Abstract
Introduction: ACE inhibitors accounts for 8% of all cases of angioneurotic edema and the overall incidence is 0.1 to 0.7% of patients on ACE inhibitors. It is a leading cause (20-40%) of emergency room visits in the US with angioedema. We report a case of angioedema caused by ACE inhibitors confined to the upper airway after four years on treatment with Lisinopril which persisted for three weeks and required endotracheal intubation and subsequent tracheostomy due to delayed resolution. This case is one of the rare cases presented as upper airway edema which persisted for a long time. Presentation: A 60-year-old Sudanese male patient with osteoarthritis in both knees underwent bilateral total knee replacement under single-shot epidural anesthesia. He had significant past medical history of type II diabetes, bipolar affective disorder and hypertension managed with Lisinopril for the past four years. Postoperatively after 10 hours the patient desaturated and developed airway obstruction requiring intubation. Laryngoscopy revealed an edematous tongue and upper airway and vocal cords were not visualized. In view of this clinical picture a provisional diagnosis of angioedema secondary to Lisinopril was made and it was discontinued. CT scan of the neck and soft tissues revealed severe airway edema with snugly fitting endotracheal tube with no peritubal air. A repeat CT neck on the tenth postoperative day showed no signs of resolution and an elective tracheostomy was performed on the eleventh postoperative day. C1 inhibitor protein and C4 levels were assayed to exclude hereditary angioedema and were found to be within normal range. Decannulation of tracheostomy was done after airway edema resolved on the twenty-fourth postoperative day as confirmed by CT scan. Subsequently he was transferred to the ward and discharged home. Conclusion: ACEI induced angioedema is a well-recognized condition. Early diagnosis based on a high index of suspicion, immediate withdrawal of the offending drug followed by supportive therapy is the cornerstone of management.
Keywords: angioedema, ACE inhibitor, life threatening.
Introduction
Angioedema is a life threatening emergency with diverse etiologies and angiotensin-converting-enzyme inhibitors (ACEI) are one among them. ACEI accounts for 8% of all angioedema cases.1 Among patients on ACEI, the incidence of angioedema is approximately 0.1 to 0.7%.2 ACEI-induced angioedema is a leading cause (20-40%) of emergency room visits in US with angioedema.3 This condition presents as asymmetric, non-pitting edema of subcutaneous or submucous tissues of nondependent areas with no itching and usually affects the lips, tongue, face and upper airway. It can present only as edema of the pharynx, larynx and subglottic area. Clinicians may fail to recognize this fatal condition. The most effective step in the management is discontinuation of the offending ACE inhibitor followed by supportive measures. The novel pharmacological agents for the management of this fatal condition are still in the experimental stage and are not easily available. Edema usually develops over minutes to hours, peaks and then resolves over 24 to 72 hours. In some cases, resolution may take days after withdrawal of ACEI.4 Two-thirds of cases of angioedema occur within the first three months of therapy. Cases have been reported even after eleven years of therapy with ACEI.5 It can recur in spite of discontinuation of ACE inhibitors and may persist for up to six months in some cases.
We report a case of ACEI induced angioedema confined to the upper airway after four years of initiation of therapy with ACEI (Lisinopril) which persisted for twenty-four days after withdrawal of the drug. This case is rare if not unique because the angioedema presented late, was confined to the upper airway and persisted for a prolonged period.
Presentation
A 60-year-old Sudanese male patient with hypertension, type II diabetes, bipolar affective disorder and bilateral osteoarthritis underwent bilateral total knee replacement. He was on the following medications: Lisinopril 10 mg once daily for the last four years, Metformin 500 mg twice daily, Amitryptiline 25 mg once daily and Clonazepam 2 mg once daily. Surgery was performed under single-shot epidural anesthesia. The duration of surgery was approximately four hours and the intraoperative period was uneventful. Continuous bilateral femoral nerve block was performed by the anesthesiologist for postoperative analgesia in the recovery room. Total intraoperative blood loss was approximately 1250 ml and he was transferred to ICU as per planned in view of his comorbid condition. On admission to ICU he was haemodynamically unstable with high output from drains which was approximately 1000 ml. He was stabilized after transfusion of two units of packed red blood cells.
After hemodynamic stabilization, he was found to be restless and agitated. In view of his psychiatric condition and his medication being on hold for the operative procedure, we administered Haloperidol 2.5 mg IV and he responded well. Eight hours after the first episode of agitation, he had a second episode of agitation. Pain, hypotension and hypoxemia were ruled out. A repeat dose of Haloperidol 2.5 mg was administered with minimal response. He was under close observation during which he became extremely restless and was complaining of a sore throat. Salivation and hoarseness of voice was noticed. After two hours, a repeat dose of Haloperidol 5 mg was administered. He responded well to the repeat medication. After 30 minutes, he started to develop partial airway obstruction along with desaturation and unresponsiveness but was haemodynamically stable.
Urgent intubation was decided. Laryngoscopy was done under succinylcholine 100 mg IV, but it was found that the upper airway along with the tongue was grossly edematous and the glottis could not be visualized. After two failed attempts, a laryngeal mask airway was inserted and he was successfully ventilated. In view of further potential threat to the airway because of edema, the patient was in need of a definite airway. Additional help from another anesthesiologist and ENT surgeon was sought. With repeat laryngoscopy and help of gum elastic bougie he was successfully intubated.
Haloperidol-induced coma and respiratory depression was considered as the cause of his deterioration, but the airway edema was unexplainable. Within 30 minutes of ventilation he became responsive and had to be sedated. Hypotension induced by sedatives was managed with ephedrine and colloids.
A search for the cause of the airway edema was performed. During the review of his past and present medications and comorbid conditions, ACEI induced angioedema was suspected as he was on Lisinopril for the last four years. Lisinopril was immediately discontinued; hydrocortisone and anti-histaminics were started as anti-edema measures. He was ventilated for 48 hours after which it was decided to wean him off the ventilator.
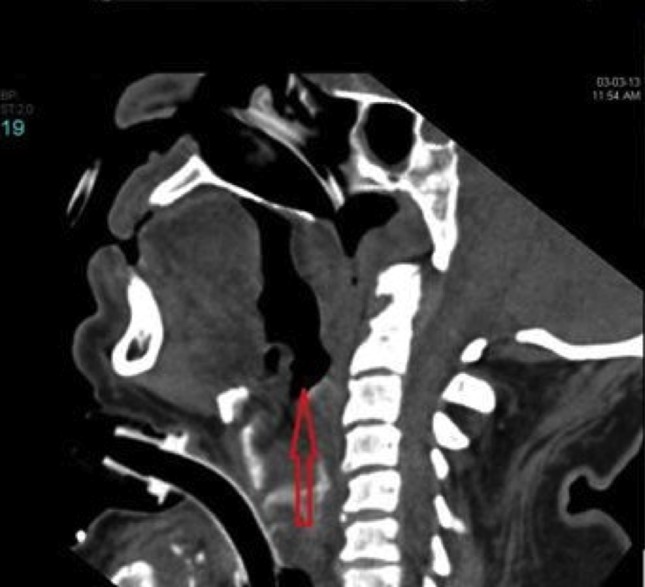
During weaning he developed increased airway pressure and supraventricular tachycardia leading to postponement of weaning and extubation. Weaning was resumed later and a nasopharyngoscopy was performed to confirm the resolution of airway edema before extubation. Fibreoptic nasopharyngoscope could not be passed beyond the soft palate resulting in inability to visualize the epiglottis or glottis confirming upper airway edema. To get a better picture of the supraglottic, glottic and subglottic area, a CT scan of the soft tissues of the neck (Figures 1 and 2) was performed which revealed airway edema from the base of the tongue up to the cricoid cartilage and snugly fitting endotracheal tube (ETT) with no peritubal air. As an empirical therapy, two units of fresh frozen plasma were administered. To rule out hereditary angioedema C3, C4 levels and C1 esterase inhibitor protein level were requested. Due to persistent leukocytosis he was started on prophylactic antibiotic empirically and septic workup was done. Antibiotic was discontinued when septic workup was found to be negative and procalcitonin levels were within normal range. EEG ruled out any seizure activity. A soft tissue neck CT was repeated on the tenth postoperative day which showed similar findings as before. Because of the need for prolonged intubation in view of persistent airway edema, tracheostomy was performed on the eleventh postoperative day. C1 inhibitor protein and C4 levels level were reported within normal range.
Figure 1.
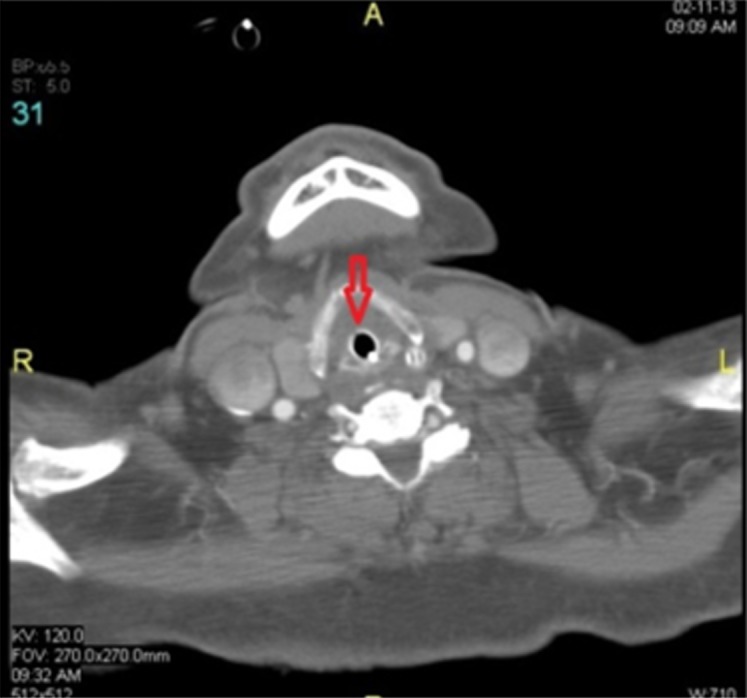
Transverse section of a soft tissue neck CT scan 48 hours after onset showing edema around ETT with no peritubal air.
Figure 2.
Sagittal plane of a soft tissue neck CT scan 48 hours after onset showing edema around ETT with no peritubal air.
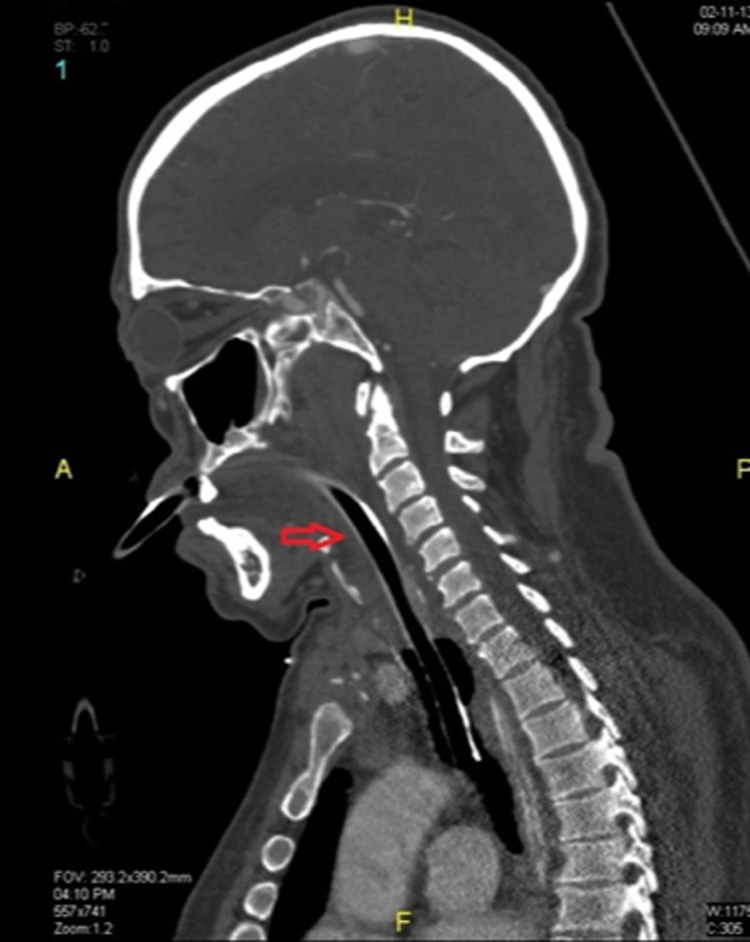
After tracheostomy, he was gradually weaned off and disconnected from the ventilator. A repeat soft tissue neck CT scan on the twenty-fourth day showed good resolution of edema (3). On the twenty-fifth day he was decannulated and was observed for another 48 hrs. On the twenty-seventh day, he was transferred to the ward and later discharged from hospital.
Figure 3.
CT scan soft tissue neck on the twenty-fourth day of onset showing resolution of edema.
Discussion
Angioedema is a non-inflammatory disease characterized by episodes of increased capillary permeability, with extravasations of intravascular fluid into skin, subcutaneous tissue and submucous tissue. It results in edema of the skin of the face, hands, feet, and mucosa of the lips and tongue. Involvement of the upper airway (larynx and pharynx) is not rare and can lead to respiratory distress. Rarely the gastrointestinal system and genitalia can be involved.6 It was first described by J.L. Milton in 1876,7 later in 1882 Quincke named it as angioneurotic edema. Edema of the mucous membranes of the mouth or throat may result in airway obstruction. The majority of angioedema cases are idiopathic. Around 8-10% are hereditary or acquired angioedema and 8% are caused by ACE inhibitors. ACEIs are responsible for the majority of cases of drug induced angioedema and around 20-40% of emergency room visits due to angioedema are caused by ACEIs. However, the overall incidence of angioedema related to ACE inhibitors has been estimated between 0.1 percent and 0.7 percent.2 In this particular case, the skin was spared and the angioedema involved only the mucosa of the upper airway, probably due to more loose areolar tissue in the mucosa in this patient compared to the skin. This makes sense if we consider that it can present as only gastrointestinal angioedema in some patients.8 However, the exact cause is unknown. Although the patient was on amytryptilene, and metformin, they are less known to cause angioedema than Lisinopril. This particular patient was from an African race, which is one of the known risk factors for ACEI induced angioedema.
Pathophysiology
ACE inhibitors block the effects of the enzyme ACE, and influence the renin-angiotensin-aldosterone pathway (RAA) and the degradation of bradykinin. Normally, angiotensinogen produced in the liver is converted by renin in the kidney to produce angiotensin I, which is then metabolized to angiotensin II in the lungs by the enzyme ACE (kininase II). Angiotensin II acts as a vasoconstrictor through stimulation of angiotensin I and II receptors. Angiotensin II is also responsible for inactivating bradykinin while ACE (kininase II) is the primary peptidase involved in the degradation of bradykinin. Bradykinin increases capillary permeability, it also acts as a potent vasodilator. It has a very short half-life of approximately 17 seconds, and is metabolized primarily by ACE (kininase II), neutral endopeptidase (NEP), and aminopeptidase P (APP) and secondarily by the enzymes dipeptidyl peptidase IV (DPPIV) and kininase I. Des–Arg9–BK is an active metabolite of bradykinin formed primarily due to the kininase I enzyme. The pharmacological activities of des-Arg9-BK, similar to those of bradykinin, are short-lived because of its breakdown by ACE. By these mechanisms, ACE inhibitors cause abundance of bradykinin and its active metabolite which are potent vasodilators responsible for the clinical manifestation of angioedema.
Presentation
Unlike allergic angioedema or NSAID-induced angioedema, angioedema as a result of ACEIs is not associated with urticaria.9 Although angioedema may occur during the first week of therapy, some patients (30%) may take the ACEI without any problem for months or years before angioedema develops.10 Therefore, ACEIs are often overlooked as a cause of angioedema and this leads to unfortunate consequences, because continuing administration tends to lead to more severe attacks.11 Angioedema usually presents as asymmetric, non-pitting edema of subcutaneous or submucous tissues of nondependent areas with no itching. It affects the lips, tongue, face and upper airway in the majority of cases or, in some cases with involvement of the pharynx, larynx and subglottic area only. Laryngeal involvement in the early stage presents with hoarseness of voice and stridor. In 10% of cases it can progress to life threatening airway obstruction and endotracheal intubation.12 Swelling usually develops over minutes to hours, peaks, and then resolves over 24 to 72 hours, although complete resolution may take many days in some cases, even if the ACE inhibitor has been discontinued.4 It is not surprising that this patient presented after four years on Lisinopril if we consider other case reports including that of Johanna et al., where Lisinopril induced angioedema occurred after eleven years on the offending drug, which is the longest time known so far.5
The diagnosis of ACEI-related angioedema is mainly by the exclusion of other pathologies such as allergic, non-allergic reactions due to antigens, C1 inhibitor deficiency (C1INH) and infections. Furthermore, no reliable tests to date can differentiate ACEI-related angioedema from angioedema due to other causes.13
Treatment is emergency management of the threatened airway, hemodynamic stabilization and discontinuation of the offending ACE inhibitor. Current evidence suggests that glucocorticoids and antihistaminics are ineffective.14,15 The edema will usually subside within 72 hours with or without treatment. In 1993, Thompson and Frable reported that in 36 patients with angioedema due to ACEIs who visited their hospital in the US, two patients were intubated, one was managed with the nasopharyngeal airway, and three required tracheostomies although, thirty patients were successfully managed with medical therapy. The author emphasized that the angioedema resulting from ACEIs is probably not IgE mediated and that antihistamines and steroids may not alleviate the airway obstruction.16 Weber et al.,17 recently proposed the introduction of a bradykinin inhibitor icatibant as a new treatment for ACEI-associated angioedema. As bradykinin is a major mediator of ACEI-associated angioedema, they opined that, icatibant, presently used in patients with hereditary angioedema (HAE) could be effective for ACEI induced angioedema. In a study by Bas et al., a single dose of icatibant resulted in symptom improvement in 51 ± 21 minutes and complete relief in 4.4 hours and no tracheostomy or intubation in the study group of eight patients. In the control group comprised of 47 patients treated with steroid and antihistamines, it took 33 hours for symptom relief, three patients required tracheostomy and two required intubation.18 The recommended dose is 30 mg subcutaneously as a single dose. New approaches targeting the kalliklein-kinin system, such as kalliklein inhibitors and bradykinin type-2 receptor antagonists might improve treatment for ACEI-induced angioedema in the near future.
Drugs under research:
a) Ecallantide is a recombinant protein used for treatment of HAE. It inhibits conversion of high molecular weight kininogen to bradykinin by inhibiting plasma kallikrein. There is no definite data on its effectiveness so far.
b) Fresh frozen plasma (FFP): two units can result in prompt resolution of angioedema in adults as per some case reports.19
c) Purified C1 INH concentrate: it inhibits kallikrein and may be helpful according to a few case reports.20
d) These therapeutic agents are yet to be proved cost-effective before recommending their routine use.
Conclusion
A high index of suspicion, early diagnosis, followed by prompt and timely intervention are the cornerstones of management of ACEI induced angioedema and this demands further research into the novel therapies and their validation in the future.
References
- 1.Hide M, Hiragun T. Japanese guidelines for diagnosis and treatment of urticaria in comparison with other countries. Allergology International. 2012;61:517–527. doi: 10.2332/allergolint.12-RAI-0497. [DOI] [PubMed] [Google Scholar]
- 2.Messerli FH, Nussberger J. Vasopeptidase inhibition and angio-oedema. Lancet. 2000;356(9230):608–609. doi: 10.1016/S0140-6736(00)02596-4. [DOI] [PubMed] [Google Scholar]
- 3.Banerji A, Clark S, Blanda M, LoVecchio F, Snyder B, Camargo CA., Jr Multicenter study of patients with angiotensin-converting enzyme inhibitor-induced angioedema who present to the emergency department. Ann Allergy Asthma Immunol. 2008;100(4):327–332. doi: 10.1016/S1081-1206(10)60594-7. [DOI] [PubMed] [Google Scholar]
- 4.Chiu AG, Newkirk KA, Davidson BJ, Burningham AR, Krowiak EJ, Deeb ZE. Angiotensin-converting enzyme inhibitor-induced angioedema: A multicenter review and an algorithm for airway management. Ann Otol Rhinol Laryngol. 2001;110(9):834–840. doi: 10.1177/000348940111000906. [DOI] [PubMed] [Google Scholar]
- 5.Norman JL, Holmes WL, Bell WA, Finks SW. Life-threatening ACE inhibitor-induced angioedema after eleven years on lisinopril. J Pharm Pract. 2013;26(4):382–388. doi: 10.1177/0897190012465990. [DOI] [PubMed] [Google Scholar]
- 6.Morimoto T, Gandhi TK, Fiskio JM, Seger AC, So JW, Cook EF, Fukui T, Bates DW. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J Eval Clin Pract. 2004;10:499–509. doi: 10.1111/j.1365-2753.2003.00484.x. [DOI] [PubMed] [Google Scholar]
- 7.Cicardi M, Agostini A. Hereditary angioedema. N Engl J Med. 1996;334:1666–1667. doi: 10.1056/NEJM199606203342510. [DOI] [PubMed] [Google Scholar]
- 8.Vasekar M, Craig TJ. ACE inhibitor-induced angioedema. Curr Allergy Asthma Rep. 2012;12(1):72–78. doi: 10.1007/s11882-011-0238-z. [DOI] [PubMed] [Google Scholar]
- 9.Kanani A, Schellenberg R, Warrington R. Urticaria and angioedema. Allergy Asthma Clin Immunol. 2011;7(Suppl 1):S9. doi: 10.1186/1710-1492-7-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chin HL, Buchan DA. Severe angioedema after long-term use of an angiotensin-converting enzyme inhibitor. Ann Intern Med. 1990;112(4):312–313. doi: 10.7326/0003-4819-112-4-312_2. Feb 15. [DOI] [PubMed] [Google Scholar]
- 11.Slater EE, Merrill DD, Guess HA, Roylance PJ, Cooper WD, Inman WH, Ewan PW. Clinical profile of angioedema associated with angiotensin converting enzyme inhibition. JAMA. 1988;260:967–970. [PubMed] [Google Scholar]
- 12.Grant NN, Deeb ZE, Chia SH. Clinical experience with angiotensin-converting enzyme inhibitor induced angioedema. Otolaryngol Head Neck Surg. 2007;137(6):931–935. doi: 10.1016/j.otohns.2007.08.012. Dec. [DOI] [PubMed] [Google Scholar]
- 13.Inomata N. Recent advances in drug-induced angioedema. Allergol Int. 2012;61(4):545–557. doi: 10.2332/allergolint.12-RAI-0493. [DOI] [PubMed] [Google Scholar]
- 14.Shiber JR. Images in clinical medicine. Angioedema of the arytenoids. N Engl J Med. 2005;353(17):e15. doi: 10.1056/NEJMicm040964. [DOI] [PubMed] [Google Scholar]
- 15.Agostoni A, Cicardi M. Hereditary and acquired C1-inhibitor deficiency: Biological and clinical characteristics in 235 patients. Medicine (Baltimore) 1992;71:206–215. doi: 10.1097/00005792-199207000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Thompson T, Frable MA. Drug-induced, life-threatening angioedema revisited. Laryngoscope. 1993;103:10–12. doi: 10.1288/00005537-199301000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Weber MA, Messerli FH. Angiotensin-converting enzyme inhibitors and angioedema: Estimating the risk. Hypertension. 2008;51:1465–1467. doi: 10.1161/HYPERTENSIONAHA.108.111393. [DOI] [PubMed] [Google Scholar]
- 18.Bas M, Greve J, Stelter K, Bier H, Stark T, Hoffmann TK, Kojda G. Therapeutic efficacy of icatibant in angioedema induced by angiotensin-converting enzyme inhibitors: A case series. Ann Emerg Med. 2010;56(3):278–282. doi: 10.1016/j.annemergmed.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 19.Karim MY, Masood A. Fresh-frozen plasma as a treatment for life-threatening ACE-inhibitor angioedema. J Allergy Clin Immunol. 2002;109(2):370–371. doi: 10.1067/mai.2002.121313. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen EW, Gramstad S. Angioedema from angiotensin-converting enzyme (ACE) inhibitor treated with complement 1 (C1) inhibitor concentrate. Acta Anaesthesiol Scand. 2006;50(1):120–122. doi: 10.1111/j.1399-6576.2005.00819.x. [DOI] [PubMed] [Google Scholar]