Abstract
Background
Echinococcosis is a zoonotic parasitic disease of humans and various herbivorous domestic animals transmitted by the contact with domestic and wild carnivores, mainly dogs and foxes. The aim of this study is the production, purification and evaluation immunogenicity of new construction of EG95 protein.
Methods
The recombinant plasmid pET32-a+ used for Eg95 expression was constructed with the EG95 gene of Echinococcus granulosus fused with the thioredoxin tag. This recombinant clone was over expressed in Escherichia coli BL-21 (DE-3). The expressed fusion protein was found almost entirely in the insoluble form (inclusion bodies) in cell lysate. The purification was performed under denaturing conditions in the presence of 8M urea by Ni–NTA column and dialysis. The purified recombinant proteins were confirmed with western blot analysis using polyclonal antiserum. To find out the immunogenicity of the purified protein, the BALB/c mice (10 mice/group) were immunized by injecting 20 μg rEG95 protein formulated in Freund’s and alum adjuvant.
Results
Immunization of mice with rEG95 using CFA/IFA and alum adjuvant generated high level of total antibody. In proliferation assay, the lymphocytes were able to mount a strong proliferative response with related production of IFN-γ, IL-12 and TNF-α but with low secretion of either IL-4 or IL-10. The humoral and cellular immune responses against rEG95 suggested a mixed Th1/Th2 response with high intensity toward Th1.
Conclusion
Our findings suggest that new construct of rEG95 formulated with CFA/IFA and alum adjuvant elicited strong cellular and humoral responses supporting further development of this vaccine candidate.
Keywords: Echinococcus granulosus, New isoform, EG95 protein, Immunogenicity
Introduction
Cystic hydatid disease (CHD) that caused by Echinococcus granulosus, is most often characterized by transmission between the domestic dog and sheep (1). Echinococcosis classed as neglected infectious diseases or neglected tropical diseases (NTDs), and neglected zoonotic diseases (NZDs) (2). In humans and domestic livestock, y sheep, larval infection typified by long-term growth of hydatid cysts that contain brood capsules and protoscoleces (PSCs) (3). Definitive hosts are carnivores, such as dogs, wolves, and foxes; sexual maturity of adult E. granulosus take place in the small intestine within 4-5 weeks after eating offal containing PSCs. Gravid proglottids and released eggs pass with feces; after ingestion by an intermediate host, they hatch to release an oncosphere, which develops a hydatid cyst in various tissues such as liver (4).
E. granulosus is geographically worldwide and its scattering closely resembles the regions of the world where grazing of livestock is the main job. Infection of the intermediate hosts establishes a major public health problem and is a cause of important economic loss in areas of endemicity (5). CHD is an important zoonotic disease overlarge area of South America, East Africa, Australia, Central Europe, Central Asia and the Mediterranean shore including North Africa (6). Among all NZDs, Asia currently has the greatest burden of CHD in humans with these accounting for over 75% of global disability adjusted life years (DALYs) lost, and primarily occurring in west and north China and the Central Asian Republics (7-10). Most countries are now considering control of CHD through education of the dog owner and regular anthelmintic treatment of the dog as definitive host, collecting stray dogs and sanitary disposal of offal in slaughterhouses. Despite success of control programs in a few countries, these programs not could be change in endemicity of CHD (11).
An effective vaccine for either the definitive host (dogs) or the intermediate host (sheep) should be an important way of breaking the cycle (3). In recent years, several studies have conducted about the vaccination against echinococcosis. A recombinant protein vaccine may supply a new tool for breaking transmission cycle of hydatid disease between intermediate and definitive host (12, 13). Among of antigens of E. granulosus, EG95 protein considered as an antigen for prevention of disease that used from oncosphere of E. granulosus (12, 14, 15). The full-length EG95 protein has a predicted molecular mass of ∼17 kDa, which contain a secretory signal sequence, a carboxy terminal Glycosyl Phosphatidyl Inositol (GPI) hydrophobic anchor motif and a fibronectin type III domain. The EG95 protein expressed by a gene family and is omnipresent in modular proteins involved in cell adhesion (13). The EG95-1-4 was expressed in oncosphere and EG95-5 and 6 were expressed in protoscolex stage. There is substantial genetic variability within the E. granulosus genotypes (16) and antigenic variability in the EG95 protein has the potential to limit the effectiveness of the EG95-based vaccine (17). Nevertheless, studies on the EG95 recombinant protein showed that this protein has high protective against echinococcosis in intermediate host (95-100%). This vaccine has been proven effective in challenge trials conducted in Australia, New Zealand, Argentina, China and Iran (18, 19).
However, development of effective vaccine for application in definitive hosts has been disregarded. Canids have a critical role in the transmission of hydatid disease; therefore abeyance of the parasite life cycle in dogs provides a cost-effective, complementary method for control. A canine vaccine could be of considerable benefit in reducing the effective period (currently more than ten years) needed to stop transmission of E. granulosus (20).
Thus, in this study we report the cloning, expression and purification of new construct and novel isoform of the EG95 proteins that expressed in protoscolex of E. granulosus. The aim of the present study was to assess the antigenicity, immunogenicity of recombinant EG95-5G1 (rEG95-5G1) in mice as new candidate vaccine for definitive host, when being administered with both aluminum hydroxide and Freund’s adjuvant.
Material and methods
Mice
Six-week-old female BALB/c mice obtained from Iran’s Razi Serum and Vaccine Production Research Institute. All mice kept under conventional conditions with free access to food and water.
Parasite
The protoscoleces were aspirated from the hydatid cysts of liver sheep, washed by sterile phosphate buffered saline (PBS), and then suspended in sterile PBS and stored liquid nitrogen overnight.
Bacterial strains and plasmid vectors
The cloning and expression hosts were E. coli GM2163 (Thermo Scientific) and BL21 (DE3) (Novagen®), respectively. pJET1.2, included in CloneJET® PCR cloning kit (Thermo Scientific), was applied for cloning and sequencing of the target DNA fragment. The expression plasmid vector was pET32a (+) (Novagen®).
RNA isolation, construction of cDNA and cloning strategies
Total RNA was isolated from the protoscoleces of E. granulosus using TRIzol reagent (Invitrogen). The purified total RNA was quantified with a spectrophotometer (Nano-Drop) at wavelengths of 230, 260, and 280 nm as described previously (Wang et al. 2008). The integrity of the total RNA was verified by running samples on 1.5 % agarose gels. First strand synthesis of cDNA was performed using the AccuPower® RocketScript™ Cycle RT PreMix (BIONEER).
Complete coding region of EG95-5 was amplified by using a specific primer pair, F: 5’- CGGAATTCATGGCATTCCAGTTATGTCTC-3’ (sense) and R: 5’- GCCTCGAGTCAAGTAAGGACAAC -3’ (antisense). Primers have restriction site sequences at the 5’ ends (Bolded), EcoRI for sense primers and XhoI for antisense primers (21). EG95 gene was amplified by pfu DNA polymerase (Thermo Scientific) and procedure included an initial denaturation at 94°C for 5 min, 35 cycles of 94°C for 30 s, 53°C for 30 s, 72°C for 30 s, final extension at 72°C for 10 min.
The ∼ 500 (492bp) blunt ended PCR products were cloned in pJET1.2 plasmid vector from CloneJET™ PCR Cloning Kit (Thermo Scientific). pJET-EG95 was sequenced with an ABI 3730xl DNA Analyzer machine (Applied Biosystems™) using pJET1.2 sequencing primers. Sequencing results were aligned with a reference sequence of EG95 (accession no. AF199350) by Vector NTI Advanced™ 11.0 software (Invitrogen). EG95 coding DNA was sub-cloned by insertion between EcoRI and XhoI sites in pET32a (+) (designated as pET32-EG95). Constructed recombinant plasmids were subsequently verified by PCR, restriction analysis and DNA sequencing.
Expression and purification of the recombinant proteins
Recombinant vector, pET32-Eg95 transformed into E. coli BL21 (DE3) expression host and was grown in Luria-Bertani (LB) broth medium. All cultures were incubated at 180 rpm and 37°C. Cultures were grown until (and if) an optical density (OD) of 0.6 at 600 nm was reached. Then, expression of rEG95 was induced by adding 1 mM IPTG (isopropyl-l-thio-fl-D-galactopyranoside) to medium. Induction time was 4 hours which the expression levels were assessed at one-hour intervals of induction. Induced samples were screened and analyzed by SDS-PAGE (Mini-PROTEAN® 3 system; Bio-Rad) with 12.5% resolving gel, followed by Coomassie Brilliant Blue G-250 staining.
Cultures (200 ml of 4-hour induced) were pelleted, washed with PBS, and suspended in lysis buffer (50mM NaH2PO4, 300mM NaCl, 10mM imidazole and 1mM PMSF; pH 8.0). Suspensions were sonicated for 3×5 minutes in 0.6 second pulses of 80% amplitude while they were kept on ice-water. The lysate centrifuged at 18,000 × g for 20 minutes at 4°C to separate soluble (clear lysate) and solid materials (inclusions) and analyzed by SDS-PAGE to detect the recombinant protein. Purification of rEG95 was performed under denaturing conditions according to the manufacturer’s instructions (The QIAexpressionist™, QIAGEN; 2003).
Briefly, for purification of the rEG95 from inclusions each pellet was dissolved in buffer B (100mM NaH2PO4, 10mM Tris-HCl, and 8M urea; pH 8) and the debris was removed by centrifugation (18000×g, 30min, 25°C). Ni-NTA agarose (50%, 1ml) was added to each 5ml of lysate and incubated on a rotary shaker at room temperature for one hour. The mixture was loaded on a column and resin was washed twice with four volumes of buffer C (100mM NaH2PO4, 10mM Tris-HCl, and 8M urea; pH 6.3) and buffer D (100mM NaH2PO4, 10mM Tris-HCl, and 8M urea; pH 5.9). The rEG95 was finally eluted with buffer E (100mM NaH2PO4, 10mM Tris-HCl, and 8M urea; pH 4.5). The eluted proteins were analyzed by SDS-PAGE and quantified using a NanoDrop spectrophotometer (NanoDrop™ 2000; Thermo Scientific).
Renaturing and re-solubilization of rEG95
To attain a higher concentration of the purified rEG95 and decrease protein loss and purification time, re-solubilization was applied by a series of decreasing urea concentration wash buffers. After absorption of urea-dissolved rEG95 to Ni-NTA resin, the column washed by buffer C, two volumes of re-solubilization buffers (50 mM NaH2PO4, 300 mM NaCl, 5% ethanol, pH 8) containing 8, 6, 4, 2, 1, and 0mM urea, sequentially. Then the column washed with 4 volumes of native wash buffer (50 mM NaH2PO4, 300 mM NaCl, 20mM imidazole, pH 8). 6His-taged protein was eluted with 250mM imidazole (50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole, pH 8) and eluted fractions were analyzed by SDS-PAGE and NanoDrop spectrophotometer. Imidazole was removed by dialysis against PBS.
Western blot analysis
Western blot analysis was performed on crude cells and purified protein samples. The crude - cell lysate was prepared by boiling cell suspension containing SDS-PAGE sample buffer (Ambresco) for 5 min. Proteins were electrotransferred to nitrocellulose membranes (Sigma-Aldrich®) in a semi-dry transfer cell at 15 volts/10 minutes (Trans-Blot® SD, Bio-Rad). After electrophoresis in 12.5% polyacrylamide mini gels, the proteins were electrotransferred onto nitrocellulose membrane for immunoblotting with and mouse polyclonal CHD antiserum. Detection of antigens was performed by an indirect antibody immunoassay using HRP-labeled anti-mouse IgG (Sigma) diluted 1:5000 in PBS with 0.05% Tween 20 (PBS-T) and DAB staining.
Formulation of rEG95 with adjuvants
rEG95 was formulated with the following adjuvants, complete and incomplete Freund’s adjuvant (CFA and IFA) and alum according to manufacturer’s instructions. Briefly, formulations with different adjuvants were prepared as follows. In case of FA, one parts FA (Iran’s Razi Serum and Vaccine Production Research Institute) were emulsified with one part of antigen (v/v). For formulation with alum hydroxide, the pH of alum (18 mg/ml) was first adjusted to 7.0 with NaOH and then mixed with rEG95 in the ratio of one part adjuvant: two part antigen (v/v). The formulation was incubated for 1 hr at 37°C on a gently rocking nutator, centrifuged at 3000 rpm for 10 min. The pellet resuspended in 0.9% NaCl. The supernatant collected and tested for the presence of any unabsorbed protein. Greater than 90% of rEG95 was absorbed by the alum. The final concentration of rEG95 in each of the formulations was 100 μg/ml.
Mouse immunization
Immunogenicity of rEG95 formulated with FA and alum was tested in BALB/c mice. Groups of ten BALB/c mice were immunized subcutaneously with 20 μg of rEG95 formulated with the different adjuvants described above. Two control groups of five mice were administered with PBS and 5μg of Trx formulated in Freund’s adjuvant, separately. The total volume used per mouse at each immunization was 200 μl. Priming was followed by two booster immunizations on days 14 and 28. Sera were collected on ten days post final immunization. All sera were stored at -70°C until used.
ELISA assay to determine isotypes of rEG95 specific IgG
Microtiter plates (Maxisorp, Nunc) were coated with 100μl of rEG95 (10μg/ml) in bicarbonate coating buffer overnight at 4°C. Plates were washed with PBS containing 0.05% Tween 20 (PBST) and blocked with 2% BSA in PBS (PBSB) (200 μl/well) for 2 hrs at 37°C. After blocking, the plates were washed with PBST and sera were diluted 1/100 in 1% BSA-PBST (PBSTB). Following overnight incubation at 4°C, the plates were washed and incubated for 1 hr at 37°C with goat anti-mouse IgG1, IgG2a, IgG2b and IgG3 (ISO-2, Sigma) at 1/2000 dilution in 1% BSA-PBST (50μl per well). After washing, plates were incubated with anti-goat HRP-conjugated antibodies diluted 1:5000. Peroxidase activity was revealed by adding 50 μl/well of a solution containing 12.5% H2O2, 0.1M citrate phosphate pH 4 and 10 mg/ml of TMB. The reaction was stopped adding 50 μl of 2M H2SO4 and the optical density (OD) was read at 450 nm in an ELISA micro-plate reader (Bio-Rad, USA). All samples were run in triplicates.
In vitro spleen cell proliferation
Spleen lymphocytes from immunized were cultured in 96-well microtitre plates at 2.5×105 per well in RPMI 1640 (Gibco®) supplemented with 10% FCS (Gibco®), Pen-Sterp (Gibco®) and non-essential amino acids (Gibco®). Cultures were incubated in the medium alone (control group), in the presence of 10 μg/ml of rEG95 or 10 μg/ml (immunized groups) in 37°C and 5% CO2 for 72 hrs. After these times 20 μl of tetrazolium (Sigma) (5 mg/ml) was added to each well and incubated in 37°C for 4 hrs then centrifuged in 1000×g for 10 min, the supernatant was discarded and 100 μl of dimethyl sulfoxide (DMSO) was added to each wells and resuspended. The OD was read by ELISA reader in 540 nm.
Cytokine assay
For the detection of cytokines, splenocytes from immunized mice were cultured in 24 well-plates at 4 × 106 using RPMI 1640 (Gibco®) four weeks after the final immunization. The medium was supplemented as described above. Cultures were stimulated with 10 μg/ml of rEG95 as described for the lymphocyte proliferation assay. Cell-free supernatants were collected and assayed for IL-4, IL-10, IFN-γ and TNF-α activity at 72 hrs. The concentrations of cytokines were determined by ELISA kit (R&D Systems, Minneapolis, MN, USA), according to manufacturer’s instructions.
Statistical analysis
The differences in the level of cytokines and antibodies production were determined by one way ANOVA. All statistical analyses were performed using SPSS 16.0 for windows (statistical package for the social sciences, SPSS Inc., IL, USA). Differences were considered significant when P < 0.05.
Results
Cloning, sequencing of rEG95 and expression plasmid construction
Total RNA was extracted from protoscoleces derived from sheep hydatid cysts and CDNA were synthesized. In RT-PCR, EG95 gene (492 bp) successfully amplified by proofreading DNA polymerase. Blunt-ended PCR products were successfully cloned in pJET1.2 vector and the target DNA fragment was inserted in a multiple cloning site. Sequence analysis of recombinant pJET-EG95 plasmids revealed complete identity with a reference sequence (accession no. AF199350). EG95 gene was sub-cloned in pET32a(+) expression vector followed by conventional PCR screening and digestion (data not shown). Sequencing results of recombinant expression plasmid vectors showed entire similarity with reference sequences and confirmed the accuracy of in-frame insertion of target ORF in multiple cloning sites of the vector.
Expression Analysis of recombinant EG95 (rEG95)
SDS-PAGE analysis of E. coli BL21 (DE3), which was transformed with pET32-EG95 (designated as BL21pET32-EG95) and induced with IPTG, showed resulted in the expression of a non-glycosylated ∼36 kDa protein1 as expected. Figure 1 shows the expression of rEG95pET32 induced by IPTG 1mM. There was a difference in quantitative expression of protein among 1- to 4-hrs induced cells. The quantity of expressing protein was increased after the 2 hrs induction and maximized in 3 hrs induced cells 1.
Fig.1.
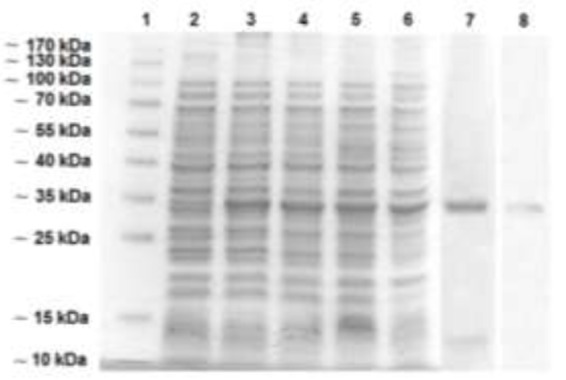
Expression of rEg95pET32 in E. coli BL21 (DE3)
1: molecular weight marker; 2: Non-induced BL21pET32-EG95; 3: 1-hour induced BL21pET32-EG95; 4: 2-hour induced BL21pET32-EG95; 5: 3-hour induced BL21pET32-EG95; 6: 4-hour induced BL21pET32-EG95; 7: Purified rEG95pET32; 8: Purified rEG95pET32 detected by polyclonal mouse CHD antiserum
Purification, renaturing and Western blot analysis of rEG95
The use of an expression vector adding a six-histidine tag at the protein’s N-terminus allowed detecting the recombinant protein through the use of mouse polyclonal antibody and subsequently purifying it by affinity chromatography. This led to detecting rEG95 as insoluble aggregate forming inclusion bodies, thus requiring high concentrations of denaturing agents (8M urea) to solubilize it. The recombinant protein was then purified by affinity chromatography using a Ni+2-NTA resin. The absorbed recombinant proteins to resin washed by buffer with decreasing gradient of urea in order to removing and resolubilizing. Finally, collected fractions were individually analyzed by SDS-PAGE and Western blot. The recombinant protein was then thoroughly dialyzed against PBS 1×, pH 7.2 to ensure the removal of all denaturing agents and to allow the protein to acquire a similar conformation to that of the native one. Finally, purified rEG95 were detected using mouse polyclonal CHD antiserum (Fig. 1).
Subtypes of Immunoglobulin G
To monitor antigen-specific response provided by immunization with rEG95, both humoral and cell mediated responses were evaluated in immunized mice. The antibody levels against EG95 were determined in sera obtained from control and vaccinated mice after 10th day of second booster injection. Fig. 2 depicts the antibody response in control and immunized mice. The levels of specific EG95/IgG were significantly higher in immunized group (P < 0.001) than in control group. To reveal the type of immune response, immunized mice were analyzed for anti-EG95 antibodies of IgG1, IgG2a, IgG2b and IgG3 isotypes (Fig. 2). The mice immunized with Eg95 formulated with CFA/IFA showed higher level of IgG2a compared to those immunized with alum (P = 0.000).
Fig.2.
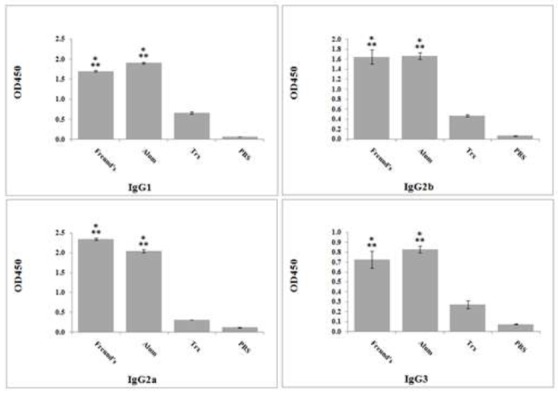
Results for immunoglubin G subtypes evaluation
IgG subtypes in mice serum samples of different experimental groups were assayed against rEG95 by ELISA. Data from each group was compared by other groups with LSD test via one-way analysis of variance (one-way ANOVA). P-values less than 0.05 were recognized as significant.
Freund’s: sera from mice immunized with rEG95 formulated with FA. Alum: sera from mice immunized with rEG95 formulated with alum. Trx: sera from mice immunized with TrxpET32
*: significant difference with non-immunized (negative control) group (P < 0.05).
**: significant difference between two test groups, “rEG95” and “Trx” (P < 0.05)
Altogether, the results revealed a predominant IgG2a response as compared to IgG1 levels (Fig. 2). IgG2a/IgG1 ratio in immunized mice showed a cellular mediated immunity schema (Table 1). The rEG95 formulated with freund and alum have not significant difference in the level of antibody production (P = 0.69).
Table 1.
Ratio of specific IgG1 and IgG2a directed to rEG95 formulated with adjuvant
| Test groups | Mean | IgG2a / IgG1/Std. Deviation | Std. Error |
|---|---|---|---|
| Freund * | 1.2696 | 0.1116 | 0.0322 |
| Alum* | 1.0443 | 0.0716 | 0.0206 |
| Trx | 0.5708 | 0.2167 | 0.0279 |
| PBS | 0.4311 | 0.2568 | 0.0741 |
Significant difference with Trx and PBS groups (one-way ANOVA; P < 0.05)
Measurement of the cytokine pattern in the immunized Animals
The stimulation indices for the mice immunized were high and comparable (P = 0.27). The antigens induced high proliferation as compared to the control (P < 0.001) with adjuvant formulation suggesting antigen specificity of the proliferation.
To analyze whether splenocytes from vaccinated mice were able to secrete cytokines, four weeks after the final immunization spleen cell suspensions from individual mice were stimulated in vitro with rEG95. Mice, which were immunized with rEG95, exhibited higher levels of TNF-α IFN-γ IL-12, IL-10 and IL-4 in response to stimulation with rEG95 than control group (P ≤ 0.05).
Mice immunized with rEG95 formulated with both adjuvants elicited strong Th1 type immune responses than those immunized with PBS and TrxpET32 (high level of IFN-γ, TNF-α or IL-12 and low-level of IL-4 or IL-10). Interestingly, lymphocyte cultures of rEG95pET32-immunized mice did not show significant level for any of the cytokines in response to stimulation with TrxpET32 (Fig. 3).
Fig. 3.
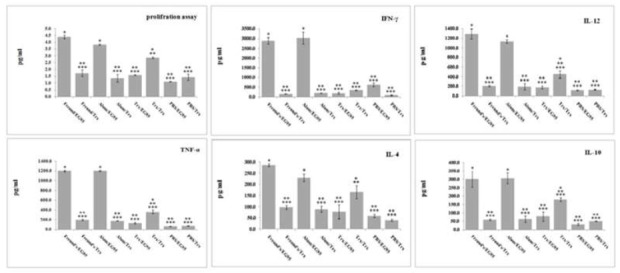
Cytokine assay results
Mean ± SD the OD (540 nm) of MTT lymphocyte proliferation assay of spleen cells stimulated with EG95 and Trx by MTT method in group of immunized mice. Cytokine levels (IFN-γ IL-12, TNF-α IL-4, IL-10) in spleen lymphocyte culture experiments groups were evaluated by ELISA. In each experiment, data from each group was compared with other groups by LSD test via one-way analysis of variance (one-way ANOVA). P-values less than 0.05 were recognized as significant.
Freund’s/EG95: spleen lymphocytes from mice immunized with rEG95 formulated with FA and stimulated with the same protein. Freund’s /Trx: spleen lymphocytes from mice immunized with rEG95 formulated with FA and stimulated with Trx. Alum/EG95: spleen lymphocytes from mice immunized with rEG95 formulated with alum and stimulated with the same protein. Alum /Trx: spleen lymphocytes from mice immunized with rEG95 formulated with alum and stimulated with Trx. Trx/EG95: spleen lymphocytes from mice immunized with Trx and stimulated with rEG95. Trx/Trx: spleen lymphocytes from mice immunized with Trx and stimulated with the same protein. PBS/EG95: spleen lymphocytes from PBS immunized mice stimulated with rEG95. PBS/Trx: spleen lymphocytes from PBS immunized mice stimulated with Trx.
*: significant difference with PBS (negative control) groups (P < 0.05)./ **: significant difference between “Freund’s/EG95” and other test groups (P < 0.05)./ ***: significant difference between “Alum/EG95” and other test groups (P < 0.05)
Discussion
The evolution of Echinococcus spp. strongly is affected by human societies and domestication of animal. Divergent strains and species of Echinococcus spp. were adapted to domestic animal, and subsequently disseminated worldwide with the distribution of human communities and animal husbandry (13, 22).
A serious feature in life cycle of Echinococcus, and in the alteration to different intermediate host species, is the ability of activated oncospheres to pierce the epithelial cell of intestine and to reach the target organ of the herbivorous host via blood and lymph vessels. Few countries have been able to control this disease (23). As mentioned above, the appropriate approach to control CHD includes the use of a vaccine against infection. An effective vaccine for either the definitive host (dogs) or the intermediate host (sheep) should be an important way of breaking the cycle (3).
Recently, EG95 antigen was characterized as a highly immunogenic protein, which encoded by a multigene family (16, 24, 25). The full length EG95 protein has a predicted molecular mass of ∼17 kDa which contain a secretory signal sequence, a carboxy terminal Glycosyl Phosphatidyl Inositol (GPI) hydrophobic anchor motif and a fibronectin type III domain (Fn3) (13), upregulated during oncosphere activation (15, 26), and probably involved in cell adhesion (27). Fn3 domains are usually involved in cell surface binding. Variations in the Fn3-domain folding, allowing oncospheres to bind variant host cell receptors, might have been adaptive for the specific invasion of a wide range of intermediate hosts (28). The interest on EG95 comes largely from its high potential as a vaccine to protect against Echinococcus infection. Protection obtained with the recombinant antigen ranges from 95% to 100% (29), and the elicited immune response effectively kills oncospheres in vitro (30). The fact that EG95-related antigens are so immunogenic is puzzling, since the Fn3 module contains highly conserved features, and is one of the most widespread domains of mosaic proteins, being present in both the parasite and the host (31).
In this study, we successfully cloned the whole coding sequence of the Eg95 protein of E. granulosus which is encoded by gene eg95-5 (isoform A) (13). For purification of His-tagged rEG95 in the form of inclusion bodies, we used buffers containing high concentrations of urea (8M).
Since the recombinant EG95 is expressed in a heterogeneous host, which lacks echinococcal chaperons, it does not gain its native echinococcal-state configuration; this may result in its accumulation as insoluble inclusions. When the denaturing agent is removed gradually, it is possible for hydrophobic/hydrophilic domains to locate themselves at a biophysically proper state according to the aqueous environment so that the whole configured structure is soluble. We hypothesize that fusion of Trx-tag as a solubility enhancer along with gradually decreasing urea concentration while rEG95 is physically fixed to Ni-NTA resin, greatly helps the internal localization of hydrophobic domains and efficient superficial positioning of hydrophilic parts in the configured protein. Presence of Trx-tag led to solubility and stability of recombinant protein. Recognition of the purified rEG95 by mouse polyclonal CHD antiserum supports that our procedure ensures partial refolding of the protein to some degrees that it can react with antibodies produced against the native antigen from protoscoleces of Echinococcus granulosus. Evaluation of IgG subtypes shows that rEG95 elicits antibodies from all subtypes and IgG2a/IgG1 ratio implies a cell-mediated immunity. In addition, raised TNF-α IL-12 and IFN-γ against low levels of IL-4 and IL-10 supports the development of cell-mediated rather than humoral response to rEG95 in spleen lymphocyte culture experiments. We hypothesize that the configuration of purified rEG95 may be suitable for eliciting cellular and humoral immunity. There were no significant differences between rEG95 formulated with CFA/IFA and alum in level of antibody and secretion of cytokines.
Based on sequence, EG95 protein is nonpolar and has isoelectric pH of 9.6, which not easily expressed in a prokaryotic host. Until now, GST tag has used for expression of EG95 in prokaryotic host. In general, it is difficult to decide on the best fusion system for a specific protein of interest. This depends on the target protein itself (e.g. stability, hydrophobicity), the expression system, and the application of the purified protein. However, fusion fragment should not interfere in the biological properties of fused protein (32). Previous studies have shown fusion of EG95 and GST increases the immunogenicity of recombinant proteins and immunogenic properties of the protein cannot be fully accounted for EG95. Evaluation of IgG subtypes in sera from immunized mice against TrxpET32 and cytokine responses in lymphocyte cultures of mice, which were immunized with rEG95pET32 and stimulated with TrxpET32, indicate that fusion of thioredoxin with EG95 does not significantly alter the type of immune responses (33).
Results of this study revealed that protein encoded by eg95-5 gene have also immunological characteristic and elicited humoral and cellular immune responses. Previous studies revealed that EG95 derived from activated oncosphere (eg95-1) have high efficacy for vaccination in the intermediate hosts that is not effective in the prevention of echinococcosis in the final hosts. Hence, the use of proteins derived from protoscolex stage is appropriate strategy to prevent infection in the final hosts. The protein has been encoded by members of a small family of related genes in this genotype. Subsequent study revealed substantial nucleotide substitutions (encoding amino acid substitutions) for EG95 in the G6/G7 genotypes (16, 34). Considerable differences were found between the EG95 related proteins from the G6 genotype compared with the EG95 protein from the G1 genotype. These data have implications for future vaccine design and provide information that would enable a G6 genotype-specific vaccine to be developed against E. granulosus, should this be considered a desirable addition to the available tools for control of cystic echinococcosis transmission (17).
Hypothetical analysis of secondary structure of EG95 protein shown the isoform A contain 9 beta sheets and 2 alpha-helices are encoded by gene eg95-5. This isoform have seen in E. granulosus, E. equinus, E. ortleppi, E. canadensis and E. multilocularis. However, isoform D with 10 beta sheets is encoded by gene eg95-1, which appears in E. vogeli, E. multilocularis, E. granulosus, E. ortleppi and E. canadensis. If Echinococcus strains or species from different geographic areas contain positively selected amino acids within the protective epitopes, one would expect a lower efficacy, because good vaccine epitopes should be rich in negatively selected sites (35). Great flexibility in the eg95 gene family based on the evolutionary pattern revealed that maximum efficacy of vaccine could possibly be obtained by a mixture of both isoforms. Therefore, the existence of high genetic diversity among human and animal isolates in endemic areas was necessitated using of different EG95 protein isoforms encoded by strains of Echinococcus. The results presented here provide a basis for further researches towards the use of new isoform of rEG95 in protection studies against echinococcosis.
Conclusion
Our findings suggest that new construct of rEG95 formulated with CFA/IFA and alum adjuvant elicited strong cellular and humoral responses supporting further development of this vaccine candidate.
Acknowledgments
This work is supported financially by Tarbiat Modares University and Iranian National Science Foundation. The authors wish to thank Research Deputy of University and Medical Committee of Iranian National Science Foundation for their kind cooperation. The authors declare that there is no conflict of interests.
Footnotes
35.77 Da protein results by fusing the full length EG95 (17.78 kDa) with 17.72 kDa Trx-tag containing peptide which is added by pET32a(+) plasmid vector.
References
- 1.Moro P, Schantz PM. Echinococcosis: a review. Int J Infec Dis. 2009;13(2):125–33. doi: 10.1016/j.ijid.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 2.WHO. The control of neglected zoonotic diseases: A route to poverty alleviation. Report of a joint WHO/DFID-AHP meeting, 20 and 21 September 2005, WHO Headquarters, Geneva, with the participation of FAO and OIE. 2009 [Google Scholar]
- 3.McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. 2003;362(9392):1295–304. doi: 10.1016/S0140-6736(03)14573-4. [DOI] [PubMed] [Google Scholar]
- 4.Heath D, Yang W, Li T, Xiao Y, Chen X, Huang Y, et al. Control of hydatidosis. Parasitol Int. 2006;55:S247–S52. doi: 10.1016/j.parint.2005.11.052. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins D, Romig T, Thompson R. Emergence/re-emergence of Echinococcus spp.—a global update. Int J Parasitol. 2005;35(11):1205–19. doi: 10.1016/j.ijpara.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Dakkak A. Echinococcosis/hydatidosis: a severe threat in Mediterranean countries. Vet Parasitol. 2010;174(1):2–11. doi: 10.1016/j.vetpar.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Budke CM, Deplazes P, Torgerson PR. Global socioeconomic impact of cystic echinococcosis. Emerg Infect Dis. 2006;12(2):296. doi: 10.3201/eid1202.050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budke CM, Jiamin Q, Zinsstag J, Qian W, Torgerson PR. Use of disability adjusted life years in the estimation of the disease burden of echinococcosis for a high endemic region of the Tibetan plateau. Am J Trop Med Hyg. 2004;71(1):56–64. [PubMed] [Google Scholar]
- 9.Torgerson PR, Oguljahan B, Muminov AE, Karaeva RR, Kuttubaev OT, Aminjanov M, et al. Present situation of cystic echinococcosis in Central Asia. Parasitol Int. 2006;55:S207–S12. doi: 10.1016/j.parint.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 10.Yang YR, Williams GM, Craig PS, McManus DP. Impact of increased economic burden due to human echinococcosis in an underdeveloped rural community of the People’s Republic of China. PLoS Negl Trop Dis. 2010;4(9):e801. doi: 10.1371/journal.pntd.0000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craig PS, Larrieu E. Control of cystic echinococcosis/hydatidosis: 1863-2002. Adv Parasitol. 2006;61:443–508. doi: 10.1016/S0065-308X(05)61011-1. [DOI] [PubMed] [Google Scholar]
- 12.Gauci C, Heath D, Chow C, Lightowlers MW. Hydatid disease: vaccinology and development of the EG95 recombinant vaccine. Expert Rev Vaccines. 2005;4(1):103–12. doi: 10.1586/14760584.4.1.103. [DOI] [PubMed] [Google Scholar]
- 13.Haag KL, Gottstein B, Ayala FJ. The EG95 antigen of Echinococcus spp. contains positively selected amino acids, which may influence host specificity and vaccine efficacy. PloS ONE. 2009;4(4):e5362. doi: 10.1371/journal.pone.0005362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow C, Gauci CG, Cowman AF, Lightowlers MW. A gene family expressing a host-protective antigen of Echinococcus granulosus. Mol Biocheml Parasitol. 2001;118(1):83–8. doi: 10.1016/s0166-6851(01)00373-5. [DOI] [PubMed] [Google Scholar]
- 15.Chow C, Gauci CG, Cowman AF, Lightowlers MW. Echinococcus granulosus: oncosphere-specific transcription of genes encoding a host-protective antigen. Exp Parasitol. 2004;106(3):183–6. doi: 10.1016/j.exppara.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez Rojas CA, Gauci CG, Nolan MJ, Harandi MF, Lightowlers MW. Characterization of the eg95 gene family in the G6 genotype of Echinococcus granulosus. Mol Biochem Parasitol. 2012;183(2):115–21. doi: 10.1016/j.molbiopara.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez Rojas C, Gauci CG, Lightowlers MW. Antigenic differences between the EG95-related proteins from Echinococcus granulosus G1 and G6 genotypes: implications for vaccination. Parasite Immunol. 2013;35(2):99–102. doi: 10.1111/pim.12009. [DOI] [PubMed] [Google Scholar]
- 18.Lightowlers M, Jensen O, Fernandez E, Iriarte J, Woollard D, Gauci C, et al. Vaccination trials in Australia and Argentina confirm the effectiveness of the EG95 hydatid vaccine in sheep. Int J Parasitol. 1999;29(4):531–4. doi: 10.1016/s0020-7519(99)00003-x. [DOI] [PubMed] [Google Scholar]
- 19.Heath D, Lightowlers M. Vaccination on hydatidology—state of the art. Arch Int Hidatid. 1997;33:14–6. [Google Scholar]
- 20.Craig PS, McManus DP, Lightowlers MW, Chabalgoity JA, Garcia HH, Gavidia CM, et al. Prevention and control of cystic echinococcosis. Lancet Infect Dis. 2007;7(6):385–94. doi: 10.1016/S1473-3099(07)70134-2. [DOI] [PubMed] [Google Scholar]
- 21.Sarvi S, Dalimi A, Ghafarifar F. Molecular Cloning and Expression of EG95 Gene of Iranian Isolates of Echinococcus granulosus. Iran J Parasitol. 2012;7(2):1–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Haag KL, Gottstein B, Ayala FJ. Taeniid history, natural selection and antigenic diversity: evolutionary theory meets helminthology. Trends Parasitol. 2008;24(2):96–102. doi: 10.1016/j.pt.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Bergquist R, Whittaker M. Control of neglected tropical diseases in Asia Pacific: implications for health information priorities. Infect Dis Poverty. 2012;1(1):3. doi: 10.1186/2049-9957-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma X, Zhou X, Zhu Y, Li Y, Wang H, Mamuti W, et al. The prediction of T and B combined epitope and tertiary structure of the Eg95 antigen of Echinococcus granulosus. Exp Ther Med. 2013;6(3):657–62. doi: 10.3892/etm.2013.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hewitson JP, Maizels RM. Vaccination against helminth parasite infections. Expert Review of Vaccines. 2014;13(4):473–87. doi: 10.1586/14760584.2014.893195. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Li J, You H, Zhang Z, Turson G, Loukas A, et al. Short report: Echinococcus granulosus from Xinjiang, PR China: cDNAs encoding the EG95 vaccine antigen are expressed in different life cycle stages and are conserved in the oncosphere. Am J Trop Med Hyg. 2003;68(1):40–3. [PubMed] [Google Scholar]
- 27.Bonay P, González LM, Benítez L, Foster M, Harrison LJ, Parkhouse RME, et al. Genomic and functional characterisation of a secreted antigen of Taenia saginata oncospheres. Mol Biochem Parasitol. 2002;121(2):269–73. doi: 10.1016/s0166-6851(02)00037-3. [DOI] [PubMed] [Google Scholar]
- 28.Bork P, Doolittle RF. Fibronectin type III modules in the receptor phosphatase CD45 and tapeworm antigens. Protein Sci. 1993;2(7):1185–7. doi: 10.1002/pro.5560020714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lightowlers MW. Vaccines against cysticercosis and hydatidosis: foundations in taeniid cestode immunology. Parasitol Int. 2006;55:S39–S43. doi: 10.1016/j.parint.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Woollard DJ, Gauci CG, Heath DD, Lightowlers MW. Protection against hydatid disease induced with the EG95 vaccine is associated with conformational epitopes. Vaccine. 2000;19(4):498–507. doi: 10.1016/s0264-410x(00)00192-4. [DOI] [PubMed] [Google Scholar]
- 31.Bork P, Doolittle RF. Proposed acquisition of an animal protein domain by bacteria. Proc Natl Acad Sci U S A. 1992;89(19):8990–4. doi: 10.1073/pnas.89.19.8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiarella P, Edelmann B, Fazio VM, Sawyer AM, de Marco A. Antigenic features of protein carriers commonly used in immunisation trials. Biotechnol Let. 2010;32(9):1215–21. doi: 10.1007/s10529-010-0283-z. [DOI] [PubMed] [Google Scholar]
- 33.LaVallie ER, DiBlasio EA, Kovacic S, Grant KL, Schendel PF, McCoy JM. A thioredoxin gene fusion expression system that circumvents inclusion body formation in the E. coli cytoplasm. Nat Biotechnol. 1993;11(2):187–93. doi: 10.1038/nbt0293-187. [DOI] [PubMed] [Google Scholar]
- 34.Chow C, Gauci CG, Vural G, Jenkins DJ, Heath DD, Rosenzvit MC et al. Echinococcus granulosus: Variability of the host-protective EG95 vaccine antigen in G6 and G7 genotypic variants. Exp Parasitol. 2008;119(4):499–505. doi: 10.1016/j.exppara.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki Y. Negative selection on neutralization epitopes of poliovirus surface proteins: implications for prediction of candidate epitopes for immunization. Gene. 2004;328:127–33. doi: 10.1016/j.gene.2003.11.020. [DOI] [PubMed] [Google Scholar]


