Abstract
Background
Cystic echinococcosis (CE), a zoonotic parasitic infection caused by the metacestode (larvae) stage of dog tapeworm Echinococcus granulosus and recognized as a major economic and public health concern in the world. This study aimed to investigate the in vitro scolicidal effect of methanolic extract of Berberis vulgaris L. roots and its main compound, berberine against protoscoleces of hydatid cysts.
Methods
For this purpose, protoscoleces were aseptically aspirated from sheep livers having hydatid cysts. Various concentrations of the methanolic extract (0.25-2 mg/ml) and berberine (0.062- 0.5 mg/ml) were used for 5 to 30 min. Viability of protoscoleces was confirmed by eosin exclusive test.
Results
In the present study, all of the various concentrations of the B. vulgaris methanolic extract (0.25, 0.5, 1 and 2 mg/ml) and berberine (0.062, 0.125, 0.25 and 0.5 mg/ml) revealed significant (P<0.05) scolicidal effects against protoscoleces of E. granulosus in a dose-dependent manner. Both berberine and methanolic extract exhibited 100% inhibition against protoscoleces of E. granulosus at the concentration of 2.0 and 0.5 mg/ml after 10 min incubation, respectively.
Conclusion
According to the results, both B. vulgaris methanolic extract and berberine alone demonstrated high scolicidal activities against protoscoleces of hydatid cysts in low concentration and short exposure time on in vitro model. However, in vivo efficacy of B. vulgaris and berberine also requires to be evaluated using an animal model with hydatid infection.
Keywords: Hydatid cyst, European barberry, Scolicidal, Echinococcus granulosus
Introduction
Hydatidosis (cystic echinococcosis) is a chronic infection of medical and veterinary importance caused by the larval stage of a cosmopolitan parasitic cestode Echinococcus granulosus (dog tapeworm). Cystic echinococcosis (CE) in humans and livestock indicates a major public health and economic problem with worldwide distribution (1). Human infection may happen after ingestion of infective eggs, which are passed in the feces of dogs through direct contact or environmental contamination (2). The released embryos penetrate the intestinal wall and via the portal system, get mainly into the liver (50-70%), lungs (20-30%), or other organs where the hydatid cysts grow up (3). At present, surgery remains the most efficient treatment for hydatid disease in many parts of the world, including Iran (4). However, recurring (secondary) CE after operation due to rupture of the cyst and/or spillage of the cyst contents (protoscoleces) during surgery is one of the major surgical complications of hydatidosis (5, 6).
Currently, many scolicidal agents including hypertonic saline, Ag-nitrate, cetrimide, and ethanol, which used for inactivation of the cyst contents present some complications such as sclerosane colangititis (biliary tract fibrosis), liver necrosis and methaemoglobinaemia (4,5,7). Hence, enormous efforts have been made to reach new scolicidal agents especially from natural resources, animals and microorganisms for treatment of CE (8, 9). Recent investigations on plant extracts and plant-derived compounds due to having less side effects, low cost and high availability have been shown a successful approach to treat a wide range of diseases (10). European barberry, Berberis vulgaris L. (family Berberidaceae), grows in Asia and Europe, is well known in Iran and most countries in the world. The different parts of this plant including root, leaf, bark and fruit have been used widely as folk medicine for the treatment and prevention of various diseases including cardiovascular, gastrointestinal, respiratory, skin, renal and infectious diseases (11). Previous studies have also been carried out on chemical composition of the B. vulgaris that showed the most important constituents of this plant are isoquinoline alkaloids such as berberine (12, 13). Up to now, various studies have been demonstrated antibacterial and antifungal effects of B. vulgaris and its main constituent berberine (14-16). Furthermore, in the other studies high antiparasitic potential of B. vulgaris and berberine against Entamoeba histolytica, Giardia lamblia, Trichomonas vaginalis and some Leishmania spp have been proven (17-20).
This study was aimed to investigate the in vitro scolicidal effects of B. vulgaris as well as its main component, berberine against protoscoleces of hydatid cysts.
Materials and Methods
Plant materials
B. vulgaris roots were gathered from Baft district in September 2012, Kerman Province, Iran. The identities were confirmed by the botanist at the Botany Department of Shahid Bahonar University, Kerman, Iran. A voucher specimen of the plant materials was deposited at the Herbarium of Department of Pharmacognosy of School of Pharmacy, Kerman University of Medical Science, Kerman, Iran (KF769).
Preparation of the methanolic extract
The dried plant materials (200 g) were grinded into powder with an electric mill and extracted by percolation method by methanol for 72 h. in room temperature. The extract was passed through filter paper (Whatman No.3, Sigma, Germany) to remove plant debris. Then concentrated in vacuum at 50 °C using a rotary evaporator (Heidolph, Germany) and stored at -20°C, until testing (21).
Preparation of berberine
Berberine, obtained from Sigma-Aldrich, (St. Louis, MO, USA), was dissolved in the dimethyl sulfoxide (DMSO). Final concentration of DMSO was never exceeded 1% in either control or treated samples.
Drug dilutions
An amount of 200 mg of berberine dissolved in 4 ml of dimethyl sulphoxide (DMSO) and serial dilution was subsequently made to obtain berberine at 0.5, 0.25, 0.125 and 0.062 mg/ml concentrations. Furthermore, 2 g of the methanolic extract of B. vulgaris was dissolved in 10 ml of distilled water and serial dilutions were then made to obtain the concentrations of 2, 1, 0.5 and 0.25 mg/ml. It should be mentioned that the selection of appropriate dilutions of the methanolic extract and berberine was based on initial experiments, which also showed that DMSO below 1.5% had no effect on the growth of protoscoleces. In the present investigation the concentration of DMSO in various dilutions was 1% and below.
Collection of protoscoleces
Hydatid cysts from livers of naturally infected sheep were obtained from Kerman slaughterhouse, southeastern Iran. The hydatid fluid of cysts aspirated by a 20 ml syringe and were aseptically transferred into the glass cylinders and left for 30 min to allow the protoscoleces to settle to the bottom of the cylinder. Then, the supernatant was removed and the protoscoleces were washed three times with PBS (pH 7.4) solution. The concentration of protoscoleces was confirmed as the number of protoscoleces per ml of the hydatid fluid in a saline solution (0.9% NaCl solution) containing 2× 103 protoscoleces in 1 ml with more than 90% viability was used for further experimentations. The viability of the protoscoleces was confirmed by 0.1% eosin exclusive test as well as flame cell motility under a light microscope (22).
Scolicidal assay
To investigate the scolicidal effects of B. vulgaris and berberine against protoscoleces of E. granulosus, we used various concentrations of the methanolic extract and berberine for 5, 10, 20 and 30 min. In each experiment, 0.5 ml of the protoscoleces solution (containing at least 1000 protoscoleces) was placed in test tubes using a Pasteur pipette. Then 0.5 ml of various concentrations of the B. vulgaris and berberine were added to each test tube. The contents of the tubes were gently mixed and then incubated at 37°C for 5, 10, 20 and 30 min. At the end of incubation, the upper phase was carefully removed. One hundred μl of 0.1% eosin stain was then added to the remaining settled protoscoleces and gently mixed. The upper portion of the solution was removed after 10 min of incubation. The remaining pellet of protoscoleces was then smeared on a glass slide, covered with a coverslip and examined under a light microscope. The percentage of dead protoscoleces was determined by counting 300 protoscoleces (23). In addition, normal saline and 20% hypertonic saline were used as control groups.
Viability test
In order to evaluate the viability of protoscoleces, eosin solution with a concentration of 0.1% (1 g of eosin powder in 1000 ml distilled water) was used.
Ten minutes after exposure, dead protoscoleces absorbed eosin and colored red (Fig. 1), but alive protoscoleces remained colorless and showed characteristic muscular movements and flame cell activity (Fig. 2). Mortality rate of protoscoleces was determined, as the percent of dead protoscoleces to the total number of protoscoleces.
Fig. 1.
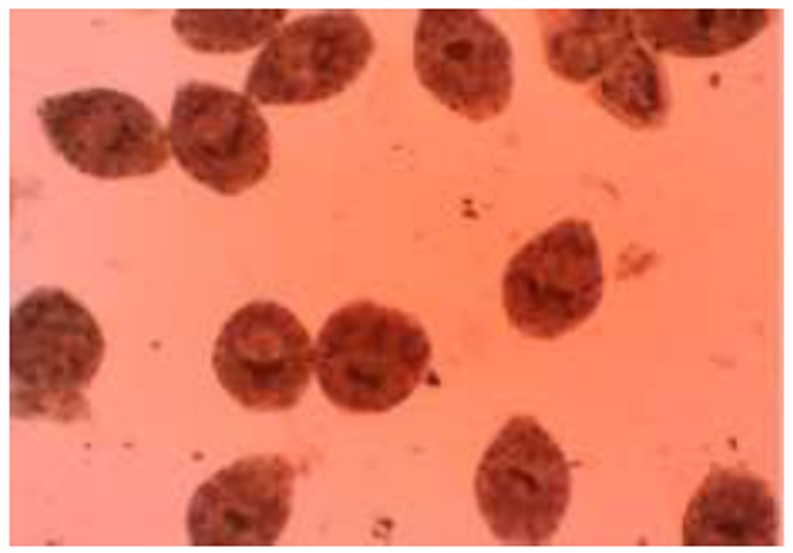
Dead protoscoleces of Echinococcus granulosus after exposure to B. vulgaris and berberine staining with 0.1% eosin
Fig. 2.
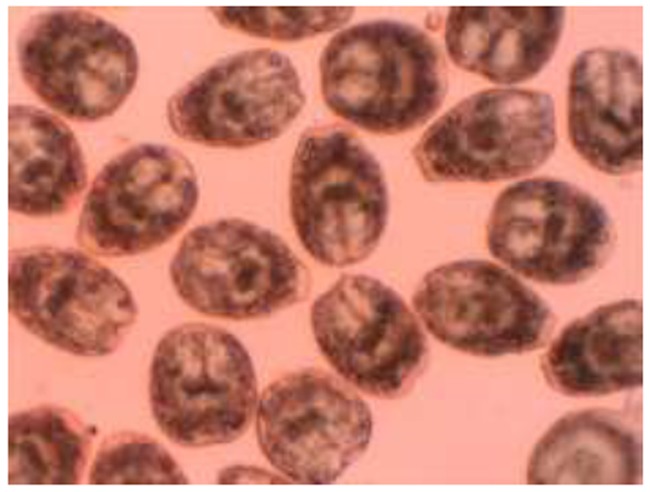
Live protoscoleces of Echinococcus granulosus after exposure with 0.1% eosin
Statistical analysis
In this investigation, all tests were performed in triplicate. Data analysis was carried out by using SPSS statistical package version 17.0 (SPSS Inc., Chicago, IL, USA). Differences between test and control groups were analyzed by Chi-square test and P<0.05 was considered statistically significant.
Results
All of the various concentrations of the B. vulgaris methanolic extract and berberine revealed significant (P<0.05) scolicidal effects against protoscoleces of E. granulosus in a dose-dependent manner as compared with the control groups.
The scolicidal effects of B. vulgaris methanolic extract at various concentrations and different exposure times are shown in Table 1.
Table 1.
Scolicidal effects of Berberis vulgaris root extract against protoscoleces of Echinococcus granulosus at various concentrations following different exposure times
| Mean of mortality rate (%) | Exposure time (min) | Concentration (mg/ml) |
|---|---|---|
| 8.6 | 5 | |
| 13.6 | 10 | |
| 20.3 | 20 | 0.25 |
| 28.3 | 30 | |
| 15.6 | 5 | |
| 22.3 | 10 | 0.5 |
| 31.3 | 20 | |
| 48.6 | 30 | |
| 54.3 | 5 | |
| 76.3 | 10 | 1 |
| 96.6 | 20 | |
| 100 | 30 | |
| 91.3 | 5 | |
| 100 | 10 | 2 |
| 100 | 20 | |
| 100 | 30 | |
| 0 | 5 | |
| 1.3 | 10 | Normal saline |
| 2.6 | 20 | |
| 5.3 | 30 | |
| 83.6 | 5 | Hypertonic saline |
| 100 | 10 | |
| 100 | 20 | |
| 100 | 30 |
When protoscoleces were exposed to methanolic extract at the concentration of 2 mg/ml, the mortality rate was 100% after 10 min of incubation. Similarly, the methanolic extract at concentration of 1 mg/ml caused 56.3, 79.3, 89.3 and 100% protoscoleces mortality after 5, 10, 20 and 30 min incubation, respectively. In contrast, methanolic extract at concentration 0.5 mg/ml killed 15.6, 22.3, 31.4 and 48.6% of the protoscoleces and at the concentration of 0.25 mg/ml killed 8.5, 13.6, 20.2 and 28.3% of the protoscoleces after 5, 10, 20 and 30 min incubation, respectively.
As shown in Table 2, berberine exhibited much more scolicidal effects against protoscoleces of E. granulosus in comparison to methanolic extract. While, the mortality rate of protoscoleces in the control groups was 6.1% after 30 min (normal saline) and 100% after 10 min (20% hypertonic saline). When protoscoleces were exposed to berberine at the concentrations of 0.5 and 0.25 mg/ml, the mortality rate was 100% after 10 and 20 min application, respectively. Moreover, berberine at the concentration of 0.125 mg/ml exhibited 28.3, 48.6, 76.7 and 88.3% protoscoleces mortality and at the concentration of 0.062 mg/ml killed 9.4, 26.6, 44.3 and 51.2 % of the protoscoleces after 5, 10, 20 and 30 min of incubation, respectively.
Table 2.
Scolicidal effects of berberine against protoscoleces of Echinococcus granulosus at various concentrations following different exposure times
| Mean of mortality rate (%) | Exposure time (min) | Concentration (mg/ml) |
|---|---|---|
| 9.6 | 5 | 0.062 |
| 26.6 | 10 | |
| 44.3 | 20 | |
| 51.3 | 30 | |
| 28.3 | 5 | |
| 48.6 | 10 | 0.125 |
| 76.6 | 20 | |
| 88.3 | 30 | |
| 53.3 | 5 | |
| 91.3 | 10 | 0.25 |
| 100 | 20 | |
| 100 | 30 | |
| 84.6 | 5 | |
| 100 | 10 | 0.5 |
| 100 | 20 | |
| 100 | 30 | |
| 0 | 5 | |
| 1.3 | 10 | Normal saline |
| 2.6 | 20 | |
| 5.3 | 30 | |
| 83.6 | 5 | Hypertonic saline |
| 100 | 10 | |
| 100 | 20 | |
| 100 | 30 |
Discussion
This investigation was designed to determine the in vitro effect of methanolic extract of B. vulgaris as well as its main compound, berberine against protoscoleces of E. granulosus. Findings revealed high protoscolicidal effects of B. vulgaris methanolic extract in all concentrations especially at the concentration of 2 mg/ml and after 10 min (100% mortality rate). In addition, berberine concentration of 0.5 mg/ml killed 100% protoscoleces after 10 min incubation.
Surgery is the preferred treatment for particular WHO stage disease. Chemotherapy with benzimidazoles and PAIR (puncture, aspiration, injection and reaspiration) are recommended as alternative treatments to surgery, especially for the patients who cannot tolerate surgery (24). The main surgical complications of CE are recurrence, secondary CE and anaphylactic shock due to rupture of the cyst and spillage of the cyst contents (protoscoleces) during surgery, which is observed in nearly 10% of the cases. Thus, the use of effective scolicidal agents during surgery is necessary (5, 6). So far, scolicidal activity of hypertonic saline (25), silver nitrate and mannitol (26), cetrimide (27), ethyl alcohol (95%) (28), H2O2 and 10% povidone iodine (29), albendazole (30), chlorhexidine gluconate (31), honey (32), selenium nanoparticles (33) and some plant extracts (9, 22) in various studies have been proven. While, they are associated with adverse effects and their efficacy is controversial (7). Therefore, development of new scolicidal agents, especially from natural sources, is of great interest.
In the past centuries, plants extracts and plant-derived compounds have been widely used as a valuable natural resource of traditional medicine (34). In the past decades advent of synthetic antimicrobial drugs led to reluctance in plants as a rich source of antimicrobial agents (35). Recently, emergence of some limitations in the use of these synthetic drugs caused a shift in the interest to ethnobotanical research (36).
Our findings indicated that B. vulgaris methanolic extract at the concentration 2 mg/ml (10 min) and berberine at the concentration of 0.5 mg/ml (10 min) were comparable with scolicidal activity of 20% hypertonic saline (15 min), 20% silver nitrate (20 min), 0.5–1% cetrimide (10 min), H2O2 3% (15 min) and 95% ethyl alcohol (15 min). Recently, in the study conducted by Rouhani et al (37) it has been shown that aqueous extract of B. vulgaris at the concentration of 4 mg/ml indicated scolicidal activity of 100% after 5 min. Whereas at the concentration of 2 mg/ml kills 96, 98, and 98.7% of protoscoleces after 5, 15, and 30 min of application, respectively. These different results might be due to the type of solvent, which has been use for extraction. Our results show that berberine could be responsible for scolicidal effect of B. vulgaris root against protoscoleces of E. granulosus. This alkaloid has been sparingly soluble in water and it is expected that the liquid extraction contain negligible amounts of berberine, whereas methanolic extract can draw this alkaloid from plant roots. In the case of toxicity of B. vulgaris and berberine, previous studies proved that they are not considered toxic at the doses used in clinical situations, nor have they been shown to be cytotoxic or mutagenic, whereas, their side effects can be pertained to dose enhancement (38, 39).
Conclusion
B. vulgaris methanolic extract and its main compound, berberine in particular demonstrated high scolicidal activities against protoscoleces of E. granulosus in low concentration and short exposure time on in vitro model. However, in vivo efficacy of B. vulgaris and berberine requires to be evaluated using an animal model of hydatid infection.
Acknowledgments
We would like to thank you Dr. Ghasemi Kia for preparation of hydatid cysts. The authors declare that there is no conflict of interest in this study.
References
- 1.Fasihi Harandi M, Budke CM, Rostami S. The Monetary Burden of Cystic Echinococcosis in Iran. PLOS Negl Trop Dis. 2012;6(11):e1915. doi: 10.1371/journal.pntd.0001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torgerson PR, Budke CM. Echinococcosis – an international public health challenge. Res Vet Sci. 2003;74:191–202. doi: 10.1016/s0034-5288(03)00006-7. [DOI] [PubMed] [Google Scholar]
- 3.Eckert J, Deplazes P. Biological, epidemio-logical, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17:107–35. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunetti E, Kern P, Vuitton DA Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Tro. 2010;114(1):1–16. doi: 10.1016/j.actatropica.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Rajabi MA. Fatal reactions and methae-moglobinaemia after silver nitrate irrigation of hydatid cyst. Surg Pract. 2009;13:2–7. [Google Scholar]
- 6.Topcu O, Sumer Z, Tuncer E, Aydin C, Koyuncu A. Efficacy of chlorhexidine gluconate during surgery for hydatid cyst. World J Surg. 2009;33:1274–80. doi: 10.1007/s00268-009-9971-z. [DOI] [PubMed] [Google Scholar]
- 7.McManus DP, Zhang W, Li J. Echinococcosis. Lancet. 2003;362:1295–304. doi: 10.1016/S0140-6736(03)14573-4. [DOI] [PubMed] [Google Scholar]
- 8.Adas G, Arikan S, Kemik O, Oner A, Sahip N, Karatepe O. Use of albendazole sulfoxide, albendazole sulfone, and combined solutions as scolicidal agents on hydatid cysts (in vitro study) World J Gastroenterol. 2009;15:112–6. doi: 10.3748/wjg.15.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zibaei M, Sarlak A, Delfan B, Ezatpour B, Azargoon A. Scolicidal effects of Olea europaea and Satureja khuzestanica extracts on protoscolices of hydatid cysts. Korean J Parasitol. 2012;50(1):53–6. doi: 10.3347/kjp.2012.50.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocha LG, Almeida JR, Macedo RO, Barbosa-Filho JM. A review of natural products with antileishmanial activity. Phytomedicin. 2005;12:514–35. doi: 10.1016/j.phymed.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Imanshahidi H, Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, Berberine. Phytother Res. 2008;22:999–1012. doi: 10.1002/ptr.2399. [DOI] [PubMed] [Google Scholar]
- 12.Ivanovska N, Philipov S. Study of the anti-inflammatory action of Berberis vulgaris root extract, alkaloid fractions and pure alkaloids. Int J Immunopharmacol. 1996;18:553–61. doi: 10.1016/s0192-0561(96)00047-1. [DOI] [PubMed] [Google Scholar]
- 13.Küpeli E, Koar M, Yeilada E, Hüsnü K, Baer C. A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species. Life Sci. 2002;72:645–7. doi: 10.1016/s0024-3205(02)02200-2. [DOI] [PubMed] [Google Scholar]
- 14.Nakamoto K, Sadamori S, Hamada T. Effects of crude drugs and berberine hydrochloride on the activities of fungi. J Prosthet Dent. 1990;64(6):691–4. doi: 10.1016/0022-3913(90)90298-q. [DOI] [PubMed] [Google Scholar]
- 15.Freile ML, Giannini F, Pucci G, Sturniolo A, Rodero L, Pucci O. Antimicrobial activity of aqueous extracts and of berberine isolated from Berberis heterophylla. Fitoterapia. 2003;74:702–5. doi: 10.1016/s0367-326x(03)00156-4. [DOI] [PubMed] [Google Scholar]
- 16.Ghaderi R, Maleki Nejad P. evaluation of anticandidal effects of Berberis vulgaris root extracts (methanolic and aqueous) and comparing their effects with those clotrimazole. J Birjand Univ Med Sci. 2006;13(2):42–8. [Google Scholar]
- 17.Vennerstrom JL, Lovelace JK, Waits VB, Hanson WL, Klayman DL. Berberine derivatives as anti-leishmanial drugs. Antimicrob Agents Chemother. 2005;34(5):198–211. doi: 10.1128/aac.34.5.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaneda Y, Torii M, Tanaka T, Aikawa M. In vitro effects of berberine sulphate on the growth and structure of Entamoeba histolytica, Giardia lamblia and Trichomonas vaginalis. Ann Trop Med Parasitol. 1991;85:417–25. doi: 10.1080/00034983.1991.11812586. [DOI] [PubMed] [Google Scholar]
- 19.Fata A, Rakhshandeh H, Berenji F, Jalalifard A. Ttreatment of cutaneous leishmaniasis in murine model by alcoholic extract of Berberis vulgaris. Iran J Parasitol. 2006;1(1):39–42. [Google Scholar]
- 20.Mahmoudvand H, Sharififar F, Sharifi I, Ezatpour B, Fasihi Harandi M, Makki M, Ziaali N, Jahanbakhsh S. In vitro inhibitory effect of Berberis vulgaris (Berberidaceae) and its main component, berberine against different Leishmania species. Iran J Parasitol. 2014;9(1):28–36. [PMC free article] [PubMed] [Google Scholar]
- 21.Sharififar F, Mozaffarian V, Moshafi MH. Chemical composition and biological activities of Zhumeria majdae Resh. f. &Wendelbo. Jundishapur J Nat Pharm Prod. 2008;3(1):8–18. [Google Scholar]
- 22.Moazeni M, Saharkhiz MJ, Hosseini AA. In vitro lethal effect of ajowan (Trachyspermum ammi L.) essential oil on hydatid cyst protoscoleces. Vet Parasitol. 2012;187:203–8. doi: 10.1016/j.vetpar.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 23.Smyth JD, Barrett NJ. Procedures for testing the viability of human hydatid cysts following surgical removal, especially after chemotherapy. Trans R Soc Trop Med Hyg. 1980;74:649–52. doi: 10.1016/0035-9203(80)90157-1. [DOI] [PubMed] [Google Scholar]
- 24.Moro PL, Schantz PM. Echinococcosis: historical landmarks and progress in research and control. Ann Trop Med Parasitol. 2006;100:703–14. doi: 10.1179/136485906X112257. [DOI] [PubMed] [Google Scholar]
- 25.Kayaalp C, Balkan M, Aydin C, Ozgurtas T, Tanyuksel M, Kirimlioglu V, Akoglu M, Oner K, Pekcan M. Hypertonic saline in hydatid disease. World J Surg. 2001;25:975–9. doi: 10.1007/s00268-001-0065-9. [DOI] [PubMed] [Google Scholar]
- 26.Caglar R, Yuzbasioglu MF, Bulbuloglu E, Gul M, Ezberci F, Kale I. In vitro effectiveness of different chemical agents on scolices of hydatid cyst. J Invest Surg. 2001;21:71–5. doi: 10.1080/08941930701883640. [DOI] [PubMed] [Google Scholar]
- 27.Besim H, Karayalcin K, Hamamci O, Güngör C, Korkmaz A. Scolicidal agents in hydatid cyst surgery. HPB Surg. 1998;10:347–51. doi: 10.1155/1998/78170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erzurumlu K, Hokelek M, Baris S, Sahin M, Birinci A, Amanvermez R, Tac K. Effect of albendazole sulfoxide solution on the scolices and the hepatobiliary system. Eur Surg Res. 1998;30:433–8. doi: 10.1159/000008610. [DOI] [PubMed] [Google Scholar]
- 29.Landa Garcı′a JI, Alonso E, Gonzalez-Uriarte J. Evaluation of scolicidal agents in an experimental hydatid disease model. Eur Surg Res. 1997;29:202–8. doi: 10.1159/000129525. [DOI] [PubMed] [Google Scholar]
- 30.Paksoy Y, Odev K, Sahin M. Percutaneous treatment of hydatid cysts: comparison of direct injection of albendazole and hypertonic saline solution. AJR Am J Roentgenol. 2005;185(3):727–34. doi: 10.2214/ajr.185.3.01850727. [DOI] [PubMed] [Google Scholar]
- 31.Puryan K, Karadayi K, Topcu O. Chlorhexidine gluconate: an ideal scolicidal agent in the treatment of intraperitoneal hydatidosis. World J Surg. 2005;29:227–30. doi: 10.1007/s00268-004-7587-x. [DOI] [PubMed] [Google Scholar]
- 32.Kilicoglu B, Kismet K, Koru O, Tanyuksel M, Oruc MT, Sorkun K, Akkus MA. The scolicidal effects of honey. Adv Ther. 2006;23:1077–83. doi: 10.1007/BF02850228. [DOI] [PubMed] [Google Scholar]
- 33.Mahmoudvand H, Fasihi Harandi M, Shakibaie M, Aflatoonian MR, Makki MS, Jahanbakhsh S. Scolicidal effects of biogenic selenium nanoparticles against protoscolices of hydatid cysts. Int J Surg. 2014 Mar 28; doi: 10.1016/j.ijsu.2014.03.017. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Cowan MM. Plant products as antimicrobial agents. Clin Microb Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones FA. Herbs-useful plants. Their role in history and today. Eur J Gastroenterol Hepatol. 1996;8:1227–31. doi: 10.1097/00042737-199612000-00018. [DOI] [PubMed] [Google Scholar]
- 36.McCutcheon AR, Ellis SM, Hancock REW, Tower GN. Antibiotic screening of medicinal plants of the British Columbian native peoples. J Ethnopharmacol. 1992;37:213–23. doi: 10.1016/0378-8741(92)90036-q. [DOI] [PubMed] [Google Scholar]
- 37.Rouhani S, Salehi N, M Kamalinejad M, Zayeri F. Efficacy of Berberis vulgaris aqueous extract on viability of Echinococcus granulosus protoscolices. J Invest Surg. 2013;26(6):347–51. doi: 10.3109/08941939.2013.818746. [DOI] [PubMed] [Google Scholar]
- 38.Peychev L. Pharmacological investigation on the cardiovascular effects of Berberis vulgaris on tested animals. Pharmacia. 2005;52:118–21. [Google Scholar]
- 39.Lin CC, Ng LT, Hsu FF, Shieh DE, Chiang LC. Cytotoxic effects of Coptis chinensis and Epimedium sagittatum extracts and their major constituents (berberine, coptisine and icariin) on hepatoma and leukaemia cell growth. Clin Exp Pharmacol Physiol. 2004;31:65–9. doi: 10.1111/j.1440-1681.2004.03951.x. [DOI] [PubMed] [Google Scholar]


