with MUO. Triglycerides, LDL-cholesterol, fasting glucose, and blood pressure were higher in metabolically unhealthy persons, while HDL-cholesterol was higher in MHO. During a median follow-up of 17.4 years, 828 incident CVD (4.5% in the MHO and 8% in the MUO) and 798 type 2 diabetes (6.0% in the MHO and 16.0% in the MUO) events occurred; 58.2% of CVD and 42.1% of type 2 diabetes cases occurred in the first 10 years of follow-up.
Table 1.
Sample characteristics at baseline (1991–93) as a function of metabolic health status and body mass index
| Metabolically healthy (n = 5015) |
Metabolically unhealthy (n = 2107) |
|||||
|---|---|---|---|---|---|---|
| Normal weight | Overweight | Obese | Normal weight | Overweight | Obese | |
| n (%) | 3100 (43.5) | 1636 (23.0) | 279 (3.9) | 649 (9.1) | 1080 (15.2) | 378 (5.3) |
| Male, % | 67.1 | 66.4 | 34.8 | 86.3 | 84.1 | 62.4 |
| Age, years | 48.6 (6.0) | 49.4 (5.9) | 49.7 (5.9) | 50.1 (6.0) | 50.5 (6.0) | 49.9 (5.8) |
| White, % | 93.1 | 90.2 | 82.4 | 89.7 | 91.7 | 91.0 |
| Married/cohabiting, % | 75.2 | 78.2 | 67.7 | 79.8 | 80.4 | 74.6 |
| High SES, % | 42.5 | 35.6 | 25.2 | 45.4 | 39.5 | 33.2 |
| Never smokers, % | 53.8 | 50.4 | 49.6 | 49.2 | 46.1 | 40.3 |
| No alcohol, % | 17.8 | 17.4 | 31.5 | 20.0 | 15.8 | 24.1 |
| Physically active, % | 45.7 | 43.6 | 34.1 | 48.2 | 45.1 | 41.0 |
| ≥1 fruit and vegetable/day, % | 64.7 | 60.4 | 65.2 | 59.8 | 56.3 | 54.4 |
| Triglycerides, mmol/L | 1.0 (0.4) | 1.2 (0.5) | 1.2 (0.5) | 2.1 (1.4) | 2.3 (1.4) | 2.4 (1.6) |
| HDL-cholesterol, mmol/L | 1.6 (0.4) | 1.5 (0.3) | 1.5 (0.3) | 1.2 (0.4) | 1.2 (0.3) | 1.2 (0.3) |
| LDL-cholesterol, mmol/L | 4.2 (0.9) | 4.5 (1.0) | 4.3 (1.1) | 4.6 (1.0) | 4.7 (1.1) | 4.6 (1.0) |
| Fasting glucose, mmol/L | 5.1 (0.4) | 5.1 (0.4) | 5.0 (0.4) | 5.5 (0.5) | 5.5 (0.5) | 5.5 (0.5) |
| Systolic blood pressure, mmHg | 115.6 (12.0) | 118.6 (11.3) | 121.8 (13.4) | 127.7 (14.4) | 128.4 (12.8) | 130.8 (12.3) |
| Diastolic blood pressure, mmHg | 75.9 (8.4) | 78.9 (8.1) | 81.0 (9.1) | 83.9 (8.9) | 85.9 (8.5) | 87.2 (8.6) |
Values are mean (SD) unless otherwise indicated.
SES, socioeconomic status.
Table 2 presents the associations of BMI categories, ATP-III components, and metabolic status with incident CVD and type 2 diabetes. Compared with normal weight individuals, both the overweight (HR = 1.36, 95% CI: 1.17, 1.57) and obese (HR = 1.89, 95% CI: 1.51, 2.37) had an increased risk of incident CVD; the same was true for type 2 diabetes, albeit the associations were stronger. All components of the ATP-III criteria were associated with incident CVD and type 2 diabetes, blood pressure being most strongly associated with CVD and fasting glucose most strongly with type 2 diabetes. Compared with metabolically healthy individuals (irrespective of obesity), metabolically unhealthy individuals had an increased risk of incident CVD (HR = 1.97, 95% CI: 1.72, 2.27) and type 2 diabetes (HR = 3.22, 95% CI: 2.79, 3.73).
Table 2.
The association of body mass index, Adult Treatment Panel-III components and metabolic status (1991–93) with cardiovascular disease and type 2 diabetes over the follow-up (until 2007–09)
| CVD HR (95% CI) (n = 828) | Type 2 diabetes HR (95% CI) (n = 798) | |
|---|---|---|
| Body mass index | ||
| Normal weight | 1.0 (ref) | 1.0 |
| Overweight | 1.36 (1.17–1.57) | 1.75 (1.49–2.05) |
| Obese | 1.89 (1.51–2.37) | 3.90 (3.19–4.76) |
| ATP-III components | ||
| Blood pressure ≥130/85 mmHg or medication | 1.74 (1.51–2.00) | 1.60 (1.39–1.85) |
| Triglycerides ≥1.7 mmol/L or medication | 1.66 (1.44–1.91) | 2.51 (2.17–2.90) |
| HDL-cholesterol <1.03 (<1.29) mmol/L (women) | 1.68 (1.44–1.97) | 2.15 (1.84–2.51) |
| Fasting glucose ≥5.6 mmol/L or medication | 1.25 (1.06–1.47) | 2.64 (2.27–3.06) |
| Metabolic status (defined using ATP-III criteria) | ||
| Healthy | 1.0 | 1.0 |
| Unhealthy | 1.97 (1.72–2.27) | 3.22 (2.79–3.73) |
Analyses adjusted for sex, socioeconomic status, marital status, ethnicity physical activity, smoking, alcohol, fruits and vegetables consumption, CVD medication and procedures.
HR, hazard ratio; CI, confidence interval.
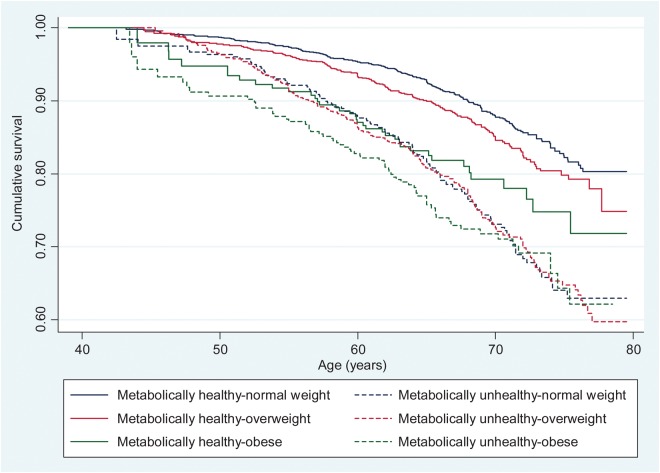
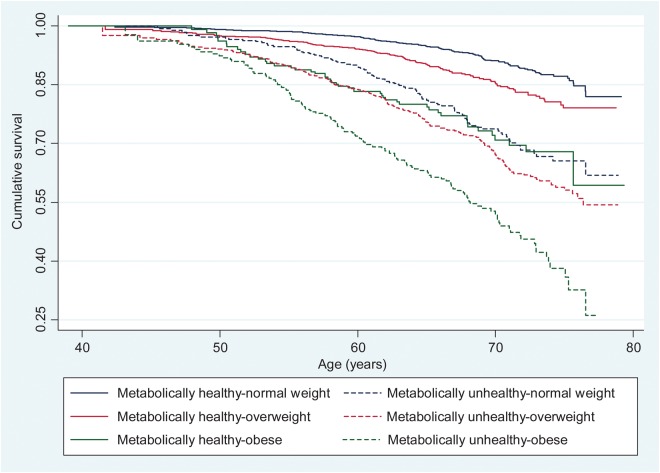
The Kaplan–Meier survival curves for cumulative survival free from, respectively, incident CVD and type 2 diabetes as a function of BMI-metabolic status phenotypes are shown in Figures 1 and 2. These survival curves differ significantly from each other over the follow-up (log-rank test, P < 0.001). The HR and CI of the association between the BMI-metabolic status phenotypes and incident CVD and type 2 diabetes are shown in Table 3; Supplementary material online, Figure S1 summarizes these findings. Compared with the MH-NW group, the five other groups were at an increased risk of incident CVD and type 2 diabetes with the associations being consistently stronger for type 2 diabetes.
Figure 1.
Kaplan–Meier survival curves showing the association between body mass index-metabolic status phenotypes and cardiovascular disease events.
Figure 2.
Kaplan–Meier survival curves showing the association between body mass index-metabolic status phenotypes and type 2 diabetes.
Table 3.
The association of body mass index -metabolic health status (1991–93) with cardiovascular disease and type 2 diabetes at the end of follow-up (2007–09)
| Events/n | Ratea | Model 1 HR (95% CI) | Model 2 HR (95% CI) | |
|---|---|---|---|---|
| CVD | ||||
| Metabolically healthy normal weight | 239/3100 | 4.81 | 1.0 (ref) | 1.0 |
| Metabolically healthy overweight | 164/1636 | 6.38 | 1.24 (1.01–1.51) | 1.24 (1.01–1.51) |
| Metabolically healthy obese | 37/279 | 8.93 | 1.99 (1.40–2.83) | 1.95 (1.37–2.77) |
| Metabolically unhealthy normal weight | 116/649 | 12.20 | 2.14 (1.71–2.68) | 2.08 (1.66–2.60) |
| Metabolically unhealthy overweight | 206/1080 | 13.00 | 2.25 (1.86–2.72) | 2.23 (1.84–2.70) |
| Metabolically unhealthy obese | 66/378 | 11.95 | 2.49 (1.89–3.27) | 2.44 (1.85–3.21) |
| Type 2 diabetes | ||||
| Metabolically healthy normal weight | 154/3100 | 3.37 | 1.0 (ref) | 1.0 |
| Metabolically healthy overweight | 136/1636 | 5.75 | 1.57 (1.24–1.98) | 1.56 (1.24–1.97) |
| Metabolically healthy obese | 48/279 | 12.96 | 3.21 (2.29–4.49) | 3.22 (2.30–4.51) |
| Metabolically unhealthy normal weight | 110/649 | 12.46 | 3.24 (2.52–4.15) | 3.20 (2.49–4.10) |
| Metabolically unhealthy overweight | 222/1080 | 15.29 | 3.96 (3.21–4.89) | 3.90 (3.16–4.82) |
| Metabolically unhealthy obese | 128/378 | 26.58 | 7.12 (5.60–9.06) | 6.92 (5.43–8.81) |
Model 1 is adjusted for sex, socioeconomic status, marital status, and ethnicity.
Model 2 = Model 1 + physical activity, smoking, alcohol, fruits and vegetables consumption, CVD medication and procedures.
HR, hazard ratio; CI, confidence interval.
aPer 1000 person-years.
In Table 4, we present the association between metabolic status and incident CVD and type 2 diabetes in analysis stratified by BMI category; the metabolically healthy group was the reference within each strata of BMI. In normal weight and overweight individuals, the metabolically unhealthy had a higher risk of CVD and type 2 diabetes. However, in obese individuals the metabolically unhealthy were not at an increased risk of incident CVD (HR = 1.26, 95% CI: 0.83, 1.92) but had a higher risk of type 2 diabetes (HR = 1.99, 95% CI: 1.39, 2.84). Supplementary material online, Tables S1 and S2 show that when angina was removed from the definition of CVD, the results were similar to those reported in the main analysis (Tables 3 and 4).
Table 4.
The association of metabolic health status (1991–93) with cardiovascular disease and type 2 diabetes in analyses stratified by body mass index categories
| Events/n | Ratea | CVD HR (95% CI) | Events/n | Ratea | Type 2 diabetes HR (95% CI) | |
|---|---|---|---|---|---|---|
| Normal weight (BMI: 18.5–24.9 kg/m2) | ||||||
| Metabolically healthy | 239/3100 | 4.81 | 1.0 (ref) | 154/3100 | 3.37 | 1.0 |
| Metabolically unhealthy | 116/649 | 12.20 | 2.04 (1.62–2.56) | 110/649 | 12.46 | 3.12 (2.42–4.02) |
| Overweight (BMI: 25–29.9 kg/m2) | ||||||
| Metabolically healthy | 164/1636 | 6.38 | 1.0 | 136/1636 | 5.75 | 1.0 |
| Metabolically unhealthy | 206/1080 | 13.00 | 1.81 (1.47–2.24) | 222/1080 | 15.29 | 2.58 (2.07–3.22) |
| Obese (BMI ≥ 30 kg/m2) | ||||||
| Metabolically healthy | 37/279 | 8.93 | 1.0 | 48/279 | 12.96 | 1.0 |
| Metabolically unhealthy | 66/378 | 11.95 | 1.26 (0.83–1.92) | 128/378 | 26.58 | 1.99 (1.39–2.84) |
HR, hazard ratio; CI, confidence interval.
Analyses adjusted for sex, socioeconomic status, marital status, ethnicity, physical activity, smoking, alcohol, fruits and vegetables consumption, CVD medication, and procedures.
aPer 1000 person-years.
Results of analyses with CHD and stroke analysed separately rather than a composite CVD outcome (Supplementary material online, Table S3) were consistent across both these outcomes and similar to those for CVD. Analyses that treated BMI-metabolic status phenotype and all over covariates (including CVD medication and procedures administered to persons over the course of the follow-up) as time varying covariates (Supplementary material online, Tables S4–S6) yielded similar results to those reported, respectively, in Tables 2–4.
The final analysis on a subset of metabolically unhealthy overweight/obese in 1991–93 compared those who remained metabolically unhealthy until 1997–99 (n = 89, rate = 15.34 per 1000 person-years for CVD and n = 128, rate = 23.82 per 1000 person-years for type 2 diabetes) to those became metabolically healthy over this period (n = 45, rate = 13.16 per 1000 person-years for CVD; n = 32, rate = 9.62 per 1000 person-years for type 2 diabetes). These results suggest that becoming metabolically healthy did not lead to significantly lower risk of CVD (HR = 0.89, 95% CI: 0.62, 1.28) but was associated with a lower risk of type 2 diabetes (HR = 0.39, 95% CI: 0.26, 0.58). Of the 574 metabolically unhealthy overweight/obese subjects in 1991–93, only 13 lost weight to become normal weight in 1997–99. No further analysis could be undertaken as there were 3 CVD events and 1 case of type 2 diabetes in this group of 13 individuals.
Discussion
In this prospective study of over 7000 adults, 9.2% of the population was obese and nearly half of them were ‘metabolically healthy’, using the ATP-III criteria to define metabolic health. The main findings are that MHO persons were at an increased risk of incident CVD and type 2 diabetes, followed-up over 17 years, compared with the MH-NW individuals. However, compared with the MUO, MHO individuals were not at a lower risk of incident CVD although their risk of type 2 diabetes was lower. These findings were replicated when CVD endpoints were defined excluding angina and when BMI-metabolic status phenotypes were treated as time varying covariates. Although risk factors for stroke and coronary heart disease do not overlap completely, we found similar patterns of associations when stroke and CHD were analysed as separate outcomes rather than a composite CVD outcome.
The finding that MHO individuals were at an increased risk of incident CVD compared with MH-NW is consistent with some,11,13,15,16 but not all previous studies.24,25 Like our study, the first set of studies had a large number of events in the analysis and a long-follow-up period (average of >10 years) while the latter studies had relatively few events in the analysis and shorter follow-up (average of <10 years). The inconsistency in these results may be due to the fact that there may be as long as a 10–15-year time lag before the evidence of the effect of the metabolic health becomes apparent.26 However, a recent large-scale study (n = 71 527) reported increased risk of MI and ischaemic heart disease in MHO compared with normal weight subjects without MetS even with a short-follow-up period (median of 3.6 years).16
Our findings show that the prognosis of MHO is outcome-specific. We considered two incident chronic diseases, CVD and type 2 diabetes, in order to examine this issue. There is no ambiguity in the results obtained for CVD compared with the MH-NW group; the MHO have higher risk of CVD and this risk is no different with that in the MUO. This suggests that obesity outweighs the impact of metabolic status for risk of CVD. Our finding is quite consistent with data from a recently published study which concluded that MetS is no more valuable than BMI in identifying individuals at risk for CVD.16 However, the pattern is different for type 2 diabetes where although the MHO group had higher risk compared with the normal weight metabolically healthy phenotype, as reported previously,24,25 their risk was significantly lower than that in the MUO. Thus for type 2 diabetes, where metabolic health is an important predictor, the risk in the MHO group is lower than that in the MUO group.
Multiple definitions of metabolic health have been used in defining the MHO phenotype.10 In a previous study, we showed that the prevalence of the MHO phenotype varies as a function of the definition used,9 contributing in part to the inconsistencies found in the association of this phenotype with health outcomes. We used the ATP-III definition of MetS2 which is based on established cut-off and not dependent on risk distribution in the population under study to define metabolic health as in some definitions.24,27,28
Given the prevalence of the MHO phenotype in obese populations, emphasis is increasingly placed on understanding the characteristics and potential mechanisms underlying their healthy metabolic profile. It has been suggested that there are hormones that play a role in allowing certain obese individuals to maintain a healthy profile; and that location, metabolic activity and histological characteristics rather than amount of adipose tissue may partially determine metabolic health among obese individuals.29 Results from previous studies suggest that MHO individuals have been obese for fewer years compared with their MUO counterparts,30 they display a lower level of C-reactive protein,7 high adiponectin concentrations,31 high levels of insulin sensitivity despite having a high accumulation of body fat.4 There is also some evidence that MHO individuals are fitter than their metabolically unhealthy counterparts.12 This favourable profile might reduce their risk of type 2 diabetes but the risk of CVD is no different with that observed in the MUO.
Notable strengths of this study include its large sample size and the assessment of outcomes over a 17-year follow-up period. We were able to show consistency in results in analyses that took into account change in metabolic health status and/or BMI and all other covariates in the analysis (including treatments and procedures administered) over the course of the follow-up. We were also able to show consistency in the results in analyses when stroke and CHD were analysed separately rather than a composite CVD outcome. To minimize the potential for confounding, we adjusted for several covariates, including SES, ethnic group, alcohol consumption, marital status, physical activity, smoking, dietary behaviour, treatments, and procedures. Loss to follow-up for the outcomes is low because data come from linkage to mortality and hospital episodes database for 10 297 of the 10 308 participants at study inception. The study also has some limitations. First, the occupational nature of the cohort of civil servants which did not include blue-collar workers and unemployed individuals and may thus not be representative of the general population limiting the generalizability of our findings. However, our sample covers a wide socioeconomic range with >10-fold difference between the highest and lowest salaries. Secondly, despite the size of the sample, there is lack of ethnic diversity in the cohort, although tests suggested no heterogeneity in associations as a function of ethnicity. A further caveat is the extent to which changes in treatments and medical intervention to reduce risk of CVD in studies with a long follow-up can be accounted for in the analyses. We adjusted for these aspects using time varying covariates with results, suggesting that changes in treatment do not affect the results. However, we recognize that it is not possible to fully control for changes in treatment over time by covariate adjustment. Finally, we examined only type 2 diabetes and CVD in the present analysis and it is possible that the associations with other health outcomes, cancer, for example, follow a different pattern.
In conclusion, we show that one source of inconsistency in the prognosis of metabolically healthy, but obese phenotype is the specific health outcome under consideration. The MHO phenotype carries less risk for type 2 diabetes when compared with MUO even though the risk is greater than that in the MH-NW persons. However, for CVD the prognosis in MHO individuals is as poor as that in the MUO. Better understanding of the health risks associated with the MHO phenotype requires closer attention to how metabolic health is defined and certainly the health outcome under consideration. Metabolically healthy obesity does not appear to be a benign condition, making it important to promote prudent weight loss in overweight/obese individuals and control of modifiable risk factors.
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors’ contributions
G-M.H. performed statistical analyses and drafted the manuscript. A.S-M. and S.C contributed to interpretation of results and revised the manuscript. A.D. helped with the statistical analyses. H.N., E.J.B., and M.K edited and reviewed the manuscript.
Funding
This research is supported by the US National Institutes of Health (R01AG013196 to A.S.M.; R01AG034454 to A.S.M. and M.K., R01HL036310 to M.K.), the UK Medical Research Council (K013351 to M.K.), the Economic and Social Research Council (to M.K.) and the British Heart Foundation (to E.J.B.). Funding to pay the Open Access publication charges for this article was provided by the UK Medical Research Council.
Conflict of interest: none declared.
Acknowledgements
We thank all of the participating civil service departments and their welfare, personnel, and establishment officers; the British Occupational Health and Safety Agency; the British Council of Civil Service Unions; all participating civil servants in the Whitehall II study; and all members of the Whitehall II study team.
References
- 1.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 3.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 4.Brochu M, Tchernof A, Dionne IJ, Sites CK, Eltabbakh GH, Sims EA, Poehlman ET. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab. 2001;86:1020–1025. doi: 10.1210/jcem.86.3.7365. [DOI] [PubMed] [Google Scholar]
- 5.Sims EA. Are there persons who are obese, but metabolically healthy? Metabolism. 2001;50:1499–1504. doi: 10.1053/meta.2001.27213. [DOI] [PubMed] [Google Scholar]
- 6.Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab. 2004;89:2569–2575. doi: 10.1210/jc.2004-0165. [DOI] [PubMed] [Google Scholar]
- 7.Karelis AD, Faraj M, Bastard JP, St-Pierre DH, Brochu M, Prud'homme D, Rabasa-Lhoret R. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005;90:4145–4150. doi: 10.1210/jc.2005-0482. [DOI] [PubMed] [Google Scholar]
- 8.Karelis AD. Metabolically healthy but obese individuals. Lancet. 2008;372:1281–1283. doi: 10.1016/S0140-6736(08)61531-7. [DOI] [PubMed] [Google Scholar]
- 9.Hinnouho GM, Czernichow S, Dugravot A, Batty GD, Kivimaki M, Singh-Manoux A. Metabolically healthy obesity and risk of mortality: does the definition of metabolic health matter? Diabetes Care. 2013;36:2294–2300. doi: 10.2337/dc12-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Primeau V, Coderre L, Karelis AD, Brochu M, Lavoie ME, Messier V, Sladek R, Rabasa-Lhoret R. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 2011;35:971–981. doi: 10.1038/ijo.2010.216. [DOI] [PubMed] [Google Scholar]
- 11.Ogorodnikova AD, Kim M, McGinn AP, Muntner P, Khan U, Wildman RP. Incident cardiovascular disease events in metabolically benign obese individuals. Obesity (Silver Spring) 2012;20:651–659. doi: 10.1038/oby.2011.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortega FB, Lee DC, Katzmarzyk PT, Ruiz JR, Sui X, Church TS, Blair SN. The intriguing metabolically healthy but obese phenotype: cardiovascular prognosis and role of fitness. Eur Heart J. 2013;34:389–397. doi: 10.1093/eurheartj/ehs174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ärnlöv J, Ingelsson E, Sundström J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation. 2010;121:230–236. doi: 10.1161/CIRCULATIONAHA.109.887521. [DOI] [PubMed] [Google Scholar]
- 14.Kuk JL, Ardern CI. Are metabolically normal but obese individuals at lower risk for all-cause mortality? Diabetes Care. 2009;32:2297–2299. doi: 10.2337/dc09-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song Y, Manson JE, Meigs JB, Ridker PM, Buring JE, Liu S. Comparison of usefulness of body mass index versus metabolic risk factors in predicting 10-year risk of cardiovascular events in women. Am J Cardiol. 2007;100:1654–1658. doi: 10.1016/j.amjcard.2007.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomsen M, Nordestgaard BG. Myocardial infarction and ischemic heart disease in overweight and obesity with and without metabolic syndrome. JAMA Intern Med. 2014;174:15–22. doi: 10.1001/jamainternmed.2013.10522. [DOI] [PubMed] [Google Scholar]
- 17.Abdullah A, Wolfe R, Stoelwinder JU, de Court M, Stevenson C, Walls HL, Peeters A. The number of years lived with obesity and the risk of all-cause and cause-specific mortality. Int J Epidemiol. 2011;40:985–996. doi: 10.1093/ije/dyr018. [DOI] [PubMed] [Google Scholar]
- 18.Marmot M, Brunner E. Cohort profile: the Whitehall II study. Int J Epidemiol. 2005;34:251–256. doi: 10.1093/ije/dyh372. [DOI] [PubMed] [Google Scholar]
- 19.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:1–253. i-xii. [PubMed] [Google Scholar]
- 20.Cooper GR. Methods for determining the amount of glucose in blood. CRC Crit Rev Clin Lab Sci. 1973;4:101–145. doi: 10.3109/10408367309151554. [DOI] [PubMed] [Google Scholar]
- 21.Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90:583–612. doi: 10.1161/01.cir.90.1.583. [DOI] [PubMed] [Google Scholar]
- 22.Rose G, Hamilton PS, Keen H, Reid DD, McCartney P, Jarrett RJ. Myocardial ischaemia, risk factors and death from coronary heart-disease. Lancet. 1977;1:105–109. doi: 10.1016/s0140-6736(77)91701-9. [DOI] [PubMed] [Google Scholar]
- 23.Tabak AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimaki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373:2215–2221. doi: 10.1016/S0140-6736(09)60619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D'Agostino RB. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 25.Appleton SL, Seaborn CJ, Visvanathan R, Hill CL, Gill TK, Taylor AW, Adams RJ. Diabetes and cardiovascular disease outcomes in the metabolically healthy obese phenotype: a cohort study. Diabetes Care. 2013;36:2388–2394. doi: 10.2337/dc12-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundstrom J, Riserus U, Byberg L, Zethelius B, Lithell H, Lind L. Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: prospective, population based cohort study. BMJ. 2006;332:878–882. doi: 10.1136/bmj.38766.624097.1F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 28.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 29.Wildman RP. Healthy obesity. Curr Opin Clin Nutr Metab Care. 2009;12:438–443. doi: 10.1097/MCO.0b013e32832c6db7. [DOI] [PubMed] [Google Scholar]
- 30.Janssen I, Katzmarzyk PT, Ross R. Duration of overweight and metabolic health risk in American men and women. Ann Epidemiol. 2004;14:585–591. doi: 10.1016/j.annepidem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Aguilar-Salinas CA, Garcia EG, Robles L, Riano D, Ruiz-Gomez DG, Garcia-Ulloa AC, Melgarejo MA, Zamora M, Guillen-Pineda LE, Mehta R, Canizales-Quinteros S, Tusie Luna MT, Gomez-Perez FJ. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J Clin Endocrinol Metab. 2008;93:4075–4079. doi: 10.1210/jc.2007-2724. [DOI] [PubMed] [Google Scholar]