Abstract
Background
Heartworm (Dirofilaria immitis) is mosquito-borne filarial nematode capable of causing serious cardiopulmonary disease in canines and felines, and pulmonary dirofilariasis in man. This research was conducted with the objectives of determining the incidence and assessing possible risk factors of canine heartworm in the southeast of Iran.
Methods
From October 2012 to September 2013, blood samples from 87 dogs from Zabol area in Sistan and Baluchestan and 33 dogs from Bam area in Kerman Province were examined for detection of Dirofilaria immitis using modified knott test and serology.
Results
Out of 120 dogs, 29 (24.2%; 95%CI: 16.6-31.8%) were positive, serologically. The overall seroprevalence of D. immitis in dog in Zabol and Bam was 27.5% (95% CI: 24.7-32.5%) and 15.15% (95% CI: 12.3-20.7%), respectively. 28.8% of stray dogs and 20.6% of housed dogs in the study areas were seropositive. Seroprevalence of D. immitis was not significantly different between stray and housed dogs (P = 0.295). Investigation of seasonal dynamic of infection with D. immitis in stray and housed dog showed that the proportion of infected dog in spring and summer was greater than colder season (autumn and winter) which was not significant. The prevalence of infection with D. immitis in >5 years old stray dogs (53.8%) was greater than other age categories while in housed dogs infection rate was greater in 3-5 years old (27.3%).
Conclusion
It is important to point out the increased incidence of canine heatrworm in Iran. In order to stop the spread of canine heartworm, preventive measures must be taken now.
Keywords: Dirofilaria immitis, Prevalence, Dog, Iran
Introduction
Dirofilaria immitis, the causal agents of cardiopulmonary, affect canine, feline and human populations with an increasing incidence in temperate and tropical areas of the world. Adult worm lives in the pulmonary artery of dogs and cats, resulting in the production of blood-circulating microfilariae in dogs, while amicrofilaremic infections are common in felines. In humans, worm cannot reach maturity, and preadult worm is responsible for pulmonary dirofilariosis (1). Cardiopulmonary dirofilariosis caused by adult worms and microfilariae in dog usually displays a progressive damage of the pulmonary blood vessels, parenchyma, and the right side of the heart. Alterations initially occur in pulmonary arteries, with pathological relaxation of the artery wall, endarteritis and perivascular inflammation, all key features leading towards the chronic pathology. These alterations have been attributed to both immunopathological and mechanical events elicited by the parasite. In human dirofilariosis, the development of pulmonary is mainly attributed to the inflammatory reaction against dying worms that cannot reach maturity in this host (2). The presence of infected dogs in which adult Dirofilaria survive for years producing microfilariae, and the adequate environmental conditions for the development of vector populations are key factors for the distribution of dirofilariosis in specific geographical areas (3). Since some mosquito species transmitting dirofilariosis feed indiscriminately on different animal species, humans, the occurrence of D. immitis in canine populations imply a risk of infection for the feline, and human populations in areas were canine dirofilariosis is found (4). Until 1999, most epidemiological studies in dogs and cats and reports of human cases were concentrated in the United States, Japan, Mediterranean countries of Europe, Russia and Australia in which this disease was considered a health problem, from both the veterinary and medical point of view. In the past ten years, D. immitis from dog have been detected with increasing frequency in countries considered non-endemic until now (5).
The prevalence and spread of heartworm infection in Iran has been studied by several researchers (6-17). The disease is diagnosed mainly in the northern and northwestern provinces (Gilan, Mazandaran, Golestan, East Azerbaijan, West Azerbaijan, Ardebil) with scattered reports from Tehran and Garmsar city in the central, shiraz and Kerman city in the south and Ahvaz city in the southwestern of country but not in Khorasan Razavi Province where until now only Acanthocheilonema (previously Dipetalonema) reconditum has been found in dogs (18) (Fig.1). An increasing number of cases are now being diagnosed in Northern Province such as Gilan. The area of highest prevalence values for dogs is along the Caspian Sea in northern of Iran, from where the first observation of the worm was made at necropsy of stray dogs in 1969 (6, 15). However, the spread of vectors of D. immitis in Iran could enhance the risk of transmission from dogs to humans.
Fig 1.
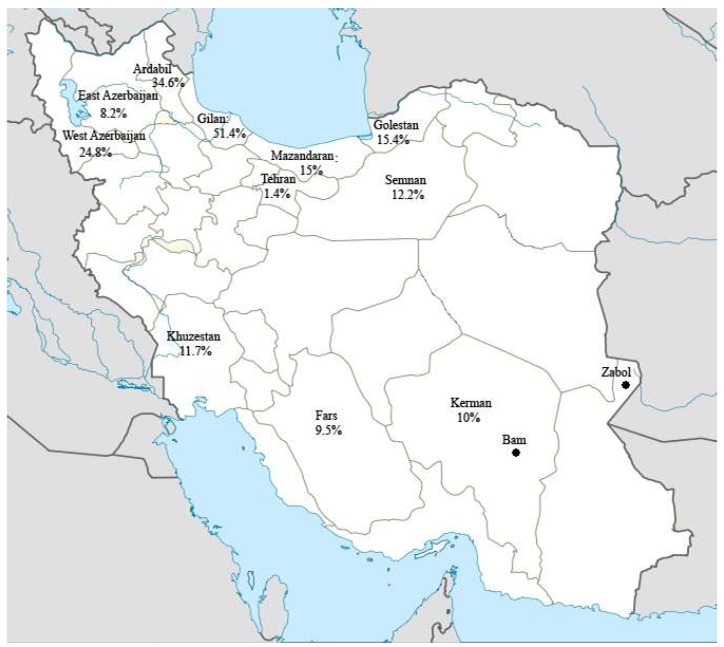
Geographical distribution of canine heartworm infection in Iran with showing investigated area in this study
Human infection with D. immitis has been reported in many countries of the world and is possible wherever the parasite is endemic in dogs. Retrospectively reviews of human dirofilariosis in the world, indicate that around 300 cases of pulmonary dirofilariosis have been reported to date. Most cases of human pulmonary dirofilariosis have been detected in the United States and Japan (19, 20). Due to the difficulty of diagnosing pulmonary dirofilariasis, published case reports likely do not reflect the true risk that D. immitis poses for human health. Furthermore, D. repens has been reported to infect occasionally humans. The public health significance of this species depends largely on its geographic distribution. For instance, in Europe, the main concern is about D. repens.
Recently, Fok (21) reviewed human dirofilariosis in Hungary and concluded that all the autochthonous infections were caused by D. repens. The vast majority of cases of D. repens infection in humans report the detection of nodule in the tissues. Indeed, ‘aberrant’ localization of D. repens has also been reported in humans. In spite of high prevalence of canine heartworm in Iran, human pulmonary dirofilariosis was not reported in the country yet, however, human subcutaneous and orbital and periocular dirofilariasis due to D. repens has been reported (22-25).Absence of human pulmonary dirofilariosis in Iran may be due to lack of available human serological test and patients must be undergoing invasive procedures to differentiate heartworm from other more serious diseases. It should be noted that human heartworm infection is incidental and is typically not associated with severe clinical disease. The authors concluded that a subpleural, non-calcified pulmonary nodule in the appropriate clinical and epidemiological setting should alert the clinician to the possibility of Dirofilaria infection and that human pulmonary dirofilariois should be considered in the differential diagnosis of pulmonary nodules. Noticeably, human cases have been reported mainly in areas of high canine prevalence, highlighting the importance of heartworm testing and chemoprophylaxis in all dogs to reduce transmission.
Considering aspects related to public health, study of the prevalence of canine dirofilariosis should therefore, be a continuous task, with the most relevant aim being the establishment of control measures. More interesting and informative than the prevalence of canine heartworm is the evolution of the frequency with which new area is detected. Taking all of this into account, the objective of the present study was to determine the incidence of canine heartworm in the southeastern of Iran, based on modified knott test and serology and to assess the risk factors associated with the presence of heartworm disease.
Materials and Methods
This study was conducted in two different regions in the southeastern of Iran. From October 2012 to September 2013, blood samples from 120 dogs from Zabol area (87 dogs) in Sistan and Baluchestan and Bam area (33 dogs) in Kerman Province were used for detection of D. immitis. Approximately 3-5 ml of blood was withdrawn from the cephalic vein of each dog, collected in sodium citrate vacuum tubes and stored under refrigeration (+4°C) in local laboratories.
All blood samples were analyzed for the presence of peripheral blood microfilariae using the modified knott test and for circulating D. immitis antigens using a commercial kit according to manufacturers’ instructions (SnapTM Canine Heartworm PF, IDEXX Laboratories Inc., MA, USA). Two dogs that had died spontaneously from heartworm disease in Zabol area were used for tissue localization of parasite.
Subsequent identification to the species level was based on morphological and morphometric characteristics (26). In order to evaluate the role of different risk factors for infection, sex, age and season of inspection of these was animals were recorded in a sheet.
Association of prevalence a of antibody against D. immitis with sex, season and age categories was evaluated using chi square test with SPSS software version 16 and P< 0.05 was considered as significant. The agreement between serological and modified knott test was performed using Kappa test.
Results
Out of 120 dogs, 29 (24.2%; 95%CI: 16.6-31.8%) were positive, serologically. The overall seroprevalence of D.immitis in dog in Zabol and Bam were 27.5% (95% CI: 24.7-32.5%) and 15.15% (95% CI: 12.3-20.7%), respectively. Seroprevalence of D. immitis in dog in Zabol and Bam for stray and housed dogs is reported in Table 1. 28.8% of stray dogs and 20.6% of housed dogs in the study areas were seropositive. Seroprevalence of D. immitis was not significantly different between stray and housed dogs (P=0.295).
Table 1.
Prevalence and 95% confidence interval of antibody against D. immitis in stray and housed dogs of Zabol and Bam district
| Area | Type | No. of animal tested | Seropositive, N (%) | 95% confidence interval |
|---|---|---|---|---|
| Zabol | ||||
| Stray | 38 | 13 (34.2) | 19.2-49.2 | |
| Housed | 49 | 11 (22.4) | 10.8-34.1 | |
| Total | 87 | 24 (27.5) | 24.4-32.5 | |
| Bam | ||||
| Stray | 14 | 2 (14.3) | 0-32.6 | |
| Housed | 19 | 3 (15.8) | 0-32.2 | |
| Total | 33 | 5 (15.2) | 12.3-20.7 |
Based on modified knott test 6 (11.5%) and 4 (5.9%) dogs were positive in stray and housed dogs, respectively. There was a moderate agreement between serology and modified knott test (Kappa = 0.42). Additionally, necropsy finding in two infected dogs in Zabol area revealed presence of adult worm of D. immitis in left ventricle.
Investigation of seasonal dynamic of infection with D. immitis in stray and housed dog showed that the proportion of infected dog in spring and summer was greater than colder season (autumn and winter) which was not significant. The prevalence of infection with D. immitis in >5 years old stray dogs (53.8%) was greater than other age categories while in housed dogs infection rate was greater in 3-5 years old (27.3%) (Table 2). In addition, the infection rate between male (33.3%) and female (24%) stray dogs did not show significant difference (Table 2). In housed dogs, there were no significant differences in prevalence of infection with D. immitis between male (22.5%) and female (17.9%) (Table 2).
Table 2.
Univariate analysis showing the relationship between sex, season and age categories with seroprevalence of D. immitis in stray (N= 52) and housed dogs (N=68)
| Stray dogs | Housed dogs | ||||||
|---|---|---|---|---|---|---|---|
| Variables | Levels | No. of animal tested | Seropositive, N (%) | Chi- square test, P-value | No. of animal tested | Seropositive N (%) | Chi- square test, P-value |
| Sex | |||||||
| Male | 27 | 9 (33.3) | 0.458 | 40 | 9 (22.5) | 0.641 | |
| Female | 25 | 6 (24) | 28 | 5 (17.9) | |||
| Season | |||||||
| Spring | 15 | 5 (33.3) | 0.905 | 16 | 3 (18.8) | 0.554 | |
| Summer | 12 | 4 (33.3) | 19 | 6 (31.6) | |||
| Autumn | 12 | 3 (25) | 18 | 3 (16.7) | |||
| Winter | 13 | 3 (23.1) | 15 | 2 (13.3) | |||
| Age category | |||||||
| 1-2 | 14 | 3 921.4) | 0.135 | 17 | 2 (11.8) | 0.533 | |
| 2-3 | 12 | 3 (25) | 19 | 3 (15.8) | |||
| 3-5 | 13 | 2 (15.4) | 11 | 3 (27.3) | |||
| >5 | 13 | 7 (53.8) | 21 | 6 (18.6) | |||
Discussion
The epidemiological situation of dirofilariosis is currently undergoing accelerated changes. In spite of efforts made to prevent and control the infection in dogs, canine dirofilariosis is rising in endemic areas as well as spreading into new areas reported as dirofilariosis-free until now (5, 6). Consequently, an increasing number of human and feline dirofilariosis cases are currently being reported in both endemic and neighboring areas where this disease was not previously detected (5). Moreover, a renewed interest in this zoonosis is also demonstrated by the worldwide reports of D. immitis in their different hosts in the past ten years, with new reports of human cases concentrated in Eastern Europe and the Middle East (1-3).
Our examination shows that D. immitis is present in the both area. Our results in Bam area are in agreement with previous study from Kerman province (13), while in Zabol area this is for the first time that D. immitis is identified. Canine heartworm has become widespread in many parts of the world, and its range varies between 0.24% and more than 50% in different areas of the world (27). Comparatively, infection rate of canine heartworm in Iran was ranged from 1.4%-51.42% (7-16). Several factors might be responsible for such an increase and for the change in the distribution of canine dirofilariasis. One of these factors is the spread of animal dirofilariasis into areas previously considered non-endemic. This fact is attributed mainly to climatic changes that facilitate the introduction of competent vectors in areas in which they were not previously present and to the lack of control measures in animal reservoirs. Another important point to consider is the role of wildlife reservoirs in perpetuating heartworm transmission. Considerably, D. immitis have been reported from wildlife carnivores such as jackals, foxes, wolves in Iran (17). These wildlife reservoirs could constitute a significant source of infection for dogs and humans, especially in areas where reservoir density is high.
Based on our data, the age of the dog was found to be an important risk factor associated with D.immitis infection. The result of current study revealed that infection rate in >5 years old stray dogs is more prevalent than younger animals. Similar findings were reported previously by several researchers (10, 12, 15, 28). This might be associated with the higher possibility of the biting of host by intermediate host so that adult animals have larger infection rates and the highest prevalence.
The present study indicated that male dogs are more susceptible to D. immitis infection than female, which was not significant. Finding non-significant difference in prevalence by sex among dogs is in agreement with other investigators (10, 12, 15, 28, 29). This difference could be confounded by keeping place so that most of male dogs are kept outdoors for their use to defend safety and property.
The seasonal dynamics of canine heatrworm showed that prevalence was highest in spring with a remarkable decline during the colder seasons that this difference was not significant. Due to the necessity of the intermediate host in dirofilariosis, influencing epidemiology of the intermediate host, determine epidemiology of the parasite indirectly. Moisture and moderate temperature is considered an important factor in determining the survival and availability of mosquitoes. Thus, the relatively higher record of canine heatrworm during spring could also be because the survival and development of intermediate host is favored by moderate temperature and high humidity. Moreover, development of D. immitis to the infective stage (L3) in mosquitoes occurs at a rate that is dependent on ambient temperature, and development may not occur at a threshold temperature below 14 °C (30). Importantly, the model of heartworm seasonality can be used for timing of heartworm chemoprophylaxis and scheduling of diagnostic testing.
As observed by our study in canine dirofilariasis, there was a moderate agreement between serology and modified knott test. It should be noted that the modified knott test is invaluable for identification of parasites when there is no microfiler. Serology is an alternative to this method. In spite of the small number of worms that cause canine infections, there is a considerable antibody response, which can be used for diagnosis. Different antigenic complexes and molecules derived from Dirofilaria spp have been used in ELISA or Western blot (31).
Human and animal reservoir population dynamics and climate change could facilitate the introduction of new competent Dirofilaria vectors in specific regions and this could be one cause for the epidemiological modifications. Besides, in regards to vector competence, studies on the variation of Dirofilaria transmission capacity from different haplotypes of a given mosquito species should be undertaken, since recent data have pointed out this possibility in Culex pipiens (32).
The changing geographical distribution of dirofilariosis could be influenced by several factors, including new human and pet population dynamics and global warming, among others. In this respect, both the use of GIS and RS-based techniques, and potentially the new serologically based epidemiological tools, could contribute to building more efficient epidemiological prediction models. From the applied point of view, an adequate knowledge of the epidemiology of canine heartworm will open the pathway to designing new strategies for the control of this zoonosis.
Conclusion
The geographic range of D. immitis will almost assuredly continue to expand with the effects of global warming, as more habitats suitable for the introduction and establishment of vector mosquitoes are created. In order to stop the spread of canine heartworm, preventive measures must be taken. Furthermore, given the high frequency of travel within and between nations for business and leisure, exposure and infection at locations other than the primary residence are feasible. This further supports the recommendation of routine heartworm testing and prophylaxis in dogs, with the aim of protecting people as well as the dogs themselves.
Acknowledgments
This study was supported by Shahid bahonar University of Kerman and Ferdowsi university of Mashhad. The authors declare that there is no conflict of interests.
Reference
- 1.Simo′n F, Genchi C. Dirofilariosis and other zoonotic filarioses: An emerging public health problem in developed countries. Res Rew Parasitol. 2000;60:1–16. [Google Scholar]
- 2.McCall JW. Heartworm disease in animals and humans. Adv Parasitol. 2008;66:193–285. doi: 10.1016/S0065-308X(08)00204-2. [DOI] [PubMed] [Google Scholar]
- 3.Genchi C. Is heartworm disease really spreading in Europe? Vet Parasitol. 2005;133:137–148. doi: 10.1016/j.vetpar.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Simo′n F. What is happening outside North America regarding human dirofilariasis? Vet Parasitol. 2005;132:181–189. doi: 10.1016/j.vetpar.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 5.Simon F, Morcho R, Gonza′ lez-Miguel J, Marcos-Atxutegi C, Lucas MS. What is new about animal and human dirofilariosis? Trends in Parasitol. 2009;25(9):404–409. doi: 10.1016/j.pt.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Sadighian A. Helminth Parasites of stray dog and Jackal in Shahsavar area, Caspian region, Iran. J Helminthol. 1969;2:372–374. [PubMed] [Google Scholar]
- 7.Jafari S, Gaur SNS, Khaksar Z. Prevalence of Dirofilaria immitis in dogs of Fars province of Iran. J Appl Anim Res. 1996;9:27–31. [Google Scholar]
- 8.Bokaie S, Mobed I, Mohebali M, Hoseini SH, Nadim A. A study of dirofilariasis prevalence in dogs in Meshkin- Shahr area, northwest Iran. J Fac Vet Med. 1998;53:23–29. in Persian with English abst. [Google Scholar]
- 9.Meshgi B, Eslami A. Study on filariasis of sheepdogs around of Tehran. J Fac Vet Med Tehran Univ. 2001;55:53–56. [Google Scholar]
- 10.Meshgi B, Eslami A, Ashrafi Helan J. Epidemiological survey of blood filariae in rural and urban dogs of Tabriz. J Fac Vet Med Tehran Univ. 2002;57:59–63. in Persian. [Google Scholar]
- 11.Ranjbar-Bahadori SH, Hekmatkhah A. A study on filariosis of stray dogs in Garmsar, Iran. J Vet Res. 2007;62:83–86. [Google Scholar]
- 12.Razi Jalali MH, Alborzi A, Avizeh R, Mosallanejad B. A study on Dirofilaria immitis in healthy urban dogs from Ahvaz, Iran. Iran J Vet Res. 2010;11:357–362. [Google Scholar]
- 13.Akhtardanesh B, Radfar MH, Voosough D, Darijani D. Seroprevalence of canine heartworm disease in Kerman, southeastern Iran. Comp Clin Pathol. 2011;20:573–577. [Google Scholar]
- 14.Ranjbar-Bahadori SH, Veshgini A, Shirani D, Eslami A, Mohieddin H, Shemshadi B, Masooleh R. Epidemiological Aspects of Canine Dirofilariasis in the North of Iran. Iran J Parasitol. 2010;(6):73–80. [PMC free article] [PubMed] [Google Scholar]
- 15.Malmasi A, Hosseini SH, Aramoon M, Bahonar A, Seifi HA. Survey of canine Dirofilaria immitis infection in Caspian provinces of Iran. Iran J Vet Res. 2011;12(4):340–344. [Google Scholar]
- 16.Javadi SH, Hanifeh M, Tavassoli M, Dalir- Naghadeh B, Khezri A, Hadian M. Dirrofilariasis in Shepherd Dogs of High Altitudes Areas in West Azerbaijan- Iran. Vet Res Forum. 2011;2(1):53–57. [Google Scholar]
- 17.Azari-Hamidian S, Yaghoubi Ershadi MR, Javadian E, Moubedi I, Abai MR. Review of Dirofilariasis in Iran. J Med Fac Guilan Univ Med Sci. 2006;15(60):102–113. [Google Scholar]
- 18.Razmi Gh. Study on situation of infection to dogs of Mashhad to types of filaria. J Fac Vet Med Tehran Univ. 1999;54(1):5–7. in Persian. [Google Scholar]
- 19.Theis JH. Public health aspects of dirofilariasis in the United States. Vet Parasitol. 2005;133:157–180. doi: 10.1016/j.vetpar.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Pampiglione S. Dirofilarial human cases in the Old World, attributed to Dirofilaria immitis: a critical analysis. Histopathol. 2009;54:192–204. doi: 10.1111/j.1365-2559.2008.03197.x. [DOI] [PubMed] [Google Scholar]
- 21.Fok E. The importance of dirofilariosis in carnivores and humans in Hungary, past and present. In: Genchi C, Rinaldi L, Cringoli G, editors. “Dirofilaria immitis and Dirofilaria repens in dog and cat and human infections”. Rolando Editore; Napoli, Italy: 2007. pp. 181–188. [Google Scholar]
- 22.Ashrafi K, Golchai J, Geranmayeh S. Human Subcutaneous Dirofilariasis due to Dirofilaria (Nochtiella) repens: Clinically Suspected as Cutaneous Fascioliasis. Iran J Public Health. 2010;39(1):105–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Jamshidi A, Jamshidi M, Mobedi I, Khosroara M. Periocular dirofilariasis in a young woman: a case report. Korean J Parasitol. 2008;46(4):265–7. doi: 10.3347/kjp.2008.46.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Negahban S, Daneshbod Y, Atefi S, Daneshbod K, Sadjjadi SM, Hosseini SV, Bedayat GR, Abidi H. Dirofilaria repens diagnosed by the presence of microfilariae in fine needle aspirates: a case report. Acta Cytol. 2007;51(4):567–70. doi: 10.1159/000325796. [DOI] [PubMed] [Google Scholar]
- 25.Tavakolizadeh S, Mobedi I. Orbital dirofilariasis in Iran: a case report. Korean J Parasitol. 2009;47(4):397–9. doi: 10.3347/kjp.2009.47.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowman DD. Georgis’ parasitology for veterinarians. 9. St. Louis: Saunders, Elsevier; 2009. pp. 318–319. [Google Scholar]
- 27.Genchi C. Epidemiology and prevention of Dirofilaria infections in dogs and cats. In: Genchi C, Rinaldi L, Cringoli G, editors. Dirofilaria immitis and D. repens in dog and cat and human infections. 2007. pp. 145–161. [Google Scholar]
- 28.Reifur L, Thomaz-Soccol V, Montiani- Ferreira F. Epidemiological aspects of filariosis in dogs on the coast of Parana state, Brazil: with emphasis on Dirofilaria immitis. Vet Parasitol. 2004;122:273–286. doi: 10.1016/j.vetpar.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Simsek S, Utuk AE, Koroglu E, Rishniw M. Serological and molecular studies on Dirofilaria immitis in dogs from Turkey. J Helminthol. 2008;82:181–186. doi: 10.1017/S0022149X0896079X. [DOI] [PubMed] [Google Scholar]
- 30.Fortin JF, Slocombe JOD. Temperature requirements for the development of Dirofilaria immitis in Aedes triseriatus and Ae. vexans. Mosq News. 1981;41:625–633. [Google Scholar]
- 31.Lee AC1, Bowman DD, Lucio-Forster A, Beall MJ, Liotta JL, Dillon R. Evaluation of a new inclinic method for the detection of canine heartworm antigen. Vet Parasitol. 2011;177(3-4):387–91. doi: 10.1016/j.vetpar.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 32.Morchon R, Bargues MD, Latorre JM, Melero-Alcibar R, Pou-Barreto C, Mas-Coma S, Simon F. Culex pipiens is a natural vector of Dirofilaria immitis in an endemic area of Western Spain. Vect Born Zoon Dis. 2007;7:653–658. doi: 10.1089/vbz.2007.0124. [DOI] [PubMed] [Google Scholar]


