Abstract
Background
Giardia lamblia is one of the most prevalent intestinal flagellate protozoa that infects a wide range of vertebrate hosts causing severe intestinal disorder in children.This study was performed to determine subspecies of G.lamblia by the PCR-RFLP method, targeting the glutamate dehydrogenase(gdh)locus, in hospitalized children at Urmia Mutahhari Hospital, West Azerbaijan Province,Iran and determining the infection transformational storages in this area.
Methods
Overall, 720 stool specimens were collected from the hospitalized children, 34 samples were positive and Giardia cysts were detected under the microscope. Cysts were partially purified by the sucrose density gradient method and then washed with sterile distilled water to remove effectively the PCR inhibitors. Genomic DNA of G. lamblia isolates was extracted by freeze-thaw cycles followed by phenol/ chloroform/isoamyl alcohol method. The single step PCR-RFLP assay was used to differentiate the assemblages between A and B, which were found in humans. In this method, 432 bp expected size was amplified, and then for detection of subspecies, specific restriction RsaI and BspLI enzymes were used.
Results
Totally 34 samples were positive in terms of Giardia cyst out of 720 examined samples microscopically, so the parasite spread rate is reported 4.72%. Analysis PCR-RFLP on these samples revealed that 28 samples (93.3%) have the genotype BIII and 2 samples (6.7%) belong to the subgroup BIV.
Conclusion
PCR-RFLP is a proper analytical method for determining the genotype among parasite types, using the glutamate dehydrogenizes zone’s genes. Based on the results, an animal origin of infection cycle is suggested.
Keywords: Giardia lamblia, Glutamate dehydrogenase, Children, PCR-RFLP, Iran
Introduction
Giardiasis, a common intestinal protozoan infection caused by Giardia lamblia(also known as G. duodenalis or G.intestinalis), has gained attention as a neglected disease in both developed and developing countries (1). Prevalence of G. lamblia is found in all age groups, but children are at the greatest risk for contracting clinical giardiasis. Clinical presentations of giardiasis differ from asymptomatic carriage to acute and chronic diarrhea (2, 3). Giardia isolates based on morphological criterion which six species, namely: G. agilis,G.ardeae,G.lamblia, G.microti, G.muris, and G.psittaci, vary significantly in their biology, host specificity, and genetics (4).
Among the 6 species, G.lamblia infects humans and numerous other mammals. Isolation of G.lambliais classified under 7 assemblages (A-G), based on the characterization of the glutamate dehydrogenase, small-subunit rRNA, and triose phosphate isomerase (tpi) genes (5, 6). Assemblage of A isolates has been placed into subgroups I and II. Assemblage of B isolates has been separated into subgroups III and IV. “Genetic assemblages C, D, E, F, and G seem to be restricted to domestic animals, livestock, and wild animals” (4, 7). Although all human-derived Giardia isolates belong to assemblages A and B, these assemblages have also been found in isolation from the other domestic and wild animals such as dogs, cats, and cattle (8). Some researchers consider that presenting of G. lambliais reflected on a risk of zoonosis from cattle (9), dogs (10-12), wild moose, reindeer (13), farm, and wild animals (14). The gdh gene is proven useful for the genotyping of Giardia isolated from mammals. PCR-RFLP has successfully been used by a number of researchers to differentiate between Giardia genotypes for humans and animals (4, 5, 8). This infection is diversely dispersed throughout all over Iran, such as West Azerbaijan Province. Incidence in this province is varied from 10.3% (15, 16) to 43.8% (17). However, most studies do not evaluate the risk factors for acquiring G. lamblia infection, which are essential for prevention and control strategies.
The primary goal of this study was to determine the genotypes of G. lamblia isolates (17) and identification of potential zoonotic reservoir in this area, with used sucrose density gradient, DNA extraction by phenol/ chloroform/isoamylalcohol, PCR RFLP method to acquire high sensitivity result in fecal samples.
Materials and Methods
Sample collection
Overall, 720 stool specimens were collected from the hospitalized children, between June 2011 and February 2012. All samples were tested by light microscopy. Giardia cysts were isolated and partially purified by sucrose flotation (18, 19). The semi filtered and concentrated cysts were stored in sterile distilled water without adding any preservatives, up to two weeks at -20 °C.
DNA extraction
According to repeated freezing and thawing method, this process was performed by 6 times freezing and thawing in liquid nitrogen for 60 seconds and in 65°C water bath for 60 seconds, respectively (20).
Then, DNA extraction was performed based on glass beads and phenol-chloroform extraction assay (21). DNA presented in the supernatant was precipitated with 0.1 volumes 3M sodium acetate (pH 5.2), and 2-propanol. The precipitant had been washed with 70% ethanol and then the purified DNA was resuspended in 30 μl of distilled water.
PCR amplification
Amplification of the gdh genes was accomplished as the single PCR. In the PCR reaction, the 432 bp fragment was amplified by using the forward primer (GDHiF) 5-CAG TAC AAC TCY GCT CTC GG-3 and the reverse primer (GDHiR), 5-GTT RTC CTT GCA CAT CTC C-3 (4). Amplification reaction was modified as follows- the PCR mix consisted of 1X buffer containing1.5 mM MgCl2 (Cinaclon, Iran), each deoxynucleotide triphosphate at the concentration of 100μM (Cinaclon, Iran), each primer at a concentration of 0.5 μM, 10 ng of DNA and 2.5 U of HotStarTaq DNA polymerase (Cinaclon, Iran). Cycling parameters were 10 min at 94°C (initial heat activation step), followed by 50 cycles of 35 s at 94°C, 35 s at61°C, and 50 s at 72°C, with a final extension of 7 min at 72°C (4). Both positive and negative controls were included in each PCR to validate results. Cysts were utilized as the templates for the positive controls, and distilled water was utilized as the template for negative controls throughout.
RFLP analysis
RFLP analysis was performed by digesting 8 μl of PCR products with 1.5 U of RsaI (vivantis) or 0.8 U of BspLI (vivantis) in 2 μl of 10X enzyme buffer in a final volume of 20 μl for 3 h at 37°C (18). The RsaI digestion allowed the distinction between the assemblage of B group III and group IV after amplification. The BspLI digestion was employed for the distinction between assemblage A group I, assemblage A group II after amplification with the GDHiF and GDHiRprimers (4).
PCR product and restriction fragment detection. PCR products and restriction fragments were separated by horizontal electrophoresis in 1.5 and 2% agarose gels, respectively, with ethidium bromide (0.6 μg/ml) staining. A 100-bpDNA ladder (Fermentas, Lithuania) was included as the size marker. PCR products and restriction fragments were recorded by UV transillumination (4).
Results
DNA extraction
After DNA extraction, we ran samples on agarose gels (1.5%) to confirm DNA extraction, but in most cases, there was either a paucity or absence of DNA. In these circumstances, the causative agent could have low parasite numbers of isolates. However, using PCR amplification, extracted DNA of positive samples is confirmed.
PCR amplification
In 34 samples, gdh gene was intensified by using freeze-thaw technique and phenol/chloroform/isoamylalcohol method, 30 samples (88.2%) with the use of primers GDHiF, GDHiR, a432bp expected size was amplified (Fig. 1).
Fig.1.
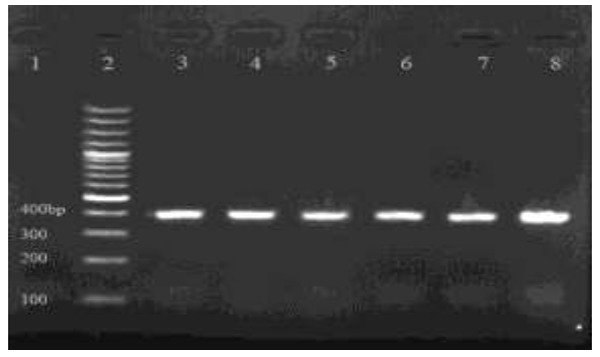
Electrophoretic separation of PCR product from DNA amplified at the gdh locus of G. lamblia, on an ethidium bromide stained 1.5% agarose gel. Lane 1, negative control; lane 2, 100 bp, Plus molecular weight marker(Fermentas, Lithuania); Lanes 3-8, PCR products (432 bp fragment)
RFLP method
RFLP assay on 30 samples, with using RsaI, BspLI enzymes. The genotyping results are summarized in Table 1.
Table 1.
Genotypes of G. lamblia determined by PCR-RFLP of gdh locus
| Isolate code | Genotype | Isolate code | Genotype |
|---|---|---|---|
| 1 | BIII | 16 | BIII |
| 2 | BIII | 17 | BIII |
| 3 | BIII | 18 | BIII |
| 4 | BIII | 19 | BIII |
| 5 | BIII | 20 | BIII |
| 6 | BIII | 21 | BIII |
| 7 | BIII | 22 | BIII |
| 8 | BIII | 23 | BIII |
| 9 | BIII | 24 | BIII |
| 10 | BIV | 25 | BIII |
| 11 | BIII | 26 | BIII |
| 12 | BIII | 27 | BIV |
| 13 | BIII | 28 | BIII |
| 14 | BIII | 29 | BIII |
| 15 | BIII | 30 | BIII |
Out of 30 samples isolates, 28 samples (93.3%) were found as G.lamblia (genotype BIII), 2(6.7%) belonged to assemblage BIV (Fig. 2).
Fig. 2.
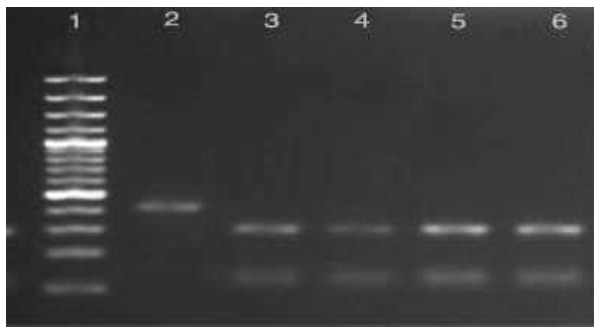
RsaI and BspLI digestion of PCR products on an ethidium bromide –stained 2% high resolution agarosegel. Line 2, G.lamblia assemblage BIV, (RsaI digestion): line 3, G. lamblia assemblage BIII (RsaI digestion), line 4-6, G.lambliaassemblage B (BspLI digestion) and line 1, 100bp plus molecular weight marker (Fermentas, Lithuania)
Risk Factors
Table 2 shows analysis of the risk factors for giardiasis in this population; it pointed at children ranging in age from 3 to 5 years old which had a superior risk of acquiring giardiasis.
Table 2.
Characteristics of hospitalized children and prevalence of Giardia lamblia infection
| Study group | no. examined | No. infected(%) | P. value |
|---|---|---|---|
| Male | 374 | 19(5.08) | 0.580 |
| Female | 349 | 15 (4.29) | 0.580 |
| <1 | 32 | 0 (0) | 0.001 |
| 1-3 | 38 | 1 (2.6) | 0.001 |
| > 3-5 | 215 | 17(7.9) | 0.001 |
| >5-8 | 185 | 11 (5.9) | 0.001 |
| >8-11 | 121 | 2(1.6) | 0.001 |
| >11-14 | 129 | 3 (2.3) | 0.001 |
| total | 720 | 34 (4.72) |
The results show that there is no significant relationship between infection and sex. (P.value=0.580).
Discussion
Giardiasis is a common intestinal protozoan infection caused by Giardia lamblia. Infection with G.lamblia is widespread in both humans and animals and multiple transmission routes exist, with water and food playing an increasingly recognized role worldwide (20). To understand the epidemiology of the infection and to implement control measures, it is important to determine genotype of G.lamblia. For this reason, to use advanced tools such as PCR-RFLP (21).In this study, molecular analysis on these samples revealed that 28 samples (93.3%) have the genotype BIII and 2 samples (6.7%) belong to the subgroup BIV. Based on the results, an animal origin of infection cycle is suggested.
As it has been mentioned, G. lamblia is the most crucial parasitic disease that is spread in various parts of Iran, especially in Urmia, which is an endemic region. Wide epidemiological studies have been conducted in this area by Dr. Hazrati et al., during 2008-2009 (15, 16) which proved that the occurrence of Giardiosis among elementary school students in the district had been 10.3%. But in the current study the outcome is 4.72%, which indicates that the level of people’s consciousness, knowledge of the public health, personal hygiene, use of safe water, health facilities, etc. have the positive effects on the decrease of the rate of giardiasis infection.
PCR-RFLP is a sensitive and powerful analytical tool which is capable of providing the level genotyping discrimination among assemblages by targeting some loci such as gdh and tpi, making it possible to identify the presence of mixed genotypes (5, 8, 23). For direct use of stool and existing PCR inhibitors, there were purified and concentrated by flotation on sucrose with specific gravity of 0.85 M.
Moreover, direct amplification of cysts DNA from feces assisted PCR inhibitors (e.g. lipids, hemoglobin, bile, salts, polysaccaharides from mucus, bacteria and food degradation product) which could affect the result of amplification (21), but with the use of sucrose density gradient centrifugation and washing with sterile distilled water in order to be effectively removed(21). The cyst walls are resistant to DNA extraction, therefore DNA extraction was ineffective. This became possible with repeated freezing and thawing, glass beads and phenol/chloroform/isoamylalcohol method (4).
All G.lamblia assemblages attained from humans were identified as assemblage B group, due to assemblage B more frequency than assemblage A in this region, corresponding to the findings of an Indian study that examined 10 clinical individual samples and found 100% assemblage B (23) and it was different from an Iranian study which found G.lamblia detected in humans (87%) of assemblage A(21). This study provides, for the first time, information on the distribution of the genotype of G.lamblia from humans with sporadic giardiasis in West Azerbaijan Province of Iran.
The difference between the occurrence of assemblages A and B may be attributed to the geographic locations of the patient studies.
Given information has evolved livestock in Urmia and surrounding and considered the obtained molecular results which indicate the dominant subspecies parasites in this area BIII is often more prevalent in humans, livestock; according to the study conducted on calves by Dalir Naghadeh et al. 2006-2007 during the infection rate reported by approximately twelve percent (24). The most likely hypothesis is that calves are the source of infection in this region. To prove this theory, a molecular epidemiological study is recommended for livestock, particularly cattle and calves infected with Giardia, which are important to understand the epidemiology and infection control procedures; cycle of transmission of diseases to humans; and to identify reservoirs.
The current study demonstrated that assemblage B, subgenotype BIII was widespread in Urmia, Iran.
Serve notice of the prevalence of assemblage B and none of sub assemblage AII, an animal source of infection is suggested. This information will be advantageous to the effective prevention and control program of giardiasis in this population.
Therefore, to discover the role of domestic animals and livestock as a potential source of infection for humans in the community, a research on molecular was advised, and secondly, to guarantee that there is no parasite genetic diversity in this region, it has been suggested that other molecular researches should beconducted on people whom are older than 14 years.
The consequence of PCR-RFLP in this region, which is mostly BIII evidenced that there is no relationship among genders, ages, and subtypes (Table 2).
According to Chi Square Test, there is a significant connection between age groups and parasitic infections that indicates that in the age group 3 to 5 years, the prevalence of the parasite occurs (P = 0.001).
Given the absence of positive samples in less than 1-year-old offspring, it seems that this age group has no direct contact with parasites. Besides, an unusual lipase enzyme in human milk, which is effective in preventing giardiosis in this age range, had been considered. It is essential to mention that the sample of individuals with gastrointestinal symptoms had been conducted. Thus, the study of parasites and subtype disease has been inevitably cancelled by itself.
Conclusion
According to the achieved outcomes, it can be concluded that:
The Phenol chloroform assay using frozen- thaw and the use of glass beads are the best and cheapest method of extracting DNA from G. lamblia cysts.
Isolating of G. lamblia is similar in terms of morphology, and genomic variation.
There are the subspecies B G. lamblia in West Azerbaijan that biotype dominant is BIII.
G.lamblia, subspecies BIII, normally is in livestock, and according to high infections of Giardia in this region, a zoonotic origin of the infection route is suggested.
The logical association between assemblageswith age and admissions were not observed.
Acknowledgments
The authors are grateful to all the staff of the center of Public Health Motahhari Hospital, especially Miss. Hossenzadeh and Mrs. Hashemiat sample collection section at the study site. In addition, we thank Research Deputy of Urmia University of Medical Sciences for providing financial support for this project. The authors declare that there is no conflict of interests.
References
- 1.Savioli L, Smith H, Thompson A. Giardia and Cryptosporidiumjoin the “Neglected Diseases Initiative”. Trends Parasitol. 2006;22:203–8. doi: 10.1016/j.pt.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Ali SA, Hill DR. Giardia intestinalis. Curr Opin Infect Dis. 2003;16:453–60. doi: 10.1097/00001432-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Ajjampur SSR, Sankaran P, Kannan A, Sathyakumar K, Sarkar R, Beryl P, et al. Giardia duodenalis assemblages associated with Diarrhea in Children in South India Identified by PCR-RFLP. Am J Trop Med Hyg. 2009;80(1):16–19. [PMC free article] [PubMed] [Google Scholar]
- 4.Fallah E, Nahavandi k, Jamali R, MahdaviPoor B, Asgharzadeh M. Molecular identification of Giardia duodenalis isolates from human and animal reservoirs by PCR-RFLP. J Biol Sci. 2008;2:172–84. [Google Scholar]
- 5.Read CM, Monis PT, Thompson RC. Discrimination of all genotypes of Giardia duodenalis at the glutamate dehydrogenaselocus using PCR-RFLP. Infect Genet. 2004;4:125–30. doi: 10.1016/j.meegid.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Helmy Moshira MF, Abdel-Fattah Hisham S, Rashed L. Real-time PCR/RFLP assay to detect Giardia intestinalis genotypes in human isolates with diarrhea in Egypt. J Parasitol. 2009;95(4):1–5. doi: 10.1645/GE-1670.1. [DOI] [PubMed] [Google Scholar]
- 7.Monis PT, Andrews RH, Mayrhofer G, Ey PL. Genetic diversity within the morphological species Giardia intestinalisand its relationship to host origin. Infect Genet. 2003;3:29–38. doi: 10.1016/s1567-1348(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 8.Itagaki T, Kinoshita S, Aoki M, Itoh N, Saeki H, Sato N, et al. Genotyping of Giardia intestinal is from domesticated wild animals in Japan using glutamate dehydrogenase gene sequencing. Vet Parasitol. 2005;133(4):283–87. doi: 10.1016/j.vetpar.2005.05.061. [DOI] [PubMed] [Google Scholar]
- 9.O’Handley RM, Olson ME, Fraser D, Adams P, Thompson RC. Prevalence and genotypic characterization of Giardia in dairy calves from Western Australia and Western Canada. Vet Parasitol. 2000;90:193–200. doi: 10.1016/s0304-4017(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 10.Eligio-Garcia L, Cortes-Campos A, Jimenez-Cardosoe E. Genotype of Giardia intestinalis isolates from children and dogs and its relationship to host origin. Parasitol Res. 2005;97(1):1–6. doi: 10.1007/s00436-005-1368-9. [DOI] [PubMed] [Google Scholar]
- 11.Leonhard S, Pfister K, Beelitz P, Wielinga C, Thompson RC. The molecular characterization of Giardia from dogs in southern Germany. Vet Parasitol. 2007;30(1):33–8. doi: 10.1016/j.vetpar.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 12.Traub RJ, Monis PT, Robertson I, Irwin P, Mencke N, Thompson RC. Epidemiological and molecular evidence support the zoonotic transmission of Giardia among humans and dogs. 149 living in the same community. Parasitology. 2004;128(3):253–62. doi: 10.1017/s0031182003004505. [DOI] [PubMed] [Google Scholar]
- 13.Robertson LJ, Forberg T, Hermansen L, Hamnes IS, Gjerde B. Giardia duodenalis cysts isolated from wild moose and reindeer in Norway: genetic characterization by PCR-RFLP and sequence analysis at two genes. J Wildl Dis. 2007;43(4):576–85. doi: 10.7589/0090-3558-43.4.576. [DOI] [PubMed] [Google Scholar]
- 14.Van Keulen H, Macechko PT, Wade S, Schaaf S, Wallis PM, Erlandsen SL. Presence of human Giardia in domestic, farm and wild animals, and environmental samples suggest a zoonotic potential for giardiasis. Vet Parasitol. 2002;108:97–107. doi: 10.1016/s0304-4017(02)00181-4. [DOI] [PubMed] [Google Scholar]
- 15.Hazrati Tappeh Kh, Mohammadzadeh H, Khashaveh Sh, Rezapor B. Prevalence of intestinal parasitic infections among primary school attending students in Nazloo-Chay rural region of Urmia, West Azerbaijan province, Iran in 2008. Urmia Journal of Medical Science. 2011;4(16):18–21. [Google Scholar]
- 16.Hazrati Tappeh Kh, Mohammadzadeh H, Khashaveh Sh, Rezapor B. Prevalence of intestinal parasitic infections among primary school attending students in Barandooz-Chay rural region of Urmia, West Azerbaijan province, Iran in 2008. Afr J Microbiol Res. 2011;5(5):28–32. [Google Scholar]
- 17.Saebi E. Protozoal diseases in Iran. 4. Textbook of Clinical Parasitology; Ayexh: Tehran: 2005. [Google Scholar]
- 18.Luchtel DL, Lawrence W, Dewalle FB. Electron microscopy of Giardia lamblia cycts. Applied Env Microbiol. 1980;40(4):821–32. doi: 10.1128/aem.40.4.821-832.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertrand I, Albertini L, Schwartzbrod J. Comparison of two target genes lamblia in human feces by PCR and PCR-Restriction Fragment Length Polymorphism. J Clin Microbiol. 2005;43(12):5940–4. doi: 10.1128/JCM.43.12.5940-5944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fallah E, Nahavandi KH, Jamali R, Mahdavi B, Asgharzadeh M. Genetic characterization of Giardia intestinalis strains from patients having sporadic giardiasis by using PCR assay. J Med Sci. 2008;4:1727–48. [Google Scholar]
- 21.Babaei Z, Oormazdi H, Akhlaghi A, Rezaie S, Razmjou1 E, Soltani- Arabshahi SK, et al. Molecular characterization of the Iranian isolates of Giardia lamblia:application of the glutamate dehydrogenase gene. Iran J Public Health. 2008;37(2):75–82. [Google Scholar]
- 22.Boontanom P, Mungthin M, Tan-ariya P, Naaglor T, Yoova L. Epidemiology of giardiasis and genotypic characterization of Giardia duodenalisin preschool children of a rural community, central Thailand. Trop Biomed. 2011;28(1):32–9. [PubMed] [Google Scholar]
- 23.Sulaiman IM, Fayer R, Bern C, Gilman RH, Trout JM, Schantz PM et al. Triose phosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis. Emerg Infect Dis. 2003;9(11):1444–52. doi: 10.3201/eid0911.030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalir-Naghadeh B, Tavassoli M, Hatefinia AZ. Study of epidemiologic measures of association between Giardia sp. infection with occurrence of diarrhea in calves. J Vet Res. 2008;62(6):363–66. [Google Scholar]


